Bestimmung des Tau und Tau/ABeta Verhältnisses im Liquor cerebrospinalis zur Diagnose der Alzheimer‐Demenz und anderer Demenzformen bei Menschen mit leichter kognitiver Beeinträchtigung
Appendices
Appendix 1. Sources searched and search strategies
The MEDLINE search strategy below was created to optimise sensitivity. The strategy utilises a number of concepts:
Concept A: lines 1 to 21 health condition/s of interest
Concept B: lines 23 to 42 what is being measured by the index test/s/the index test/s
Concept C: lines 44 to 49 method of measurement (i.e. CSF)
The main yield is created by combining A AND B AND C
However, in order to try to capture those records that perhaps do not mention one or more of the three concepts above, some additional combinations were added to the strategy. For example: In the MEDLINE strategy below, lines 51 and 52 (which identify records in Medline with the dementia MeSH subheading of diagnosis and those with a subheading of cerebrospinal fluid) were combined with the concept for the index test(s). This approach identified unique records and an examination of the first 50 of these records resulted in two further citations for possible inclusion within the review.
| Source | Search strategy | Hits retrieved |
| 1. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946 to present (Ovid SP) | 1. exp Dementia/ 2. Cognition Disorders/ 3. (alzheimer* or dement* or AD or lewy* or VaD or frontotemporal or 'vascular cognit* impair*').ti,ab. 4. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 5. (forgetful* or confused or confusion).ti,ab. 6. MCI.ti,ab. 7. ACMI.ti,ab. 8. ARCD.ti,ab. 9. SMC.ti,ab. 10. CIND.ti,ab. 11. BSF.ti,ab. 12. AAMI.ti,ab. 13. LCD.ti,ab. 14. QD.ti,ab. 15. AACD.ti,ab. 16. MNCD.ti,ab. 17. MCD.ti,ab. 18. (nMCI or aMCI or mMCI).ti,ab. 19. ('N‐MCI' or 'A‐MCI' or 'M‐MCI').ti,ab. 20. 'Petersen”.ab. 21. ((CDR adj2 '0.5') or ('clinical dementia rating' adj3 '0.5')).ab. 22. or/1‐21 23. (neurofibril* adj3 tangle*).ti,ab. 24. (neurofilament adj3 protein*).ti,ab. 25. (neuropil adj3 thread*).ti,ab. 26. ((senile or amyloid or neuritic) adj3 plaque*).ti,ab. 27. Neuropil Threads/ 28. Senile Plaques/ 29. exp Neurofibrils/ 30. Neurofilament Proteins/ 31. tau Proteins/ 32. tau*.ti,ab. 33. hyperphosphorylation.ti,ab. 34. pTau181.ti,ab. 35. *peptide fragments/cf 36. pTau*.ti,ab. 37. ('t‐tau*' or 'p‐tau*').ti,ab. 38. (innotest or inno‐bia or Alzbio3).ti,ab. 39. ((abeta* or ab42 or ab40 or 'amyloid‐beta' or 'beta‐amyloid' or 'a?42' or 'a?40' or 'a beta') adj4 (ratio or ratios)).ti,ab. 40. ('phospho‐tau*' or 'total‐tau*').ti,ab. 41. or/23‐40 42. (cerebrospinal fluid* or csf or 'spinal fluid*').ti,ab. 43. (blood or plasma).ti,ab. 44. Cerebrospinal Fluid/ 45. Blood‐Brain Barrier/ 46. or/42‐45 47. (cf or bl or di or du).fs. 48. or/46‐47 49. 48 and 41 and 22 50. exp *Dementia/cf [Cerebrospinal Fluid] 51. exp Dementia/di [Diagnosis] 52. cf.fs. 53. 41 and 51 and 52 54. Cerebrospinal Fluid Proteins/ 55. Biological Markers/cf [Cerebrospinal Fluid] 56. or/54,55 57. 56 and 22 and 41 58. or/49,50,53,57 59. (animals not (humans and animals)).sh. 60. 58 not 59 | July 2012: 7718 Jan 2013: 480 |
| 2. Embase 1980 to 2012 week 29 (Ovid SP) | 1. dement*.ti. 2. alzheimer*.ti. 3. (AD or VaD or lewy or frontotemporal or 'vascular cognit* impair*').ti. 4. Dementia/di 5. dementia/ep [Epidemiology] 6. (('conversion to' or 'conversion from') adj4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*')).ab. 7. ((endpoint* or 'end point*' or outcome*) adj5 (dement* or alzheimer* or AD or VaD or lewy)).ab. 8. (predict* adj5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*')).ab. 9. ((convert or converted) adj4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*')).ab. 10. (progress* adj5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*')).ab. 11. or/1‐10 12. exp dementia/ 13. (alzheimer* or dement* or AD or lewy* or VaD or frontotemporal or 'vascular cognit* impair*').ti,ab. 14. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 15. (forgetful* or confused or confusion).ti,ab. 16. MCI.ti,ab. 17. ACMI.ti,ab. 18. ARCD.ti,ab. 19. SMC.ti,ab. 20. CIND.ti,ab. 21. BSF.ti,ab. 22. AAMI.ti,ab. 23. LCD.ti,ab. 24. QD.ti,ab. 25. AACD.ti,ab. 26. MNCD.ti,ab. 27. MCD.ti,ab. 28. (nMCI or aMCI or mMCI).ti,ab. 29. ('N‐MCI' or 'A‐MCI' or 'M‐MCI').ti,ab. 30. 'Petersen criteria'.ab. 31. ((CDR adj2 '0.5') or ('clinical dementia rating' adj3 '0.5')).ab. 32. (neurofibril* adj3 tangle*).ti,ab. 33. (neurofilament adj3 protein*).ti,ab. 34. (neuropil adj3 thread*).ti,ab. 35. ((senile or amyloid or neuritic) adj3 plaque*).ti,ab. 36. neuropil thread/ 37. senile plaque/ 38. neurofilament/ 39. neurofilament protein/ 40. or/12‐39 41. (cerebrospinal fluid* or csf or 'spinal fluid*').ti,ab. 42. (blood or plasma).ti,ab. 43. cerebrospinal fluid/ 44. blood brain barrier/ 45. or/41‐44 46. tau protein/ 47. tau.ti,ab. 48. hyperphosphorylation.ti,ab. 49. pTau181.ti,ab. 50. tau181.ti,ab. 51. peptide fragment/ 52. ('abeta*/tau' and ratio).ab. 53. pTau*.ti,ab. 54. ('t‐tau*' or 'p‐tau*').ti,ab. 55. (innotest or inno‐bia or Alzbio3).ti,ab. 56. ((abeta* or ab42 or ab40 or 'amyloid‐beta' or 'beta‐amyloid' or (amyloid and 'β') or 'aβ' or 'aβ42' or 'aβ40' or 'a beta') adj4 ratio).ti,ab. 57. ('phospho‐tau*' or 'total‐tau*').ti,ab. 58. tau231.ti,ab. 59. or/46‐58 60. 40 and 45 and 59 61. sensitivit*.ab. 62. specificit*.ab. 63. (ROC or 'receiver operat*').ab. 64. area under the curve/ 65. ('Area under curve' or AUC).ab. 66. (detect* adj3 (dement* or AD or alzheimer*)).ti,ab. 67. sROC.ab. 68. accura*.ti,ab. 69. (likelihood adj3 (ratio* or function*)).ab. 70. (conver* adj3 (dement* or AD or alzheimer*)).ti,ab. 71. ((true or false) adj3 (positive* or negative*)).ab. 72. ((positive* or negative* or false or true) adj3 rate*).ti,ab. 73. reproducibility/ 74. diagnos*.ti. 75. diagnostic accuracy/ 76. or/61‐75 77. 11 and 59 and 76 78. 60 or 77 | July 2012: 3692 Jan 2013: 732 |
| 3. PsycINFO 1806 to July week 1 2012 (Ovid SP) | 1. dement*.ti,ab. 2. alzheimer*.ti,ab. 3. (AD or VaD or lewy or frontotemporal or 'vascular cognit* impair*').ti,ab. 4. exp Dementia/ 5. (('conversion to' or 'conversion from') adj4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*')).ab. 6. ((endpoint* or 'end point*' or outcome*) adj5 (dement* or alzheimer* or AD or VaD or lewy)).ab. 7. (predict* adj5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*')).ab. 8. ((convert or converted) adj4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*')).ab. 9. (progress* adj5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*')).ab. 10. or/1‐9 11. Prediction/ or Diagnosis/ 12. (dement* or alzheimer* or AD or VaD or lewy or frontotemporal or 'vascular cognit* impair*').ab. 13. exp *Dementia/ 14. or/11‐13 15. 10 or 14 16. exp Dementia/ 17. exp Cognitive Impairment/ 18. (alzheimer* or dement* or AD or lewy* or VaD or frontotemporal or 'vascular cognit* impair*').ti,ab. 19. (forgetful* or confused or confusion).ti,ab. 20. MCI.ti,ab. 21. ACMI.ti,ab. 22. ARCD.ti,ab. 23. SMC.ti,ab. 24. CIND.ti,ab. 25. BSF.ti,ab. 26. AAMI.ti,ab. 27. LCD.ti,ab. 28. QD.ti,ab. 29. AACD.ti,ab. 30. MNCD.ti,ab. 31. MCD.ti,ab. 32. (nMCI or aMCI or mMCI).ti,ab. 33. ('N‐MCI' or 'A‐MCI' or 'M‐MCI').ti,ab. 34. 'Petersen criteria'.ab. 35. ((CDR adj2 '0.5') or ('clinical dementia rating' adj3 '0.5')).ab. 36. (neurofibril* adj3 tangle*).ti,ab. 37. (neurofilament adj3 protein*).ti,ab. 38. (neuropil adj3 thread*).ti,ab. 39. ((senile or amyloid or neuritic) adj3 plaque*).ti,ab. 40. exp Neurofibrillary Tangles/ 41. exp Senile Plaques/ 42. or/16‐41 43. (cerebrospinal fluid* or csf or 'spinal fluid*').ti,ab. 44. (blood or plasma).ti,ab. 45. exp Cerebrospinal Fluid/ 46. exp Blood Brain Barrier/ 47. or/43‐46 48. tau.ti,ab. 49. hyperphosphorylation.ti,ab. 50. pTau181.ti,ab. 51. tau181.ti,ab. 52. ('abeta*/tau' and ratio).ab. 53. pTau*.ti,ab. 54. ('t‐tau*' or 'p‐tau*').ti,ab. 55. (innotest or inno‐bia or Alzbio3).ti,ab. 56. ((abeta* or ab42 or ab40 or 'amyloid‐beta' or 'beta‐amyloid' or (amyloid and 'β') or 'aβ' or 'aβ42' or 'aβ40' or 'a beta') adj4 ratio).ti,ab. 57. ('phospho‐tau*' or 'total‐tau*').ti,ab. 58. tau231.ti,ab. 59. or/48‐58 60. 42 and 59 61. 47 and 60 62. 15 and 59 63. 61 or 62 | July 2012: 2645 Jan 2013: 464 |
| 4. BIOSIS Previews (Thomson Reuters Web of Science) | Topic = (tau OR p‐tau OR t‐tau OR pTau OR tTau OR hyperphosphorylation OR pTau181 OR 'phospho‐tau*' or 'total‐tau*' OR tau231) AND Topic = (dement* OR alzheimer* OR MCI OR 'cognit* impair*' OR 'CDR 0.5' OR 'petersen criteria' OR aMCI OR nMCI OR mMCI) AND Topic = (diagnosis OR sensitiv* OR specificit* OR ROC OR 'receiver operat*' OR 'Area under curve' or AUC OR sROC OR accura* OR 'follow*‐up' OR 'positive predictive value*' OR 'negative predictive value*' OR longitudinal OR longitudinally) Timespan = All Years. Databases = BIOSIS Previews. Lemmatization = On | July 2012: 1775 Jan 2013: 206 |
| 5. Web of Science Core Collection, including Conference Proceedings Citation Index (Thomson Reuters Web of Science) (1945‐present) | Topic = (tau OR p‐tau OR t‐tau OR pTau OR tTau OR hyperphosphorylation OR pTau181 OR 'phospho‐tau*' or 'total‐tau*' OR tau231) AND Topic = (dement* OR alzheimer* OR MCI OR 'cognit* impair*' OR 'CDR 0.5' OR 'petersen criteria' OR aMCI OR nMCI OR mMCI) AND Topic = (diagnosis OR sensitiv* OR specificit* OR ROC OR 'receiver operat*' OR 'Area under curve' or AUC OR sROC OR accura* OR 'follow*‐up' OR 'positive predictive value*' OR 'negative predictive value*' OR longitudinal OR longitudinally) Timespan = All Years. Databases = Web of Science Core Collection. Lemmatization = On | July 2012: 2205 Jan 2013: 234 |
| 6. LILACS (BIREME) | Hiperfosforilación OR hyperphosphorylation OR tau OR fosfo‐tau OR phosphor‐tau OR p‐tau OR pTau181 OR tau181 OR tau231 | July 2012: 126 Jan 2013: 3 |
| 7. CINAHL (EBSCOhost) | S1 TX dement* S2 TX AD OR VaD OR lewy OR frontotemporal OR 'vascular cognit* impair*' S3 TX alzheimer* S4 (MH 'Dementia/DI') S5 (MH 'Dementia/ET') S6 TX 'conversion to' N2 dement* S7 TX ('conversion from') N4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*') S8 TX (endpoint* or 'end point*' or outcome*) N5 (dement* or alzheimer* or AD or VaD or lewy) S9 TX predict* N5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*') S10 TX (convert or converted) N4 (dement* or alzheimer* or AD or lewy or VaD or 'vascular cognit* impair*') S11 TX progress* N5 (dement* or alzheimer* or AD or VaD or lewy or 'vascular cognit* impair*') S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 (MH 'Predictive Value of Tests') S14 TX dement* or alzheimer* or AD or VaD or lewy or frontotemporal or 'vascular cognit* impair*' S15 (MM 'Dementia+') S16 S14 or S15 S17 S13 and S16 S18 S12 or S17 S19 (MH 'Dementia+') S20 (MH 'Cognition Disorders') S21 TX alzheimer* or dement* or AD or lewy* or VaD or frontotemporal or 'vascular cognit* impair*' S22 TX (cognit* or memory or cerebr* or mental*) N3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*) S23 TX forgetful* or confused or confusion S24 TX MCI S25 TX ACMI S26 TX ARCD S27 TX SMC S28 TX CIND S29 TX LCD S30 TX AACD S31 TX MNCD S32 TX MCD S33 TX nMCI or aMCI or mMCI S34 TX 'N‐MCI' or 'A‐MCI' or 'M‐MCI' S35 TX 'Petersen criteria' S36 TX CDR N2 '0.5' S37 TX 'clinical dementia rating' N3 '0.5' S38 TX neurofibril* N3 tangle* S39 TX neurofilament N3 protein* S40 TX neuropil N3 thread* S41 TX (senile or amyloid or neuritic) N3 plaque* S42 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 S43 TX cerebrospinal fluid* or csf or 'spinal fluid*' S44 (MH 'Cerebrospinal Fluid') S45 S43 or S44 S46 TX tau S47 TX hyperphosphorylation S48 TX pTau181 S49 TX tau181 S50 TX (abeta* N3 tau) N4 ratio S51 TX (amyloid* N3 tau) N4 ratio S52 TX (ab42 N3 tau) N4 ratio S53 TX (ab40 N3 tau) N4 ratio S54 TX ('a beta' N3 tau) N4 ratio S55 TX pTau* S56 TX 't‐tau*' or 'p‐tau*' S57 TX ('aβ40' or 'a beta') N4 ratio S58 TX 'phospho‐tau*' or 'total‐tau*' S59 TX tau231 S60 S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 S61 S42 and S60 S62 S12 and S60 S63 S17 and S45 S64 S61 or S62 or S63 | July 2012: 591 Jan 2013: 59 |
| TOTAL before de‐duplication and first assessment | July 2012: 18752 Jan 2013: 1694 | |
Appendix 2. Cross classification of test results and disease status (2X2)
Table 1: Conversion from MCI to Alzheimer’s disease dementia
| Index test information | Reference standard information | |
| ADD present | ADD absent | |
| Index test positive | Index test + who convert to ADD (TP) | Index test + who remain MCI (FP) & Index test + who convert to non‐ADD (FP) |
| Index test negative | Index test ‐ who convert to ADD (FN) | Index test ‐ who remain MCI (TN) & Index test ‐ who convert to non‐ADD (TN) |
Table 2: Conversion from MCI to non‐Alzheimer’s disease dementia
| Index test information | Reference standard information | |
| Non‐ADD present | Non‐ADD absent | |
| Index test positive | Index test + who convert to non‐ADD (TP) | Index test + who remain MCI (FP) & Index test + who convert to ADD (FP) |
| Index test negative | Index test ‐ who convert to non‐ADD (FN) | Index test ‐ who remain MCI (TN) & Index test ‐ who convert to ADD (TN) |
Table 3: Conversion from MCI to any form of dementia
| Index test information | Reference standard information | |
| Any forms of dementia present | Dementia absent | |
| Index test positive | Index test + who convert to any form of dementia (TP) | Index test + who remain MCI (FP) |
| Index test negative | Index test ‐ who convert to any form of dementia (FN) | Index test ‐ who remain MCI (TN) |
Appendix 3. Assessment of methodological quality table QUADAS‐2 tool
| DOMAIN | PARTICIPANT SELECTION | INDEX TEST | REFERENCE STANDARD | FLOW AND TIMING |
| Description | Describe methods of participant selection: Describe included participants (prior testing, presentation, intended use of index test, and setting) | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted | Describe any participants who did not receive the index test(s) and/or reference standard or who were excluded from the 2 x 2 table (refer to flow diagram): Describe the time interval and any interventions between index test(s) and reference standard |
| Signalling questions: (yes/no/unclear) | Was a consecutive or random sample of participants enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it prespecified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all participants receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all participants receive the same reference standard? | |||
| Were all participants included in the analysis? | ||||
| Risk of bias: High/low/unclear | Could the selection of participants have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow have introduced bias? |
| Concerns regarding applicability: High/low/unclear | Are there concerns that the included participants do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
|
Appendix 4. Anchoring statements for quality assessment of CSF tau and tau/ABeta ratio biomarkers diagnostic studies
| Category | Review question | Inclusion criteria |
| Participants | Participants with mild cognitive impairment, no dementia | Participants fulfilling the criteria for the clinical diagnosis of MCI at baseline |
| Index test | CSF t‐tau; CSF p‐tau; CSF t‐tau/ABeta ratio | CSF t‐tau; CSF p‐tau; CSF t‐tau/ABeta ratio |
| Target condition | Alzheimer’s disease dementia (conversion from MCI to Alzheimer’s disease dementia) Any other forms of dementia (conversion from MCI to any other forms of dementia) | Alzheimer’s disease dementia (conversion from MCI to Alzheimer’s disease dementia) Any other forms of dementia (conversion from MCI to any other forms of dementia) |
| Reference standard | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; NINDS‐ARIEN criteria | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; NINDS‐ARIEN criteria |
| Outcome | N/A | Data to construct 2 X 2 table |
| Study design | N/A | Longitudinal cohort studies and nested case‐control studies if they incorporate a delayed verification design (case‐control nested in cohort studies) |
Anchoring statements for quality assessment CSF tau and CSF tau/ABeta ratio
We provide some core anchoring statements for quality assessment of diagnostic test accuracy review of CSF tau and CSF tau/ABeta ratio biomarkers in dementia. These statements are designed for use with the QUADAS‐2 tool and are based on the guidance for quality assessment of diagnostic test accuracy reviews of IQCODE in dementia (Quinn 2014).
During a two‐day, multidisciplinary focus group and the piloting/validation of the guidance, it was clear that certain issues were key to assessing quality, while other issues were important to record but less important for assessing overall quality. To assist, we describe a 'weighting' system. Where an item is weighted 'high risk' then that section of the QUADAS‐2 results table is likely to be scored as 'high risk of bias'. For example, in dementia diagnostic test accuracy studies, ensuring that clinicians performing dementia assessment are blinded to results of index test is fundamental. If this blinding was not present, then the item on the reference standard should be scored 'high risk of bias', regardless of the other contributory elements.
In assessing individual items, the score of 'unclear' should only be given if there is genuine uncertainty. In these situations, review authors will contact the relevant study teams for additional information.
Anchoring statements to assist with assessment for risk of bias
Patient selection
Was the sampling method appropriate?
Where sampling is used, the designs least likely to cause bias are consecutive sampling or random sampling. Sampling that is based on volunteers or selecting subjects from a clinic or research resource is prone to bias.
Weighting: High risk of bias (‘no’)
Was a case‐control or similar design avoided?
Designs similar to case‐control that may introduce bias are those designs in which the study team deliberately increase or decrease the proportion of subjects with the target condition, which may not be representative. For example, a population study may be enriched with extra dementia subjects from a secondary care setting, who are typically more diseased. Some case‐control methods may already be excluded if they mix subjects from various settings.
Weighting: High risk of bias (‘no’)
Are exclusion criteria described and appropriate?
The study will be automatically graded as unclear if exclusions are not detailed (pending contact with study authors). Where exclusions are detailed, the study will be graded as 'low risk' if exclusions are felt to be appropriate by the review authors. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative condition. Exclusions are not felt to be appropriate if ‘difficult to diagnose’ participants are excluded.
Post hoc and inappropriate exclusions will be labelled 'high risk' of bias.
Weighting: High risk (‘no’)
Index test
Was CSF tau and CSF tau/ABeta ratio biomarkers' assessment/interpretation performed without knowledge of clinical dementia diagnosis?
Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the index test may be influenced by knowledge of the results of reference standard. If the index test is always interpreted prior to the reference standard, then the person interpreting the index test cannot be aware of the results of the reference standard and so this item could be rated as ‘yes’.
For certain index tests the result is objective and knowledge of reference standard should not influence result, for example level of protein in cerebrospinal fluid, in this instance the quality assessment may be 'low risk' even if blinding was not achieved.
Weighting: High risk (‘no’)
Were CSF tau and CSF tau/ABeta ratio biomarkers’ thresholds prespecified?
For scales and biomarkers there is often a reference point (in units or categories) above which subjects are classified as 'test positive'; this may be referred to as threshold; clinical cutoff or dichotomisation point. A study is classified 'high risk of bias' if the authors define the optimal cut‐off post‐hoc based on their own study data because selecting the threshold to maximise sensitivity and/specificity may lead to overoptimistic measures of test performance.
Certain papers may use an alternative methodology for analysis that does not use thresholds and these papers should be classified as not applicable.
Weighting: High risk (‘no’)
Reference standard
Is the assessment used for clinical diagnosis of dementia acceptable?
Commonly used international criteria to assist with clinical diagnosis of dementia include those detailed in DSM‐IV and ICD‐10. Criteria specific to dementia subtypes include but are not limited to NINCDS‐ADRDA criteria for Alzheimer’s dementia; McKeith criteria for Lewy Body dementia; Lund criteria for frontotemporal dementias; and the NINDS‐AIREN criteria for vascular dementia. Where the criteria used for assessment is not familiar to the review authors or the Cochrane Dementia and Cognitive Improvement group (‘unclear’), this item should be classified as 'high risk of bias'.
Weighting: High risk (‘no’)
Was clinical assessment for dementia performed without knowledge of the CSF tau and CSF tau/ABeta ratio biomarkers?
Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the reference standard may be influenced by knowledge of the results of index test.
Weighting: High risk (‘no’)
Patient flow
Was there an appropriate interval between CSF tau and CSF tau/ABeta ratio biomarkers and clinical dementia assessment?
As we test the accuracy of the CSF tau and CSF tau/ABeta ratio biomarkers for MCI conversion to dementia, there will always be a delay between the index test and the reference standard assessments. The time between reference standard and index test will influence the accuracy (Geslani 2005;Okello 2009;Visser 2006), and therefore we will note time as a separate variable (both within and between studies) and will test its influence on the diagnostic accuracy. We have set a minimum mean time to follow‐up assessment of one year. If more than 16% of subjects of subjects have assessment for MCI conversion before nine months this item will score ‘no’.
Weighting: High risk (‘no’)
Did all subjects get the same assessment for dementia regardless of CSF tau and CSF tau/ABeta ratio biomarkers?
There may be scenarios where subjects who score 'test positive' on index test have a more detailed assessment. Where dementia assessment differs between subjects, this should be classified as 'high risk of bias'.
Weighting: High risk ('no')
Were all participants who received CSF tau and CSF tau/ABeta ratio biomarkers’ assessment included in the final analysis?
If the number of participants enrolled differs from the number of participants included in the 2 X 2table, then there is the potential for bias. If participants lost to dropout differ systematically from those who remain, then estimates of test performance may differ.
If dropouts, these should be accounted for; a maximum proportion of dropouts to remain 'low risk of bias' has been specified as 20%.
Weighting: High risk (‘no’)
Were missing or uninterpretable CSF tau and CSF tau/ABeta ratio biomarkers results reported?
Where missing or uninterpretable results are reported, and if there is substantial attrition (we have set an arbitrary value of 50% missing data); this should be scored as ‘no’. If those results are not reported, this should be scored as ‘unclear’ and authors will be contacted.
Weighting: High risk (‘no’ and ‘unclear’)
Anchoring statements to assist with assessment for applicability
Patient selection
Were included participants representative of the general population of interest?
The included participants should match the intended population as described in the review question. The review authors should consider population in terms of: symptoms; pretesting; potential disease prevalence; setting.
If there is a clear ground for suspecting an unrepresentative spectrum the item should be rated 'poor applicability'.
Index test
Were sufficient data on CSF tau and CSF tau/ABeta ratio biomarkers’ application given for the test to be repeated in an independent study?
Variation in technology, test execution, and test interpretation may affect estimate of accuracy. In addition, the background, and training/expertise of the assessor should be reported and taken in consideration. If CSF tau and CSF tau/ABeta ratio biomarkers were not performed consistently, this item should be rated 'poor applicability'.
Reference standard
Was clinical diagnosis of dementia made in a manner similar to current clinical practice?
For many reviews, inclusion criteria and assessment for risk of bias will already have assessed the dementia diagnosis. For certain reviews, an applicability statement relating to reference standard may not be applicable. There is the possibility that a form of dementia assessment, although valid, may diagnose a far larger proportion of subjects with disease than usual clinical practice. In this instance, the item should be rated 'poor applicability'.
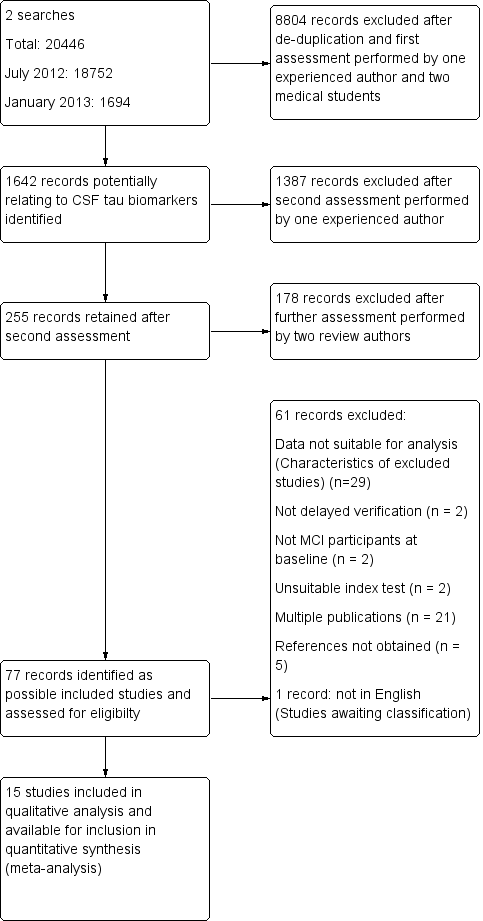
Study flow diagram
Note: a top‐up search performed in December 2015 revealed 6134 records
85 records retained after de‐duplication and assessment by one experienced reviewer
81 records excluded after further assessment performed by two review authors
4 studies identified for possible inclusion (Characteristics of studies awaiting classification)

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Forest plot of 1 CSF t‐tau conversion to AD dementia.

Summary ROC Plot of 1 CSF t‐tau conversion to AD dementia.

Post‐test probability plots (Analysis 1): Conversion from MCI to Alzheimer’s disease for CSF t‐tau as a diagnostic test

Forest plot of 2 CSF p‐tau conversion to AD dementia.

Summary ROC Plot of 2 CSF p‐tau conversion to AD dementia.

Post‐test probability plots (Analysis 2): Conversion from MCI to Alzheimer’s disease for CSF p‐tau as a diagnostic test
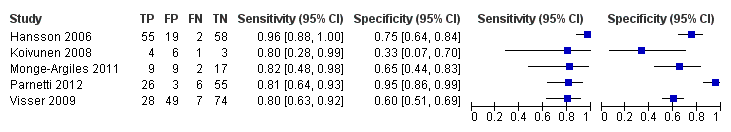
Forest plot of 3 CSF p‐tau/ABeta ratio to AD dementia.

Summary ROC Plot of 3 CSF p‐tau/ABeta ratio to AD dementia.
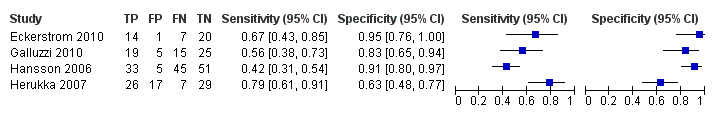
Forest plot of 4 CSF t‐tau conversion to all forms of dementia.
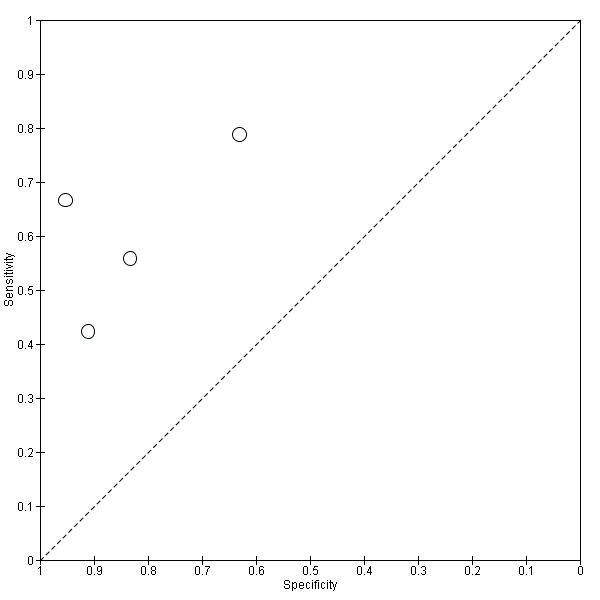
Summary ROC Plot of 4 CSF t‐tau conversion to All dementias.

CSF t‐tau conversion to AD dementia.

CSF p‐tau conversion to AD dementia.

CSF p‐tau/ABeta ratio to AD dementia.

CSF t‐tau conversion to All dementias.
| What is the diagnostic accuracy of CSF biomarker levels for detecting Alzheimer's disease pathology in people with mild cognitive impairment (MCI), and identifying those MCI participants who would convert to Alzheimer’s disease dementia or other forms of dementia over time | |||||||
| Descriptive | |||||||
| Patient population | Participants diagnosed with MCI at baseline using any of the Petersen criteria or CDR = 0.5 or any 16 definitions included by Matthews (Matthews 2008) | ||||||
| Sampling procedure | Consecutive or random (n = 5) Not consecutive or random (n = 3) Unclear (n = 7) | ||||||
| Sources of recruitment | University memory clinic (n = 8); European multicentre memory clinics (n = 2); inpatients (n = 2); General Hospital memory clinic (n = 1); Research centre outpatient memory clinic (n = 1); not reported (n = 1) | ||||||
| Prior testing | The only testing prior to performing the plasma and CSF biomarkers was the application of diagnostic criteria for identifying participants with MCI. | ||||||
| MCI criteria | Petersen criteria (n = 14) Global Deterioration Scale (GDS) (n = 1) | ||||||
| Index tests | CSF t‐tau or CSF p‐tau or CSF p‐tau/ABeta ratio or CSF t‐tau/ABeta ratio | ||||||
| Reference standard | NINCDS‐ADRDA and/or DSM and/or ICD criteria for Alzheimer's disease dementia (n = 12); Global Dementia Scale (GDS) & Research criteria (n = 1); CDR = 1 criteria (n = 1); not specified (n = 1) McKeith criteria for Lewy body dementia; Lund criteria for frontotemporal dementia; and NINDS AIREN criteria for vascular dementia | ||||||
| Target condition | Alzheimer’s disease dementia or any other types of dementia | ||||||
| Included studies | Prospectively well‐defined cohorts of MCI participants (n = 7), nested case‐control studies with a prospectively defined MCI group (n = 6) and studies with a retrospectively defined MCI group with longitudinal data (n = 2). Fifteen studies (N = 1282 participants) were included. Number included in analysis: 1172 | ||||||
| Quality concerns | Patient selection and conduct of the reference standard were poorly reported. Applicability concerns were generally low. Regarding the inclusion criteria set in the review, the majority of included studies did match the review question: 'Could CSF t‐tau and CSF t‐tau/ABetaratio biomarkers identify those MCI participants with Alzheimer’s disease pathology at baseline who would convert clinically to dementia at follow up?' However, due to a limited number of included studies and levels of heterogeneity, it is difficult to determine to what extent the findings from a meta‐analysis can be applied to clinical practice. | ||||||
| Limitations | Limited investigation of heterogeneity due to insufficient number of studies. There was a lack of common thresholds. | ||||||
| Test Median percentage converting (range) 2 | Studies | Cases/participants | Median specificity from included studies | Sensitivity (95% CI)1 at median specificity | Consequences in a cohort of 100 | ||
| Median percentage converting2 | Missed cases | Overdiagnosed | |||||
| Alzheimer's disease dementia | |||||||
| CSF t‐tau | 7 | 436/709 | 72 | 77 (67, 85) | 37 | 9 | 18 |
| Alzheimer's disease dementia | |||||||
| CSF p‐tau | 6 | 164/492 | 47.5 | 81 (64, 91.5) | 37 | 7 | 33 |
| Alzheimer's disease dementia | |||||||
| CSF p‐tau/ ABeta ratio | 5 | 140/433 | No meta‐analysis | No meta‐analysis | |||
| All types of dementia | |||||||
| CSF t‐tau | 4 | 166/319 | No meta‐analysis | No meta‐analysis | |||
| Investigation of heterogeneity: the planned investigations were not possible due to the limited number of studies available for each analysis. We were unable to investigate the effect of duration of follow‐up due to substantial variation in length and reporting. | |||||||
| Conclusions: Given the insufficient evidence to evaluate the diagnostic value in MCI of CSF t‐tau, CSF p‐tau, CSF t‐tau/ABeta ratio and CSF p‐tau/ABeta ratio for Alzheimer's disease dementia and other forms of dementias examined in this review, particular attention should be paid to the risk of misdiagnosis and overdiagnosis of dementia (and therefore overtreatment) in clinical practice. Future studies with more uniform approaches to thresholds, analysis and study conduct may provide a more homogenous estimate than the one that has been available from the included studies we have identified. | |||||||
| 1Meta‐analytic estimate of sensitivity derived from the HSROC model at a fixed value of specificity. Summary estimates of sensitivity and specificity were not computed because the studies that contributed to the estimation of the summary ROC curve used different thresholds. 2The median percentage converting was calculated using all the studies that reported 'conversion from MCI to Alzheimers' disease dementia' (Table 2) | |||||||
| Conversion from MCI to Alzheimer’s disease dementia | |||||||
| Study | Participants n/N (included in analysis) | Index test (number and % of positive tests) | Threshold (test abnormal) (prespecified Yes/No) | Number of converters (%) FP and FN | Test accuracy at study level | Duration of follow‐up | |
| Sensitivity | Specificity | ||||||
| *Balasa 2014 | 51/51 | CSF ABeta42/p‐tau ratio 25/51 (49%) | < 6.43 (Yes) | 24/51 (47%) FP =1; FN =0 | 100% | 96% | 41 months for MCI‐AD; 30 months for MCI‐MCI |
| *Ewers 2012 | 130/130 | CSF t‐tau 65/130 (50%) | Not reported | 58/130 (45%) FP = 30; FN = 23 | 60.7% | 58.9% | 24 months |
| CSF p‐tau 67/130 (51.5%) | Not reported | 58/130 (45%) FP = 30; FN = 21 | 63.9% | 58.9% | |||
| *Leuzy 2015 | 33/33 | CSF t‐tau 15/33 (45%) | ˃ 400 pg/mL(Yes) | 12/33 (36%) FP = 7; FN = 4 | 67% | 67% | Not reported |
| CSF t‐tau/ABeta ratio 12/33 (36%) | < 1.14 (Yes) | 12/33 (36%) FP = 6; FN = 6 | 50% | 71% | |||
| Conversion from MCI to all dementias | |||||||
| *Eckerstrom 2015 | 73/73 | CSF p‐tau 15/73 (20.5%) | 73 pg/mL (No) | 27/73 (36.9%) FP = 3; FN = 15 | 75% | 92% | 43.1 ± 23 months MCI‐stable; 33.7 ± 24 months MCI converters |
| Study awaiting translation | |||||||
| Urakami 2004 | |||||||
| AD: Alzheimer's disease; FN: false negative; FP: false positive; MCI: mild cognitive impairment *Authors need to be contacted in order to obtain missing data/relevant information. Data presented are provisional. | |||||||
| Included studies, index test and test accuracy at study level for conversion from MCI to Alzheimer’s disease dementia | |||||||
| Study | Participants n/N (included in analysis) | Index test (number and % of positive tests) | Threshold (test abnormal) (prespecified Yes/No) | Number of converters (%) FP and FN | Test accuracy at study level | Duration of follow‐up | |
| Sensitivity | Specificity | ||||||
| Amlien 2013 | 49/39 | CSF t‐tau 9/39 (23%) | ≥ 300 ng/L for age younger than 50 years; ≥ 450 ng/L for age 50 to 69 years; ≥ 500 ng/L for age older than 70 years (Sjogren 2001) (Yes) | 9/39 (23%); FP = 4; FN = 4 | 56% | 87% | mean 2.6 ± 0.5 years (range 1.6 to 4 years) |
| Buchhave 2012* | 137/134 | CSF p‐tau/ABeta ratio 69/134 (51%) | ˂ 6.2 ng/L (No) | 72/134 (54%) FP = 6; FN = 9 | 88% | 90% | median: 9.2 years (range 4 to 12 years) |
| Fellgiebel 2007 | 16/16 | CSF p‐tau 12/16 (75%) | ≥ 50 pg/mL (No) | 4/16 (25%) FP = 8; FN = 0 | 100% | 33% | mean 19.6 ± 9.0 months |
| Hampel 2004 | 52/52 | CSF t‐tau 38/52 (73%) | ≥ 479 ng/L (No) | 29/52 (56%); FP = 12; FN = 3 | 90% | 48% | mean 8.4 ± 5.1 months (range 2 to 24 months) |
| Hansson 2006* | 137/134 | CSF t‐tau 38/134 (28%) | > 350 ng/L (No) | 57/134 (42%); FP = 9; FN = 28 | 51% | 88% | Total sample: median 5.2 years (range 4.0 to 6.8 years); MCI‐AD: median: 4.3 years (range 1.1 to 6.7 years) MCI‐other dementias: median 4.2 years (range 1.5 to 3 years) |
| CSF p‐tau 50/134 (37%) | ≥ 60 ng/L (No) | 57/134 (42%); FP = 11; FN = 18 | 68% | 86% | |||
| CSF p‐tau/ABeta ratio 74/134 (55%) | ˂ 6.5 ng/L (No) | 57/134 (42%); FP = 19; FN = 2 | 96% | 75% | |||
| Kester 2011 | 153/100 | CSF t‐tau 64/100 (64%) | > 356 pg/mL (Yes) | 42/100 (42%) FP = 29; FN = 7 | 83% | 50% | median 18 months (IQR 13 ‐ 24) |
| Koivunen 2008 | 15/14 | CSF p‐tau 9/14 (64%) | ≥ 70 pg/mL (Yes) | 5/14 (36%) FP = 7; FN = 3 | 40% | 22% | 2 years |
| CSF p‐tau/ABeta ratio 9/14 (64%) | ˂ 6.5 pg/mL (yes) | 5/14 (36%) FP = 6; FN = 1 | 80% | 33% | |||
| Monge‐Argiles 2011 | 37/37 | CSF t‐tau 16/37 (43%) | ≥ 77.5 pg/mL (No) | 11/37 (28%) FP = 8; FN = 3 | 73% | 69% | 6 months |
| CSF p‐tau 20/37 (54%) | ≥ 54.5 pg/mL (No) | 11/37 (28%) FP = 11; FN = 2 | 82% | 58% | |||
| CSF p‐tau/ABeta ratio 18/37 (49%) | 0.17 (No) | 11/37 (28%) FP = 9; FN = 2 | 82% | 66% | |||
| CSF t‐tau/ABeta ratio 23/37 (62%) | 0.18 (No) | 11/37 (28%) FP = 13; FN = 1 | 91% | 50% | |||
| Palmqvist 2013 | 133/133 | CSF t‐tau 65/133 (49%) | > 87 pg/mL (No) | 52/133 (39%) FP = 23; FN = 10 | 81% | 72% | mean 5.9 years (range 3.2 to 8.8 years) |
| CSF p‐tau 46/133 (34%) | > 39 pg/mL (No) | 52/133 (39%) FP = 11; FN = 17 | 67% | 86% | |||
| Parnetti 2012 | 90/90 | CSF p‐tau/ABeta ratio 29/90 (32%) | 1074.0 (No) | 32/90 (35%) FP = 3; FN = 6 | 81% | 95% | maximum: 4 years; mean 3.40 ± 1.01 years |
| Visser 2009 | 168/158 | CSF p‐tau 108/158 (68%) | ≥ 51 pg/mL (used in clinical practice) (No) | 35/158 (22%) FP = 77; FN = 4 | 88% | 37% | range 1 to 3 for MCI |
| CSF p‐tau 45/158 (28%) | ≥ 85pg/mL (> 90th percentile of controls after correction for age) (No) | 35/158 (22%) FP = 25; FN = 15 | 57% | 80% | |||
| CSF p‐tau/ABeta ratio 77/158 (49%) | ˂ 9.92 (< 10th percentile of reference group after correction for age) (No) | 35/158 (22%); FP = 49; FN = 7 | 80% | 60% | |||
| Vos 2013 | 231/214 | CSF t‐tau 93/214 (43%) | > 450 pg/mL for age less than 70 years; > 500 pg/mL for age older than 70 years (Yes) | 91/214 (42%) FP = 28; FN = 26 | 71% | 77% | mean 2.5 ± 1.0 years |
| CSF t‐tau/ABeta ratio 147/214 (69%) | ABeta1–42/(240 1 [1.18 3 t‐tau]) ˂ 1.0 (Yes) | 91/214 (42%) FP = 60; FN = 4 | 96% | 51% | |||
| AD: Alzheimer's disease; FN: false negative; FP: false positive; MCI: mild cognitive impairment *Studies involved the same participants. Only Hansson 2006 is included in the meta‐analysis | |||||||
| Included studies, index test and test accuracy at study level for conversion from MCI to All dementias | |||||||
| Study | Participants n/N (included in analysis) | Index test (Number and % of positive tests) | Threshold (test abnormal) (pre‐specified Yes / No) | Number of converters (%) FP and FN | Test accuracy at study level | Duration of follow‐up | |
| Sensitivity | Specificity | ||||||
| Eckerstrom 2010 | 42/42 | CSF t‐tau 15/42 (36%) | ≥ 500 ng/L (No) | 21/42 (50%) FP = 1 FN = 7 | 67% | 95% | Total sample: 19.6 ± 9.0 months; MCI‐MCI: 19.5 ± 9.3 months; MCI‐progressive: 17.6 ± 8.8 months (4/8 MCI‐AD: 23.7 ± 2.0 months) |
| Galluzzi 2010 | 90/64 | CSF t‐tau 24/64 (37.5%) | > 450 pg/mL for subjects with an age range between 51 and 70 determined; > 500 pg/mL for subjects with an age range between 71 and 93 (Yes) | 34/64 (53%) FP = 5 FN = 15 | 56% | 83% | Total sample: 8.4 ± 5.1 months (range 2 to 24 months); follow‐up interval for converters was 9.6 ± 5.4, and for non‐converters 7.0 ± 4.3 months |
| Hansson 2006 | 137/134 | CSF t‐tau 38/134 (28%) | > 350 pg/mL (No) | 78/134 (58%) FP = 5 FN = 45 | 42% | 91% | Total sample: median 5.2 years (range 4.0 to 6.8); MCI‐AD: median: 4.3 years (range 1.1 to 6.7); MCI‐other dementias: median 4.2 (1.5 to 6.3) |
| Herukka 2007 | 79/79 | CSF t‐tau 43/79 (54%) | > 400 pg/mL (Yes) | 33/79 (42%) FP = 17 FN = 7 | 79% | 63% | Mean 3.52 ± 1.95 years in MCI converters; mean 4.56 ± 3.09 years in MCI‐stable |
| AD: Alzheimer's disease; FN: false positive; FP: false negative; MCI: mild cognitive impairment | |||||||
| Test | No. of studies | No. of participants |
| 1 CSF t‐tau conversion to AD dementia Show forest plot | 7 | 709 |
| 2 CSF p‐tau conversion to AD dementia Show forest plot | 6 | 492 |
| 3 CSF p‐tau/ABeta ratio to AD dementia Show forest plot | 5 | 433 |
| 4 CSF t‐tau conversion to All dementias Show forest plot | 4 | 319 |

