طراحی قطر (gauge) و سر سوزن برای پیشگیری از سردرد متعاقب بیحسی نخاعی (PDPH)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010807.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 07 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ingrid Arevalo‐Rodriguez: (IA‐R), Luis Muñoz (LM), Natalia Godoy‐Casasbuenas (NG‐C), Jimmy J Arevalo (JJA), Sabine Boogaard (SB), Agustín Ciapponi (AC), Marta Roqué i Figul (MRF)
Conceiving the review: IA‐R, LM
Designing the review: IA‐R, LM, JJA, AC, MRF
Co‐ordinating the review: IA‐R
Undertaking manual searches: IA‐R, NG‐C
Screening search results: IA‐R, LM, JJA, NG‐C
Organizing retrieval of papers: LM, NG‐C
Screening retrieved papers against inclusion criteria: IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Appraising quality of papers: IA‐R, LM, SB, AC, MRF, NG‐C
Abstracting data from papers: LM, JJA, NG‐C, IAR
Writing to authors of papers for additional information: IAR
Providing additional data about papers: IA‐R, AC
Obtaining and screening data on unpublished studies: IA‐R, LM
Providing data management for the review: RM, IAR
Entering data into Review Manager (RevMan 5.3): IA‐R, LM, NG‐C
Managing RevMan statistical data: MRF, IAR
Performing other statistical analyses not using RevMan: MR
Ensuring double entry of data (data entered by person one: MRF; data entered by person two: IA‐R)
Interpreting data: IA‐R, LM, AC, MRF, NG‐C
Making statistical inferences: IA‐R, LM, AC, MRF
Writing the review: IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Providing guidance on the review: AC, MRF
Securing funding for the review: IA‐R
Performing previous work that served as the foundation of the present study: IA‐R, LM, AC, MRF
Serving as guarantor for the review (one author): IA‐R
Taking responsibility for reading and checking the review before submission:IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Sources of support
Internal sources
-
Fundación Universitaria de Ciencias de la Salud, Bogotá D.C., Colombia.
-
Institute for Clinical Effectiveness and Health Policy IECS, Buenos Aires, Argentina.
-
Iberoamerican Cochrane Centre, Barcelona, Spain.
-
Universidad El Bosque, Bogotá, Colombia.
Luis Muñoz is a Master of Science degree student at the Department of Clinical Epidemiology of El Bosque University, Bogotá, Colombia.
External sources
-
Agencia de Calidad del Sistema Nacional de Salud, Ministry of Health, Spain.
Declarations of interest
Ingrid Arevalo‐Rodriguez: none known.
Luis Muñoz: none known.
Natalia Godoy‐Casasbuenas: none known.
Jimmy J Arevalo: none known.
Sabine Boogaard: none known
Agustín Ciapponi: none known.
Marta Roqué i Figuls: none known.
Acknowledgements
We would like to thank Bronagh Blackwood (content editor), Vibeke E Horstmann (statistical editor), Stephen Halpern and James D Griffiths (peer reviewers), and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Mathew Zacharias (content editor), Marialena Trivella (statistical editor) and Andrew Moore, Stephen Halpern and Polpun Boonmak (peer reviewers) for their help and editorial advice during the preparation of the protocol for this systematic review. The review authors would also like to thank Arturo Marti‐Carvajal for his support and assistance in the design of this review's search strategies and for giving feedback on this protocol (Arevalo‐Rodriguez 2013a).
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 07 | Needle gauge and tip designs for preventing post‐dural puncture headache (PDPH) | Review | Ingrid Arevalo‐Rodriguez, Luis Muñoz, Natalia Godoy‐Casasbuenas, Agustín Ciapponi, Jimmy J Arevalo, Sabine Boogaard, Marta Roqué i Figuls | |
| 2013 Oct 29 | Needle gauge and tip designs for preventing post‐dural puncture headache (PDPH) | Protocol | Ingrid Arevalo‐Rodriguez, Luis Muñoz, Jimmy J Arevalo, Agustín Ciapponi, Marta Roqué i Figuls | |
Differences between protocol and review
We made the following changes to the published protocol (Arevalo‐Rodriguez 2013a).
-
Due to heterogeneity in the reporting of adverse events, we chose paraesthesia and backache as the most important adverse events(additional to PDPH) related to needle gauge and tip designs. We extracted all numerical information related to these two events and we reported the results in the corresponding sections.
-
In order to make a comprehensive 'Risk of bias' assessment, we considered seven domains (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) instead of the six domains planned in our protocol (Arevalo‐Rodriguez 2013a). However, we did not consider blinding of personnel because of the nature of the intervention (lumbar puncture).
-
We did not expect to encounter any unit of analysis issues, as we do not expect to find cross‐over studies or cluster‐randomized trials. However, we did identify four such studies (one cross‐over trial and three parallel‐group studies with punctures instead of patients as the unit of analysis) with our search strategies. We included these trials in our review in the qualitative report, but we did not include their results in our main analyses.
-
Subgroup analysis for age (younger than 18 years of age, older than 65 years of age and 18 to 65 years of age). Due to heterogeneity in the reporting of age, we classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more. We analysed the numerical information into these three categories.
-
Subgroup analysis by type of surgery: in participants receiving anaesthesia, we analysed the primary outcome by type of surgical procedure in order to explain all sources of heterogeneity. We identified at least three groups: caesarean section, orthopaedic surgeries and other surgeries. It has been reported that some subgroups of patients, such as obstetric women, have an increased risk of PDPH.
-
We did not use number needed to treat to harm (NNTH) figures to illustrate the harms or benefits of interventions, taking into account the quality of evidence and its limitations.
-
In order to consider all possible studies, we performed a sensitivity analysis to measure the risk difference (RD) in those analyses that presented zero events in both treatment arms; they were then not included in the risk ratio analysis.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Needles;
- Back Pain [epidemiology, etiology];
- Equipment Design;
- Headache [epidemiology, etiology];
- Paresthesia [epidemiology, etiology];
- Post‐Dural Puncture Headache [epidemiology, *prevention & control];
- Randomized Controlled Trials as Topic;
- Sensitivity and Specificity;
- Spinal Puncture [*adverse effects, instrumentation];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.

Funnel plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.

Funnel plot of comparison: 3 Atraumatic needles: different gauges, outcome: 3.1 PDPH major gauge versus minor gauge by number.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 1 PDPH by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 2 PDPH by gauge.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 3 PDPH by gender.
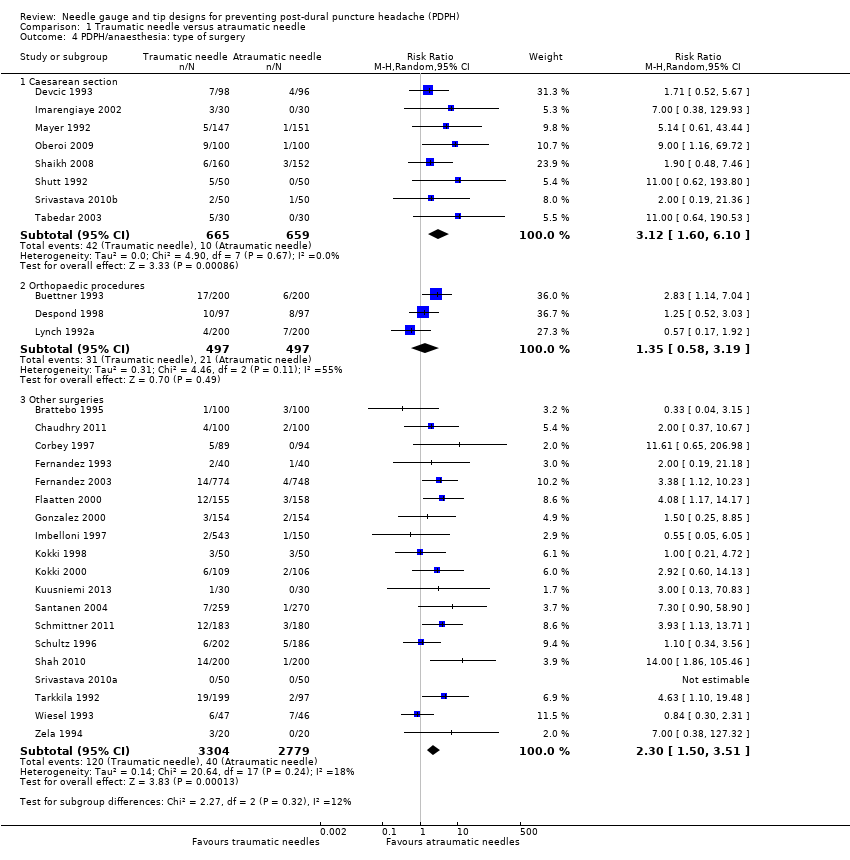
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 4 PDPH/anaesthesia: type of surgery.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 5 PDPH by position.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 6 PDPH by age.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 7 AE: paraesthesia.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 8 AE: backache.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 9 Severe PDPH by indication.
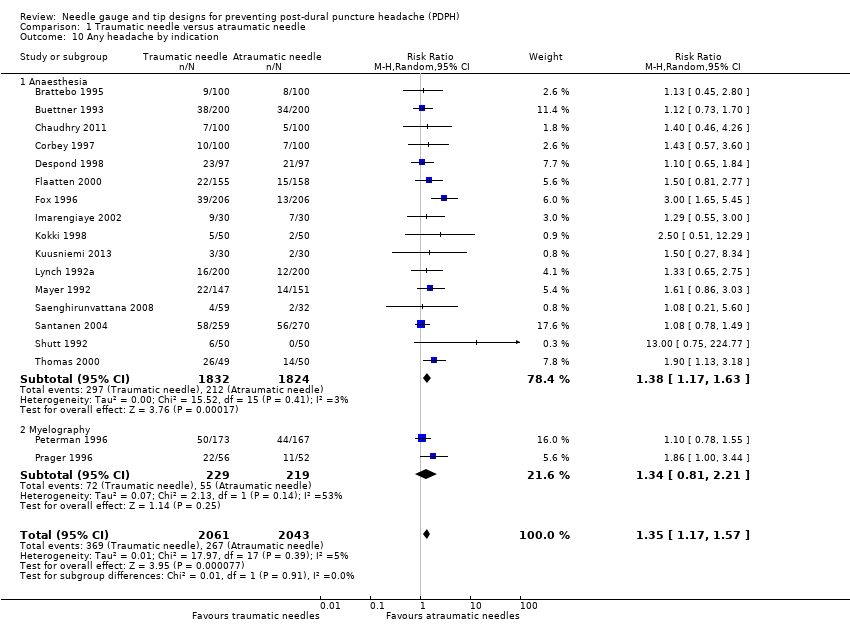
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 10 Any headache by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 11 PDPH sensitivity analysis.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 3 PDPH by age.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 4 PDPH by position.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 5 AE: backache.
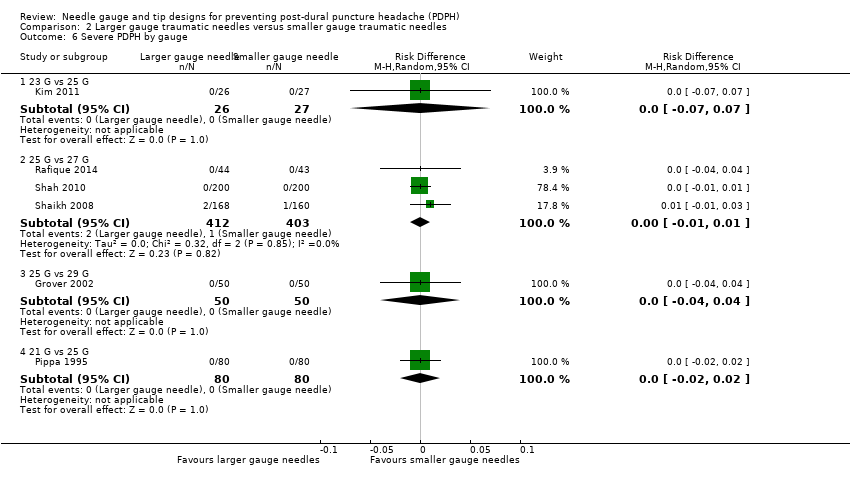
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 6 Severe PDPH by gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 7 Any headache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 3 PDPH by gender.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 4 PDPH by position.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 5 AE: paraesthesia.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 6 AE: backache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 7 Severe PDPH by gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 8 Any headache by gauge.
| Traumatic needles compared to atraumatic needles for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needles | Traumatic needles | |||||
| Onset of PDPH | 30 per 1000 | 64 per 1000 | RR 2.14 | 9378 | ⊕⊕⊕⊝ | — |
| Adverse events: paraesthesia | 52 per 1000 | 50 per 1000 | RR 0.96 | 573 | ⊕⊕⊕⊝ | — |
| Adverse events: backache | 155 per 1000 | 147 per 1000 | RR 0.94 | 3027 | ⊕⊕⊕⊝ | — |
| Severe PDPH | 0 per 1000 | 10 per 1000 | RD 0 | 6420 | ⊕⊕⊝⊝ | — |
| Any headache | 221 per 1000 | 290 per 1000 | RR 1.35 | 4104 | ⊕⊕⊕⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Traumatic needle(major gauge) compared to traumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Traumatic needle ‐ smaller gauge | Traumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.86 to 6.47 | 2288 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Adverse events: paraesthesia ‐ not reported | See comment | See comment | Not estimable | — | See comment | We did not identify any studies reporting this outcome. |
| Adverse event: backache | — | — | RR ranged | 948 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Severe PDPH | — | — | RD ranged | 1128 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Any headache | — | — | RR ranged | 771 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Imprecision downgraded by one level due to few events reported in each arm. 3Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit. | ||||||
| Atraumatic needle (major gauge) compared to atraumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needle ‐ smaller gauge | Atraumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.38 to 9.3 | 3134 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: paraesthesia | — | — | RR ranged from 1.03 to 7.61 | 439 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: backache | — | — | RR ranged | 526 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Severe PDPH | — | — | RD ranged | 1983 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Any headache | — | — | RR ranged | 1791 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH by indication Show forest plot | 36 | 9378 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.72, 2.67] |
| 1.1 Anaesthesia only | 30 | 8401 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.60, 3.04] |
| 1.2 Myelography only | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.34, 3.00] |
| 1.3 Diagnostic lumbar puncture only | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.38, 3.58] |
| 2 PDPH by gauge Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 22 gauge | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.56, 2.97] |
| 2.2 25 gauge | 5 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.56, 3.95] |
| 2.3 27 gauge | 11 | 4076 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.81, 4.53] |
| 3 PDPH by gender Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 9 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.62, 4.17] |
| 4 PDPH/anaesthesia: type of surgery Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Caesarean section | 8 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.60, 6.10] |
| 4.2 Orthopaedic procedures | 3 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.58, 3.19] |
| 4.3 Other surgeries | 19 | 6083 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.50, 3.51] |
| 5 PDPH by position Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Lateral position | 9 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.39, 9.24] |
| 5.2 Sitting position | 11 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.52, 2.94] |
| 6 PDPH by age Show forest plot | 36 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 No distinctions by age | 34 | 9063 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.73, 2.73] |
| 6.2 Only < 18 years | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.56, 5.12] |
| 7 AE: paraesthesia Show forest plot | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.96] |
| 8 AE: backache Show forest plot | 12 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 9 Severe PDPH by indication Show forest plot | 24 | 6420 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.20, 2.94] |
| 9.1 Anesthesia | 19 | 5542 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.88, 3.53] |
| 9.2 Myelography | 4 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.68, 4.28] |
| 9.3 Diagnostic lumbar puncture | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.18, 7.63] |
| 10 Any headache by indication Show forest plot | 18 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.17, 1.57] |
| 10.1 Anaesthesia | 16 | 3656 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 10.2 Myelography | 2 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.21] |
| 11 PDPH sensitivity analysis Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.26, 6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 23 G vs 25 G | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 1.2 25 G vs 27 G | 4 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.39] |
| 1.3 25 G vs 29 G | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.46, 9.78] |
| 1.4 26 G vs 27 G | 1 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.55, 16.43] |
| 1.5 21 G vs 25 G | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 2 PDPH by type of surgery Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.64, 2.57] |
| 2.2 Orthopaedic surgeries | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.58] |
| 2.3 Other surgeries | 6 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.23, 7.03] |
| 3 PDPH by age Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 No distinctions about age | 8 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.11, 3.95] |
| 3.2 Only children | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 3.3 Only > 60 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 4 PDPH by position Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Lateral position | 5 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.98, 3.16] |
| 4.2 Sitting position | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.56] |
| 5 AE: backache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Severe PDPH by gauge Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 23 G vs 25 G | 1 | 53 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.2 25 G vs 27 G | 3 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 6.3 25 G vs 29 G | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.4 21 G vs 25 G | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7 Any headache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 22 G vs 24 G | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.81] |
| 1.2 22 G vs 25 G | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.50] |
| 1.3 24 G vs 25 G | 2 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [1.00, 31.67] |
| 1.4 25 G vs 26 G | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.90] |
| 1.5 25 G vs 27 G | 2 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.59, 23.64] |
| 1.6 26 G vs 27 G | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.30, 10.73] |
| 1.7 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.58, 4.37] |
| 2 PDPH by type of surgery Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 6 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.64, 5.79] |
| 2.2 Orthopaedic procedures | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.30, 5.07] |
| 2.3 Other surgeries | 5 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.83] |
| 3 PDPH by gender Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 8 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.51, 2.20] |
| 4 PDPH by position Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Sitting position | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.06] |
| 4.2 Lateral position | 5 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.65, 5.41] |
| 5 AE: paraesthesia Show forest plot | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.31, 15.30] |
| 6 AE: backache Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Severe PDPH by gauge Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 22 G vs 24 G | 1 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7.2 22 G vs 25 G | 1 | 234 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 24 G vs 25 G | 1 | 304 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.4 25 G vs 26 G | 2 | 311 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.5 25 G vs 27 G | 1 | 212 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.6 26 G vs 27 G | 1 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.7 27 G vs 29 G | 1 | 389 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Any headache by gauge Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 22 G vs 25 G | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.85, 5.51] |
| 8.2 24 G vs 25 G | 2 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.49, 2.77] |
| 8.3 25 G vs 26 G | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.99] |
| 8.4 25 G vs 27 G | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.65, 5.39] |
| 8.5 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |

