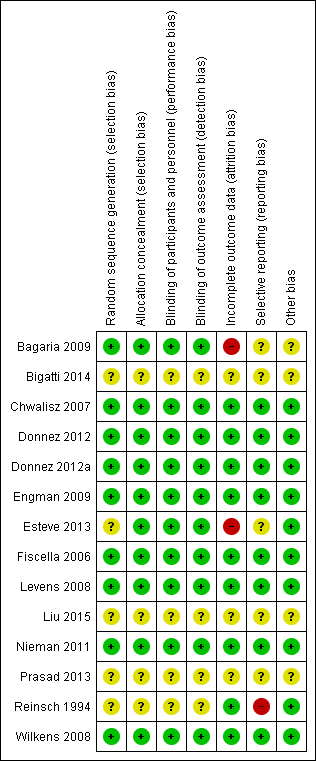
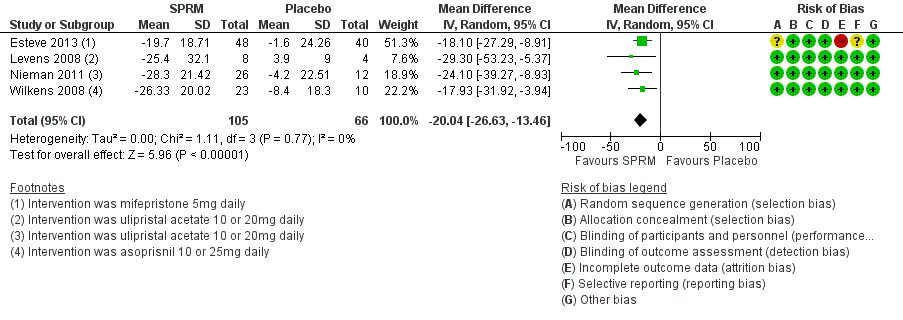
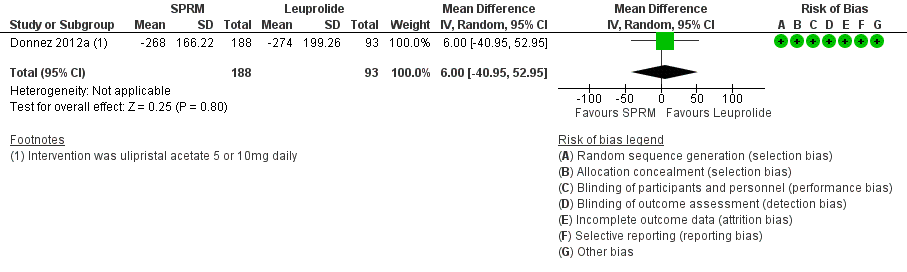
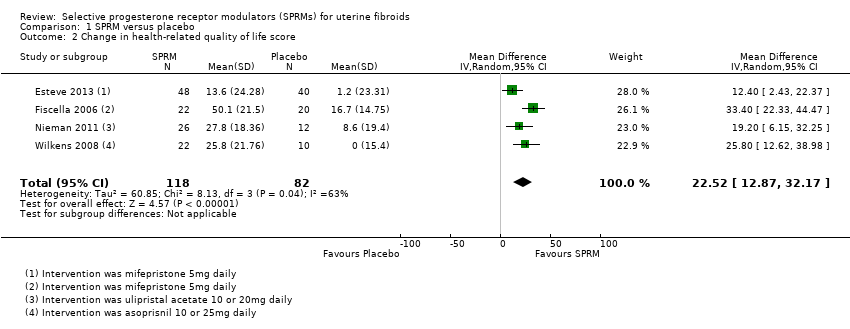
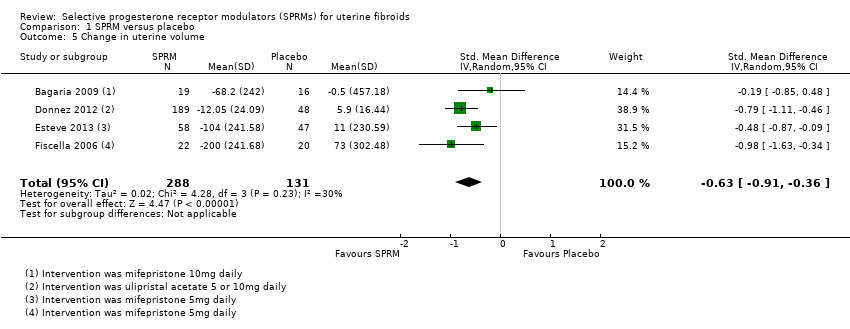
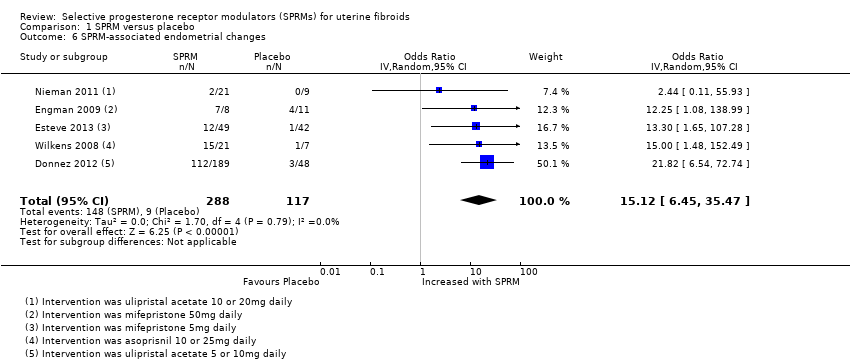
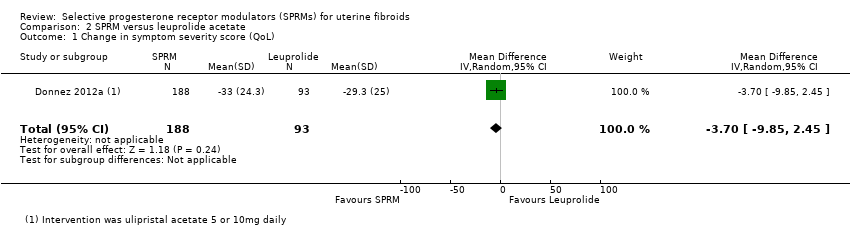
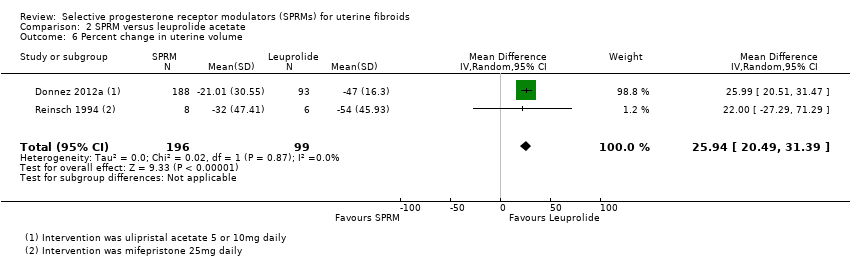
Contenido relacionado
Revisiones y protocolos relacionados
Mario Tristan, Leonardo J Orozco, Antonia Steed, Anggie Ramirez-Morera, Peter Stone | 15 agosto 2012
Jing Fu, Hao Song, Min Zhou, Huili Zhu, Yuhe Wang, Hengxi Chen, Wei Huang | 25 julio 2017
Linyu Deng, Taixiang Wu, Xiao Y Chen, Lingxia Xie, Jinrong Yang | 17 octubre 2012
Rafael M Moroni, Wellington P Martins, Rui A Ferriani, Carolina S Vieira, Carolina O Nastri, Francisco José Candido Dos Reis, Luiz Gustavo Brito | 20 marzo 2015
Anne Lethaby, Lucian Puscasiu, Beverley Vollenhoven | 15 noviembre 2017
Jian Ping Liu, Hong Yang, Yun Xia, Francesco Cardini | 30 abril 2013
Ussanee S Sangkomkamhang, Pisake Lumbiganon, Porjai Pattanittum | 23 noviembre 2020
Huan Song, DongHao Lu, Kate Navaratnam, Gang Shi | 23 octubre 2013
Lin ‐qiu Ke, Kun Yang, Chun‐Mei Li, Jing Li | 8 julio 2009
Hunain Shiwani, Naomi S Clement, Jane P Daniels, William Atiomo | 2 mayo 2024
Respuestas clínicas Cochrane
Jane Burch, Mohammed R Houda | 19 diciembre 2017