Вмешательства по улучшению функции верхних конечностей после инсульта
Abstract
Background
Improving upper limb function is a core element of stroke rehabilitation needed to maximise patient outcomes and reduce disability. Evidence about effects of individual treatment techniques and modalities is synthesised within many reviews. For selection of effective rehabilitation treatment, the relative effectiveness of interventions must be known. However, a comprehensive overview of systematic reviews in this area is currently lacking.
Objectives
To carry out a Cochrane overview by synthesising systematic reviews of interventions provided to improve upper limb function after stroke.
Methods
Search methods: We comprehensively searched the Cochrane Database of Systematic Reviews; the Database of Reviews of Effects; and PROSPERO (an international prospective register of systematic reviews) (June 2013). We also contacted review authors in an effort to identify further relevant reviews.
Selection criteria: We included Cochrane and non‐Cochrane reviews of randomised controlled trials (RCTs) of patients with stroke comparing upper limb interventions with no treatment, usual care or alternative treatments. Our primary outcome of interest was upper limb function; secondary outcomes included motor impairment and performance of activities of daily living. When we identified overlapping reviews, we systematically identified the most up‐to‐date and comprehensive review and excluded reviews that overlapped with this.
Data collection and analysis: Two overview authors independently applied the selection criteria, excluding reviews that were superseded by more up‐to‐date reviews including the same (or similar) studies. Two overview authors independently assessed the methodological quality of reviews (using a modified version of the AMSTAR tool) and extracted data. Quality of evidence within each comparison in each review was determined using objective criteria (based on numbers of participants, risk of bias, heterogeneity and review quality) to apply GRADE (Grades of Recommendation, Assessment, Development and Evaluation) levels of evidence. We resolved disagreements through discussion. We systematically tabulated the effects of interventions and used quality of evidence to determine implications for clinical practice and to make recommendations for future research.
Main results
Our searches identified 1840 records, from which we included 40 completed reviews (19 Cochrane; 21 non‐Cochrane), covering 18 individual interventions and dose and setting of interventions. The 40 reviews contain 503 studies (18,078 participants). We extracted pooled data from 31 reviews related to 127 comparisons. We judged the quality of evidence to be high for 1/127 comparisons (transcranial direct current stimulation (tDCS) demonstrating no benefit for outcomes of activities of daily living (ADLs)); moderate for 49/127 comparisons (covering seven individual interventions) and low or very low for 77/127 comparisons.
Moderate‐quality evidence showed a beneficial effect of constraint‐induced movement therapy (CIMT), mental practice, mirror therapy, interventions for sensory impairment, virtual reality and a relatively high dose of repetitive task practice, suggesting that these may be effective interventions; moderate‐quality evidence also indicated that unilateral arm training may be more effective than bilateral arm training. Information was insufficient to reveal the relative effectiveness of different interventions.
Moderate‐quality evidence from subgroup analyses comparing greater and lesser doses of mental practice, repetitive task training and virtual reality demonstrates a beneficial effect for the group given the greater dose, although not for the group given the smaller dose; however tests for subgroup differences do not suggest a statistically significant difference between these groups. Future research related to dose is essential.
Specific recommendations for future research are derived from current evidence. These recommendations include but are not limited to adequately powered, high‐quality RCTs to confirm the benefit of CIMT, mental practice, mirror therapy, virtual reality and a relatively high dose of repetitive task practice; high‐quality RCTs to explore the effects of repetitive transcranial magnetic stimulation (rTMS), tDCS, hands‐on therapy, music therapy, pharmacological interventions and interventions for sensory impairment; and up‐to‐date reviews related to biofeedback, Bobath therapy, electrical stimulation, reach‐to‐grasp exercise, repetitive task training, strength training and stretching and positioning.
Authors' conclusions
Large numbers of overlapping reviews related to interventions to improve upper limb function following stroke have been identified, and this overview serves to signpost clinicians and policy makers toward relevant systematic reviews to support clinical decisions, providing one accessible, comprehensive document, which should support clinicians and policy makers in clinical decision making for stroke rehabilitation.
Currently, no high‐quality evidence can be found for any interventions that are currently used as part of routine practice, and evidence is insufficient to enable comparison of the relative effectiveness of interventions. Effective collaboration is urgently needed to support large, robust RCTs of interventions currently used routinely within clinical practice. Evidence related to dose of interventions is particularly needed, as this information has widespread clinical and research implications.
Резюме на простом языке
Мероприятия по улучшению функции рук у людей после инсульта
Вопрос исследования
Какие вмешательства способствуют восстановлению руки и кисти после перенесенного инсульта?
Актуальность
Очень часто после инсульта возникают проблемы с функцией руки (нарушения верхних конечностей). Нарушения верхних конечностей обычно включают в себя нарушения движения и координации рук, кистей и пальцев, что часто приводит к сложностям выполнения повседневных функций, таких как прием пищи, одевание и умывание. Более чем у половины людей с нарушениями в верхних конечностях после инсульта, проблемы всё ещё будут продолжаться от нескольких месяцев до нескольких лет после инсульта. Улучшение функции руки является ключевым элементом реабилитации. Было разработано множество возможных вмешательств; они включают различные упражнения или тренировки, специальное оборудование или технологии, или они могут быть представлять собой лекарство (таблетка или инъекция), назначаемое, чтобы улучшить движения руки.
Реабилитация верхних конечностей после инсульта часто включает в себя несколько различных вмешательств и обычно требует сотрудничества пациента, ухаживающих за ним и реабилитационной команды.
Мы разработали Кокрейновский обзор, чтобы помочь людям легко получить доступ к информации об эффективных вмешательствах, и помочь им сравнить эффекты различных вмешательств. Мы стремились объединить все систематические обзоры вмешательств, осуществляемых для улучшения функции верхней конечности (руки) после инсульта.
Характеристики исследований
Мы провели поиск Кокрейновских и не‐Кокрейновских обзоров по эффективности вмешательств для улучшения функции руки после инсульта. Мы включили 40 систематических обзоров (19 Кокрейновских обзоров и не‐Кокрейновские обзоры ‐числом 21). Доказательства актуальны по июль 2013 года.
Обзоры рассмотрели 18 различных типов вмешательств, а также дозу вмешательства и условия, в которых вмешательство было предоставлено. Эти обзоры различались по включенной популяции (начальное ухудшение верхней конечности и тяжесть инсульта), и по включенным группам сравнения (контрольные вмешательства, отсутствие лечения и обычная терапия).
Мы извлекли информацию о 127 сравнениях, которые были изучены в рамках обзоров. Они показали, в какой степени различные вмешательства оказывали эффект на функции верхней конечности, ухудшение [функции] верхней конечности и способность выполнять повседневную работу.
Основные результаты
В настоящее время нет доступных доказательств высокого качества для любых вмешательств, которые используются в настоящее время в повседневной практике. Доказательств недостаточно, чтобы показать, какие являются самыми эффективными для улучшения функции верхней конечности.
Доказательства среднего качества позволяют предполагать, что следующие вмешательства могут быть эффективными: двигательная терапия, индуцированная ограничением (напряжением), психоневрологическая практика, зеркальная терапия, вмешательства для сенсорных нарушений, виртуальная реальность и относительно высокие дозы повторных практических заданий. Доказательства умеренного качества также показывают, что одностороннее обучение руки (упражнения для поврежденной руки) может быть более эффективным, чем двустороннее обучение рук (выполнение того же самого упражнения обеими руками одновременно).
Некоторые свидетельства показывают, что большая доза любого вмешательства лучше, чем меньшая. Необходимы дополнительные исследования, чтобы определить оптимальную дозу вмешательства для восстановления руки.
Объединение доказательств всех доступных систематических обзоров помогло нам сделать конкретные рекомендации для будущих исследований. Эти рекомендации включают (но не ограничиваются этим) крупные рандомизированные контролируемые испытания ТИО, психоневрологической практики, зеркальной терапии и виртуальной реальности. Мы рекомендуем высоко‐качественные современные обзоры и дальнейшие первичные исследования для ряда конкретных вмешательств.
Качество доказательств
Мы оценили качество доказательств как высокое в отношении одного вмешательства: вариант стимуляции мозга, называемый транскраниальная стимуляция постоянным током (микрополяризация) (tDCS), которая в настоящее время не используется повседневной практике. Эти высококачественные данные показывают, что tDCS не улучшает способность людей выполнять повседневную работу.
Мы оценили качество доказательств как умеренное для 48 сравнений (по семи отдельным вмешательствам), и как низкое или очень низкое для 76 сравнений. Причины понижения качества доказательств до среднего, низкого или очень низкого включают малые числа исследований и участников, плохое качество методологии или представления результатов исследований, включенных в обзоры, значительную неоднородность (гетерогенность, вариацию) между результатами обучения и низкое качество обзора или сообщения о методах.
Мы пришли к выводу, что настоятельно необходимы доказательства высокого качества в отношении эффективности вмешательств, позволяющих улучшить функции верхних конечностей, в частности тех вмешательств, для которых свидетельства умеренного качества позволяют в настоящее время предполагать их благоприятный эффект.
Authors' conclusions
Background
Stroke is the third most common cause of death and the main cause of acquired adult disability in high‐income countries (Warlow 2008). This affects from 112 to 223 per 100,000 people in high‐income countries, and from 73 to 165 per 100,000 in low‐income countries (Feigin 2009). The annual incidence of stroke is 795,000 people in the USA (Go 2013), more than 110,000 in England (NHS Choices) and around 15,000 in Scotland (Stroke in Scotland 2010). Motor impairment, typically affecting movement of the face, arm and leg of one side of the body, affects about 80% of stroke survivors (Langhorne 2009). Upper limb (i.e. arm, hand and/or finger) motor impairments are often persistent and disabling (Lai 2002); only half of all stroke survivors with an initial plegic (paralysed) upper limb regain some useful upper limb function after six months (Kwakkel 2003), and, of those with initial arm impairment, 50% have problems with arm function four years post stroke (Broeks 1999). Activities of daily living (ADLs) largely depend on arm function (Sveen 1999), particularly for personal activities such as feeding, dressing and grooming. One year after stroke, arm motor impairment is associated with anxiety (Morris 2013) and poorer perception of health‐related quality of life (Franceschini 2010) and subjective well‐being (Wyller 1997). Therefore, improving upper limb function is a core element of rehabilitation after stroke to maximise recovery (Langhorne 2003). Therapists have developed many diverse techniques that aim to rehabilitate arm function after stroke. Evidence on the effects of individual treatment techniques/modalities has been synthesised in a large number of reviews, including at least 11 Cochrane reviews. Most Cochrane reviews compare an intervention versus a placebo intervention, no intervention or usual care, whereas, in practice, clinicians need information to judge the relative effectiveness of different interventions when selecting the most effective treatment. Therefore, this Cochrane overview will draw together information from systematic reviews of all interventions to improve arm function after stroke to help inform clinicians and policy makers.
Description of the condition
A stroke causes damage within the brain that can directly affect movement and sensation of the arm. Damage to the sensory motor cortex, subcortical areas and/or cerebellum can result in the following.
-
Loss of motor control, which causes difficulties with, or prevents, the voluntary production of movement, and compromises dexterity and co‐ordination of the fingers, hands and arms.
-
Sensory and proprioceptive deficits, which reduce awareness of limb position and movement.
The reduced level of movement predisposes to changes in muscle, connective and neural tissues, resulting in several secondary problems, which may include the following.
-
Shortening of muscles ('contracture') and weakening of muscles ('paresis').
-
Disordered muscle contraction ('spasticity').
-
Compromised motor and sensory nerve function, as unused neural pathways lose connectivity.
-
Shoulder subluxation (partial, temporary dislocation of the shoulder joint), caused by lack of motor control and muscle weakness in the rotator cuff muscles.
-
Pain, which is a common complication, often secondary to shoulder subluxation, but also commonly associated with the musculoskeletal changes caused by immobility.
These impairments make many ADLs difficult, especially those activities that depend on co‐ordination between both upper limbs or fine finger movements. With time, the tendency is to use the unaffected limb predominantly and to disregard the affected limb, thereby developing learned non‐use (Taub 2006). Mood and cognitive ability can be adversely affected by stroke, further diminishing functional abilities, and arm motor impairment itself can impact well‐being. The ensuing loss of meaningful activity tends to reduce participation in society.
Description of the interventions
Professionals responsible for the delivery of upper limb rehabilitation interventions most commonly consist of physical therapists and occupational therapists. However, other health professionals (e.g. nurses, doctors) and non‐health professionals (e.g. exercise professionals, carers, family members) may also contribute to the delivery of interventions (Coupar 2012; Harris 2010a). Therapy is usually provided to patients during their period of hospitalisation, during early supported discharge at home or in outpatient settings. In some countries, patients are admitted to rehabilitation centres once they are medically stable. Therapy may be provided individually or to groups of stroke survivors in classes.
Patients and carers frequently report that they feel they would benefit from continued rehabilitation: Results of a survey of UK stroke survivors indicate that 43% wanted additional therapy, most commonly more physiotherapy (Stroke 2012). Similar unmet needs have been reported for upper limb rehabilitation by Canadian stroke survivors (Duxbury 2012; Vincent 2007). After discharge from formal rehabilitation, stroke survivors may enrol in fitness centres (Best 2012) or may utilise commercially available gaming products to continue exercising for therapeutic purposes (Anderson 2010; Elsworth 2008; Saposnik 2010; Yavuzer 2008). Effective upper limb interventions that can be delivered across the stroke pathway—in hospitals and rehabilitation, outpatient and community settings—are clearly needed. In addition to interventions that can be delivered by healthcare professionals, self‐management strategies must be available to promote more independent recovery among stroke survivors.
Generally, the interventions used by rehabilitation professionals will consider each patient's goals and will be selected after assessment of a patient's upper limb impairments, together with their effects on activity and level of participation (Langhorne 2011). However, upper limb rehabilitation interventions could also be delivered as part of a group exercise class or circuit training. Additional interventions may be selected by patients, for example, commercial gaming devices or fitness equipment that can be used at home or in fitness centres.
A wide range of interventions can be delivered in an attempt to improve the function of upper limbs after stroke. Such interventions may be aimed at particular impairments (e.g. muscle weakness) or functional movements (e.g. grasp and release). Upper limb interventions may be used separately or may be combined so that treatment addresses the multi‐factorial nature of the deficits that may follow stroke, integrating a number of techniques to address problems and secondary complications. Therefore upper limb rehabilitation after stroke is likely to involve a complex intervention that requires the co‐operation of patient, carers and the rehabilitation team.
Upper limb rehabilitation interventions may be delivered at different doses, with 'dose' referring to the intensity (effort), frequency and duration (time) of an intervention (Bosch 2014; Cooke 2010; Kwakkel 2006; Page 2012). The dose of an intervention is likely to affect the outcome (Cooke 2010; Kwakkel 2006). (See Published notes for full definitions of doses used within this Overview.)
Interventions relevant to this Cochrane overview include but are not limited to the following, which are listed here in alphabetical order.
Bilateral arm training
Simultaneous bilateral arm training uses activities for which both arms perform identical movements at the same time (McCombe Waller 2008; Mudie 2000; Stewart 2006). Different forms of simultaneous bilateral arm training are available. Some use 'free' arm movements, and others use mechanical or robotic devices to drive active or passive movement of the affected limb through identical movement of the less‐affected upper limb. The key ingredient of this form of intervention is interlimb coupling, which is thought to rebalance interhemispheric inhibition, activate the affected hemisphere (Stinear 2008) and improve motor control within the affected limb (McDermott 2012).
Biofeedback
Biofeedback provides enhanced awareness of movement or function, with the goal of improving voluntary control of that movement or function. Electromyographic (EMG) biofeedback provides information about muscle activity, which is detected through surface electrodes placed on the skin, or through needle or fine‐wire electrodes inserted into the muscle, and is fed back to the patient via electrical activity displayed on a visual display unit or by an auditory signal (Crow 1989; Wolf 1983).
Bobath approach
The Bobath approach, which is classed as a 'neurodevelopmental technique,' was originally thought to reduce abnormal tone by positioning, while handling techniques are used to facilitate normal movement (Bobath 1990; Davies 1985; Davies 1990; Raine 2009). This approach has evolved over time (Lennon 2000) and has recently been defined as "a problem solving approach to the assessment and treatment of individuals with disturbances of function, movement, and postural control due to a lesion of the central nervous system" (Kollen 2009). The content of interventions based on the Bobath approach has been widely debated, and lack of agreement on what constitutes 'Bobath' poses challenges (DeJong 2004; Langhammer 2012; Mayston 2008; Tyson 2009).
Brain stimulation
Transcranial direct current stimulation (tDCS)
This is thought to have an effect similar to that of TMS (above), but it is applied through two surface electrodes placed on the skull (Dayan 2013; Hummel 2006).
Transcranial magnetic stimulation (TMS)
TMS involves stimulation of the brain applied via a wired coil positioned on the head over the sensory motor area (Dayan 2013; Hummel 2005). Rapidly changing magnetic fields, initiated by a brief high‐intensity electrical current, stimulate the central nervous system. Repetitive pulse TMS (rTMS) is proposed as a treatment for people with stroke, as it can be used to modulate excitability in the cerebral cortex over longer periods of time than are required by other types of TMS (Kagan 2012a).
Complementary interventions
Complementary therapies that can be used to promote upper limb function after stroke include traditional Chinese therapies, acupuncture and homeopathy. With acupuncture, needles are inserted at meridian points or trigger points with the objective of improving neurological function after stroke (Wu 2009).
Constraint‐induced movement therapy (CIMT)
In CIMT, or 'forced use therapy,' the non‐affected hand is placed in an arm sling or, more commonly, in a mitt that prevents its use during fine movement (Page 2001; Page 2002; Taub 1993; Uswatte 2006; Wolf 2006). With the non‐affected hand 'constrained,' operant conditioning (i.e. learning through consequences) is used to increase task difficulty for the affected hand in small amounts, so the stroke survivor can succeed in using the affected limb. Progression is therapeutically directed by using these shaping techniques, thereby reducing learned non‐use.
Electrical stimulation
Electrical stimulation involves stimulation applied to muscles through surface electrodes or percutaneous electrodes (which penetrate the skin). Electrical stimulation is usually delivered with the aim of strengthening a muscle contraction or improving voluntary motor control, or both. Functional electrical stimulation (FES) involves stimulation aimed at replacing or assisting a voluntary muscle contraction during a functional task (Roy 2010). Several stimulators are available; these provide single‐channel or multi‐channel stimulation that can be programmed to an appropriate frequency, bandwidth and strength, to control the duration of stimulation and the duration of intervals between stimulation. Muscles can be stimulated cyclically, triggered by movement or triggered electromyographically (by initiation of muscle activity within the muscle to be stimulated). Electrical stimulation applied to the whole hand through a glove may provide sensory stimulation (Dimitrijevic 1996; Pomeroy 2006).
'Hands‐on' therapy (manual therapy techniques)
The arm and hand joints may be moved by a therapist, who may provide partial or full assistance if the patient's active control is inadequate: Such movement may be aimed at maintaining joint and soft tissue mobility. Passive or active movements of the wrist and interphalangeal and metacarpophalangeal joints of the fingers and thumb can be used to stretch the wrist and finger muscles to their maximum pain‐free range. Mobilisation of an accessory movement of a small joint by a therapist may be applied to maintain or increase movement of these joints, or to treat joint pain.
Mental practice
Exercise‐based and functional movement–based interventions can involve overt as well as covert techniques to promote skill acquisition (Jeannerod 2005). Covert techniques commonly involve observational learning and mental practice. Mental practice, sometimes called mental imagery or motor imagery, is a training method that involves no actual movement. However, during mental practice training, mental rehearsal is often combined with (or followed by) physical practice when possible. Mental practice training may focus on goal attainment or anxiety management, but the type used most often in stroke rehabilitation involves cognitive rehearsal of specific activities by imagining task performance (Page 2007).
Mirror therapy
Exercise‐based interventions can use stimulation of other (non‐motor) pathways to promote functional movement (Johannson 2012). Mirror therapy is based on visual stimulation. In mirror therapy, a mirror is placed in the patient's sagittal plane, thus reflecting the non‐affected side as if it were the affected side, so that movements of the non‐affected limb give the illusion that the affected limb is moving (Michielsen 2010).
Music therapy
Music therapy may be used to stimulate movement, cognition and speech, to enhance relaxation or to reduce pain; it is generally delivered by certified/registered music therapists. Music therapy interventions may include listening and moving to music, performing, improvising or composing music, singing or performing vocal activities. Music may be combined with other modalities. Music can be used to cue rhythmical functional movement: This is known as rhythmical auditory stimulation (Bradt 2010).
Pharmacological interventions
A number of systemic drugs (drugs that affect the whole body) are generally used to reduce spasticity, including baclofen, diazepam and dantrolene. Botulinum toxin can be injected to provide a focal treatment when spasticity in a specific muscle or muscle group is the cause of problems (Cousins 2010; Shaw 2011).
Repetitive task training
Repetitive task training involves the repeated practice of functional tasks (whole task practice when possible), combining elements of intensity of practice and functional relevance (French 2007) (see also 'Task‐specific training,' below). Repetitive task training—when progressed appropriately—is thought to reduce muscle weakness and to form the physiological basis of motor learning (Butefisch 1995). Key components of skill acquisition, such as active cognitive involvement, functional relevance of the task and knowledge of results and performance, are hypothesised to enhance learning during repetitive task training (Schmidt 2014). These components are central to the so‐called 'movement science' approach to stroke rehabilitation (Carr 1987; Carr 1990; Carr 1998).
Findings from animal research have shown that neuroplastic changes emerge only after new skills are learned—not after repetitive movement (Nudo 2000; Nudo 2003a; Nudo 2003b). Hence, it is important to emphasise that the 'repetition' within repetitive task training refers to repeated practice of new functional skills—not to the reproduction of identical movements per se.
Robotics
Electromechanical and robotic devices are devices that can move passive limbs, while providing assistance or resistance to movement of a single joint or control of intersegmental co‐ordination (Mehrholz 2012). Robotic devices may be used to deliver or enhance repetitive task training or task‐specific training, and are thought to support motor learning and increase motor control and strength.
Sensory interventions (interventions to improve sensory function)
Movement and somatosensory awareness can be enhanced in several ways, including techniques such as sensory reeducation, tactile kinaesthetic guiding, repetitive sensory practice or desensitisation (Doyle 2010). Sensory and positional awareness may be stimulated by passive or active‐assisted movement, as well as by stimulatory techniques such as stroking and tapping.
Strength training
Muscle strength training is directed at working a specific muscle, or group of muscles, by using voluntary control. Movement may be assisted or resisted by a therapist or by gym equipment (Harris 2010b). Alternatively, exercises may be done in classes directed by a therapist or exercise professional, may utilise various exercise machines or may involve circuit training.
Stretching and positioning
Several techniques may be used to optimise joint position and to maintain or regain soft tissue length. These techniques often involve the use of assistive devices, such as supportive devices, splints and orthoses. Shoulder subluxation has traditionally been treated with supportive devices (Ada 2005). Splints are external devices used to fix a joint in one position, often used to support the hand or fingers in an optimal position. Orthoses are external devices (similar to splints) applied to elbow, wrist and/or finger joints to optimise position, provide stability and prevent, limit or assist movement (Hoffman 2011; Lannin 2007). These may be used alone or with electrical stimulation in a neuroprosthesis (an orthotic device with prepositioned electrodes that assist function) (Hendricks 2001).
Surgical interventions
Several different surgical interventions could be used to promote upper limb function after stroke. For example, tendon surgery can relieve shoulder pain and reduce spasticity in the upper limb after stroke (Namdari 2012; Pomerance 1996), but it is not part of routine clinical practice in the UK.
Task‐specific training
Task‐specific training, also referred to as functional task training, involves practice of tasks relevant to daily life, including part‐ and whole‐task practice (Van Peppen 2004). The 'motor learning,' 'motor relearning' or 'movement science' approach involves functional or task‐specific training (Carr 1987; Carr 1990; Carr 1998) and is often supplemented by other modalities, such as assistive technologies (Timmermans 2009). Task‐specific training may be carried out as a form of repetitive task training (see above).
Reach‐to‐grasp exercise is a form of task‐specific training, as reach‐to‐grasp is a common functional task performed by the upper limb.
Virtual reality
Virtual reality involves interactive simulations created with computer hardware and software to provide a simulated practice environment, as well as feedback on movement execution or goal attainment, or both (Laver 2011; Merians 2006). Virtual reality enables people to engage in activities within an environment that appears and feels similar to real‐world objects and events, using devices such as a keyboard and a mouse, or through multi‐modal devices such as a wired glove (Kagan 2012b). Virtual reality may also be used with robotic devices that assist or resist movement (see above).
How the intervention might work
Rehabilitation of the arm following stroke is a complex intervention that integrates different modalities to address deficits that are often multi‐factorial, with clinicians individualising treatment programmes in an attempt to optimise outcomes for patients. Understanding of the precise mechanisms of action for many of the interventions delivered by clinicians is limited. The ways that interventions are thought to work can be described by using several different frameworks. The International Classification of Functioning, Disability and Health, known more commonly as the ICF, can be used to describe whether treatments are aimed at reducing impairments, increasing activity or increasing participation (ICF 2001). Alternatively, treatments can be described as being used to prevent or reduce the development of complications (e.g. shortening of muscles (contractures)); to restore original status or to substitute with compensatory mechanisms (altered neural pathways or movements); or to utilise compensatory devices (e.g. neuroprostheses) (Dobkin 2005). Treatments may also prime (act to prepare the sensory motor system for practice) or augment (enhance sensorimotor function during practice), thereby maximising the benefits derived from task‐specific practice (Pomeroy 2011).
For the purposes of this review, we have used a taxonomy of rehabilitation interventions based on work arising from a major multi‐site stroke rehabilitation study (DeJong 2004). This taxonomy provides a model that describes key types of rehabilitation interventions (Figure 1) and attempts to encapsulate the diversity and complexity of rehabilitation treatments. This taxonomy shows that neuromuscular and musculoskeletal interventions may work by leading to and supporting the practice of functional activities. Additional interventions using cognitive, perceptual and sensory attributes can be used to enhance skill acquisition. Such interventions may be delivered by the therapist with or without devices (e.g. orthoses) or additional modalities (e.g. electrical stimulation). These interventions may be delivered in various settings that may impact the people available to provide the intervention or the setting (e.g. hospital or home) of such work, and may influence motivation and integration with ADLs.
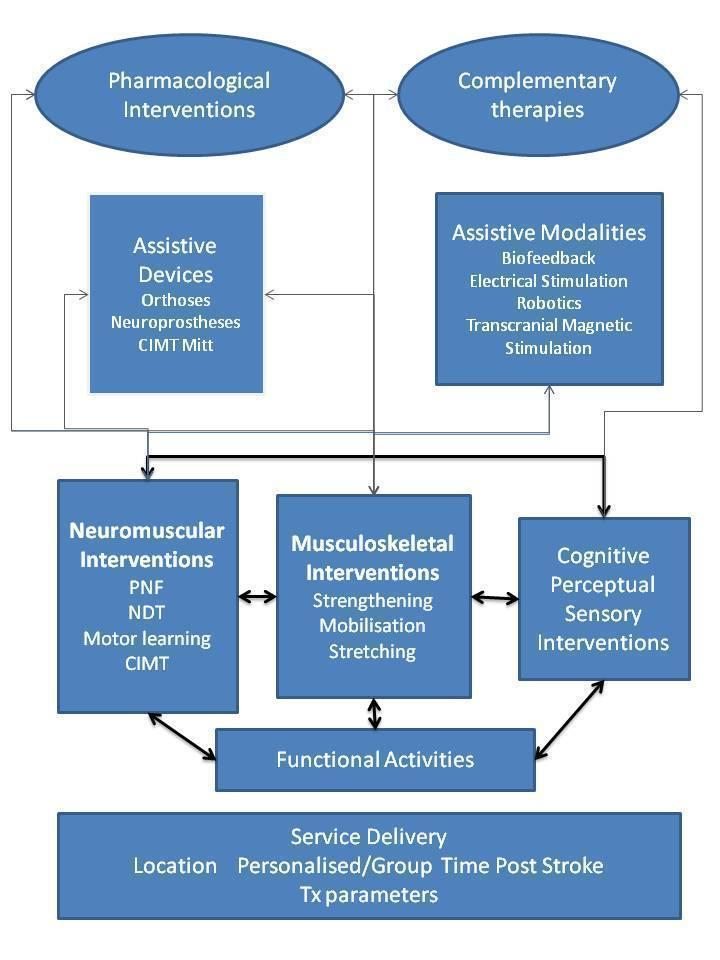
Taxonomy of rehabilitation interventions used within this overview.
Key: CIMT: constraint‐induced movement therapy; NDT: neurodevelopmental treatment; PNF: proprioceptive neuromuscular facilitation; Tx: treatment.
References relevant to the intervention mechanisms explained in this section are cited within Description of the interventions. The ways in which individual treatment components may work are briefly outlined below.
Musculoskeletal interventions
Joint contractures and reduced range of motion at joints can result from various factors, including reduced muscle length and increased stiffness of muscle and connective tissue. The tendency toward progressive loss of range may be reduced by moving the joints through a full range of motion with pressure at the end of the range; stiffness may be reduced by repetitive movements. Such motion can be delivered by manual therapy or self‐stretching. Mechanical and electromechanical devices may provide or assist movement, and electrical stimulation can cause muscle contractions that may also have the effect of lengthening the antagonist of the stimulated muscles and causing joint motion.
Muscle weakness may be reduced through exercises that utilise muscles or by electrical stimulation of muscles. Muscle can be strengthened by graduated resistance exercises. When muscles are unable to move the limb against gravity, manual support provided by the therapist or a weight‐relieving system (e.g. robot) allows weakened muscles to produce limb movement. Electrical stimulation can be used to strengthen muscles when the muscle contraction produced by stimulation is of adequate intensity. Some improvements in muscle strength and endurance may be gained during repetitive task training.
Neuromuscular interventions
Normal co‐ordination can be impeded by stroke. Abnormal movement synergies may be seen (e.g. wrist flexion with finger flexion when attempting to grasp), thus some practitioners consider that movement needs reeducation.
Bilateral training is thought to utilise interlimb coupling, so that the intact brain hemisphere facilitates activation of the damaged hemisphere.
Repetitve task training may augment the activity of neural pathways that underlie specific functions and promote acquisition of the tasks practised.
CIMT is used to overcome the acquired behaviour of non‐use of the affected arm after stroke. It focuses movement practice on the affected arm and hand during prolonged periods of intense, progressively structured activities, for which success is rewarded with enthusiastic praise. Use of the non‐affected arm and hand is inhibited by use of a constraining device, such as a mitt or an arm sling.
In mirror therapy, the same cortical areas of the brain are active during action—and observation of action—of the reflected image of unimpaired arm movement: This affects the excitability of the motor area of the affected limb and limits the development of learned non‐use.
Mental practice has been used to enhance elite performance in sports, dance and music, and thus has potential for benefit in the rehabilitation context. A considerable body of evidence from non‐impaired people shows that similar areas of the brain are active whether movement is actual, observed or imagined, with the exception of the areas responsible for execution of actual movement.
Assistive devices
A wrist orthosis can support the wrist in an extended position; this may facilitate gripping. A neuroprosthesis comprises an orthosis together with prepositioned electrodes that are stimulated to assist grasp and release.
Assistive modalities
Proprioceptive and other sensory deficits reduce 'normal feedback.' Biofeedback systems utilise signals produced by muscle activity to inform the user about the extent and timing of muscle activity by means of a visual or auditory display, or both. Electromechanical (robotic) systems use actuators (complex control mechanisms) to assist and to provide feedback on limb movement visual display units. Alternatively, a game scenario is used to provide feedback.
Electrical stimulation may be used to reeducate movement when the stimulator has a number of channels that can be programmed to stimulate muscles in the desired sequence.
Sensory awareness may be increased by tactile stimulation. Electrical stimulation at a sensory level can be applied via a glove, again increasing awareness.
Non‐invasive brain stimulation (TMS and tDCS) can be used to enhance motor skills, although the specific underlying mechanisms of stimulation‐induced effects remain largely unknown (Dayan 2013).
Virtual reality can offer the motivation for practising specific actions at the intensity required to induce cortical reorganisation. Most systems provide knowledge of the result (i.e. whether or not the outcome was successful), although there is the potential for knowledge of performance (i.e. details of the effectiveness of a movement, for example, through provision of kinematic feedback). Tasks can be graded by clinicians to provide a progressively challenging practice that can be performed without direct clinical supervision.
Such technologies may be used individually or integrated with other therapeutic modalities (Burridge 2010).
Pharmacological interventions
Systemic antispasticity medications, such as baclofen and diazepam, act on the nervous system to reduce nerve signals to muscles, thereby reducing spasticity. Dantrolene acts within the muscle by interfering with calcium release from the sarcoplasmic reticulum, weakening muscle contractile function and thus acting as a muscle relaxant. Spasticity can also be treated focally with injections of botulinum neurotoxin. Within muscles, this neurotoxin inhibits the release of acetylcholine, thereby blocking nerve impulses and limiting hyperactivity in treated muscles.
Complementary medicine
Acupuncture is thought to cause biological responses within a person's biochemistry or circulation. Sensory neurons may transmit effects distal to the needle insertion site, thus affecting various physiological systems.
Treatment setting
Services can be delivered at different locations that may affect treatment through environmental and societal factors. Some stroke survivors may be motivated by group sessions. In early supported discharge, the rehabilitation team may be able to advise on how to integrate rehabilitation activities into home life. Accessibility to some interventions may be restricted within some treatment settings as the result of resource issues such as equipment availability or staff training or skills.
Why it is important to do this overview
Identifying the most effective upper limb rehabilitation interventions is a recognised priority for stroke research. The Chartered Society of Physiotherapy used a modified Delphi technique, which identified the top priority question for physiotherapy research in the field of neurology as this: "What is best practice in the rehabilitation of the upper limb in patients with stroke with respect to timing, content and dosage?" (Rankin 2012). Furthermore, in our James Lind Alliance priority setting project, which involved equal involvement among stroke survivors, carers and healthcare professionals, the question "What are the best treatments for arm recovery and function?" was included in the Top 10 agreed upon research priorities, out of 226 unanswered research questions identified as relating to life after stroke (Pollock 2012).
Given the importance of upper limb rehabilitation and associated research, it is not surprising that a substantive and growing number of randomised controlled trials (RCTs) are examining the effectiveness of rehabilitation interventions aimed at promoting upper limb recovery (Langhorne 2009). Evidence of the effectiveness of many of these interventions has been synthesised and summarised within several systematic reviews. The rapidly growing body of systematic reviews can be overwhelming for decision makers and healthcare practitioners who do not have time to keep up‐to‐date with this evidence base (Bastian 2010). Furthermore, although Cochrane systematic reviews have synthesised available RCT evidence, these reviews of upper limb interventions generally explore the effects of specific, single, interventions compared with placebo or control interventions (e.g. French 2007; Laver 2011; Pomeroy 2006; Sirtori 2009). Arguably, synthesis of evidence related to single, specific upper limb interventions fails to facilitate translation of evidence into clinical practice or decision making for which the relative effectiveness of different treatment options must be considered (Jansen 2013). A Cochrane overview of upper limb rehabilitation reviews will synthesise all high‐quality evidence about upper limb rehabilitation interventions into an accessible, comprehensive document, thus supporting clinicians and policy makers in decision making for stroke rehabilitation (Becker 2011).
Objectives
To carry out a Cochrane overview by synthesising systematic reviews of interventions provided to improve upper limb function after stroke.
Methods
Criteria for considering reviews for inclusion
We included all reviews that met our selection criteria and that are published in the Cochrane Database of Systematic Reviews (CDSR) or the Database of Abstracts of Reviews of Effects (DARE).
It has been argued that, as the quality of Cochrane systematic reviews has consistently been found to be better than that of non‐Cochrane reviews (Delaney 2007; Farmer 2012; Jadad 1998; Jørgensen 2008; Moher 2007; Moja 2005; Olsen 2001), the primary aim of a Cochrane overview should be to summarise multiple Cochrane intervention reviews (Becker 2011). However, as some time has passed since some Cochrane reviews were updated, we anticipated that some non‐Cochrane reviews may be more current. We therefore believed it was essential to consider other high‐quality reviews to ensure that our overview is as comprehensive and current as possible. Systematic reviews included in DARE, which comprises the results of extensive searches carried out by the Centre for Reviews and Dissemination, at the University of York (DARE), have been independently assessed by two overview authors to confirm that a number of key quality criteria are met. This application of quality criteria ensures that systematic reviews in DARE have (1) reported inclusion or exclusion criteria, (2) employed an adequate search strategy and (3) synthesised included studies. In addition, to be included on DARE, a review must be considered to have assessed the quality of the included studies or provided sufficient details about individual included studies to enable assessment of quality by a reader.
To be eligible for inclusion, reviews had to meet the following criteria.
-
Included RCTs. If a review included quasi‐RCTs (QRCTs) as well as RCTs, we included data from the QRCTs if they had been pooled with data from the RCTs. However, if it was possible to extract data pertaining only to the RCTs, we did this in preference to including data from QRCTs. In the event that we included evidence from QRCTs, we planned to highlight and discuss the implications of including this evidence. If a review included other studies in addition to RCTs (e.g. before‐and‐after studies), we included the review, but did not include the evidence from these other study types. We excluded reviews of other study designs or of qualitative studies.
-
Included studies in which the participants are adults with a clinical diagnosis of stroke. We included reviews that included studies with other participants in addition to people with stroke (e.g. adults with other neurological diseases or traumatic brain injury) when at least 75% of the participants were stroke patients, or when data on stroke patients had been presented and analysed as a separate subgroup; we will highlight when data are reported from a mixed population.
-
Investigated an intervention for which the primary aim is to improve functional recovery or to reduce impairment—or both—of the upper limb.
-
Investigated the effects of interventions for the upper limb. This may include comparisons of interventions with control, placebo or standard care; comparisons of one active treatment versus another active treatment; and comparisons of different doses, intensities or timing of delivery of the same intervention.
When we identified overlapping reviews (i.e. reviews exploring the same participants, interventions, comparisons and outcomes), we systematically identified the most up‐to‐date and comprehensive review and excluded reviews that overlapped with this. When it was unclear whether reviews overlapped, we systematically explored methodological features of the reviews and reached consensus on which reviews should be included or excluded to avoid overlap (see Data extraction and management for additional details).
We included any review for which the primary aim of the intervention was to improve functional recovery, or reduce impairment, of the upper limb, regardless of the outcome measures reported.
Primary and secondary outcomes of interest to this overview are as follows.
Primary outcome
The primary outcome for the overview involved upper limb function, including measures that examine active function, dexterity, object manipulation and reach‐to‐grasp, grip or pinch. For synthesis and analysis within the overview, we planned to group measures of upper limb function according to whether, primarily, they assess function of the arm (including shoulder, elbow and wrist) or function of the hand (including fingers). This outcome can be measured by using a range of measures including, but not limited to, those that follow.
Arm function
-
Action Research Arm Test (ARAT) (Lyle 1981) or Upper Extremity Function Test (Carroll 1967).
-
Box and Block Test (Desrosiers 1994; Mathiowetz 1985).
-
Wolf Motor Function Test (WMFT) (Wolf 2001).
-
Frenchay Arm Test (Heller 1987).
-
Functional Test of the Hemiparetic Upper Extremity (Wilson 1984).
-
Upper Extremity Performance Test for the Elderly (TEMPA) (Desrosiers 1993).
-
Sodring Motor Evaluation of Stroke Patients—arm section (Sodring 1995).
-
Chedoke Arm and Hand Activity Inventory (Barreca 2005).
-
Motor Assessment Scale—hand movement or advanced hand movement scores (Carr 1985).
Hand function
-
ABILHAND (Gustafsson 2004).
-
Jebsen Hand Function Test (Jebsen 1969).
-
Nine‐Hole Peg Test (Kellor 1971).
-
Purdue Peg Test (Desrosiers 1995).
-
Stroke Impact Scale (Duncan 1999).
Secondary outcomes
Secondary outcomes include measures of motor impairment, active movement and co‐ordination and performance of ADLs and extended ADLs.
Motor impairment (including deficits in active movement and co‐ordination)
A wide range of methods, measures and tools can be used to assess motor impairment. We planned to include assessments that could be categorised into the following four motor impairment outcomes, using one of the measures listed.
-
Motor impairment scales.
-
Fugl‐Meyer Assessment of Sensorimotor Recovery after Stroke (upper limb section) (Fugl‐Meyer 1975).
-
Wolf Motor Function Test (WMFT) (Wolf 2001).
-
Motricity Index (Demeurisse 1980).
-
Rivermead Motor Assessment (arm section) (Lincoln 1979).
-
Motor Club Assessment (Ashburn 1982).
-
Motor Status Score (Ferraro 2002).
-
-
Measures of movement and co‐ordination.
-
Temporal measures.
-
Movement time for completion of various tasks.
-
Number of movements executed within stated time.
-
Movement speed/velocity.
-
-
Spatial outcomes.
-
Kinematic measures.
-
Spatial accuracy.
-
-
-
Strength outcomes.
-
Grip strength.
-
Medical Research Council (MRC) Scale (MRC 1975).
-
Dynamometer scores (including Jamar) (Bohannon 1987).
-
-
Muscle tone/spasticity.
-
Ashworth Scale (Ashworth 1964), or Modified Ashworth Scale (Bohannon 1987).
-
Electromyographic (EMG) activity.
-
Reflex activity (e.g. H reflex).
-
Performance of activities of daily living
We included measures of performance of ADLs including feeding, dressing, bathing, toileting, simple mobility and transfers. Common outcome measures include global measures of ADLs, such as Barthel ADL Index (Mahoney 1965), Rivermead ADL Assessment (Whiting 1980), Rivermead Motor Ability Scale (Collen 1991), Rankin Scale (Bonita 1988), Functional Independence Measure (FIM) (Keith 1987), Katz Index of Activities of Daily Living (Katz 1970) and Rehabilitation Activities Profile (Van Bennekom 1995).
Performance of extended activities of daily living (ADLs)
We planned to include measures of performance of extended ADLs including shopping and household tasks. Common outcome measures can be assessed by using the following tools.
-
Nottingham Extended Activities of Daily Living (Nouri 1987).
-
Rivermead Extended Activities of Daily Living (Rossier 2001).
-
Frenchay Activities Index (Holbrook 1983).
We also documented other outcomes reported in included reviews, including measures of participation, mood, adverse events and quality of life.
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Reviews of Effects (DARE) (The Cochrane Library; searched 14 June 2013).
We developed a sensitive search strategy for The Cochrane Library with the help of the Cochrane Stroke Group Trials Search Co‐ordinator (Appendix 1).
In an effort to identify ongoing systematic reviews, we searched for protocols of Cochrane reviews in the CDSR (The Cochrane Library; searched 14 June 2013) and PROSPERO, an international prospective register of systematic reviews (www.crd.york.ac.uk/prospero/; searched 11 June 2013) (Appendix 2). We contacted the authors of protocols meeting our selection criteria and included any reviews that were completed before the end of February 2014. When protocol or review authors had indicated when a review should be finished, we sent reminder emails in advance of this date to check on progress.
To ensure that data included in the overview were as current as possible, we contacted authors of relevant reviews to ascertain details of planned updates. We also contacted authors of all relevant Cochrane reviews, Cochrane protocols and other reviews in an effort to identify additional relevant systematic reviews.
We searched for relevant reviews in all languages and arranged translation when necessary.
Data collection and analysis
Selection of reviews
Two overview authors (SF and AP) independently assessed titles and abstracts of records identified by the electronic searches and excluded obviously irrelevant reviews. We obtained the full text of the remaining reviews, then two overview authors (SF and AP) independently selected systematic reviews including trials that met the following criteria.
-
Included adults with a clinical diagnosis of stroke.
-
Investigated any intervention targeted at improving functional recovery of the upper limb.
-
Assessed outcomes of upper limb motor function, ADLs, motor impairment, extended ADLs, participation, quality of life or adverse events.
If disagreement arose between these two overview authors, they consulted a third overview author (FvW) to reach consensus through discussion.
Two overview authors (FvW and JM) independently assessed articles published in German, and we assessed articles published in Chinese with the assistance of a Chinese speaker with experience in appraising stroke rehabilitation trials (Pei Ling Choo). We planned to seek translations of publications in other languages if this was required.
Data extraction and management
Two overview authors (SF and AP) extracted data independently. They resolved disagreements by discussion, with assistance from a third overview author (FvW), if necessary. We used a data collection form that was specifically designed and piloted by the overview author team.
Onto this form, we extracted and recorded key features of each review including details of the aims and rationale, types of studies, participants, interventions, comparisons, outcomes assessed and date of last search.
We systematically synthesised, using a spreadsheet, the studies included within all identified reviews to explore whether any reviews covered the same studies. When overlap between reviews was noted, two overview authors (SF and AP) discussed the overlap with consideration of each review question and comparisons explored, the date of the last search and key aspects of methodological quality (e.g. types of studies included, risk of bias assessment). We used these details to reach agreement regarding which of the reviews should contribute data to the results (e.g. if two reviews of similar methodological quality and with similar trials addressed the same question, we would extract data only from the review with the more up‐to‐date search strategy that had identified trials published more recently).
For each comparison reported in each included review, one overview author (SF) systematically extracted data on the risk of bias (as documented in the published review) of trials within the comparison and the results of any meta‐analyses performed. These data were then checked by a second overview author (AP) with reference to the published review.
Assessment of methodological quality of included reviews
Quality of included reviews
Two overview authors (SF and AP, FvW, JM or MB) independently assessed the methodological quality of included reviews, basing this assessment on the AMSTAR measurement tool (Shea 2007; Shea 2009) and considering the following key domains.
-
Clarity of review objective.
-
Description of trial eligibility criteria.
-
Extent of searching undertaken.
-
Transparency of assessment process.
-
Assessment of publication bias.
-
Assessment of heterogeneity.
The AMSTAR measurement tool has been demonstrated to be valid and reliable (Shea 2009). However, questions within the AMSTAR tool are often multi‐faceted, which complicates the rating process. Univariable questions derived from these multi‐faceted questions have previously been used effectively to assess risk of bias in review articles (Farmer 2012). Therefore we formulated simple univariable questions for each of the AMSTAR questions/criteria, so that we have an item‐specific record of information obtained from each review that we assessed. These questions are outlined in Table 1, and additional clarification notes are provided in Appendix 3. For each of the questions within our modified AMSTAR (mAMSTAR) tool, two overview authors independently documented each answer as 'yes,' 'no,' 'unsure' or 'not applicable,' and provided relevant comments (in a similar format to that used with the Cochrane 'Risk of bias' tool). We developed and implemented an objective algorithm to determine responses to the original AMSTAR questions based on agreed mAMSTAR responses (Appendix 3).
| AMSTAR questions/criteria | Dichotomous questions used to assess quality of reviews |
| 1. Was an 'a priori' design provided? The research question and inclusion criteria should be established before the conduct of the review. | 1.1 Were review subjects clearly defined? |
| 1.2 Were review interventions described? | |
| 1.3 Were review comparisons specified? | |
| 1.4 Were review outcomes specified? | |
| 2. Was there duplicate study selection and data extraction? There should be at least two independent data extractors, and a consensus procedure for disagreements should be in place. | 2.1 Were studies assessed for inclusion by two independent review authors? |
| 2.2 Were data extracted by two independent review authors? | |
| 2.3 Was there a clear procedure for resolving any disagreements? | |
| 3. Was a comprehensive literature search performed? At least two electronic sources should be searched. The report must include years and databases used (e.g. CENTRAL, EMBASE, MEDLINE). Key words and/or MeSH terms must be stated and, where feasible, the search strategy should be provided. All searches should be supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found. | 3.1 Were at least two major databases searched? |
| 3.2 Were dates searched reported? | |
| 3.3 Were key words stated? | |
| 3.4 Were MeSH terms stated? | |
| 3.5 Was the search strategy provided or available on request? | |
| 3.6 Were searches supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found? | |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? The review authors should state that they searched for reports regardless of their publication type. The review authors should state whether or not they excluded any reports (from the systematic review), based on their publication status, language, etc. | 4.1 Were studies searched for and included regardless of their publication type? |
| 4.2 Were papers included regardless of language of publication? | |
| 5. Was a list of studies (included and excluded) provided? A list of included and excluded studies should be provided. | 5.1 Was there a list of included studies? |
| 5.2 Was there a list of excluded studies? | |
| 5.4 Was there a flow diagram? | |
| 6. Were the characteristics of the included studies provided? In an aggregated form such as a table, data from the original studies should be provided on the participants, interventions and outcomes. The ranges of characteristics in all the studies analysed (e.g. age, race, sex, relevant socioeconomic data, disease status, duration, severity, other diseases) should be reported. | 6.1 Were details provided on the participants of included studies (including age, gender, severity of stroke, time since stroke)? |
| 6.2 Were details provided on the interventions of included studies? | |
| 6.3 Were details provided on the outcomes reported by included studies? | |
| 7. Was the scientific quality of the included studies assessed and documented? 'A priori' methods of assessment should be provided (e.g. for effectiveness studies if the author(s) chose to include only randomised, double‐blind, placebo‐controlled studies, or allocation concealment as inclusion criteria); for other types of studies, alternative items will be relevant.
| 7.1 Was the scientific quality of included studies assessed? |
| 7.2 Was this done by at least two independent review authors? | |
| 7.3 Was the scientific quality of studies documented? | |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? The results of the methodological rigour and scientific quality should be considered in the analysis and the conclusions of the review, and explicitly stated in formulating recommendations.
| 8.1 Were the results of methodological rigour of the included studies considered in the analysis of the review? |
| 8.2 Were the results of the scientific quality of the included studies considered in the conclusions and/or recommendations of the review? | |
| 9. Were the methods used to combine the findings of studies appropriate? For the pooled results, a test should be done to ensure the studies were combinable, to assess their homogeneity (i.e. Chi2 test for homogeneity, I2). If heterogeneity exists, a random‐effects model should be used and/or the clinical appropriateness of combining should be taken into consideration (i.e. Is it sensible to combine?).
| 9.1 Were the methods used to combine the findings of studies clearly described or referenced to appropriate text, or both? |
| 9.2 If results are pooled, are the mean and confidence intervals (or equivalent data) reported? | |
| 9.3 If results are pooled, is a test of heterogeneity reported? | |
| 9.4 Have the review authors stated a definition of statistical heterogeneity? | |
| 9.5 If statistical heterogeneity is present or suspected, has a random‐effects model been used? | |
| 10. Was the likelihood of publication bias assessed? An assessment of publication bias should include a combination of graphical aids (e.g. funnel plot, other available tests) and/or statistical tests (e.g. Egger regression test).
| 10. Was the likelihood of publication bias assessed? |
| 11. Was the conflict of interest stated? Potential sources of support should be clearly acknowledged in both the systematic review and the included studies. | 11.1 Was there a conflict of interest statement?
|
| 11.2 Was the review free of any conflicts of interest?
|
When overview authors were authors of an included review, they were not involved in assessment of methodological quality of that review, and this was done independently by two other overview authors.
Note: See Differences between protocol and review for a description of amendments made to our modified AMSTAR during the review process, including the introduction of objective criteria to determine answers to the original AMSTAR questions based on responses to our modified AMSTAR responses.
Quality of evidence in included reviews
We did not reassess the quality of individual studies included within reviews but reported the quality of individual studies according to the review authors' assessment. We documented the quality of evidence synthesised within the reviews based on criteria considered within the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (Guyatt 2008), which includes the following.
-
Risk of bias due to flawed design or conduct of studies.
-
Imprecision (e.g. when confidence intervals for treatment effect are wide).
-
Inconsistency (e.g. when point estimates vary widely, I² is large).
-
Indirectness (e.g. variations in participants, interventions, comparisons and outcomes).
-
Publication bias (may be explored with the use of funnel plots and classed as not suspected, suspected, strongly suspected or very strongly suspected).
Two overview authors (SF and AP) assessed and documented risk of bias related to study design, imprecision, inconsistency, indirectness and publication bias for each outcome within comparisons presented in included reviews. Owing to the degree of subjectivity required when the criteria above are considered and the GRADE level of evidence determined, we developed objective criteria to enable transparent, reproducible assignment of GRADE levels of evidence. The criteria we used in our judgement of each comparison presented within every included review were based on systematic assessment of:
-
the number of participants within the analysis;
-
the risk of bias of trials contributing participants to the analysis;
-
heterogeneity within the analysis, as determined by I²; and
-
the methodological quality of the review.
Two overview authors (SF and AP) worked together to ensure consensus and consistency of entry of objective data pertaining to these criteria onto a spreadsheet, and we used an objective algorithm to determine whether evidence arising from each comparison was classed as high, moderate, low or very low within GRADE, based on the following definitions (Guyatt 2008).
-
High quality: when further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate quality: when further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low quality: when further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low quality: when we are very uncertain about the estimate.
Details of the objective criteria and algorithm that we used to determine the GRADE level of evidence are provided in Appendix 4.
Note: See Differences between protocol and review for a more detailed description of why we developed these objective criteria.
Data synthesis
Two overview authors (SF and AP) independently extracted relevant data from the reviews and systematically synthesised these data within tables. In these tables, we documented the primary and secondary outcomes of each intervention comparison in an included review, as well as the number of studies and the number of participants included in the comparison, and (when available from the reviews) the mean difference (or standardised mean difference), 95% confidence intervals and I² statistic for heterogeneity (Deeks 2001). Comparisons were determined by data provided in the included reviews. This table also synthesised key information related to the quality of evidence, and documented eligibility criteria, study characteristics and the primary outcome of each review.
Statistical analyses
Indirect comparisons are those made between interventions that have not been compared directly with each other within the same trial (Becker 2011). We had planned to complete statistical analysis using indirect comparisons of interventions included in different reviews only if it was judged that trials included in the reviews had a low level of clinical and methodological heterogeneity. To judge clinical heterogeneity, we considered factors that are known to predict upper limb recovery or response to rehabilitation after stroke (Coupar 2011; Sunderland 1989).
If indirect comparisons had been possible, we had planned to evaluate differences between treatment and placebo/control/usual care interventions, while preserving randomisation of the originally assigned participant groups. We planned to use the test for differences between subgroups in RevMan (RevMan 2012), with subgroups defined by the different comparisons made, and will estimate differences between subgroups and will determine statistical significance (Becker 2011). Differences between summary effects in the two subgroups would have provided an estimate of the indirect comparison of two interventions. We planned to not perform indirect comparisons when studies performed direct comparisons, or when the same studies were included within more than one review. As indirect comparisons are not randomly assigned comparisons, we planned to apply caution when interpreting the results of statistical analyses.
Note: Although we had planned for potential indirect comparisons, no indirect comparisons have been carried out. All available outcome data comprised continuous data, generally pooling results from a variety of different outcome measures using standardised mean differences, and statistical advice suggested that consequently indirect comparisons were not appropriate. Subsequently, we did not formally explore clinical and methodological heterogeneity with a view toward indirect comparisons; however in general, we judged that levels of clinical and methodological heterogeneity within trials included in the reviews were high. Rather than performing indirect comparisons, when a comparison was judged to have moderate‐quality evidence related to the effect on our primary outcome of upper limb function, and the review reported a standardised mean difference and 95% confidence intervals, we plotted these results on a graph to provide a visual representation of effect sizes.
Sensitivity analysis
We planned, when possible, to conduct sensitivity analyses based on the methodological quality of included reviews, by comparing results when all studies are included against those obtained when evidence assessed to be of low quality or at high risk of bias is excluded. We also planned to explore the results when only Cochrane reviews are included versus when reviews from DARE are included. (See Differences between protocol and review.)
Results
Note: The main results, including a summary of included reviews, interventions covered and implications for practice and research, are summarised in Table 2 and Figure 2.

Summary of findings.
| Intervention | Included reviews | Moderate‐quality evidence of effect on upper limb function | Moderate‐quality evidence of effect on upper limb impairment | Moderate‐quality evidence of effect on ADL outcomes | Low‐ or very low‐quality evidence | Implications for clinical practice | Recommendations for research |
| Bilateral arm training | Coupar 2010 (vs usual care or control) van Delden 2012 (vs unilateral arm training) | Unilateral arm training more effective than bilateral arm training (6 trials, n = 375) | No difference between unilateral arm training and bilateral arm training (4 trials, n = 228) | Unilateral arm training more effective than bilateral arm training | Low‐quality evidence for bilateral arm training compared with usual care or other interventions | Evidence does not support bilateral arm training as a replacement for unilateral arm training | A sound theoretical rationale is essential to justify further research into bilateral arm training |
| Biofeedback | Woodford 2007 (EMG biofeedback) Molier 2010 (qualitative data only) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Up‐to‐date reviews required | |||
| Bobath therapy | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Up‐to‐date reviews required | ||||
| Brain stimulation: tDCS | tDCS beneficial for impairment (7 trials, n = 304) | High‐quality evidence of no benefit or harm of tDCS (5 trials, n = 286) | Evidence insufficient to support introduction into routine clinical practice | High‐quality RCTs required | |||
| Brain stimulation: rTMS | Current evidence of low quality | Insufficient evidence to support introduction into routine clinical practice | High‐quality RCTs required | ||||
| Constraint‐induced movement therapy (CIMT) | Corbetta 2010 (subgroup analyses) | CIMT beneficial when compared with control (14 trials, n = 477) | Evidence of low quality for measures of ADLs (because of methodological limitations within review) | Moderate‐quality evidence that CIMT may be effective intervention for selected patients | Phase III RCTs recommended Dose must be considered | ||
| Electrical stimulation | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Meta‐analysis of current trials/completion of ongoing review required (Howlett) | ||||
| "Hands‐on" therapy (manual therapy techniques) | Winter 2011 (qualitative data only) | in | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | High‐quality RCTs required | ||
| Mental practice | Barclay‐Goddard 2011 (subgroup analyses) Wang 2011 (includes Chinese trials) | Mental practice beneficial when given in addition to conventional interventions (7 trials, n = 197) | Mental practice beneficial when given in addition to conventional interventions (5 trials, n = 216) | No benefit or harm of mental practice | Moderate‐quality evidence that mental practice may be effective intervention for some patients | Phase III RCTs recommended | |
| Mirror therapy | Mirror therapy beneficial (10 trials, n = 421): combined upper limb function and impairment outcomes | (see upper limb function) | Mirror therapy beneficial (4 trials, n = 217) | Moderate‐quality evidence that mirror therapy may be effective intervention for some patients | Phase III RCTs recommended | ||
| Music therapy | Lack of trial evidence | Insufficient evidence to support any change in current clinical practice | High‐quality RCTs required | ||||
| Pharmacological interventions | Elia 2009 (botulinum toxin for spasticity) Olvey 2010 (botulinum toxin for spasticity; qualitative data only) Demetrios 2013 (multi‐disciplinary rehabilitation following pharmacological interventions; qualitative data only) Singh 2010 (pharmacological interventions for shoulder pain) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Reviews require updating High‐quality RCTs required | |||
| Repetitive task training (RTT) | No benefit or harm of RTT (8 trials, n = 412) Beneficial effect when dose > 20 hours (3 trials, n = 113) | Moderate‐quality evidence that a higher dose of RTT may be beneficial | Review requires updating Large‐scale RCTs to explore dose is a research priority, including number of repetitions during RTT | ||||
| Robotics | Beneficial effect of robotics as compared with any comparison on impairment scales (16 trials, n = 586) No benefit or harm as compared with the same duration of conventional therapy (6 trials, n = 204) No benefit or harm on measures of strength (10 trials, n = 321) | Beneficial effect of robotics as compared with any comparison on ADLs (13 trial, n = 552) | Current evidence does not support Introduction into routine clinical practice | High‐quality RCTs required, including consideration of dose | |||
| Sensory interventions | Schabrun 2009 (qualitative data only) | Beneficial effect of sensory stimulation as compared with no treatment (1 trial, n = 29) | Beneficial effect of sensory stimulation as compared with no treatment (1 trial, n = 29) | Low‐quality evidence for all other interventions | Current evidence does not support any change in current clinical practice | High‐quality RCTs required | |
| Strength training | Low‐quality evidence of a beneficial effect on upper limb function (11 trials, n = 465) and grip strength (6 trials, n = 306). (Quality judgement influenced by poor reporting within review) | Insufficient evidence to support any change in current clinical practice | High‐quality up‐to‐date review required High‐quality RCTs required | ||||
| Stretching and positioning | Katalinic 2010 (stretching and positioning) Borisova 2009 (positioning of shoulder) Ada 2005 (shoulder supports) Lannin 2003 (hand splinting) Hijmans 2004 (elbow orthoses; qualitative data only) | No benefit or harm of stretching as compared with any other intervention on joint mobility and spasticity | No benefit or harm of stretching as compared with any other intervention on ADLs | Low‐quality evidence of no benefit of shoulder supports | Current evidence does not support any change in current clinical practice | High‐quality up‐to‐date review required Essential that research protocols comprise doses that are theoretically predicted to effect change | |
| Task‐specific training (reach‐to‐grasp exercise) | Pelton 2012 (qualitative data only) Urton 2007 (qualitative data only) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | High‐quality, up‐to‐date review required | |||
| Virtual reality | Virtual reality beneficial (7 trials, n = 205): combined upper limb function and impairment outcomes | (see upper limb function) No benefit or harm for grip strength (2 trials, n = 44) | Moderate‐quality evidence that virtual reality may be effective intervention for some patients | Phase III RCTs recommended, including consideration of dose |
Summary of results and implications related to individual interventions.
ADLs: Activities of daily living.
EMG: Electromyography.
RCTs: Randomised controlled trials.
rTMS: Repetitive transcranial magnetic stimulation.
tDCS: Transcranial direct current stimulation.
Results of the search
Our search identified 1840 possible records (1451 from CDSR, 277 from DARE, 109 from PROSPERO and three from other sources). After eliminating 1700 obviously irrelevant records on the basis of titles, two independent overview authors assessed abstracts for the remaining 140 reviews (Figure 3). They agreed that 35 studies did not meet the inclusion criteria, leaving 105, for which we obtained full texts and then assessed for inclusion. We excluded 52 of these: 37 because the review had clearly been superseded by a more up‐to‐date review addressing the same question, or because the review clearly contained the same (or fewer) studies of another review of similar (or better) quality; 14 because they did not meet the selection criteria for the Overview; and one because we were unable to locate the full‐text paper. The remaining 53 reviews were eligible for inclusion in this Overview; however, 11 of these were identified to be ongoing, and two were references to duplicate publications: Foongchomchaey 2005 was a duplicate publication of Ada 2005; French 2010 was a duplicate publication of French 2007, leaving 40 reviews to be included within the qualitative synthesis of reviews (Table 3). Nineteen of the 40 reviews were Cochrane reviews, and 21 were non‐Cochrane reviews.

Study flow diagram.
| Review (source) | Intervention | Date of search | Objective (as stated within review) | Types of studies included | Participants included | Interventions included | Comparisons included | Outcomes (as defined within review) | Number of studies included (number of participants included) |
| Ada 2005 (CDSR); Foongchomchaey 2005 (DARE) | Stretch or positioning | 22/03/2004 | To investigate the effects of supportive devices in preventing subluxation, repositioning the head of the humerus in the glenoid fossa, | RCTs, quasi‐randomised and controlled trials | Stroke | Supportive devices | Alternative supportive device or no support | Distance of subluxation (from x‐ray), pain, function, contracture | 4 (142) |
| Barclay‐Goddard 2011 (CDSR) | Mental practice | 24/11/2010 | To determine whether mental practice improves the outcomes of upper extremity rehabilitation for individuals living with the effects of stroke | RCTs | Stroke, UL functional deficits | Mental practice of upper extremity | No intervention; conventional intervention; placebo mental practice; or other novel therapies | Upper | 6 (119) |
| Borisova 2009 (DARE) | Stretch or positioning | 30/06/2005 | To assess the effectiveness of positioning on range of motion of the paretic shoulder following stroke | RCTs | Stroke | Positioning | Control | Range of motion | 5 (126) |
| Bradt 2010 (CDSR) | Music therapy | 25/02/2010 | To examine the effects of music therapy with standard care versus standard care alone or standard care combined with other therapies | RCTs, quasi‐RCTs | Acquired brain injury | Music therapy | Standard care or standard care with other therapies | Upper extremity function:measured by Secondary outcomes: Adverse events (e.g. death, fatigue, falls) | 7 (184) |
| Braun 2013 (DARE) | Mental practice | 01/06/2012 | To investigate the beneficial and adverse effects of a mental practice intervention on activities, cognition and emotion in patients after stroke, patients with Parkinson’s disease or multiple sclerosis | RCTs | Stroke, Parkinson's or multiple sclerosis (but no studies with participants with multiple sclerosis found) | Mental practice as therapy or embedded in therapy | Control that allows assessment of the possible effects of mental practice | Measures of function, activity and participation | Stroke—14 (421) Parkinson's—2(60) |
| Cooke 2010 (DARE) | Exercise therapy | 01/10/2009 | To determine the strength of current evidence for provision of a | RCTs or quasi‐RCTs | Stroke | Experimental and control group interventions identical | See previous column—exercises but without increased duration | Motor impairment: Motor activity:Modified | 7 (680) |
| Corbetta 2010 (DARE) | CIMT (constraint‐ induced movement therapy) | 01/04/2010 | This article aims to present an update of the Cochrane review and to assess the effects of CIMT, modified CIMT and forced use on disability and arm motor function | RCTs and quasi‐RCTs | Stroke | CIMT, modified CIMT or forced use | Usual care | Disability:Functional Independence measure, Barthel Index Arm motor function:Action Research Arm Test, Wolf Motor Function Test, Emory Function Test, Motor Assessment Scale | 18 (674) |
| Coupar 2010 (CDSR) | Bilateral arm training | 28/08/2009 | To determine the effects of simultaneous bilateral training for improving arm function after stroke | RCTs | Stroke | Simultaneous bilateral training | Control, usual care | Performance in ADLs: functional movement of the upper limb; | 18 (549) |
| Coupar 2012 (CDSR) | Service delivery | 21/05/2011 | To determine the effects of home‐based therapy programmes for upper limb recovery in patients with upper limb impairment following | RCTs | Stroke | Home‐based therapy for UL rehabilitation | Placebo, no intervention or usual care | ADLs and functional movement, extended ADLs, motor impairment | 4 (166) |
| Demetrios 2013 (CDSR) | Pharmacological | 01/09/2012 | To assess the effectiveness of multi‐disciplinary rehabilitation, following botulinum neurotoxin and other focal intramuscular treatments such as phenol, in improving | RCTs | Adults and children with poststroke spasticity (clinical diagnosis) | Multi‐disciplinary rehabilitation after botulinum neurotoxin or other focal intramuscular treatments | Multi‐disciplinary rehabilitation | Passive function: Leeds Arm Spasticity Impact Scale, Disability Assessment Scale, Active function of the upper limb: e.g. Motor Activity Log (MAL) or Action Research Arm Test Active function of the lower limb: e.g. tests of walking speed, balance and gait Impairments—pain, spasm frequency, joint range Participation and impact on caregivers: WHO QoL‐BREF, Caregiver Strain Adverse events | 3 (91) |
| Doyle 2010 (CDSR) | Sensory intervention | 16/09/2009 | To determine the effects of interventions that target upper limb sensory impairment after stroke | RCTs and controlled clinical trials | Stroke | For sensory impairment | No treatment, | Functional use of the upper limb: Activity limitations: Participation: Stroke Impact Scale, | 13 (467) |
| Elia 2009 (DARE) | Botulinum toxin injection by any route, including but not limited | 01/09/2006 | The aim of this systematic review was to determine | All levels of evidence | Stroke | Intramuscular injections, botulinum neurotoxin A or botulinum neurotoxin B | Ashworth Scale, Improvement of Global Assessment Scale (area under the curve of Ashworth scores), functional | 11 (782) | |
| Elsner 2013 (CDSR) | Transcranial direct current stimulation (tDCS) | May 2013 | To assess the effects of tDCS on generic activities of daily living and motor function in people with stroke | RCTs, first period of randomised cross‐over trials | Stroke | Active tDCS | Placebo, sham tDCS, no intervention or conventional rehabilitation | Primary outcome: Activities of daily living—Frenchay Activities Index, Barthel Index, Rivermead Activities of Daily Living Assessment, Modified Rankin Scale and Functional Independence Measure Secondary outcomes: Upper limb function—Action Research Arm Test, Fugl‐Meyer Score, Nine‐Hole Peg Test or Jebsen Taylor Hand Function Test. Muscle strength—grip force or motricity index Lower limb function | 15 (455) |
| Farmer 2014 (personal communication) | Assistive technologies, including electrical stimulation | 01/09/2011 | To identify and explore evidence for use of assistive technologies in poststroke upper limb rehabilitation | RCTs | Stroke | Assistive technologies including electrical stimulation (An assistive technology was defined as "a mechanical | Placebo, alternative treatments, usual care | Impairment: Range of motion, grip strength, subjective assessment of strength, Fugl‐Meyer Activity: Action Research Arm Test and Wolf Motor Function Test Partcipation: Motor Activity Log Amount of Use; Motor Activity Log Quality of Movement; Functional Independence Measure; Barthel index; Rankin Score and Stroke Impact Scale | 11 (474) |
| French 2007 (CDSR); French 2010 (DARE) | Repetitive task training | 16/10/2006 | To determine whether repetitive task training after stroke improves global, upper or lower limb function, and whether treatment effects are dependent on the amount, type or timing of practice | RCTs, controlled clinical trials | Stroke | Repetitive tasks training; an active motor sequence (multi‐joint motion) performed repetitively | Attention control, recreation, cognitive therapy, upper limb versus lower limb | Arm function: Motor Assessment Scale—Upper Limb Component, Action Research Arm Test, Frenchay Arm Test, Wolf Motor Function Test, Functional Test of the Hemiparetic Upper Extremity, Box and Block Test, Southern Motor Group Assessment Hand function: Motor Assessment Scale Hand; Jebsen Test of Hand Function, Peg Test Sitting balance/reach: Reaching Performance Scale, Functional Reach. | 14 (659) |
| French 2008 (DARE) | Repetitive task training | 01/09/2006 | To determine whether repetitive | RCTs, quasi‐RCTs, cross‐over trials (first part) | Stroke | Repetitive task training | Usual practice or attention control, alternative training | Arm function: Action Research Arm Test; Motor Assessment Scale—Upper Sitting Balance/Reach—Reaching Performance | 31 (1078) |
| Hao 2013 (CDSR) | Repetitive transcranial magnetic stimulation (rTMS) | 23/04/2012 | To assess the efficacy and safety of rTMS for improving function in people with stroke | RCTs | Stroke (any age) | rTMS, rTMS added to standard treatment | Sham treatment, sham treatment added to baseline treatment, baseline treatment alone | ADLs: Barthel Index, Functional Independence Measure, Modified Rankin Motor function:Upper limb function—Motor Assessment Scale, Action Research Arm Test, Nine‐Hole Peg Test. Lower limb function—changes in stride length or speed, Timed Up and Go Test, Rivermead Motor Assessment Scale. Global motor function—Motor Assessment Scale, Rivermead Motor Death or disability Any other impairment improvement (e.g. visual, perceptual, depression, cognition, etc) | 19 (588) |
| Harris 2010 (DARE) | Exercise | 04/2009 | To examine the evidence | RCTs | Stroke | Strength training (voluntary exercise against resistance) | No treatment, placebo, non‐strengthening intervention | Upper limb strength, upper limb function or ADLs | 13 (517) |
| Hijmans 2004 (DARE) | Stretch or positioning | 01/06/2003 | To assess the scientific base of elbow | All designs considered | Elbow condition | Splinting | No comparisons prespecified | Range of motion, pain, grip strength | Stroke RCT—1 (18), Cohort—1 (16) |
| Katalinic 2010 (CDSR) | Stretch or positioning | 01/04/2009 | To determine the effects of stretch on contractures in people with, or at risk of, contractures | RCTs and controlled | • Neurological conditions (e.g. stroke, multiple sclerosis, | Stretch, stretch plus co‐intervention | No stretch, placebo or sham stretch, co‐intervention | Range of motion, torques, QoL, SF‐36, Tardieu, Modified Ashworth Scale, Functional Independence Measure, motor ability score | 35 (1391) |
| Lannin 2003 (DARE) | Stretch or positioning | 26/05/2003 | To assess the effectiveness of hand splinting on the hemiplegic | All designs considered | Stroke | Splinting | No comparisons prespecified | Functional use of hand, range of motion, tone, spasticity, oedema, pain | 21 (230) |
| Laver 2011 (CDSR) | Virtual reality | 30/03/2010 | To evaluate the effects of virtual reality and interactive video gaming on upper limb, lower limb and global motor function after stroke | RCTs | Stroke | Immersive or non‐immersive virtual reality | Alternative intervention, no Intervention | Upper limb function and activity: Gait and balance function and activity: Global motor function: Motor Assessment Scale Secondary outcomes: Cognitive function—Trail Making Test, Useful Field of View Test; Activity limitation— Adverse events: motion sickness, pain, injury, falls | 19 (565) |
| Laver 2013 (CDSR) | Service delivery | 09/07/2013 | To evaluate the effects of telerehabilitation, in comparison with in‐person or no rehabilitation, on activities of daily living for people after | RCTs | Stroke | Telerehabilitation | "In‐Person Rehabilitation" or no rehabilitation or alternative method of delivering telerehabilitation | Primary outcomes: Activities of daily living—Functional Independence Measure; Nottingham Extended Activities of Daily Living Secondary outcomes: Self‐care and domestic life; Patient satisfaction with the intervention—self‐reported health‐related quality of life; upper limb function (e.g. Action Research Arm Test, Wolf Motor Function Test, Fugl‐Meyer Upper Extremity Adverse events | 10 (933) |
| Luke 2004 (DARE) | Exercise therapy | 2003 | To determine the effectiveness of the Bobath concept in reducing upper limb impairments, activity limitations and participation restrictions after stroke | RCTs, cross‐over and single case series | Stroke | Stated use of the Bobath concept | A control for Bobath | Any outcome | RCTs—5 (209) Cross‐over—1 (131) Single‐case study—2 (34) |
| Mehrholz 2012 (CDSR) | Robotics | 01/08/2011 | To assess the effectiveness of electromechanical and robot‐assisted arm training in improving generic activities of daily living, arm function and arm muscle strength in patients after stroke | RCTs | Stroke | Electromechanical and robot‐assisted arm training for recovery of arm function | Other rehabilitation or placebo interventions, or no treatment | ADLs (Barthel Index, Functional Independence Measure), Fugl‐Meyer, Motricity Index and other measures of arm function or strength | 19 (666) |
| Meilink 2008 (DARE) | Electrostimulation | 01/06/2006 | To assess whether EMG‐triggered neuromuscular electrical | RCTs | Stroke | EMG‐NMES | Usual care | Reaction time, Fugl‐Meyer Assessment, Box and Block Test, peak velocity, deceleration time, Functional Independence Measure (self‐care), Action Research Arm Test, grip strength, Motricity Index, pinch and grip strength, elbow flexion/shoulder abduction, goniometry | 8 (157) |
| Molier 2010 (DARE) | Biofeedback | 01/03/2009 | To investigate the effects of different aspects and types of augmented feedback on motor functions and motor | All levels of evidence | Stroke | Intervention augmented by biofeedback | Comparisons were not defined a priori but included practising movements without feedback or robotic guidance | Fugl‐Meyer, Composite Spasticity Index, Ashworth Scale, Test Evaluant des | 23 (328): RCTs—8 (148) Cohort—10 (106) Matched pairs—2 (52) Not randomised—1 (16) Observational study—1 (5) Single case study—1 (1) |
| Nascimento 2014 (PROSPERO) | Electrical stimulation | December 2012 | To determine whether electrical stimulation is effective in increasing strength after stroke, and whether any benefits are maintained beyond the intervention period or carried over to activity | RCTs and controlled trials | Stroke | Cyclical electrical stimulation for strengthening | 1. No treatment, placebo, non‐strengthening interventions 2. Other strengthening interventions 3. Different modes of electrical stimulation | Strength: peak force generation Activity: Block and Box Test, Action Research Arm Test General activity: Barthel Index | 16 (638) |
| Norouzi‐Gheidari 2012 (DARE) | Robotics | 01/07/2010 | To find evidence regarding the effectiveness | RCTs | Stroke | Robot therapy | Conventional therapy | Fugl‐Meyer, Functional Independence Measure, Motor Power Scale, Motor Status Scale | 12 (383) |
| Olvey 2010 (DARE) | Pharmacological | 01/07/2010 | To review | RCTs, open‐labelled non‐randomised or observational studies | Stroke | Pharmacological treatments for spasticity | Dose comparisons or placebo with or without other treatment | Spasticity (Ashworth Scale or Modified Ashworth Scale or Tardieu Scale), pain, Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Disability Assessment Scale, range of motion, health‐related quality of life | RCTs—23 (1039) Other—31 (1288) |
| Pelton 2012 (DARE) | Exercise | 04/2010 | To identify all existing interventions targeted at co‐ordination of arm and hand segments for reach‐to‐grasp | All types of study design | Stroke | Treatment to develop co‐ordination of hand and arm during reach‐to‐grasp | Specific measures of co‐ordination such | 8 (155) | |
| Schabrun 2009 (DARE) | Sensory training | Not reported | We examined the volume and quality of the evidence available for | All types of study design | Stroke | Sensory retraining | Jebsen Taylor Hand Function, Action Research Arm Test, Modified Ashworth Assessment Scale, Modified Motor Assessment Scale | 14 (296) | |
| Singh 2010 (CDSR) | Pharmacological | 22/01/2010 | To assess the benefits and safety of botulinum toxin compared with placebo or alternative treatments in adults with shoulder pain | RCTs | Shoulder pain | Botulinum toxin injection by any route, including but not limited | Placebo injection or another active treatment | Pain: measured on a visual analogue scale, numerical Adverse effects Function or disability: measured using validated shoulder‐specific General Quality of life: e.g. | 6 (164) |
| Sirtori 2009 (CDSR) | Constraint‐induced movement therapy (CIMT) | 01/06/2008 | To assess the efficacy of CIMT, modified CIMT (mCIMT) or forced use (FU) for arm management in hemiparetic patients | RCTs and quasi‐RCTs | Stroke | CIMT, mCIMT or forced use | Other rehabilitation techniques or none | Arm motor function: perceived arm motor function, arm impairment, dexterity, quality of life | 19 (619) |
| Thieme 2012 (CDSR) | Sensory intervention | 08/06/2011 | To summarise the effectiveness of mirror therapy for improving motor function, activities of daily living, pain and visuospatial neglect in patients after stroke | RCTs and randomised cross‐over trials | Stroke | Mirror therapy | Any control intervention | Upper limb and hand function: Fugl‐Meyer, Action Reseach Arm Test, Wolf Motor Function Test, Brunnstrom Stages of Upper Extremity, Motricity Index Lower limb function: Functional Independence Measure, Barthel Index | 14 (567) |
| Urton 2007 (DARE) | Mixed | 06/2005 | To critically analyse the literature on effective interventions for upper extremity | RCTs and CCTs | Stroke | For upper limb hemiparesis | Fugl‐Meyer Assessment, Box and Block Test, Purdue Pegboard Test, Action Research Arm Test, Functional Independence Measure, TEMPA, Wolf Motor Function Test, grip strength, Caregiver Strain Index, Geriatric Depression Score, Ashworth Scale, Motor Activity Log and other study‐specific measures | 11 (269) | |
| van Delden 2012 (DARE) | Bilateral arm training | 01/06/2011 | To compare the effects of unilateral and bilateral | RCTs | Stroke up to 1 month = acute, 1‐6 months = subacute, after 6 months = chronic | Unilateral arm training; bilateral arm training | Alternative treatment | Wolf Motor Function Measure, Canadian Occupational Performance Measure, Fugl‐Meyer Assessment, Functional Independence Measure, Motor Activity Log, Stroke Impact Scale, Action Research Arm Test, Rivermead Motor Assessment, Nine‐Hole Peg Test, Modified Barthel Index, Nottingham Health Profile, Hospital Anxiety and Depression Scale, Motor Status Scale, Motor Assessment Scale, dynamometry, Rehabilitation Activities Profile, fMRI, additional kinematics | 9 (452) |
| Wang 2011 (DARE) | Mental practice | 10/2010 | To evaluate mental imagery on rehabilitation of functions in patients with stroke | RCTs | Stroke | Mental practice or mental imagery. Other clinical and rehabilitative treatments were the same as control group | Conventional stroke rehabilitation methods (such as physiotherapy and occupational therapy) | Upper limb function: Upper Limb Section of Fugl‐Meyer Assessment of Motor Recovery, Action Research Arm Test Other outcomes: Motor Assessment Scale, Modified Ashworth Scale, Upper Extremity Function Test, Functional independence Measure, Motor Activity Log, Color Trails Test, Task Performance Test, Motricity Index, The Arm Functional Test, simple test for evaluating hand function, Modified Barthel Index | 16 (652) (191 total participants for English papers, 461 total participants for Chinese papers) |
| Winter 2011 (CDSR) | Stretch or positioning | 22/03/2010 | To identify whether specific hands‐on therapeutic interventions enhance motor activity and function of the upper limb post stroke | RCTs | Stroke | Manual therapy techniques | Unclear | UL function: Action Research Arm Test, Motricity Index, Functional Independence Measure, Barthel Index | 3 (86) |
| Woodford 2007 (CDSR) | Biofeedback | 29/03/2006 | To assess the effects of EMG‐BFB for motor function recovery following stroke | RCTs and quasi‐RCTs | Stroke | EMG biofeedback with standard physiotherapy | Standard physiotherapy or standard physiotherapy and sham feedback | Range of motion, improvement in gait (stride length, speed, need for ambulation aids), | 13 (269) |
ABILHAND: Assessment tool that measures a patient's perceived difficulty using his/her hands to perform manual activities in daily life.
ADLs: Activities of daily living.
CDSR: Cochrane Database of Systematic Reviews.
CIMT: Constraint‐induced movement therapy.
DARE: Database of Reviews of Effectiveness.
EMG‐BF: Electromyographic biofeedback.
EMG‐NMES: Electromyographic neuromuscular electrical stimulation.
EQ5D: A questionnaire to measure health‐related quality of life.
FIM: Functional Independence Measure.
FU: Forced use.
MAL: Motor Activity Log.
mCIMT: Modified constraint‐induced movement therapy.
MRI: Magnetic resonance imaging.
QoL: Quality of life.
RCT: Randomised controlled trial.
RFTP: Repetitive functional task practice.
RTMS: Repetitive transcranial magnetic stimulation.
SF‐36: Short Form 36 questionnaire.
TEMPA: Test d'Evaluation de la performance des Membres Supérieurs des Personnes Agées.
tDCS: Transcranial direct current stimulation.
UL: Upper limb.
WHO QoL‐BREF: World Health Organisation Quality of Life short instrument
Details of the 11 ongoing reviews are provided in Table 4, and reasons for exclusion of the 52 excluded reviews are provided in the 'Characteristics of excluded reviews' section (Table 5).
| Reference | Brief description of review/review aim | Dates/Notes |
| Effects of reach‐to‐grasp training using trunk restraint in individuals with hemiparesis post stroke: a systematic review | Anticipated publication stated as May 2013. Personal communication with author: completion date currently unknown PROSPERO 2012: CRD42012003464 | |
| To assess whether additional exercise therapy has an impact on recovery following stroke when compared with routine exercise therapy | Protocol published June 2012 | |
| Systematic review of functional electrical stimulation to improve activity and participation after stroke | Anticipated publication stated as October 2013. Personal communication with author: February 2014 in final stages PROSPERO 2012: CRD42012003054 | |
| Systematic review of self‐management interventions for stroke survivors | Protocol published February 2013 PROSPERO 2013: CRD42013003592 | |
| Physical therapies as an adjunct to botulinum toxin—injection to the upper or lower limb for the treatment of spasticity following neurological impairment: a systematic review | Personal communication with author: August 2013, in press PROSPERO 2011: CRD42011001491 | |
| To assess the efficacy and possible adverse effects of acupuncture for the treatment of poststroke upper limb pain | Protocol published April 2011 | |
| To determine whether pharmacological interventions for spasticity are more effective than no intervention, normal practice or control in improving function following stroke | Protocol published February 2013 | |
| To assess the effects of assistive technologies for the management of contractures in people with stroke | Protocol published October 2013 | |
| To determine whether physical treatment interventions are effective in preventing or minimising activity limitation and participation restrictions in patients developing spasticity post stroke | Protocol published July 2011 | |
| Intensive treatment versus normal treatment for improved motor recovery after stroke: a systematic review | Personal communication with author: Publication date anticipated around May 2014 PROSPERO 2012: CRD42012003221 | |
| The role of transcranial direct current stimulation (tDCS) in motor rehabilitation in stroke survivors: a systematic review | Protocol published May 2013 PROSPERO 2013: CRD42013003970 |
| Review (source) | Intervention | Reason for exclusion | Date of search | Objective (as stated within review) | Types of studies included | Participants included | Interventions included | Comparisons included | Outcomes (as defined within review) | Number of studies included (number of participants included) |
| Ada 2002 (DARE) | Electrical stimulation | Superseded by more up‐to‐date review | July 2002 | A meta‐analysis of all eligible randomised or quasi‐randomised trials of electrical stimulation for the treatment of shoulder subluxation | RCTs and quasi‐randomised trials | Stroke | Surface electrical stimulation with motor response | Conventional therapy | Subluxation, pain or function | 5 (183) |
| Aziz 2008 (CDSR) | Home rehabilitation therapy | No UL specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bjorklund 2006 (DARE) | CIMT | Superseded by more up‐to‐date review | 2004 | To investigate the outcomes of numerous CIMT trials to gauge improvement in upper extremity motor function among individuals suffering from hemiparesis experienced after stroke | RCTs, controlled trials, pre/post cohorts | Ischaemic or haemorrhagic stroke | CIMT | Self as control, conventional therapy | Fugl‐Meyer Assessment, Action Research Arm Test, Wolf Motor Function Test, | 11 (179) |
| Bolton 2004 (DARE) | EMG‐triggered electrical stimulation | Superseded by more up‐to‐date review | 3rd quarter 2003 | To assess the mean effect size of EMG‐triggered neuromuscular | RCTs, controlled trials | Stroke | EMG‐triggered | Usual therapy, stretching | Fugl‐Meyer Assessment, Block and Box Test, Rivermead Motor Assessment | 5 (86) |
| Bonaiuti 2007 (DARE) | CIMT | Superseded by up‐to‐date review | July 2004 | To analyse the evidence of effectiveness on adult stroke patients of CIMT | RCTs | Stroke | CIMT | Conventional therapy | Measures of impairment | 9 (243) |
| Braun 2006 (DARE) | Mental practice | Superseded by up‐to‐date review | August 2005 | To assess the effects of a mental practice intervention | RCTs, controlled trials | Stroke | Mental practice | Not clear | Measures of activity limitation or impairment | 4 RCTs ("Study sizes were small |
| Cardoso 2005 (DARE) | Botulinum toxin A | Superseded by up‐to‐date review | 2004 | To assess whether botulinum toxin is an adequate treatment for spasticity due to stroke | RCT | Stroke | Botulinum toxin A injections for upper limb spasticity | Placebo | Modified Ashworth Scale, Global Assessment Scale | 5 (329) |
| Crosbie 2007 (DARE) | Virtual reality | Superseded by up‐to‐date review | February 2005 | To assess the utility of virtual reality (VR) in stroke rehabilitation | RCTs, pre/post case series | Stroke | Virtual reality intervention | Self as control, healthy controls, age‐matched controls | Impairment or activity measurement | 5 (30) |
| Galvin 2008 (DARE) | Exercise therapy | Superseded by up‐to‐date review (3 trials included in this review were excluded from more up‐to‐date review) | Not reported (2006) | This article focuses on the impact of increased duration of exercise therapy on functional recovery after stroke | RCTs | Stroke | "Additional," "augmented" or "increased Exercise therapy was defined as | The same exercise therapy, but a lesser duration or dose | Fugl‐Meyer Upper Limb, Action Research Arm Test, Dynamometer, Functional Test | 8 (863) |
| Glanz 1996 (DARE) | Biofeedback | Superseded by up‐to‐date review | Not reported | To assess the efficacy of biofeedback therapy in poststroke rehabilitation | RCTs | Stroke | Biofeedback for upper extremity paresis | Conventional therapy | Impairment | 3 (82) |
| Glanz 1996 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | 1994 | To assess the efficacy of functional electrical | RCT | Stroke | Functional electrical stimulation | Control | Wrist torque | 1 UL ( 30) |
| Green 2003 (CDSR) | Interventions for shoulder pain | Not stroke | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hakkennes 2005 (DARE) | CIMT | Superseded by up‐to‐date review | March 2005 | To investigate the effects on function, quality of life, healthcare costs and patient/carer satisfaction of constraint‐induced movement therapy (CIMT) for upper limb hemiparesis following stroke | RCTs and systematic reviews | Stroke | CIMT or mCIMT | Alternative therapy, control, dose‐matched therapy or comparison between CIMT and mCIMT | Action Research Arm Test, Functional Independence Measure, Fugl‐Meyer Assessment, Motor Activity Log and Wolf Motor Function Test | 14 (292) |
| Handy 2003 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | 2002 | To examine the effectiveness of electrical stimulation in treating the upper extremities of patients who suffer cerebrovascular | RCTs and quasi‐experimental studies | Stroke | Functional electrical stimulation or transcutaneous electrical nerve stimulation | Control or alternative therapy | Subluxation, pain, range of motion or function | 5 (224) |
| Hayward 2010 (DARE) | Mixed | No additional studies | March 2009 | To investigate the effects of interventions that promote UL recovery in stroke survivors with severe paresis | RCTs | Stroke with severe UL paresis | Interventions that enabled stroke | Alternative or control treatment | Outcomes for impairment, activity and/or participation | 17 (486) |
| Henderson 2007 (DARE) | Virtual reality | Superseded by more up‐to‐date review | January 2006 | To evaluate scientific evidence for the effectiveness of virtual reality in rehabilitation of the UL post stroke | RCTs, | Stroke (acute, subacute and chronic) | Immersive or non‐immersive virtual reality | Conventional therapy or no therapy | Fugl‐Meyer Assessment, Functional Independence Measure, Wolf Motor Function Test | 6 (95) |
| Koog 2010 (DARE) | Treatment for shoulder pain | No UL function–specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| de Kroon 2002 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | December 2001 | Assessment of available evidence on the effects of therapeutic electrical stimulation of the affected upper extremity in improving motor control and functional abilities after stroke | RCTs | Stroke | Therapeutic electrical stimulation | Standard therapy, sensory stimulation, dose‐matched therapy | Measurement of motor control or functional abilities | 6 (207) |
| de Kroon 2005 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | December 2003 | To explore the relationship between | Clinical trials | Stroke | Surface electrical stimulation | No treatment, exercises, placebo, dose‐matched therapy | Range of motion, grip strength, Fugl‐Meyer Assessment, Motor Assessment Scale, Box and Block Test, Motor Activity Log, Ashworth Scale, Barthel Index, Rankin Scale, Pain | 19 (578) |
| Kwakkel 2008 (DARE) | Robotics | Superseded by more up‐to‐date review | October 2006 | The aim of the study was to present a systematic | RCTs | Stroke | Robot‐assisted therapy for the upper limb | Control (robot exposure), neurodevelopmental therapy, electrical stimulation | Fugl‐Meyer, Chedoke‐ McMaster | 10 (218) |
| Latimer 2010 (DARE) | Bilateral arm training | Superseded by more up‐to‐date review | Before 2008 | To determine the evidence for bilateral therapy interventions aimed at improving upper limb function in | RCTs and cohort studies | 6 months post stroke | Bilateral upper limb intervention | RCTs:
| Upper Extremity of Fugl‐Meyer Assessment, Frenchay Arm Test, Rivermead Motor Assessment, Wolf Motor Function Test, Modified Motor Assessment Scale | 9 (166): RCTs—4 (85) Cohort studies—5 (81) |
| Legg 2006 (CDSR) | Therapy for ADL | No UL‐specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ma 2002 (DARE) | Therapy | No UL outcome measure | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| McIntyre 2012 (DARE) | CIMT | No additional studies | July 2012 | To determine the effectiveness of constraint‐induced | RCTs | Over 50% stroke; ≥ 6 months post stroke | CIMT | Traditional rehabilitation therapy | Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement, Wolf Motor Function Test, Fugl‐Meyer Assessment, Action Research Arm Test, Functional Independence Measure | 16 (572) |
| Mehrholz 2011 (CDSR) | Exercise | No UL outcome measure | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moreland 1994 (DARE) | Biofeedback | Superseded by more up‐to‐date review | 1992 | To examine the efficacy | RCTs | Stroke | EMG biofeedback alone or with | Conventional physical | Any functional measure | 6 RCTs |
| Moreland 1998 (DARE) | Biofeedback | LL outcomes only | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortenson 2003 (DARE) | Positioning and stretching | Brain injury and stroke outcomes combined | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nijland 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | No additional studies | January 12, 2010 | To examine the literature | RCTs | Acute or subacute stroke (within 10 weeks of stroke) | High‐intensity CIMT, low‐intensity CIMT | Usual care implied | Fugl‐Meyer Assessment, Action Research Arm Test, Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement | 5 (106) |
| Nilsen 2010 (DARE) | Mental practice | Superseded by more up‐to‐date review | February 2009 | To determine whether mental practice is an effective intervention to improve upper limb | Categorised all levels of evidence, RCTs to case reports | Stroke | Mental practice alone or in combination with other therapies | Other therapies | Fugl‐Meyer Assessment, Wolf Motor Function Test, Action Research Arm Test, Motor Activity Log, Motricity Index, Pegboard Test, Dynamometer, position sense, 2‐point discrimination, Recovery Locus of Control Scale, Barthel Index, Functional Limitations Profile, kinemetics of reaching and grasping, Jebsen Hand Function Test, pinch strength, grip strength, Chedoke McMaster, range of motion | 15 (145) RCTs—4 (72) Cohort 3 (43) Case series or single case studies—8 (30) |
| Therapy‐based rehabilitation services | No UL‐specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Peurala 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | Superseded by more up‐to‐date review | January 5, 2011 | To examine the effects of constraint‐induced movement therapy and modified constraint‐induced | RCTs | Stroke | CIMT; modified CIMT (not forced use) | Usual care implied | Motor Activity Log, Action Research Arm Test, Wolf Motor Function Test, Functional Independence Measure, Stroke Impact Scale, Barthel Index | 27 RCTs |
| Platz 2003 (DARE) | Mixed | Superseded by more up‐to‐date review | October 2002 | To identify all studies providing evidence to support interventions comprising exercise therapy or neuromuscular electrical stimulation to improve arm paresis or ensuing activity limitations after stroke | Systematic reviews, meta‐analyses, RCTs, controlled cohort studies | Stroke with hemiparesis/ hemiplegia affecting the upper limb | Exercise therapy or neuromuscular electrical stimulation aimed at improving arm paresis or ensuing activity limitations after stroke. This included physiotherapy approaches, arm ability training, CIMT, repetitive sensorimotor training, EMG biofeedback, kinesthetic feedback, electrostimulation, robot‐assisted arm rehabilitation. Training intensity was also investigated | Not stated | No measures or scores reported, only generic terms, e.g. "strength," "function," "selectivity," "efficiency of arm function" | 30 references |
| (PROSPERO) | No intervention | No upper limb interventions | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pomeroy 2006 (CDSR) | Electrostimulation | Superseded by more up‐to‐date review | January 1, 2004 | To find whether electrostimulation improved functional motor ability and the ability to undertake activities of daily living | RCTs or quasi‐RCTs | Stroke | Electrostimulation | No treatment, placebo, conventional therapy | Functional motor ability—included the following: Measures of ADL—included the following: Barthel Index, Functional Independent Measure Measures | 24 (888) |
| Prange 2006 (DARE) | Robotics | Superseded by more up‐to‐date review | August 1, 2005 | To investigate the effects of robot‐aided therapy on upper limb motor control and functional abilities of stroke patients | Pre/post studies and RCTs | Stroke | Robot therapy | Conventional therapy | Fugl‐Meyer Assessment, Motor Status Score, Motor Power, Functional Independence Measure, Barthel Index | 8 (246) |
| Price 2000 (CDSR) | Electrical stimulation | Pain outcomes, no functional UL outcomes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Péter 2011 (DARE) | Robotics | Superseded by more up‐to‐date review | January 11, 2010 | To review robot‐supported upper limb physiotherapy focusing on | Clinical trial (randomised or non‐randomised, self‐controlled or with control group) | Hemiparesis due to upper motor neuron lesion | Shoulder, elbow and/or wrist robot‐mediated therapy | Conventional therapy or electrical stimulation | Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Motor Power Scale, Motor Status Scale, Medical Research Council Muscle Grading, Wolf Motor Function Test, range of motion, spasticity, Arm Motor Ability Test, Rancho Los Amigos Functional Test | 30 (393) |
| Richards 2008 (DARE) | TMS | No functional outcomes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Saposnik 2010 (DARE) | Virtual reality | Superseded by more up‐to‐date review | January 7, 2010 | To determine the added benefit of virtual reality technology for arm motor recovery after stroke | RCTs and pre/post design | Stroke | Immersive or non‐immersive virtual reality | Conventional rehabilitation, sham virtual reality, recreational activities or orthoses | Fugl‐Meyer Assessment, Wolf Motor Function Test, Box and Block Test, Jebsen Hand Function Test | 12 (195) |
| Shi 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | Superseded by more up‐to‐date review | January 4, 2010 | To compare the effectiveness of modified constraint‐ | RCTs | Stroke | Modified CIMT | Traditional rehabilitation therapy | Fugl‐Meyer Assessment, Action Research Arm Test, Functional Independence Measure, Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement, reaction time, peak velocity | 13 (278) |
| Stevenson 2012 (DARE) | Constraint‐induced movement therapy (CIMT) | No additional studies | January 2, 2011 | To examine constraint‐induced movement therapy, relative to dose‐matched control interventions, for | RCTs or cross‐over design | Stroke | CIMT | Dose‐matched control | Motor capacity—Fugl‐Meyer Assessment, kinematics, indirect indicators of neurophysiological mechanisms UL ability—Action Research Arm Test, Nine‐Hole Peg Test, Wolf Motor Function Measure Comprehensive—Functional Independence Measure, Barthel Index, self‐report (Motor Activity Log, Stroke Impact Scale) | Motor capacity—15 (432) UL ability—14 (351) FIM 6 (182) Motor Activity Log 12 (352) |
| Stewart 2006 (DARE) | Bilateral arm training | Superseded by more up‐to‐date review | 2nd quarter 2005 | To determine the overall effectiveness of | Pre/post and RCTs | Upper extremity stroke hemiparesis, with enough | Bilateral movement training or bilateral training with auditory cuing or active neuromuscular stimulation | Pretreatment/single‐arm tasks | Kinematic performance, Fugl‐Meyer Upper Extremity Test, Motor Assessment Scale, Box and Block Test | 11 (171) |
| Tang 2012 (DARE) | Repetitive transcranial magnetic stimulation (rTMS) | Unable to find full paper | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Timmermanns 2009 (DARE) | No intervention | Scoping review of treatment rationale | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tyson 2011 (DARE) | Stretching (orthoses) | No additional studies | July 2009 | To establish whether an orthosis can improve function and/or impairments | RCTs | Adults with stroke or the stable non‐progressive sequelae of a brain lesion (such as infection or traumatic brain injury) that resulted in motor impairments | Orthoses to manage upper limb motor impairments (The following types of orthoses were excluded: splinting during constraint‐induced movement therapy, devices to prevent shoulder subluxation, orthoses that were part of a hybrid device to deliver functional electrical stimulation, taping, strapping, air‐pressure splints, serial casting) | Comparison of an orthosis with no treatment, normal care, placebo treatment. Or comparison of an orthosis plus normal management versus normal management alone | Upper limb impairments, activity limitations or incidence of adverse events | 4 (126) |
| van der Lee 2001 (DARE) | Exercise therapy | Superseded by more up‐to‐date review | August 2000 | Assessment of available evidence for the effectiveness of exercise therapy | RCTs | Stroke | Exercise therapy | Other treatment or no treatment | Barthel Index, Action Research Arm Test, Fugl‐Meyer Assessment | 13 (939) |
| van Dijk 2005 (DARE) | Biofeedback | Superseded by more up‐to‐date review | December 2004 | Assessment of available evidence regarding | RCTs | Upper limb rehabilitation patients (Parkinson's disease—3 studies; spinal cord injury—2 studies; cerebral palsy—1 study, traumatic brain injury—1 study; stroke and traumatic brain injury—2 studies, stroke—16 studies | Augmented feedback | Placebo, conventional therapy, no treatment | Active range of motion, Brunnstrom's stages of recovery, electromyographical activity, Upper Extremity Functional Scale, Nine‐Hole Peg Test, est Evaluant des | 26 (927) |
| van Kuijk 2002 (DARE) | Botulinum toxin injection by any route, including but not limited | Superseded by more up‐to‐date review | January 10, 2000 | The goal of this | Excluded studies with fewer than 10 participants, but included case series (for phenol or alcohol injections) | Stroke | Botulinum toxin | Placebo but not always specified | Modified Ashworth Scale, grip strength, Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Frenchay Arm Test, Motricity Index | 12 (not reported) |
| van Peppen 2004 (DARE) | Physical therapy interventions | Superseded by more up‐to‐date review | January 2004 | To determine the evidence for physical therapy interventions aimed at | RCTs and CCTs | Stroke | Exercises for upper limb | Control | Action Research Arm Test, Arm Motor Activity Test, Motor Activity Log | 5 (104) |
| Wu 2006 (CDSR) | Acupuncture | No UL‐specific intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zimmermann‐Schlatter 2008 (DARE) | Mental practice | Superseded by more up‐to‐date review | January 8, 2005 | To evaluate how motor imagery and conventional therapy (physiotherapy or | RCTs | Stroke | Motor imagery plus conventional therapy | Conventional therapy | Fugl‐Meyer Upper Extremity Score; Action Research Arm Test | 4 (86) |
CCT: Controlled clinical trial.
CIMT: Constraint‐induced movement therapy.
CDSR: Cochrane Database of Systematic Reviews.
DARE: Database of Reviews of Effectiveness.
EMG: Electromyography.
FES: Functional electrical stimulation.
LL: Lower limb.
mCIMT: Modified constraint‐induced movement therapy.
RCT: Randomised controlled trial.
rTMS: Repetitive transcranial magnetic stimulation.
UE: Upper extremity.
UL: Upper limb.
Thirty‐one of the 40 included reviews contained data suitable for inclusion within the quantitative synthesis of reviews; however four of these contributed only data from subgroup analyses, as the main analyses overlapped with other included reviews. Table 6 provides a summary of which reviews are included within the qualitative and quantitative syntheses.
| Intervention | Reviews included in qualitative synthesis | Reviews included in quantitative synthesis | Ongoing reviews | Excluded, as superseded by more up‐to‐date review/ contains no additional studies |
| Acupuncture | ||||
| Bilateral arm training | ||||
| Biofeedback | ||||
| Bobath therapy | ||||
| Brain stimulation | ||||
| CIMT | Corbetta 2010 (SG only) | (Farmer 2014*) | ||
| Electrical stimulation | (Farmer 2014a) | (Farmer 2014a) | ||
| "Hands‐on" therapy | ||||
| Mental practice | Barclay‐Goddard 2011 (SG only) | |||
| Mirror therapy | ||||
| Music therapy | ||||
| Pharmacological interventions | ||||
| Repetitive task training | ||||
| Robotics | Norouzi‐Gheidari 2012 (SG only) | (Farmer 2014*) | ||
| Self‐management | ||||
| Sensory interventions | ||||
| Strength training | ||||
| Stretching and positioning | Borisova 2009 (SG only) | |||
| Task‐specific training (reach‐to‐grasp exercise) | (Urton 2007a) | |||
| Virtual reality | ||||
| Mixed | ||||
| Factors in service delivery: dose of intervention | ||||
| Factors in service delivery: location of intervention | ||||
| Numbers of reviews | 40 | 31 of 40 reviews 27 of 31 reviews—data from main comparisons included; 4 of 31 reviews—overlap with trials included in main comparisons: data from subgroup comparisons included only (marked as "SG only") | 11 | 37 |
CIMT: Constraint‐induced movement therapy.
SG: Subgroup.
aReviews covering a mixture of different interventions (listed under 'Mixed').
Description of included reviews
Types of studies
Thirty‐one of the 40 reviews included RCTs and quasi‐RCTs, and nine of the reviews included non‐randomised trials and other designs as well as RCTs. Additional details are provided in Table 3. The 40 reviews contain 503 studies (18,078 participants), although some overlap is evident in studies included in some of the reviews, and some of these studies are non‐randomised studies or included populations other than people with stroke with upper limb impairment. These overlaps and the types of studies included are explored further in the description of types of interventions provided in the included reviews.
Participants
Thirty‐four of the 40 reviews included only participants with stroke. Six reviews included mixed populations of participants: Three included populations with neurological conditions (Bradt 2010 acquired brain injury; Braun 2013 stroke, Parkinson's disease, multiple sclerosis; Demetrios 2013 adults and children with stroke), and three included mixed neurological and non‐neurological populations (Hijmans 2004 elbow conditions of any cause; Katalinic 2010 contractures due to neurological conditions, advanced age, trauma or surgery, joint or muscle pathology; Singh 2010 shoulder pain of any cause).
Interventions
Thirty‐seven of the 40 reviews focused on different types of interventions for the upper limb. Thirty‐five of these are focused on single types of interventions, and two reviews (Farmer 2014; Urton 2007) included a mixture of different single interventions. Exploration of overlap between the reviews of single interventions and these reviews, which include several different interventions, led to the inclusion of each of these mixed intervention reviews under one intervention only, with Farmer 2014 contributing data related to electrical stimulation and Urton 2007 contributing data related to reach‐to‐grasp exercise.
We identified reviews related to a total of 20 individual intervention types (some with additional subcategories). However, for two of these intervention types (acupuncture and self‐management), we identified no completed reviews (ongoing reviews only). Therefore, we have identified evidence related to 18 different types of interventions from these 37 reviews.
The remaining three of the 40 reviews examined factors in service delivery, focusing on the dose or location of the intervention.
Comparisons
Included reviews explored comparisons of interventions with no treatment, placebo, control, usual care, other interventions and different doses of interventions. Comparison groups included within each review are summarised in the 'Characteristics of included reviews' section (Table 3), and relevant comparison groups are described in relation to the included reviews in the sections below.
Outcomes
Included reviews covered a wide range of outcomes; these are summarised in the 'Characteristics of included reviews' section (Table 3). Further details of available data related to these outcomes are provided below.
Description of included reviews related to individual interventions
The 37 reviews related to each of the 18 types of interventions are described below, in relation to each intervention.
Bilateral arm training
Two included reviews focused on bilateral training (Coupar 2010; van Delden 2012). However, the focus of these reviews differed, with Coupar 2010 comparing simultaneous bilateral arm training versus usual care or control intervention, while van Delden 2012 included only studies that directly compared bilateral arm training with unilateral arm training. The methodological quality of these two reviews was quite similar. The most recent search date was provided by van Delden 2012 (June 2011), which also identified two RCTs that were published after the search of Coupar 2010 (August 2009). However, the wider scope of Coupar 2010 meant that a larger number of trials (18 trials, 549 participants) were included compared with van Delden 2012 (nine trials, 452 participants).
Therefore, data from Coupar 2010 contributed to comparisons of bilateral arm training versus usual care, and bilateral arm training versus other interventions (with data available for arm function, hand function, impairment and ADL outcomes), and data from van Delden 2012 contributed to comparisons of unilateral arm training versus bilateral arm training (with data available for arm function and impairment outcomes).
In addition, van Delden 2012 reported subgroup analyses exploring the impact of severity of stroke on arm function outcomes.
Biofeedback
Two included reviews explored biofeedback (Molier 2010; Woodford 2007). However, Molier 2010 investigated the effects of any type of biofeedback on arm function (eight trials, 148 participants), while Woodford 2007 included only studies of EMG biofeedback (13 trials, 269 participants). Molier 2010 did not carry out any meta‐analysis and did not present data on effect sizes; therefore this study contributed only qualitative information (Table 7).
| Review | Intervention | Brief description of review | Results: effects of interventions | Reasons for not including quantitative data from review |
| Bradt 2010 (CDSR) | Music therapy | The aim of this review was to examine the effects of music therapy with standard care versus standard care alone or standard care combined with other therapies on gait, upper extremity function, communication, mood and emotions, social skills, pain, behavioural outcomes, activities of daily living and adverse events in participants with brain injury. A total of 7 studies (184 participants) were included, but only 2 (41 participants) were relevant to the upper limb: “Two trials measured the effects of music therapy on upper extremity function in hemispheric stroke patients. Elbow extension angle was the only common outcome measure in these two studies. However, because of the significant clinical heterogeneity of the studies, their effect sizes were not pooled” | Narrative descriptions of the results of the trials are provided: One trial: “examined the effects of RAS on spatiotemporal control of reaching movements of the paretic arm in 21 patients. Results indicated that RAS increased the elbow extension angle by 13.8% compared to the non‐rhythmic trial, and this difference was statistically significant (P = 0.007). Results further indicated that variability of timing and reaching trajectories were reduced significantly (35% and 40.5%, respectively, P < 0.05).” One trial: “evaluated the effects of music‐making activity on elbow extension in 20 participants with hemiplegia. The elbow extension (measured from 135 to 0 with negative numbers expressing limitations) post‐intervention was ‐29.4 (SD 29.49) for the experimental group and ‐39.2 (SD 38.19) for the control group. This difference was not statistically significant. Post‐test shoulder flexion data indicated no statistically significant difference (P = 0.44) between the music therapy group (85.6°, SD 26.71) and the control group (71.8°, SD 39)” | Data from the 2 trials were not pooled |
| Demetrios 2013 (CDSR) | Multi‐disciplinary rehabilitation following botulinum toxin or other focal neuromuscular treatment | The aim of this review was to assess the effectiveness of multi‐disciplinary rehabilitation, following botulinum neurotoxin and other focal intramuscular treatments such as phenol, in improving activity limitations and other outcomes in adults and children with post‐stroke spasticity. Three RCTs (91 participants), all classed as 'low quality,' were included. "All studies investigated various types and intensities of outpatient rehabilitation programmes following botulinum neurotoxin for upper limb spasticity in adults with chronic stroke. Rehabilitation programmes included: modified constraint‐induced movement therapy (mCIMT) compared with a neurodevelopmental therapy programme; task practice therapy with cyclic functional electrical stimulation (FES) compared with task practice therapy only; and occupational, manual therapy with dynamic elbow extension splinting compared with occupational therapy only." "Due to the limited number of included studies, with clinical, methodological and statistical heterogeneity, quantitative meta‐analysis was not possible" | Descriptions of the results from the 3 included studies are provided. The review authors classify all evidence as "low quality" and conclude: "At best there was 'low level' evidence for the effectiveness of outpatient MD rehabilitation in improving active function and impairments following botulinum neurotoxin for upper limb spasticity in adults with chronic stroke." The review authors conclude that there is a need for "robust trials" | Data from the 3 trials were not pooled |
| French 2008 (DARE) | “Repetitive functional task practice,” including repetitive task training (RTT), constraint‐induced movement therapy (CIMT) and treadmill training | The aim was to determine whether repetitive functional task practice (RFTP) after stroke improves limb‐specific or global function or activities of daily living and whether treatment effects are dependent on the amount of practice, or the type or timing of the intervention. Also to provide estimates of the cost effectiveness of RFTP. Eighteen trials (634 participants) measured arm function. These included 8 RTT trials (467 participants) and 10 CIMT trials (167 participants) | Arm function Data from 8 RTT trials (412 participants) and 7 CIMT trials (285 participants) were pooled. The pooled effect for the impact of RFTP on arm function was as follows: SMD 0.24, 95% CI 0.06 to 0.42; I2 = 22% Hand function Data from 5 RTT trials (281 participants) and 2 CIMT trials (27 participants) were pooled. The pooled effect for RFTP on hand function was as follows: SMD 0.19, 95% CI –0.03 to 0.42; I2 = 0% | This review pools the data from 2 interventions: RTT and CIMT. Data from these interventions are included from French 2010 (RTT) and Corbetta 2010 and Sirtori 2009 (CIMT). The French 2010 RTT data are exactly the same as these French 2008 data. The Corbetta 2010 and Sirtori 2009 data are more comprehensive than the French 2008 data; this review has a much earlier search date and includes far fewer trials Including the data from this French 2008 review would effectively result in “double‐counting” of the data presented under the separate intervention headings of RTT and CIMT |
| Hijmans 2004 (DARE) | Elbow orthoses | The aim was to review papers related to the use of elbow Only 2 studies included participants with stroke. One was an RCT (18 participants), and one used a cross‐over design (16 participants) | No data are provided. The review authors state that (based on the cross‐over study) “wrist function and elbow range of movement seem to benefit from custom made Lycra garments applied at the elbow,” but (based on the RCT) probably no benefits are associated with an inflatable pressure splint | No data are available for inclusion |
| Molier 2010 (DARE) | Augmented feedback | The aim was to investigate the effects of different aspects and types of augmented feedback on motor functions and motor activities of the hemiparetic arm after stroke. 8 RCTs, 4 non‐randomised studies, 9 pre/post treatment design, 1 observational study and 1 single case study were included | For each study, it was stated whether beneficial effect, no effect or inconclusive effect was found for each outcome assessed. No data were provided. The results are discussed in the text. The authors state: “There are some trends in favour of providing augmented knowledge of performance feedback, augmented auditory and combined sensory and visual feedback. No consistent effects on motor relearning were observed for summary or faded, terminal or concurrent, solely visual or solely sensory augmented feedback.” And conclude that “ it was not possible to determine which combinations of aspects and types of augmented feedback are most essential for a beneficial effect on motor activities and motor functions of the hemiparetic arm after stroke. This was due to the combination of multiple aspects and types of augmented feedback in the included studies. This systematic review indicates that augmented feedback in general has an added value for stroke rehabilitation” | No data are available for inclusion |
| Olvey 2010 (DARE) | Pharmacological therapies for upper limb spasticity | The aim was to review studies of “contemporary pharmacological therapies” for upper limb spasticity after stroke. 54 studies were included: 23 RCTs and 31 non‐randomised studies. 51 of these investigated botulinum toxin | The results of the included studies are tabulated, with data from individual studies described. 23 studies assessed functional ability: FIM—6 studies. 5 found no benefit; 1 significant benefit. Fugl‐Meyer—5 studies. 2 found significant benefit. Barthel Index—8 studies. 2 report improvement; 6 no improvement. Disability Assessment Scale—5 studies. All report some benefit. Results from measures of upper limb function “were inconsistent.” 26 studies evaluated range of motion; 15 reported a significant improvement in 1 or more parameters after treatment | No data are available for inclusion |
| Pelton 2012 (DARE) | Any intervention targeted at co‐ordination of arm and hand segment for reach to grasp after stroke | The aim was to determine the effectiveness of current treatments for improving co‐ordination of reach to grasp following stroke. 7 studies were included: 1 RCT, 2 case‐control studies, 2 pre/post tests, 1 cross‐over, 1 observational. Interventions identified fell into 3 categories: “functional therapy, biofeedback or electrical stimulation and robot or computerised training” | The results of each study are tabulated, and the effect is reported as positive, negative or no effect. “Four studies (one RCT and three experimental studies without controls) report a result in favour of the experimental intervention for improved hand and arm coordination, whereas one experimental study without controls found no benefit. Two experimental studies with controls did not report specific training effects for hand and arm coordination after stroke” | No data are available for inclusion |
| Urton 2007 (DARE) | Any interventions for upper extremity hemiparesis following stroke | The aim was to critically analyse the literature on effective interventions for upper extremity hemiparesis following stroke. 11 experimental studies that evaluated interventions for upper extremity hemiparesis after stroke were included. Interventions included augmented exercise therapy, electrical stimulation, goal‐directed reaching and reach‐to‐grasp movements | Study details are tabulated, and the results of each study are described narratively | No data are available for inclusion |
| Winter 2011 (CDSR) | Hands‐on physical interventions (manual therapy techniques) | The aim was to explore the effectiveness of "clearly described hands‐on physical intervention (manual therapy techniques), or treatment component schedules, for the upper limb following stroke, either as the experimental intervention or as the control group." Pharmacological, electrical or psychological (e.g. mental imagery, relaxation) techniques were excluded, and only trials with interventions that addressed physical impairment were included. Three trials (86 participants) were included, each of which investigated different interventions (including manual stretch, passive extension and hands‐on therapy). Note: In the trial of passive extension (22 participants), passive extension was actually delivered as the control intervention and electrostimulation as the experimental intervention | Because of the heterogeneity between studies, no meta‐analysis is performed. The results of each of the 3 studies are described narratively. Methodological limitations are identified for all 3 studies The study authors conclude: "The findings of the review demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization when applied to the hemiplegic upper limb following stroke merits further research." | No data are available for inclusion |
CDSR: Cochrane Database of Systematic Reviews.
CI: Confidence interval.
CMIT: Constraint‐induced movement therapy.
DARE: Database of Reviews of Effectiveness.
FES: Functional electrical stimulation.
mCIMT: Modified constraint‐induced movement therapy.
MD: Medical Department
RAS: Rhythmic auditory stimulation.
RCT: Randomised controlled trial.
RFTP: Repetitive functional task practice.
RTT: Repetitive task training.
SD: Standard deviation.
SMD: Standardised mean difference.
Woodford 2007 compared EMG biofeedback (combined with physiotherapy) versus physiotherapy alone and provided data for arm function, impairment and ADL outcomes. The search date for Woodford 2007 was March 2006.
Bobath approach
One review investigated the effectiveness of the Bobath approach, including five RCTs (209 participants), which compared upper limb therapy based on the Bobath concept versus control intervention (Luke 2004). Effect sizes were presented for two individual studies for upper limb function outcomes. The search date for this review (2003) is considerably out‐of‐date.
(Note: We are aware of another review investigating the effectiveness of the Bobath approach (Kollen 2009). At the time of our search, this review was presented as a record only (i.e. no structured abstract) within DARE; subsequently this review was not identified during the search and is not included in this overview. In August 2013, a structured abstract was published on DARE (DARE). This review included seven RCTs (392 participants) that reported upper limb outcomes. No meta‐analyses were reported, and the abstract published by the Centre for Reviews and Dissemination (CRD Kollen 2009) highlights issues related to methodological limitations of the review and the included RCTs.)
Brain stimulation
Two up‐to‐date reviews, both judged to be of high methodological quality, investigated different types of brain stimulation. Elsner 2013 explored the effects of tDCS compared with sham tDCS, no intervention or conventional therapy and included 15 trials (455 participants). Hao 2013 investigated rTMS compared with sham rTMS, sham rTMS plus other baseline intervention or baseline intervention only and included 19 trials (588 participants).
Constraint‐induced movement therapy (CIMT)
Among 11 systematic reviews related to CIMT, Corbetta 2010 (last search April 2010; 18 trials, 674 participants) was judged to provide the most up‐to‐date and comprehensive inclusion of relevant trials. However, Corbetta 2010 included only data on two main comparisons: CIMT versus control for arm function and ADL outcomes. Sirtori 2009 (last search June 2008; 19 trials, 619 participants), although not as up‐to‐date as Corbetta 2010, provided subgroup comparisons related to time post stroke and dose of intervention. We therefore planned to include data from these subgroup analyses. However, only subgroup data related to ADL outcomes were available; for our primary outcome of upper limb function, these data could not be obtained.
Electrical stimulation
We included three reviews related to electrical stimulation (Farmer 2014; Meilink 2008; Nascimento 2014). Farmer 2014 focused on neuromuscular electrical stimulation, Meilink 2008 on EMG‐triggered electrical stimulation and Nascimento 2014 on electrical stimulation for improving muscle strength. Relatively few overlaps were noted between trials included in these three reviews, and a total of 37 electrical stimulation trials were included between the reviews (13 of 18 electrical stimulation trials included by Farmer 2014 were 'unique'; six of eight included by Meilink 2008 were 'unique'; and 13 of 16 trials included by Nascimento 2014 were 'unique').
Farmer 2014 (last search September 2011) provided data related to neuromuscular electrical stimulation versus control for outcomes of upper limb function, impairment and ADLs. However, Farmer 2014 provides the effect sizes of individual trials and has pooled no data: 18 trials (706 participants) related to electrical stimulation were included.
Meilink 2008 (last search June 2006) compared EMG‐triggered electrical stimulation versus cyclical electrical stimulation for outcomes of arm function and impairment; investigators also compared EMG‐triggered electrical stimulation versus no treatment for arm function outcomes. A total of eight trials (157 participants) were included.
Nascimento 2014 is the most up‐to‐date of these reviews (last search December 2012) and was judged to be of high methodological quality. The primary aim of this review was to explore the effect of electrical stimulation on muscle strength, but outcomes related to arm function and ADLs were also included. Sixteen trials (638 participants) were included.
'Hands‐on' therapy (manual therapy techniques)
One review investigated the effectiveness of hands‐on therapeutic interventions or manual therapy techniques, including three trials (86 participants), each exploring different interventions (Winter 2011; last search March 2010). Data were not pooled, and this review is included within qualitative syntheses only. The small number of studies and the methodological limitations make it inappropriate to draw conclusions from this review.
Mental practice
Three reviews related to mental practice were included (Barclay‐Goddard 2011; Braun 2013; Wang 2011). All investigated trials that delivered mental practice as an addition to conventional exercise, compared with conventional exercise alone, or conventional exercise plus a control or placebo intervention. We identified Braun 2013 as the most up‐to‐date review related to mental practice (last search June 2012). However, we found that Wang 2011 (last search October 2010) included several Chinese language publications that were not included by Braun 2013. Braun 2013 included 14 trials with stroke participants (421 participants), and Wang 2011 included 16 trials (652 participants), of which eight trials (461 participants) were published in Chinese language journals and were not included within Braun 2013. We therefore extracted data from both of these trials but explored where there was overlap; we did not include any of the analyses presented by Wang 2011 that did not include data from Chinese trials. Thus, we extracted from Braun 2013 data related to the effects of mental practice on arm function and activities of daily living and from Wang 2011 data related to impairment .
Barclay‐Goddard 2011 (last search November 2010; six trials, 119 participants) was superseded by Braun 2013. However, as neither Braun 2013 nor Wang 2011 carried out any subgroup analyses, we extracted from Barclay‐Goddard 2011 data from the subgroup analyses related to time post stroke and dose of intervention.
Mirror therapy
One Cochrane review, of high methodological quality, assessed the effects of mirror therapy, compared with any other intervention, for improving motor function, ADLs, pain and visuospatial neglect (Thieme 2012; last search June 2011); it included 14 trials (567 participants). Data were pooled from 10 trials (421 participants), combining upper limb function and impairment outcomes. Data from four trials (217 participants) related to measures of ADL outcomes were pooled.
Music therapy
We found one review related to the effectiveness of music therapy on a range of outcomes in participants with brain injury (Bradt 2010; last search February 2010). Only two trials (41 participants) explored effectiveness on upper limb recovery, and data from these studies were not pooled because of clinical differences between these studies.
Pharmacological interventions
Pharmacological interventions for spasticity
Two reviews explored the effects of pharmacological interventions on spasticity in participants with stroke (Elia 2009; Olvey 2010). Elia 2009 (last search September 2006; 11 trials, 782 participants) included only studies that investigated botulinum neurotoxin A or B but included any studies (regardless of type of evidence) aimed at improving spasticity (not limited to upper limb). Olvey 2010 (last search July 2010; 54 studies, 2327 participants, of which 23 studies (1039 participants) are trials) included any pharmacological treatment for upper limb spasticity. However, almost all studies included in Olvey 2010 investigated botulinum neurotoxin (51 of 54 included studies); subsequently substantial overlap is evident between the studies included in these two reviews. No data were pooled within Olvey 2010; therefore only data from Elia 2009 are included within the quantitative results. Of 11 trials included by Elia 2009, spasticity in the upper limb was measured by the Ashworth Scale in two trials (142 participants) of botulinum toxin (Dysport), and three trials (185 participants) investigated the effects of botulinum toxin (Botox). Nine trials measured disability, but no meta‐analysis was carried out because of the nature of the measurement scales reported.
Multi‐disciplinary rehabilitation following pharmacological interventions
One review investigated the effects of multidisciplinary rehabilitation following botulinum toxin, compared with multi‐disciplinary rehabilitation alone, in improving activity limitation (Demetrios 2013; last search September 2012). This review included both adults and children with poststroke spasticity. Three trials (91 participants) were included, but no data were pooled because of heterogeneity. This review is therefore included within the qualitative synthesis only.
Pharmacological interventions for shoulder pain
One review investigated the effects of botulinum toxin on shoulder pain, spasticity and shoulder range of movement, in participants with shoulder pain (including poststroke shoulder pain) (Singh 2010; last search January 2010). Six studies (164 participants) were included, of which five (109 participants) examined poststroke shoulder pain.
Repetitive task training
The same group of authors published two reviews (French 2007 with dual publication French 2010; and French 2008), both of which explored the effects of repetitive task training on functional ability in people with stroke. French 2007 (last search October 2006) defined repetitive task training as "an active motor sequence (multi joint motion) performed repetitively" and identified 14 trials (659 participants), of which eight trials (412 participants) assessed the impact of repetitive task training on upper limb function. Six (274 participants) of these eight trials explored upper limb training, and two (138 participants) investigated global functional activities. Data related to effects on measures of arm and hand function were pooled, and subgroup analyses related to time post stroke and dose of intervention were carried out.
French 2008 (last search September 2006) combined trials of repetitive upper limb training identified in French 2007 with trials of constraint‐induced movement therapy. As a result of overlap between included trials, this review is included within our qualitative synthesis only (Table 7). French 2008 pooled data from 18 trials (634 participants) related to the effects of repetitive functional task practice on arm function; eight of these (467 participants) are trials of repetitive task training, and 10 (167 participants) are trials of CIMT.
Robotics
Two reviews explored the effects of robot‐assisted arm training (Mehrholz 2012; Norouzi‐Gheidari 2012). Mehrholz 2012 (last search August 2011) was judged to be most up‐to‐date and to be of the highest methodological quality. However, Norouzi‐Gheidari 2012 (last search July 2010) reported a number of subgroup analyses that we considered relevant to this overview. Therefore, we extracted data from Mehrholz 2012 (19 trials, 666 participants) for the main analyses but used data from Norouzi‐Gheidari 2012 (12 trials, 383 participants) in relation to the subgroup analyses. However, although Norouzi‐Gheidari 2012 explored different subgroups on the basis of time after stroke (acute and subacute or chronic) and the comparison investigated (additional robotic therapy or same duration of conventional therapy), no tests for subgroup differences were provided, and differences must be inferred from reported effect sizes. Mehrholz 2012 compared robotic therapy versus any comparator, including other rehabilitation, placebo or no treatment, although Norouzi‐Gheidari 2012 included only comparisons with conventional rehabilitation.
Sensory interventions (interventions to improve sensory function)
Two reviews investigated the effectiveness of interventions that aim to train sensory function in participants with stroke (Doyle 2010; Schabrun 2009). Doyle 2010 (last search September 2009) investigated "interventions hypothesised to remediate sensory impairment after stroke," and divided included trials into those investigating "sensory re‐training" (which included active training or exercises, such as mirror therapy, discrimination activities, tactile recognition tasks and motor imagery) and "sensory stimulation" (which included interventions such as electrical stimulation, magnetic stimulation, intermittent pneumatic compression, tensive mobilisations of peripheral nerves). Schabrun 2009 (search date not reported) similarly defined two groups of interventions: "active sensory training" ("exercises specifically designed to train sensory function, for example, practice localising and detecting position of body parts in space") and "passive sensory training" ("electrical stimulation to produce activation of cutaneous nerves in the absence of muscle contraction"). Doyle 2010 included 13 trials (467 participants), three (71 participants) of which investigated sensory retraining and 10 (396 participants) of which investigated sensory stimulation. Schabrun 2009 included 14 studies (of any design; 296 participants), six (101 participants) of which were classed as active sensory retraining and eight (195 participants) as passive sensory retraining. Despite the similarity of interventions included within these two reviews, no overlap of trials was noted. Doyle 2010 is focused specifically on the upper limb and includes only RCTs, and nine of the 14 studies included by Schabrun 2009 were specific to the upper limb (four lower limb; and one both upper and lower limb); only five were assessed to be "properly designed" RCTs.
Doyle 2010 presented effect sizes related to arm function and impairment outcomes from trials that compared treatment for sensory impairment versus no additional treatment (both treatment groups could receive conventional or routine therapy) and from trials that compared treatment for sensory impairment versus placebo or attention control treatment. However, no data were pooled "due to clinical and methodological diversity." Data were extracted from individual trials for reported effect sizes. One of the included trials investigated mirror therapy; as the effect of mirror therapy has been investigated by another review (Thieme 2012; see 'Mirror Therapy'), data from this single trial were not extracted. Schabrun 2009 states that data were insufficient to enable pooling of data related to active sensory training, and no effect sizes were presented for any of the studies focused on the upper limb. Therefore, no data from Schabrun 2009 related to active sensory training interventions were extracted. Schabrun 2009 presents pooled data for three studies (participant numbers unclear) that investigate passive sensory training (electrical stimulation), although the comparison group is not clearly reported. Only one of these three studies was assessed by the review authors to be a "properly designed" RCT.
Strength training
One non‐Cochrane review investigated the effect of strength training for the affected upper limb, in which strength training was defined as voluntary exercise against resistance (Harris 2010; last search April 2009). Thirteen trials (517 participants) were included, and data were pooled for outcomes of upper limb function, grip strength and ADLs. Subgroup analyses are reported for subgroups of participants with subacute or chronic stroke, with mild or moderate impairment. However, no test for subgroup differences was reported.
(Note: We are aware of another review investigating the effectiveness of strength training interventions (Ada 2006). This review was presented as a record with DARE only (i.e. no structured abstract); subsequently this review was not identified during the search and is not included in this overview. However, peer review comments related to this overview have highlighted this review. This review does not present information related to upper limb interventions separately, so it would be difficult to extract data related to effects of strength training on the upper limb. This review is considered as 'awaiting assessment' for inclusion within this overview.)
Stretching and positioning
Katalinic 2010 (last search April 2009) investigated the effects of stretch for contractures, defined a stretching intervention as one that "aimed to maintain or increase the mobility of any synovial joint," with a criterion for study inclusion stating: "the stretch needed to sustain the soft tissues in a lengthened position for a minimum of 20 seconds on more than one occasion." This review included participants from a wide range of populations at risk of muscle contracture at the shoulder; a total of 35 trials (1391 participants) were included; 24 of these trials (782 participants) included populations of people with neurological conditions, including stroke. The interventions were focused on a range of joints, both lower limb (eight trials) and upper limb (16 trials). Types of stretch administered included "passive stretching (self‐administered, therapist‐administered and device‐administered), positioning, splinting and serial casting." Meta‐analyses within this review present data from the subgroup of participants with neurological conditions, but data related to limb or type of intervention are pooled and no subgroups are presented. Furthermore, pooled comparisons included trials with any type of control group intervention, including no intervention, usual care or other active interventions such as physiotherapy, passive stretching or botulinum toxin.
Positioning of the shoulder
Borisova 2009 (last search June 2005) reviewed trials that investigated positioning of the shoulder. All included trials had to have a measure of shoulder range of motion as an outcome measure. All of the five trials (126 participants) included in Borisova 2009 are also included in the review of stretching interventions by Katalinic 2010. However, pooled analysis of the trials of positioning presented by Borisova 2009 effectively forms a subgroup (based on joint and type of intervention) of the trials included by Katalinic 2010; therefore we extracted data from the range of movement outcome presented by Borisova 2009.
Hand splinting
One review synthesised studies, of any methodological design, involving hand splinting to prevent contracture and reduce spasticity (Lannin 2003; last search May 2003). Twenty‐one studies (230 participants) were included, of which five (participant numbers unclear) were RCTs. No overlap was noted between the trials included by Lannin 2003 and those included within Katalinic 2010, although one of the included trials was excluded from Katalinic 2010, and Katalinic 2010 includes several more recently published trials that investigate wrist and hand splints. However, Katalinic 2010 does not present these data as a separate subgroup analysis. Lannin 2003 presents data for comparisons of hand splints versus no splint or hand splint versus a 30‐minute stretch, and we extracted these data. Comparisons of dorsal and volar hand splints and length of time wearing finger spreaders are also presented within the review, but we did not extract these data.
Elbow orthoses
One non‐Cochrane review, which is considerably out of date (Hijmans 2004; last search June 2003), investigated elbow orthoses. However, only one trial (18 participants) was included, and this trial was excluded from the review by Katalinic 2010 (which also identified more recent trials focused on the elbow). No data were presented within this review, and it is included within the qualitative synthesis only.
Shoulder supports
One Cochrane review, which is considerably out‐of‐date (Ada 2005; last search March 2004), investigated the effectiveness of supportive devices in preventing subluxation, repositioning the head of the humerus, decreasing pain or increasing function following stroke. Four trials (142 participants) were included, all of which investigated shoulder strapping or the hemi‐sling for preventing or reducing shoulder subluxation. No overlap is evident between the trials included by Ada 2005 and those included within Katalinic 2010.
Task‐specific training
Reach‐to‐grasp exercise
Two reviews that investigated reach‐to‐grasp exercise–related interventions were included (Pelton 2012; Urton 2007). However, both are included within the qualitative synthesis only, as neither provided data suitable for extraction. Pelton 2012 (last search April 2010) investigated interventions aimed at improving co‐ordination of the arm and hand during the reach‐to‐grasp movement. Eight studies (155 participants) were included, but these included a variety of study designs. Urton 2007 (last search June 2005; 11 studies, 269 participants) included a mixture of different interventions, including 'goal‐directed reaching' and 'reach‐to‐grasp' interventions. However, the methodological quality of this review was judged to be poor, so it is not appropriate to draw conclusions from it.
Virtual reality
One Cochrane review investigated the effects of virtual reality and interactive video‐gaming on function after stroke (Laver 2011; last search March 2010). Of a total of 19 included trials (565 participants), eight (240 participants) were focused on upper limb function. Data were extracted for comparisons of virtual reality versus any other intervention, for a mixed upper limb function and impairment outcome and for grip strength. Subgroup analyses based on time post stroke (more or less than 6 months post stroke) and length of intervention (more or less than 15 hours) are presented, along with a test for subgroup differences.
Description of included reviews related to factors in service delivery
The three reviews related to different factors in service delivery are described below.
Dose of intervention
Cooke 2010 (last search October 2009) was judged to be the review with the most up‐to‐date evidence related to intensity of the intervention. It included trials that investigated the effects of additional, augmented or increased duration or effort of exercise therapy compared with a lesser dose. Seven trials (680 participants) were included; however, only three of these trials (258 participants) investigated increased intensity of dose for the upper limb.
Service location
Home‐based therapy
One Cochrane review, of high methodological quality, investigated the effects of home‐based therapy programmes on upper limb recovery (Coupar 2012; last search May 2011); it included four trials (166 participants). Three of the four trials (156 participants) compared home‐based therapy versus usual care; pooled data related to outcomes of arm function, ADLs, extended ADLs and impairment were extracted; the intervention in two of these three trials consisted of an upper limb programme of exercise, and in the other trial, the intervention comprised virtual reality delivered via telerehabilitation. One of the four trials (10 participants) compared upper limb therapy (based on virtual reality) provided at home (delivered via telerehabilitation) versus the same intervention provided in hospital; data were available for measures of impairment only.
Telerehabilitation
One Cochrane review, of high methodological quality, investigated the effects of telerehabilitation services for people with stroke (Laver 2013; last search July 2013). This review included 10 trials (933 participants) covering a wide range of telerehabilitation services; only four of these trials (87 participants) investigated interventions that aimed to improve upper limb function, all of which comprised customised computer‐based training programmes. Data could be pooled for only two of these trials (46 participants) for a measure of upper limb function. Both of these trials were also included in the review of home‐based therapy (Coupar 2012; see above) and contributed to pooled data related to impairment outcomes (but not to the outcome of arm function, ADLs or extended ADLs).
Reviews incorporating evidence related to a mixture of different interventions
As described above, two reviews incorporated evidence related to a mixture of different interventions (Farmer 2014; Urton 2007). A brief description of these reviews is provided below.
Farmer 2014 included trials of 'assistive technologies' including studies of electrical stimulation (17 RCTs), CIMT (12 RCTs), biofeedback (two RCTs), robotics (seven RCTs), brain stimulation (one RCT), virtual reality (one RCT) and stochastic resonance (one RCT). This review did not pool data from any of the trials but presented effect sizes for individual trials and outcomes; this limited our ability to extract data from this review. Eleven trials that were not included within other reviews of single interventions were included in Farmer 2014; 10 of these trials investigated electrical stimulation (of which one described 'stochastic resonance,' rather than 'electrical stimulation') and one CIMT. As we had identified a large number of reviews of CIMT and two high‐quality reviews that pooled the data from trials of CIMT (Corbetta 2010; Sirtori 2009), we made the decision to not extract from Farmer 2014 data related to CIMT. However, it is important to note that Farmer 2014, which includes a more recent search, did identify one additional trial of CIMT. We had identified two other trials related to electrical stimulation; however, each of these trials had a different focus, and Farmer 2014 explored trials of neuromuscular electrical stimulation and stochastic resonance; data related to trials have therefore been included within the section on electrical stimulation.
Urton 2007 stated that these investigators included studies of "effective interventions for upper extremity hemiparesis following stroke." The 11 included trials investigated augmented exercise therapy, electrical stimulation, goal‐directed reaching and reach‐to‐grasp movements. As a result of poor methodological quality and absence of data presented within the review, this review is included only within the qualitative synthesis. It was considered to contribute unique trial data related to 'reach‐to‐grasp' exercise only, and therefore is discussed under this heading only. Interventions of augmented exercise therapy and electrical stimulation were judged to be covered more comprehensively by other reviews.
Methodological quality of included reviews
Figure 4 provides details of judgements for the modified AMSTAR and AMSTAR assessment questions, and summarises the responses arising from Cochrane and non‐Cochrane reviews for each of these questions; Table 8 provides results for the AMSTAR assessment only.

AMSTAR and mAMSTAR results (AMSTAR in shaded columns; mAMSTAR in unshaded columns).
| Review source | Author | 1. Was an 'a priori' design provided? The research question and inclusion criteria should be established before the conduct of the review | 2. Was there duplicate study selection and data extraction?There should be at least two independent data extractors, and a consensus procedure for disagreements should be in place | 3. Was a comprehensive literature search performed? At least two electronic sources should be searched. The report must include years and databases used (e.g. CENTRAL, EMBASE, and MEDLINE). Key words and/or MeSH terms must be stated and where feasible the search strategy should be provided. All searches should be supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found | 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? The authors should state that they searched for reports regardless of their publication type. The authors should state whether or not they excluded any reports (from the systematic review), based on their publication status, language, etc | 5. Was a list of studies (included and excluded) provided? A list of included and excluded studies should be provided | 6. Were the characteristics of the included studies provided? In an aggregated form such as a table, data from the studies should be provided on the participants, interventions and outcomes. The ranges of characteristics in all the studies analysed (e.g. age, race, sex, relevant socioeconomic data, disease status, duration, severity) or other diseases should be reported | 7. Was the scientific quality of the included studies assessed and documented? 'A priori' methods of assessment should be provided (e.g. for effectiveness studies if the author(s) chose to include only randomised, double‐blind, placebo‐ controlled studies, or allocation concealment as inclusion criteria); for other types of studies, alternative items will be relevant | 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? The results of the methodological rigour and scientific quality should be considered in the analysis and the conclusions of the review, and explicitly stated in formulating recommendations | 9. Were the methods used to combine the findings of studies appropriate? For the pooled results, a test should be done to ensure the studies were combinable, to assess their homogeneity (i.e. Chi2 test for homogeneity, I2). If heterogeneity exists, a random‐effects model should be used and/or the clinical appropriateness of combining should be taken into consideration (i.e. is it sensible to combine?) | 10. Was the likelihood of publication bias assessed? An assessment of publication bias should include a combination of graphical aids (e.g. funnel plot, other available tests) and/or statistical tests (e.g. Egger regression test) | 11. Was the conflict of interest stated? Potential sources of support should be clearly acknowledged in both the systematic review and the included studies |
| CDSR | Y | U | Y | Y | Y | Y | U | N | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | |
| DARE | Y | N | N | U | N | N | Y | N | U | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| OTHER | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | |
| DARE | Y | Y | Y | N | Y | N | Y | N | Y | N | Y | |
| DARE | Y | N | U | Y | N | N | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | U | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| DARE | Y | U | N | U | Y | Y | U | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| OTHER | Y | N | N | N | N | N | U | N | N/A | N | Y | |
| DARE | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | n | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | Y | U | N | N | N | Y | N | Y | Y | N | Y | |
| DARE | N | N | Y | U | Y | N | N | N | N/A | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | Y | N | Y | N | Y | N | Y | Y | N/A | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | y | N | N | N | N | Y | Y | Y | N | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | N | U | Y | N | N | N | Y | Y | Y | N | N | |
| DARE | Y | Y | Y | N | N | Y | N | N | N/A | N | Y | |
| PROSPERO | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | Y | U | Y | N | Y | N | U | U | Y | N | Y | |
| DARE | N | N | Y | N | N | N | N | Y | N/A | N | Y | |
| DARE | Y | Y | Y | N | N | Y | Y | Y | N/A | N | U | |
| DARE | Y | U | N | N | Y | Y | N | Y | Y | N | Y | |
| CDSR | Y | U | Y | U | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | U | Y | Y | U | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | N | N | N | N | N | N | N | N | N/A | N | N | |
| DARE | Y | Y | Y | N | Y | Y | Y | U | Y | N | N | |
| DARE | Y | U | U | N | N | N | Y | U | Y | Y | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| CDSR | Y | U | Y | Y | Y | U | Y | Y | Y | N | Y | |
| Number of responses: all reviews | YES | 36 | 23 | 31 | 19 | 29 | 27 | 29 | 29 | 27 | 8 | 30 |
| NO | 4 | 8 | 7 | 15 | 11 | 12 | 6 | 8 | 1 | 32 | 9 | |
| UNCLEAR | 0 | 9 | 2 | 6 | 0 | 1 | 5 | 3 | 1 | 0 | 1 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | |
| Number of responses: CDSR reviews | YES | 19 | 16 | 19 | 16 | 19 | 18 | 17 | 17 | 15 | 5 | 19 |
| NO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 14 | 0 | |
| UNCLEAR | 0 | 3 | 0 | 3 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | |
| Number of responses: other reviews | YES | 17 | 7 | 12 | 3 | 10 | 9 | 12 | 12 | 12 | 3 | 11 |
| NO | 4 | 8 | 7 | 15 | 11 | 12 | 6 | 6 | 1 | 18 | 9 | |
| UNCLEAR | 0 | 6 | 2 | 3 | 0 | 0 | 3 | 3 | 1 | 0 | 1 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
CDSR: Cochrane Database of Systematic Reviews.
DARE: Database of Reviews of Effectiveness.
N: No.
N/A: Not applicable.
U: Unclear.
Y: Yes.
See Figure 4 for results of mAMSTAR.
The table below summarises the number of 'yes' responses assigned to each of the 40 included reviews for the 11 AMSTAR questions, where 11 'yes' responses represent a judgement of the highest methodological quality.
| AMSTAR number of 'yes' responses | Cochrane reviews | Non‐Cochrane reviews |
| 11 | ||
| 10 | ||
| 9 | ||
| 8 | ||
| 7 | ||
| 6 | ||
| 5 | ||
| 4 | ||
| 3 | ||
| 2 | ||
| 1 | ||
| 0 |
*Reviews included in qualitative synthesis only.
1. Was an 'a priori' design provided?
We judged 36 of the 40 reviews to have provided 'a priori' design, establishing the research question before the review was conducted. The four reviews judged not to provide 'a priori' design were all non‐Cochrane reviews, with three judged not to pre‐describe the outcomes (Hijmans 2004; Meilink 2008; Olvey 2010) and one judged not to pre‐describe the intervention (Urton 2007). In addition, nine of the non‐Cochrane reviews were judged not to specify the comparison of interest, and this was judged to be unclear for a further two reviews (one Cochrane; one non‐Cochrane).
2. Was there duplicate study selection and data extraction?
We judged that 23 of the 40 reviews had appropriate study selection and data extraction; eight reviews were judged not to have appropriate study selection and data extraction; and this was unclear for nine reviews. All eight of the reviews judged not to have appropriate study selection and data extraction were non‐Cochrane reviews, with three reviews judged not to have two independent review authors for study selection or data extraction (Lannin 2003; Olvey 2010; Urton 2007); two judged not to have two independent review authors for data extraction (Borisova 2009; Hijmans 2004); and three judged to have two independent review authors but no clear procedure for resolving disagreements (Corbetta 2010; Farmer 2014; Luke 2004). Three of the nine reviews that judged this to be unclear were Cochrane reviews, with one unclear for all parameters (Singh 2010); one unclear in relation to the use of two independent review authors for data extraction (Ada 2005); and one unclear in relation to the procedure for resolving disagreements (Woodford 2007). The six non‐Cochrane reviews judged to be unclear were all—at a minimum—unclear in relation to whether two independent review authors were involved in data extraction (Elia 2009; Harris 2010; Meilink 2008; Norouzi‐Gheidari 2012; Schabrun 2009; Wang 2011).
3. Was a comprehensive literature search performed?
We judged that 31 of the 40 reviews performed a comprehensive literature search; seven non‐Cochrane reviews were judged not to report a comprehensive literature search; and this was unclear for two non‐Cochrane reviews. The most common reason for not being judged to report a comprehensive literature search was that the search strategy was not available, or it was unclear (Borisova 2009; Corbetta 2010; Elia 2009; Harris 2010; Urton 2007; Wang 2011). Three reviews did not supplement electronic searches with searching of other resources (Farmer 2014; Luke 2004; Urton 2007), and one did not report dates of searches (Schabrun 2009).
4. Was the status of publication (i.e. grey literature) used as an inclusion criterion?
We judged that 19 of the 40 reviews searched for reports regardless of publication type or language; 16 of these were Cochrane reviews and three were non‐Cochrane reviews (Corbetta 2010; French 2008; Nascimento 2014). This information was unclear for three of the 19 Cochrane reviews, primarily because of the absence of a statement related to language of publication (Coupar 2010; Singh 2010; Sirtori 2009). For most (19/21) of the non‐Cochrane reviews, this information was not provided or was unclear.
5. Was a list of studies (included and excluded) provided?
Twenty‐nine of the 40 reviews provided a list of included and excluded studies. All 40 of the included reviews provided a list of included studies, but 11 non‐Cochrane reviews did not provide a list of excluded studies (Borisova 2009; Corbetta 2010; Farmer 2014; Harris 2010; Luke 2004; Meilink 2008; Molier 2010; Olvey 2010; Pelton 2012; Urton 2007; Wang 2011). Thirteen of the 19 Cochrane reviews (Ada 2005; Barclay‐Goddard 2011; Bradt 2010; Coupar 2010; Coupar 2012; Doyle 2010; Hao 2013; Katalinic 2010; Laver 2011; Mehrholz 2012; Sirtori 2009; Thieme 2012; Woodford 2007) and eight of the 21 non‐Cochrane reviews (Borisova 2009; Corbetta 2010; Elia 2009; Lannin 2003; Luke 2004; Olvey 2010; Schabrun 2009; Urton 2007) did not provide a flow diagram illustrating study selection.
6. Were the characteristics of included studies provided?
We judged that 27 of the 40 reviews provided adequate details of the characteristics of included studies. Eighteen of the 19 Cochrane reviews were judged to provide adequate details, and details were unclear for one Cochrane review (Woodford 2007). Eleven of the 12 non‐Cochrane reviews were judged not to provide adequate details related to the included participants (Borisova 2009; Braun 2013; Cooke 2010; Farmer 2014; Hijmans 2004; Lannin 2003; Meilink 2008; Norouzi‐Gheidari 2012; Olvey 2010; Urton 2007; Wang 2011), and one was judged not to provide adequate details related to outcomes (Corbetta 2010).
7. Was the scientific quality of included studies assessed and documented?
We judged that 30 of the 40 reviews adequately assessed and documented the scientific quality of included studies; these included 17 Cochrane reviews and 13 non‐Cochrane reviews. Information about whether scientific quality was assessed by two independent review authors was unclear for two of the 19 Cochrane reviews (Ada 2005; Sirtori 2009), was not reported or was judged to be unclear for seven of the non‐Cochrane reviews (Elia 2009; Harris 2010; Hijmans 2004; Molier 2010; Norouzi‐Gheidari 2012; Olvey 2010; Urton 2007) and was judged to have been assessed but not documented for two of the non‐Cochrane reviews (Farmer 2014; Schabrun 2009).
8. Was the scientific quality of included studies used appropriately in formulating conclusions?
We judged that 29 of the 40 reviews used the scientific quality of studies appropriately in formulating conclusions; these included 17 Cochrane reviews and 12 non‐Cochrane reviews. Two Cochrane reviews were judged not to appropriately consider the methodological rigour of the included studies in the review analyses (Ada 2005; Barclay‐Goddard 2011); nine non‐Cochrane reviews were judged to not use this appropriately or to be unclear in the use of scientific quality within the analyses or in formulating conclusions (Borisova 2009; Cooke 2010; Farmer 2014; Hijmans 2004; Molier 2010; Norouzi‐Gheidari 2012; Urton 2007; van Delden 2012; Wang 2011).
9. Were methods used to combine the findings of studies appropriate?
We judged that 27 of the 40 reviews used appropriate methods for combining the results of studies; however, 11 were judged not to have combined the results of studies, and one was unclear on this. One review was judged not to have reported appropriate methods for combining the results of studies (Luke 2004).
10. Was the likelihood of publication bias assessed?
We judged that eight of 40 reviews assessed publication bias. Five of these were Cochrane reviews (Elsner 2013; French 2010; Hao 2013; Katalinic 2010; Mehrholz 2012), and three were non‐Cochrane reviews (Braun 2013; French 2008; Wang 2011).
11. Was the conflict of interest stated?
Thirty of 40 reviews included a conflict of interest statement; these included all 19 Cochrane reviews and 11 of 21 non‐Cochrane reviews. Eleven of the non‐Cochrane reviews were judged not to have a conflict of interest statement or to provide unclear information on this. The Cochrane review of virtual reality was judged to have a potential conflict of interest, as one of the review authors was a "co‐owner of a company that develops virtual reality for rehabilitation" (Laver 2011).
Reviews included in data synthesis
Nine of the 40 reviews (three Cochrane and six non‐Cochrane) are included within a synthesis of qualitative data only. Details of these reviews, their reported results and the reasons for inclusion only in qualitative synthesis are provided in Table 7.
Data from the remaining 31 reviews are included in our synthesis of quantitative data. However, some overlap was noted between data included within some reviews; to avoid inclusion of duplicate comparisons, only subgroup comparisons were considered for four reviews (see Table 9 for further information).
| Intervention | Reviews contributing data to main comparisons | Reviews contributing data to subgroup comparisons only | Justification for decisions |
| Constraint‐induced movement therapy (CIMT) | Studies included in these 2 reviews overlap. Corbetta 2010 was judged to be most up‐to‐date and comprehensive. Corbetta 2010 pools data comparing CIMT with control. However, no sub‐group analyses are reported. Sirtori 2009 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Corbetta 2010, and data related to subgroup comparisons are extracted from Sirtori 2009 | ||
| Mental practice | Braun 2013 has the most up‐to‐date search and includes trials that are not included within (or considered for inclusion in) Barclay‐Goddard 2011. Methodological quality is similar. Data from Braun 2013 are therefore extracted for the main comparisons. However, no subgroup analyses are reported. Barclay‐Goddard 2011 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Braun 2013, and data related to subgroup comparisons are extracted from Barclay‐Goddard 2011. Additional impairment data were extracted for the main comparisons from Wang 2011, as this included Chinese language trials not included within Braun 2013 | ||
| Robotics | Mehrholz 2012 has the most up‐to‐date search and includes trials that are included within (or considered for inclusion in) Norouzi‐Gheidari 2012. Methodological quality of Mehrholz 2012 was judged to be considerably greater than that of Norouzi‐Gheidari 2012. Data from Mehrholz 2012 are therefore extracted for the main comparisons. However, no subgroup analyses are reported. Norouzi‐Gheidari 2012 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Mehrholz 2012, and data related to subgroup comparisons are extracted from Norouzi‐Gheidari 2012 | ||
| Stretching and positioning | Katalinic 2010 is a review of stretching interventions, including positioning interventions. Borisova 2009 includes positioning interventions only. The methodological quality of Katalinic 2010 is judged to be considerably greater than that of Borisova 2009, and data from Katalinic 2010 are therefore extracted for the main comparisons. All trials included in Borisova 2009 are also included in Katalinic 2010; however, as this is a subgroup of a particular type of stretching intervention, data from Borisova 2009 have been included as a subgroup analysis |
Data from two reviews related to mental practice were included, as considerable differences were noted in the included trials, with Wang 2011 including several non‐English papers not included by Braun 2013, and Braun 2013 including some English‐language publications not included in Wang 2011. It should be noted that some overlap is evident in the trials contributing data within these reviews.
Outcome comparisons included in data synthesis
From the 31 reviews within our synthesis of quantitative data, we extracted data related to the results of 127 comparisons of measures of upper limb function, impairment or ADLs. Ninety‐one of these comparisons were performed immediately at the end of the intervention, and 20 at a follow‐up assessment; 16 of these 127 comparisons were subgroup comparisons. Further details related to the number of reviews contributing to these comparisons and the outcome data extracted are briefly described as follows.
Upper limb function: immediate outcome
We extracted data related to our primary outcome of upper limb function as related to 29 comparisons, presented by 19 reviews, immediately at the end of intervention. Of these 29 comparisons, 18 comprised outcomes within our prestated category of 'arm function' and five outcomes within our prestated category of 'hand function.' Two combined both arm and hand function outcomes, which we refer to as 'upper limb function.' Two comparisons combined arm function outcomes with measures of ADL, and two combined upper limb function outcomes with measures of motor impairment, but we judged these combined outcomes to be most relevant to our upper limb function category.
Upper limb function: follow‐up outcome
Data suitable for extraction related to follow‐up outcomes of upper limb function were available from only two reviews (three comparisons). Two of these comparisons were related to measures of arm function, assessed at less than and more than six months post stroke (Cooke 2010). The third comparison combined both arm and hand function outcomes to form a pooled measure of upper limb function (French 2007).
Impairment: immediate outcome
We extracted data from 21 reviews related to 44 comparisons as related to measures of impairment immediately at the end of the intervention. Of these 44 comparisons, 19 were measures of 'motor impairment' (16 were assessed using the Fugl‐Meyer Assessment), nine were measures of range of movement, eight were measures of spasticity, seven were measures of strength and one was a measure of sensory impairment.
Impairment: follow‐up outcome
Seven reviews contributed follow‐up data related to 11 comparisons with measures of impairment: three motor impairment, one strength, two spasticity and five range of movement. Most reviews pooled data from any follow‐up period, which generally occurred over three months following intervention, although Katalinic 2010 presented data for follow‐up measures of 24 hours to one week and longer than one week.
Activities of daily living: immediate outcome
We extracted data related to 18 comparisons from 13 reviews as related to measures of ADLs. Thirteen of the comparison outcomes comprised generic ADL assessments, most commonly Barthel Index and Functional Independence Measure. Four comprised assessments of activity, as measured by the Motor Activity Log. One comparison pooled data from generic ADL assessments with measures of upper limb function (Mehrholz 2012); as these were principally measures of ADLs, this information is presented with the ADL outcomes.
Activities of daily living: follow‐up outcome
Four reviews contributed follow‐up data related to five comparisons, all of which included measures of generic ADLs. As in the follow‐up assessment of impairment, most reviews pooled data from any follow‐up period, but Katalinic 2010 presented data for follow‐up measures of 24 hours to one week and longer than one week.
Quality of evidence within reviews included in data synthesis
This section describes judgement of the quality of evidence for each of the 127 comparisons for which data were extracted. Quality is described as high, moderate, low or very low, as derived using the objective criteria and algorithm presented in Assessment of methodological quality of included reviews and Appendix 4. It is important to note that this statement and categorisation of the quality of evidence do not reflect the effectiveness of the interventions in any way. The effect of interventions is reported in the section Effects of interventions.
Table 10, Table 11, Table 12, Table 13, Table 14 and Table 15 detail comparisons judged to provide moderate‐ (or high‐) quality evidence; Table 16, Table 17, Table 18, Table 19, Table 20 and Table 21 detail comparisons judged to provide low‐ or very low‐quality evidence. Table 22 presents data for the subgroup comparisons.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Arm function | ARAT, WMFT | 6 | 375 | SMD 0.20 | (‐0.00 to 0.41) | Favours unilateral exercise training | |
| CIMT | Control | Arm function | ARAT, WMFT, EFT, MAS | 14 | 477 | SMD 0.44 | (0.03 to 0.84) | Beneficial effect | |
| Repetitive task training | Any control | Arm function | ARAT, WMFT, BBT, FTHUE, SMGA | 8 | 412 | SMD 0.17 | (‐0.03 to 0.36) | No benefit or harm | |
| Hand function | 9HPT, 10HPT, MAS | 5 | 281 | SMD 0.16 | (‐0.07 to 0.40) | No benefit or harm | |||
| Mental practice | Any control | Arm function | ARAT | 7 | 197 | SMD 0.62 | (0.05 to 1.19) | Beneficial effect | |
| Mirror therapy | Any other intervention | UL function + impairment | ARAT, MAS, FMA | 10 | 421 | SMD 0.53 | (0.04 to 1.01) | Beneficial effect | |
| Sensory impairment | No treatment | Arm function | mMAS | 1 | 29 | MD 1.58 | (0.98 to 2.18) | Beneficial effect | |
| Virtual reality | Other treatment | UL function + impairment | ARAT, WMFT, FMA | 7 | 205 | SMD 0.53 | (0.25 to 0.81) | Beneficial effect | |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 3 | 258 | ES 0.1 | (‐5.7 to 6.0) | No benefit or harm | |
| Factors in service delivery: location: home‐based therapy | Usual care | Arm function | WMFT | 1 | 100 | MD 2.25 | (‐0.24 to 4.73) | No benefit or harm |
10HPT: Ten‐Hole Peg Test.
9HPT: Nine‐Hole Peg Test.
ARAT: Action Research Arm Test.
BBT: Box and Block Test.
EFT: Emory Function Test.
ES: Effect size.
FMA: Fugl‐Meyer Assessment/
FTHUE: Functional Test of the Hemiparetic Upper Extremity.
MAS: Motor Assessment Scale.
MD: Mean difference.
mMAS: Modified Motor Assessment Scale.
SMD: Standardised mean difference.
SMGA: Southern Motor Group Assessment.
WMFT: Wolf Motor Function Test.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Repetitive task training | Any control | Upper limb function | ARAT, WMFT, BBT, FTHUE, SMGA, JTHF, SMES, 10HPT | 6 | 246 | SMD 0.08 | (‐0.17 to 0.33) | No benefit or harm | All FU outcomes | |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 3 | 319 | ES ‐6.4 | (‐12.8 to 0.0) | No benefit or harm | 6 months |
10HPT: Ten‐Hole Peg Test.
ARAT: Action Research Arm Test.
BBT: Box and Block Test.
ES: Effect size.
FTHUE: Functional Test of the Hemiparetic Upper Extremity.
JTHF: Jebsen Taylor Hand Function Test.
SMD: Standardised mean difference.
SMES: Sodring Motor Evaluation Scale.
SMGA: Southern Motor Group Assessment.
WMFT: Wolf Motor Function Test.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Motor impairment | FMA, MSS | 4 | 228 | SMD 0.06 | (‐0.20 to 0.33) | No difference | |
| Brain stimulation: tDCS | Placebo or control | Motor impairment | FMA | 7 | 304 | SMD 3.45 | (1.24 to 5.67) | Beneficial effect | |
| Mental practice | Conventional treatment | Motor impairment | FMA | 5 | 216 | MD 7.81 | (1.96 to 13.65) | Beneficial effect | |
| Robotics | Any other intervention | Motor impairment | FMA | 16 | 586 | SMD 0.45 | (0.2 to 0.69) | Beneficial effect | |
| Strength | Strength | 10 | 321 | SMD 0.48 | (‐0.06 to 1.03) | No benefit or harm | |||
| Sensory impairment | No treatment | Motor impairment | BMR | 1 | 29 | MD 0.19 | (0.09 to 0.29) | Beneficial effect | |
| Stretching and positioning (stretch) | Any | Range of movement | Joint mobility | 7 | 193 | MD 2.17 | (‐1.63 to 5.97) | No benefit or harm | |
| Spasticity | Spasticity | 4 | 109 | SMD 0.08 | (‐0.30 to 0.45) | No benefit or harm | |||
| Virtual reality | Other treatment | Motor impairment | FMA | 5 | 171 | MD 4.43 | (1.98 to 6.88) | Beneficial effect | |
| Strength | Grip strength | 2 | 44 | MD 3.55 | (‐0.20 to 7.30) | No benefit or harm | |||
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Strength | Hand grip force/strength | 2 | 195 | ES ‐10.1 | (‐19.1 to ‐1.2) | Benefit of standard therapy dose | |
| Factors in service delivery: location: home‐based therapy | Usual care | Motor impairment | FMA | 3 | 156 | MD 1.46 | (‐0.58 to 3.51) | No benefit or harm | |
| Factors in service delivery: location: telemedicine | Usual care | Motor impairment | FMA | 2 | 46 | MD 3.65 | (‐0.26 to 7.57) | No benefit or harm |
BMR: Brunnstrom motor recovery.
FMA: Fugl‐Meyer Assessment.
MSS: Motor status score.
tDCS: Transcranial direct current stimulation.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Stretching and positioning (stretch) | Any | Range of movement | Joint mobility | 3 | 77 | MD ‐0.09 | (‐3.58 to 3.40) | No benefit or harm | 24 hours to 1 week | |
| 4 | 134 | MD ‐0.32 | (‐4.09 to 3.44) | No benefit or harm | > 1 week | |||||
| Spasticity | Spasticity | 1 | 42 | SMD ‐0.5 | (‐1.12 to 0.11) | No benefit or harm | > 1 week | |||
| Factors in service delivery: location: home‐based therapy | Usual care | Motor impairment | FMA | 1 | 36 | MD 4.3 | (0.19 to 8.41) | Beneficial effect | Any FU |
FMA: Fugl‐Meyer Assessment.
FU: Follow‐up.
MD: Mean difference.
SMD: Standardised mean difference.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Activity | MAL: AOU | 3 | 146 | SMD 0.42 | (0.09 to 0.76) | Favours unilateral exercise training | |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Activity | MAL: QOM | 3 | 146 | SMD 0.45 | (0.12 to 0.78) | Favours unilateral exercise training | |
| Brain stimulation: tDCS | Placebo or control | Generic ADL | BI | 5 | 286 | SMD 5.31 | (‐0.52 to 11.14) | No benefit or harm | |
| Mental practice | Any control | Generic ADL | BI | 3 | 135 | MD 0.87 | (‐0.8 to 2.53) | No benefit or harm | |
| Mirror therapy | Any other intervention | Generic ADL | BI, FIM | 4 | 217 | SMD 0.33 | (0.05 to 0.60) | Beneficial effect | |
| Robotics | Any other intervention | Generic ADL + UL function | BI, FIM, ABILHAND, SIS, FAT | 13 | 552 | SMD 0.43 | (0.11 to 0.75) | Beneficial effect | |
| Stretching and positioning (stretch) | Any | Generic ADL | MAS, mBI, DASH | 4 | 130 | SMD 0.2 | (‐0.24 to 0.65) | No benefit or harm | |
| Factors in service delivery: location: home‐based therapy | Usual care | Generic ADL | BI | 2 | 113 | MD 2.85 | ([‐1.43 to 7.14) | No benefit or harm |
ABILHAND: Assessment tool that measures a patient's perceived difficulty using his/her hands to perform manual activities in daily life.
ADL: Activity of daily living.
BI: Barthel Index.
CI: Confidence interval.
DASH: Disabilities of the Arm Shoulder and Hand outcome.
FAT: Frenchay Arm Test.
FIM: Functional Independence Measure.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MAL: AOU: Motor Activity Log: Amount of Use.
MAL: QOM: Motor Activity Log: Quality of Movement.
MAS: Motor Assessment Scale.
mBI: Modified Barthel Index.
MD: Mean difference.
SIS: Stroke Impact Scale.
SMD: Standardised mean difference.
tDCS: Transcranial direct current stimulation.
UL: Upper limb.
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Stretching and positioning (stretch) | Any | Generic ADL | MAS | 1 | 40 | MD 1.7 | (‐0.40 to 3.80) | No benefit or harm | 24 hours to 1 week | |
| MAS, DASH | 4 | 136 | SMD 0.14 | (‐0.29 to 0.58) | No benefit or harm | > 1 week | ||||
| Factors in service delivery: location: home‐based therapy | Usual care | Generic ADL | BI | 1 | 80 | MD ‐1.70 | (‐5.51 to 2.11) | No benefit or harm | Any |
ADL: Activity of daily living.
BI: Barthel Index.
DASH: Disabilities of the Arm Shoulder and Hand outcome.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MAS: Motor Assessment Scale.
MD: Mean difference.
SMD: Standardised mean difference.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Arm function + ADL | BBT, WMFT, MAL: AOU | 4 | 127 | SMD ‐0.07 | (‐0.42 to 0.28) | 1 | 1 | 0 | 1 | Low | |
| Other upper limb intervention | Arm function | BBT, ARAT, MAS, mMAS | 5 | 189 | SMD ‐0.2 | (‐0.49 to 0.09) | 1 | 1 | 0 | 1 | Low | ||
| Usual care | Hand function | PPT | 2 | 73 | SMD ‐0.04 | (‐0.5 to 0.42) | 2 | 1 | 0 | 1 | Low | ||
| Other upper limb intervention | Hand function | MAS, mMAS, SIS (hand function), 9HPT | 4 | 173 | SMD ‐0.21 | (‐0.51 to 0.09) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Arm function | UEFT | 1 | 29 | SMD ‐0.17 | (‐0.9 to 0.56) | 2 | 1 | 0 | 1 | Low | |
| Bobath therapy | Control | Arm function | UEFT | 1 | 29 | ES 0.17 | (‐0.56 to 0.90) | 2 | 1 | 0 | 2 | Low | |
| MAS | 1 | 61 | ES ‐0.29 | (‐0.80 to 0.21) | 2 | 1 | 0 | 2 | Low | ||||
| Arm function | SMES | 1 | 61 | ES ‐0.32 | (‐0.83 to 0.19) | 2 | 1 | 0 | 2 | Very low | |||
| Brain stimulation: rTMS | Control | Upper limb function | JTHF, PPT, WMFT, ARAT | 4 | 73 | SMD 0.51 | (‐0.99 to 2.01) | 2 | 1 | 1 | 0 | Low | |
| Electrical stimulation (for strength) | Control | Arm function + ADL | ARAT, BBT, BI | 3 | 122 | SMD 0.79 (random effects) | (‐0.11 to 1.69) | 1 | 1 | 0 | 1 | Low | |
| Electrical stimulation (NMES) | No treatment | Arm function | ARAT | 4 | 319 | 0.04 to 0.5 | (‐0.35 to 0.44) to (‐0.69 to 1.68) | 0 | 0 | 1 | 2 | Low | |
| Electrical stimulation (stochastic resonance) | Control | Arm function | ARAT | 1 | 30 | 0.15 | (‐0.66 to 0.96) | 2 | 0 | 0 | 2 | Low | |
| Electrical stimulation (EMG‐triggered) | No treatment | Arm function | BBT | 3 | 42 | 0.37 | (‐0.27 to 1.01) | 2 | 1 | 0 | 2 | Very low | |
| Cyclical electrical stimulation | Arm function | ARAT | 2 | 48 | 0 | (‐0.56 to 0.57) | 2 | 0 | 0 | 2 | Low | ||
| Sensory impairment (interventions for sensory impairment) | No treatment | Hand function | Hand FunctionTest | 1 | 36 | MD ‐1.16 | (‐2.10 to ‐0.22) | 2 | 1 | 0 | 0 | Low | |
| Placebo or attention control | Arm function | ARAT | 1 | 21 | MD 12.9 | (5.65 to 20.15) | 2 | 1 | 0 | 0 | Low | ||
| Sensory impairment (passive sensory retraining) | Not reported | Hand function | JTHF | 3 | Unclear | MD 8.72 | (2.48 to 14.95) | 2 | 1 | 1 | 2 | Very low | |
| Strength training | Control | Upper limb function | MAS, TEMPA, RMA, PPB, WMFT, BBT, ARAT, FTHUE | 11 | 465 | SMD 0.21 | (0.03 to 0.39) | 0 | 1 | 0 | 2 | Low | |
| Stretching and positioning: shoulder support | Control | Arm function | MAS | 1 | 83 | MD 0.83 | (‐1.46 to 3.12) | 2 | 0 | 0 | 1 | Low |
9HPT: Nine‐Hole Peg Test.
ADL: Activity of daily living.
ARAT: Action Research Arm Test.
BBT: Box and Block Test.
BI: Barthel Index.
EMG BF: Electromyographic biofeedback.
ES: Effect size.
FTHUE: Functional Test of the Hemiparetic Upper Extremity.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
JTHF: Jebsen Taylor Hand Function Test.
JTHF: Jebsen Test of Hand Function.
MAL: AOU Motor Activity Log: Amount of Use.
MAS: Motor Assessment Scale.
MD: Mean difference.
mMAS: Modified Motor Assessment Scale.
NMES: Neuromuscular electrical stimulation.
PPB: Purdue Peg Board.
PPT: Purdue Peg Test.
RMA: Rivermead Motor Assessment.
rTMS: Repetitive transcranial magnetic stimulation.
SIS: Stroke Impact Scale.
SMD: Standardised mean difference.
SMES: Sodring Motor Evaluation Scale.
TEMPA: Test d'Evaluation des Membres Superieurs de Personnes Agees.
TEMPA: Upper Extremity Performance Test for Elderly (Test d’Evaluation des Membres Supérieurs de Personnes Agées).
UEFT: Upper Extremity Function Test.
WMFT: Wolf Motor Function Test.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | Follow‐up |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 2 | 168 | 2.2 | (‐6.0 to 10.4) | 1 | 1 | 1 | 1 | Low | FU1 |
ARAT: Action Research Arm Test.
FU: Follow‐up.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Motor impairment | FMA | 4 | 127 | SMD 0.67 | (‐0.43 to 1.77) | 1 | 1 | 1 | 1 | Low | |
| Other upper limb intervention | Motor impairment | FMA, RMA | 4 | 175 | SMD ‐0.25 | (‐0.55 to 0.05) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Range of movement | Wrist ROM | 1 | 9 | SMD 0.96 | (‐0.48 to 2.40) | 2 | 1 | 0 | 1 | Low | |
| Shoulder ROM | 1 | 26 | SMD 0.88 | (0.07 to 1.70) | 2 | 1 | 0 | 1 | Low | ||||
| Motor impairment | BMR | 2 | 57 | SMD 0.69 | (0.15 to 1.23) | 2 | 1 | 0 | 1 | Low | |||
| FMA | 1 | 29 | SMD 0.44 | (‐0.19 to 1.07) | 2 | 1 | 0 | 1 | Low | ||||
| Electrical stimulation (for strength) | Control | Strength | Strength | 6 | 162 | SMD 0.55 | (0.23 to 0.86) | 1 | 1 | 0 | 1 | Low | |
| Electrical stimulation (NMES) | Control | Motor impairment | FMA | 5 | 152 | ES 0.01 to 2.43 | (‐0.8 to 0.81) to (‐0.74 to 5.59) | 1 | 0 | 1 | 2 | Low | |
| Strength | Grip strength, power | 3 | 93 | ES 0.00 to 0.38 | (‐0.88 to 0.88) (to ‐0.16 to 0.93) | 2 | 0 | 1 | 2 | Very low | |||
| Electrical stimulation (EMG‐triggered) | Cyclical electrical stimulation | Motor impairment | FMA | 3 | 57 | SMD 0.1 | (0.43 to 0.64) | 2 | 0 | 0 | 2 | Low | |
| Botulinum neurotoxin | Placebo | Spasticity | Spasticity | 2 | 45 | MD ‐0.62 | (‐1.40 to 0.17) | 2 | 1 | 0 | 2 | Very low | |
| Pharmacological interventions: botulinum neurotoxin | Placebo | Range of movement | Shoulder flexion | 1 | 29 | MD 3 | (‐15.54 to 21.54) | 2 | 0 | 0 | 2 | Low | |
| Shoulder abduction | 3 | 65 | MD 8.49 | (‐2.40 to 19.39) | 2 | 1 | 0 | 2 | Very low | ||||
| Shoulder external rotation | 3 | 70 | MD 9.84 | (0.20 to 19.49) | 2 | 1 | 0 | 2 | Very low | ||||
| Spasticity | Area under curve of Ashworth score: elbow | 2 | 101 | MD ‐6.28 | (‐16.02 to ‐3.47) | 1 | 1 | 1 | 2 | Very low | |||
| Area under curve of Ashworth score: wrist | 2 | 101 | MD ‐11.71 | (‐16.72 to 6.71) | 1 | 1 | 0 | 2 | Low | ||||
| Area under curve of Ashworth score: fingers | 2 | 101 | MD ‐7.79 | (‐13.44 to 2.74) | 1 | 1 | 0 | 2 | Low | ||||
| Pharmacological interventions: botulinum toxin type A: Dysport 500U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 1 | 41 | OR 0.22 | (0.06 to 0.81) | 2 | 1 | 0 | 2 | Very low | |
| Pharmacological interventions: botulinum toxin type A: Dysport 1000U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 2 | 100 | OR 0.22 | (0.09 to 6.52) | 1 | 1 | 0 | 2 | Low | |
| Pharmacological interventions: botulinum toxin type A: Dysport 1500U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 1 | 36 | OR 0.42 | (0.11 to 1.56) | 2 | 1 | 0 | 2 | Very low | |
| Sensory impairment (interventions for) | No treatment | Motor impairment | FMA—upper limb | 1 | 18 | MD ‐6 | (‐16.58 to 4.58) | 2 | 1 | 0 | 0 | Low | |
| FMA—wrist and hand | 1 | 18 | MD ‐0.12 | (‐9.06 to 8.82) | 2 | 1 | 0 | 0 | Low | ||||
| Placebo or attention control | Motor impairment | FMA | 1 | 23 | MD 11.5 | (‐5.45 to 28.45) | 2 | 1 | 0 | 0 | Low | ||
| Sensory interventions: passive sensory retraining | Not reported | Strength | Muscle Strength | 1 | Unclear | MD ‐3.5 | (‐8.13 to 1.13) | 2 | 1 | 1 | 2 | Very low | |
| Sensory interventions: active sensory retraining | Not reported | Sensory | Proprioception | 1 | Unclear | MD 0.14 | (‐2.77 to 3.05) | 2 | 1 | 1 | 2 | Very low | |
| Strength training | Control | Strength | Grip strength | 6 | 306 | SMD 0.95 | (0.05 to 1.85) | 0 | 1 | 1 | 2 | Low | |
| Stretching and positioning: shoulder support | Control | Range of movement | Contracture | 1 | 81 | MD ‐1.2 | (‐10.90 to 8.10) | 2 | 1 | 0 | 1 | Low | |
| Loss of shoulder external rotation | 1 | 14 | OR 1 | (0.11 to 9.34) | 2 | 0 | 0 | 1 | Low | ||||
| Stretching and positioning: inflatable splint | No splint | Motor impairment | FMA | 1 | 18 | MD ‐0.12 | (‐9.8 to 9.6) | 2 | 1 | 0 | 2 | Very low | |
| Stretching and positioning: hand splint (12 hours at night) | 30‐minute stretch | Range of movement | Contracture | 1 | 28 | MD 1 degree | (‐3.7 to 6.1 degrees) | 2 | 0 | 0 | 2 | Low | |
| Factors in service delivery: location: home‐based therapy | Same treatment in hospital | Motor impairment | FMA | 1 | 10 | MD 0.6 | (‐8.94 to 10.14) | 2 | 1 | 0 | 0 | Low |
BMR: Brunnstrom motor recovery.
EMG BF: Electromyographic biofeedback.
ES: Effect size.
FMA: Fugl‐Meyer Assessment.
MD: Mean difference.
NMES: Neuromuscular electrical stimulation.
OR: Odds ratio.
SMD: Standardised mean difference.
RMA: Rivermead Motor Assessment.
ROM: Range of movement.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | Time of FU |
| Brain stimulation: tDCS | Placebo or control | Motor impairment | FMA | 2 | 68 | SMD 9.22 | (‐13.47 to 31.90) | 2 | 1 | 1 | 0 | Low | ||
| Electrical stimulation | Control | Strength | Strength | 2 | 89 | SMD 0.38 | (‐0.04 to 0.80) | 2 | 0 | 0 | 1 | Low | ||
| Pharmacological interventions: botulinum neurotoxin | Placebo | Spascitity | Spasticity | 2 | 45 | MD ‐0.13 | (‐0.65 to 0.38) | 2 | 1 | 0 | 2 | Very low | ||
| Range of movement | Shoulder flexion | 1 | 29 | MD 1 | (‐17.87 to 19.87) | 2 | 0 | 0 | 2 | Low | ||||
| Range of movement | Shoulder abduction | 2 | 45 | MD 17.72 | (‐9.61 to 45.04) | 2 | 1 | 0 | 2 | Very low | ||||
| Range of movement | Shoulder external rotation | 2 | 50 | MD 11.86 | (‐0.61 to 24.33) | 2 | 1 | 0 | 2 | Very low | ||||
| Stretching and positioning: hand splint (12 hours at night) | 30‐minute stretch | Range of movement | Contracture | 1 | 28 | ‐2 degrees | (‐7.2 to 3.2 degrees) | 2 | 0 | 0 | 2 | Low | ||
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Motor impairment | Motricity | 2 | 168 | 10.7 | (1.7 to 19.8) | 1 | 1 | 1 | 1 | Low |
AMSTAR: Measurement tool to assess the methodological quality of systematic reviews.
FMA: Fugl‐Meyer Assessment.
FU: Follow‐up.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MD: Mean difference.
ROB: Risk of bias.
SMD: Standardised mean difference.
tDCS: Transcranial direct current stimulation.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Generic ADL | FIM | 3 | 106 | SMD 0.25 | (‐0.14 to 0.63) | 1 | 1 | 0 | 1 | Low | |
| Other upper limb intervention | Generic ADL | FIM, BI | 3 | 151 | SMD ‐0.25 | (‐0.57 to 0.08) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Generic ADL | BI | 1 | 16 | SMD ‐0.21 | (‐1.20 to 0.77) | 2 | 1 | 0 | 1 | Low | |
| Brain stimulation: rTMS | Control | Generic ADL | BI | 2 | 183 | SMD 15.92 | (‐2.11 to 33.95) | 2 | 1 | 1 | 0 | Low | |
| CIMT | Control | Generic ADL | FIM, BI | 8 | 276 | SMD 0.21 | (‐0.08 to 0.50) | 0 | 1 | 0 | 2 | Low | |
| Electrical stimulation (NMES) | Control | Generic ADL | FIM, BI | 4 | 112 | ES 0.15 to 1.78 | (‐0.61 to 0.91) to (0.00 to 3.56) | 1 | 0 | 1 | 2 | Low | |
| Activity | MAL: AOU | 1 (2 comparisons in one study) | 28 | ES 2.24 to 2.52 | (‐3.24 to 7.72) to (‐8.09 to 13.13) | 2 | 0 | 1 | 2 | Very low | |||
| Activity | MAL: QOM | 1 (2 comparisons in one study) | 28 | ES 2.09 to 2.48 | (‐1.76 to 5.94) to (‐7.03 to 11.99) | 2 | 0 | 1 | 2 | Very low | |||
| Electrical stimulation (stochastic resonance) | Control | Generic ADL | SIS | 1 | 30 | ES ‐0.03 | (‐0.77 to 0.71) | 2 | 0 | 0 | 2 | Low | |
| Strength training | Control | Generic ADL | SF36, FIM, BI | 5 | 210 | SMD 0.26 | (‐0.10 to 0.63) | 0 | 1 | 0 | 2 | Low |
ADL: Activity of daily living.
AMSTAR: Measurement tool to assess the methodological quality of systematic reviews.
BI: Barthel Index.
ES: Effect size.
FIM: Functional Independence Measure.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MAL: AOU: Motor Activity Log: Amount of Use.
MAL: QOM: Motor Activity Log: Quality of Movement.
NMES: Neuromuscular electrical stimulation.
ROB: Risk of bias.
SF36: Short Form (36) Health Survey.
SIS: Stroke Impact Scale.
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | FU time |
| Brain stimulation: tDCS | Placebo or control | Generic ADL | BI | 3 | 99 | SMD 11.16 | (2.89 to 19.43) | 2 | 1 | 0 | 0 | Low | ||
| Mental practice | Any control | Generic ADL | BI | 2 | 57 | MD 0.46 | (‐2.36 to 3.27) | 2 | 0 | 1 | 1 | Low |
ADL: Activity of daily living.
AMSTAR: Measurement tool to assess the methodological quality of systematic reviews.
BI: Barthel Index.
FU: Follow‐up.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MD: Mean difference.
ROB: Risk of bias.
SMD: Standardised mean difference.
tDCS: Transcranial direct current stimulation.
| Details of subgroup | Intervention | Comparison | Review | Outcome category | Number of trials | Number of participants | Effect size | 95% CI | P value | Evidence of effect? | |
| Severity | Mild | Bilateral arm training | Unilateral arm training | Arm function | 5 | 203 | SMD 0.3 | (0.02 to 0.58) | 0.60 | Low quality | |
| Moderate | 3 | 137 | SMD 0.08 | (‐0.25 to 0.42) | No benefit or harm | ||||||
| Severe | 1 | 35 | SMD 0.11 | (‐0.58 to 0.81) | Low quality | ||||||
| Time post stroke | > 6 months post stroke | Mental practice + other treatment | Other treatment | Arm function | 4 | 66 | SMD 1.55 | (0.38 to 2.72) | 0.78 | Low | |
| < 6 months post stroke | 1 | 36 | SMD 1.35 | (0.62 to 2.08) | Low | ||||||
| 0 to 15 days post stroke | Repetitive task training | Any control | Upper limb function | 4 | 239 | SMD 0.21 | (‐0.04 to 0.47) | 0.98 | No benefit or harm | ||
| 16 days to 6 months post stroke | 4 | 105 | Low‐quality evidence | Low‐quality evidence | No benefit or harm | ||||||
| > 6 months post stroke | 3 | 140 | SMD 0.25 | (‐0.08 to 0.59) | No benefit or harm | ||||||
| < 6 months post stroke | Virtual reality | Other treatment | Upper limb function | 2 | 54 | SMD 0.76 | (0.18 to 1.34) | 0.37 | Beneficial effect | ||
| > 6 months post stroke | 5 | 151 | SMD 0.46 | (0.13 to 0.78) | Beneficial effect | ||||||
| Dose | < 360 minutes | Mental practice + other treatment | Other treatment | Arm function | 2 | 46 | SMD 2.79 | (‐0.60 to 1.60) | 0.30 | Low | |
| > 360 minutes | 3 | 56 | SMD 0.95 | (0.31 to 1.60) | Low | ||||||
| 0 to 20 hours | Repetitive task training | Any control | Upper limb function | 8 | 371 | SMD 0.18 | (‐0.02 to 0.39) | 0.31 | No benefit or harm | ||
| > 20 hours | 3 | 113 | SMD 0.40 | (0.03 to 0.78) | Beneficial effect | ||||||
| < 15 hours | Virtual reality | Other treatment | Upper limb function | 2 | 31 | SMD 0.58 | (‐0.12 to 1.29) | 0.87 | No benefit or harm | ||
| > 15 hours | 5 | 171 | SMD 0.52 | (0.21 to 0.83) | Beneficial effect |
CI: Confidence interval.
GRADE: Grades of Recommendation, Assessment, Development and Evaluation.
MD: Mean difference.
SMD: Standardised mean difference.
Quality of evidence related to effects of interventions on upper limb function
High‐quality evidence: upper limb function
No high‐quality evidence was related to the effects of intervention on upper limb function.
Moderate‐quality evidence: upper limb function
Twelve of the 127 comparisons provided moderate‐quality GRADE evidence related to the effects of intervention on upper limb function. These 12 comparisons came from nine different reviews: five Cochrane reviews and four non‐Cochrane reviews.
Ten of these comparisons related to the effects of intervention on upper limb function immediately at the end of intervention, providing moderate‐quality evidence in relation to:
-
bilateral arm training (compared with unilateral arm training) (van Delden 2012);
-
CIMT (Corbetta 2010);
-
repetitive task training (French 2010);
-
mental practice (Braun 2013);
-
mirror therapy (Thieme 2012);
-
treatment for sensory impairment (Doyle 2010);
-
virtual reality (Laver 2011);
-
factors in service delivery: dose of intervention (augmented exercise) (Cooke 2010); and
-
factors in service delivery: location (home‐based therapy) (Coupar 2012).
(See Table 10.)
Two of these comparisons were related to follow‐up measures of upper limb function, providing moderate‐quality evidence in relation to repetitive task training (French 2010) and factors in service delivery: dose of intervention (augmented exercise) (Cooke 2010) (Table 11).
Low‐ or very low‐quality evidence: upper limb function
Twenty of the 127 comparisons provided low‐ or very low‐quality GRADE evidence related to the effects of intervention on upper limb function. These 20 comparisons came from 11 different reviews. Nineteen comparisons were related to outcomes immediately at the end of the intervention (Table 16), and one to a follow‐up assessment (Table 17). A summary of the quality criteria that led to the downgrading of each comparison to low or very low is provided in these tables.
Quality of evidence related to effects of interventions on upper limb impairment
High‐quality evidence: upper limb impairment
No high‐quality evidence was related to the effects of intervention on upper limb impairment.
Moderate‐quality evidence: upper limb impairment
Seventeen of the 127 comparisons provided moderate‐quality GRADE evidence related to the effects of intervention on measures of upper limb impairment. These 17 comparisons came from 10 different reviews: seven Cochrane reviews and three non‐Cochrane reviews.
Thirteen of these comparisons were related to the effects of intervention on upper limb impairment immediately at the end of intervention, providing moderate‐quality evidence in relation to:
-
bilateral arm training (compared with unilateral arm training) (van Delden 2012);
-
brain stimulation—tDCS (Elsner 2013);
-
mental practice (Wang 2011);
-
robotics (Mehrholz 2012);
-
treatment for sensory impairment (Doyle 2010);
-
stretching and positioning (Katalinic 2010);
-
virtual reality (Laver 2011);
-
factors in service delivery: dose of intervention (augmented exercise) (Cooke 2010);
-
factors in service delivery: location (home‐based therapy) (Coupar 2012); and
-
factors in service delivery: location (telemedicine) (Laver 2013).
(See Table 12.)
Four comparisons were related to follow‐up measures of upper limb impairment, providing moderate‐quality evidence in relation to stretching and positioning (Katalinic 2010; three comparisons) and factors in service delivery: location (home‐based therapy) (Coupar 2012; one comparison) (Table 13).
Low‐ or very low‐quality evidence: upper limb impairment
Thirty‐nine of the 127 comparisons provided low‐ or very low‐quality GRADE evidence related to the effects of interventions on upper limb impairment. These 39 came from 13 different reviews, with 31 related to outcomes measured immediately at the end of intervention (Table 18) and eight related to outcomes measured at follow‐up (Table 19). A summary of the quality criteria that led to downgrading of each comparison to low or very low is provided in these tables.
Quality of evidence related to effects of interventions on ADL outcomes
High‐quality evidence: ADL outcomes
High‐quality evidence was related to one comparison exploring the effects of tDCS on ADLs (Elsner 2013; a Cochrane review). No other high‐quality evidence was related to ADL outcomes.
Moderate‐quality evidence: ADL outcomes
Ten comparisons provided moderate‐quality GRADE evidence related to the effects of intervention on measures of ADLs. These 10 comparisons came from six reviews: four Cochrane reviews and two non‐Cochrane reviews.
Seven of these comparisons were related to the effects of intervention on ADL outcomes immediately at the end of intervention, providing moderate‐quality evidence in relation to:
-
bilateral arm training (compared with unilateral arm training) (van Delden 2012);
-
mental practice (Braun 2013);
-
mirror therapy (Thieme 2012);
-
robotics (Mehrholz 2012);
-
stretching and positioning (Katalinic 2010); and
-
factors in service delivery: location (home‐based therapy) (Coupar 2012).
(See Table 14.)
Three comparisons were related to follow‐up measures of ADL outcomes, providing moderate‐quality evidence in relation to stretching and positioning (Katalinic 2010; two comparisons) and factors in service delivery: location (home‐based therapy) (Coupar 2012, one comparison) (Table 15).
Low‐ or very low‐quality evidence: ADL outcomes
Twelve of the 127 comparisons provided low‐ or very low‐quality GRADE evidence related to the effects of intervention on measures of ADL. These 12 comparisons came from eight different reviews, with 10 comparisons related to ADL measures immediately at the end of intervention (Table 20) and two related to follow‐up ADL assessments (Table 21). A summary of the quality criteria that led to downgrading of each comparison to low or very low is provided in these tables.
Quality of evidence related to other outcomes
Few data related to other outcomes defined as of interest to this review were available; consequently these are not reported in tables, but when quality of evidence is judged to be moderate, these data are described in relation to each individual intervention or factor in service delivery.
Quality of evidence related to subgroup analyses
Data from 16 subgroup comparisons were extracted; these were related to severity of stroke (three subgroup comparisons; van Delden 2012); time post stroke (seven subgroup comparisons; Barclay‐Goddard 2011; French 2007; Laver 2011); and dose of intervention (six subgroup comparisons; Barclay‐Goddard 2011; French 2007; Laver 2011). Ten of these subgroup comparisons were judged to provide moderate‐quality GRADE evidence, and six to provide low‐quality GRADE evidence (Table 22).
Effect of interventions
Table 2 and Figure 2 provide a summary of the evidence of effects of interventions; further details on the effects of each individual intervention and factors in service delivery are provided as follows.
Individual interventions
Bilateral arm training
Moderate‐quality evidence shows that unilateral arm training was more beneficial than bilateral arm training for improving upper limb function (six trials, 375 participants) and ADLs (three trials, 146 participants), but no difference was noted between unilateral and bilateral arm training for measures of impairment (four trials, 228 participants) (van Delden 2012).
Only low‐quality evidence was related to bilateral arm training compared with usual care or other interventions for upper limb function, impairment and ADL outcomes (Coupar 2010).
Biofeedback
Up‐to‐date data related to biofeedback were absent. Low‐quality evidence compared EMG biofeedback with physiotherapy (Woodford 2007). Qualitative information suggests that low‐quality evidence was related to biofeedback, with some suggestion that biofeedback may have some beneficial impact (Molier 2010).
Bobath approach
Only very low‐quality evidence was related to the effectiveness of the Bobath approach, and data from individual trials had not been pooled (Luke 2004). The review search is considerably out‐of‐date (last search 2003). (Note: Evidence from Kollen 2009, which was not identified for inclusion in this overview, would also be judged to be of low or very low quality.)
Brain stimulation
tDCS
No evidence related to the impact of tDCS on measures of upper limb function was available.
High‐quality evidence indicated that tDCS resulted in no benefit or harm for ADL outcomes compared with placebo or control intervention; this was based on a pooled analysis of five trials (286 participants). Moderate‐quality evidence showed a beneficial impact on measures of impairment, based on data from seven trials (304 participants) comparing tDCS versus placebo or control intervention (Elsner 2013).
Some evidence was related to follow‐up ADL and impairment outcomes, but this evidence was of low quality, primarily because of the small number of trials that provided follow‐up data (Elsner 2013).
rTMS
Data from upper limb function outcomes were combined with pooled data from four trials (73 participants), providing low‐quality evidence related to the impact of rTMS on upper limb function. However, data from one trial (15 participants) related to the ARAT were classed as providing moderate‐quality evidence and demonstrated no significant benefit or harm of rTMS (standardised mean difference (SMD) 0.19, 95% confidence interval (CI) ‐0.84 to 1.23) (not shown in tables) (Hao 2013).
Data from two trials (183 participants) measuring ADL outcomes were pooled, providing low‐quality evidence related to the impact of rTMS on ADL outcomes (Hao 2013).
Constraint‐induced movement therapy (CIMT)
Moderate‐quality evidence showed a beneficial effect of CIMT on measures of upper limb function; this evidence came from data pooled from 14 trials (477 participants) comparing CIMT versus any control. Evidence related to measures of ADL outcome was classed as of low quality, although the low‐quality grading was largely influenced by methodological limitations within the systematic review (Corbetta 2010).
Electrical stimulation
Only low‐quality evidence was related to the effectiveness of electrical stimulation. Despite relatively large numbers of trials, differences between interventions and outcomes prevented pooling of a large portion of the data. Farmer 2014 made the decision to not pool data from any included trials; Nascimento 2014 pooled available data, but most studies were judged to be at high risk of bias. Small study size and limitations with the systematic review contributed to low‐quality GRADE evidence from Meilink 2008.
Hands‐on therapy (manual therapy techniques)
Lack of trial evidence means that evidence was insufficient to permit any conclusions related to the effectiveness of hands‐on therapy techniques (Winter 2011; qualitative synthesis only).
Mental practice
Moderate‐quality evidence showed a beneficial effect of mental practice (provided in addition to conventional exercise‐based interventions) on arm function (data from Braun 2013; seven trials, 197 participants) and impairment (data from Wang 2011; five trials, 216 participants). The impairment outcome was based on an analysis of trials that delivered a four‐week intervention; pooled evidence related to the effects of a six‐week or eight‐week intervention was of very low quality (four trials, 90 participants) and of low quality (six trials, 282 participants), respectively. Moderate‐quality evidence showed no benefit or harm of mental practice for ADL measures, but evidence related to follow‐up measures was of low quality, largely because of low participant numbers at follow‐up (Braun 2013).
Mirror therapy
Moderate‐quality evidence showed a beneficial effect of mirror therapy compared with any other treatment on a combined upper limb function and impairment outcome (10 trials, 421 participants) and on ADL outcomes (four trials, 217 participants) (Thieme 2012).
Music therapy
Lack of trial evidence means that evidence was insufficient to permit any conclusions related to the effectiveness of music therapy on upper limb outcomes (Bradt 2010).
Pharmacological interventions
Pharmacological interventions for spasticity
Low‐ and very low‐quality evidence was related to the effects of botulinum toxin on measures of spasticity after stroke (data from Elia 2009). This is supported by evidence, which has been synthesised narratively only, from Olvey 2010, which concludes that findings related to the effects on upper limb function of botulinum toxin in participants with spasticity are "inconsistent." Methodological limitations are seen in these reviews, and available trial evidence is limited.
Multi‐disciplinary rehabilitation following pharmacological interventions
Demetrios 2013, a review included within the qualitative synthesis only, concludes that evidence is of low quality and that high‐quality trials are needed.
Pharmacological interventions for shoulder pain
Low‐ and very low‐quality evidence was related to the effectiveness of pharmacological interventions (botulinum toxin) on measures of spasticity and shoulder range of movement in participants with poststroke shoulder pain (data from Singh 2010; five trials, 109 participants with stroke). The methodological quality of the review and the volume of participants were key contributors to the quality of the evidence.
Repetitive task training
Moderate‐quality evidence showed no benefit or harm for upper limb function as a result of repetitive task training, immediately at the end of intervention or at longer‐term follow‐up (data from French 2007; eight trials, 412 participants). Subgroup analyses revealed differences between subgroups related to time post stroke and dose of intervention (see below).
'Repetitive functional task practice' (repetitive task training and constraint‐induced movement therapy)
Pooling of data from trials of repetitive task training and constraint‐induced movement therapy provides moderate‐quality evidence of a beneficial effect of repetitive functional task practice on arm function (SMD 0.24, 95% CI 0.06 to 0.42) and a non‐significant trend towards benefit for hand function (SMD 0.19, 95% CI ‐0.03 to 0.42) (French 2008; data not reported in tables).
Robotics
Moderate‐quality evidence showed a beneficial effect of robotics compared with any comparison intervention (other rehabilitation, placebo or no treatment) on measures of impairment (Fugl‐Meyer) (16 trials, 586 participants) and ADLs (13 trials, 552 participants), and moderate‐quality evidence indicated no benefit or harm for measures of strength (10 trials, 321 participants) (data from Mehrholz 2012).
In contrast, subgroup analyses reported by Norouzi‐Gheidari 2012 demonstrated moderate‐quality evidence of no benefit or harm of robotics, compared with the same duration of conventional rehabilitation, on the Fugl‐Meyer Assessment (six trials, 204 participants; SMD 0.17, 95% CI ‐0.14 to 0.48). Evidence related to the effects of additional robotic therapy (delivered in addition to conventional rehabilitation), compared with conventional rehabilitation, demonstrated benefit but was judged to be of low quality (four trials, 158 participants; SMD 0.46, 95% CI 0.14 to 0.78) (data not reported in tables). Norouzi‐Gheidari 2012 reported the same subgroup comparisons (i.e. same duration or additional robotic therapy) for outcomes of ADLs and motor power, but these comparisons are judged to be of low quality, and no tests for subgroup differences are reported. The methodological quality of this review is judged to have a key impact on the quality of this evidence.
Sensory interventions (interventions to improve sensory function)
Moderate‐quality evidence, arising from one single, small, well‐conducted RCT (29 participants), showed that sensory stimulation (thermal stimulation) had a beneficial effect on arm function, when compared with no treatment. Moderate‐quality evidence arising from the same RCT suggested that sensory stimulation was more beneficial than no treatment in improving impairment as measured by the recovery rate of the Brunnstrom assessment (Doyle 2010).
Evidence from other small single trials of sensory stimulation or passive sensory training was judged to be of low or very low quality (Doyle 2010; Schabrun 2009; qualitative analysis only).
Strength training
Data for pooled comparisons of all outcomes for comparisons of strength training with control interventions were judged to be of low quality. However, it is important to note that the quality judgement was downgraded for risk of bias of included trials, but that this was based on absence of information rather than evidence of high risk within included trials, as the review provided only total Physiotherapy Evidence Database (PEDro) scores, and the component scores were not available.
Low‐quality evidence showed a beneficial effect of strength training on upper limb function, based on data from 11 trials (465 participants), and of a beneficial effect on grip strength, based on data from six trials (306 participants). Low‐quality evidence showed no benefit or harm of strength training on ADLs (five trials, 210 participants) (Harris 2010).
(Note: Ada 2006 is currently awaiting assessment for inclusion within this review and contains evidence related to the effects of strength training.)
Stretching and positioning
One high‐quality review (Katalinic 2010) provided moderate‐quality evidence suggesting no benefit or harm of stretching compared with any other intervention for measures of impairment (joint mobility and spasticity) and ADLs. This finding pertains to measures taken within 24 hours of the end of the intervention, those taken between 24 hours and one week after the intervention and those taken more than one week after the intervention. However, this review pools data from trials including a wide range of populations, interventions and comparison groups, and—other than presenting data from the subgroup of trials with participants with neurological conditions—no subgroup data related to these variables are presented. Three other reviews (Borisova 2009; Hijmans 2004; Lannin 2003) provide what is effectively subgroup comparisons of the populations, interventions and comparisons included by Katalinic 2010; however, all of these reviews are out‐of‐date (search dates May 2003 to June 2005) and have several methodological limitations. Evidence arising from these reviews is judged to be of low or very low quality, and evidence is limited by the small numbers of participants within the comparisons explored and by the methodological quality of the reviews.
Shoulder supports
Low‐quality evidence, derived from an out‐of‐date review, show no benefit of shoulder supports on arm function (one trial, 83 participants), shoulder external rotation (one trial, 14 participants) and contracture (one trial, 81 participants) (Ada 2005).
Task‐specific training
Reach‐to‐grasp exercise
Evidence was insufficient to permit conclusions related to the effectiveness of reach‐to‐grasp exercise, as no high‐quality systematic review has explored this intervention (Pelton 2012; Urton 2007; both in qualitative synthesis only).
Virtual reality
Moderate‐quality evidence shows a beneficial effect of virtual reality from a pooled analysis including measures of both upper limb function (ARAT, WMFT) and impairment (Fugl‐Meyer outcome) (seven trials, 205 participants). Moderate‐quality evidence also suggests a beneficial effect on the Fugl‐Meyer outcome alone (five trials; 171 participants; all of these data were included within the pooled analysis of upper limb function and impairment). Moderate‐quality evidence, based on two trials (44 participants), further shows no benefit or harm of virtual reality on grip strength (Laver 2011).
Factors in service delivery
Dose of intervention
Moderate‐quality evidence from three trials (258 to 319 participants) showed no benefit or harm of increased dose of intervention for arm function or strength. Moderate‐quality evidence also suggested no benefit or harm for arm function at six‐month follow‐up, although evidence at shorter follow‐up length was of low quality. Evidence related to impairment outcomes at follow‐up was of low quality (Cooke 2010).
Evidence from subgroup analyses
Evidence related to dose of intervention was extracted from subgroup analyses within reviews related to CIMT (Sirtori 2009), mental practice (Barclay‐Goddard 2011), repetitive task training (French 2007) and virtual reality (Laver 2011).
Moderate‐quality evidence was related to the subgroups of trials that delivered between 0 and 20 hours or more than 20 hours of repetitive task training, with evidence that the subgroup receiving more than 20 hours had a beneficial effect (three trials, 113 participants). However, a significant subgroup difference between these groups based on dose of intervention was not reported (P value 0.31) (data from French 2007). Similarly, moderate‐quality evidence was related to the subgroups of trials that delivered more or less than 15 hours of virtual reality, with evidence that the subgroup receiving more than 15 hours had a beneficial effect (five trials, 171 participants), but no significant subgroup difference between these groups was reported (P value 0.87) (data from Laver 2011).
Subgroup analyses related to the dose of CIMT were extracted only for our secondary outcome of ADL measures. A non‐significant (P value 0.07) trend towards a greater effect was noted with a CIMT dose less than or equal to 30 hours (Sirtori 2009; data not entered in tables; more than 30 hours of exercise, two trials, 73 participants; SMD 0.02, 95% CI ‐0.44 to 0.49; 30 hours or less of exercise, four trials, 111 participants; SMD 0.58, 95% CI 0.20 to 0.97).
Subgroup analyses related to dose of mental practice were of low quality and did not indicate a difference between participants who received more or less than 360 minutes (P value 0.30) (Barclay‐Goddard 2011).
Service location
Home‐based therapy
For the comparison of home‐based therapy programmes for upper limb recovery versus usual care, moderate‐quality evidence showed no benefit or harm for measures of upper limb function immediately after intervention (one trial, 100 participants); ADL outcomes, both immediately after intervention (two trials, 113 participants) and at longer‐term follow‐up (one trial, 80 participants); and extended ADL outcomes, both immediately after intervention (two trials, 113 participants; mean difference (MD) 0.83, 95% CI ‐0.51 to 2.17) and at longer‐term follow‐up (one trial, 80 participants; MD 0.80, 95% CI ‐0.96 to 2.56) (data from extended ADLs not provided in data tables). For measures of impairment based on the Fugl‐Meyer Assessment, moderate‐quality evidence similarly showed no benefit or harm immediately after intervention (three trials, 156 participants); however, at follow‐up moderate‐quality evidence of benefit favoured home‐based therapy (one trial, 36 participants) (Coupar 2012).
Evidence related to the comparison of upper limb therapy delivered at home versus in hospital was of low quality. Data were available for only one trial (10 participants), which was judged to be at high risk of bias (Coupar 2012).
Telerehabilitation
No data related to the primary outcome of upper limb function were presented in the available review (Laver 2013). Data from two small trials (46 participants) provided moderate‐quality evidence related to upper limb impairment, as measured by the Fugl‐Meyer Scale, demonstrating that telerehabilitation (comprising a computer‐based training programme) resulted in no benefit or harm when compared with usual care (MD 3.65, 95% CI ‐0.26 to 7.57). These data also contribute to the moderate‐quality evidence showing no benefit or harm of home‐based upper limb therapy for measures of impairment (see above).
Severity of stroke
Evidence from subgroup analyses
Evidence related to severity of stroke was extracted from subgroup analyses within reviews related to bilateral arm training (van Delden 2012) and strength training (Harris 2010). No significant subgroup differences related to stroke severity in terms of improvements in upper limb function occurred as a result of bilateral arm training versus unilateral arm training (P value 0.60).
Harris 2010 presents a meta‐analysis for subgroups of participants who have moderate or mild impairment after stroke. However, no test for subgroup differences is reported, limiting the ability to draw conclusions from these data (data not provided in tables).
Time post stroke
Evidence from subgroup analyses
Evidence related to time post stroke was extracted from subgroup analyses within reviews related to CIMT (Sirtori 2009), mental practice (Barclay‐Goddard 2011), repetitive task training (French 2007), robotics (Norouzi‐Gheidari 2012), strength training (Harris 2010) and virtual reality (Laver 2011).
Subgroup analyses related to the effects of time post stroke following CIMT were extracted only for our secondary outcome of ADL measures. No significant subgroup differences related to time post stroke were noted for improvements in ADL outcomes as a result of CIMT (P value 0.39) (Sirtori 2009; data not presented in tables).
Subgroup analyses related to effects of time post stroke on mental practice outcomes were of low quality and showed no differences between participants who were more and less than six months post stroke (P value 0.78) (Barclay‐Goddard 2011).
Subgroup analyses comparing trials with participants who were zero to 15 days, 16 days to six months or more than six months post stroke found no significant subgroup differences between groups for measures of arm function (P value 0.98), and all groups demonstrated no benefit or harm from the repetitive task training intervention (data from French 2007). Similarly, subgroup analyses of participants who were more or less than six months post stroke found no significant subgroup differences between groups for a composite upper limb function and impairment measure (P value 0.37), although both groups demonstrated a beneficial effect of the virtual reality intervention (data from Laver 2011).
Both Norouzi‐Gheidari 2012 and Harris 2010 reported meta‐analysis results for subgroups of participants in the acute/subacute or chronic phase after stroke. However, no test for subgroup differences is reported in either of these trials, limiting the ability to draw conclusions from these data, which relate to robotics and strength training, respectively (data not provided in tables).
Farmer 2014 explored intervention effect size (from trials of assistive technologies, which primarily included electrical stimulation, CIMT, biofeedback and robotics) in relation to time post stroke, providing some very limited evidence that the greatest effects are achieved with treatment in the acute phase after stroke. Farmer 2014 also reported evidence from an individual trial that early treatment with CIMT may cause adverse effects in some groups of stroke patients.
Indirect comparisons between interventions
As stated in the methods (Data collection and analysis), although we had planned for potential indirect comparisons, no indirect comparisons have been carried out because different outcome measures were combined with the use of SMDs and levels of heterogeneity were judged to be high between trials within reviews. Instead, when a comparison was judged to yield moderate‐quality evidence related to the effects on our primary outcome of upper limb function, and when the review reported an SMD and 95% CIs, we plotted these results on a graph to provide a visual representation of effect sizes. This is presented in Figure 5. We recommend that no conclusions should be drawn related to differences in effect sizes between these interventions, as evidence varies in relation to key parameters such as dose of intervention and time post stroke.

Effects of interventions: upper limb function. Moderate‐level GRADE evidence (comparisons reporting standardised mean differences only).
Comparison of intervention versus any other control (including no treatment, control or usual care), unless otherwise stated (as in the comparison of bilateral arm training vs unilateral arm training).
Favours intervention if to the right of the zero line (for comparison of bilateral vs unilateral arm training—favours unilateral arm training).
Discussion
This overview included 40 reviews related to interventions for improving upper limb function after stroke, with some areas of overlap noted between the trials included within these reviews. However, it is important to note that we specifically excluded 37 additional reviews because they had been superseded by a later review, or contained no additional trials compared with a review of similar, or higher, methodological quality. Identifying the most up‐to‐date evidence related to interventions to improve upper limb function is clearly challenging because of overlap between reviews. The quality of the included reviews varied substantially.
Overlap between reviews and methodological limitations within some reviews present significant challenges to clinicians and policy makers seeking synthesised evidence to aid clinical decision making.
We identified reviews related to 18 different individual interventions, many of which included several subcategories of intervention types; this further confirms the challenges involved in identifying the best intervention for an individual patient. This overview, therefore, has an important role in synthesising best evidence on upper limb rehabilitation interventions into a single, accessible, comprehensive document, thus supporting clinicians and policy makers in clinical decision making for stroke rehabilitation.
Summary of main results
High‐quality evidence
High‐quality evidence related to the effectiveness of interventions in improving upper limb function is absent, and evidence is insufficient to permit confident recommendations regarding specific interventions for routine use in clinical practice.
The only high‐quality evidence identified within this overview demonstrated that tDCS had no beneficial effect (or harm) for ADL outcomes. This finding leads us to recommend that tDCS should not be introduced into routine clinical practice. However, moderate‐quality evidence of a beneficial effect of tDCS on upper limb impairment indicates that tDCS does merit further investigation within clinical trials.
Moderate‐quality evidence related to a relatively small number of interventions can be used to support clinical decision making. Current evidence is insufficient to enable indirect comparisons of the relative effects of different interventions; consequently, selection of interventions must be based on expert clinical reasoning and judgement following assessment of an individual patient and with due consideration for the patient and patient goals, preferences and setting.
Individual interventions with moderate‐quality evidence of effect
Moderate‐quality evidence suggests that CIMT, mental practice, mirror therapy and virtual reality may be beneficial in the treatment of upper limb function after stroke, but adequately powered, high‐quality RCTs are required to confirm the benefits of these interventions.
Moderate‐quality evidence suggests that robotics may be effective in improving upper limb impairment and ADL outcomes. However, robotics may not be more beneficial than conventional therapy at the same dose. Further research is required to explore this, and all trials should be careful to control for the effects of dose when exploring novel interventions and assistive modalities. We recommend further research into robotics before robotic devices are introduced into routine clinical practice.
In relation to the dose of intervention, moderate‐quality evidence indicates that repetitive task training provided no benefit or harm; however, the subgroup with the greatest number of repetitions showed beneficial effects. We recommend that the current review of repetitive task training be updated and large‐scale RCTs carried out to explore the effects of dose, including number of repetitions during repetitive task training. Further research may be required to explore the impact of different treatment parameters to inform the development of large‐scale RCTs related to the effects of dose.
Some moderate‐quality evidence is related to one form of intervention for sensory impairment; however, this evidence came from just one high‐quality RCT, and further high‐quality trials are therefore recommended. We do not recommend changes to clinical practice based on this single RCT; however, interventions for sensory impairment are already used widely within routine clinical practice.
Moderate‐quality evidence suggests that bilateral arm training is not as effective as unilateral arm training. This evidence shows that further research investigating bilateral arm training as a generic intervention for the population of people with impaired arm function after stroke may not represent an efficient use of resources. However, current reviews synthesise a clinically diverse range of bilateral arm training interventions, tend to use outcome measures designed to assess unilateral arm function (i.e. function of the impaired limb) and tend to not assess function using both arms together. Consequently, future research into bilateral arm training interventions may be justified if a sound theoretical rationale can be provided for both the intervention and the outcome measure.
Individual interventions with moderate‐quality evidence of no benefit or harm
Moderate‐quality evidence shows no benefit or harm associated with repetitive task training. However, as stated above, evidence shows a dose response, and further research into the issue of dose is essential (see below). It is essential that future trials of repetitive task training achieve what is proposed to be minimum numbers of repetitions for successful skill acquisition. Current evidence shows that an average of more than 300 repetitions per practice session may be required to achieve improvements in arm function (Birkenmeier 2010).
Moderate‐quality evidence also shows no benefit or harm associated with stretching and positioning interventions. However, this evidence was derived from a wide range of populations with varied intervention and comparison groups. We recommend further exploration to investigate the effects of clearly targeted interventions on specific groups of participants. The issue of dose of intervention (including duration, frequency and joint angle) is likely to be central to the effect of stretching and positioning interventions, and we urge researchers to ensure that research protocols comprise doses that are theoretically predicted to effect change. High‐quality up‐to‐date reviews are required for all stretching and positioning interventions.
Individual interventions with low‐quality evidence
Up‐to‐date reviews required
Evidence related to the following interventions is currently of low quality; high‐quality, up‐to‐date reviews are recommended to adequately inform the current state of evidence.
-
Biofeedback.
-
Bobath therapy. (Note: A Cochrane review is currently exploring the effectiveness of the Bobath approach but excludes trials focused only on the upper limb (Pollock 2014). A review similar to this is needed but should include upper limb trials. As in Pollock 2014, the challenge of defining the Bobath concept would have to be addressed within any review of upper limb trials.)
-
Electrical stimulation. (Note: An ongoing review is related to functional electrical stimulation (Howlett (Ongoing)), but this is unlikely to cover all evidence related to electrical stimulation.)
-
Strength training. (See also recommendations for high‐quality RCTs, below.)
-
Task‐specific training. (Note: An ongoing review is related to reach‐to‐grasp exercise—Diermayr (Ongoing).)
-
Pharmacological interventions. (Note: We are aware of at least one phase III RCT that has not been included within current reviews (Shaw 2011); updating of current reviews is required to include this trial evidence.)
In addition, subgroup analyses are recommended to explore different populations, interventions and comparisons in relation to stretching and positioning interventions. An ongoing review is related to assistive devices for contractures and may explore some of these recommended subgroups (Meeran (Ongoing)). An update of the review of repetitive task training is recommended, as are high‐quality analyses related to the effects of CIMT on measures of impairment and ADL outcomes.
High‐quality RCTs required
Despite high‐quality systematic reviews, evidence in relation to many interventions remains of low quality, and high‐quality RCTs are recommended. We support recommendations for further high‐quality RCTs, as provided within up‐to‐date high‐quality systematic reviews. Interventions for which further RCTs are recommended include rTMS (Hao 2013), hands‐on therapy (Winter 2011), music therapy (Bradt 2010) and pharmacological interventions (Demetrios 2013).
For interventions for sensory impairment (Doyle 2010), we determined that some moderate‐quality evidence currently shows benefit in trials synthesised within high‐quality reviews, but further high‐quality RCTs with appropriate attention controls are recommended.
Although we recommend an up‐to‐date systematic review related to upper limb strength training (see above), we do consider that current evidence is sufficient to justify (see, for example, Ada 2006) support of recommendations for high‐quality RCTs.
Factors in service delivery
Dose of intervention
Moderate‐quality evidence from a systematic review of trials of increased dose of exercise shows that increased dose of intervention provides no benefit or harm (Cooke 2010). However, some evidence from subgroup analyses indicates that a greater effect size may occur with increased dose of an individual intervention. Moderate‐quality evidence from subgroup analyses comparing greater and lesser doses of mental practice (Barclay‐Goddard 2011), repetitive task training (French 2007) and virtual reality (Laver 2011) demonstrates a beneficial effect for the group given the greater dose, but not for the group given the smaller dose. However, in none of these cases does a test for subgroup differences suggest a statistically significant difference between groups. The issue of dose is central to establishing meaningful high‐quality evidence related to rehabilitation interventions, and we recommend that:
-
all reviews of upper limb interventions should explore subgroups based on dose of intervention;
-
RCTs of upper limb interventions should consider the impact of dose and, when appropriate, ensure that control interventions are matched for dose; and
-
RCTs related to dose of upper limb intervention should be carried out. These should consider length of treatment sessions, number of treatment sessions and length of treatment period, as well as intensity of interventions (including number of repetitions and, when appropriate, resistance applied).
We identified two ongoing reviews that may further inform the evidence base related to dose of intervention (Galvin 2012 (Ongoing); Schneider (Ongoing)).
Location of intervention
Some evidence has been found for the effectiveness of home‐based therapy programmes (Coupar 2012) and telemedicine (Laver 2013) in improving upper limb function. However, interpretation of this evidence is limited by the intervention delivered to the control group, which was often "usual care," rather than a comparison with another service location. Furthermore, the evidence base is limited by the fact that overlap is evident between the trials included within the reviews of home‐based therapy and telemedicine, with both including the same trials of a computer‐based intervention. If trials, or reviews, related to the location of the intervention are to be carried out, we recommend that the question to be answered should be clearly defined, and if the question relates to comparison of outcomes when rehabilitation is provided in one setting (e.g. home) versus another (e.g. hospital), trials/reviews should be planned accordingly.
Time post stroke and severity of impairment
All evidence related to the influence of time post stroke and severity of initial upper limb impairment on the effect of interventions is of low quality. We recommend that the issue of the best time at which to offer rehabilitation interventions for the upper limb, and to which participants, is explored within high‐quality RCTs, and that all RCTs of upper limb interventions consider the impact of these issues. We also recommend that all reviews of upper limb interventions, when possible, explore subgroups based on time post stroke and severity of initial upper limb impairment.
Overall completeness and applicability of evidence
Completeness of evidence within reviews
Review evidence related to interventions to improve upper limb function after stroke is not complete. We have identified several reviews that require updating and for which methodological limitations need to be addressed. We did identify some ongoing reviews that were relevant to these areas. In addition, we identified two individual interventions (acupuncture and self‐management) for which there is currently only an ongoing review (Kidd (Ongoing); Liang 2011 (Ongoing)). There may be additional interventions for which no review, or no registered ongoing review, has been conducted; it is therefore impossible to be entirely confident that all relevant upper limb interventions are covered by at least one review and included within this overview. However, we did identify some reviews that covered a broad mixture of different interventions; we considered all interventions covered by these reviews, and we believe this increases the chance that we will have successfully identified all interventions for which some primary research evidence is available in the form of RCTs.
The search dates of included reviews, as illustrated in Figure 6, range from December 2003 to July 2013. The mean search date is around February 2010. We recommend urgent updating for reviews on three topics for which the longest time since last search has passed; these include biofeedback, Bobath therapy and repetitive task training. Updating of reviews is clearly a challenge, but it is essential to the completeness of the evidence base. Decisions to update must be made with consideration of the priority of the review topic, the likelihood of new high‐quality trials and the current quantity and quality of evidence within the review. A high‐quality up‐to‐date review of an intervention should be prepared before any further RCTs are undertaken, so that primary research can be appropriately informed by the current evidence base.
Date of last search for evidence for identified interventions.
For some interventions and topics, we have identified a large number of overlapping reviews, and determining the most comprehensive and up‐to‐date review was complex. We urge researchers to take action to avoid publication of overlapping or similar reviews by searching for reviews and protocols before initiating a review, by publishing review protocols and by clearly highlighting when a new publication supersedes previous publications. Registration and publication of Cochrane reviews is designed to avoid the challenges associated with overlapping reviews, and the Cochrane Stroke Review Group takes steps to ensure that no overlap occurs between Cochrane reviews. When a Cochrane review is out‐of‐date, researchers interested in an updated review on that topic or intervention are encouraged to contact the Cochrane Stroke Review Group to discuss collaboration on updating the review, rather than preparing an alternative journal publication.
Applicability of evidence
The aim of this overview was to synthesise best evidence on upper limb rehabilitation interventions into a single, accessible, comprehensive document, thus supporting clinicians and policy makers in clinical decision making for stroke rehabilitation. However, the aim was not to bring together all evidence required to make an individual treatment decision about an individual patient within a specific setting. This overview serves to signpost clinicians and policy makers toward relevant systematic reviews to support clinical decisions. It is the nature of stroke rehabilitation research and clinical practice that the application of evidence to an individual patient or healthcare setting will depend on the specific details of that patient or setting, and that clinical decisions require expert clinical reasoning and judgement if available evidence is to be interpreted and applied effectively. Before any evidence is applied, we therefore recommend that clinicians and policy makers are guided to the appropriate review, and that they consider carefully the details of the trials synthesised within that review, specifically reflecting on the relevance of the participant population, trial setting and context, interventions delivered and outcomes assessed in relation to the clinical decision to be made. We believe that, given the large volume of overlapping evidence and the variable quality of this evidence, this overview can serve to efficiently guide clinicians and policy makers to the most appropriate review evidence.
Within this overview, in addition to variations among participants, interventions, setting and context, we specifically found that the dose of interventions, outcomes and comparisons were central to assessment of the potential applicability of evidence. Further discussion related to the impact of these on the applicability of evidence is provided in Appendix 5.
Quality of the evidence
Assessment of quality of included reviews
We assessed the quality of included reviews using a modified version of the AMSTAR tool to derive answers to the original AMSTAR questions (Table 1). Despite a number of challenges associated with development and use of the mAMSTAR and AMSTAR tools (see Appendix 3 for further discussion and details), we believe that our use of mAMSTAR questions has provided substantial benefit, and that our clear reporting of agreed upon responses (in Figure 4) enhances the transparency of our judgements and provides the reader with a detailed overview of methodological components of each review.
Quality of included reviews
We have provided a detailed, transparent assessment of the quality of included reviews in Figure 4 and Table 8 and have described issues related to each of the 11 AMSTAR questions in Methodological quality of included reviews. There is clearly a difference in the number of 'yes' responses between Cochrane reviews and non‐Cochrane reviews. However, the data demonstrate that many of these differences are accounted for by poor reporting of information within some of the non‐Cochrane reviews (i.e. lack of 'yes' responses reflects an absence of, or unclear, information, rather than reflecting poor methods per se).
Within the included reviews, we have identified various methods of assessing and reporting the quality of included studies. These are briefly summarised and discussed in Table 23.
| Method of assessment/reporting quality | Discussion |
| Cochrane 'Risk of bias' tool | This is used within all Cochrane reviews; however this tool has developed over time. Some of the reporting within earlier Cochrane reviews is limited primarily to an assessment of concealed allocation, whereas more recent reviews tend to have assessed random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Developments in the Cochrane 'Risk of bias' tool therefore contribute toward improved reporting over time |
| PEDro scale (Maher 2003; PEDro) | This scale assesses reporting of absence or presence of eligibility criteria; random allocation; allocation concealment; baseline similarity; participant, therapist and assessor blinding; dropouts/follow‐up; intention‐to‐treat; statistical comparisons and variability. However within some reviews, only the total PEDro 'score' was given, limiting our ability to judge specific issues related to risk of bias associated with randomisation, allocation concealment, etc. When reviews reported responses to the PEDro scale for each study, we had sufficient information to judge risk of bias for key criteria. Decisions around reporting this information within a published journal article are likely to be influenced by publication restrictions related to article length and number of tables |
| 'Levels of evidence' (Levels of Evidence) | These levels of evidence are based primarily on the methodological design of a study. Some reviews based their reports of quality on the types of study designs of included studies, using these levels of evidence. Often these were reviews that included a variety of different study types (i.e. were not limited to RCTs). These levels of evidence did not provide us with any information relatedto the issues associated with risk of bias, such as randomisation method, participant blinding or how incomplete data were managed |
| Assessment of study quality as part of review inclusion criteria | Some non‐Cochrane reviews (e.g. Farmer 2014) used an assessment of quality of studies as part of the eligibility criteria, including only studies that were judged to be at low risk of bias. Application of quality assessment in this way clearly has consequent implications related to the need to consider the scientific quality of included studies. The AMSTAR tool does not necessarily enable acknowledgement of the fact that all included studies had been judged to be at low risk of bias, and such reviews may be 'marked down' when this is, arguably, not appropriate |
AMSTAR: Measurement tool to assess the methodological quality of systematic reviews.
PEDro: Physiotherapy Evidence Database.
RCT: Randomised controlled trial.
In the past, full and adequate reporting of methodological details of reviews has been challenging because of the word restrictions of a journal publication. However, this should no longer be a limitation of adequate reporting, now that most journals provide opportunities for publication of online supplementary material (Hoffmann 2014a). Despite opportunities for online material, we found less comprehensive reporting in non‐Cochrane reviews, which, for example, rarely reported details of excluded studies. For reviews to be useful and inform clinical decisions, adequate reporting of methods is essential. We urge review authors and journal editors to ensure that minimum reporting standards are achieved. As guidelines and checklists are increasingly used by journal editors in considering study and review methodology, this endeavour should support improved reporting.
Many reviews of stroke rehabilitation interventions will include trials that explore a wide range of diverse interventions, participants and outcome measures. This diversity presents additional challenges to review quality. If reviews are to inform clinical practice, it is essential that they contain adequate descriptions of interventions investigated and participants included. We believe that further work is required to enhance reporting and assessment of these details in a systematic and clinically relevant way, and that this will be supported by the use of tools such as the recently developed template for intervention description and replication (TIDieR) checklist (Hoffmann 2014b). Often review authors make important decisions related to whether to pool (or to not pool) data arising from relatively diverse trials. Such decisions should always be fully explored and discussed to highlight the benefits and limitations associated with the decision, and appropriate steps should be taken by review authors to avoid the introduction of bias at this stage of the review process. We believe that further work is required to establish transparent methods designed to avoid introduction of bias at the stage of decision making related to meta‐analyses of data related to diverse interventions.
Assessment of quality of evidence in included reviews
Systematically establishing the quality of evidence has been central to this overview, and considerable work has gone into ensuring objective and consistent application of GRADE levels of evidence to all comparisons contributing data to this review. Our methods of objectively determining GRADE levels of evidence, based on assessment of the quality of included reviews and the quality of trials within the included reviews, are described in the methods section, and further details are provided in Appendix 4.
Further work is clearly required to explore our methods of applying GRADE levels of evidence. However, in the absence of this, we believe that our objective application and determination of GRADE levels of evidence provide substantial benefit to our overview. We have assessed the quality of evidence using a transparent, objective process, with consideration of both the quality of the review and the trials included within the review, while removing potential risk of bias associated with subjective interpretation and application of this evidence. Further discussion related to our method of objectively determining GRADE levels of evidence is provided in Appendix 4.
Quality of evidence in included reviews: GRADE levels of evidence
Details of GRADE levels of evidence applied to comparisons within this overview are presented in the section 'Quality of the evidence within reviews included in data synthesis' within Methodological quality of included reviews. Only one of the 127 included comparisons was judged to provide high‐level GRADE evidence. Just over one‐third of the comparisons were judged to provide moderate‐level GRADE evidence (49/127), and the remaining two‐thirds (77/127) were judged to present low‐ or very low‐quality GRADE evidence. Reasons for judging evidence as low or very low were related to all criteria judged in assessment of the evidence—number of participants, risk of bias of included trials, heterogeneity within analyses and methodological quality of the review. As most evidence related to interventions to improve upper limb function after stroke is of low quality, this will have a significant impact on clinical decision making; consequently, considerable expertise is required to enable clinical decisions. Expert clinical judgement will be a key component of any decision‐making process. Further research is urgently required to improve the quality of evidence available to support clinical decisions related to upper limb rehabilitation after stroke. Specific recommendations have been made regarding future research related to the individual interventions assessed. These recommendations include full‐scale definitive RCTs for those interventions for which current evidence of benefit is of moderate quality; and for interventions with low‐quality evidence, updated high‐quality reviews or further primary research will contribute to an existing review.
Potential biases in the overview process
We identified reviews for inclusion by searching CDSR, DARE and PROSPERO. We also included other relevant reviews of which review team members were aware. We agreed on a cutoff point and did not include reviews published after this date. The extent of our search, the inclusion of reviews of which review team members were aware and the introduction of a cutoff potentially introduced biases to the reviews selected for inclusion. However, we did ensure that the decision to include any identified reviews was based on an independent assessment by two overview authors, with discussion involving a third overview author when disagreement arose. We also used two independent review authors at all stages of assessment of the quality of included reviews. When one member of our overview team was an author of an identified review, that person was not involved in assessment of that review. When data were extracted from a review, one overview author extracted these onto a spreadsheet, and a second overview author checked each entry against the original review. We used objective criteria to (1) determine the AMSTAR responses from the mAMSTAR, and (2) allocate a GRADE level of evidence from quality assessment of the review and of the trials included in the review. Although we recognise that potential biases exist at all stages of the overview process, we believe that we have taken appropriate steps to reduce these biases throughout the process. In particular, we believe that our use of objective criteria to apply the GRADE level of evidence has substantially reduced potential subjectivity and bias at this stage, has resulted in a transparent and reproducible system and is a key strength of this overview.
Our search of DARE may have failed to reveal some potentially relevant non‐Cochrane reviews; this was particularly the case for reviews for which a record but no structured abstract was available. In these cases, our search was limited to the review title and assigned medical subject heading (MeSH) terms. When reviews were not specifically focused on the upper limb, it is likely that our search strategy will have failed to identify them as potential reviews. Subsequent to our search, we have identified two reviews for which records, but no structured abstracts, were available on DARE at the time of our search (Ada 2006; Kollen 2009). Neither of these reviews included terms relevant to the upper limb within the review title or assigned MeSH terms, yet they are potentially relevant to this overview. We will assess these reviews for inclusion in future updates of this overview. Ideally, non‐Cochrane reviews would be identified through complete searches of electronic databases (including MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Physiotherapy Evidence Database (PEDro)), using a comprehensive systematic review methodology filter.
The quality of the reviews included in this overview and the quality of the studies included within these reviews have varied substantially. We did not exclude reviews on the basis of methodological quality or the quality of included studies. However, we have taken steps to reflect any limitations in the quality of the evidence by considering key quality components in our assessment and judgement of the quality of evidence. It is clear that methodological limitations within both the reviews and the studies included within the reviews mean that all evidence within this overview should be interpreted with caution, as several biases may exist.
We systematically explored overlap between the studies included within reviews; we then made judgements as to which was the most up‐to‐date or comprehensive review in relation to each intervention. We attempted to do this in a rigorous, transparent manner; however, we were required to make a number of complex decisions. The decision whether one review was more up‐to‐date or more comprehensive than another was often complicated by the fact that some reviews included studies other than RCTs, some included participant populations other than those with stroke and some included studies related to a mixture of different interventions (so the number of included studies alone was not a reflection of the relevant high‐quality evidence associated with one intervention). In making decisions about whether one review had been superseded by another, we considered the mAMSTAR assessment, but we did not have objective criteria on which to base these decisions, and each decision was made through discussion between overview authors. Therefore, potential risk of bias was associated with decisions made by the overview authors in relation to which reviews were included.
Several potential biases were associated with data related to comparisons presented within the tables of 'Effects of interventions' (Tables 9 to 21) and summarised within Figure 2 and Table 2. In particular, we have provided few details related to the content of the comparison group, and these details are often unclear within the reviews. Many review authors make decisions to pool data from trials that include a range of diverse comparison groups, including no treatment, control and attention control, usual care and other alternative interventions. This introduces a potential risk of bias, and the 'alternative' intervention should always be considered when clinical decisions are made on the basis of available evidence. In addition, although we took substantial steps to avoid inclusion of reviews with overlapping studies, there remain some studies that contribute to more than one included review. We have attempted to highlight all situations in which this occurred, but this remains a potential bias within this overview.
Agreements and disagreements with other studies or reviews
We are unaware of any other overviews or reviews of reviews exploring the evidence related to upper limb rehabilitation.
After the cutoff date for inclusion within this overview, a large review of RCTs of physical therapy interventions was published (Veerbeek 2014). The aim of that review was to synthesise evidence and carry out meta‐analyses related to "stroke rehabilitation interventions in the domain of physical therapy." This review covers physical interventions to improve upper limb function that are also included in this overview. Pharmacological and brain stimulation interventions included within this overview are not included.
A summary of the characteristics of this review is available in Appendix 6, along with our assessment (based on assessments by two independent overview authors) of the methodological quality of this review using mAMSTAR.
The key difference between our overview and this large review by Veerbeek 2014 is that Veerbeek 2014 has based assessments of the evidence on RCTs, but we have used only reviews of RCTs. Furthermore, Veerbeek 2014 assessed the quality of RCTs using the PEDro score and considered any trial with a score greater than or equal to 4 to be of "high quality." This assessment of "high quality" does not take into consideration criteria such as volume of evidence or heterogeneity of pooled data (although this information is reported). Clearly some advantages are associated with using RCT evidence directly, rather than reviews of RCTs, as this avoids the potential risks of bias associated with review methods and reporting.
The conclusions from both our overview and the review of Veerbeek 2014 are in agreement that evidence suggests a beneficial effect (on outcomes of upper limb function, impairment and/or ADLs) for CIMT, mental practice, robotics, interventions for sensory impairment and virtual reality.
It is important to note that Veerbeek 2014 reported a significant increase in upper limb muscle tone among participants receiving virtual reality interventions.
Our overview of evidence also concluded that moderate‐quality evidence suggests a beneficial effect of mirror therapy. In contrast, meta‐analyses performed by Veerbeek 2014 demonstrated a non‐significant effect on outcomes or motor function and arm‐hand activities. Veerbeek 2014 reported no significant effect of bilateral training; this is consistent with our findings when bilateral arm training was compared with usual care or other control, but we also concluded that moderate‐quality evidence shows that unilateral arm training was more beneficial than bilateral arm training.
We concluded that an up‐to‐date systematic review of RCTs related to electrical stimulation is needed, and, based on the lack of review evidence, we judged evidence related to electrical stimulation to be low‐quality GRADE evidence. Veerbeek 2014 has carried out a series of meta‐analyses of RCT data related to electrical stimulation, which demonstrate that neuromuscular stimulation of the wrist/finger flexors/extensors has a significant beneficial effect on measures of upper limb function, motor function (impairment) and muscle strength (22 trials, 894 participants). Electromyography‐triggered neuromuscular stimulation of the wrist/finger extensors showed a significant beneficial effect on measures of upper limb impairment (25 trials, 492 participants). No evidence revealed an effect of transcutaneous electrical nerve stimulation (TENS) (four trials, 484 participants).
Veerbeek 2014 concluded that significant benefit is associated with high‐intensity exercise or practice (effect size 0.21, 95% CI 0.02 to 0.39). We found evidence from subgroup analyses of benefit associated with higher doses or greater intensity of interventions. However, we found no evidence of a beneficial effect in reviews that provided pooled data related to intensity or dose.
In conclusion, comparison of this overview with the review of RCTs by Veerbeek 2014 revealed the following.
-
Broad agreement regarding the level of evidence, and, when evidence of benefit is apparent, for most interventions, it is agreed that CIMT, mental practice, robotics, interventions for sensory impairment and virtual reality are potentially beneficial interventions.
-
Broad agreement regarding evidence demonstrating the benefit of increased dose of intervention, although our overview is cautious about drawing conclusions based on this evidence.
-
Disagreement regarding evidence related to mirror therapy, with our overview concluding that there is evidence of benefit, and Veerbeek 2014 concluding that there is no evidence of benefit;we recommend further exploration of RCT data related to mirror therapy.
-
We have recommended an updated review and meta‐analysis of evidence related to electrical stimulation; Veerbeek 2014 reports the results of analysis of evidence related to electrical stimulation, suggesting that this intervention may provide beneficial effects.
-
Broad agreement regarding interventions for which low‐quality evidence is currently available and further research is required.
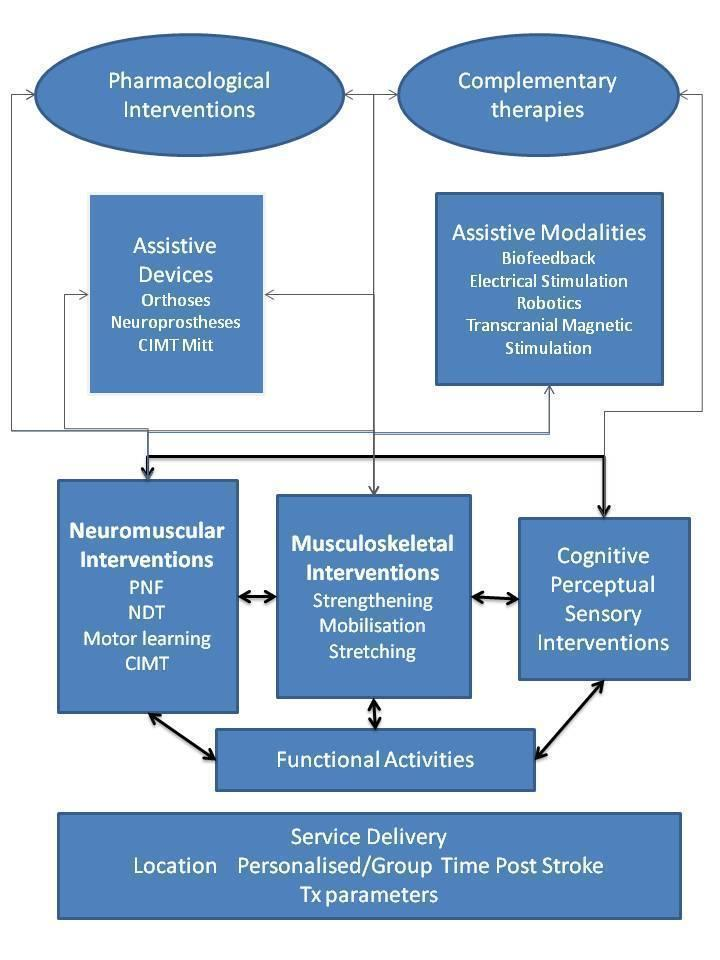
Taxonomy of rehabilitation interventions used within this overview.
Key: CIMT: constraint‐induced movement therapy; NDT: neurodevelopmental treatment; PNF: proprioceptive neuromuscular facilitation; Tx: treatment.
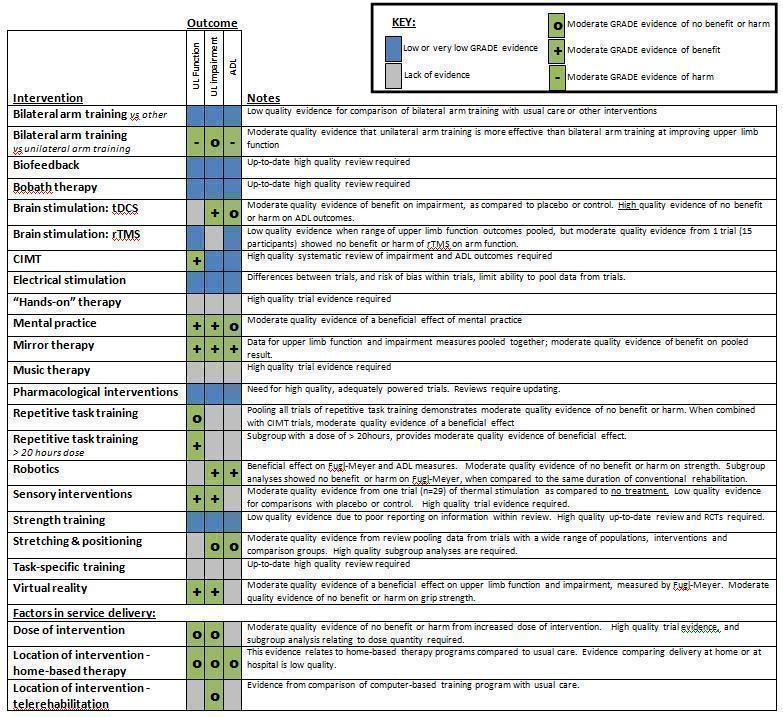
Summary of findings.
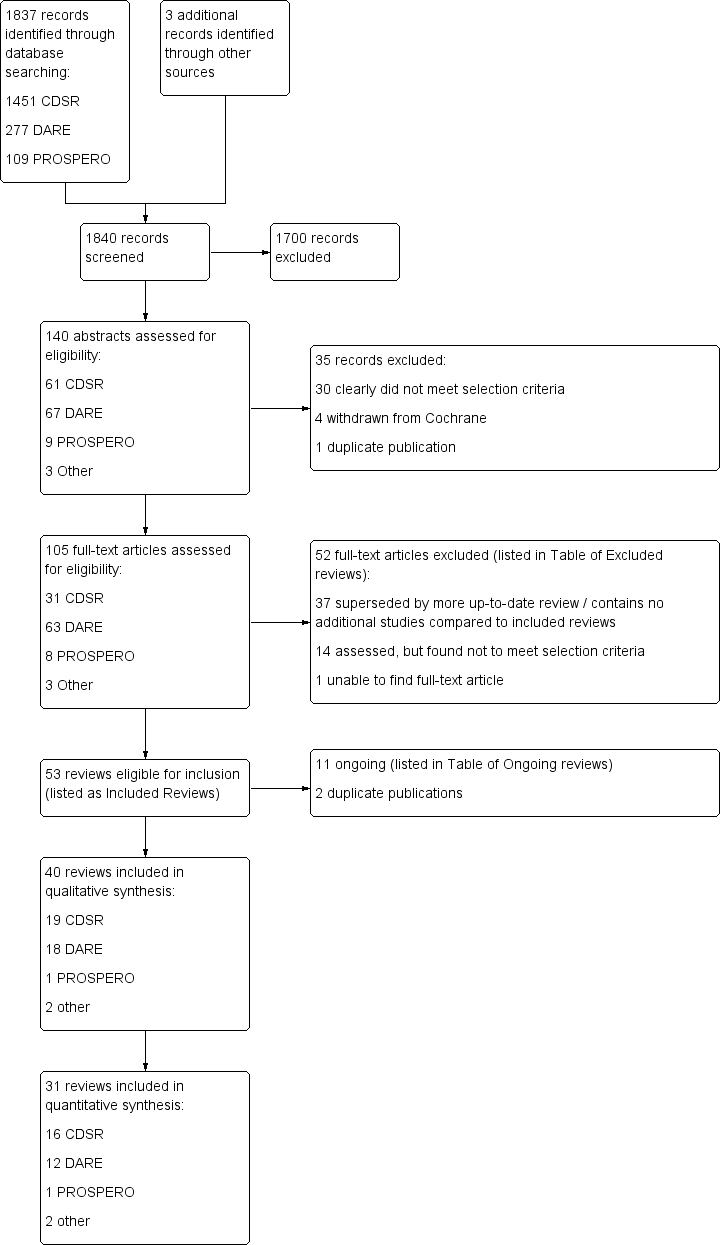
Study flow diagram.
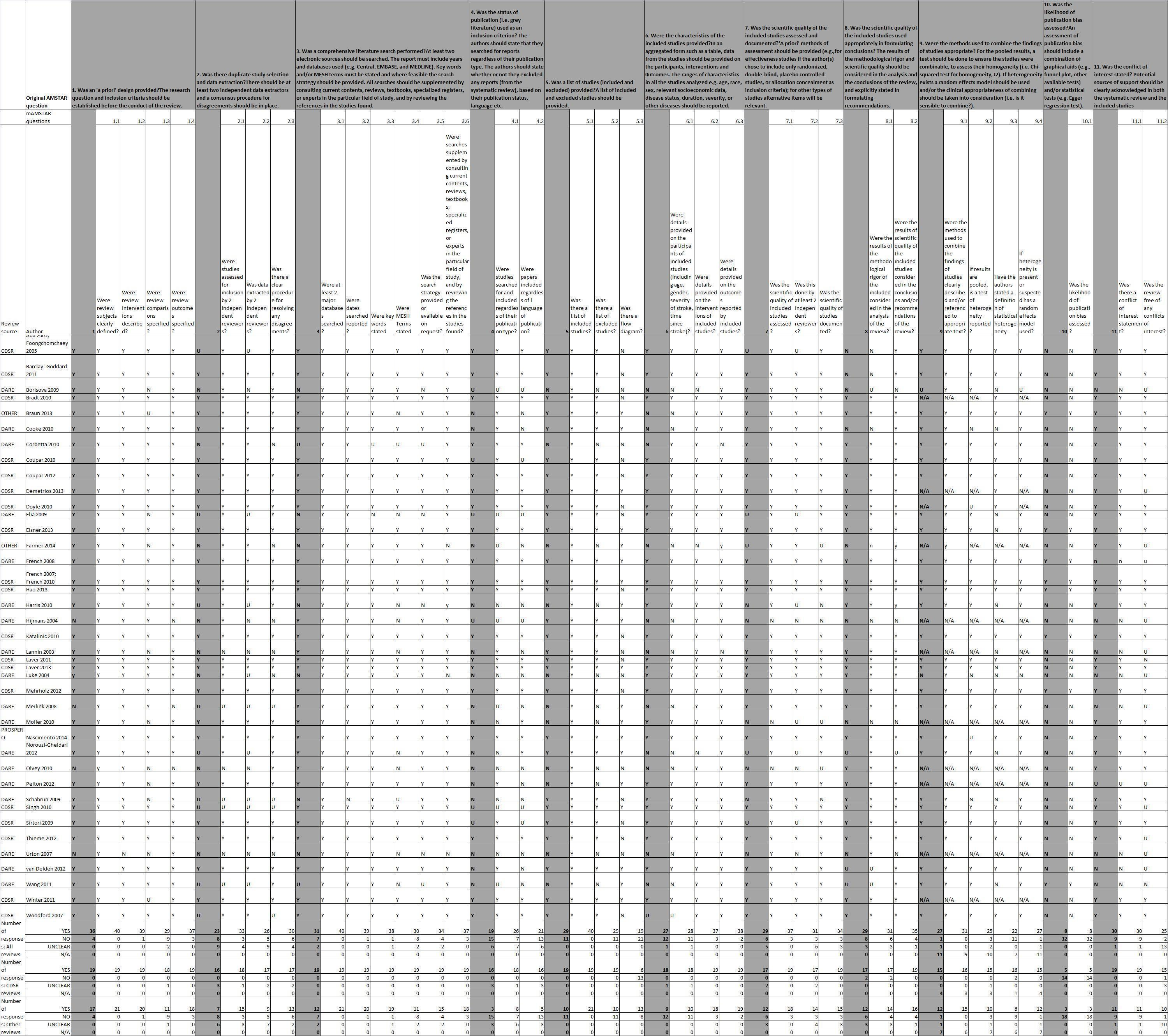
AMSTAR and mAMSTAR results (AMSTAR in shaded columns; mAMSTAR in unshaded columns).

Effects of interventions: upper limb function. Moderate‐level GRADE evidence (comparisons reporting standardised mean differences only).
Comparison of intervention versus any other control (including no treatment, control or usual care), unless otherwise stated (as in the comparison of bilateral arm training vs unilateral arm training).
Favours intervention if to the right of the zero line (for comparison of bilateral vs unilateral arm training—favours unilateral arm training).

Date of last search for evidence for identified interventions.
| AMSTAR questions/criteria | Dichotomous questions used to assess quality of reviews |
| 1. Was an 'a priori' design provided? The research question and inclusion criteria should be established before the conduct of the review. | 1.1 Were review subjects clearly defined? |
| 1.2 Were review interventions described? | |
| 1.3 Were review comparisons specified? | |
| 1.4 Were review outcomes specified? | |
| 2. Was there duplicate study selection and data extraction? There should be at least two independent data extractors, and a consensus procedure for disagreements should be in place. | 2.1 Were studies assessed for inclusion by two independent review authors? |
| 2.2 Were data extracted by two independent review authors? | |
| 2.3 Was there a clear procedure for resolving any disagreements? | |
| 3. Was a comprehensive literature search performed? At least two electronic sources should be searched. The report must include years and databases used (e.g. CENTRAL, EMBASE, MEDLINE). Key words and/or MeSH terms must be stated and, where feasible, the search strategy should be provided. All searches should be supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found. | 3.1 Were at least two major databases searched? |
| 3.2 Were dates searched reported? | |
| 3.3 Were key words stated? | |
| 3.4 Were MeSH terms stated? | |
| 3.5 Was the search strategy provided or available on request? | |
| 3.6 Were searches supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found? | |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? The review authors should state that they searched for reports regardless of their publication type. The review authors should state whether or not they excluded any reports (from the systematic review), based on their publication status, language, etc. | 4.1 Were studies searched for and included regardless of their publication type? |
| 4.2 Were papers included regardless of language of publication? | |
| 5. Was a list of studies (included and excluded) provided? A list of included and excluded studies should be provided. | 5.1 Was there a list of included studies? |
| 5.2 Was there a list of excluded studies? | |
| 5.4 Was there a flow diagram? | |
| 6. Were the characteristics of the included studies provided? In an aggregated form such as a table, data from the original studies should be provided on the participants, interventions and outcomes. The ranges of characteristics in all the studies analysed (e.g. age, race, sex, relevant socioeconomic data, disease status, duration, severity, other diseases) should be reported. | 6.1 Were details provided on the participants of included studies (including age, gender, severity of stroke, time since stroke)? |
| 6.2 Were details provided on the interventions of included studies? | |
| 6.3 Were details provided on the outcomes reported by included studies? | |
| 7. Was the scientific quality of the included studies assessed and documented? 'A priori' methods of assessment should be provided (e.g. for effectiveness studies if the author(s) chose to include only randomised, double‐blind, placebo‐controlled studies, or allocation concealment as inclusion criteria); for other types of studies, alternative items will be relevant.
| 7.1 Was the scientific quality of included studies assessed? |
| 7.2 Was this done by at least two independent review authors? | |
| 7.3 Was the scientific quality of studies documented? | |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? The results of the methodological rigour and scientific quality should be considered in the analysis and the conclusions of the review, and explicitly stated in formulating recommendations.
| 8.1 Were the results of methodological rigour of the included studies considered in the analysis of the review? |
| 8.2 Were the results of the scientific quality of the included studies considered in the conclusions and/or recommendations of the review? | |
| 9. Were the methods used to combine the findings of studies appropriate? For the pooled results, a test should be done to ensure the studies were combinable, to assess their homogeneity (i.e. Chi2 test for homogeneity, I2). If heterogeneity exists, a random‐effects model should be used and/or the clinical appropriateness of combining should be taken into consideration (i.e. Is it sensible to combine?).
| 9.1 Were the methods used to combine the findings of studies clearly described or referenced to appropriate text, or both? |
| 9.2 If results are pooled, are the mean and confidence intervals (or equivalent data) reported? | |
| 9.3 If results are pooled, is a test of heterogeneity reported? | |
| 9.4 Have the review authors stated a definition of statistical heterogeneity? | |
| 9.5 If statistical heterogeneity is present or suspected, has a random‐effects model been used? | |
| 10. Was the likelihood of publication bias assessed? An assessment of publication bias should include a combination of graphical aids (e.g. funnel plot, other available tests) and/or statistical tests (e.g. Egger regression test).
| 10. Was the likelihood of publication bias assessed? |
| 11. Was the conflict of interest stated? Potential sources of support should be clearly acknowledged in both the systematic review and the included studies. | 11.1 Was there a conflict of interest statement?
|
| 11.2 Was the review free of any conflicts of interest?
|
| Intervention | Included reviews | Moderate‐quality evidence of effect on upper limb function | Moderate‐quality evidence of effect on upper limb impairment | Moderate‐quality evidence of effect on ADL outcomes | Low‐ or very low‐quality evidence | Implications for clinical practice | Recommendations for research |
| Bilateral arm training | Coupar 2010 (vs usual care or control) van Delden 2012 (vs unilateral arm training) | Unilateral arm training more effective than bilateral arm training (6 trials, n = 375) | No difference between unilateral arm training and bilateral arm training (4 trials, n = 228) | Unilateral arm training more effective than bilateral arm training | Low‐quality evidence for bilateral arm training compared with usual care or other interventions | Evidence does not support bilateral arm training as a replacement for unilateral arm training | A sound theoretical rationale is essential to justify further research into bilateral arm training |
| Biofeedback | Woodford 2007 (EMG biofeedback) Molier 2010 (qualitative data only) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Up‐to‐date reviews required | |||
| Bobath therapy | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Up‐to‐date reviews required | ||||
| Brain stimulation: tDCS | tDCS beneficial for impairment (7 trials, n = 304) | High‐quality evidence of no benefit or harm of tDCS (5 trials, n = 286) | Evidence insufficient to support introduction into routine clinical practice | High‐quality RCTs required | |||
| Brain stimulation: rTMS | Current evidence of low quality | Insufficient evidence to support introduction into routine clinical practice | High‐quality RCTs required | ||||
| Constraint‐induced movement therapy (CIMT) | Corbetta 2010 (subgroup analyses) | CIMT beneficial when compared with control (14 trials, n = 477) | Evidence of low quality for measures of ADLs (because of methodological limitations within review) | Moderate‐quality evidence that CIMT may be effective intervention for selected patients | Phase III RCTs recommended Dose must be considered | ||
| Electrical stimulation | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Meta‐analysis of current trials/completion of ongoing review required (Howlett) | ||||
| "Hands‐on" therapy (manual therapy techniques) | Winter 2011 (qualitative data only) | in | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | High‐quality RCTs required | ||
| Mental practice | Barclay‐Goddard 2011 (subgroup analyses) Wang 2011 (includes Chinese trials) | Mental practice beneficial when given in addition to conventional interventions (7 trials, n = 197) | Mental practice beneficial when given in addition to conventional interventions (5 trials, n = 216) | No benefit or harm of mental practice | Moderate‐quality evidence that mental practice may be effective intervention for some patients | Phase III RCTs recommended | |
| Mirror therapy | Mirror therapy beneficial (10 trials, n = 421): combined upper limb function and impairment outcomes | (see upper limb function) | Mirror therapy beneficial (4 trials, n = 217) | Moderate‐quality evidence that mirror therapy may be effective intervention for some patients | Phase III RCTs recommended | ||
| Music therapy | Lack of trial evidence | Insufficient evidence to support any change in current clinical practice | High‐quality RCTs required | ||||
| Pharmacological interventions | Elia 2009 (botulinum toxin for spasticity) Olvey 2010 (botulinum toxin for spasticity; qualitative data only) Demetrios 2013 (multi‐disciplinary rehabilitation following pharmacological interventions; qualitative data only) Singh 2010 (pharmacological interventions for shoulder pain) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | Reviews require updating High‐quality RCTs required | |||
| Repetitive task training (RTT) | No benefit or harm of RTT (8 trials, n = 412) Beneficial effect when dose > 20 hours (3 trials, n = 113) | Moderate‐quality evidence that a higher dose of RTT may be beneficial | Review requires updating Large‐scale RCTs to explore dose is a research priority, including number of repetitions during RTT | ||||
| Robotics | Beneficial effect of robotics as compared with any comparison on impairment scales (16 trials, n = 586) No benefit or harm as compared with the same duration of conventional therapy (6 trials, n = 204) No benefit or harm on measures of strength (10 trials, n = 321) | Beneficial effect of robotics as compared with any comparison on ADLs (13 trial, n = 552) | Current evidence does not support Introduction into routine clinical practice | High‐quality RCTs required, including consideration of dose | |||
| Sensory interventions | Schabrun 2009 (qualitative data only) | Beneficial effect of sensory stimulation as compared with no treatment (1 trial, n = 29) | Beneficial effect of sensory stimulation as compared with no treatment (1 trial, n = 29) | Low‐quality evidence for all other interventions | Current evidence does not support any change in current clinical practice | High‐quality RCTs required | |
| Strength training | Low‐quality evidence of a beneficial effect on upper limb function (11 trials, n = 465) and grip strength (6 trials, n = 306). (Quality judgement influenced by poor reporting within review) | Insufficient evidence to support any change in current clinical practice | High‐quality up‐to‐date review required High‐quality RCTs required | ||||
| Stretching and positioning | Katalinic 2010 (stretching and positioning) Borisova 2009 (positioning of shoulder) Ada 2005 (shoulder supports) Lannin 2003 (hand splinting) Hijmans 2004 (elbow orthoses; qualitative data only) | No benefit or harm of stretching as compared with any other intervention on joint mobility and spasticity | No benefit or harm of stretching as compared with any other intervention on ADLs | Low‐quality evidence of no benefit of shoulder supports | Current evidence does not support any change in current clinical practice | High‐quality up‐to‐date review required Essential that research protocols comprise doses that are theoretically predicted to effect change | |
| Task‐specific training (reach‐to‐grasp exercise) | Pelton 2012 (qualitative data only) Urton 2007 (qualitative data only) | Current evidence of low quality | Insufficient evidence to support any change in current clinical practice | High‐quality, up‐to‐date review required | |||
| Virtual reality | Virtual reality beneficial (7 trials, n = 205): combined upper limb function and impairment outcomes | (see upper limb function) No benefit or harm for grip strength (2 trials, n = 44) | Moderate‐quality evidence that virtual reality may be effective intervention for some patients | Phase III RCTs recommended, including consideration of dose | |||
| Summary of results and implications related to individual interventions. ADLs: Activities of daily living. EMG: Electromyography. RCTs: Randomised controlled trials. rTMS: Repetitive transcranial magnetic stimulation. tDCS: Transcranial direct current stimulation. | |||||||
| Review (source) | Intervention | Date of search | Objective (as stated within review) | Types of studies included | Participants included | Interventions included | Comparisons included | Outcomes (as defined within review) | Number of studies included (number of participants included) |
| Ada 2005 (CDSR); Foongchomchaey 2005 (DARE) | Stretch or positioning | 22/03/2004 | To investigate the effects of supportive devices in preventing subluxation, repositioning the head of the humerus in the glenoid fossa, | RCTs, quasi‐randomised and controlled trials | Stroke | Supportive devices | Alternative supportive device or no support | Distance of subluxation (from x‐ray), pain, function, contracture | 4 (142) |
| Barclay‐Goddard 2011 (CDSR) | Mental practice | 24/11/2010 | To determine whether mental practice improves the outcomes of upper extremity rehabilitation for individuals living with the effects of stroke | RCTs | Stroke, UL functional deficits | Mental practice of upper extremity | No intervention; conventional intervention; placebo mental practice; or other novel therapies | Upper | 6 (119) |
| Borisova 2009 (DARE) | Stretch or positioning | 30/06/2005 | To assess the effectiveness of positioning on range of motion of the paretic shoulder following stroke | RCTs | Stroke | Positioning | Control | Range of motion | 5 (126) |
| Bradt 2010 (CDSR) | Music therapy | 25/02/2010 | To examine the effects of music therapy with standard care versus standard care alone or standard care combined with other therapies | RCTs, quasi‐RCTs | Acquired brain injury | Music therapy | Standard care or standard care with other therapies | Upper extremity function:measured by Secondary outcomes: Adverse events (e.g. death, fatigue, falls) | 7 (184) |
| Braun 2013 (DARE) | Mental practice | 01/06/2012 | To investigate the beneficial and adverse effects of a mental practice intervention on activities, cognition and emotion in patients after stroke, patients with Parkinson’s disease or multiple sclerosis | RCTs | Stroke, Parkinson's or multiple sclerosis (but no studies with participants with multiple sclerosis found) | Mental practice as therapy or embedded in therapy | Control that allows assessment of the possible effects of mental practice | Measures of function, activity and participation | Stroke—14 (421) Parkinson's—2(60) |
| Cooke 2010 (DARE) | Exercise therapy | 01/10/2009 | To determine the strength of current evidence for provision of a | RCTs or quasi‐RCTs | Stroke | Experimental and control group interventions identical | See previous column—exercises but without increased duration | Motor impairment: Motor activity:Modified | 7 (680) |
| Corbetta 2010 (DARE) | CIMT (constraint‐ induced movement therapy) | 01/04/2010 | This article aims to present an update of the Cochrane review and to assess the effects of CIMT, modified CIMT and forced use on disability and arm motor function | RCTs and quasi‐RCTs | Stroke | CIMT, modified CIMT or forced use | Usual care | Disability:Functional Independence measure, Barthel Index Arm motor function:Action Research Arm Test, Wolf Motor Function Test, Emory Function Test, Motor Assessment Scale | 18 (674) |
| Coupar 2010 (CDSR) | Bilateral arm training | 28/08/2009 | To determine the effects of simultaneous bilateral training for improving arm function after stroke | RCTs | Stroke | Simultaneous bilateral training | Control, usual care | Performance in ADLs: functional movement of the upper limb; | 18 (549) |
| Coupar 2012 (CDSR) | Service delivery | 21/05/2011 | To determine the effects of home‐based therapy programmes for upper limb recovery in patients with upper limb impairment following | RCTs | Stroke | Home‐based therapy for UL rehabilitation | Placebo, no intervention or usual care | ADLs and functional movement, extended ADLs, motor impairment | 4 (166) |
| Demetrios 2013 (CDSR) | Pharmacological | 01/09/2012 | To assess the effectiveness of multi‐disciplinary rehabilitation, following botulinum neurotoxin and other focal intramuscular treatments such as phenol, in improving | RCTs | Adults and children with poststroke spasticity (clinical diagnosis) | Multi‐disciplinary rehabilitation after botulinum neurotoxin or other focal intramuscular treatments | Multi‐disciplinary rehabilitation | Passive function: Leeds Arm Spasticity Impact Scale, Disability Assessment Scale, Active function of the upper limb: e.g. Motor Activity Log (MAL) or Action Research Arm Test Active function of the lower limb: e.g. tests of walking speed, balance and gait Impairments—pain, spasm frequency, joint range Participation and impact on caregivers: WHO QoL‐BREF, Caregiver Strain Adverse events | 3 (91) |
| Doyle 2010 (CDSR) | Sensory intervention | 16/09/2009 | To determine the effects of interventions that target upper limb sensory impairment after stroke | RCTs and controlled clinical trials | Stroke | For sensory impairment | No treatment, | Functional use of the upper limb: Activity limitations: Participation: Stroke Impact Scale, | 13 (467) |
| Elia 2009 (DARE) | Botulinum toxin injection by any route, including but not limited | 01/09/2006 | The aim of this systematic review was to determine | All levels of evidence | Stroke | Intramuscular injections, botulinum neurotoxin A or botulinum neurotoxin B | Ashworth Scale, Improvement of Global Assessment Scale (area under the curve of Ashworth scores), functional | 11 (782) | |
| Elsner 2013 (CDSR) | Transcranial direct current stimulation (tDCS) | May 2013 | To assess the effects of tDCS on generic activities of daily living and motor function in people with stroke | RCTs, first period of randomised cross‐over trials | Stroke | Active tDCS | Placebo, sham tDCS, no intervention or conventional rehabilitation | Primary outcome: Activities of daily living—Frenchay Activities Index, Barthel Index, Rivermead Activities of Daily Living Assessment, Modified Rankin Scale and Functional Independence Measure Secondary outcomes: Upper limb function—Action Research Arm Test, Fugl‐Meyer Score, Nine‐Hole Peg Test or Jebsen Taylor Hand Function Test. Muscle strength—grip force or motricity index Lower limb function | 15 (455) |
| Farmer 2014 (personal communication) | Assistive technologies, including electrical stimulation | 01/09/2011 | To identify and explore evidence for use of assistive technologies in poststroke upper limb rehabilitation | RCTs | Stroke | Assistive technologies including electrical stimulation (An assistive technology was defined as "a mechanical | Placebo, alternative treatments, usual care | Impairment: Range of motion, grip strength, subjective assessment of strength, Fugl‐Meyer Activity: Action Research Arm Test and Wolf Motor Function Test Partcipation: Motor Activity Log Amount of Use; Motor Activity Log Quality of Movement; Functional Independence Measure; Barthel index; Rankin Score and Stroke Impact Scale | 11 (474) |
| French 2007 (CDSR); French 2010 (DARE) | Repetitive task training | 16/10/2006 | To determine whether repetitive task training after stroke improves global, upper or lower limb function, and whether treatment effects are dependent on the amount, type or timing of practice | RCTs, controlled clinical trials | Stroke | Repetitive tasks training; an active motor sequence (multi‐joint motion) performed repetitively | Attention control, recreation, cognitive therapy, upper limb versus lower limb | Arm function: Motor Assessment Scale—Upper Limb Component, Action Research Arm Test, Frenchay Arm Test, Wolf Motor Function Test, Functional Test of the Hemiparetic Upper Extremity, Box and Block Test, Southern Motor Group Assessment Hand function: Motor Assessment Scale Hand; Jebsen Test of Hand Function, Peg Test Sitting balance/reach: Reaching Performance Scale, Functional Reach. | 14 (659) |
| French 2008 (DARE) | Repetitive task training | 01/09/2006 | To determine whether repetitive | RCTs, quasi‐RCTs, cross‐over trials (first part) | Stroke | Repetitive task training | Usual practice or attention control, alternative training | Arm function: Action Research Arm Test; Motor Assessment Scale—Upper Sitting Balance/Reach—Reaching Performance | 31 (1078) |
| Hao 2013 (CDSR) | Repetitive transcranial magnetic stimulation (rTMS) | 23/04/2012 | To assess the efficacy and safety of rTMS for improving function in people with stroke | RCTs | Stroke (any age) | rTMS, rTMS added to standard treatment | Sham treatment, sham treatment added to baseline treatment, baseline treatment alone | ADLs: Barthel Index, Functional Independence Measure, Modified Rankin Motor function:Upper limb function—Motor Assessment Scale, Action Research Arm Test, Nine‐Hole Peg Test. Lower limb function—changes in stride length or speed, Timed Up and Go Test, Rivermead Motor Assessment Scale. Global motor function—Motor Assessment Scale, Rivermead Motor Death or disability Any other impairment improvement (e.g. visual, perceptual, depression, cognition, etc) | 19 (588) |
| Harris 2010 (DARE) | Exercise | 04/2009 | To examine the evidence | RCTs | Stroke | Strength training (voluntary exercise against resistance) | No treatment, placebo, non‐strengthening intervention | Upper limb strength, upper limb function or ADLs | 13 (517) |
| Hijmans 2004 (DARE) | Stretch or positioning | 01/06/2003 | To assess the scientific base of elbow | All designs considered | Elbow condition | Splinting | No comparisons prespecified | Range of motion, pain, grip strength | Stroke RCT—1 (18), Cohort—1 (16) |
| Katalinic 2010 (CDSR) | Stretch or positioning | 01/04/2009 | To determine the effects of stretch on contractures in people with, or at risk of, contractures | RCTs and controlled | • Neurological conditions (e.g. stroke, multiple sclerosis, | Stretch, stretch plus co‐intervention | No stretch, placebo or sham stretch, co‐intervention | Range of motion, torques, QoL, SF‐36, Tardieu, Modified Ashworth Scale, Functional Independence Measure, motor ability score | 35 (1391) |
| Lannin 2003 (DARE) | Stretch or positioning | 26/05/2003 | To assess the effectiveness of hand splinting on the hemiplegic | All designs considered | Stroke | Splinting | No comparisons prespecified | Functional use of hand, range of motion, tone, spasticity, oedema, pain | 21 (230) |
| Laver 2011 (CDSR) | Virtual reality | 30/03/2010 | To evaluate the effects of virtual reality and interactive video gaming on upper limb, lower limb and global motor function after stroke | RCTs | Stroke | Immersive or non‐immersive virtual reality | Alternative intervention, no Intervention | Upper limb function and activity: Gait and balance function and activity: Global motor function: Motor Assessment Scale Secondary outcomes: Cognitive function—Trail Making Test, Useful Field of View Test; Activity limitation— Adverse events: motion sickness, pain, injury, falls | 19 (565) |
| Laver 2013 (CDSR) | Service delivery | 09/07/2013 | To evaluate the effects of telerehabilitation, in comparison with in‐person or no rehabilitation, on activities of daily living for people after | RCTs | Stroke | Telerehabilitation | "In‐Person Rehabilitation" or no rehabilitation or alternative method of delivering telerehabilitation | Primary outcomes: Activities of daily living—Functional Independence Measure; Nottingham Extended Activities of Daily Living Secondary outcomes: Self‐care and domestic life; Patient satisfaction with the intervention—self‐reported health‐related quality of life; upper limb function (e.g. Action Research Arm Test, Wolf Motor Function Test, Fugl‐Meyer Upper Extremity Adverse events | 10 (933) |
| Luke 2004 (DARE) | Exercise therapy | 2003 | To determine the effectiveness of the Bobath concept in reducing upper limb impairments, activity limitations and participation restrictions after stroke | RCTs, cross‐over and single case series | Stroke | Stated use of the Bobath concept | A control for Bobath | Any outcome | RCTs—5 (209) Cross‐over—1 (131) Single‐case study—2 (34) |
| Mehrholz 2012 (CDSR) | Robotics | 01/08/2011 | To assess the effectiveness of electromechanical and robot‐assisted arm training in improving generic activities of daily living, arm function and arm muscle strength in patients after stroke | RCTs | Stroke | Electromechanical and robot‐assisted arm training for recovery of arm function | Other rehabilitation or placebo interventions, or no treatment | ADLs (Barthel Index, Functional Independence Measure), Fugl‐Meyer, Motricity Index and other measures of arm function or strength | 19 (666) |
| Meilink 2008 (DARE) | Electrostimulation | 01/06/2006 | To assess whether EMG‐triggered neuromuscular electrical | RCTs | Stroke | EMG‐NMES | Usual care | Reaction time, Fugl‐Meyer Assessment, Box and Block Test, peak velocity, deceleration time, Functional Independence Measure (self‐care), Action Research Arm Test, grip strength, Motricity Index, pinch and grip strength, elbow flexion/shoulder abduction, goniometry | 8 (157) |
| Molier 2010 (DARE) | Biofeedback | 01/03/2009 | To investigate the effects of different aspects and types of augmented feedback on motor functions and motor | All levels of evidence | Stroke | Intervention augmented by biofeedback | Comparisons were not defined a priori but included practising movements without feedback or robotic guidance | Fugl‐Meyer, Composite Spasticity Index, Ashworth Scale, Test Evaluant des | 23 (328): RCTs—8 (148) Cohort—10 (106) Matched pairs—2 (52) Not randomised—1 (16) Observational study—1 (5) Single case study—1 (1) |
| Nascimento 2014 (PROSPERO) | Electrical stimulation | December 2012 | To determine whether electrical stimulation is effective in increasing strength after stroke, and whether any benefits are maintained beyond the intervention period or carried over to activity | RCTs and controlled trials | Stroke | Cyclical electrical stimulation for strengthening | 1. No treatment, placebo, non‐strengthening interventions 2. Other strengthening interventions 3. Different modes of electrical stimulation | Strength: peak force generation Activity: Block and Box Test, Action Research Arm Test General activity: Barthel Index | 16 (638) |
| Norouzi‐Gheidari 2012 (DARE) | Robotics | 01/07/2010 | To find evidence regarding the effectiveness | RCTs | Stroke | Robot therapy | Conventional therapy | Fugl‐Meyer, Functional Independence Measure, Motor Power Scale, Motor Status Scale | 12 (383) |
| Olvey 2010 (DARE) | Pharmacological | 01/07/2010 | To review | RCTs, open‐labelled non‐randomised or observational studies | Stroke | Pharmacological treatments for spasticity | Dose comparisons or placebo with or without other treatment | Spasticity (Ashworth Scale or Modified Ashworth Scale or Tardieu Scale), pain, Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Disability Assessment Scale, range of motion, health‐related quality of life | RCTs—23 (1039) Other—31 (1288) |
| Pelton 2012 (DARE) | Exercise | 04/2010 | To identify all existing interventions targeted at co‐ordination of arm and hand segments for reach‐to‐grasp | All types of study design | Stroke | Treatment to develop co‐ordination of hand and arm during reach‐to‐grasp | Specific measures of co‐ordination such | 8 (155) | |
| Schabrun 2009 (DARE) | Sensory training | Not reported | We examined the volume and quality of the evidence available for | All types of study design | Stroke | Sensory retraining | Jebsen Taylor Hand Function, Action Research Arm Test, Modified Ashworth Assessment Scale, Modified Motor Assessment Scale | 14 (296) | |
| Singh 2010 (CDSR) | Pharmacological | 22/01/2010 | To assess the benefits and safety of botulinum toxin compared with placebo or alternative treatments in adults with shoulder pain | RCTs | Shoulder pain | Botulinum toxin injection by any route, including but not limited | Placebo injection or another active treatment | Pain: measured on a visual analogue scale, numerical Adverse effects Function or disability: measured using validated shoulder‐specific General Quality of life: e.g. | 6 (164) |
| Sirtori 2009 (CDSR) | Constraint‐induced movement therapy (CIMT) | 01/06/2008 | To assess the efficacy of CIMT, modified CIMT (mCIMT) or forced use (FU) for arm management in hemiparetic patients | RCTs and quasi‐RCTs | Stroke | CIMT, mCIMT or forced use | Other rehabilitation techniques or none | Arm motor function: perceived arm motor function, arm impairment, dexterity, quality of life | 19 (619) |
| Thieme 2012 (CDSR) | Sensory intervention | 08/06/2011 | To summarise the effectiveness of mirror therapy for improving motor function, activities of daily living, pain and visuospatial neglect in patients after stroke | RCTs and randomised cross‐over trials | Stroke | Mirror therapy | Any control intervention | Upper limb and hand function: Fugl‐Meyer, Action Reseach Arm Test, Wolf Motor Function Test, Brunnstrom Stages of Upper Extremity, Motricity Index Lower limb function: Functional Independence Measure, Barthel Index | 14 (567) |
| Urton 2007 (DARE) | Mixed | 06/2005 | To critically analyse the literature on effective interventions for upper extremity | RCTs and CCTs | Stroke | For upper limb hemiparesis | Fugl‐Meyer Assessment, Box and Block Test, Purdue Pegboard Test, Action Research Arm Test, Functional Independence Measure, TEMPA, Wolf Motor Function Test, grip strength, Caregiver Strain Index, Geriatric Depression Score, Ashworth Scale, Motor Activity Log and other study‐specific measures | 11 (269) | |
| van Delden 2012 (DARE) | Bilateral arm training | 01/06/2011 | To compare the effects of unilateral and bilateral | RCTs | Stroke up to 1 month = acute, 1‐6 months = subacute, after 6 months = chronic | Unilateral arm training; bilateral arm training | Alternative treatment | Wolf Motor Function Measure, Canadian Occupational Performance Measure, Fugl‐Meyer Assessment, Functional Independence Measure, Motor Activity Log, Stroke Impact Scale, Action Research Arm Test, Rivermead Motor Assessment, Nine‐Hole Peg Test, Modified Barthel Index, Nottingham Health Profile, Hospital Anxiety and Depression Scale, Motor Status Scale, Motor Assessment Scale, dynamometry, Rehabilitation Activities Profile, fMRI, additional kinematics | 9 (452) |
| Wang 2011 (DARE) | Mental practice | 10/2010 | To evaluate mental imagery on rehabilitation of functions in patients with stroke | RCTs | Stroke | Mental practice or mental imagery. Other clinical and rehabilitative treatments were the same as control group | Conventional stroke rehabilitation methods (such as physiotherapy and occupational therapy) | Upper limb function: Upper Limb Section of Fugl‐Meyer Assessment of Motor Recovery, Action Research Arm Test Other outcomes: Motor Assessment Scale, Modified Ashworth Scale, Upper Extremity Function Test, Functional independence Measure, Motor Activity Log, Color Trails Test, Task Performance Test, Motricity Index, The Arm Functional Test, simple test for evaluating hand function, Modified Barthel Index | 16 (652) (191 total participants for English papers, 461 total participants for Chinese papers) |
| Winter 2011 (CDSR) | Stretch or positioning | 22/03/2010 | To identify whether specific hands‐on therapeutic interventions enhance motor activity and function of the upper limb post stroke | RCTs | Stroke | Manual therapy techniques | Unclear | UL function: Action Research Arm Test, Motricity Index, Functional Independence Measure, Barthel Index | 3 (86) |
| Woodford 2007 (CDSR) | Biofeedback | 29/03/2006 | To assess the effects of EMG‐BFB for motor function recovery following stroke | RCTs and quasi‐RCTs | Stroke | EMG biofeedback with standard physiotherapy | Standard physiotherapy or standard physiotherapy and sham feedback | Range of motion, improvement in gait (stride length, speed, need for ambulation aids), | 13 (269) |
| ABILHAND: Assessment tool that measures a patient's perceived difficulty using his/her hands to perform manual activities in daily life. ADLs: Activities of daily living. CDSR: Cochrane Database of Systematic Reviews. CIMT: Constraint‐induced movement therapy. DARE: Database of Reviews of Effectiveness. EMG‐BF: Electromyographic biofeedback. EMG‐NMES: Electromyographic neuromuscular electrical stimulation. EQ5D: A questionnaire to measure health‐related quality of life. FIM: Functional Independence Measure. FU: Forced use. MAL: Motor Activity Log. mCIMT: Modified constraint‐induced movement therapy. MRI: Magnetic resonance imaging. QoL: Quality of life. RCT: Randomised controlled trial. RFTP: Repetitive functional task practice. RTMS: Repetitive transcranial magnetic stimulation. SF‐36: Short Form 36 questionnaire. TEMPA: Test d'Evaluation de la performance des Membres Supérieurs des Personnes Agées. tDCS: Transcranial direct current stimulation. UL: Upper limb. WHO QoL‐BREF: World Health Organisation Quality of Life short instrument | |||||||||
| Reference | Brief description of review/review aim | Dates/Notes |
| Effects of reach‐to‐grasp training using trunk restraint in individuals with hemiparesis post stroke: a systematic review | Anticipated publication stated as May 2013. Personal communication with author: completion date currently unknown PROSPERO 2012: CRD42012003464 | |
| To assess whether additional exercise therapy has an impact on recovery following stroke when compared with routine exercise therapy | Protocol published June 2012 | |
| Systematic review of functional electrical stimulation to improve activity and participation after stroke | Anticipated publication stated as October 2013. Personal communication with author: February 2014 in final stages PROSPERO 2012: CRD42012003054 | |
| Systematic review of self‐management interventions for stroke survivors | Protocol published February 2013 PROSPERO 2013: CRD42013003592 | |
| Physical therapies as an adjunct to botulinum toxin—injection to the upper or lower limb for the treatment of spasticity following neurological impairment: a systematic review | Personal communication with author: August 2013, in press PROSPERO 2011: CRD42011001491 | |
| To assess the efficacy and possible adverse effects of acupuncture for the treatment of poststroke upper limb pain | Protocol published April 2011 | |
| To determine whether pharmacological interventions for spasticity are more effective than no intervention, normal practice or control in improving function following stroke | Protocol published February 2013 | |
| To assess the effects of assistive technologies for the management of contractures in people with stroke | Protocol published October 2013 | |
| To determine whether physical treatment interventions are effective in preventing or minimising activity limitation and participation restrictions in patients developing spasticity post stroke | Protocol published July 2011 | |
| Intensive treatment versus normal treatment for improved motor recovery after stroke: a systematic review | Personal communication with author: Publication date anticipated around May 2014 PROSPERO 2012: CRD42012003221 | |
| The role of transcranial direct current stimulation (tDCS) in motor rehabilitation in stroke survivors: a systematic review | Protocol published May 2013 PROSPERO 2013: CRD42013003970 |
| Review (source) | Intervention | Reason for exclusion | Date of search | Objective (as stated within review) | Types of studies included | Participants included | Interventions included | Comparisons included | Outcomes (as defined within review) | Number of studies included (number of participants included) |
| Ada 2002 (DARE) | Electrical stimulation | Superseded by more up‐to‐date review | July 2002 | A meta‐analysis of all eligible randomised or quasi‐randomised trials of electrical stimulation for the treatment of shoulder subluxation | RCTs and quasi‐randomised trials | Stroke | Surface electrical stimulation with motor response | Conventional therapy | Subluxation, pain or function | 5 (183) |
| Aziz 2008 (CDSR) | Home rehabilitation therapy | No UL specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bjorklund 2006 (DARE) | CIMT | Superseded by more up‐to‐date review | 2004 | To investigate the outcomes of numerous CIMT trials to gauge improvement in upper extremity motor function among individuals suffering from hemiparesis experienced after stroke | RCTs, controlled trials, pre/post cohorts | Ischaemic or haemorrhagic stroke | CIMT | Self as control, conventional therapy | Fugl‐Meyer Assessment, Action Research Arm Test, Wolf Motor Function Test, | 11 (179) |
| Bolton 2004 (DARE) | EMG‐triggered electrical stimulation | Superseded by more up‐to‐date review | 3rd quarter 2003 | To assess the mean effect size of EMG‐triggered neuromuscular | RCTs, controlled trials | Stroke | EMG‐triggered | Usual therapy, stretching | Fugl‐Meyer Assessment, Block and Box Test, Rivermead Motor Assessment | 5 (86) |
| Bonaiuti 2007 (DARE) | CIMT | Superseded by up‐to‐date review | July 2004 | To analyse the evidence of effectiveness on adult stroke patients of CIMT | RCTs | Stroke | CIMT | Conventional therapy | Measures of impairment | 9 (243) |
| Braun 2006 (DARE) | Mental practice | Superseded by up‐to‐date review | August 2005 | To assess the effects of a mental practice intervention | RCTs, controlled trials | Stroke | Mental practice | Not clear | Measures of activity limitation or impairment | 4 RCTs ("Study sizes were small |
| Cardoso 2005 (DARE) | Botulinum toxin A | Superseded by up‐to‐date review | 2004 | To assess whether botulinum toxin is an adequate treatment for spasticity due to stroke | RCT | Stroke | Botulinum toxin A injections for upper limb spasticity | Placebo | Modified Ashworth Scale, Global Assessment Scale | 5 (329) |
| Crosbie 2007 (DARE) | Virtual reality | Superseded by up‐to‐date review | February 2005 | To assess the utility of virtual reality (VR) in stroke rehabilitation | RCTs, pre/post case series | Stroke | Virtual reality intervention | Self as control, healthy controls, age‐matched controls | Impairment or activity measurement | 5 (30) |
| Galvin 2008 (DARE) | Exercise therapy | Superseded by up‐to‐date review (3 trials included in this review were excluded from more up‐to‐date review) | Not reported (2006) | This article focuses on the impact of increased duration of exercise therapy on functional recovery after stroke | RCTs | Stroke | "Additional," "augmented" or "increased Exercise therapy was defined as | The same exercise therapy, but a lesser duration or dose | Fugl‐Meyer Upper Limb, Action Research Arm Test, Dynamometer, Functional Test | 8 (863) |
| Glanz 1996 (DARE) | Biofeedback | Superseded by up‐to‐date review | Not reported | To assess the efficacy of biofeedback therapy in poststroke rehabilitation | RCTs | Stroke | Biofeedback for upper extremity paresis | Conventional therapy | Impairment | 3 (82) |
| Glanz 1996 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | 1994 | To assess the efficacy of functional electrical | RCT | Stroke | Functional electrical stimulation | Control | Wrist torque | 1 UL ( 30) |
| Green 2003 (CDSR) | Interventions for shoulder pain | Not stroke | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hakkennes 2005 (DARE) | CIMT | Superseded by up‐to‐date review | March 2005 | To investigate the effects on function, quality of life, healthcare costs and patient/carer satisfaction of constraint‐induced movement therapy (CIMT) for upper limb hemiparesis following stroke | RCTs and systematic reviews | Stroke | CIMT or mCIMT | Alternative therapy, control, dose‐matched therapy or comparison between CIMT and mCIMT | Action Research Arm Test, Functional Independence Measure, Fugl‐Meyer Assessment, Motor Activity Log and Wolf Motor Function Test | 14 (292) |
| Handy 2003 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | 2002 | To examine the effectiveness of electrical stimulation in treating the upper extremities of patients who suffer cerebrovascular | RCTs and quasi‐experimental studies | Stroke | Functional electrical stimulation or transcutaneous electrical nerve stimulation | Control or alternative therapy | Subluxation, pain, range of motion or function | 5 (224) |
| Hayward 2010 (DARE) | Mixed | No additional studies | March 2009 | To investigate the effects of interventions that promote UL recovery in stroke survivors with severe paresis | RCTs | Stroke with severe UL paresis | Interventions that enabled stroke | Alternative or control treatment | Outcomes for impairment, activity and/or participation | 17 (486) |
| Henderson 2007 (DARE) | Virtual reality | Superseded by more up‐to‐date review | January 2006 | To evaluate scientific evidence for the effectiveness of virtual reality in rehabilitation of the UL post stroke | RCTs, | Stroke (acute, subacute and chronic) | Immersive or non‐immersive virtual reality | Conventional therapy or no therapy | Fugl‐Meyer Assessment, Functional Independence Measure, Wolf Motor Function Test | 6 (95) |
| Koog 2010 (DARE) | Treatment for shoulder pain | No UL function–specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| de Kroon 2002 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | December 2001 | Assessment of available evidence on the effects of therapeutic electrical stimulation of the affected upper extremity in improving motor control and functional abilities after stroke | RCTs | Stroke | Therapeutic electrical stimulation | Standard therapy, sensory stimulation, dose‐matched therapy | Measurement of motor control or functional abilities | 6 (207) |
| de Kroon 2005 (DARE) | Electrical stimulation | Superseded by up‐to‐date review | December 2003 | To explore the relationship between | Clinical trials | Stroke | Surface electrical stimulation | No treatment, exercises, placebo, dose‐matched therapy | Range of motion, grip strength, Fugl‐Meyer Assessment, Motor Assessment Scale, Box and Block Test, Motor Activity Log, Ashworth Scale, Barthel Index, Rankin Scale, Pain | 19 (578) |
| Kwakkel 2008 (DARE) | Robotics | Superseded by more up‐to‐date review | October 2006 | The aim of the study was to present a systematic | RCTs | Stroke | Robot‐assisted therapy for the upper limb | Control (robot exposure), neurodevelopmental therapy, electrical stimulation | Fugl‐Meyer, Chedoke‐ McMaster | 10 (218) |
| Latimer 2010 (DARE) | Bilateral arm training | Superseded by more up‐to‐date review | Before 2008 | To determine the evidence for bilateral therapy interventions aimed at improving upper limb function in | RCTs and cohort studies | 6 months post stroke | Bilateral upper limb intervention | RCTs:
| Upper Extremity of Fugl‐Meyer Assessment, Frenchay Arm Test, Rivermead Motor Assessment, Wolf Motor Function Test, Modified Motor Assessment Scale | 9 (166): RCTs—4 (85) Cohort studies—5 (81) |
| Legg 2006 (CDSR) | Therapy for ADL | No UL‐specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ma 2002 (DARE) | Therapy | No UL outcome measure | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| McIntyre 2012 (DARE) | CIMT | No additional studies | July 2012 | To determine the effectiveness of constraint‐induced | RCTs | Over 50% stroke; ≥ 6 months post stroke | CIMT | Traditional rehabilitation therapy | Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement, Wolf Motor Function Test, Fugl‐Meyer Assessment, Action Research Arm Test, Functional Independence Measure | 16 (572) |
| Mehrholz 2011 (CDSR) | Exercise | No UL outcome measure | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moreland 1994 (DARE) | Biofeedback | Superseded by more up‐to‐date review | 1992 | To examine the efficacy | RCTs | Stroke | EMG biofeedback alone or with | Conventional physical | Any functional measure | 6 RCTs |
| Moreland 1998 (DARE) | Biofeedback | LL outcomes only | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortenson 2003 (DARE) | Positioning and stretching | Brain injury and stroke outcomes combined | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nijland 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | No additional studies | January 12, 2010 | To examine the literature | RCTs | Acute or subacute stroke (within 10 weeks of stroke) | High‐intensity CIMT, low‐intensity CIMT | Usual care implied | Fugl‐Meyer Assessment, Action Research Arm Test, Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement | 5 (106) |
| Nilsen 2010 (DARE) | Mental practice | Superseded by more up‐to‐date review | February 2009 | To determine whether mental practice is an effective intervention to improve upper limb | Categorised all levels of evidence, RCTs to case reports | Stroke | Mental practice alone or in combination with other therapies | Other therapies | Fugl‐Meyer Assessment, Wolf Motor Function Test, Action Research Arm Test, Motor Activity Log, Motricity Index, Pegboard Test, Dynamometer, position sense, 2‐point discrimination, Recovery Locus of Control Scale, Barthel Index, Functional Limitations Profile, kinemetics of reaching and grasping, Jebsen Hand Function Test, pinch strength, grip strength, Chedoke McMaster, range of motion | 15 (145) RCTs—4 (72) Cohort 3 (43) Case series or single case studies—8 (30) |
| Therapy‐based rehabilitation services | No UL‐specific outcome | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Peurala 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | Superseded by more up‐to‐date review | January 5, 2011 | To examine the effects of constraint‐induced movement therapy and modified constraint‐induced | RCTs | Stroke | CIMT; modified CIMT (not forced use) | Usual care implied | Motor Activity Log, Action Research Arm Test, Wolf Motor Function Test, Functional Independence Measure, Stroke Impact Scale, Barthel Index | 27 RCTs |
| Platz 2003 (DARE) | Mixed | Superseded by more up‐to‐date review | October 2002 | To identify all studies providing evidence to support interventions comprising exercise therapy or neuromuscular electrical stimulation to improve arm paresis or ensuing activity limitations after stroke | Systematic reviews, meta‐analyses, RCTs, controlled cohort studies | Stroke with hemiparesis/ hemiplegia affecting the upper limb | Exercise therapy or neuromuscular electrical stimulation aimed at improving arm paresis or ensuing activity limitations after stroke. This included physiotherapy approaches, arm ability training, CIMT, repetitive sensorimotor training, EMG biofeedback, kinesthetic feedback, electrostimulation, robot‐assisted arm rehabilitation. Training intensity was also investigated | Not stated | No measures or scores reported, only generic terms, e.g. "strength," "function," "selectivity," "efficiency of arm function" | 30 references |
| (PROSPERO) | No intervention | No upper limb interventions | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pomeroy 2006 (CDSR) | Electrostimulation | Superseded by more up‐to‐date review | January 1, 2004 | To find whether electrostimulation improved functional motor ability and the ability to undertake activities of daily living | RCTs or quasi‐RCTs | Stroke | Electrostimulation | No treatment, placebo, conventional therapy | Functional motor ability—included the following: Measures of ADL—included the following: Barthel Index, Functional Independent Measure Measures | 24 (888) |
| Prange 2006 (DARE) | Robotics | Superseded by more up‐to‐date review | August 1, 2005 | To investigate the effects of robot‐aided therapy on upper limb motor control and functional abilities of stroke patients | Pre/post studies and RCTs | Stroke | Robot therapy | Conventional therapy | Fugl‐Meyer Assessment, Motor Status Score, Motor Power, Functional Independence Measure, Barthel Index | 8 (246) |
| Price 2000 (CDSR) | Electrical stimulation | Pain outcomes, no functional UL outcomes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Péter 2011 (DARE) | Robotics | Superseded by more up‐to‐date review | January 11, 2010 | To review robot‐supported upper limb physiotherapy focusing on | Clinical trial (randomised or non‐randomised, self‐controlled or with control group) | Hemiparesis due to upper motor neuron lesion | Shoulder, elbow and/or wrist robot‐mediated therapy | Conventional therapy or electrical stimulation | Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Motor Power Scale, Motor Status Scale, Medical Research Council Muscle Grading, Wolf Motor Function Test, range of motion, spasticity, Arm Motor Ability Test, Rancho Los Amigos Functional Test | 30 (393) |
| Richards 2008 (DARE) | TMS | No functional outcomes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Saposnik 2010 (DARE) | Virtual reality | Superseded by more up‐to‐date review | January 7, 2010 | To determine the added benefit of virtual reality technology for arm motor recovery after stroke | RCTs and pre/post design | Stroke | Immersive or non‐immersive virtual reality | Conventional rehabilitation, sham virtual reality, recreational activities or orthoses | Fugl‐Meyer Assessment, Wolf Motor Function Test, Box and Block Test, Jebsen Hand Function Test | 12 (195) |
| Shi 2011 (DARE) | Constraint‐induced movement therapy (CIMT) | Superseded by more up‐to‐date review | January 4, 2010 | To compare the effectiveness of modified constraint‐ | RCTs | Stroke | Modified CIMT | Traditional rehabilitation therapy | Fugl‐Meyer Assessment, Action Research Arm Test, Functional Independence Measure, Motor Activity Log Amount of Use, Motor Activity Log Quality of Movement, reaction time, peak velocity | 13 (278) |
| Stevenson 2012 (DARE) | Constraint‐induced movement therapy (CIMT) | No additional studies | January 2, 2011 | To examine constraint‐induced movement therapy, relative to dose‐matched control interventions, for | RCTs or cross‐over design | Stroke | CIMT | Dose‐matched control | Motor capacity—Fugl‐Meyer Assessment, kinematics, indirect indicators of neurophysiological mechanisms UL ability—Action Research Arm Test, Nine‐Hole Peg Test, Wolf Motor Function Measure Comprehensive—Functional Independence Measure, Barthel Index, self‐report (Motor Activity Log, Stroke Impact Scale) | Motor capacity—15 (432) UL ability—14 (351) FIM 6 (182) Motor Activity Log 12 (352) |
| Stewart 2006 (DARE) | Bilateral arm training | Superseded by more up‐to‐date review | 2nd quarter 2005 | To determine the overall effectiveness of | Pre/post and RCTs | Upper extremity stroke hemiparesis, with enough | Bilateral movement training or bilateral training with auditory cuing or active neuromuscular stimulation | Pretreatment/single‐arm tasks | Kinematic performance, Fugl‐Meyer Upper Extremity Test, Motor Assessment Scale, Box and Block Test | 11 (171) |
| Tang 2012 (DARE) | Repetitive transcranial magnetic stimulation (rTMS) | Unable to find full paper | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Timmermanns 2009 (DARE) | No intervention | Scoping review of treatment rationale | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tyson 2011 (DARE) | Stretching (orthoses) | No additional studies | July 2009 | To establish whether an orthosis can improve function and/or impairments | RCTs | Adults with stroke or the stable non‐progressive sequelae of a brain lesion (such as infection or traumatic brain injury) that resulted in motor impairments | Orthoses to manage upper limb motor impairments (The following types of orthoses were excluded: splinting during constraint‐induced movement therapy, devices to prevent shoulder subluxation, orthoses that were part of a hybrid device to deliver functional electrical stimulation, taping, strapping, air‐pressure splints, serial casting) | Comparison of an orthosis with no treatment, normal care, placebo treatment. Or comparison of an orthosis plus normal management versus normal management alone | Upper limb impairments, activity limitations or incidence of adverse events | 4 (126) |
| van der Lee 2001 (DARE) | Exercise therapy | Superseded by more up‐to‐date review | August 2000 | Assessment of available evidence for the effectiveness of exercise therapy | RCTs | Stroke | Exercise therapy | Other treatment or no treatment | Barthel Index, Action Research Arm Test, Fugl‐Meyer Assessment | 13 (939) |
| van Dijk 2005 (DARE) | Biofeedback | Superseded by more up‐to‐date review | December 2004 | Assessment of available evidence regarding | RCTs | Upper limb rehabilitation patients (Parkinson's disease—3 studies; spinal cord injury—2 studies; cerebral palsy—1 study, traumatic brain injury—1 study; stroke and traumatic brain injury—2 studies, stroke—16 studies | Augmented feedback | Placebo, conventional therapy, no treatment | Active range of motion, Brunnstrom's stages of recovery, electromyographical activity, Upper Extremity Functional Scale, Nine‐Hole Peg Test, est Evaluant des | 26 (927) |
| van Kuijk 2002 (DARE) | Botulinum toxin injection by any route, including but not limited | Superseded by more up‐to‐date review | January 10, 2000 | The goal of this | Excluded studies with fewer than 10 participants, but included case series (for phenol or alcohol injections) | Stroke | Botulinum toxin | Placebo but not always specified | Modified Ashworth Scale, grip strength, Fugl‐Meyer Assessment, Functional Independence Measure, Barthel Index, Frenchay Arm Test, Motricity Index | 12 (not reported) |
| van Peppen 2004 (DARE) | Physical therapy interventions | Superseded by more up‐to‐date review | January 2004 | To determine the evidence for physical therapy interventions aimed at | RCTs and CCTs | Stroke | Exercises for upper limb | Control | Action Research Arm Test, Arm Motor Activity Test, Motor Activity Log | 5 (104) |
| Wu 2006 (CDSR) | Acupuncture | No UL‐specific intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zimmermann‐Schlatter 2008 (DARE) | Mental practice | Superseded by more up‐to‐date review | January 8, 2005 | To evaluate how motor imagery and conventional therapy (physiotherapy or | RCTs | Stroke | Motor imagery plus conventional therapy | Conventional therapy | Fugl‐Meyer Upper Extremity Score; Action Research Arm Test | 4 (86) |
| CCT: Controlled clinical trial. CIMT: Constraint‐induced movement therapy. CDSR: Cochrane Database of Systematic Reviews. DARE: Database of Reviews of Effectiveness. EMG: Electromyography. FES: Functional electrical stimulation. LL: Lower limb. mCIMT: Modified constraint‐induced movement therapy. RCT: Randomised controlled trial. rTMS: Repetitive transcranial magnetic stimulation. UE: Upper extremity. UL: Upper limb. | ||||||||||
| Intervention | Reviews included in qualitative synthesis | Reviews included in quantitative synthesis | Ongoing reviews | Excluded, as superseded by more up‐to‐date review/ contains no additional studies |
| Acupuncture | ||||
| Bilateral arm training | ||||
| Biofeedback | ||||
| Bobath therapy | ||||
| Brain stimulation | ||||
| CIMT | Corbetta 2010 (SG only) | (Farmer 2014*) | ||
| Electrical stimulation | (Farmer 2014a) | (Farmer 2014a) | ||
| "Hands‐on" therapy | ||||
| Mental practice | Barclay‐Goddard 2011 (SG only) | |||
| Mirror therapy | ||||
| Music therapy | ||||
| Pharmacological interventions | ||||
| Repetitive task training | ||||
| Robotics | Norouzi‐Gheidari 2012 (SG only) | (Farmer 2014*) | ||
| Self‐management | ||||
| Sensory interventions | ||||
| Strength training | ||||
| Stretching and positioning | Borisova 2009 (SG only) | |||
| Task‐specific training (reach‐to‐grasp exercise) | (Urton 2007a) | |||
| Virtual reality | ||||
| Mixed | ||||
| Factors in service delivery: dose of intervention | ||||
| Factors in service delivery: location of intervention | ||||
| Numbers of reviews | 40 | 31 of 40 reviews 27 of 31 reviews—data from main comparisons included; 4 of 31 reviews—overlap with trials included in main comparisons: data from subgroup comparisons included only (marked as "SG only") | 11 | 37 |
| CIMT: Constraint‐induced movement therapy. SG: Subgroup. aReviews covering a mixture of different interventions (listed under 'Mixed'). | ||||
| Review | Intervention | Brief description of review | Results: effects of interventions | Reasons for not including quantitative data from review |
| Bradt 2010 (CDSR) | Music therapy | The aim of this review was to examine the effects of music therapy with standard care versus standard care alone or standard care combined with other therapies on gait, upper extremity function, communication, mood and emotions, social skills, pain, behavioural outcomes, activities of daily living and adverse events in participants with brain injury. A total of 7 studies (184 participants) were included, but only 2 (41 participants) were relevant to the upper limb: “Two trials measured the effects of music therapy on upper extremity function in hemispheric stroke patients. Elbow extension angle was the only common outcome measure in these two studies. However, because of the significant clinical heterogeneity of the studies, their effect sizes were not pooled” | Narrative descriptions of the results of the trials are provided: One trial: “examined the effects of RAS on spatiotemporal control of reaching movements of the paretic arm in 21 patients. Results indicated that RAS increased the elbow extension angle by 13.8% compared to the non‐rhythmic trial, and this difference was statistically significant (P = 0.007). Results further indicated that variability of timing and reaching trajectories were reduced significantly (35% and 40.5%, respectively, P < 0.05).” One trial: “evaluated the effects of music‐making activity on elbow extension in 20 participants with hemiplegia. The elbow extension (measured from 135 to 0 with negative numbers expressing limitations) post‐intervention was ‐29.4 (SD 29.49) for the experimental group and ‐39.2 (SD 38.19) for the control group. This difference was not statistically significant. Post‐test shoulder flexion data indicated no statistically significant difference (P = 0.44) between the music therapy group (85.6°, SD 26.71) and the control group (71.8°, SD 39)” | Data from the 2 trials were not pooled |
| Demetrios 2013 (CDSR) | Multi‐disciplinary rehabilitation following botulinum toxin or other focal neuromuscular treatment | The aim of this review was to assess the effectiveness of multi‐disciplinary rehabilitation, following botulinum neurotoxin and other focal intramuscular treatments such as phenol, in improving activity limitations and other outcomes in adults and children with post‐stroke spasticity. Three RCTs (91 participants), all classed as 'low quality,' were included. "All studies investigated various types and intensities of outpatient rehabilitation programmes following botulinum neurotoxin for upper limb spasticity in adults with chronic stroke. Rehabilitation programmes included: modified constraint‐induced movement therapy (mCIMT) compared with a neurodevelopmental therapy programme; task practice therapy with cyclic functional electrical stimulation (FES) compared with task practice therapy only; and occupational, manual therapy with dynamic elbow extension splinting compared with occupational therapy only." "Due to the limited number of included studies, with clinical, methodological and statistical heterogeneity, quantitative meta‐analysis was not possible" | Descriptions of the results from the 3 included studies are provided. The review authors classify all evidence as "low quality" and conclude: "At best there was 'low level' evidence for the effectiveness of outpatient MD rehabilitation in improving active function and impairments following botulinum neurotoxin for upper limb spasticity in adults with chronic stroke." The review authors conclude that there is a need for "robust trials" | Data from the 3 trials were not pooled |
| French 2008 (DARE) | “Repetitive functional task practice,” including repetitive task training (RTT), constraint‐induced movement therapy (CIMT) and treadmill training | The aim was to determine whether repetitive functional task practice (RFTP) after stroke improves limb‐specific or global function or activities of daily living and whether treatment effects are dependent on the amount of practice, or the type or timing of the intervention. Also to provide estimates of the cost effectiveness of RFTP. Eighteen trials (634 participants) measured arm function. These included 8 RTT trials (467 participants) and 10 CIMT trials (167 participants) | Arm function Data from 8 RTT trials (412 participants) and 7 CIMT trials (285 participants) were pooled. The pooled effect for the impact of RFTP on arm function was as follows: SMD 0.24, 95% CI 0.06 to 0.42; I2 = 22% Hand function Data from 5 RTT trials (281 participants) and 2 CIMT trials (27 participants) were pooled. The pooled effect for RFTP on hand function was as follows: SMD 0.19, 95% CI –0.03 to 0.42; I2 = 0% | This review pools the data from 2 interventions: RTT and CIMT. Data from these interventions are included from French 2010 (RTT) and Corbetta 2010 and Sirtori 2009 (CIMT). The French 2010 RTT data are exactly the same as these French 2008 data. The Corbetta 2010 and Sirtori 2009 data are more comprehensive than the French 2008 data; this review has a much earlier search date and includes far fewer trials Including the data from this French 2008 review would effectively result in “double‐counting” of the data presented under the separate intervention headings of RTT and CIMT |
| Hijmans 2004 (DARE) | Elbow orthoses | The aim was to review papers related to the use of elbow Only 2 studies included participants with stroke. One was an RCT (18 participants), and one used a cross‐over design (16 participants) | No data are provided. The review authors state that (based on the cross‐over study) “wrist function and elbow range of movement seem to benefit from custom made Lycra garments applied at the elbow,” but (based on the RCT) probably no benefits are associated with an inflatable pressure splint | No data are available for inclusion |
| Molier 2010 (DARE) | Augmented feedback | The aim was to investigate the effects of different aspects and types of augmented feedback on motor functions and motor activities of the hemiparetic arm after stroke. 8 RCTs, 4 non‐randomised studies, 9 pre/post treatment design, 1 observational study and 1 single case study were included | For each study, it was stated whether beneficial effect, no effect or inconclusive effect was found for each outcome assessed. No data were provided. The results are discussed in the text. The authors state: “There are some trends in favour of providing augmented knowledge of performance feedback, augmented auditory and combined sensory and visual feedback. No consistent effects on motor relearning were observed for summary or faded, terminal or concurrent, solely visual or solely sensory augmented feedback.” And conclude that “ it was not possible to determine which combinations of aspects and types of augmented feedback are most essential for a beneficial effect on motor activities and motor functions of the hemiparetic arm after stroke. This was due to the combination of multiple aspects and types of augmented feedback in the included studies. This systematic review indicates that augmented feedback in general has an added value for stroke rehabilitation” | No data are available for inclusion |
| Olvey 2010 (DARE) | Pharmacological therapies for upper limb spasticity | The aim was to review studies of “contemporary pharmacological therapies” for upper limb spasticity after stroke. 54 studies were included: 23 RCTs and 31 non‐randomised studies. 51 of these investigated botulinum toxin | The results of the included studies are tabulated, with data from individual studies described. 23 studies assessed functional ability: FIM—6 studies. 5 found no benefit; 1 significant benefit. Fugl‐Meyer—5 studies. 2 found significant benefit. Barthel Index—8 studies. 2 report improvement; 6 no improvement. Disability Assessment Scale—5 studies. All report some benefit. Results from measures of upper limb function “were inconsistent.” 26 studies evaluated range of motion; 15 reported a significant improvement in 1 or more parameters after treatment | No data are available for inclusion |
| Pelton 2012 (DARE) | Any intervention targeted at co‐ordination of arm and hand segment for reach to grasp after stroke | The aim was to determine the effectiveness of current treatments for improving co‐ordination of reach to grasp following stroke. 7 studies were included: 1 RCT, 2 case‐control studies, 2 pre/post tests, 1 cross‐over, 1 observational. Interventions identified fell into 3 categories: “functional therapy, biofeedback or electrical stimulation and robot or computerised training” | The results of each study are tabulated, and the effect is reported as positive, negative or no effect. “Four studies (one RCT and three experimental studies without controls) report a result in favour of the experimental intervention for improved hand and arm coordination, whereas one experimental study without controls found no benefit. Two experimental studies with controls did not report specific training effects for hand and arm coordination after stroke” | No data are available for inclusion |
| Urton 2007 (DARE) | Any interventions for upper extremity hemiparesis following stroke | The aim was to critically analyse the literature on effective interventions for upper extremity hemiparesis following stroke. 11 experimental studies that evaluated interventions for upper extremity hemiparesis after stroke were included. Interventions included augmented exercise therapy, electrical stimulation, goal‐directed reaching and reach‐to‐grasp movements | Study details are tabulated, and the results of each study are described narratively | No data are available for inclusion |
| Winter 2011 (CDSR) | Hands‐on physical interventions (manual therapy techniques) | The aim was to explore the effectiveness of "clearly described hands‐on physical intervention (manual therapy techniques), or treatment component schedules, for the upper limb following stroke, either as the experimental intervention or as the control group." Pharmacological, electrical or psychological (e.g. mental imagery, relaxation) techniques were excluded, and only trials with interventions that addressed physical impairment were included. Three trials (86 participants) were included, each of which investigated different interventions (including manual stretch, passive extension and hands‐on therapy). Note: In the trial of passive extension (22 participants), passive extension was actually delivered as the control intervention and electrostimulation as the experimental intervention | Because of the heterogeneity between studies, no meta‐analysis is performed. The results of each of the 3 studies are described narratively. Methodological limitations are identified for all 3 studies The study authors conclude: "The findings of the review demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization when applied to the hemiplegic upper limb following stroke merits further research." | No data are available for inclusion |
| CDSR: Cochrane Database of Systematic Reviews. CI: Confidence interval. CMIT: Constraint‐induced movement therapy. DARE: Database of Reviews of Effectiveness. FES: Functional electrical stimulation. mCIMT: Modified constraint‐induced movement therapy. MD: Medical Department RAS: Rhythmic auditory stimulation. RCT: Randomised controlled trial. RFTP: Repetitive functional task practice. RTT: Repetitive task training. SD: Standard deviation. SMD: Standardised mean difference. | ||||
| Review source | Author | 1. Was an 'a priori' design provided? The research question and inclusion criteria should be established before the conduct of the review | 2. Was there duplicate study selection and data extraction?There should be at least two independent data extractors, and a consensus procedure for disagreements should be in place | 3. Was a comprehensive literature search performed? At least two electronic sources should be searched. The report must include years and databases used (e.g. CENTRAL, EMBASE, and MEDLINE). Key words and/or MeSH terms must be stated and where feasible the search strategy should be provided. All searches should be supplemented by consulting current contents, reviews, textbooks, specialised registers or experts in the particular field of study, and by reviewing the references in the studies found | 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? The authors should state that they searched for reports regardless of their publication type. The authors should state whether or not they excluded any reports (from the systematic review), based on their publication status, language, etc | 5. Was a list of studies (included and excluded) provided? A list of included and excluded studies should be provided | 6. Were the characteristics of the included studies provided? In an aggregated form such as a table, data from the studies should be provided on the participants, interventions and outcomes. The ranges of characteristics in all the studies analysed (e.g. age, race, sex, relevant socioeconomic data, disease status, duration, severity) or other diseases should be reported | 7. Was the scientific quality of the included studies assessed and documented? 'A priori' methods of assessment should be provided (e.g. for effectiveness studies if the author(s) chose to include only randomised, double‐blind, placebo‐ controlled studies, or allocation concealment as inclusion criteria); for other types of studies, alternative items will be relevant | 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? The results of the methodological rigour and scientific quality should be considered in the analysis and the conclusions of the review, and explicitly stated in formulating recommendations | 9. Were the methods used to combine the findings of studies appropriate? For the pooled results, a test should be done to ensure the studies were combinable, to assess their homogeneity (i.e. Chi2 test for homogeneity, I2). If heterogeneity exists, a random‐effects model should be used and/or the clinical appropriateness of combining should be taken into consideration (i.e. is it sensible to combine?) | 10. Was the likelihood of publication bias assessed? An assessment of publication bias should include a combination of graphical aids (e.g. funnel plot, other available tests) and/or statistical tests (e.g. Egger regression test) | 11. Was the conflict of interest stated? Potential sources of support should be clearly acknowledged in both the systematic review and the included studies |
| CDSR | Y | U | Y | Y | Y | Y | U | N | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | |
| DARE | Y | N | N | U | N | N | Y | N | U | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| OTHER | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | |
| DARE | Y | Y | Y | N | Y | N | Y | N | Y | N | Y | |
| DARE | Y | N | U | Y | N | N | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | U | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| DARE | Y | U | N | U | Y | Y | U | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| OTHER | Y | N | N | N | N | N | U | N | N/A | N | Y | |
| DARE | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | n | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | Y | U | N | N | N | Y | N | Y | Y | N | Y | |
| DARE | N | N | Y | U | Y | N | N | N | N/A | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | Y | N | Y | N | Y | N | Y | Y | N/A | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | y | N | N | N | N | Y | Y | Y | N | N | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| DARE | N | U | Y | N | N | N | Y | Y | Y | N | N | |
| DARE | Y | Y | Y | N | N | Y | N | N | N/A | N | Y | |
| PROSPERO | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | Y | U | Y | N | Y | N | U | U | Y | N | Y | |
| DARE | N | N | Y | N | N | N | N | Y | N/A | N | Y | |
| DARE | Y | Y | Y | N | N | Y | Y | Y | N/A | N | U | |
| DARE | Y | U | N | N | Y | Y | N | Y | Y | N | Y | |
| CDSR | Y | U | Y | U | Y | Y | Y | Y | Y | N | Y | |
| CDSR | Y | Y | Y | U | Y | Y | U | Y | Y | N | Y | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | |
| DARE | N | N | N | N | N | N | N | N | N/A | N | N | |
| DARE | Y | Y | Y | N | Y | Y | Y | U | Y | N | N | |
| DARE | Y | U | U | N | N | N | Y | U | Y | Y | N | |
| CDSR | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | |
| CDSR | Y | U | Y | Y | Y | U | Y | Y | Y | N | Y | |
| Number of responses: all reviews | YES | 36 | 23 | 31 | 19 | 29 | 27 | 29 | 29 | 27 | 8 | 30 |
| NO | 4 | 8 | 7 | 15 | 11 | 12 | 6 | 8 | 1 | 32 | 9 | |
| UNCLEAR | 0 | 9 | 2 | 6 | 0 | 1 | 5 | 3 | 1 | 0 | 1 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | |
| Number of responses: CDSR reviews | YES | 19 | 16 | 19 | 16 | 19 | 18 | 17 | 17 | 15 | 5 | 19 |
| NO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 14 | 0 | |
| UNCLEAR | 0 | 3 | 0 | 3 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | |
| Number of responses: other reviews | YES | 17 | 7 | 12 | 3 | 10 | 9 | 12 | 12 | 12 | 3 | 11 |
| NO | 4 | 8 | 7 | 15 | 11 | 12 | 6 | 6 | 1 | 18 | 9 | |
| UNCLEAR | 0 | 6 | 2 | 3 | 0 | 0 | 3 | 3 | 1 | 0 | 1 | |
| N/A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | |
| CDSR: Cochrane Database of Systematic Reviews. DARE: Database of Reviews of Effectiveness. N: No. N/A: Not applicable. U: Unclear. Y: Yes. See Figure 4 for results of mAMSTAR. | ||||||||||||
| Intervention | Reviews contributing data to main comparisons | Reviews contributing data to subgroup comparisons only | Justification for decisions |
| Constraint‐induced movement therapy (CIMT) | Studies included in these 2 reviews overlap. Corbetta 2010 was judged to be most up‐to‐date and comprehensive. Corbetta 2010 pools data comparing CIMT with control. However, no sub‐group analyses are reported. Sirtori 2009 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Corbetta 2010, and data related to subgroup comparisons are extracted from Sirtori 2009 | ||
| Mental practice | Braun 2013 has the most up‐to‐date search and includes trials that are not included within (or considered for inclusion in) Barclay‐Goddard 2011. Methodological quality is similar. Data from Braun 2013 are therefore extracted for the main comparisons. However, no subgroup analyses are reported. Barclay‐Goddard 2011 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Braun 2013, and data related to subgroup comparisons are extracted from Barclay‐Goddard 2011. Additional impairment data were extracted for the main comparisons from Wang 2011, as this included Chinese language trials not included within Braun 2013 | ||
| Robotics | Mehrholz 2012 has the most up‐to‐date search and includes trials that are included within (or considered for inclusion in) Norouzi‐Gheidari 2012. Methodological quality of Mehrholz 2012 was judged to be considerably greater than that of Norouzi‐Gheidari 2012. Data from Mehrholz 2012 are therefore extracted for the main comparisons. However, no subgroup analyses are reported. Norouzi‐Gheidari 2012 includes subgroup analyses to explore time post stroke and extent of treatment. Data related to main comparisons are therefore extracted from Mehrholz 2012, and data related to subgroup comparisons are extracted from Norouzi‐Gheidari 2012 | ||
| Stretching and positioning | Katalinic 2010 is a review of stretching interventions, including positioning interventions. Borisova 2009 includes positioning interventions only. The methodological quality of Katalinic 2010 is judged to be considerably greater than that of Borisova 2009, and data from Katalinic 2010 are therefore extracted for the main comparisons. All trials included in Borisova 2009 are also included in Katalinic 2010; however, as this is a subgroup of a particular type of stretching intervention, data from Borisova 2009 have been included as a subgroup analysis |
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Arm function | ARAT, WMFT | 6 | 375 | SMD 0.20 | (‐0.00 to 0.41) | Favours unilateral exercise training | |
| CIMT | Control | Arm function | ARAT, WMFT, EFT, MAS | 14 | 477 | SMD 0.44 | (0.03 to 0.84) | Beneficial effect | |
| Repetitive task training | Any control | Arm function | ARAT, WMFT, BBT, FTHUE, SMGA | 8 | 412 | SMD 0.17 | (‐0.03 to 0.36) | No benefit or harm | |
| Hand function | 9HPT, 10HPT, MAS | 5 | 281 | SMD 0.16 | (‐0.07 to 0.40) | No benefit or harm | |||
| Mental practice | Any control | Arm function | ARAT | 7 | 197 | SMD 0.62 | (0.05 to 1.19) | Beneficial effect | |
| Mirror therapy | Any other intervention | UL function + impairment | ARAT, MAS, FMA | 10 | 421 | SMD 0.53 | (0.04 to 1.01) | Beneficial effect | |
| Sensory impairment | No treatment | Arm function | mMAS | 1 | 29 | MD 1.58 | (0.98 to 2.18) | Beneficial effect | |
| Virtual reality | Other treatment | UL function + impairment | ARAT, WMFT, FMA | 7 | 205 | SMD 0.53 | (0.25 to 0.81) | Beneficial effect | |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 3 | 258 | ES 0.1 | (‐5.7 to 6.0) | No benefit or harm | |
| Factors in service delivery: location: home‐based therapy | Usual care | Arm function | WMFT | 1 | 100 | MD 2.25 | (‐0.24 to 4.73) | No benefit or harm | |
| 10HPT: Ten‐Hole Peg Test. 9HPT: Nine‐Hole Peg Test. ARAT: Action Research Arm Test. BBT: Box and Block Test. EFT: Emory Function Test. ES: Effect size. FMA: Fugl‐Meyer Assessment/ FTHUE: Functional Test of the Hemiparetic Upper Extremity. MAS: Motor Assessment Scale. MD: Mean difference. mMAS: Modified Motor Assessment Scale. SMD: Standardised mean difference. SMGA: Southern Motor Group Assessment. WMFT: Wolf Motor Function Test. | |||||||||
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Repetitive task training | Any control | Upper limb function | ARAT, WMFT, BBT, FTHUE, SMGA, JTHF, SMES, 10HPT | 6 | 246 | SMD 0.08 | (‐0.17 to 0.33) | No benefit or harm | All FU outcomes | |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 3 | 319 | ES ‐6.4 | (‐12.8 to 0.0) | No benefit or harm | 6 months | |
| 10HPT: Ten‐Hole Peg Test. ARAT: Action Research Arm Test. BBT: Box and Block Test. ES: Effect size. FTHUE: Functional Test of the Hemiparetic Upper Extremity. JTHF: Jebsen Taylor Hand Function Test. SMD: Standardised mean difference. SMES: Sodring Motor Evaluation Scale. SMGA: Southern Motor Group Assessment. WMFT: Wolf Motor Function Test. | ||||||||||
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Motor impairment | FMA, MSS | 4 | 228 | SMD 0.06 | (‐0.20 to 0.33) | No difference | |
| Brain stimulation: tDCS | Placebo or control | Motor impairment | FMA | 7 | 304 | SMD 3.45 | (1.24 to 5.67) | Beneficial effect | |
| Mental practice | Conventional treatment | Motor impairment | FMA | 5 | 216 | MD 7.81 | (1.96 to 13.65) | Beneficial effect | |
| Robotics | Any other intervention | Motor impairment | FMA | 16 | 586 | SMD 0.45 | (0.2 to 0.69) | Beneficial effect | |
| Strength | Strength | 10 | 321 | SMD 0.48 | (‐0.06 to 1.03) | No benefit or harm | |||
| Sensory impairment | No treatment | Motor impairment | BMR | 1 | 29 | MD 0.19 | (0.09 to 0.29) | Beneficial effect | |
| Stretching and positioning (stretch) | Any | Range of movement | Joint mobility | 7 | 193 | MD 2.17 | (‐1.63 to 5.97) | No benefit or harm | |
| Spasticity | Spasticity | 4 | 109 | SMD 0.08 | (‐0.30 to 0.45) | No benefit or harm | |||
| Virtual reality | Other treatment | Motor impairment | FMA | 5 | 171 | MD 4.43 | (1.98 to 6.88) | Beneficial effect | |
| Strength | Grip strength | 2 | 44 | MD 3.55 | (‐0.20 to 7.30) | No benefit or harm | |||
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Strength | Hand grip force/strength | 2 | 195 | ES ‐10.1 | (‐19.1 to ‐1.2) | Benefit of standard therapy dose | |
| Factors in service delivery: location: home‐based therapy | Usual care | Motor impairment | FMA | 3 | 156 | MD 1.46 | (‐0.58 to 3.51) | No benefit or harm | |
| Factors in service delivery: location: telemedicine | Usual care | Motor impairment | FMA | 2 | 46 | MD 3.65 | (‐0.26 to 7.57) | No benefit or harm | |
| BMR: Brunnstrom motor recovery. FMA: Fugl‐Meyer Assessment. MSS: Motor status score. tDCS: Transcranial direct current stimulation. | |||||||||
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Stretching and positioning (stretch) | Any | Range of movement | Joint mobility | 3 | 77 | MD ‐0.09 | (‐3.58 to 3.40) | No benefit or harm | 24 hours to 1 week | |
| 4 | 134 | MD ‐0.32 | (‐4.09 to 3.44) | No benefit or harm | > 1 week | |||||
| Spasticity | Spasticity | 1 | 42 | SMD ‐0.5 | (‐1.12 to 0.11) | No benefit or harm | > 1 week | |||
| Factors in service delivery: location: home‐based therapy | Usual care | Motor impairment | FMA | 1 | 36 | MD 4.3 | (0.19 to 8.41) | Beneficial effect | Any FU | |
| FMA: Fugl‐Meyer Assessment. FU: Follow‐up. MD: Mean difference. SMD: Standardised mean difference. | ||||||||||
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Activity | MAL: AOU | 3 | 146 | SMD 0.42 | (0.09 to 0.76) | Favours unilateral exercise training | |
| Bilateral arm training (bilateral exercise training) | Unilateral exercise training | Activity | MAL: QOM | 3 | 146 | SMD 0.45 | (0.12 to 0.78) | Favours unilateral exercise training | |
| Brain stimulation: tDCS | Placebo or control | Generic ADL | BI | 5 | 286 | SMD 5.31 | (‐0.52 to 11.14) | No benefit or harm | |
| Mental practice | Any control | Generic ADL | BI | 3 | 135 | MD 0.87 | (‐0.8 to 2.53) | No benefit or harm | |
| Mirror therapy | Any other intervention | Generic ADL | BI, FIM | 4 | 217 | SMD 0.33 | (0.05 to 0.60) | Beneficial effect | |
| Robotics | Any other intervention | Generic ADL + UL function | BI, FIM, ABILHAND, SIS, FAT | 13 | 552 | SMD 0.43 | (0.11 to 0.75) | Beneficial effect | |
| Stretching and positioning (stretch) | Any | Generic ADL | MAS, mBI, DASH | 4 | 130 | SMD 0.2 | (‐0.24 to 0.65) | No benefit or harm | |
| Factors in service delivery: location: home‐based therapy | Usual care | Generic ADL | BI | 2 | 113 | MD 2.85 | ([‐1.43 to 7.14) | No benefit or harm | |
| ABILHAND: Assessment tool that measures a patient's perceived difficulty using his/her hands to perform manual activities in daily life. ADL: Activity of daily living. BI: Barthel Index. CI: Confidence interval. DASH: Disabilities of the Arm Shoulder and Hand outcome. FAT: Frenchay Arm Test. FIM: Functional Independence Measure. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MAL: AOU: Motor Activity Log: Amount of Use. MAL: QOM: Motor Activity Log: Quality of Movement. MAS: Motor Assessment Scale. mBI: Modified Barthel Index. MD: Mean difference. SIS: Stroke Impact Scale. SMD: Standardised mean difference. tDCS: Transcranial direct current stimulation. UL: Upper limb. | |||||||||
| Intervention | Comparison | Review | Outcome category | Outcome measures | Number of trials | Number of participants | Effect size | 95% CI | Evidence of effect? | FU time |
| Stretching and positioning (stretch) | Any | Generic ADL | MAS | 1 | 40 | MD 1.7 | (‐0.40 to 3.80) | No benefit or harm | 24 hours to 1 week | |
| MAS, DASH | 4 | 136 | SMD 0.14 | (‐0.29 to 0.58) | No benefit or harm | > 1 week | ||||
| Factors in service delivery: location: home‐based therapy | Usual care | Generic ADL | BI | 1 | 80 | MD ‐1.70 | (‐5.51 to 2.11) | No benefit or harm | Any | |
| ADL: Activity of daily living. BI: Barthel Index. DASH: Disabilities of the Arm Shoulder and Hand outcome. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MAS: Motor Assessment Scale. MD: Mean difference. SMD: Standardised mean difference. | ||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Arm function + ADL | BBT, WMFT, MAL: AOU | 4 | 127 | SMD ‐0.07 | (‐0.42 to 0.28) | 1 | 1 | 0 | 1 | Low | |
| Other upper limb intervention | Arm function | BBT, ARAT, MAS, mMAS | 5 | 189 | SMD ‐0.2 | (‐0.49 to 0.09) | 1 | 1 | 0 | 1 | Low | ||
| Usual care | Hand function | PPT | 2 | 73 | SMD ‐0.04 | (‐0.5 to 0.42) | 2 | 1 | 0 | 1 | Low | ||
| Other upper limb intervention | Hand function | MAS, mMAS, SIS (hand function), 9HPT | 4 | 173 | SMD ‐0.21 | (‐0.51 to 0.09) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Arm function | UEFT | 1 | 29 | SMD ‐0.17 | (‐0.9 to 0.56) | 2 | 1 | 0 | 1 | Low | |
| Bobath therapy | Control | Arm function | UEFT | 1 | 29 | ES 0.17 | (‐0.56 to 0.90) | 2 | 1 | 0 | 2 | Low | |
| MAS | 1 | 61 | ES ‐0.29 | (‐0.80 to 0.21) | 2 | 1 | 0 | 2 | Low | ||||
| Arm function | SMES | 1 | 61 | ES ‐0.32 | (‐0.83 to 0.19) | 2 | 1 | 0 | 2 | Very low | |||
| Brain stimulation: rTMS | Control | Upper limb function | JTHF, PPT, WMFT, ARAT | 4 | 73 | SMD 0.51 | (‐0.99 to 2.01) | 2 | 1 | 1 | 0 | Low | |
| Electrical stimulation (for strength) | Control | Arm function + ADL | ARAT, BBT, BI | 3 | 122 | SMD 0.79 (random effects) | (‐0.11 to 1.69) | 1 | 1 | 0 | 1 | Low | |
| Electrical stimulation (NMES) | No treatment | Arm function | ARAT | 4 | 319 | 0.04 to 0.5 | (‐0.35 to 0.44) to (‐0.69 to 1.68) | 0 | 0 | 1 | 2 | Low | |
| Electrical stimulation (stochastic resonance) | Control | Arm function | ARAT | 1 | 30 | 0.15 | (‐0.66 to 0.96) | 2 | 0 | 0 | 2 | Low | |
| Electrical stimulation (EMG‐triggered) | No treatment | Arm function | BBT | 3 | 42 | 0.37 | (‐0.27 to 1.01) | 2 | 1 | 0 | 2 | Very low | |
| Cyclical electrical stimulation | Arm function | ARAT | 2 | 48 | 0 | (‐0.56 to 0.57) | 2 | 0 | 0 | 2 | Low | ||
| Sensory impairment (interventions for sensory impairment) | No treatment | Hand function | Hand FunctionTest | 1 | 36 | MD ‐1.16 | (‐2.10 to ‐0.22) | 2 | 1 | 0 | 0 | Low | |
| Placebo or attention control | Arm function | ARAT | 1 | 21 | MD 12.9 | (5.65 to 20.15) | 2 | 1 | 0 | 0 | Low | ||
| Sensory impairment (passive sensory retraining) | Not reported | Hand function | JTHF | 3 | Unclear | MD 8.72 | (2.48 to 14.95) | 2 | 1 | 1 | 2 | Very low | |
| Strength training | Control | Upper limb function | MAS, TEMPA, RMA, PPB, WMFT, BBT, ARAT, FTHUE | 11 | 465 | SMD 0.21 | (0.03 to 0.39) | 0 | 1 | 0 | 2 | Low | |
| Stretching and positioning: shoulder support | Control | Arm function | MAS | 1 | 83 | MD 0.83 | (‐1.46 to 3.12) | 2 | 0 | 0 | 1 | Low | |
| 9HPT: Nine‐Hole Peg Test. ADL: Activity of daily living. ARAT: Action Research Arm Test. BBT: Box and Block Test. BI: Barthel Index. EMG BF: Electromyographic biofeedback. ES: Effect size. FTHUE: Functional Test of the Hemiparetic Upper Extremity. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. JTHF: Jebsen Taylor Hand Function Test. JTHF: Jebsen Test of Hand Function. MAL: AOU Motor Activity Log: Amount of Use. MAS: Motor Assessment Scale. MD: Mean difference. mMAS: Modified Motor Assessment Scale. NMES: Neuromuscular electrical stimulation. PPB: Purdue Peg Board. PPT: Purdue Peg Test. RMA: Rivermead Motor Assessment. rTMS: Repetitive transcranial magnetic stimulation. SIS: Stroke Impact Scale. SMD: Standardised mean difference. SMES: Sodring Motor Evaluation Scale. TEMPA: Test d'Evaluation des Membres Superieurs de Personnes Agees. TEMPA: Upper Extremity Performance Test for Elderly (Test d’Evaluation des Membres Supérieurs de Personnes Agées). UEFT: Upper Extremity Function Test. WMFT: Wolf Motor Function Test. | |||||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | Follow‐up |
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Arm function | ARAT | 2 | 168 | 2.2 | (‐6.0 to 10.4) | 1 | 1 | 1 | 1 | Low | FU1 | |
| ARAT: Action Research Arm Test. FU: Follow‐up. | ||||||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Motor impairment | FMA | 4 | 127 | SMD 0.67 | (‐0.43 to 1.77) | 1 | 1 | 1 | 1 | Low | |
| Other upper limb intervention | Motor impairment | FMA, RMA | 4 | 175 | SMD ‐0.25 | (‐0.55 to 0.05) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Range of movement | Wrist ROM | 1 | 9 | SMD 0.96 | (‐0.48 to 2.40) | 2 | 1 | 0 | 1 | Low | |
| Shoulder ROM | 1 | 26 | SMD 0.88 | (0.07 to 1.70) | 2 | 1 | 0 | 1 | Low | ||||
| Motor impairment | BMR | 2 | 57 | SMD 0.69 | (0.15 to 1.23) | 2 | 1 | 0 | 1 | Low | |||
| FMA | 1 | 29 | SMD 0.44 | (‐0.19 to 1.07) | 2 | 1 | 0 | 1 | Low | ||||
| Electrical stimulation (for strength) | Control | Strength | Strength | 6 | 162 | SMD 0.55 | (0.23 to 0.86) | 1 | 1 | 0 | 1 | Low | |
| Electrical stimulation (NMES) | Control | Motor impairment | FMA | 5 | 152 | ES 0.01 to 2.43 | (‐0.8 to 0.81) to (‐0.74 to 5.59) | 1 | 0 | 1 | 2 | Low | |
| Strength | Grip strength, power | 3 | 93 | ES 0.00 to 0.38 | (‐0.88 to 0.88) (to ‐0.16 to 0.93) | 2 | 0 | 1 | 2 | Very low | |||
| Electrical stimulation (EMG‐triggered) | Cyclical electrical stimulation | Motor impairment | FMA | 3 | 57 | SMD 0.1 | (0.43 to 0.64) | 2 | 0 | 0 | 2 | Low | |
| Botulinum neurotoxin | Placebo | Spasticity | Spasticity | 2 | 45 | MD ‐0.62 | (‐1.40 to 0.17) | 2 | 1 | 0 | 2 | Very low | |
| Pharmacological interventions: botulinum neurotoxin | Placebo | Range of movement | Shoulder flexion | 1 | 29 | MD 3 | (‐15.54 to 21.54) | 2 | 0 | 0 | 2 | Low | |
| Shoulder abduction | 3 | 65 | MD 8.49 | (‐2.40 to 19.39) | 2 | 1 | 0 | 2 | Very low | ||||
| Shoulder external rotation | 3 | 70 | MD 9.84 | (0.20 to 19.49) | 2 | 1 | 0 | 2 | Very low | ||||
| Spasticity | Area under curve of Ashworth score: elbow | 2 | 101 | MD ‐6.28 | (‐16.02 to ‐3.47) | 1 | 1 | 1 | 2 | Very low | |||
| Area under curve of Ashworth score: wrist | 2 | 101 | MD ‐11.71 | (‐16.72 to 6.71) | 1 | 1 | 0 | 2 | Low | ||||
| Area under curve of Ashworth score: fingers | 2 | 101 | MD ‐7.79 | (‐13.44 to 2.74) | 1 | 1 | 0 | 2 | Low | ||||
| Pharmacological interventions: botulinum toxin type A: Dysport 500U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 1 | 41 | OR 0.22 | (0.06 to 0.81) | 2 | 1 | 0 | 2 | Very low | |
| Pharmacological interventions: botulinum toxin type A: Dysport 1000U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 2 | 100 | OR 0.22 | (0.09 to 6.52) | 1 | 1 | 0 | 2 | Low | |
| Pharmacological interventions: botulinum toxin type A: Dysport 1500U | Placebo | Spasticity | Number of participants with reduction in Ashworth score of at least 2 points | 1 | 36 | OR 0.42 | (0.11 to 1.56) | 2 | 1 | 0 | 2 | Very low | |
| Sensory impairment (interventions for) | No treatment | Motor impairment | FMA—upper limb | 1 | 18 | MD ‐6 | (‐16.58 to 4.58) | 2 | 1 | 0 | 0 | Low | |
| FMA—wrist and hand | 1 | 18 | MD ‐0.12 | (‐9.06 to 8.82) | 2 | 1 | 0 | 0 | Low | ||||
| Placebo or attention control | Motor impairment | FMA | 1 | 23 | MD 11.5 | (‐5.45 to 28.45) | 2 | 1 | 0 | 0 | Low | ||
| Sensory interventions: passive sensory retraining | Not reported | Strength | Muscle Strength | 1 | Unclear | MD ‐3.5 | (‐8.13 to 1.13) | 2 | 1 | 1 | 2 | Very low | |
| Sensory interventions: active sensory retraining | Not reported | Sensory | Proprioception | 1 | Unclear | MD 0.14 | (‐2.77 to 3.05) | 2 | 1 | 1 | 2 | Very low | |
| Strength training | Control | Strength | Grip strength | 6 | 306 | SMD 0.95 | (0.05 to 1.85) | 0 | 1 | 1 | 2 | Low | |
| Stretching and positioning: shoulder support | Control | Range of movement | Contracture | 1 | 81 | MD ‐1.2 | (‐10.90 to 8.10) | 2 | 1 | 0 | 1 | Low | |
| Loss of shoulder external rotation | 1 | 14 | OR 1 | (0.11 to 9.34) | 2 | 0 | 0 | 1 | Low | ||||
| Stretching and positioning: inflatable splint | No splint | Motor impairment | FMA | 1 | 18 | MD ‐0.12 | (‐9.8 to 9.6) | 2 | 1 | 0 | 2 | Very low | |
| Stretching and positioning: hand splint (12 hours at night) | 30‐minute stretch | Range of movement | Contracture | 1 | 28 | MD 1 degree | (‐3.7 to 6.1 degrees) | 2 | 0 | 0 | 2 | Low | |
| Factors in service delivery: location: home‐based therapy | Same treatment in hospital | Motor impairment | FMA | 1 | 10 | MD 0.6 | (‐8.94 to 10.14) | 2 | 1 | 0 | 0 | Low | |
| BMR: Brunnstrom motor recovery. EMG BF: Electromyographic biofeedback. ES: Effect size. FMA: Fugl‐Meyer Assessment. MD: Mean difference. NMES: Neuromuscular electrical stimulation. OR: Odds ratio. SMD: Standardised mean difference. RMA: Rivermead Motor Assessment. ROM: Range of movement. | |||||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | Time of FU |
| Brain stimulation: tDCS | Placebo or control | Motor impairment | FMA | 2 | 68 | SMD 9.22 | (‐13.47 to 31.90) | 2 | 1 | 1 | 0 | Low | ||
| Electrical stimulation | Control | Strength | Strength | 2 | 89 | SMD 0.38 | (‐0.04 to 0.80) | 2 | 0 | 0 | 1 | Low | ||
| Pharmacological interventions: botulinum neurotoxin | Placebo | Spascitity | Spasticity | 2 | 45 | MD ‐0.13 | (‐0.65 to 0.38) | 2 | 1 | 0 | 2 | Very low | ||
| Range of movement | Shoulder flexion | 1 | 29 | MD 1 | (‐17.87 to 19.87) | 2 | 0 | 0 | 2 | Low | ||||
| Range of movement | Shoulder abduction | 2 | 45 | MD 17.72 | (‐9.61 to 45.04) | 2 | 1 | 0 | 2 | Very low | ||||
| Range of movement | Shoulder external rotation | 2 | 50 | MD 11.86 | (‐0.61 to 24.33) | 2 | 1 | 0 | 2 | Very low | ||||
| Stretching and positioning: hand splint (12 hours at night) | 30‐minute stretch | Range of movement | Contracture | 1 | 28 | ‐2 degrees | (‐7.2 to 3.2 degrees) | 2 | 0 | 0 | 2 | Low | ||
| Factors in service delivery: dose of intervention (augmented therapy) | Standard therapy | Motor impairment | Motricity | 2 | 168 | 10.7 | (1.7 to 19.8) | 1 | 1 | 1 | 1 | Low | ||
| AMSTAR: Measurement tool to assess the methodological quality of systematic reviews. FMA: Fugl‐Meyer Assessment. FU: Follow‐up. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MD: Mean difference. ROB: Risk of bias. SMD: Standardised mean difference. tDCS: Transcranial direct current stimulation. | ||||||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence |
| Bilateral arm training | Usual care | Generic ADL | FIM | 3 | 106 | SMD 0.25 | (‐0.14 to 0.63) | 1 | 1 | 0 | 1 | Low | |
| Other upper limb intervention | Generic ADL | FIM, BI | 3 | 151 | SMD ‐0.25 | (‐0.57 to 0.08) | 1 | 1 | 0 | 1 | Low | ||
| Biofeedback: EMG BF | Physiotherapy | Generic ADL | BI | 1 | 16 | SMD ‐0.21 | (‐1.20 to 0.77) | 2 | 1 | 0 | 1 | Low | |
| Brain stimulation: rTMS | Control | Generic ADL | BI | 2 | 183 | SMD 15.92 | (‐2.11 to 33.95) | 2 | 1 | 1 | 0 | Low | |
| CIMT | Control | Generic ADL | FIM, BI | 8 | 276 | SMD 0.21 | (‐0.08 to 0.50) | 0 | 1 | 0 | 2 | Low | |
| Electrical stimulation (NMES) | Control | Generic ADL | FIM, BI | 4 | 112 | ES 0.15 to 1.78 | (‐0.61 to 0.91) to (0.00 to 3.56) | 1 | 0 | 1 | 2 | Low | |
| Activity | MAL: AOU | 1 (2 comparisons in one study) | 28 | ES 2.24 to 2.52 | (‐3.24 to 7.72) to (‐8.09 to 13.13) | 2 | 0 | 1 | 2 | Very low | |||
| Activity | MAL: QOM | 1 (2 comparisons in one study) | 28 | ES 2.09 to 2.48 | (‐1.76 to 5.94) to (‐7.03 to 11.99) | 2 | 0 | 1 | 2 | Very low | |||
| Electrical stimulation (stochastic resonance) | Control | Generic ADL | SIS | 1 | 30 | ES ‐0.03 | (‐0.77 to 0.71) | 2 | 0 | 0 | 2 | Low | |
| Strength training | Control | Generic ADL | SF36, FIM, BI | 5 | 210 | SMD 0.26 | (‐0.10 to 0.63) | 0 | 1 | 0 | 2 | Low | |
| ADL: Activity of daily living. AMSTAR: Measurement tool to assess the methodological quality of systematic reviews. BI: Barthel Index. ES: Effect size. FIM: Functional Independence Measure. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MAL: AOU: Motor Activity Log: Amount of Use. MAL: QOM: Motor Activity Log: Quality of Movement. NMES: Neuromuscular electrical stimulation. ROB: Risk of bias. SF36: Short Form (36) Health Survey. SIS: Stroke Impact Scale. | |||||||||||||
| Intervention | Outcome | Review | Outcome category | Outcome measure | Studies | Participants | Effect size | 95% confidence interval | Study size downgrades | ROB downgrades | I2 | AMSTAR downgrades | GRADE level of evidence | FU time |
| Brain stimulation: tDCS | Placebo or control | Generic ADL | BI | 3 | 99 | SMD 11.16 | (2.89 to 19.43) | 2 | 1 | 0 | 0 | Low | ||
| Mental practice | Any control | Generic ADL | BI | 2 | 57 | MD 0.46 | (‐2.36 to 3.27) | 2 | 0 | 1 | 1 | Low | ||
| ADL: Activity of daily living. AMSTAR: Measurement tool to assess the methodological quality of systematic reviews. BI: Barthel Index. FU: Follow‐up. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MD: Mean difference. ROB: Risk of bias. SMD: Standardised mean difference. tDCS: Transcranial direct current stimulation. | ||||||||||||||
| Details of subgroup | Intervention | Comparison | Review | Outcome category | Number of trials | Number of participants | Effect size | 95% CI | P value | Evidence of effect? | |
| Severity | Mild | Bilateral arm training | Unilateral arm training | Arm function | 5 | 203 | SMD 0.3 | (0.02 to 0.58) | 0.60 | Low quality | |
| Moderate | 3 | 137 | SMD 0.08 | (‐0.25 to 0.42) | No benefit or harm | ||||||
| Severe | 1 | 35 | SMD 0.11 | (‐0.58 to 0.81) | Low quality | ||||||
| Time post stroke | > 6 months post stroke | Mental practice + other treatment | Other treatment | Arm function | 4 | 66 | SMD 1.55 | (0.38 to 2.72) | 0.78 | Low | |
| < 6 months post stroke | 1 | 36 | SMD 1.35 | (0.62 to 2.08) | Low | ||||||
| 0 to 15 days post stroke | Repetitive task training | Any control | Upper limb function | 4 | 239 | SMD 0.21 | (‐0.04 to 0.47) | 0.98 | No benefit or harm | ||
| 16 days to 6 months post stroke | 4 | 105 | Low‐quality evidence | Low‐quality evidence | No benefit or harm | ||||||
| > 6 months post stroke | 3 | 140 | SMD 0.25 | (‐0.08 to 0.59) | No benefit or harm | ||||||
| < 6 months post stroke | Virtual reality | Other treatment | Upper limb function | 2 | 54 | SMD 0.76 | (0.18 to 1.34) | 0.37 | Beneficial effect | ||
| > 6 months post stroke | 5 | 151 | SMD 0.46 | (0.13 to 0.78) | Beneficial effect | ||||||
| Dose | < 360 minutes | Mental practice + other treatment | Other treatment | Arm function | 2 | 46 | SMD 2.79 | (‐0.60 to 1.60) | 0.30 | Low | |
| > 360 minutes | 3 | 56 | SMD 0.95 | (0.31 to 1.60) | Low | ||||||
| 0 to 20 hours | Repetitive task training | Any control | Upper limb function | 8 | 371 | SMD 0.18 | (‐0.02 to 0.39) | 0.31 | No benefit or harm | ||
| > 20 hours | 3 | 113 | SMD 0.40 | (0.03 to 0.78) | Beneficial effect | ||||||
| < 15 hours | Virtual reality | Other treatment | Upper limb function | 2 | 31 | SMD 0.58 | (‐0.12 to 1.29) | 0.87 | No benefit or harm | ||
| > 15 hours | 5 | 171 | SMD 0.52 | (0.21 to 0.83) | Beneficial effect | ||||||
| CI: Confidence interval. GRADE: Grades of Recommendation, Assessment, Development and Evaluation. MD: Mean difference. SMD: Standardised mean difference. | |||||||||||
| Method of assessment/reporting quality | Discussion |
| Cochrane 'Risk of bias' tool | This is used within all Cochrane reviews; however this tool has developed over time. Some of the reporting within earlier Cochrane reviews is limited primarily to an assessment of concealed allocation, whereas more recent reviews tend to have assessed random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Developments in the Cochrane 'Risk of bias' tool therefore contribute toward improved reporting over time |
| PEDro scale (Maher 2003; PEDro) | This scale assesses reporting of absence or presence of eligibility criteria; random allocation; allocation concealment; baseline similarity; participant, therapist and assessor blinding; dropouts/follow‐up; intention‐to‐treat; statistical comparisons and variability. However within some reviews, only the total PEDro 'score' was given, limiting our ability to judge specific issues related to risk of bias associated with randomisation, allocation concealment, etc. When reviews reported responses to the PEDro scale for each study, we had sufficient information to judge risk of bias for key criteria. Decisions around reporting this information within a published journal article are likely to be influenced by publication restrictions related to article length and number of tables |
| 'Levels of evidence' (Levels of Evidence) | These levels of evidence are based primarily on the methodological design of a study. Some reviews based their reports of quality on the types of study designs of included studies, using these levels of evidence. Often these were reviews that included a variety of different study types (i.e. were not limited to RCTs). These levels of evidence did not provide us with any information relatedto the issues associated with risk of bias, such as randomisation method, participant blinding or how incomplete data were managed |
| Assessment of study quality as part of review inclusion criteria | Some non‐Cochrane reviews (e.g. Farmer 2014) used an assessment of quality of studies as part of the eligibility criteria, including only studies that were judged to be at low risk of bias. Application of quality assessment in this way clearly has consequent implications related to the need to consider the scientific quality of included studies. The AMSTAR tool does not necessarily enable acknowledgement of the fact that all included studies had been judged to be at low risk of bias, and such reviews may be 'marked down' when this is, arguably, not appropriate |
| AMSTAR: Measurement tool to assess the methodological quality of systematic reviews. PEDro: Physiotherapy Evidence Database. RCT: Randomised controlled trial. | |

