Anti‐IL5‐Therapien für Asthma
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel, double‐blind RCT with a 16‐week treatment phase | |
| Participants | 315 participants (42 male) with moderate‐severe asthma, with airway reversibility, blood eosinophilia, ACQ score of at least 1.5, and taking ICS
| |
| Interventions | IV infusion of reslizumab 0.3 mg/kg, reslizumab 3.0 mg/kg, or placebo once every 4 weeks (total of 4 doses) | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | 68 locations across 13 countries Funded by Teva Branded Pharmaceutical Products R&D, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) | Low risk | Slightly more withdrawals in placebo group (20/105, 19%) than treatment arms (12‐17%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial run over 48 weeks | |
| Participants | 1204 participants with symptomatic asthma were randomised to 1 of 3 groups (benralizumab 30 mg 4 weeks, benralizumab 30 mg 8 weeks, or placebo)
| |
| Interventions | SC benralizumab 30 mg/mL every 4 weeks or every 8 weeks versus placebo | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Multi‐centre trial in 374 centres from 17 countries Funded by AstraZeneca and Kyowa Hakko Kirin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was assigned a unique enrolment number and randomisation code by an interactive web‐based voice response system |
| Allocation concealment (selection bias) | Low risk | The identity of the treatment allocation was not made available to the participants, investigators involved in participant treatment or clinical assessment, or study funder |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind (participant, caregiver and investigator) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawal rates were relatively low (10.1%‐12.8%) |
| Selective reporting (reporting bias) | Low risk | Unless otherwise specified, all results were presented for participants with baseline blood eosinophilia |
| Methods | Randomised, controlled, double‐blind, dose‐ranging trial | |
| Participants | 606 participants with uncontrolled asthma randomised and 535 completed
| |
| Interventions | 6 arms: benralizumab 2 mg or benralizumab 20 mg or benralizumab 100 mg or placebo delivered by 2 SC injections every 4 weeks for the first 3 doses (weeks 1, 4, and 8), then every 8 weeks (weeks 16, 24, 32, and 40) | |
| Outcomes | Primary outcomes
Secondary outcomes in eosinophilic individuals
| |
| Notes | 52‐year multi‐national study with sites in 10 countries. The study protocol was developed by MedImmune and the corresponding author. The investigators collected and had full access to all study data, which were analysed by the funding source. The analysis was done solely by MedImmune; however, study authors helped determine which analyses were done and could request further ad‐hoc analyses. The report was written by the study authors with a medical writer funded by the funding source. The corresponding author had final responsibility for decision to submit for publication. Funding: MedImmune | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive web/voice‐response system for random assignment |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by the vendor systems and no study personnel or site had access to the system. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, treating physicians, study investigators, and study statisticians were masked to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rates were even across groups |
| Selective reporting (reporting bias) | Low risk | Results for most but not all listed primary and secondary outcomes were reported (e.g. symptoms score, AQLQ – shown in supplementary material in graphs only) |
| Methods | Double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 489 participants with moderate‐severe asthma (medium dose of ICS, inadequate control ACQ ≥ 1.5, and at least 1 exacerbation in the past 12 months)
| |
| Interventions | IV infusion of reslizumab 3 mg/kg or matching placebo every 4 weeks (13 doses with last dose in week 48) | |
| Outcomes | Primary outcomes (per protocol)
Secondary outcomes (per protocol):
| |
| Notes | 128 clinical research centres. The research was funded by Teva Branded Pharmaceutical Products R&D. Teva employees were involved in the study design, data collection and analysis, and in the writing of this manuscript. All study authors had full access to all study data and had final responsibility for the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with use of interactive response technology with computerised central randomisation. |
| Allocation concealment (selection bias) | Low risk | The funder’s clinical personnel involved in the study were also masked to the study drug identity until the database was locked for analysis and the treatment assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rates were relatively low and even across the groups (11%‐14%) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were reported. |
| Methods | Double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 464 participants with moderate‐severe asthma (medium does of ICS, inadequate control ACQ ≥1.5 and at least 1 exacerbation in the past 12 months).
| |
| Interventions | IV infusion of reslizumab 3 mg/kg or matching placebo every 4 weeks (13 doses with last dose in week 48) | |
| Outcomes | Primary outcomes (per protocol):
Secondary outcomes (per protocol):
| |
| Notes | Funding: Teva Branded Pharmaceutical Products R&D. Teva employees were involved in the study design, data collection and analysis, and in the writing of this manuscript. All study authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with use of interactive response technology with computerised central randomisation. |
| Allocation concealment (selection bias) | Low risk | The funder’s clinical personnel involved in the study were also masked to the study drug identity until the database was locked for analysis and the treatment assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rates were relatively low and even across the groups (11%‐14%) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were reported |
| Methods | Multicentre, placebo‐controlled, double‐blind, parallel‐group study | |
| Participants | 551 participants with severe eosinophilic asthma Males (%): mepolizumab 125 (46); placebo, 101 (36)
| |
| Interventions | Mepolizumab 100 mg SC every 4 weeks for a period of 24 weeks (total of 6 doses) along with their respective standard care of treatment, versus placebo (0.9% sodium chloride) SC every 4 weeks for a period of 24 weeks (total of 6 doses) along with their respective standard care of treatment | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Funding: GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an interactive voice‐response system and a centralised, computer‐generated, permuted‐block design of block size six |
| Allocation concealment (selection bias) | Low risk | Participants, investigators, other site staff, and the entire study team including those assessing outcomes data were masked to treatment assignment. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and investigators remained masked to treatment assignment during the study. |
| Incomplete outcome data (attrition bias) | Low risk | In the treatment arm 5 participants were withdrawn from the study: 2 withdrew consent, 2 experienced an adverse event |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
| Methods | Parallel, double‐blind | |
| Participants | 496 participants with moderate‐severe asthma (based on at least medium‐dose ICS, inadequate control ACQ ≥ 1.5)
| |
| Interventions | IV reslizumab 3.0 mg/kg or placebo once every 4 weeks (total of 4 doses) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | 66 study locations across the USA Funding: Teva Branded Pharmaceutical Products R&D, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double blind |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts comparable in each group (16/98, 16%, placebo vs 58/398, 15%, reslizumab) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported with numbers, except blood eosinophil counts only shown as a chart |
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | 1306 participants with moderate‐severe (medium‐high‐dose ICS + LABA, ≥ 2 asthma exacerbations last 12 months, FEV1 < 80% predicted), ACQ‐6 ≥ 1.5 at enrolment
| |
| Interventions | 56 weeks (final follow‐up at 60 weeks). SC benralizumab 30 mg every 4 weeks for 56 weeks or every 4 weeks for 3 doses then 8 weeks thereafter for 56 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Funding: AstraZeneca and Kyowa Hakko Kirin. 303 clinical research centres in 11 countries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using an interactive web‐based voice‐response system. Randomisation was stratified by ICS dosage at enrolment (high or medium), geographic region, age group (adult or adolescent), and peripheral blood eosinophil count at enrolment (< 300 cells per μL or ≥ 300 cells per μL) |
| Allocation concealment (selection bias) | Low risk | The study investigator assigned randomisation codes sequentially in each stratum as participants became eligible for randomisation, until each stratum was full |
| Blinding of participants and personnel (performance bias) | Low risk | To preserve blinding, participants and study centre staff were masked to treatment allocation, placebo solution was visually matched with benralizumab solution, and both placebo and benralizumab were provided in accessorised (needle guards and finger phalanges), prefilled syringes. |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rates were relatively low: placebo 11.1% (49/440); benralizumab 30 mg every four weeks 9.6% (41/425); benralizumab 30 mg every eight weeks 13.4% (59/441) |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | 61 participants had refractory eosinophilic asthma and a history of recurrent severe exacerbations.
| |
| Interventions | Intravenous mepolizumab (750 mg) versus matched placebo (150 mL of 0.9% saline) at monthly intervals for 1 year | |
| Outcomes | Reported as: "[P]rimary outcome measure was the number of severe exacerbations per participant during the 50‐week treatment phase. Secondary outcomes included a change in asthma symptoms, scores on the Asthma Quality of Life Questionnaire (AQLQ, in which scores range from 1 to 7, with lower values indicating more severe impairment and a change of 0.5 unit considered to be clinically important), forced expiratory volume in 1 second (FEV1) after use of a bronchodilator, airway hyperresponsiveness, and eosinophil counts in the blood and sputum." | |
| Notes | Single centre trial conducted at Institute for Lung Health, Leicester, UK Supported by GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as: "Stratified randomisation with use of the minimisation method, which was performed by an independent clinician. Participants were randomly assigned with the use of the minimisation method to receive 12 infusions of either 750 mg of mepolizumab delivered intravenously or matched placebo (150 mL of 0.9% saline) at monthly intervals between visits 3 and 14. The criteria used for minimisation were the frequency of exacerbations in the previous 12 months, the baseline eosinophil count in the sputum and the number of participants taking oral corticosteroids." |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as double blind |
| Blinding of outcome assessment (detection bias) | Low risk | Reported as double blind |
| Incomplete outcome data (attrition bias) | Low risk | Reported as: "A total of 61 of the 63 participants ( one required and operation and one withdrew consent) who were screened started treatment and constituted the modified intention‐to‐treat population. Thirty‐two participants were randomly assigned to receive placebo. Overall, 94.9% of treatment visits were completed. Participants who withdrew completed a mean of 4.6 treatment visits (38.3%)." |
| Selective reporting (reporting bias) | Low risk | No apparent indication of reporting bias |
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled, phase 3 efficacy and safety study | |
| Participants | 13 participants with uncontrolled asthma taking medium‐dose ICS plus long‐acting beta2 agonist (LABA)
| |
| Interventions | Fixed 30 mg dose of benralizumab every 4 weeks or fixed 30 mg dose of benralizumab, every 4 weeks for the first 3 doses and then every 8 weeks thereafter versus placebo | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Study terminated due to sponsor decision after recruitment of 13 participants. No participant completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported as double blind, but blinding of outcome assessment not specifically described |
| Incomplete outcome data (attrition bias) | High risk | Study terminated due to decision of sponsor after recruitment of 13 participants. No reason given for decision to terminate |
| Selective reporting (reporting bias) | High risk | Study terminated due to decision of sponsor after recruitment of 13 participants. No reason given for decision to terminate. Original secondary outcomes listed removed from trial registration. Outcomes could not be incorporated into meta‐analysis |
| Methods | Randomised, double‐blind, double‐dummy, phase 3 study | |
| Participants | 576 participants with recurrent asthma exacerbations and evidence of eosinophilic inflammation despite high doses of inhaled glucocorticoids to 1 of 3 study groups
| |
| Interventions | Mepolizumab in a 75 mg intravenous dose versus mepolizumab in a 100 mg subcutaneous dose versus placebo every 4 weeks for 32 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes:
| |
| Notes | 32‐week treatment intervention, with 1‐6 weeks run‐in and 8‐week follow‐up. Conducted in Baltimore, Middlesex, Ghent, Vancouver, Parma, Marseille and Paris Funding: GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated permuted block schedule |
| Allocation concealment (selection bias) | Low risk | Treatment allocations will be concealed via the RandAll system |
| Blinding of participants and personnel (performance bias) | Low risk | Mepolizumab and placebo were identical in appearance and were administered by a staff member who was unaware of the study group assignments. |
| Blinding of outcome assessment (detection bias) | Low risk | The study drugs were prepared by staff members who were aware of the study group assignments but were not involved in study assessments. |
| Incomplete outcome data (attrition bias) | Low risk | 6% (placebo), 8% (IV), 5% ( SC) did not complete the study |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Methods | Parallel | |
| Participants | 103. 38 males. (age 53.2, 55.6, 51.4, 50.8 Moderate/severe (based on ICS dose (medium/high), exacerbation history, and ACQ ≥ 1.5 on at least 2 occasions) participants also had to demonstrate post‐bronchodilator FEV1 reversibility ≥ 12% and ≥ 200 mL, or a positive response to methacholine challenge (PC20 ≤ 8 mg/mL)
| |
| Interventions | Subcutaneous doses given at weeks 1, 4, 8, 16, 24, 32, 40. Benralizumab 2 mg, 20 mg or 100 mg subcutaneously | |
| Outcomes | Primary outcomes
Secondary outcomes
Exploratory endpoints included blood eosinophil counts. | |
| Notes | 32 sites in South Korea and Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eosinophilic participants were randomised using a central, interactive web‐response system |
| Allocation concealment (selection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Blinding of participants and personnel (performance bias) | Low risk | The study medication was administered … in a blinded fashion |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, no clarification available from study authors |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates relatively high but even across groups (19.2% for placebo vs 16.0%‐23.1% for treatment groups) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Multicentre, double‐blind, placebo‐controlled trial | |
| Participants | 621 participants with severe asthma despite receiving high doses of standard asthma medications
| |
| Interventions | 13 total intravenous infusions of mepolizumab (750 mg), mepolizumab (250 mg), mepolizumab (75 mg) or placebo given every 4 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | 52‐week study conducted at 81 centres in 13 countries (Argentina, Australia, Canada, Chile, France, Germany, South Korea, Poland, Romania, Russia, Ukraine, the UK and the USA) Supported by GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone‐based system and computer‐generated randomly permuted block schedule stratified by whether treatment with OCS was required |
| Allocation concealment (selection bias) | Low risk | Mepolizumab and placebo were prepared by unmasked site staff who were not involved in study assessments |
| Blinding of participants and personnel (performance bias) | Low risk | Mepolizumab and placebo were prepared by unmasked site staff who were not involved in study assessments. Both treatments were identical in appearance and were given to participants by a masked member of the site staff |
| Blinding of outcome assessment (detection bias) | Low risk | Data analysts were masked to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for with information on reasons for having withdrawn. Some participants not included in results due to ‘poor efficacy’ |
| Selective reporting (reporting bias) | Low risk | No apparent indication of reporting bias |
ACQ: Asthma Control Questionnaire; ALT: alanine aminotransferase; Alk Phos: alkaline phosphatase; AQLQ: Asthma Quality of Life Questionnaire; AST: aspartate aminotransferase; ECP: eosinophil cationic protein; ED: emergency department; FeNO: exhaled fraction of nitric oxide; FEV1 : Forced expiratory volume in 1 second; FP: fluticasone propionate; FVC: forced vital capacity; HRQoL: health‐related quality of life; ICS: inhaled corticosteroid; ICU: intensive care unit; IL: interleukin; IQR: interquartile range; IV: intravenous; JACQ: Juniper Asthma Control Questionnaire; OCS: oral corticosteroids; PC20 : histamine provocative concentration causing a 20% drop in FEV1;PEFR: peak expiratory flow rate; SC: subcutaneous; SD: standard deviation; SGRQ: St. George's Respiratory Questionnaire; ULN: Upper Limit of Normal; VC: vital capacity.
aQTc(F): a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle, corrected for the heart rate using Fredericia's formula.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Post‐hoc analysis of observational study | |
| Intervention used in study (cat extract immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Aggregation of two clinical trials | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Focus of trial is on steroid reduction and therefore does not meet our predefined inclusion criteria | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (pavilizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (anti‐IgE antibody e25) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Aggregation of two clinical trials | |
| Not a RCT | |
| Not a RCT | |
| Intervention used in study (rhumab‐25) is not anti‐IL‐5 therapy | |
| Intervention used in study (rhumab‐25) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (soybean oil) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy | |
| Treatment < 16 weeks | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Aggregation of two clinical trials | |
| < 16 weeks in length | |
| Intervention used in study (dupilumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (various foods) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (il‐4ralpha antagonist) is not anti‐IL‐5 therapy | |
| Not a RCT | |
| Participants did not have a diagnosis of asthma (COPD patients) | |
| Intervention used in study (anti‐IL‐13 mab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Not a RCT | |
| The study participants did not have asthma | |
| Treatment period < 16 weeks | |
| The study participants did not have asthma | |
| Not an RCT and endpoints are not applicable as this is a long‐term access programme | |
| Not placebo‐controlled. Single treatment arm only | |
| No placebo arm/single treatment arm and treatment duration < 16 weeks | |
| Participants do not have a diagnosis of asthma, no placebo arm, treatment duration < 16 weeks | |
| Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy | |
| Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy | |
| Treatment duration < 16 weeks in length | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Treatment < 16 weeks | |
| Treatment < 16 weeks | |
| Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study ( anti‐IL‐13) is not anti‐IL‐5 therapy | |
| Intervention used in study ( anti‐tslp) is not anti‐IL‐5 therapy | |
| Intervention used in study (ox40l antagonism) is not anti‐IL‐5 therapy | |
| Intervention used in study (quilizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (ligelizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (ligelizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study is not anti‐IL‐5 therapy | |
| Participants do not have a diagnosis of asthma | |
| Not a randomised, placebo‐controlled trial | |
| Intervention used in study ( jade screen powder) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (quilizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study ( anti‐IL‐13) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Treatment < 16 weeks | |
| Intervention used in study (anti‐cd4) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Treatment < 16 weeks | |
| Treatment < 16 weeks | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Review article, not a RCT | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Not a randomised, placebo‐controlled trial | |
| Combined secondary analysis of two trials: NCT01287039 and NCT01285323 | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (anti‐IgE) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Participants do not have diagnosis of asthma | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Focus of trial is on steroid reduction and therefore does not meet our predefined inclusion criteria | |
| All participants do not have a diagnosis of asthma | |
| Treatment duration < 16 weeks | |
| Non randomised study | |
| Study does not include a placebo arm | |
| Phase 2 study comparing three doses of mepolizumab. This trial does not have a placebo arm | |
| Focus of study was on tolerability, pharmacokinetics and pharmacodynamics of single dose SB‐240563 administered intravenously to Japanese healthy male participants. People with asthma were not included in the study. | |
| This study does not include a placebo group. Multi‐centre, open‐label, long‐term safety study with total sample receiving 100 mg mepolizumab administered subcutaneously (no control group) | |
| This study does not include a placebo group. Multi‐centre, open‐label, long‐term safety study with total sample receiving 100 mg mepolizumab administered subcutaneously (no control group) | |
| Focus of trial is on oral steroid reduction | |
| This study does not include a placebo group. Multi‐center, open‐label, long‐term study of subcutaneously (SC) administered mepolizumab 100 mg in addition to standard of care (SOC), in participants with severe eosinophilic asthma | |
| Not a RCT (an extension study with no placebo arm) | |
| Aim of study is to provide a 'reliable description of the severe asthma patient landscape with respect to the potential eligibility for treatment with mepolizumab, omalizumab, and reslizumab'. No pharmaceutical intervention in study | |
| Not a RCT | |
| Focus of trial is on oral steroid reduction | |
| Not placebo‐controlled ‐ single treatment arm only | |
| Not a RCT | |
| Treatment duration < 16 weeks | |
| Treatment duration < 16 weeks | |
| Treatment duration < 16 weeks | |
| Not a placebo‐controlled trial | |
| Not a placebo‐controlled trial and treatment duration < 16 weeks | |
| No placebo arm/single treatment arm, treatment duration < 16 weeks | |
| Not a RCT | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Treatment < 16 weeks | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (anti‐IL‐9) is not anti‐IL‐5 therapy | |
| Participants do not have a diagnosis of asthma | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Not a RCT | |
| Intervention used in study (anti‐IL‐9) is not anti‐IL‐5 therapy | |
| Intervention used in study (immunotherapy) is not anti‐IL‐5 therapy | |
| Posthoc analysis of Pavord 2012a and Ortega 2014 stratified by prior use of anti‐IgE therapy | |
| Study is not a RCT | |
| An analysis of sera collected from asthma patients enrolled in two clinical studies: NCT00659659 and NCT00783289 | |
| Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy | |
| Study has no placebo arm or clinical endpoints | |
| Aggregation of two clinical trials | |
| Study is not a randomised, placebo controlled trial | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (air/diesel exhaust +/‐ antioxidant) is not anti‐IL‐5 therapy | |
| Intervention used in study (tralokinumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (pneumococcal vaccine) is not anti‐IL‐5 therapy | |
| Not a RCT | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (lebrikizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (pneumococcal vaccine) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (pavilizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (anti‐IL‐13) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (influenza vaccine) is not anti‐IL‐5 therapy | |
| Intervention used in study (dupilumab) is not anti‐IL‐5 therapy | |
| Not a RCT | |
| Intervention used in study (influenza vaccine) is not anti‐IL‐5 therapy | |
| Study predates monoclonal treatments | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Aggregation of two clinical trials | |
| Pharmacometrics assessment of phase IIb data to characterize the exposure‐response relationship with Benralizumab in adults with asthma. | |
| Intervention used in study (itraconazole) is not anti‐IL‐5 therapy | |
| Combined secondary analysis of two trials: NCT01287039 and NCT01285323 | |
| Intervention used in study (golimumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (dupilumab) is not anti‐IL‐5 therapy | |
| Interventionused in study (dupilumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (dupilumab) is not anti‐IL‐5 therapy | |
| Participants do not have a diagnosis of asthma | |
| Participants do not all have diagnosis of asthma | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy | |
| Intervention used in study (omalizumab) is not anti‐IL‐5 therapy |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Mepolizumab and exacerbation frequency in refractory eosinophilic asthma. A randomised, double blind, placebo controlled, parallel group trial |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial |
| Participants | Target recruitment = 60 participants with refractory eosinophilic asthma Principal inclusion criteria
|
| Interventions | Mepolizumab IV Placebo |
| Outcomes | Main objective To investigate whether mepolizumab effectively suppresses the presence of eosinophils in sputum and whether this translates into a fall in the frequency of asthma exacerbations in a cohort of refractory asthmatics who otherwise require a high dose of inhaled corticosteroids and, in some cases, regular oral corticosteroids to control their asthma. Secondary objectives To assess the effects of mepolizumab on:
|
| Starting date | Date of competent authority/ethics committee decision 2005‐11‐16 |
| Contact information | (No contact details listed) Sponsored by University Hospitals of Leicester www.clinicaltrialsregister.eu/ctr‐search/trial/2005‐001932‐61/GB |
| Notes | Non‐commercial |
| Trial name or title | Mepolizumab treatment for rhinovirus‐induced asthma exacerbations (MATERIAL) |
| Methods | Randomised, double‐blind trial |
| Participants | People with mild allergic asthma with viral airway infections Target recruitment = 48 participants Inclusion criteria
Exclusion criteria:
|
| Interventions | 3 monthly intravenous infusions of 750 mg versus 3 monthly intravenous infusions with saline |
| Outcomes | Primary outcome measures
Secondary outcome measures:
|
| Starting date | January 2012 |
| Contact information | Suzanne Bal +31 205668043 [email protected] Koenraad van der Sluijs +31 205668224 [email protected] Principal Investigator: René Lutter, Academisch Medisch Centrum ‐ Universiteit van Amsterdam (AMC‐UvA) |
| Notes | Also known as "MATERIAL" study. Clinicaltrials.gov website notes "The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years." Estimated study completion date March 2014 |
| Trial name or title | A 52‐week double‐blind, placebo‐controlled, parallel‐group efficacy and safety study of reslizumab 110 mg fixed, subcutaneous dosing in patients with uncontrolled asthma and elevated blood eosinophils |
| Methods | Double‐blind, placebo‐controlled, parallel‐group study |
| Participants | 469 participants with unstable asthma Inclusion criteria
|
| Interventions | Reslizumab will be administered subcutaneously in a dose of 110 mg every 4 weeks versus placebo |
| Outcomes | The primary objective of this study is to determine the effect of reslizumab (110 mg) administered subcutaneously every 4 weeks on clinical asthma exacerbations in adults and adolescents with asthma and elevated blood eosinophils who are inadequately controlled on standard‐of‐care asthma therapy. Primary outcome measures
Secondary outcome measures
|
| Starting date | September 2015 |
| Contact information | Study Director: Teva Medical Expert, MD |
| Notes | Estimated study completion date: January 2018 Responsible party: Teva Branded Pharmaceutical Products, R&D Inc. International multicentre study with 200 centres |
| Trial name or title | Cessation versus continuation of long‐term mepolizumab in severe eosinophilic asthma patients |
| Methods | Multi‐center, randomised, double‐blind, placebo‐controlled, parallel‐group study |
| Participants | 300 participants
|
| Interventions | Mepolizumab 100 mg versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2016 |
| Contact information | US GSK Clinical Trials Call Center [email protected] |
| Notes | Estimated study completion date: January 2019 |
| Trial name or title | A randomised, double‐blind, placebo‐controlled, mono‐center study to evaluate the effects of mepolizumab on airway physiology in patients with eosinophilic asthma: the MEMORY Study |
| Methods | Randomised, double‐blind, placebo‐controlled, mono‐centre study |
| Participants | 29 participants with severe eosinophilic asthma Inclusion criteria
|
| Interventions | Mepolizumab 100 mg SC every 4 weeks for 13 injections and placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | November 2015 |
| Contact information | PI Dr. Stephanie Korn, Johannes Gutenberg University Mainz |
| Notes | GlaxoSmithKline collaborator Estimated study completion date August 2018 |
| Trial name or title | A double‐bind, randomised, parallel group, placebo‐controlled multi‐centre study to evaluate the effect of benralizumab on allergen‐induced inflammation in mild, atopic asthmatics |
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study |
| Participants | Estimated enrolment 42 participants with mild atopic asthma Inclusion criteria
|
| Interventions | Benralizumab administered subcutaneously compared with placebo administered subcutaneously Allergen challenge (all participants) |
| Outcomes | Primary outcome measures
Secondary outcome measures
Other outcome measures:
|
| Starting date | October 2016 |
| Contact information | AstraZeneca Clinical Study Information Center 1‐877‐240‐9479 [email protected] |
| Notes | Still recruiting April 2017 Estimated completion date February 2019 |
BDP: beclomethasone dipropionate; CO: carbon monoxide; ECG: electrocardiogram; ED: emergency department; eNO: exhaled nitric oxide; FEV1 : Forced expiratory volume in 1 second; FVC: forced vital capacity; GETE: global evaluation of treatment effectiveness; IC: inspiratory capacity; ICU: intensive care unit; NO: nitric oxide; PC20 : histamine provocative concentration causing a 20% drop in FEV1: RV: residual volume; TLC: total lung capacity;
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.45 [0.36, 0.55] |
| Analysis 1.1 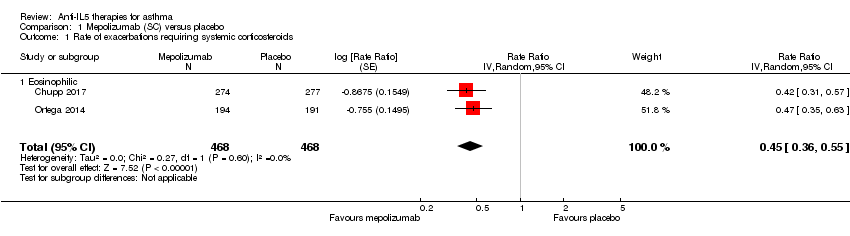 Comparison 1 Mepolizumab (SC) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids. | ||||
| 1.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.45 [0.36, 0.55] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.36 [0.20, 0.66] |
| Analysis 1.2  Comparison 1 Mepolizumab (SC) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission. | ||||
| 2.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.36 [0.20, 0.66] |
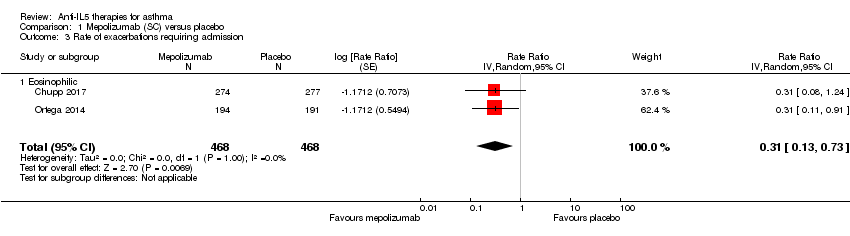
| 3 Rate of exacerbations requiring admission Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.31 [0.13, 0.73] |
| Analysis 1.3  Comparison 1 Mepolizumab (SC) versus placebo, Outcome 3 Rate of exacerbations requiring admission. | ||||
| 3.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.31 [0.13, 0.73] |
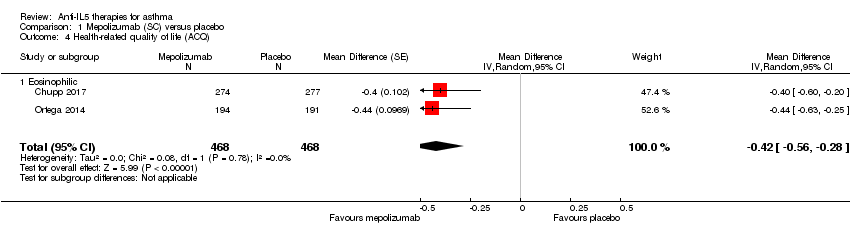
| 4 Health‐related quality of life (ACQ) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | ‐0.42 [‐0.56, ‐0.28] |
| Analysis 1.4  Comparison 1 Mepolizumab (SC) versus placebo, Outcome 4 Health‐related quality of life (ACQ). | ||||
| 4.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | ‐0.42 [‐0.56, ‐0.28] |
| 5 Health‐related quality of life (SGRQ) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | ‐7.40 [‐9.50, ‐5.29] |
| Analysis 1.5 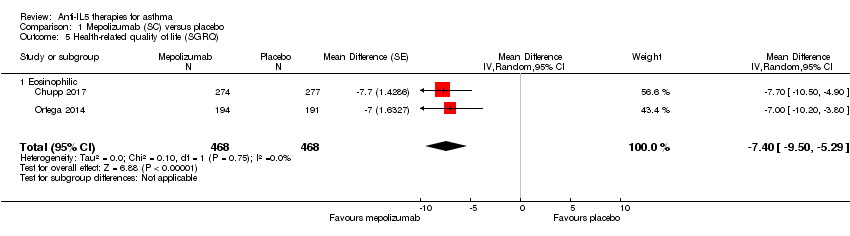 Comparison 1 Mepolizumab (SC) versus placebo, Outcome 5 Health‐related quality of life (SGRQ). | ||||
| 5.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | ‐7.40 [‐9.50, ‐5.29] |
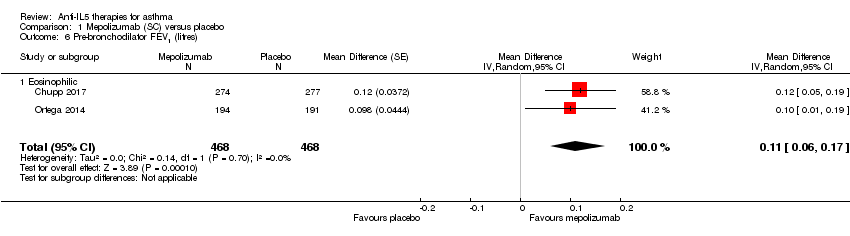
| 6 Pre‐bronchodilator FEV1 (litres) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | 0.11 [0.06, 0.17] |
| Analysis 1.6  Comparison 1 Mepolizumab (SC) versus placebo, Outcome 6 Pre‐bronchodilator FEV1 (litres). | ||||
| 6.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | 0.11 [0.06, 0.17] |
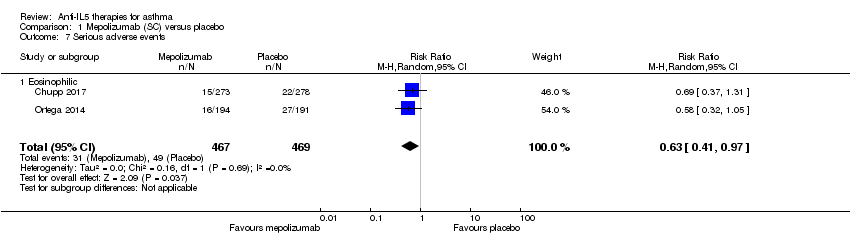
| 7 Serious adverse events Show forest plot | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.97] |
| Analysis 1.7 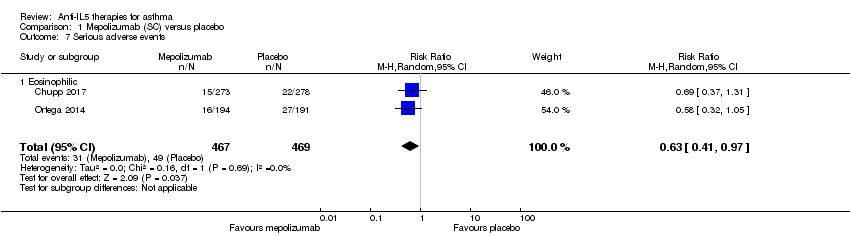 Comparison 1 Mepolizumab (SC) versus placebo, Outcome 7 Serious adverse events. | ||||
| 7.1 Eosinophilic | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.97] |
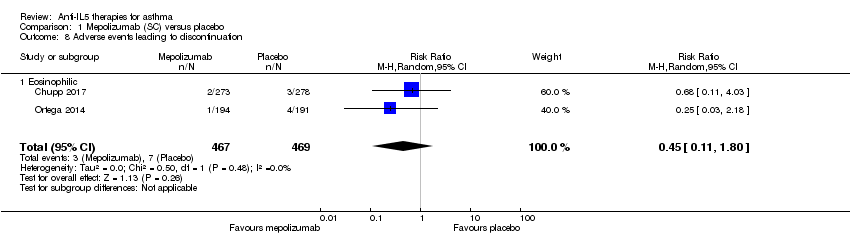
| 8 Adverse events leading to discontinuation Show forest plot | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.80] |
| Analysis 1.8  Comparison 1 Mepolizumab (SC) versus placebo, Outcome 8 Adverse events leading to discontinuation. | ||||
| 8.1 Eosinophilic | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of clinically significant exacerbations Show forest plot | 3 | 751 | Rate Ratio (Random, 95% CI) | 0.53 [0.44, 0.64] |
| Analysis 2.1 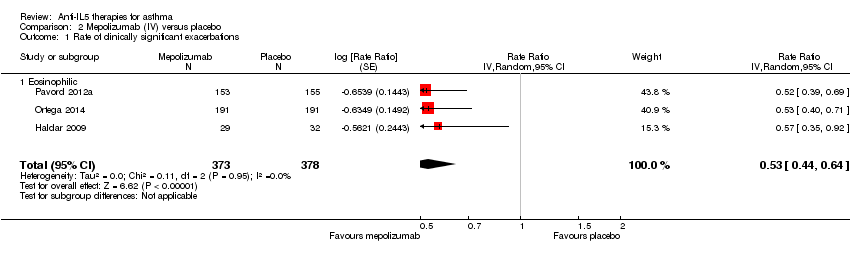 Comparison 2 Mepolizumab (IV) versus placebo, Outcome 1 Rate of clinically significant exacerbations. | ||||
| 1.1 Eosinophilic | 3 | 751 | Rate Ratio (Random, 95% CI) | 0.53 [0.44, 0.64] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.52 [0.31, 0.87] |
| Analysis 2.2  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission. | ||||
| 2.1 Eosinophilic | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.52 [0.31, 0.87] |
| 3 Rate of exacerbations requiring admission Show forest plot | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.61 [0.33, 1.13] |
| Analysis 2.3 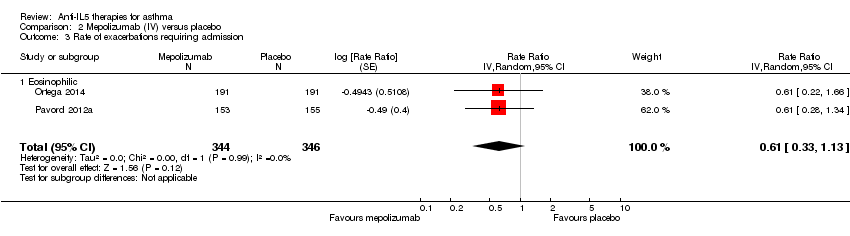 Comparison 2 Mepolizumab (IV) versus placebo, Outcome 3 Rate of exacerbations requiring admission. | ||||
| 3.1 Eosinophilic | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.61 [0.33, 1.13] |
| 4 People with one or more exacerbations Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.09] |
| Analysis 2.4 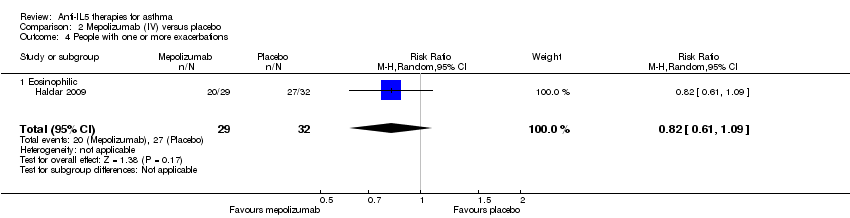 Comparison 2 Mepolizumab (IV) versus placebo, Outcome 4 People with one or more exacerbations. | ||||
| 4.1 Eosinophilic | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.09] |
| 5 Health‐related quality of life (AQLQ) Show forest plot | 2 | 369 | Mean Difference (Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| Analysis 2.5  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 5 Health‐related quality of life (AQLQ). | ||||
| 5.1 Eosinophilic | 2 | 369 | Mean Difference (Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 6 Health‐related quality of life (ACQ) Show forest plot | 2 | 369 | Mean Difference (Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
| Analysis 2.6 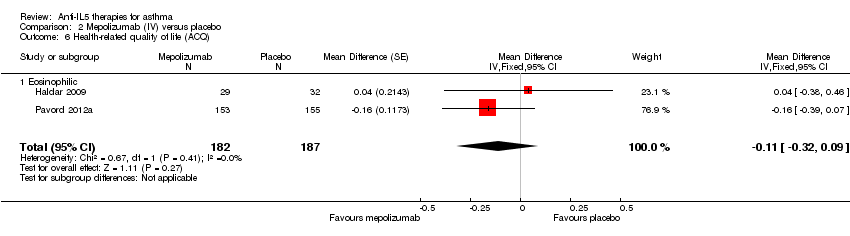 Comparison 2 Mepolizumab (IV) versus placebo, Outcome 6 Health‐related quality of life (ACQ). | ||||
| 6.1 Eosinophilic | 2 | 369 | Mean Difference (Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
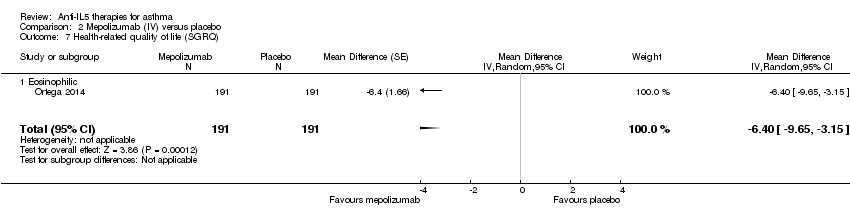
| 7 Health‐related quality of life (SGRQ) Show forest plot | 1 | 382 | Mean Difference (Random, 95% CI) | ‐6.4 [‐9.65, ‐3.15] |
| Analysis 2.7  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 7 Health‐related quality of life (SGRQ). | ||||
| 7.1 Eosinophilic | 1 | 382 | Mean Difference (Random, 95% CI) | ‐6.4 [‐9.65, ‐3.15] |
| 8 Pre‐bronchodilator FEV1 (litres) Show forest plot | 2 | 690 | Mean Difference (Random, 95% CI) | 0.08 [0.02, 0.15] |
| Analysis 2.8 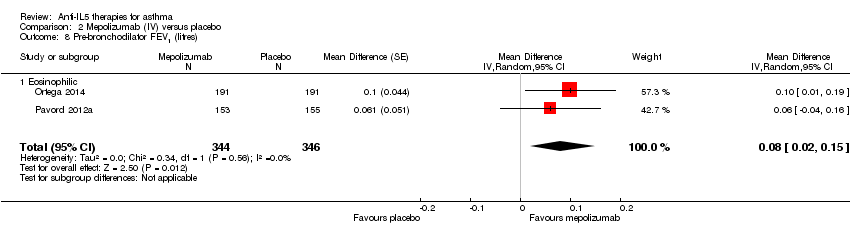 Comparison 2 Mepolizumab (IV) versus placebo, Outcome 8 Pre‐bronchodilator FEV1 (litres). | ||||
| 8.1 Eosinophilic | 2 | 690 | Mean Difference (Random, 95% CI) | 0.08 [0.02, 0.15] |
| 9 Serious adverse events Show forest plot | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| Analysis 2.9  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 9 Serious adverse events. | ||||
| 9.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 10 Adverse events leading to discontinuation Show forest plot | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
| Analysis 2.10  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 10 Adverse events leading to discontinuation. | ||||
| 10.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
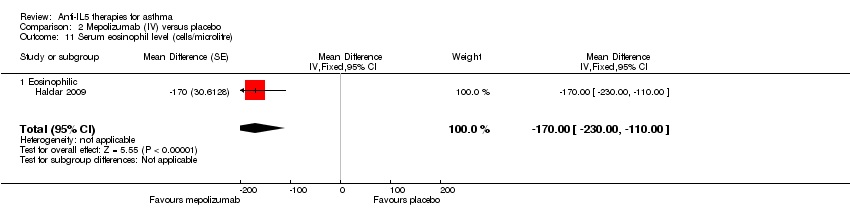
| 11 Serum eosinophil level (cells/microlitre) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐170.0 [‐228.00, ‐110.00] | |
| Analysis 2.11  Comparison 2 Mepolizumab (IV) versus placebo, Outcome 11 Serum eosinophil level (cells/microlitre). | ||||
| 11.1 Eosinophilic | 1 | Mean Difference (Fixed, 95% CI) | ‐170.0 [‐228.00, ‐110.00] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| Analysis 3.1  Comparison 3 Reslizumab (IV) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids. | ||||
| 1.1 Eosinophilic | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| Analysis 3.2  Comparison 3 Reslizumab (IV) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission. | ||||
| 2.1 Eosinophilic | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| 3 Health‐related quality of life (AQLQ) Show forest plot | 3 | 1164 | Mean Difference (Fixed, 95% CI) | 0.28 [0.17, 0.39] |
| Analysis 3.3 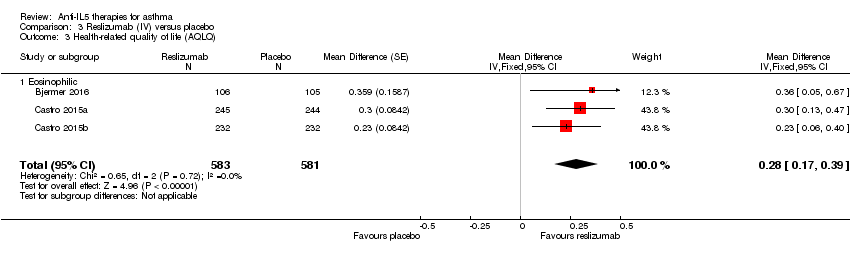 Comparison 3 Reslizumab (IV) versus placebo, Outcome 3 Health‐related quality of life (AQLQ). | ||||
| 3.1 Eosinophilic | 3 | 1164 | Mean Difference (Fixed, 95% CI) | 0.28 [0.17, 0.39] |
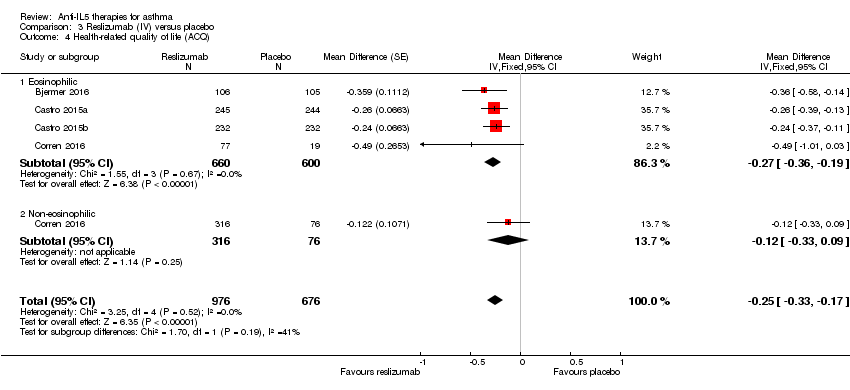
| 4 Health‐related quality of life (ACQ) Show forest plot | 4 | 1652 | Mean Difference (Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| Analysis 3.4 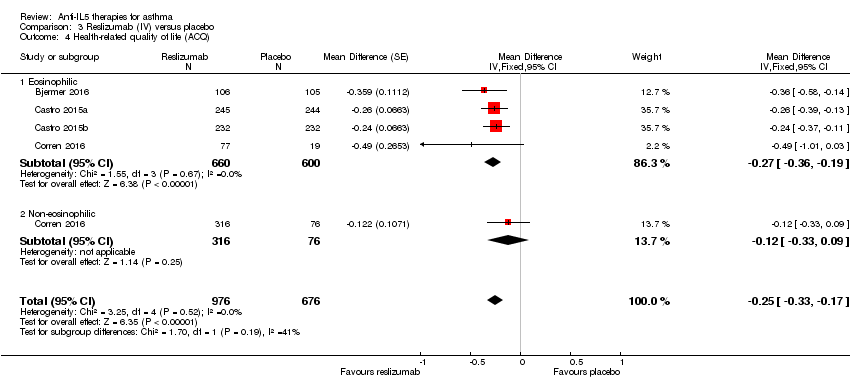 Comparison 3 Reslizumab (IV) versus placebo, Outcome 4 Health‐related quality of life (ACQ). | ||||
| 4.1 Eosinophilic | 4 | 1260 | Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.36, ‐0.19] |
| 4.2 Non‐eosinophilic | 1 | 392 | Mean Difference (Fixed, 95% CI) | ‐0.12 [‐0.33, 0.09] |
| 5 Pre‐bronchodilator FEV1 (litres) Show forest plot | 4 | 1652 | Mean Difference (Fixed, 95% CI) | 0.11 [0.07, 0.15] |
| Analysis 3.5 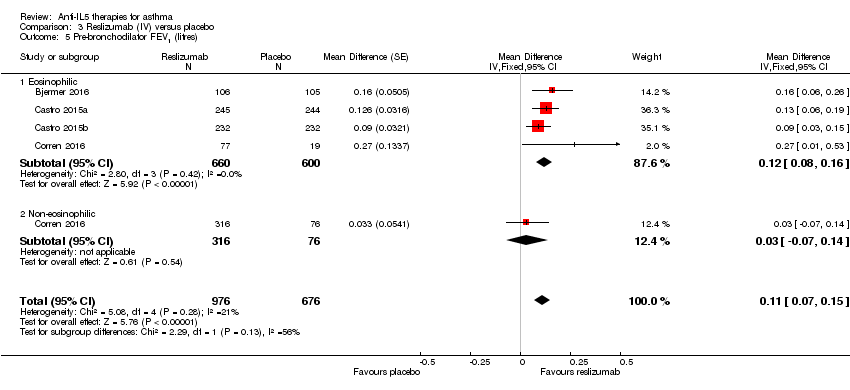 Comparison 3 Reslizumab (IV) versus placebo, Outcome 5 Pre‐bronchodilator FEV1 (litres). | ||||
| 5.1 Eosinophilic | 4 | 1260 | Mean Difference (Fixed, 95% CI) | 0.12 [0.08, 0.16] |
| 5.2 Non‐eosinophilic | 1 | 392 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
| 6 Serious adverse events Show forest plot | 4 | 1656 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.12] |
| Analysis 3.6  Comparison 3 Reslizumab (IV) versus placebo, Outcome 6 Serious adverse events. | ||||
| 6.1 Eosinophilic | 3 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| 6.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.34, 2.88] |
| 7 Adverse events leading to discontinuation Show forest plot | 4 | 1659 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.02] |
| Analysis 3.7 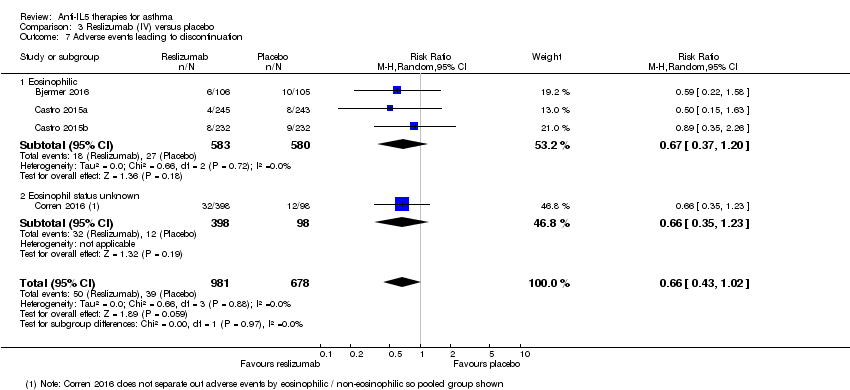 Comparison 3 Reslizumab (IV) versus placebo, Outcome 7 Adverse events leading to discontinuation. | ||||
| 7.1 Eosinophilic | 3 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.20] |
| 7.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.23] |
| 8 Serum eosinophil level (cells/microlitre) Show forest plot | 4 | 1656 | Mean Difference (Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
| Analysis 3.8 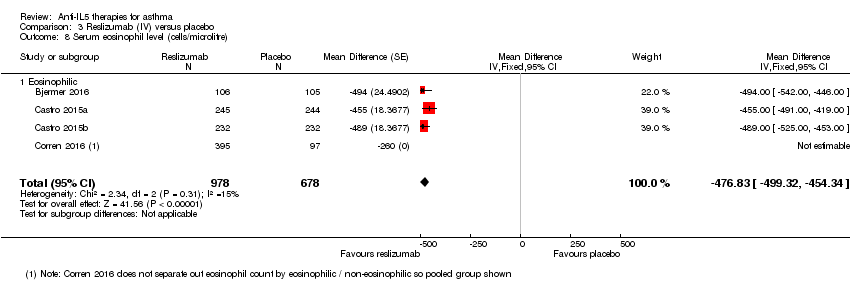 Comparison 3 Reslizumab (IV) versus placebo, Outcome 8 Serum eosinophil level (cells/microlitre). | ||||
| 8.1 Eosinophilic | 4 | 1656 | Mean Difference (Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 3 | 2456 | Rate Ratio (Fixed, 95% CI) | 0.62 [0.55, 0.70] |
| Analysis 4.1 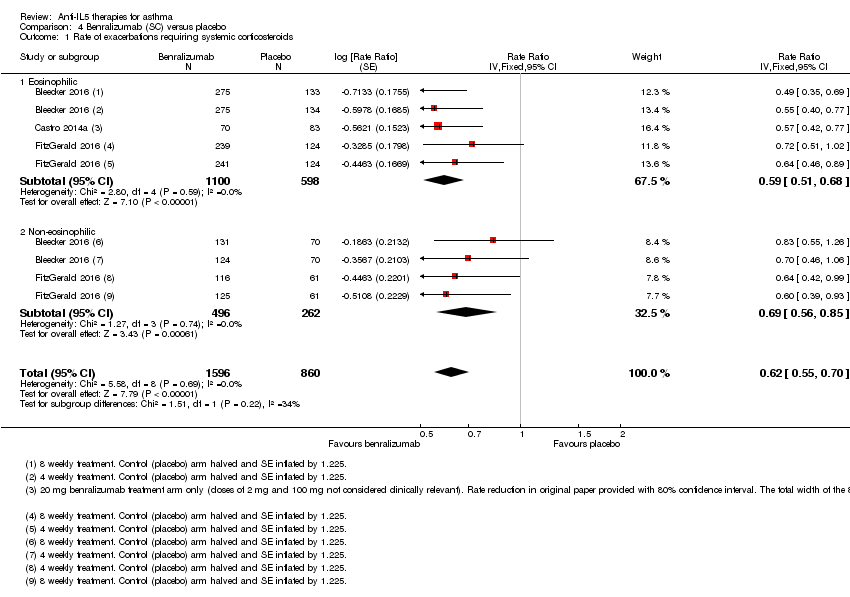 Comparison 4 Benralizumab (SC) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids. | ||||
| 1.1 Eosinophilic | 3 | 1698 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.51, 0.68] |
| 1.2 Non‐eosinophilic | 2 | 758 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 1537 | Rate Ratio (Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| Analysis 4.2  Comparison 4 Benralizumab (SC) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission. | ||||
| 2.1 Eosinophilic | 2 | 1537 | Rate Ratio (Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| 3 Health‐related quality of life (AQLQ mean difference) Show forest plot | 3 | 1541 | Mean Difference (Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| Analysis 4.3  Comparison 4 Benralizumab (SC) versus placebo, Outcome 3 Health‐related quality of life (AQLQ mean difference). | ||||
| 3.1 Eosinophilic | 3 | 1541 | Mean Difference (Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 4 Health‐related quality of life (ACQ mean difference) Show forest plot | 3 | 2359 | Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.29, ‐0.11] |
| Analysis 4.4  Comparison 4 Benralizumab (SC) versus placebo, Outcome 4 Health‐related quality of life (ACQ mean difference). | ||||
| 4.1 Eosinophilic | 3 | 1604 | Mean Difference (Fixed, 95% CI) | ‐0.23 [‐0.34, ‐0.12] |
| 4.2 Non‐eosinophilic | 2 | 755 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 5 Pre‐bronchodilator FEV1 (litres) Show forest plot | 3 | 2355 | Mean Difference (Fixed, 95% CI) | 0.10 [0.05, 0.14] |
| Analysis 4.5  Comparison 4 Benralizumab (SC) versus placebo, Outcome 5 Pre‐bronchodilator FEV1 (litres). | ||||
| 5.1 Eosinophilic | 3 | 1617 | Mean Difference (Fixed, 95% CI) | 0.13 [0.08, 0.19] |
| 5.2 Non‐eosinophilic | 2 | 738 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.03, 0.10] |
| 6 Serious adverse events Show forest plot | 4 | 2648 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 1.01] |
| Analysis 4.6  Comparison 4 Benralizumab (SC) versus placebo, Outcome 6 Serious adverse events. | ||||
| 6.1 Eosinophilic | 2 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 6.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.57, 1.27] |
| 6.3 Eosinophil status unknown | 2 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.37, 1.51] |
| 7 Adverse events leading to discontinuation Show forest plot | 3 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.02, 4.57] |
| Analysis 4.7 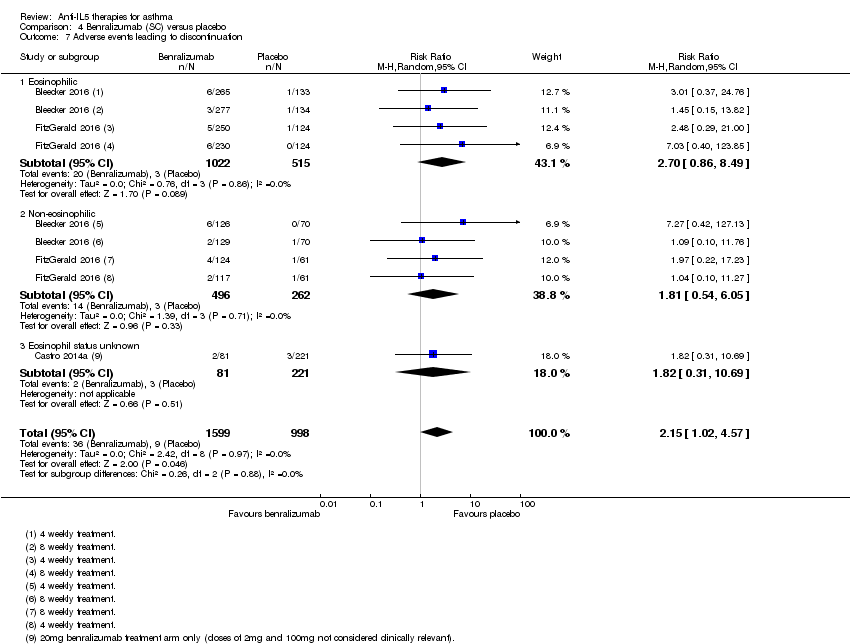 Comparison 4 Benralizumab (SC) versus placebo, Outcome 7 Adverse events leading to discontinuation. | ||||
| 7.1 Eosinophilic | 2 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.86, 8.49] |
| 7.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.54, 6.05] |
| 7.3 Eosinophil status unknown | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.31, 10.69] |
| 8 Serum eosinophil level (% change from baseline) Show forest plot | 2 | 2295 | Mean Difference (Fixed, 95% CI) | ‐104.74 [‐116.12, ‐93.35] |
| Analysis 4.8  Comparison 4 Benralizumab (SC) versus placebo, Outcome 8 Serum eosinophil level (% change from baseline). | ||||
| 8.1 Eosinophilic | 2 | 1537 | Mean Difference (Fixed, 95% CI) | ‐101.74 [‐113.27, ‐90.21] |
| 8.2 Non‐eosinophilic | 2 | 758 | Mean Difference (Fixed, 95% CI) | ‐216.81 [‐287.35, ‐146.28] |

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
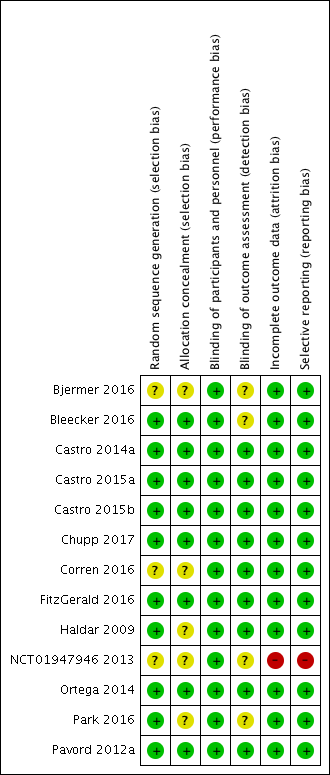
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Mepolizumab (SC) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids.

Comparison 1 Mepolizumab (SC) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission.
Comparison 1 Mepolizumab (SC) versus placebo, Outcome 3 Rate of exacerbations requiring admission.
Comparison 1 Mepolizumab (SC) versus placebo, Outcome 4 Health‐related quality of life (ACQ).

Comparison 1 Mepolizumab (SC) versus placebo, Outcome 5 Health‐related quality of life (SGRQ).
Comparison 1 Mepolizumab (SC) versus placebo, Outcome 6 Pre‐bronchodilator FEV1 (litres).
Comparison 1 Mepolizumab (SC) versus placebo, Outcome 7 Serious adverse events.
Comparison 1 Mepolizumab (SC) versus placebo, Outcome 8 Adverse events leading to discontinuation.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 1 Rate of clinically significant exacerbations.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 3 Rate of exacerbations requiring admission.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 4 People with one or more exacerbations.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 5 Health‐related quality of life (AQLQ).

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 6 Health‐related quality of life (ACQ).
Comparison 2 Mepolizumab (IV) versus placebo, Outcome 7 Health‐related quality of life (SGRQ).

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 8 Pre‐bronchodilator FEV1 (litres).

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 9 Serious adverse events.

Comparison 2 Mepolizumab (IV) versus placebo, Outcome 10 Adverse events leading to discontinuation.
Comparison 2 Mepolizumab (IV) versus placebo, Outcome 11 Serum eosinophil level (cells/microlitre).

Comparison 3 Reslizumab (IV) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids.

Comparison 3 Reslizumab (IV) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission.

Comparison 3 Reslizumab (IV) versus placebo, Outcome 3 Health‐related quality of life (AQLQ).
Comparison 3 Reslizumab (IV) versus placebo, Outcome 4 Health‐related quality of life (ACQ).

Comparison 3 Reslizumab (IV) versus placebo, Outcome 5 Pre‐bronchodilator FEV1 (litres).

Comparison 3 Reslizumab (IV) versus placebo, Outcome 6 Serious adverse events.
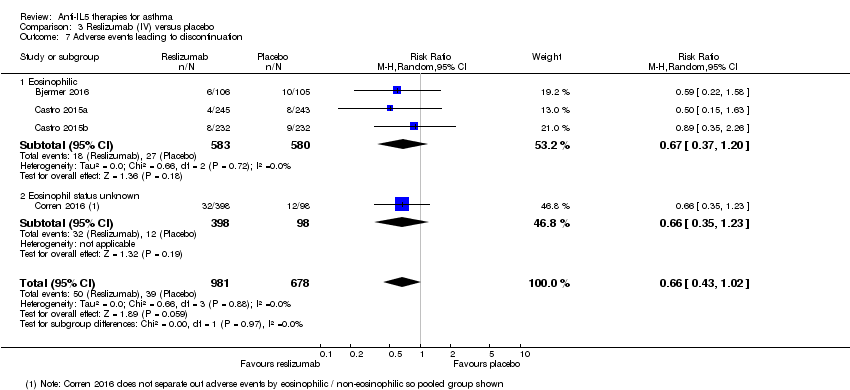
Comparison 3 Reslizumab (IV) versus placebo, Outcome 7 Adverse events leading to discontinuation.

Comparison 3 Reslizumab (IV) versus placebo, Outcome 8 Serum eosinophil level (cells/microlitre).

Comparison 4 Benralizumab (SC) versus placebo, Outcome 1 Rate of exacerbations requiring systemic corticosteroids.

Comparison 4 Benralizumab (SC) versus placebo, Outcome 2 Rate of exacerbations requiring emergency department treatment or admission.

Comparison 4 Benralizumab (SC) versus placebo, Outcome 3 Health‐related quality of life (AQLQ mean difference).

Comparison 4 Benralizumab (SC) versus placebo, Outcome 4 Health‐related quality of life (ACQ mean difference).

Comparison 4 Benralizumab (SC) versus placebo, Outcome 5 Pre‐bronchodilator FEV1 (litres).
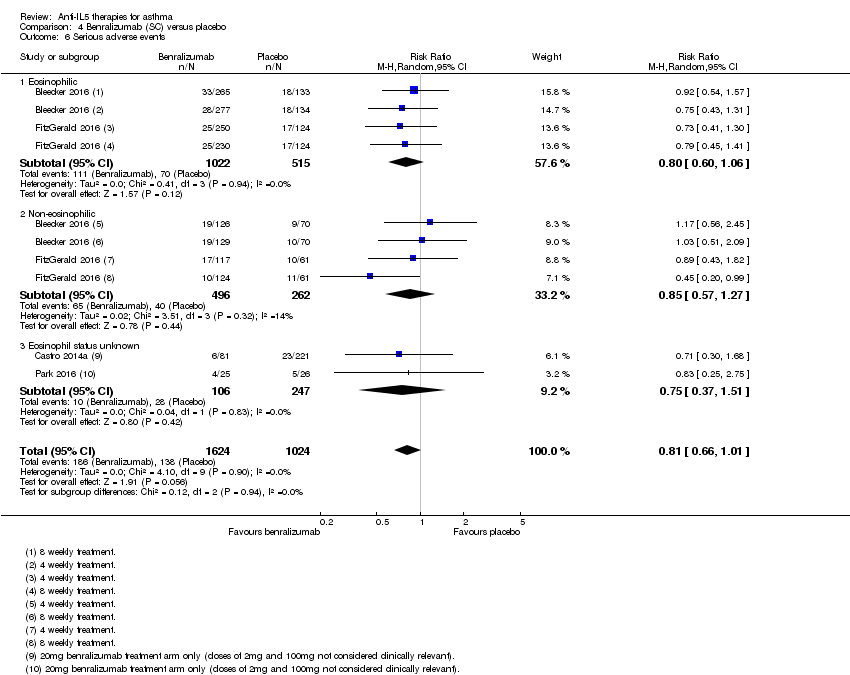
Comparison 4 Benralizumab (SC) versus placebo, Outcome 6 Serious adverse events.

Comparison 4 Benralizumab (SC) versus placebo, Outcome 7 Adverse events leading to discontinuation.

Comparison 4 Benralizumab (SC) versus placebo, Outcome 8 Serum eosinophil level (% change from baseline).
| Mepolizumab (SC) compared to placebo for asthma | ||||||
| Patient or population: people with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with mepolizumab (SC) | |||||
| Rate of exacerbations requiring systemic corticosteroids | The mean rate in the placebo group was 1.48 events per participant per yeara | The mean rate in the intervention group was 0.81 fewer events per participant per year (95% CI 0.66 fewer to 0.94 fewer) | Rate ratio 0.45 (0.36 to 0.55) | 936 | ⊕⊕⊕⊕ | |
| Rate of exacerbations requiring emergency department treatment or admission | The mean rate in the placebo group was 0.15 events per patient per yearb | The mean rate in the intervention group was 0.10 fewer events per participant per year (95% CI 0.05 fewer to 0.12 fewer) | Rate ratio 0.36 (0.20 to 0.66) | 936 | ⊕⊕⊕⊕ | |
| Health‐related quality of life (ACQ) | The mean change in the placebo group ranged from −0.4 to −0.5 units | The mean in the intervention group was ‐0.42 units fewer (‐0.56 fewer to ‐0.28 fewer) | ‐ | 936 | ⊕⊕⊕⊝ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Health‐related quality of life (SGRQ) | The mean change in the placebo group ranged from −7.9 to −9.0 units | The mean change in the intervention group was ‐7.4 units fewer (‐9.5 fewer to ‐5.29 fewer) | ‐ | 936 | ⊕⊕⊕⊕ | A change of ≥ 4 is considered the minimum clinically significant difference |
| Pre‐bronchodilator FEV1 (L) | The mean change in the placebo group ranged from 0.086 L (± 0.031 L) to 0.120 L (0.047 to 0.192 L) | The mean difference from placebo was a further 0.11 L (0.06 L to 0.17 L) | ‐ | 936 | ⊕⊕⊕⊕ | |
| Adverse events leading to discontinuation | 15 per 1000 | 7 per 1000 | Risk ratio 0.45 | 936 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRounded mean of the rate in the placebo group of the two studies: 1.21 and 1.74. | ||||||
| Mepolizumab (IV) compared to placebo for asthma | ||||||
| Patient or population: people with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with mepolizumab (IV) | |||||
| Rate of clinically significant exacerbations | The mean rate in the placebo group was 2.51 events per participant per yeara | The mean rate in the intervention groups was 1.18 fewer events per participant per year (1.41 fewer to 0.90 fewer) | Rate ratio 0.53 | 751 | ⊕⊕⊕⊝ | |
| Rate of exacerbations requiring emergency department treatment or admission | The mean rate in the placebo group was 0.32 events per participant per yearb | The mean rate in the intervention groups was 0.15 fewer events per participant per year (0.22 fewer to 0.04 fewer) | Rate ratio 0.52 | 690 | ⊕⊕⊕⊝ | |
| Health‐related quality of life (AQLQ) | The mean change in the placebo group ranged from 0.18 to 0.71 units | MD 0.21 higher | ‐ | 677 | ⊕⊕⊕⊝ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Health‐related quality of life (ACQ) | The mean change in the placebo group ranged from −0.59 to −0.50 units | MD ‐0.11 lower | ‐ | 369 | ⊕⊕⊕⊝ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Pre‐bronchodilator FEV1 (L) | The mean change in the placebo group ranged from 0.060 L (± 0.038 L) to 0.086 L (± 0.031 L) | MD 0.08 L (0.02 L higher to 0.15 L higher) | ‐ | 690 | ⊕⊕⊕⊝ | |
| Adverse events leading to discontinuation | 26 per 1000 | 19 per 1000 | RR 0.72 | 751 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRounded mean of the rate in the placebo group of the three studies: 1.74, 2.40 and 3.4. | ||||||
| Reslizumab (IV) compared to placebo for asthma | ||||||
| Patient or population: people with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with reslizumab (IV) | |||||
| Rate of exacerbations requiring systemic corticosteroids | The mean rate in the placebo group was 1.54 events per participant per year | The mean rate in the intervention groups was 0.93 fewer events per participant per year (1.09 fewer to 0.73 fewer) | Rate ratio 0.43 | 953 | ⊕⊕⊕⊕ | |
| Rate of exacerbations requiring emergency department treatment or admission | The mean rate in the placebo group was 0.12 events per participant per year | The mean rate in the intervention groups was 0.04 fewer events per participant per year (0.07 fewer to 0.02 more) | Rate ratio 0.67 | 953 | ⊕⊕⊕⊕ | |
| Health‐related quality of life (AQLQ) | The mean change in the placebo group ranged from 0.779 to 0.89 units | MD 0.28 higher | ‐ | 1164 | ⊕⊕⊕⊕ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Health‐related quality of life (ACQ) | The mean change in the placebo group ranged from −0.368 to −0.80 units | MD ‐0.25 lower | ‐ | 1652 | ⊕⊕⊕⊕ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Pre‐bronchodilator FEV1 (L) | The mean change in the placebo group ranged from 0.002 L (± 0.1216 L) to 0.215 (± 0.0484 L) | MD 0.11 L higher | ‐ | 1652 | ⊕⊕⊕⊕ | |
| Serious adverse events | 91 per 1000 | 72 per 1000 | RR 0.79 | 1656 | ⊕⊕⊕⊕ | |
| Adverse events leading to discontinuation | 58 per 1000 | 38 per 1000 | RR 0.66 | 1659 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The mean difference (0.28) is smaller than the minimum clinically significant difference (a reduction of 0.5 points). | ||||||
| Benralizumab (SC) compared to placebo for asthma | ||||||
| Patient or population: people with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with benralizumab (SC) | |||||
| Rate of exacerbations requiring systemic corticosteroids | The mean rate in the placebo group was 0.98 events per participant per yeara | The mean rate in the intervention groups was 0.37 fewer events per participant per year (0.44 fewer to 0.29 fewer) | Rate ratio 0.62 | 2456 | ⊕⊕⊕⊕ | |
| Rate of exacerbations requiring emergency department treatment or admission | The mean rate in the placebo group was 0.11 events per participant per yearb | The mean rate in the intervention groups was 0.04 fewer events per participant per year (0.06 fewer to 0.002 fewer) | Rate ratio 0.68 | 1537 | ⊕⊕⊕⊝ | There is greater heterogeneity (I² = 43%) owing to inclusion of less severe participants in FitzGerald 2016 (a larger proportion who had only suffered one exacerbation the previous year, with correspondingly less potential for exacerbation) |
| Health‐related quality of life (AQLQ) | The mean change in the placebo group ranged from 0.98 to 1.31 units | MD 0.23 higher | ‐ | 1541 | ⊕⊕⊕⊕ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Health‐related quality of life (ACQ) | The mean change in the placebo group ranged from −1.19 to −0.76 units | MD ‐0.20 lower | ‐ | 2359 | ⊕⊕⊕⊕ | A change of ≥ 0.5 is considered the minimum clinically significant difference |
| Pre‐bronchodilator FEV1 (L) | The mean change in the placebo group ranged from ‐0.01 L to 0.239 L | MD 0.10 L higher | ‐ | 2355 | ⊕⊕⊕⊕ | |
| Serious adverse events | 135 per 1000 | 109 per 1000 | RR 0.81 | 2648 | ⊕⊕⊕⊕ | |
| Adverse events leading to discontinuation | 9 per 1000 | 19 per 1000 | RR 2.15 | 2597 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Rounded mean of the rate in the placebo group of the eosinophilic and non‐eosinophilic arms (as applicable) or the three studies: 1.33, 1.21, 0.68, 0.49, 0.93, 1.21. | ||||||
| Study | Design, follow‐up (weeks) | Baseline asthma severity | Baseline treatment | Intervention (route) | Primary and secondary outcomes |
| Chupp 2017 (551) | RCT, double‐blind, placebo‐controlled (24) | Blood eosinophils ≥ 150 cells/μL at screening or ≥ 300 cells/μL in previous 12 months; and ≥ 2 exacerbations in previous 12 months; and FEV1 < 80% | High‐dose ICS for ≥ 12 months; + additional controller for ≥ 3 months; ± maintenance OCS | Mepolizumab 100 mg (SC) or placebo every 4 weeks for 24 weeks (last dose at 20 weeks) | ‐ SGRQ ‐ Mean change from baseline pre‐bronchodilator FEV1 ‐ Proportion of SGRQ total score responders at week 24 ‐ Mean change from baseline in ACQ‐5 |
| Haldar 2009 (61) | RCT, double‐blind, placebo‐controlled, parallel‐group (50) | ≥ 3% sputum eosinophils; and ≥ 2 exacerbations in previous 12 months | High‐dose ICS | Mepolizumab 75 (IV) or matched placebo (150 mL of 0.9% saline) at monthly intervals for 1 y | ‐ Severe exacerbations per person ‐ Change in AQLQ ‐ post‐bronchodilator FEV1 ‐ Airway hyperresponsiveness ‐ Blood/sputum eosinophil counts |
| Ortega 2014 (576) | RCT, double‐blind, double‐dummy, phase 3 (32) | Blood eosinophils ≥ 150 cells/μL at screening or ≥ 300 cells/μL in previous 12 months; and ≥ 2 exacerbations in previous 12 months; and FEV1 < 80% | High‐dose ICS for ≥ 12 months; + additional controller for ≥ 3 months; ± maintenance OCS | Mepolizumab 75 mg (IV) or 100 mg (SC) or placebo every 4 weeks for 32 weeks | ‐ Exacerbations per y ‐ Mean change from baseline pre‐bronchodilator FEV1 ‐ Mean change from baseline SGRQ total score |
| Pavord 2012a (621) | Multicentre, double‐blind, placebo‐controlled (52) | ≥ 3% sputum eosinophils or blood eosinophil ≥ 300 cells/μL; and ≥ 2 exacerbations in previous 12 months | High‐dose ICS (i.e. ≥ 880 μg/d FP or equivalent daily); + additional controller; ± maintenance OCS | Mepolizumab 75 mg, 250 mg or 750 mg (IV) or placebo every 4 weeks for 13 doses | ‐ Time to first clinically significant exacerbation ‐ Frequency of exacerbations requiring hospitalisation ‐ Time to first exacerbation requiring hospitalisation or ED visit ‐ Mean change from baseline pre‐bronchodilator FEV1 ‐ Mean change from baseline post‐bronchodilator FEV1 ‐ Mean change from baseline ACQ |
| Bjermer 2016 (315) | RCT, double‐blind, placebo‐controlled, parallel‐group, fixed‐dosage, multicentre phase 3 (16) | Blood eosinophils ≥ 400 cells/μL during 2‐4 weeks screening period; and ACQ‐7 score ≥ 1.5 | Medium‐dose ICS; maintenance OCS not allowed | Reslizumab 0.3 mg/kg or 3 mg/kg (IV) or placebo every 4 weeks for 4 doses | ‐ Pre‐bronchodilator FEV1, FVC, FEF25‐75 ‐ ACQ, ACQ‐6, ACQ‐5 ‐ ASUI ‐ AQLQ ‐ Rescue inhaler use ‐ Blood eosinophil levels |
| Castro 2015a (489) and Castro 2015b (464) | 2 x duplicate RCT double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3 (52) | Blood eosinophils ≥ 400 cells/μL during 2‐4 week screening period; and ACQ‐7 score ≥ 1.5 | Medium‐dose ICS (i.e. ≥ 440 μg/day FP or equivalent daily); ± additional controller or maintenance OCS | Reslizumab 3 mg/kg (IV) or matching placebo every 4 weeks for 13 doses (last dose week 48) | ‐ Annual frequency of exacerbations ‐ Change in FEV1 from baseline over 16 weeks ‐ ACQ‐7 score ‐ ASUI score ‐ Rescue use of SABA ‐ Blood eosinophil count ‐ AQLQ total score at weeks 16, 32 and 52 |
| Corren 2016 (496) | RCT double‐blind, placebo‐controlled, multicentre phase 3 (16) | ACQ‐7 score ≥ 1.5 (no selection based on blood eosinophils) | Medium‐dose ICS; maintenance OCS not allowed | Reslizumab 3 mg/kg (IV) or matching placebo every 4 weeks for 4 doses | ‐ Change in FEV1 from baseline ‐ ACQ‐7 score ‐ Rescue (SABA) use within previous 3 days ‐ FVC ‐ Blood eosinophils |
| Bleecker 2016 (1204) | RCT double‐blind, parallel‐group, placebo‐controlled multicentre (52) | ≥ 2 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at enrolment; and FEV1 < 80% (if 12‐17 years old, < 90%) | Adults (> 18 y) high‐dose (≥ 500 μg/d FP or equivalent) ICS/LABA for ≥ 12 months Children (12‐17 y) at least medium‐dose (≥ 250 μg/day FP or equivalent) ICS/LABA | Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo for 48 weeks | ‐ Annual exacerbation rate ‐ Pre‐bronchodilator FEV1 ‐ Total asthma symptom score ‐ Time to first exacerbation ‐ Annual rate of exacerbations requiring ED visit or hospital admission ‐ Post‐bronchodilator FEV1 ‐ ACQ‐6 ‐ AQLQ(S)+12 score |
| Castro 2014a (606) | RCT double‐blind, placebo‐controlled, multicentre dose‐ranging (52) | 2‐6 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at least twice during screening; and morning pre‐bronchodilator FEV1 40%‐90% | Medium‐ to high‐dose ICS in combination with LABA for ≥ 12 months | Benralizumab 2 mg, 20 mg or 100 mg (SC) or placebo every 4 weeks for the first 3 doses, then every 8 weeks (total 7 doses) | ‐ Annual exacerbation rate ‐ Change from baseline in FEV1 ‐ Mean ACQ‐6 score ‐ Overall symptom score ‐ Mean AQLQ score |
| FitzGerald 2016 (1306) | RCT, double‐blind, parallel‐group, placebo‐controlled multicentre (56) | ≥ 2 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at enrolment; and FEV1 < 80% | Medium‐ (≥ 250 μg/d FP or equivalent) to high‐dose (≥ 500 μg/d FP or equivalent) ICS/LABA for ≥ 12 months; high‐dose ICS/LABA for ≥ 3 months | Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo | ‐ Annual exacerbation rate for participants with blood eosinophils ≥ 300 cells/μL ‐ Pre‐bronchodilator FEV1 ‐ Total asthma symptom score ‐ Time to first exacerbation ‐ Annual rate of exacerbations requiring ED visit or hospital admission ‐ Post‐bronchodilator FEV1 ‐ ACQ‐6 ‐ AQLQ(S)+12 score |
| (13) | RCT double‐blind, parallel‐group, placebo‐controlled multicentre (48) | Uncontrolled asthma taking medium‐dose ICS plus LABA | Medium‐dose ICS (>250ug and ≤500ug fluticasone dry powder formulation equivalents total daily dose) and LABA for at least 3 month prior to first visit | Benralizumab 30 mg (SC) or placebo either every 4 weeks or every 4 weeks for the first 3 doses then every 8 weeks or placebo | Asthma exacerbations over 48‐week treatment period |
| Park 2016 (103) | RCT double‐blind, placebo‐controlled, dose‐ranging multicentre (52) | 2‐6 exacerbations in the previous 12 months; and ACQ‐6 score ≥ 1.5 at least twice during screening; and morning pre‐bronchodilator FEV1 40%‐90% | Medium‐ to high‐dose ICS in combination with LABA for ≥ 12 months | Benralizumab 2 mg, 20 mg or 100 mg (SC) or placebo every 4 weeks for the first 3 doses, then every 8 weeks (total 7 doses) | ‐ Annual exacerbation rate ‐ Lung function ‐ ACQ‐6 ‐ FeNO ‐ Blood eosinophil counts |
| ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ASUI: Asthma Symptom Utility Index; BDP: beclomethasone dipropionate; b: day; ECP: eosinophil cationic protein; ED: emergency department; FEF25‐75 : forced expiratory flow at 25% to 75% of FVC; FeNO: exhaled fraction of nitric oxide; FEV1 : Forced expiratory volume in 1 second; FVC: forced vital capacity; FP; fluticasone propionate; ICS; inhaled corticosteroid; IV: intravenous; LABA: long‐acting beta2 agonistOCS; oral corticosteroid; PC20 : histamine provocative concentration causing a 20% drop in FEV1; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SABA: short‐acting beta2‐agonists; SC: subcutaneous; SGRQ: St George's Respiratory Questionnaire; y: year | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.45 [0.36, 0.55] |
| 1.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.45 [0.36, 0.55] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.36 [0.20, 0.66] |
| 2.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.36 [0.20, 0.66] |
| 3 Rate of exacerbations requiring admission Show forest plot | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.31 [0.13, 0.73] |
| 3.1 Eosinophilic | 2 | 936 | Rate Ratio (Random, 95% CI) | 0.31 [0.13, 0.73] |
| 4 Health‐related quality of life (ACQ) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | ‐0.42 [‐0.56, ‐0.28] |
| 4.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | ‐0.42 [‐0.56, ‐0.28] |
| 5 Health‐related quality of life (SGRQ) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | ‐7.40 [‐9.50, ‐5.29] |
| 5.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | ‐7.40 [‐9.50, ‐5.29] |
| 6 Pre‐bronchodilator FEV1 (litres) Show forest plot | 2 | 936 | Mean Difference (Random, 95% CI) | 0.11 [0.06, 0.17] |
| 6.1 Eosinophilic | 2 | 936 | Mean Difference (Random, 95% CI) | 0.11 [0.06, 0.17] |
| 7 Serious adverse events Show forest plot | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.97] |
| 7.1 Eosinophilic | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.97] |
| 8 Adverse events leading to discontinuation Show forest plot | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.80] |
| 8.1 Eosinophilic | 2 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of clinically significant exacerbations Show forest plot | 3 | 751 | Rate Ratio (Random, 95% CI) | 0.53 [0.44, 0.64] |
| 1.1 Eosinophilic | 3 | 751 | Rate Ratio (Random, 95% CI) | 0.53 [0.44, 0.64] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.52 [0.31, 0.87] |
| 2.1 Eosinophilic | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.52 [0.31, 0.87] |
| 3 Rate of exacerbations requiring admission Show forest plot | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.61 [0.33, 1.13] |
| 3.1 Eosinophilic | 2 | 690 | Rate Ratio (Random, 95% CI) | 0.61 [0.33, 1.13] |
| 4 People with one or more exacerbations Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.09] |
| 4.1 Eosinophilic | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.09] |
| 5 Health‐related quality of life (AQLQ) Show forest plot | 2 | 369 | Mean Difference (Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 5.1 Eosinophilic | 2 | 369 | Mean Difference (Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 6 Health‐related quality of life (ACQ) Show forest plot | 2 | 369 | Mean Difference (Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
| 6.1 Eosinophilic | 2 | 369 | Mean Difference (Fixed, 95% CI) | ‐0.11 [‐0.32, 0.09] |
| 7 Health‐related quality of life (SGRQ) Show forest plot | 1 | 382 | Mean Difference (Random, 95% CI) | ‐6.4 [‐9.65, ‐3.15] |
| 7.1 Eosinophilic | 1 | 382 | Mean Difference (Random, 95% CI) | ‐6.4 [‐9.65, ‐3.15] |
| 8 Pre‐bronchodilator FEV1 (litres) Show forest plot | 2 | 690 | Mean Difference (Random, 95% CI) | 0.08 [0.02, 0.15] |
| 8.1 Eosinophilic | 2 | 690 | Mean Difference (Random, 95% CI) | 0.08 [0.02, 0.15] |
| 9 Serious adverse events Show forest plot | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 9.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.94] |
| 10 Adverse events leading to discontinuation Show forest plot | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
| 10.1 Eosinophilic | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.92] |
| 11 Serum eosinophil level (cells/microlitre) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐170.0 [‐228.00, ‐110.00] | |
| 11.1 Eosinophilic | 1 | Mean Difference (Fixed, 95% CI) | ‐170.0 [‐228.00, ‐110.00] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| 1.1 Eosinophilic | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.43 [0.33, 0.55] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| 2.1 Eosinophilic | 2 | 953 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.39, 1.17] |
| 3 Health‐related quality of life (AQLQ) Show forest plot | 3 | 1164 | Mean Difference (Fixed, 95% CI) | 0.28 [0.17, 0.39] |
| 3.1 Eosinophilic | 3 | 1164 | Mean Difference (Fixed, 95% CI) | 0.28 [0.17, 0.39] |
| 4 Health‐related quality of life (ACQ) Show forest plot | 4 | 1652 | Mean Difference (Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 4.1 Eosinophilic | 4 | 1260 | Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.36, ‐0.19] |
| 4.2 Non‐eosinophilic | 1 | 392 | Mean Difference (Fixed, 95% CI) | ‐0.12 [‐0.33, 0.09] |
| 5 Pre‐bronchodilator FEV1 (litres) Show forest plot | 4 | 1652 | Mean Difference (Fixed, 95% CI) | 0.11 [0.07, 0.15] |
| 5.1 Eosinophilic | 4 | 1260 | Mean Difference (Fixed, 95% CI) | 0.12 [0.08, 0.16] |
| 5.2 Non‐eosinophilic | 1 | 392 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
| 6 Serious adverse events Show forest plot | 4 | 1656 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.12] |
| 6.1 Eosinophilic | 3 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| 6.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.34, 2.88] |
| 7 Adverse events leading to discontinuation Show forest plot | 4 | 1659 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.02] |
| 7.1 Eosinophilic | 3 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.20] |
| 7.2 Eosinophil status unknown | 1 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.23] |
| 8 Serum eosinophil level (cells/microlitre) Show forest plot | 4 | 1656 | Mean Difference (Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
| 8.1 Eosinophilic | 4 | 1656 | Mean Difference (Fixed, 95% CI) | ‐476.83 [‐499.32, ‐454.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of exacerbations requiring systemic corticosteroids Show forest plot | 3 | 2456 | Rate Ratio (Fixed, 95% CI) | 0.62 [0.55, 0.70] |
| 1.1 Eosinophilic | 3 | 1698 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.51, 0.68] |
| 1.2 Non‐eosinophilic | 2 | 758 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] |
| 2 Rate of exacerbations requiring emergency department treatment or admission Show forest plot | 2 | 1537 | Rate Ratio (Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| 2.1 Eosinophilic | 2 | 1537 | Rate Ratio (Fixed, 95% CI) | 0.68 [0.47, 0.98] |
| 3 Health‐related quality of life (AQLQ mean difference) Show forest plot | 3 | 1541 | Mean Difference (Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 3.1 Eosinophilic | 3 | 1541 | Mean Difference (Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 4 Health‐related quality of life (ACQ mean difference) Show forest plot | 3 | 2359 | Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.29, ‐0.11] |
| 4.1 Eosinophilic | 3 | 1604 | Mean Difference (Fixed, 95% CI) | ‐0.23 [‐0.34, ‐0.12] |
| 4.2 Non‐eosinophilic | 2 | 755 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 5 Pre‐bronchodilator FEV1 (litres) Show forest plot | 3 | 2355 | Mean Difference (Fixed, 95% CI) | 0.10 [0.05, 0.14] |
| 5.1 Eosinophilic | 3 | 1617 | Mean Difference (Fixed, 95% CI) | 0.13 [0.08, 0.19] |
| 5.2 Non‐eosinophilic | 2 | 738 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.03, 0.10] |
| 6 Serious adverse events Show forest plot | 4 | 2648 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 1.01] |
| 6.1 Eosinophilic | 2 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 6.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.57, 1.27] |
| 6.3 Eosinophil status unknown | 2 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.37, 1.51] |
| 7 Adverse events leading to discontinuation Show forest plot | 3 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.02, 4.57] |
| 7.1 Eosinophilic | 2 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.86, 8.49] |
| 7.2 Non‐eosinophilic | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.54, 6.05] |
| 7.3 Eosinophil status unknown | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.31, 10.69] |
| 8 Serum eosinophil level (% change from baseline) Show forest plot | 2 | 2295 | Mean Difference (Fixed, 95% CI) | ‐104.74 [‐116.12, ‐93.35] |
| 8.1 Eosinophilic | 2 | 1537 | Mean Difference (Fixed, 95% CI) | ‐101.74 [‐113.27, ‐90.21] |
| 8.2 Non‐eosinophilic | 2 | 758 | Mean Difference (Fixed, 95% CI) | ‐216.81 [‐287.35, ‐146.28] |

