cualquierIntervención precoz (movilización o ejercicio activo) para pacientes adultos en estado crítico en la unidad de cuidados intensivos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010754.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 27 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Atención crítica y de emergencia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: Katherine A Doiron (KAD), Tammy C Hoffmann (TCH)
Co‐ordinating the review: KAD
Undertaking manual searches: KAD
Screening search results: KAD, research assistant
Organizing retrieval of papers: KAD
Screening retrieved papers against inclusion criteria: KAD, research assistant
Appraising quality of papers: KAD, Elaine M Beller (EMB), TCH
Abstracting data from papers: KAD, EMB, research assistant
Writing to authors of papers for additional information: KAD
Providing additional data about papers: KAD
Obtaining and screening data on unpublished studies: KAD
Providing data management for the review: KAD, EMB
Entering data into Review Manager 5 (RevMan 5) (RevMan 2014): KAD
Entering RevMan 5 statistical data: EMB, KAD
Performing other statistical analysis not using RevMan 5: not applicable
Interpreting data: KAD, EMB
Making statistical inferences: KAD, EMB
Writing the review: KAD, TCH, EMB
Securing funding for the review: no funding received
Performing previous work that was the foundation of the present study: none
Serving as guarantor for the review (one review author): KAD
Taking responsibility for reading and checking the review before submission: EMB
Sources of support
Internal sources
-
No sources of support supplied, Other.
External sources
-
National Health and Medical Research Council (NHMRC), Australia.
Elaine M Beller's work on this review was supported by an Australia Fellowship Grant from the NHMRC, Australia, to the Centre for Evidence‐Based Practice, Bond University.
Declarations of interest
Katherine A Doiron: none known
Tammy C Hoffmann: none known
Elaine M Beller: work on this review was supported by an Australia Fellowship Grant from the National Health and Medical Research Council (NHMRC), Australia, to the Centre for Evidence‐Based Practice, Bond University.
Acknowledgements
We would like to thank Bronagh Blackwood (content editor), Vibeke E Horstmann (statistical editor), Linda Denehy, Sue Berney, Tom J Overend, Andrea Lemos (peer reviewers) and Odie Geiger (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would like to thank Matthew Zacharias (content editor), Cathal Walsh (statistical editor), Andrea Lemos, Andrezza L Bezzera, Linda Denehy and Sue Berney (peer reviewers) for their help and editorial advice during the preparation of the Cochrane protocol (Doiron 2013).
We would like to thank Karen Hovhannisyan, former Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care (ACE), and Sarah Thorning, previous Trials Search Co‐ordinator, Cochrane Acute Respiratory Infections, for their assistance in identifying search terms for this review, and Justin Clark, Information Specialist at the Centre for Research in Evidence‐Based Practice for running search updates. We also thank Leanne McGregor and Rebecca Sims (research assistants at Centre for Research in Evidence‐Based Practice, Bond University) for their assistance with screening search results and initial data extraction.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 27 | Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit | Review | Katherine A Doiron, Tammy C Hoffmann, Elaine M Beller | |
| 2013 Oct 04 | Early intervention (mobilization or active exercise) for critically ill patients in the intensive care unit | Protocol | Katherine A Doiron, Tammy Hoffmann, Elaine M Beller | |
Differences between protocol and review
We made the following changes to the published protocol (Doiron 2013).
-
We changed the title from 'Early intervention (mobilization or active exercise) for critically ill patients in the intensive care unit' to 'Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit' because we only assessed studies investigating the adult population in this clinical setting.
-
We changed 'participants' to 'adults' in the objective section in the Abstract and the Objectives section in the review.
-
We expanded the initial search strategy because the first search (Appendix 8), did not return an expected study.
-
We removed the Acute Physiology and Chronic Health Evaluation (APACHE) score and SOFA score from the examples listed for the outcome Health‐related quality of life or well‐being (see Types of outcome measures and Data extraction and management), because they do not measure quality of life.
-
We clarified the definition of our primary outcome.
-
We removed length of stay in the ICU and hospital from the 'Summary of findings' table as they are not primary outcomes in this review (see summary of findings Table for the main comparison).
-
We added the outcome 'duration of mechanical ventilation' to Table 8 (Other outcomes not specified in this review) because most included studies reported this outcome and we felt this to be of interest to clinicians.
-
We intended to conduct intention‐to‐treat (ITT) analysis and impute missing standard deviations but this was not required.
-
If we had done a meta‐analysis, we planned to use the I2 statistic (Higgins 2003) to measure heterogeneity in the participants, interventions and outcomes. As there were insufficient studies to do a meta‐analysis, we reported possible sources of heterogeneity descriptively.
-
We planned to investigate possible sources of heterogeneity such as age group, cause of ICU stay, length of mechanical ventilation, comorbidities such as diabetes and use of corticosteroids using subgroup analyses. However, as there were insufficient studies identified, we reported these factors descriptively.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
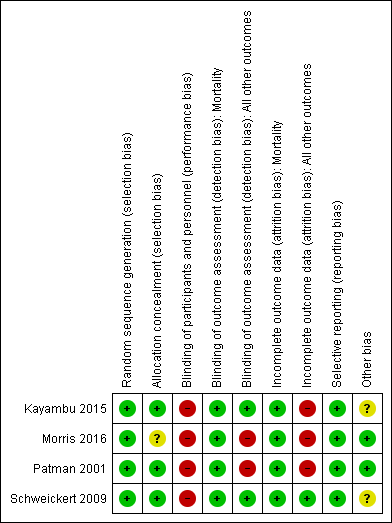
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
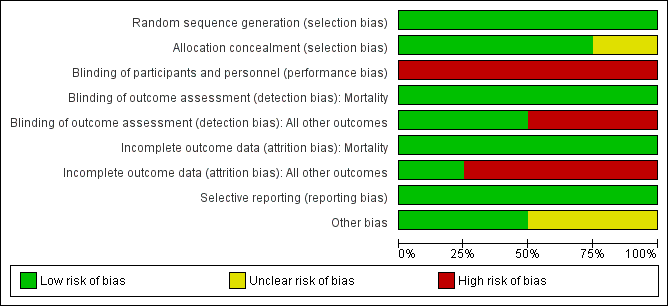
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Study flow diagram
| Early intervention (mobilization or active exercise) versus usual care for critically ill adults | ||||||
| Patient or population: critically ill, mechanically ventilated adults Control: usual care (defined as no mobilization/active exercise while in ICU, or mobilization/active exercise given later than the intervention group) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early intervention (mobilization or active exercise) | |||||
| Physical function ‐ return to independent functional status at hospital discharge | Study population | RR 1.71 | 104 | ⊕⊕⊝⊝ | ||
| 345 per 1000 | 591 per 1000 | |||||
| Physical function ‐ independent ADLs total at ICU discharge | Study population | 104 | ⊕⊕⊝⊝ | |||
| Median 0 (IQR 0 to 5) | Median 3 (IQR 0 to 5) | |||||
| Physical function ‐ Independent ADL total at hospital discharge | Study population | 104 | ⊕⊕⊝⊝ | |||
| Median 4 (IQR 0 to 6) | Median 6 (IQR 0 to 6) | |||||
| Physical function Barthel score (0 ‐ 100) | Study population | 104 (1 study) | ⊕⊕⊝⊝ | |||
| Median 55 (IQR 0 to 85) | Median 75 (IQR 7.5 to 95) | |||||
| Physical performance | Study population | 42 | ⊕⊕⊝⊝ | |||
| Mean score 55.0 | Mean score 61.1 (46.2 to 76.0) MD 6.10 (‐11.85 to 24.05) | |||||
| Physical performance Physical Function ICU Test score at ICU discharge | Study population | 42 (1 study) | ⊕⊕⊝⊝ | |||
| Mean score 5.4 (4.7 to 6.1) | Mean score 5.6 (4.7 to 6.5) MD 0.20 (‐0.98 to 1.38) | |||||
| Physical performance Short Physical Performance Battery score at ICU discharge | Study population | 184 (1 study) | ⊕⊕⊝⊝ | |||
| Mean score 1.9 (1.3 to 2.4) | Mean score 1.6 (1.0 to 2.2) MD ‐0.30 (‐1.10 to 0.50) | |||||
| Physical performance Short Physical Performance Battery score at hospital discharge | Study population | 204 (1 study) | ⊕⊕⊝⊝ | |||
| Mean score 4.7 (4.0 to 5.4) | Mean score 4.7 (4.0 to 5.4) MD 0 (‐1.00 to 0.90) | |||||
| Adverse events Proportion of participants with one or more events, or proportion of intervention sessions where an event occurred (falls, accidental dislodgement of attachments, haemodynamic instability, oxygen desaturation or any other adverse events defined by study authors) | One study reported that in the intervention group 1/49 (2%) experienced oxygen desaturation < 80% and 1/49 (2%) had accidental dislodgement of the radial catheter. This study also found cessation of therapy due to patient instability occurred in 19/498 (4%) of the intervention sessions. In another study 5/101 (5%) of the intervention group and 5/109 (4.6%) of the control group had postoperative pulmonary complications. These were deemed to be unrelated to intervention. A third study found 1/150 in the intervention group had an episode of asymptomatic bradycardia, but completed the exercise session. The fourth study reported no adverse events. | 690 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one point for high risk of bias. Risk of bias was high for blinding of participants and personnel (performance bias). | ||||||
| Item | ||||
| Brief name | Standardized rehabilitation therapy | Early physical rehabilitation in ICU | Physiotherapy | Early physical and occupational therapy in mechanically ventilated, critically ill patients |
| What and Procedures) | 3 exercise types:
Both the physiotherapy and resistance training targeted lower extremity functional tasks and ADLs. See trial protocol (supplement to article) for more details. | Specific equipment not reported. Procedures mentioned include: arm or leg ergometry; passive, active and resisted ROM exercises; bed mobility activities; sitting and standing balance exercises; transfer training; pre‐gait exercises; ambulation with assistance; electrical muscle stimulation; and Tilt table | Specific equipment was not reported. Procedures mentioned include: positioning; manual hyperinflation; endotracheal suctioning; thoracic expansion exercises; and upper limb exercises | Specific equipment was not reported. Procedures mentioned included: passive, active‐assisted and active ROM exercises; bed mobility activities; sitting balance exercises; ADL exercises to promote increased independence with functional tasks; transfer training; pre‐gait exercises; and ambulation |
| Who provided | Physiotherapist, ICU nurse, and nursing assistant | ICU research physiotherapist | Team of physiotherapists under the guidance of the principal investigator | An occupational therapist and a physical therapist |
| Where | One medical ICU, Medical Centre, North Carolina, USA | Quaternary‐level general ICU, Australia | Surgical ICU, Perth, Australia | Two medical ICUs: Chicago, USA and Iowa City, USA |
| When and how much | 3 separate sessions every day of hospitalization for 7 days per week, from enrolment through to hospital discharge | 30 min 1‐2 times/day within 48 h of diagnosis of sepsis until discharge from the ICU | 1‐2 interventions during the first 24 h of mechanical ventilation | Interventions were synchronized with daily interruption of sedation. Each morning within 48‐72 h of intubation until return to previous level of function or discharge from the ICU |
| Tailoring and progression | The participant's level of consciousness determined whether they were considered suitable to receive the physiotherapy or progressive resistance exercise, as did their ability to complete the exercises. When participants were unconscious, the 3 sessions consisted of passive ROM. As consciousness was gained, physiotherapy and progressive resistance exercise was commenced. Participants did not need to be free of mechanical ventilation to begin any of the exercise sessions | Interventions were tailored, planned, administered and progressed at the discretion of the physiotherapist, participant acuity of illness and level of co‐operation | Not reported | Progression of interventions depended on participant tolerance and stability. |
| Modification of intervention throughout trial | Not reported | Not reported | Not reported | Not reported |
| Fidelity (strategies to improve) | Not reported | Not reported | Not reported | Not reported |
| Fidelity (extent) | The mean percentage of study days that participants received therapy was 87.1% (SD 18.4%) for passive ROM; 54.6% (SD 27.2%) for physiotherapy; and 35.7% (SD 23%) for progressive resistive exercise. The median days of delivery of therapy per participant was 8.0 (IQR, 5.0‐14.0) for passive ROM, 5.0 (IQR, 3.0‐8.0) for physiotherapy, and 3.0 (IQR, 1.0‐5.0) for progressive resistance exercise | All participants adhered and remained enrolled for an average of 11.4 days. No further details. | Not reported | Therapy occurred on 87% of the days on the study for all participants in the intervention group and on 95% of the days on the study for 22/55 (40%) of participants in the control group |
| *See Hoffmann 2014 for TIDieR criteria ADLs: activities of daily living; ICU: intensive care unit; ROM: range of motion | ||||
| n (%) in intervention group | n (%) in control group | Risk ratio (95% CI) | P value | Reference of studies | |
| ICU mortality | 3/26 (12%) | 1/24 (4%) | RR 2.77 (0.31 to 24.85) | 0.36 | |
| ICU mortality | 0/101 (0%) | 3/109 (2.8%) | RR 0.16 (0.008 to 3.03) | 0.22 | |
| Hospital mortality | 9/49 (18%) | 14/55 (25%) | RR 0.72 (0.34 to 1.52) | 0.53 | |
| 90‐day mortality | 8/26 (31%) | 2/24 (8%) | RR 3.69 (0.87 to 15.69) | 0.08 | |
| 6‐month hospital‐free survival | 73/150 (48.7%) | 67/150 (44.7%) | RR 1.09 (0.86 to 1.39) | 0.69 | |
| CI: confidence interval; ICU: intensive care unit; n: number; RR: risk ratio | |||||
| Outcome measure | Intervention group | Control group | Effect size (95% CI) where possible | P value | Reference of studies |
| Return to independent functional status at hospital discharge ‐ n (%) in each group | 29/49 (59%) | 19/55 (35%) | RR 1.71 (1.11 to 2.64) | 0.01 | |
| Independent ADL total at ICU discharge ‐ median (IQR) | 3 (0‐5) | 0 (0‐5) | 0.15 | ||
| Independent ADL total at hospital discharge ‐ median (IQR) | 6 (0‐6) | 4 (0‐6) | 0.06 | ||
| Acute Care Index of Function (ACIF) at ICU discharge ‐ mean (SD) | 61.1 (33.1) | 55 (24.4) | MD 6.10 (‐11.85 to 24.05) | 0.45 | |
| Physical Function ICU Test (PFIT) at ICU discharge ‐ mean (SD) | 5.6 (2.1) | 5.4 (1.7) | MD 0.20 (‐0.98 to 1.38) | 0.61 | |
| Short Physical Performance Battery at ICU discharge ‐ mean (SD) | 1.6 (3.1) | 1.9 (2.8) | MD ‐0.3 (‐1.1 to 0.5) | 0.46 | |
| Time from intubation to out of bed (days) ‐ median (IQR) | 1.7 (1.1 to 3.0) | 6.6 (4.2‐8.3) | < 0.0001 | ||
| Time from intubation to standing (days) ‐ median (IQR) | 3.2 (1.5 to 5.6) | 6.0 (4.5‐8.9) | < 0.0001 | ||
| Time from intubation to marching in place (days) ‐ median (IQR) | 3.3 (1.6 to 5.8) | 6.2 (4.6‐9.6) | < 0.0001 | ||
| Time from intubation to transferring to a chair (days) ‐ median (IQR) | 3.1 1.8 to 4.5) | 6.2 (4.5‐8.4) | < 0.0001 | ||
| Time from intubation to walking (days) ‐ median (IQR) | 3.8 (1.9 to 5.8) | 7.3 (4.9‐9.6) | < 0.0001 | ||
| Barthel Index score at hospital discharge (score 0‐100) ‐ median (IQR) | 75 (7.5 to 95) | 55 (0‐85) | 0.05 | ||
| Greatest walking distance (metres) at hospital discharge ‐ median (IQR) (metres) | 33.4 (0 to 91.4) | 0 (0‐30.4) | 0.004 | ||
| ADL: activities of daily living; CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; n: number; RR: risk ratio; SD: standard deviation | |||||
| Outcome measure | Intervention group | Control group | Mean difference | P value | Reference of studies |
| Mean (SD) LOS (h) in ICU ‐ mean (SD) | 42.7 (42.4) | 36.7 (26.8) | 6.00 (‐3.58 to 15.58) | 0.56 | |
| Median (IQR) LOS (days) in ICU ‐ median (IQR) | 12.0 (4‐45) | 8.5 (3‐36) | 0.43 | ||
| 7.5 (4‐14) | 8.0 (4‐13) | 0.68 | |||
| 5.9 (4.5‐13.2) | 7.9 (6.1‐12.9) | 0.08 | |||
| Mean (SD) LOS (days) in hospital ‐ mean (SD) | 9.2 (4.5) | 9.6 (6.7) | ‐0.40 (‐1.97 to 1.17) | 0.25 | |
| Median (IQR) LOS (days)in hospital ‐ median (IQR) | 41 (9‐158) | 45 (14‐308) | 0.80 | ||
| 10.0 (6‐17) | 10.0 (7‐16) | 0.41 | |||
| 13.5 (8.0‐23.1) | 12.9 (8.9‐19.8) | 0.93 | |||
| CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; SD: standard deviation | |||||
| Intervention group | Control group | Effect size (95% CI) where possible | P value | Reference of studies | |
| Muscle strength (MRC score, 0‐60) at ICU discharge ‐ mean (SD) | 51.9 (10.5) | 47.3 (13.6) | MD 4.60 (‐3.11 to 12.31) | 0.24 | |
| Hand‐grip strength (kg) at ICU discharge ‐ mean (SD) | 20.0 | 20.9 | MD ‐0.8 (‐4.0 to 2.3) | 0.60 | |
| Muscle strength (MRC score, 0‐60) at hospital discharge ‐ median (IQR) | 52 (25‐58) | 48 (0‐58) | 0.38 | ||
| Hand‐grip strength (kg) at hospital discharge ‐ median (IQR) | 39 (10‐58) | 35 (0‐57) | 0.67 | ||
| Hand‐grip strength (kg) at hospital discharge ‐ mean (SD) | 22.6 (10.4) | 24.3 (16.3) | MD ‐1.7 (‐4.6 to 1.2) | 0.25 | |
| ICU‐acquired paresis at hospital discharge ‐ n (%) | 15/49 (31%) | 27/55 (49%) | RR 0.62 (0.38 to 1.03) | 0.09 | |
| CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; MRC: medical research council; SD: standard deviation | |||||
| Outcome measure at 6 months' follow‐up | Mean (SD) of intervention group | Mean (SD) of control group | Mean difference (95% CI) | P value | Reference of studies |
| SF‐36 physical function | 81.8 (22.2) | 60.0 (29.4) | 21.8 (0.81 to 42.79) | 0.04 | |
| 55.9 (27.3) | 43.6 (27.7) | 12.2 (3.9 to 20.7) | 0.001 | ||
| SF‐36 role physical | 61.4 (43.8) | 17.1 (34.4) | 44.3 (14.79 to 73.81) | 0.005 | |
| SF‐36 bodily pain | 70.9 (20.7) | 64.7 (22.5) | 6.20 (‐10.78 to 23.18) | 0.46 | |
| SF‐36 general health | 50.5 (11.9) | 41.8 (11.3) | 8.70 (‐0.24 to 17.64) | 0.06 | |
| SF‐36 vitality | 45.9 (12.0) | 39.2 (7.7) | 6.70 (‐0.22 to 13.62) | 0.07 | |
| SF‐36 social functioning | 71.6 (37.1) | 73.7 (37.2) | ‐2.10 (‐30.94 to 26.74) | 0.88 | |
| SF‐36 role emotional | 63.6 (40.7) | 33.3 (45.8) | 30.30 (‐3.88 to 64.48) | 0.08 | |
| SF‐36 mental health | 38.6 (11.5) | 37.3 (7.4) | 1.30 (‐5.75 to 8.35) | 0.71 | |
| 48.8 | 46.4 | 2.4 (‐1.2 to 6.0) | 0.19 | ||
| CI: confidence interval; SD: standard deviation; SF‐36: Short Form 36 | |||||
| Intervention group | Control group | P value | Reference of studies | |
| ICU (days) with delirium ‐ median (IQR) | 2.0 (0.0‐6.0) | 4.0 (2.0‐7.0) | 0.03 | |
| 0 (0 ‐12.5) | 0 (0‐9.1) | 0.71 | ||
| Hospital (days) with delirium ‐ median (IQR) | 2.0 (0.0‐6.0) | 4.0 (2.0‐8.0) | 0.02 | |
| ICU: intensive care unit; IQR: interquartile range | ||||
| Intervention group | Control group | Mean difference (95% CI) where possible | P value | Reference of studies | |
| Duration (h) of mechanical ventilation ‐ mean (SD) | 13 (4.8) | 12.7 (4.7) | 0.20 (‐1.1 to 1.65) | 0.85 | |
| Duration (days) of mechanical ventilation ‐ median (IQR) | 8.0 (4‐64) | 7.0 (2‐30) | 0.22 | ||
| 3.4 (2.3‐7.3) | 6.1 (4.0‐9.6) | 0.02 | |||
| Abbreviations: CI: confidence interval. IQR: interquartile range. SD: standard deviation. | |||||

