用于预防激光小梁成形术后眼压暂时升高的围手术期药物
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: vehicle‐controlled, double‐masked, multicenter, parallel‐group RCT Country: US Number randomized: Total: 232 Per group: brimonidine/brimonidine = 62, brimonidine/vehicle = 57, vehicle/brimonidine = 53, vehicle/vehicle = 60 Exclusions after randomization: none reported Number analyzed: Total: 232 Per group: brimonidine/brimonidine = 62, brimonidine/vehicle = 57, vehicle/brimonidine = 53, vehicle/vehicle = 60 Unit of analysis (participants vs eyes): eye Losses to follow‐up: 10 eyes had unacceptably high IOPs within the first 3 hours after surgery (brimonidine/brimonidine = 1, brimonidine/vehicle = 1, vehicle/vehicle = 8). These participants were released from the study and treated at the discretion of the investigator. How was missing data handled?: data from the released participants included in the analysis Reported power calculation: power calculation not reported but the authors stated, "this study had an 80% success rate in detecting a difference between treatments in the incidence of IOP elevation of approximately 21%." Unusual study design (any issues with study design)?: no | |
| Participants | Age (mean ± SD; years): brimonidine/brimonidine = 69.3 ± 1.4, brimonidine/vehicle = 63.9 ± 1.8, vehicle/brimonidine = 66.8 ± 1.5, vehicle/vehicle = 66.9 ± 1.4 Females: brimonidine/brimonidine = 47%, brimonidine/vehicle = 58%, vehicle/brimonidine = 58%, vehicle/vehicle = 53% Inclusion criteria: people with uncontrolled glaucoma whose IOPs were inadequately controlled despite maximal tolerated medication, and in whom 360° ALP was indicated Exclusion criteria: people with active ocular infection or inflammation, contraindications to alpha‐agonist treatment or hyposensitivity to alpha‐agonists or other components of the formulation, women of childbearing potential or who were nursing, people taking topical or systemic alpha‐agonists 2 weeks prior to study entry or who took systemic clonidine 4 weeks before study entry Equivalence of baseline characteristics: yes, "No significant differences were noted between treatments or sites in demographic data." | |
| Interventions | Intervention 1: brimonidine 0.5%, 30 to 45 min before and immediately after ALT Intervention 2: brimonidine 0.5%, 30 to 45 min before but vehicle immediately after ALT Intervention 3: vehicle, 30 to 45 min before but brimonidine 0.5% immediately after ALT Intervention 4: vehicle, 30 to 45 min before and immediately after ALT Length of follow‐up: Planned: 1, 2, and 3 hours, 1 to 2, and 4 to 6 weeks after ALT Actual: 1, 2, and 3 hours, 1 to 2, and 4 to 6 weeks after ALT | |
| Outcomes | Primary outcome: mean IOP Secondary outcomes: mean IOP lowering in contralateral eye, mean systolic BP after treatment, mean heart rate after treatment Adverse events reported: yes Intervals at which outcomes assessed: hourly for 3 hours; 1 to 2 weeks, and 4 to 6 weeks | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: 3 authors were employees of Allergan Pharmaceuticals, who make Alphagan, a brimonidine tartrate ophthalmic solution Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned to one of four treatment groups in a randomized, double‐masked fashion..." States randomization was done but not the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | "Patients were assigned to one of four treatment groups in a randomized, double‐masked fashion..." Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | No information provided about outcome assessors, but lid retraction and conjunctival blanching is a known adverse effect of brimonidine, which would have been obvious to a clinician at the time of IOP measurements at post‐ALT checkpoints as to whether the vehicle or study medication was used. |
| Incomplete outcome data (attrition bias) | Low risk | Participants with "unacceptably high IOPs" at 3 hours were released from further study participation but data from these participants was still included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | 3 of the authors were employees of Allergan, the company that manufactures brimonidine. |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 56 eyes of 41 participants Per group: brimonidine = 29 eyes, apraclonidine = 27 eyes Exclusions after randomization: 10/15 participants who required bilateral ALT had randomization to receive the identical medication for each eye, and for these participants, only the first eye was included in the study. Number analyzed: Total: 46 eyes of 41 participants Per group: brimonidine = 23 eyes, apraclonidine = 23 eyes Unit of analysis (participants vs eyes): eyes Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: yes, power of 80% Unusual study design (any issues with study design)?: the unit of measurement was the eye and not the participant. The second eye was treated 2 to 6 weeks after the first. | |
| Participants | Age (mean; years): brimonidine = 69.9, apraclonidine = 62.4 Females: brimonidine = 48%; apraclonidine = 30% Inclusion criteria: aged ≥ 21 years with diagnosis of POAG, pigmentary glaucoma, pseudoexfoliation syndrome, or ocular hypertension. Participants had IOP too high for their level of optic nerve cupping, no glaucoma medications were used 12 to 24 hours before ALT Exclusion criteria: people with active ocular inflammation, contraindications to treatment with alpha‐agonists, or known hypersensitivities to alpha‐agonists, women of childbearing potential, current use of either of the study medications, previous experience with ALT Equivalence of baseline characteristics: no statistical difference in the baseline IOP levels, number of laser applications, or energy level used in either group. Statistically significant difference in mean age (P = 0.008) of each group and the distribution of gender in each group; however, these were likely not clinically significant differences. | |
| Interventions | Intervention 1: brimonidine 0.2%, 30 to 45 min before and immediately after 360° ALT Intervention 2: apraclonidine 1.0%, 30 to 45 min before and immediately after 360° ALT Length of follow‐up: Planned: 4 hours after surgery Actual: 4 hours after surgery | |
| Outcomes | Primary outcome: maximum IOP change (from baseline to the highest postoperative IOP) Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, and 4 hours after ALT | |
| Notes | Trial registration: not reported Funding sources: research grant provided by Allergan, Inc Disclosures of interest: "The authors have no proprietary interest in the products described in this study." Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random‐number generator assigned patients to a treatment group before ALT." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "Both patient and physician were masked as to which agent the patient received" "…and a technician would give the appropriate medication without the physician or patients' knowledge." |
| Masking of outcome assessment (detection bias) | Unclear risk | Details about outcome assessors not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Some participants' randomization caused them to receive the same medication for each eye, so only the first eye was included in the study to account for the intra‐dependability of eyes and to prevent skewed results. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Study reported a research grant provided by Allergan, Inc, which makes the brimonidine 0.2% ophthalmic solution. |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 72 Per group: 2 doses apraclonidine = 24, 1 dose apraclonidine before surgery = 24, 1 dose apraclonidine after surgery = 24 Exclusions after randomization: none Number analyzed: Total: 72 Per group: 2 doses apraclonidine = 24, 1 dose apraclonidine before surgery = 24, 1 dose apraclonidine after surgery = 24 Unit of analysis (participants vs eyes): participants Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: potentially not double‐masked, as there was no mention of a vehicle drop given, so participant could have known which group he or she was in based on when the drops were given. | |
| Participants | Age (mean ± SD; years): 2 doses apraclonidine = 70.4 ± 10.6, 1 dose apraclonidine before surgery = 69.3 ± 10.9, 1 dose after surgery = 69.2 ± 9.6 Females: 2 doses apraclonidine = 75%, 1 dose apraclonidine before surgery = 75%, 1 dose apraclonidine after surgery = 54% Inclusion criteria: POAG, including an elevated IOP in the setting of either characteristic glaucomatous optic nerve damage on stereoscopic biomicroscopic exam or glaucomatous visual field defects on Humphrey automated field testing, or both Exclusion criteria: previous treatment over 360° of the angle, unable to return for the 24‐hour IOP check Equivalence of baseline characteristics: yes, no significant difference between the groups in the mean power setting used or in the mean number of burns, or the mean IOP at baseline and between‐group differences were not significant for race, gender, vision, age, and number of medications | |
| Interventions | Intervention 1: 2 doses apraclonidine 1.0%, 15 min before and immediately after the laser procedure Intervention 2: 1 dose apraclonidine 1.0%, 15 min before the laser procedure Intervention 3: 1 dose apraclonidine 1.0%, immediately after the laser procedure Length of follow‐up: Planned: 24 hours Actual: 24 hours | |
| Outcomes | Primary outcomes: IOP at 1 hour and 24 hours after surgery Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 24 hours | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: 1 September to 30 November 1994 Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned with the use of a random number table to one of three treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Personnel appear to be masked, "…a second investigator (D.H.S.), who was unaware of the group, assignment, performed the laser treatment…"; however, the report did not mention a vehicle drop given, so the participant could have known to which group (before or after surgery or both) they were assigned. |
| Masking of outcome assessment (detection bias) | Low risk | "…a third investigator (B.M.), who was also unaware of group assignment, measured the IOP." |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 169 undergoing 1 of 3 surgeries (trabeculoplasty, iridotomy, or capsulotomy) Per group: not reported for ALT alone; for full study population apraclonidine = 85, placebo = 84 Exclusions after randomization: 4/169 could not be evaluated due to loss to follow‐up or refusal of treatment medication (apraclonidine = 2, placebo = 2) Number analyzed (total and per group): Total: 83 who underwent trabeculoplasty (out of 165 for all lasers) Per group: apraclonidine = 41, placebo = 42 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: apraclonidine = 1 (type of surgery not given) refused to wait for the follow‐up exam How was missing data handled?: authors only analyzed the 164 who had all outcomes collected Reported power calculation: no Unusual study design (any issues with study design)?: randomization was to 3 types of lasers, but for this review we used only data from the trabeculoplasty group). | |
| Participants | Age: not reported for ALT alone, overall cohort (mean ± SD; years): apraclonidine = 64 ± 15, placebo = 65 ± 13 Females: not reported for ALT alone, overall cohort: apraclonidine = 61%, placebo = 55% Inclusion criteria: people who were about to undergo trabeculoplasty, iridotomy, or capsulotomy*, with inadequately controlled IOP despite maximum‐tolerated medical therapy, receiving 360° of angle treatment Exclusion criteria: active ocular infection or inflammation, unstable cardiovascular disease, any abnormality preventing reliable applanation tonometry, pregnant or nursing women, women of childbearing potential, participation in any other study within the past 30 days, people with vision in 1 eye only, people taking systemic clonidine, and people whose fellow eye had been enrolled previously in the study Equivalence of baseline characteristics: yes, there were no significant differences between treatment groups with respect to demographic characteristics (P < 0.05), baseline visual acuity, preoperative IOP, pulse rate, history of glaucoma or prior surgery, or number/type of antiglaucoma medications being taken at the time of laser surgery | |
| Interventions | Intervention 1: 1 drop apraclonidine (para‐amino‐clonidine(PAC)) 1% before surgery and 1 drop after surgery Intervention 2: 1 drop placebo before surgery and 1 drop after surgery Length of follow‐up: Planned: 1 week Actual: 1 week | |
| Outcomes | Primary outcome: control of IOP in the first 3 postoperative hours after laser surgery Secondary outcomes: pulse rate, diastolic BP, systolic BP Adverse events reported: yes Intervals at which outcomes assessed: 45 min; 1, 2, 3 hours; 1 week | |
| Notes | Trial registration: not reported Funding sources: supported in party by an unrestricted research grant from Research to Prevent Blindness, Inc Disclosures of interest: not reported, but 1 author worked for Alcon and the study used an Alcon product Study period: not reported Reported subgroup analyses: yes, by type of surgery *Study included trabeculoplasty, peripheral iridotomy, and capsulotomy, but we used only the trabeculoplasty results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned to either the 1% ALO 2145 [apraclonidine] or placebo groups by a randomized treatment code." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | Masking of outcome assessors was not reported, but apraclonidine has ocular effects which are difficult to mask such as conjunctival blanching and upper eyelid elevation. |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 participants who were randomized were not included in the analyses but that was due to refusal to wait for follow‐up measures or refusal of study drugs, and therefore unlikely to be due to the study drugs themselves. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: Italy Number randomized: Total: 30 total, 10 who underwent trabeculoplasty Per group: within the trabeculoplasty group, apraclonidine = 5, placebo = 5 Exclusions after randomization: none Number analyzed: Total: 30 total, 10 who underwent trabeculoplasty Per group: within the trabeculoplasty group, apraclonidine = 5, placebo = 5 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: baseline demographics and other characteristics not reported | |
| Participants | Age: not reported Females: not reported Inclusion criteria: aged ≥ 18 years, scheduled for LTP, iridotomy, or posterior capsulotomy Exclusion criteria: active ocular infection or inflammation, past or present severe ocular disease (except cataract and glaucoma, unstable cardiovascular disease, any abnormality preventing reliable applanation tonometry, pregnancy (actual or potential) or breastfeeding, 1 single seeing eye, treatment systemic clonidine, previous enrollment of the fellow eye in the study Equivalence of baseline characteristics: baseline characteristics not reported | |
| Interventions | Intervention 1: 1 drop apraclonidine 1%, 1 hour prior and 1 drop immediately after 360° ALT surgery Intervention 2: 1 drop placebo, 1 hour prior and 1 drop immediately after 360° ALT surgery Length of follow‐up: Planned: 1 week Actual: only up to 3 hours was reported | |
| Outcomes | Primary outcomes: mean IOP and IOP changes during the postoperative period, maximum IOP increases from baseline, IOP increase of 5 mmHg and 10 mmHg from baseline Secondary outcomes: heart rate, BP Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3 hours | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: yes, subgroups were different laser procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was designed as a prospective, randomized, double‐masked, and placebo‐controlled trial." States randomization was done but not the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | Masking of outcome assessors not reported; however, apraclonidine can cause conjunctival blanching and eyelid raising, which would have been visible to the person assessing IOP after the procedure. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | High risk | In the methods, the authors stated that, "Ocular examination, heart rate count and blood pressure measurement were repeated hourly during the first three postoperative hours and again one week post operatively;" however, no data were presented from the 1‐week assessments. |
| Other bias | High risk | Funding sources not reported, very small sample sizes within the different surgeries do not allow for statistical analyses for each surgery alone; authors stated, "Due to the low numbers of cases in each series, individual statistical analyses for each of the 3 series were considered inappropriate." |
| Methods | Study design: parallel‐group RCT Country: Canada Number randomized: Total: all laser treatments = 85, trabeculoplasty = 51 Per group: all laser treatments, brimonidine = 43, apraclonidine = 42; trabeculoplasty, brimonidine = 27, apraclonidine = 24 Exclusions after randomization: none reported Number analyzed: Total: all laser treatments = 85; trabeculoplasty = 51 Per group: all laser treatments, brimonidine = 43, apraclonidine = 42; trabeculoplasty, brimonidine = 27, apraclonidine = 24 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: none | |
| Participants | Age (mean ± SD; years): (only available for all laser treatments): brimonidine = 70 ± 12.0, apraclonidine = 67.3 ± 13.7 Females: (only available for all laser treatments): brimonidine = 67%, apraclonidine = 45% Inclusion criteria: medically uncontrolled IOP, any type of glaucoma, initial or repeat ALTs 180° to any quadrant Exclusion criteria: chronic topical alpha‐2 agonist therapy, use of topical alpha‐2 agonist within the past 2 weeks, active ocular infection or inflammation, abnormality precluding reliable applanation tonometry, unable to stay for the 1‐hour follow‐up IOP check Equivalence of baseline characteristics: no, "No significant difference were found among treatment groups in terms of age, race, or baseline IOP…There was a statistically significant difference in the gender distribution between groups…" Also noted that uneven distribution of pseudoexfoliation pigmentary glaucoma/mixed glaucoma may affect post laser IOP spike numbers | |
| Interventions | Intervention 1: 1 drop apraclonidine hydrochloride 0.5%, 10 min prior to laser surgery Intervention 2: 1 drop brimonidine tartrate 0.2%, 10 min prior to laser surgery Length of follow‐up: Planned: 1 hour Actual: 1 hour | |
| Outcomes | Primary outcome: IOP Secondary outcomes: mean IOP change, IOP elevation ≥ 5 mmHg change from baseline Adverse events reported: yes, reported no systemic or localized ocular reactions and no other adverse effects Intervals at which outcomes assessed: baseline; 1 hour after surgery | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: "The authors hold no proprietary interest in the drugs used in this study." Study period: January 1998 to May 1998 Reported subgroup analyses: yes, subgroups were different types of anterior segment laser procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Low risk | IOP analysis performed by the same masked observer at 1 hour post laser, every effort was made to use the same tonometer as prior to surgery. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up; all participants who were randomized completed all assessments. Exclusion criteria was for people who could stay for 1 hour post laser surgery, so there was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. Also, this study reported subgroup analysis for the ALT group but did not report baseline demographics by subgroup. |
| Methods | Study design: parallel‐group RCT Country: England Number randomized: Total: 75 eyes Per group: apraclonidine = 26 eyes, pilocarpine = 23 eyes, apraclonidine/pilocarpine = 26 eyes Exclusions after randomization: none reported Number analyzed: Total: 75 eyes Per group: apraclonidine = 26 eyes, pilocarpine = 23 eyes, apraclonidine/pilocarpine = 26 eyes Unit of analysis: eyes, if both eyes required LTP, then the first eye to be treated was entered into the study. Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: none | |
| Participants | Age (mean (range); years): apraclonidine = 72.2 (53 to 84), pilocarpine = 68.4 (53 to 86), apraclonidine/pilocarpine = 71.3 (46 to 87) Females: not reported Inclusion criteria: OAG with in IOP > 21 mmHg Exclusion criteria: regular pilocarpine to either eye, active ocular infection or inflammation present, unstable cardiovascular disease, taking systemic clonidine Equivalence of baseline characteristics: yes, "There was no statistically significant difference between the groups with respect to age, eye color, type of glaucoma, or glaucoma medication. All patients had similar disease as judged by single medication, duration of disease, and cumulative treatment." | |
| Interventions | Intervention 1: 1 drop apraclonidine 1% 1 hour before and 1 drop immediately after 180° ALT Intervention 2: 1 drop pilocarpine 4% immediately after 180° ALT Intervention 3: 1 drop of apraclonidine 1%, 1 hour before and 1 drop of apraclonidine 1%/1 drop of pilocarpine 4%, immediately after 180° ALT Length of follow‐up: Planned: 1 week Actual: 1 week | |
| Outcomes | Primary outcome: IOP Secondary outcomes: heart rate, BP Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 week following trabeculoplasty | |
| Notes | Trial registration: not reported Funding sources: Alcon Laboratories in England supported the study Disclosures of interest: no disclosures reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were then randomly allocated to one of the three treatment groups." Did not state how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | Masking of participants and personnel not reported but due to the nature of the interventions, participants would know whether or not they got medication before or after the surgery and that they received 2 drops of medication if in the combination group. |
| Masking of outcome assessment (detection bias) | Low risk | "The observer was masked to the study group of the patient." |
| Incomplete outcome data (attrition bias) | Low risk | Data available for all participants who were randomized. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Supported by Alcon Laboratories, which makes products containing apraclonidine and pilocarpine. Additionally, a difference drop regimen without use of vehicle drops would leave participants essentially unmasked. |
| Methods | Study design: parallel‐group RCT Country: Israel, US Number randomized: Total: 248 eyes Per group: not reported Exclusions after randomization: 9 were removed from the statistical analysis (2 improperly entered into the study, 7 due to protocol violations) Number analyzed: Total: 239 eyes Per group: brimonidine/brimonidine = 60, brimonidine/vehicle = 62, vehicle/brimonidine = 61, vehicle/vehicle = 56 Unit of analysis: eyes, 1 eye per participant Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design: none | |
| Participants | Age: not reported Females: not reported Inclusion criteria: aged ≥ 21 years with useful vision in both eyes Exclusion criteria: prior glaucoma surgery or intraocular surgery Equivalence of baseline characteristics: "The groups were similar with regard to the type of pressure‐lowering medications that the patients were receiving prior to enrollment in the study." "The four groups were similar with respect to demographics and iris color." | |
| Interventions | Intervention 1: brimonidine 0.5%, 30 to 45 min before and after 360° ALT Intervention 2: brimonidine 0.5%, 30 to 45 min before 360° ALT and vehicle after Intervention 3: vehicle, 30 to 45 min before and brimonidine 0.5% after ALT Intervention 4: vehicle, 30 to 45 min before and after ALT Length of follow‐up: Planned: 4 to 6 weeks Actual: 4 to 6 weeks | |
| Outcomes | Primary outcome: IOP Secondary outcomes: heart rate, BP Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 to 2 weeks after ALT; 4 to 6 weeks after ALT | |
| Notes | Type of study: published Funding sources: none reported Disclosures of interest: 3 authors were employees of Allergan Inc. The other authors had no proprietary interest in either Allergan Inc or its products. Study period: not reported Reported subgroup analyses: yes, some results were reported combining participants into those who had any brimonidine vs those in the vehicle‐only group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Description of randomization method not provided. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | High risk | Brimonidine can have ocular adverse effects of conjunctival blanching and lid retraction, which would be easy for outcome assessors to see even if they were masked. |
| Incomplete outcome data (attrition bias) | High risk | "Of the 248 patients enrolled in the study, nine were disqualified from the statistical analysis. Two subjects had been improperly entered into the study and seven were excluded due to study protocol violations." Participants with unacceptably high IOP elevations were treated and removed from the study, and not included in the final analysis, but they provided important information about which groups they initially belonged in and could alter the numbers reported of how effective brimonidine was in lowering IOP. |
| Selective reporting (reporting bias) | High risk | Participants with unacceptably high IOP elevations were treated and removed from the study, and not included in the final analysis, but they provided important information about which groups they initially belonged in and could alter the numbers reported of how effective brimonidine was in lowering IOP. |
| Other bias | High risk | 3 authors were employees of Allergan Inc, which produces ophthalmic drugs containing brimonidine. |
| Methods | Study design: intra‐individual RCT Country: not reported Number randomized: Total: 20 eyes of 10 participants Per group: brimonidine = 10 eyes, apraclonidine = 10 eyes Exclusions after randomization: none reported Number analyzed: Total: 20 eyes of 10 participants Per group: brimonidine = 10 eyes, apraclonidine = 10 eyes Unit of analysis: eyes Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: none | |
| Participants | Age: not reported Females: not reported Inclusion criteria: SLT for POAG on both eyes Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: brimonidine tartrate 0.15%, 1 hour prior to 360° LTP, 1 drop randomly assigned in the left or right eye Intervention 2: apraclonidine 0.5%, 1 hour prior to 360° LTP, 1 drop assigned in opposite eye of the brimonidine tartrate treatment Length of follow‐up: Planned: not reported Actual: 1 week | |
| Outcomes | Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: yes, stated there were no non‐ocular clinically significant symptoms in either group Intervals at which outcomes assessed: baseline; 1 hour; 1 week post‐surgery | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Both eyes of a participant were included in the study and received different medications; however, the authors did not report if and how they took into account the inter‐dependency of eyes within the same participant. |
| Methods | Study design: parallel‐group RCT Country: Norway Number randomized: Total: 50 Per group: pilocarpine pretreatment = 25, no pretreatment = 25 Exclusions after randomization: none Number analyzed: Total: 50 Per group: pilocarpine pretreatment = 25, no pretreatment = 25 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design: none | |
| Participants | Age (mean ± SD; years): pilocarpine pretreatment = 69 ± 9.9, no treatment = 71.9 ± 7.1 Females: not reported Inclusion criteria: IOP ≥ 25 mmHg measured by applanation tonometry at the initial evaluation by 1 of the authors and just before laser treatment. The mean of these 2 was taken as prelaser IOP. Glaucomatous disk damage or visual field defects (or both), defined as cupping of the optic nerve head extending to the margin of the disc, a difference of vertical cup‐disk ratio of ≥ 0.2 between the 2 eyes, and different degrees of disk pallor in the 2 eyes with no other explanation. No earlier glaucoma treatment. Exclusion criteria: not reported Equivalence of baseline characteristics: yes, "There is no evidence of dissimilarities between the two groups." | |
| Interventions | Intervention 1: 2 drops pilocarpine 2%, 1 hour before LTP Intervention 2: no pretreatment Length of follow‐up: Planned: 6 months Actual: 6 months | |
| Outcomes | Primary outcome: IOP Secondary outcomes: number of participants with change in IOP > 10 mmHg or 20 mmHg, number of participants with peak IOP ≥ 50 mmHg Adverse events reported: no Intervals at which outcomes assessed: 1, 2, 4, 6, 8, 24 hours after treatment; 1 week; 1, 3, 6 months | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: September 1989 to December 1990 Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported participants were randomly assigned to a group but did not describe how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | "The study was not masked because the pilocarpine induced miosis was very obvious to the investigators." |
| Masking of outcome assessment (detection bias) | High risk | Study was not masked because of pilocarpine's induced miosis which was very obvious to the investigators. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Low risk | IOP was the only outcome of interest and the focus of the paper; authors reported that, "visual field changes following LTP will be the subject of a separate study." |
| Other bias | Unclear risk | Funding sources not reported. |
| Methods | Study design: parallel‐group, placebo controlled, RCT Country: US (multicenter) Number randomized: Total: 122 Per group: dorozolamide = 61, placebo = 61 Exclusions after randomization: none reported Number analyzed: Total: 122 Per group: dorozolamide = 61, placebo = 61; in ALT group: dorozolamide = 17, placebo = 23 Unit of analysis: participant (1 eye per person) Losses to follow‐up: none reported How was missing data handled?: missing data were imputed by carrying forward data from the previous time point Reported power calculation: yes, "With 60 patients per group, there was 90% power to detect such a difference at the P<0.05‐level (two‐sided)." | |
| Participants | Age: not reported Females: not reported Inclusion criteria: to have posterior capsular opacity requiring Nd:YAG laser capsulotomy, OAG requiring ALT or any condition requiring ALT or Nd:YAG laser iridotomy Exclusion criteria: ocular inflammation within the past 2 months, advanced visual field defects with risk of further loss if a spike in IOP were to occur, baseline IOP > 30 mmHg, use of corticosteroid, oral beta‐blocker, or oral carbonic anhydrase inhibitor therapy Equivalence of baseline characteristics: yes, "Baseline characteristics were similar in both treatment groups." | |
| Interventions | Intervention 1: 1 drop dorozolamide hydrochloride 2%, 1 hour before and 1 drop at the end of surgery Intervention 2: 1 drop placebo, 1 hour before and 1 drop immediately after Length of follow‐up: Planned: 24 hours Actual: 24 hours | |
| Outcomes | Primary outcome: percentage of participants with an increase in IOP from the baseline of ≥ 10 mmHg during the first 4 hours after surgery Secondary outcomes: heart rate, BP, incidence of adverse effects, ocular signs Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3, 4, 24 hours | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: the Dorzolamide Laser Study Group was sponsored by pharmaceutical research corporation, and multiple authors work for Merck Research Laboratories, which makes dorozolamide. Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data imputed by carrying forward data from the previous time point, but the authors did not specify how much attrition occurred. |
| Selective reporting (reporting bias) | High risk | Did not provide table of baseline characteristics. Did not discuss attrition of study participants, and did not report data at 24 hours despite mentioning it as part of methods section, no mention of specific ocular adverse events, and did not describe number of participants with systemic adverse effects other than to say there was no difference between groups. |
| Other bias | High risk | Merck is the maker of dorozolamide, and several authors were employees of Merck. |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 60 Per group: apraclonidine before and after = 30, apraclonidine only after = 30 Exclusions after randomization: not reported Number analyzed: Total: 60 Per group: apraclonidine before and after = 30, apraclonidine only after = 30 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no | |
| Participants | Age: not reported Females: not reported Inclusion criteria: people who had POAG, defined by optic disk cupping and visual field loss, and a pretreatment IOP > 21 mmHg on maximally tolerated medical therapy Exclusion criteria: previous intraocular surgical procedures or laser treatment, people who had secondary OAG (e.g. pigmentary, exfoliative, or uveitic), and aged < 40 years Equivalence of baseline characteristics: yes, "There were no statistical differences between preoperative IOP and the number of antiglaucoma medications between the two groups of patients." | |
| Interventions | Intervention 1: 1 drop apraclonidine 1%, 1 hour before and immediately after 360° LTP Intervention 2: 1 drop apraclonidine 1%, only after 360° LTP Length of follow‐up: Planned: 2 hours Actual: 2 hours | |
| Outcomes | Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2 hours after treatment | |
| Notes | Trial registration: not reported Funding sources: "This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York." Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One of the following two apraclonidine open‐label treatment regimens was determined from a random table chart…" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | Study was open‐label and no mention of vehicle drops, so participants would be aware whether they received drops before and after surgery or only after surgery. |
| Masking of outcome assessment (detection bias) | Low risk | "Intraocular pressure was measured one and two hours after treatment by an observer who was masked to the random assignment to treatment with apraclonidine." |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: not reported Number randomized: Total: 37 Per group: apraclonidine = 21, latanoprost = 16 Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis (participants vs eyes): participant Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: unclear whether participants had only 1 eye included or if they had both eyes included | |
| Participants | Age: not reported Females: not reported Inclusion criteria: people undergoing ALT for glaucoma Exclusion criteria: not reported Equivalence of baseline characteristics: no (equivalence of baseline characteristics were not fully reported, and no demographics info were provided. Only medical history and procedure reported: "Both groups had equal types of glaucoma, pigmentation and no previous surgery.") | |
| Interventions | Intervention 1: 1 drop apraclonidine 0.5%, 45 min prior to ALT Intervention 2: 1 drop latanoprost 0.005%, 45 min prior to ALT Length of follow‐up: Planned: not reported Actual: 6 weeks | |
| Outcomes | Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: apraclonidine group = 2 hours; 1, 6 weeks; latanoprost group = 2 hours; 1 day; 1, 6 weeks | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported; unclear whether the participant or the eye was analyzed. |
| Methods | Study design: parallel‐group RCT Country: not reported Number randomized: Total: 50 Per group: apraclonidine = 28, latanoprost = 22 Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis: participants Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: unclear whether the study analyzed participants or eyes | |
| Participants | Age: not reported Females: not reported Inclusion criteria: people undergoing ALT Exclusion criteria: not reported Equivalence of baseline characteristics: no, demographic characteristics were not discussed, only medical characteristics: "Both groups had equal types of glaucoma, pigmentation, and previous surgical histories." | |
| Interventions | Intervention 1: 1 drop apraclonidine 0.5%, 1 hour prior to surgery Intervention 2: 1 drop latanoprost 0.005%, 6 hours and 1 hour prior to surgery Length of follow‐up: Planned: not reported Actual: 6 weeks | |
| Outcomes | Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: 1.5 hours; 1 day; 1, 6 weeks | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported; unclear whether the participant or the eye were analyzed. |
| Methods | Study design: parallel‐group RCT Country: not reported Number randomized: Total: 23 eyes (17 participants) Per group: not reported Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Age: not reported Females: not reported Inclusion criteria: participants with POAG undergoing ALT Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: 1 drop apraclonidine 1%, 1 hour before and immediately after ALT Intervention 2: 1 drop placebo, 1 hour before and immediately after ALT Length of follow‐up: Planned: 24 hours Actual: 24 hours | |
| Outcomes | Primary outcomes: IOP, flare intensity Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: participants observed during a 24‐hour observation period, but specific time points not described. | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "We studied the effects of this compound on the inflammatory reaction and the IOP responses to ALT in a randomized, double‐masked manner." |
| Masking of outcome assessment (detection bias) | Low risk | "We studied the effects of this compound on the inflammatory reaction and the IOP responses to ALT in a randomized, double‐masked manner." |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
| Methods | Study design: parallel‐group RCT Country: Korea Number randomized: Total: 80 Per group: brimonidine/brimonidine = 20; brimonidine/placebo = 20, placebo/brimonidine = 20, placebo/placebo = 20 Exclusions after randomization: none reported Number analyzed: Total: 80 Per group: brimonidine/brimonidine = 20; brimonidine/placebo = 20, placebo/brimonidine = 20, placebo/placebo = 20 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Age (mean ± SD; years): overall = 58.4 ± 8.9, brimonidine/brimonidine = 57.7, brimonidine/placebo = 58.0, placebo/brimonidine = 60.6, placebo/placebo = 57.5 Females: brimonidine/brimonidine = 35%, brimonidine/placebo = 55%, placebo/brimonidine = 55%, placebo/placebo = 45% Inclusion criteria: none listed Exclusion criteria: people who had glaucoma or intraocular surgery, who had already received any systemic alpha‐agonist or had a hypersensitivity to any alpha‐agonist Equivalence of baseline characteristics: yes, "No significant pretreatment differences in terms of age, sex, iris color, or baseline IOP were noted among treatment groups." | |
| Interventions | Intervention 1: brimonidine 0.2%, 30 to 60 min before and immediately after 180° ALT Intervention 2: brimonidine 0.2%, 30 to 60 min before and placebo immediately after 180° ALT Intervention 3: placebo, 30 to 60 min before and brimonidine 0.2% immediately after 180° ALT Intervention 4: placebo, 30 to 60 min before and immediately after Length of follow‐up: Planned: 4 weeks Actual: 4 weeks | |
| Outcomes | Primary outcome: IOP Secondary outcomes: IOP of the contralateral eye, mean heart rate, systolic BP Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3 hours; 1 day; 1, 4 weeks | |
| Notes | Trial registration: not reported Funding sources: "This study was supported by the Research Institute of Clinical Medicine, Chonnam University Hospital." Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective reporting. |
| Other bias | Low risk | None |
| Methods | Study design: prospective, randomized, double‐masked, parallel‐group RCT Country: UK Number randomized: Total: 100 Per group: acetazolamide = 50; placebo = 50 Exclusions after randomization: N/A Number analyzed: Total: 100 Per group: acetazolamide = 50; placebo = 50 Unit of analysis (participants vs eyes): eyes, 1 eye per participant, chosen if that eye needed laser. If both eyes were lasered, the first eye was chosen for inclusion. Losses to follow‐up: N/A How was missing data handled?: not reported Reported power calculation: no Unusual study design?: no | |
| Participants | Age (mean ± SD; years): acetazolamide = 74.0 ± 6.0, placebo = 74.6 ± 5.9 Females: acetazolamide = 54%, placebo = 52%, overall = 53% Inclusion criteria: uncontrolled OAG with IOP > 21 mmHg and progressive visual field loss, on maximum tolerated topical therapy, no previous LTP Exclusion criteria: already receiving acetazolamide Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: acetazolamide (2 × 250 mg tablets), 1 hour prior to LTP Intervention 2: placebo (2 placebo tablets), 1 hour prior to LTP Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcomes: IOP in both eyes, degree of anterior segment inflammation Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: 30 min; 1, 2, 3, 24 hours; 2 months after laser treatment | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: none Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "The medication selected was masked to both the patient and the physician." |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: France Number randomized: Total: 38 Per group: trabeculoplasty/apraclonidine = 10, capsulotomy/apraclonidine = 8, trabeculoplasty/placebo = 10; capsulotomy/placebo = 10 Exclusions after randomization: none Number analyzed: Total: 38 Per group: apraclonidine = 18 (trabeculoplasty = 10, capsulotomy = 8); placebo n = 20 (trabeculoplasty = 10, capsulotomy = 8) Unit of analysis: eyes Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Age: not reported Females: not reported Inclusion criteria: not reported Exclusion criteria: aged < 18 years, infection or eye inflammation, severe eye disease in the past or currently, with the exception of cataract and glaucoma, non‐stabilized cardiovascular disease, an abnormality preventing reliable measure of IOP tonometry, blindness, receiving general clonidine, already participated in the study with their other eye, participated in another clinical trial during the last 30 days Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: placebo Intervention 2: apraclonidine 1% Length of follow‐up: Planned: 1 week Actual: 1 week | |
| Outcomes | Primary outcomes: efficacy/efficiency with IOP (i.e. mean change in IOP) Secondary outcome: incident IOP spikes (≥ 10 mmHg) Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3 hours; 1 week | |
| Notes | Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: yes, by surgical procedure, i.e. trabeculoplasty vs capsulotomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors report the study was "double‐blinded" but did not describe who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | Authors report the study was "double‐blinded" but did not describe who was masked: participants, surgeons, or outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how the authors handled missing data; missing a "table 1" study population demographics. |
| Selective reporting (reporting bias) | Unclear risk | Authors described outcomes and how they graded/collected in the methods section; same outcomes reported in results; reported both statistically significant and non‐significant data; however, there was a protocol deviation (i.e. from iridotomies to capsulotomies) and the authors dropped 2 iridotomy cases. |
| Other bias | Unclear risk | Funding sources not reported. |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 228 Per group: apraclonidine = 114; pilocarpine = 114 Exclusions after randomization: none reported Number analyzed: Total: 228 Per group: apraclonidine = 114; pilocarpine = 114 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: yes Unusual study design?: none | |
| Participants | Age (mean ± SD; years): apraclonidine = 68.4 ± 11.4, pilocarpine = 70.3 ± 10.1 Females: apraclonidine = 62%, pilocarpine = 56% Inclusion criteria: POAG with bilateral elevation (> 21 mmHg before therapy), characteristic glaucomatous optic nerve damage on stereoscopic biomicroscopy, and glaucomatous visual field defects on Humphrey automated field testing Exclusion criteria: secondary OAG and previous intraocular surgery Equivalence of baseline characteristics: no, pre‐ALT IOP was higher in the apraclonidine group | |
| Interventions | Intervention 1: 1 drop apraclonidine 1%, 15 min before 180° LTP Intervention 2: 1 drop pilocarpine 4%, 15 min before 180° LTP Length of follow‐up: Planned: 24 hours Actual: 24 hours | |
| Outcomes | Primary outcome: IOP Secondary outcome: incidence of IOP spike Adverse events reported: yes, "There was an apparent lack of serious or longlasting side effects after single instillation of either apraclonidine or pilocarpine." Intervals at which outcomes assessed: 5 min; 1, 24 hours | |
| Notes | Trial registration: not reported Funding sources: "Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc" Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: yes, by regular medication type | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was an RCT but no description of how the randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 73 Per group: apraclonidine = 39, placebo = 34 Exclusions after randomization: none reported Number analyzed: Total: 73 Per group: apraclonidine = 39, placebo = 34 Unit of analysis (participants vs eyes): participant (1 eye per participant); if a participant required bilateral therapy, the eye treated first was selected Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Age (mean ± SD; years): apraclonidine = 60.9 ± 14.3, placebo = 68.8 ± 12.4 Females: apraclonidine = 54%, placebo = 74% Inclusion criteria: pre‐existing OAG and poor IOP control despite maximum tolerated medical therapy Exclusion criteria: prior ALT Equivalence of baseline characteristics: no, "There was no statistically significant difference in any variable except for mean patient age, (P<0.25)" | |
| Interventions | Intervention 1: topical 1% apraclonidine, 1 hour prior and immediately after 360° ALT Intervention 2: placebo, 1 hour prior and immediately after 360° ALT Length of follow‐up: Planned: 1 month Actual: 1 month | |
| Outcomes | Primary outcomes: visual acuity, IOP, anterior segment inflammation Secondary outcome: heart rate Adverse events reported: authors reported that there were no adverse events Intervals at which outcomes assessed: 1, 2, 3 hours; 1 week; 1 month | |
| Notes | Trial registration: not reported Funding sources: "This study was funded in part by a grant from Alcon Laboratories." Disclosures of interest: "Betty House is an employee of Alcon Laboratories, Fort Worth, Tex. None of the authors as any financial, commercial, or proprietary interest in ALO 2145 [apraclonidine]." Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random‐number table was utilized, and the selected medication was masked to both the physician and the patient." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "A computer‐generated random‐number table was utilized, and the selected medication was masked to both the physician and the patient." |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Study funded in part by Alcon Laboratories, who manufacture the study drug apraclonidine. |
| Methods | Study design: parallel‐group RCT Country: US Number randomized: Total: 260 Per group: apraclonidine = 125, pilocarpine = 37, timolol = 35, dipivefrin = 32, acetazolamide = 31 Exclusions after randomization: none reported Number analyzed: Total: 260 Per group: apraclonidine = 125, pilocarpine = 37, timolol = 35, dipivefrin = 32, acetazolamide = 31 Unit of analysis (participants vs eyes): participants (1 eye per participant; if both eyes had elevated IOP and required trabeculoplasty, the study included only the eye treated first) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: "To increase our experience with the use of apraclonidine, the randomization allowed about four times more eyes to receive topical 1% apraclonidine than either timolol 0.5%, pilocarpine 4%, dipivefrin 0.1%, or 250 mg acetazolamide." | |
| Participants | Age (mean ± SD; years): apraclonidine = 66.5 ± 12.2, pilocarpine = 67.6 ± 8.9, timolol = 68.4 ± 10.3, dipivefrin = 65.5 ± 14.0, acetazolamide = 63.0 ± 13.1 Females: apraclonidine = 56%, pilocarpine = 62%, timolol = 57%, dipivefrin = 50%, acetazolamide = 65% Inclusion criteria: people of legal age with various forms of glaucoma, with disk and visual field damage, poor IOP control despite maximum‐tolerated medical therapy Exclusion criteria: people with asthma, sulfa allergy, unstable cardiovascular disease, allergy to any of the test medications, and eyes that had previously undergo ALT Equivalence of baseline characteristics: yes, "There were no significant preoperative differences among the five treatment groups in terms of race, age, sex, eye color, or preoperative types of glaucoma." | |
| Interventions | Intervention 1: apraclonidine 1%, 1 hour before and immediately after LTP Intervention 2: pilocarpine hydrochloride, 4% 1 hour before and immediately after LTP Intervention 3: timolol maleate 0.5%, 1 hour before and immediately after LTP Intervention 4: dipivefrin 0.1%, 1 hour before and immediately after LTP Intervention 5: acetazolamide 250 mg, 1 hour before and immediately after LTP Length of follow‐up: Planned: 1 month Actual: 1 month | |
| Outcomes | Primary outcome: IOP changes Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 week; 1 month | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: "The author has no proprietary interests in Alcon Laboratories, Inc, or in apraclonidine hydrochloride." Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random‐number table was used to assign eyes to five treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Participants in the acetazolamide group would be aware they were taking a pill rather than receiving topical treatment, though they may not have known what the drug was. |
| Masking of outcome assessment (detection bias) | Low risk | "The investigator was masked to which medication each subject received." |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Country: Turkey Number randomized: Total: 48 Per group: apraclonidine/180° ALT = 16, apraclonidine/360° ALT = 16, placebo/180° ALT = 16 Exclusions after randomization: none reported Number analyzed: Total: 48 Per group: apraclonidine/180° ALT = 16, apraclonidine/360° ALT = 16, placebo/180° ALT = 16 Unit of analysis (participants vs eyes): participant (1 eye per person) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Age (mean ± SD; years): apraclonidine/180° ALT = 63.3 ± 8.8, apraclonidine/360° ALT = 65.5 ± 7.4, placebo/180° ALT = 62.1 ± 8.3 Females: apraclonidine/180° ALT = 25%, apraclonidine/360° ALT = 38%, placebo/180° ALT = 31% Inclusion criteria: people with POAG, defined by optic disk cupping and visual field loss and a pretreatment IOP > 21 mmHg on maximally tolerated medical therapy Exclusion criteria: previous intraocular surgical procedures or laser treatment, secondary OAG (i.e. pigmentary, exfoliative, or uveitic), and aged < 40 years Equivalence of baseline characteristics: yes, "There were no significant differences among the three groups in terms of average age, gender, preoperative IOP, or number of antiglaucoma medications (P>0.05)." | |
| Interventions | Intervention 1: apraclonidine 1%, 1 hour before and immediately after 180° ALT Intervention 2: apraclonidine 1%, 1 hour before and immediately after 360° ALT Intervention 3: placebo, 1 hour before and immediately after 180° ALT Length of follow‐up: Planned: 3 hours Actual: 3 hours | |
| Outcomes | Primary outcomes: IOP change, frequency of IOP elevation Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours after treatment | |
| Notes | Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Low risk | "IOP was measured preoperatively and 1, 2, and 3 hours after treatment by an observer who was blinded to the random assignment of treatment with the drugs." |
| Incomplete outcome data (attrition bias) | Unclear risk | Study did not address whether outcome data were complete at each time point, but study only lasted 3 hours post‐ALT. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
ALT: argon laser trabeculoplasty; BP: blood pressure; IOP: intraocular pressure; LTP: laser trabeculoplasty; min: minute; N/A: not applicable; Nd:YAG: neodymium‐doped yttrium aluminum garnet; OAG: open‐angle glaucoma; POAG: primary open‐angle glaucoma; RCT: randomized controlled trial; SD: standard deviation; SLT: selective laser trabeculoplasty.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study medication was not an IOP‐lowering drop. | |
| Non‐comparative study. | |
| Study medications were not IOP‐lowering drops. | |
| Study medications were not IOP‐lowering drops. | |
| Included participants who received laser peripheral iridotomy, ALT, and Nd:YAG laser capsulotomy; results not separated by type of surgery. | |
| Included participants who received laser peripheral iridotomy, ALT, and Nd:YAG laser capsulotomy; results not separated by type of surgery. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medication was not an IOP‐lowering drop. | |
| Dosage study; no eligible comparison group. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medication was not an IOP‐lowering drop. | |
| Not an RCT. | |
| Not an RCT. | |
| Non‐comparative study. | |
| Included only participants who received laser iridotomy. | |
| Unable to confirm whether study was randomized. | |
| Study medication was not an IOP‐lowering drop. | |
| Unable to confirm whether study was randomized; included participants who received ALT or Nd:YAG capsulotomy; results not separated by type of surgery. | |
| Study medication was not an IOP‐lowering drop. | |
| Dosage study; no eligible comparison group. | |
| Study medication was not an IOP‐lowering drop. | |
| Unable to confirm whether study was randomized; included participants who received ALT, and Nd:YAG laser iridotomy; results not separated by type of surgery. | |
| Unable to confirm whether study was randomized; categorized by handsearchers as a CCT as no randomization was reported. | |
| Unable to confirm whether study was randomized; categorized by handsearchers as a CCT as no randomization was reported. | |
| Dosage study; no eligible comparison group. | |
| 1 study arm received only medication without LTP; no eligible comparison group. | |
| Study medication was not an IOP‐lowering drop. | |
| Study medications were not IOP‐lowering drops. |
ALT: argon laser trabeculoplasty; CCT: controlled clinical trial; IOP: intraocular pressure; LTP: laser trabeculoplasty; Nd:YAG: neodymium‐doped yttrium aluminum garnet; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Serbian. Awaiting translation. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Korean. Awaiting translation. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 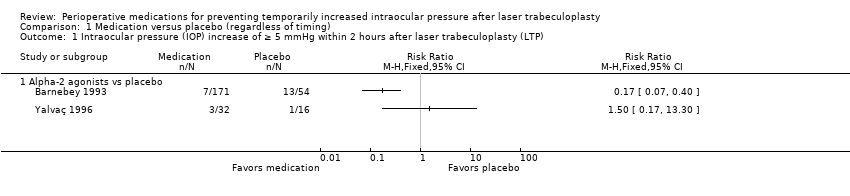 Comparison 1 Medication versus placebo (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP). | ||||
| 1.1 Alpha‐2 agonists vs placebo | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.20] |
| Analysis 1.2  Comparison 1 Medication versus placebo (regardless of timing), Outcome 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP. | ||||
| 2.1 Acetazolamide vs placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.52] |
| 2.2 Alpha‐2 agonists vs placebo | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.27] |
| 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Medication versus placebo (regardless of timing), Outcome 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP. | ||||
| 3.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐7.43 [‐10.60, ‐4.27] |
| 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 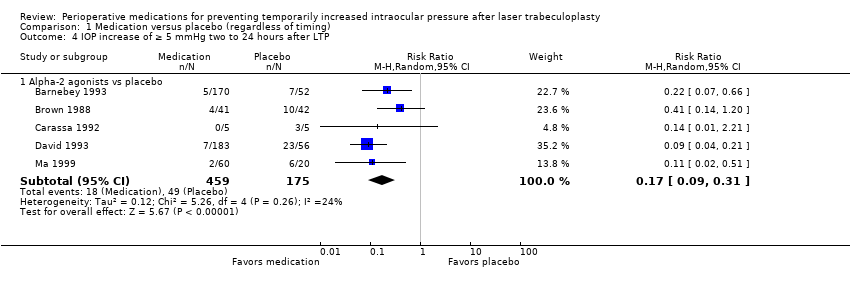 Comparison 1 Medication versus placebo (regardless of timing), Outcome 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP. | ||||
| 4.1 Alpha‐2 agonists vs placebo | 5 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.31] |
| 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 9 | 817 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] |
| Analysis 1.5 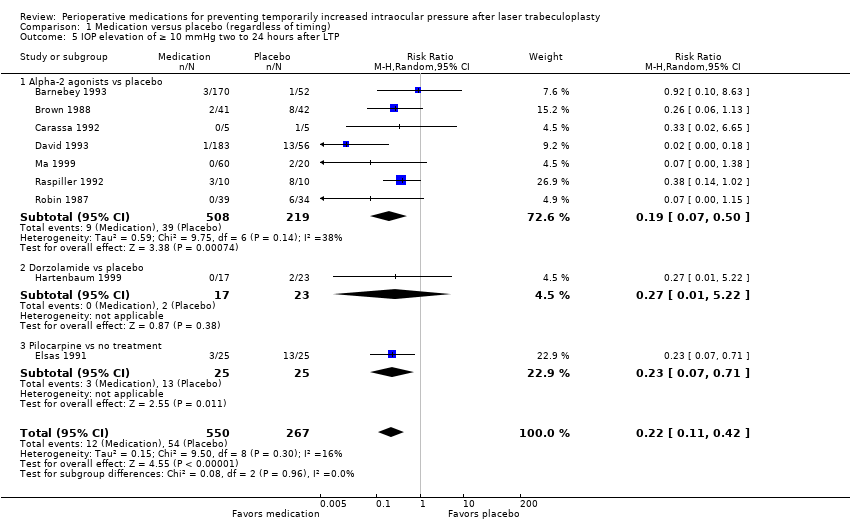 Comparison 1 Medication versus placebo (regardless of timing), Outcome 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP. | ||||
| 5.1 Alpha‐2 agonists vs placebo | 7 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] |
| 5.2 Dorzolamide vs placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 5.22] |
| 5.3 Pilocarpine vs no treatment | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.71] |
| 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Medication versus placebo (regardless of timing), Outcome 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP. | ||||
| 6.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐5.32 [‐7.37, ‐3.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) Show forest plot | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.32, 16.03] |
| Analysis 2.1 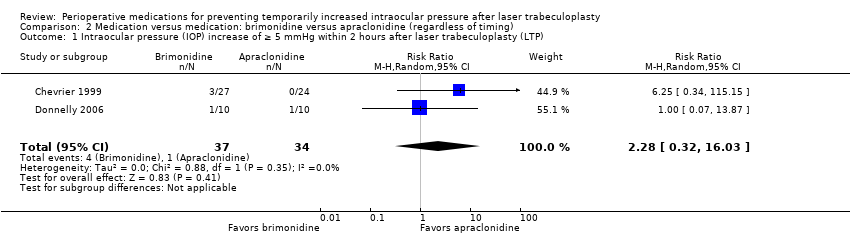 Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP). | ||||
| 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg) Show forest plot | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐2.56, 1.17] |
| Analysis 2.2  Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg) Show forest plot | 2 | 277 | Mean Difference (Random, 95% CI) | 0.61 [‐0.44, 1.66] |
| Analysis 3.1  Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg). | ||||
| 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP. | ||||
| 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.14, 5.63] |
| Analysis 3.3  Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP. | ||||
| 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 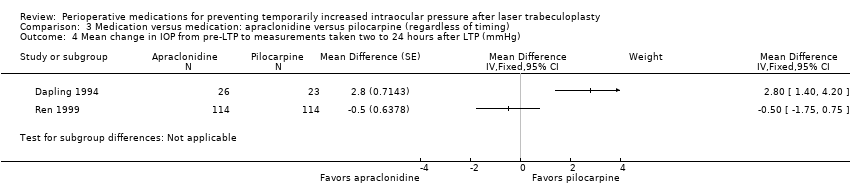 Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Timing comparison: medication before versus medication after, Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP). | ||||
| 1.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.63] |
| 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2 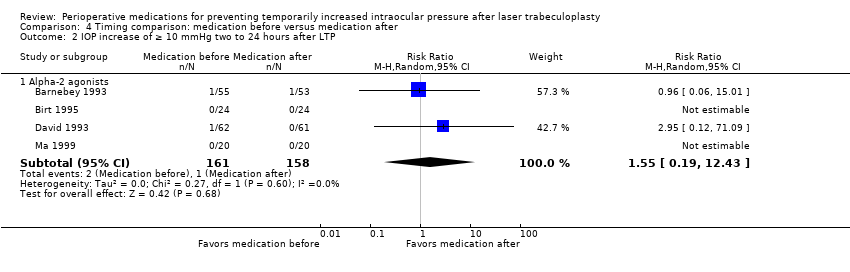 Comparison 4 Timing comparison: medication before versus medication after, Outcome 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP. | ||||
| 2.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.19, 12.43] |
| 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg) Show forest plot | 3 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Timing comparison: medication before versus medication after, Outcome 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg). | ||||
| 3.1 Alpha‐2 agonists (mmHg) | 3 | 198 | Mean Difference (Random, 95% CI) | ‐1.07 [‐2.51, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Conjunctival blanching (brimonidine vs placebo) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Adverse events, Outcome 1 Conjunctival blanching (brimonidine vs placebo). | ||||

Study flow diagram.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
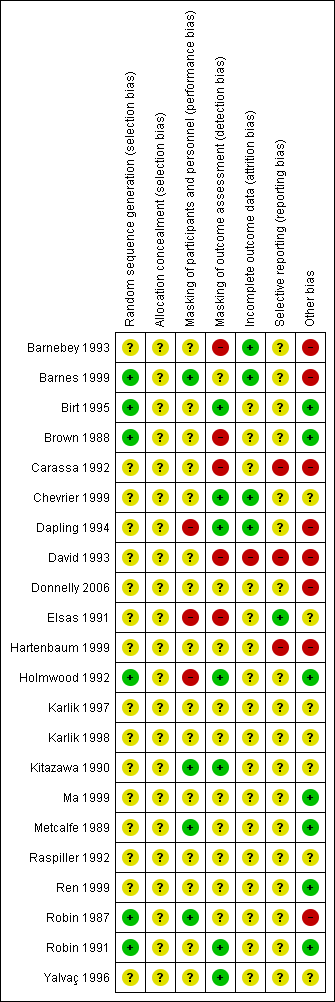
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Medication versus placebo (regardless of timing), outcome: 1.4 Intraocular pressure (IOP) increase ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).

Forest plot of comparison: 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), outcome: 3.2 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 after laser trabeculoplasty (LTP).

Forest plot of comparison: 4 Timing comparison: medication before versus medication after, outcome: 4.1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).
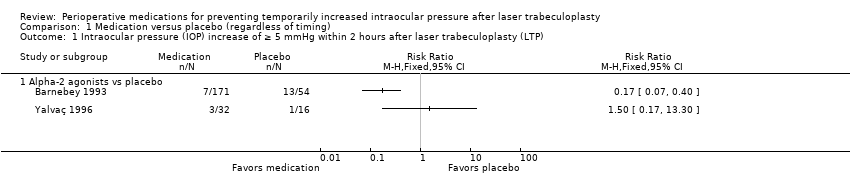
Comparison 1 Medication versus placebo (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP).

Comparison 1 Medication versus placebo (regardless of timing), Outcome 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP.

Comparison 1 Medication versus placebo (regardless of timing), Outcome 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP.

Comparison 1 Medication versus placebo (regardless of timing), Outcome 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP.

Comparison 1 Medication versus placebo (regardless of timing), Outcome 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP.
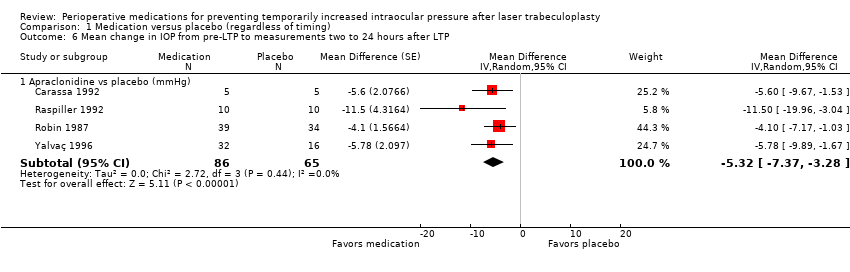
Comparison 1 Medication versus placebo (regardless of timing), Outcome 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP.

Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP).
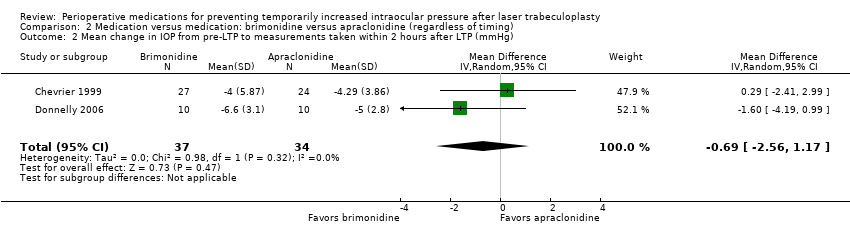
Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg).
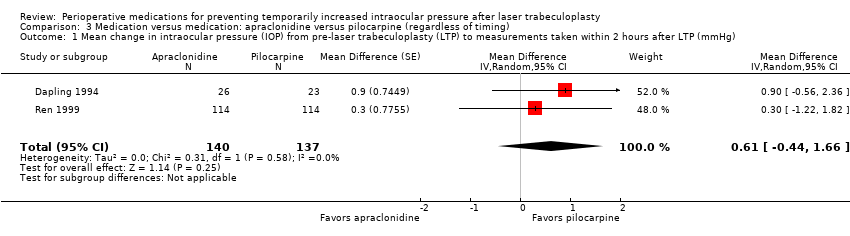
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg).
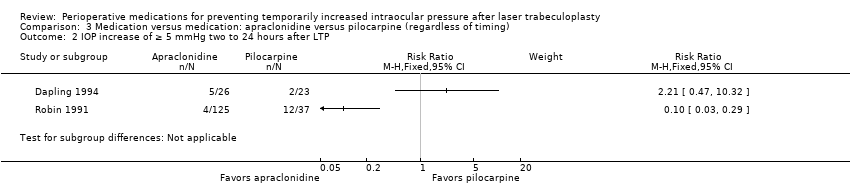
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP.
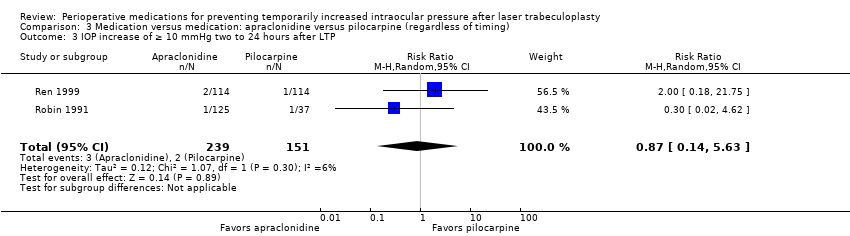
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP.

Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg).

Comparison 4 Timing comparison: medication before versus medication after, Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).

Comparison 4 Timing comparison: medication before versus medication after, Outcome 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP.

Comparison 4 Timing comparison: medication before versus medication after, Outcome 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg).
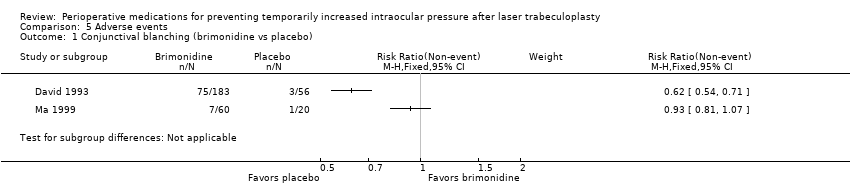
Comparison 5 Adverse events, Outcome 1 Conjunctival blanching (brimonidine vs placebo).
| Medication compared with placebo for preventing temporarily increased IOP after LTP | ||||||
| Participant or population: people with glaucoma receiving LTP Intervention: IOP‐lowering medication (apraclonidine, acetazolamide, brimonidine, pilocarpine) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Medication | |||||
| IOP increase of ≥ 5 mmHg within 2 hours | See comment | ‐ | 273 | ⊕⊕⊝⊝1,2 | Medications in this comparison were apraclonidine and brimonidine. 2 studies reported on this outcome and 1 favored the alpha‐2 agonists while the other favored placebo. Due to significant statistical heterogeneity (I2 = 70%), we did not perform a meta‐analysis. | |
| IOP increase of ≥ 10 mmHg within 2 hours | 195 per 1000 | 10 per 1000 | RR 0.05 (0.01 to 0.20) | 446 | ⊕⊕⊕⊝1 | Medications in this comparison were acetazolamide, apraclonidine, and brimonidine. |
| Mean change in IOP from pre‐LTP within 2 hours | The mean change in IOP ranged across control groups from0.4 mmHg to 4.40 mmHg, for 3 included studies | The mean change in IOP in the intervention groups was 7.43 mmHg lower | ‐ | 151 | ⊕⊕⊕⊝1 | Each of the studies included in this outcome compared apraclonidine vs placebo. |
| IOP increase of ≥ 5 mmHg between 2 and 24 hours | 280 per 1000 | 48 per 1000 | RR 0.17 (0.09 to 0.31) | 634 | ⊕⊕⊝⊝3 Low | Medications in this comparison were apraclonidine and brimonidine. |
| IOP increase of ≥ 10 mmHg between 2 and 24 hours | 202 per 1000 | 44 per 1000 | RR 0.22 (0.11 to 0.42) | 817 | ⊕⊝⊝⊝3,4 | Medications in this comparison were apraclonidine, brimonidine, dorozolamide, and pilocarpine. |
| Mean change in IOP from pre‐LTP between 2 and 24 hours | The mean change in IOP ranged across control groups from‐2.0 mmHg to 0.63 mmHg, for 3 included studies | The mean change in IOP in the intervention groups was 5.32 mmHg lower | ‐ | 151 | ⊕⊕⊕⊝1 | Each of the studies included in this outcome compared apraclonidine vs placebo. |
| Adverse events ‐ conjunctival blanching during study period | See comment | ‐ | 319 (2 studies) | ⊕⊕⊕⊝1 | 2 studies reported on conjunctival blanching; however, due to significant statistical heterogeneity (I2 = 95%), we did not perform a meta‐analysis. In both studies, conjunctival blanching was reported in more participants in the group that received an alpha‐2 agonist compared with participants who received placebo. 1 other study that reported only the range of participants who had conjunctival blanching also reported that this adverse event was more frequent in the groups receiving brimonidine vs placebo. Other adverse events reported for the comparison of medication vs placebo were lid retraction and conjunctival hyperemia, reported in 1 study each. | |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The certainty of the evidence was downgraded due to concerns of risk of bias: masking of outcomes assessors was difficult and in one study, the authors of some studies worked with the company making the study drug. 2 The certainty of the evidence was downgraded due to inconsistency of the outcome measurements in the individual studies: one favored medication and one favored placebo. 3 The certainty of the evidence was downgraded two levels due to concerns of very serious plausible bias: some studies in these analyses had issues with masking of outcomes assessors, high risk of selective reporting, and authors associated with the manufacturer of the study drug. 4 The certainty of the evidence was downgraded due to imprecision: there is a small number of events in the medication groups. | ||||||
| Brimonidine compared with apraclonidine for preventing temporarily increased IOP after LTP | ||||||
| Participant or population: people with glaucoma receiving LTP Intervention: brimonidine Comparison: apraclonidine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Apraclonidine | Brimonidine | |||||
| IOP increase of ≥ 5 mmHg within 2 hours | 29 per 1000 | 67 per 1000 | RR 2.28 (0.32 to 16.03) | 71 | ⊕⊝⊝⊝1,2,3 | 1 other study reported this outcome but found that no participants in either study group had an IOP increase of ≥ 5 mmHg. This study was not included in the meta‐analysis. |
| IOP increase of ≥ 10 mmHg within 2 hours | See comment | ‐ | ‐ | ‐ | 1 study reported that no participants given either medication had an IOP increase of ≥ 10 mmHg. Another study reported that only 1 eye that had received apraclonidine had an IOP spike > 10 mmHg, but this was not statistically significant given the size of the study (RR 0.33, 95% CI 0.02 to 7.32). | |
| Mean change in IOP from pre‐LTP within 2 hours | The mean change in IOP ranged across control groups from‐4.29 to ‐5.00 mmHg | The mean change in IOP in the intervention groups was 0.69 mmHg lower (2.56 lower to 1.17 higher) | ‐ | 71 | ⊕⊕⊕⊝3 | ‐ |
| IOP increase of ≥ 5 mmHg between 2 and 24 hours | This outcome was not reported for this comparison. | |||||
| IOP increase of ≥ 10 mmHg between 2 and 24 hours | This outcome was not reported for this comparison. | |||||
| Mean change in IOP from pre‐LTP between 2 and 24 hours | See comment | ‐ | ‐ | ‐ | 1 study reported that participants randomized to receive brimonidine had a mean (± SD) IOP reduction of 2.6 ± 3.6 mmHg, while participants randomized to receive apraclonidine had a mean IOP reduction of 2.3 ± 3.7 mmHg (MD ‐0.30 mmHg, 95% CI ‐2.41 to 1.81). | |
| Adverse events ‐ during study period | This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The certainty of the evidence was downgraded two levels due to imprecision: our effect measurement had a very wide confidence interval. 2 The certainty of the evidence was downgraded due to inconsistency: the RRs of the individual trials were very different. 3 The certainty of the evidence was downgraded due to concerns of risk of bias: masking of participants and personnel was unclear, and in one study, both eyes of the participants were included in the study and received different medications but the authors did not report if and how they took into account the interdependability of eyes. | ||||||
| Apraclonidine compared with pilocarpine for temporarily increased IOP after LTP | ||||||
| Participant or population: people with glaucoma receiving LTP Intervention: apraclonidine Comparison: pilocarpine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pilocarpine | Apraclonidine | |||||
| IOP increase of ≥ 5 mmHg within 2 hours | See comment | ‐ | ‐ | ‐ | 1 study reported that 8.8% of the apraclonidine group had an increase of ≥ 5 mmHg vs 4.4% of the pilocarpine group. These were not statistically different (RR 2.00, 95% CI 0.71 to 5.67). | |
| IOP increase of ≥ 10 mmHg within 2 hours | This outcome was not reported for this comparison. | |||||
| Mean change in IOP from pre‐LTP within 2 hours | The mean change in IOP was only reported in 1 study: ‐3.6 mmHg. The second study reported only the mean IOP at a time point rather than the mean change | The mean change in IOP in the intervention groups was 0.61 mmHg higher (0.44 lower to 1.66 higher) | ‐ | 277 | ⊕⊕⊕⊝1 | ‐ |
| IOP increase of ≥ 5 mmHg between 2 and 24 hours | See comment. | ⊕⊕⊝⊝1, 2 | 2 studies reported on this outcome and 1 favored apraclonidine while the other favored pilocarpine. Due to significant statistical heterogeneity (I2 = 91%), we did not perform a meta‐analysis. | |||
| IOP increase of ≥ 10 mmHg between 2 and 24 hours | 13 per 1000 | 12 per 1000 | RR 0.87 (0.14 to 5.63) | 390 | ⊕⊕⊝⊝2,3 | 1 additional study reported on this outcome but found that no participants in either study group had an IOP increase of ≥ 10 mmHg. This study was not included in the meta‐analysis. |
| Mean change in IOP from pre‐LTP between 2 and 24 hours | See comment. | ‐ | 277 | ⊕⊕⊝⊝1, 2 | 2 studies reported on this outcome and 1 favored apraclonidine while the other favored pilocarpine. Due to significant statistical heterogeneity (I2 = 92%), we did not perform a meta‐analysis. | |
| Adverse events ‐ during study period | This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The certainty of the evidence was downgraded due to concerns of plausible bias: one study included in the analyses had issues with masking of their participants due to the nature of the study design, and additionally the authors were employees of the company that manufactured the study drug. 2 The certainty of the evidence was downgraded due to inconsistency: in each outcome analysis, one study favored pilocarpine while the other favored apraclonidine. 3 The certainty of the evidence was downgraded due to imprecision: our effect measurement had a very wide confidence interval. | ||||||
| Medication given before LTP compared with the same medication given after LTP for temporarily increased IOP after LTP | ||||||
| Participant or population: people with glaucoma receiving LTP Intervention: medication before LTP Comparison: medication after LTP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Medication after LTP | Medication before LTP | |||||
| IOP increase of ≥ 5 mmHg within 2 hours | See comment | ‐ | ‐ | ‐ | 1 study comparing apraclonidine given before and after surgery reported no participants had an increase of ≥ 5 mmHg. Another study reported that 5.3% of participants given brimonidine before surgery had an IOP increase of ≥ 5 mmHg, compared with 7.5% of participants given brimonidine after surgery (RR 0.70, 95% CI 0.16 to 2.97). | |
| IOP increase of ≥ 10 mmHg within 2 hours | See comment | ‐ | ‐ | ‐ | 1 study comparing apraclonidine given before and after surgery reported no participants had an increase of ≥ 10 mmHg. Another study reported that only 1 study participant had this high of an increase, and they had been randomized to brimonidine before surgery. | |
| Mean change in IOP from pre‐LTP within 2 hours | See comment | ‐ | ‐ | ‐ | Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP was not reported in any study, but 1 study did report the mean IOP for the 2 study arms within 2 hours and it was not statistically different between the 2 groups. | |
| IOP increase of ≥ 5 mmHg between 2 and 24 hours | 38 per 1000 | 31 per 1000 | RR 0.82 (0.25 to 2.63) | 319 | ⊕⊕⊕⊝1 | ‐ |
| IOP increase of ≥ 10 mmHg between 2 and 24 hours | 6 per 1000 | 10 per 1000 | RR 1.55 (0.19 to 12.43) | 319 | ⊕⊝⊝⊝1,2 | ‐ |
| Mean change in IOP from pre‐LTP between 2 and 24 hours | The mean change in IOP was only reported in 1 study: ‐3.4 mmHg. The other 2 studies reported only the mean IOP at a time point rather than the mean change: range was13.0 mmHg to 18.6 mmHg | The mean change in IOP in the intervention groups was 1.07 mmHg lower (2.51 lower to 0.37 higher) | ‐ | 198 | ⊕⊕⊕⊝3 | ‐ |
| Adverse events ‐ during study period | This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The certainty of the evidence was downgraded due to concerns of plausible bias: masking of outcomes assessors was difficult and the authors of two studies work with the company making the study drug; one study had a high risk of selective reporting bias. 2 The certainty of the evidence was downgraded two levels due to imprecision: the included studies for which data were available had very wide confidence intervals. 3 The certainty of the evidence was downgraded due to inconsistency: of the three included studies, two favored medication given before surgery, and the other favored medication given after surgery. | ||||||
| Study ID | Types of participants | Number of treatment groups | Type of trabeculoplasty | Degree of laser | Comparison (baseline IOP in mmHg) |
| Uncontrolled glaucoma | 4 | ALT | 360 | Brimonidine before and after vs brimonidine before and vehicle after vs vehicle before and brimonidine after vs vehicle before and after (individual baseline IOPs not reported; range 23.4 ± 0.6 to 24.3 ± 0.7) | |
| POAG, pigmentary glaucoma, pseudoexfoliation syndrome, or ocular hypertension | 2 | ALT | 360 | Brimonidine (19.6 ± 4.5) vs apraclonidine (20.5 ± 4.6) | |
| POAG | 3 | ALT | 180 | Apraclonidine before and after (22.2 ± 3.6) vs apraclonidine before (23.9 ± 5.3) vs apraclonidine after (22.1 ± 3.2) | |
| Inadequately controlled IOP despite maximum‐tolerated medical therapy | 2 | ALT | 360 | Apraclonidine before and after vs placebo before and after (IOPs not available) | |
| Advanced glaucoma on maximal tolerated medical therapy with inadequate IOP control | 2 | ALT | 360 | Apraclonidine before and after (19.20 ± 5.95) vs placebo before and after (19.80 ± 5.23) | |
| Candidates for ALT, peripheral iridectomy, or posterior capsulotomy | 2 | ALT | 180 | Brimonidine before (20.3 ± 6) vs apraclonidine before (20.0 ± 5.1) *the reported IOPs included participants who received other types of glaucoma surgery besides ALT | |
| OAG | 3 (1 combination group not of interest in this study) | ALT | 180 | Apraclonidine before and after vs pilocarpine after ("all eyes had...an IOP greater than 21mmHg") | |
| Participants undergoing ALT | 4 | ALT | 360 | Brimonidine before and after (23.3) vs brimonidine before, placebo after (23.9) vs placebo before, brimonidine after (24.1) vs placebo before and after (24.0) | |
| POAG | 2 (opposite eyes) | SLT | 360 | Brimonidine before vs apraclonidine after (right eyes: 18, left eyes: 18.4) | |
| Exfoliative glaucoma and simple glaucoma | 2 | ALT | 360 | Pilocarpine before (34.9 ± 8.1) vs no treatment (33.3 ± 5.6) | |
| OAG requiring ALT | 2 | ALT | 180 | Dorzolamide before and after (18.3 ± 0.57) vs placebo before and after (19.6 ± 0.72) | |
| OAG | 2 | ALT | 360 | Apraclonidine before and after (22.6 ± 0.9) vs apraclonidine after (22.6 ± 0.6) | |
| Glaucoma | 2 | ALT | 180 | Latanoprost before (24.1) vs apraclonidine before (23.2) | |
| Glaucoma | 2 | ALT | 180 | Latanoprost before vs apraclonidine before | |
| POAG | 2 | ALT | 180 | Apraclonidine before and after (24.2 ± 9.0) vs placebo before and after (23.2 ± 6.8) | |
| Glaucoma | 4 | ALT | 180 | Brimonidine before and after (24.9) vs brimonidine before, placebo after (24.8) vs placebo before, brimonidine after (24.1) vs placebo before and after (24.6) | |
| Uncontrolled OAG | 2 | ALT | 180 | Acetazolamide before (23.6 ± 6.1) vs placebo before (23.7 ± 6.5) | |
| POAG | 2 | ALT | 360 | ALO 2145 (apraclonidine) before and after (20.1 ± 4.07) vs placebo before and after (25.0 ± 5.47) | |
| POAG | 2 | ALT | 180 | Apraclonidine before (23.2 ± 4.5) vs pilocarpine before (21.7 ± 3.5) | |
| OAG | 2 | ALT | 360 | ALO 2145 (apraclonidine) before and after (26.4 ± 3.0) vs placebo before and after (27.9 ± 6.9) | |
| OAG with disc and visual field damage | 5 | ALT | 360 | Apraclonidine before and after (27.2 ± 5.1) vs timolol before and after (27.6 ± 4.1) vs pilocarpine before and after (27.1 ± 5.1) vs dipivefrin before and after (25.9 ± 3.0) vs acetazolamide before and after (25.7 ± 3.9) | |
| POAG | 3 | ALT | 360 and 180 | Apraclonidine before and after, 180° ALT (26.1 ± 5.1) vs placebo before and after, 180° ALT (25.6 ± 3.4) vs apraclonidine before and after, 360° ALT (26.4 ± 3.1) | |
| ALT: argon laser trabeculoplasty; IOP: intraocular pressure; OAG: open‐angle glaucoma; POAG: primary open‐angle glaucoma; SLT: selective laser trabeculoplasty. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Alpha‐2 agonists vs placebo | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.20] |
| 2.1 Acetazolamide vs placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.52] |
| 2.2 Alpha‐2 agonists vs placebo | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.27] |
| 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐7.43 [‐10.60, ‐4.27] |
| 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Alpha‐2 agonists vs placebo | 5 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.31] |
| 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 9 | 817 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] |
| 5.1 Alpha‐2 agonists vs placebo | 7 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] |
| 5.2 Dorzolamide vs placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 5.22] |
| 5.3 Pilocarpine vs no treatment | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.71] |
| 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐5.32 [‐7.37, ‐3.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) Show forest plot | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.32, 16.03] |
| 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg) Show forest plot | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐2.56, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg) Show forest plot | 2 | 277 | Mean Difference (Random, 95% CI) | 0.61 [‐0.44, 1.66] |
| 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.14, 5.63] |
| 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.63] |
| 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.19, 12.43] |
| 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg) Show forest plot | 3 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Alpha‐2 agonists (mmHg) | 3 | 198 | Mean Difference (Random, 95% CI) | ‐1.07 [‐2.51, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Conjunctival blanching (brimonidine vs placebo) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

