குழந்தைகள் மற்றும் பெரியவர்களது பல்சொத்தையைத் தடுக்க சைலிடோல் (xylitol) கொண்ட பொருட்கள்
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: parallel (2 arms) Location: clinical centres of university dental schools, USA Number of centres: 3 Recruitment period: April 2007 to September 2008 | |
| Participants | Inclusion criteria: aged 21 to 80 years; minimum of one coronal or root surface cavitated caries lesion; minimum of 12 teeth with exposed coronal or root surfaces; ability to read and understand study materials in English (the caries criteria were meant to ensure the inclusion of participants with an elevated risk of experiencing caries) Exclusion criteria: more than 10 teeth with caries lesions; type IV periodontitis (pocket depths or attachment loss greater than 6 mm); long‐term antibiotic use; requiring antibiotic treatment before dental treatment; history of head and neck radiotherapy; history of adverse reaction to either the active or placebo ingredients; serious illness that would interfere with participation; plans to leave the area within the following 3 years; no telephone; co‐inhabiting with someone already enrolled in the study Baseline caries: (D₂FS) Gp A: mean 18.8 (SD 12.8); Gp B: mean 18.5 (SD 12.5) Age at baseline (years): Gp A: mean 46.3 (SD 13.5); Gp B: mean 47.7 (SD 13.7) Any other details of important prognostic factors: fluoride exposure (toothpaste and professionally applied topical fluoride) was similar in both groups; all 3 study areas had fluoridated water Number randomised: 691 (Gp A: 344; Gp B: 347) Number evaluated: 669 (Gp A: 331; Gp B: 338) ‐ numbers including data from all 3 centres (see comment in risk of bias table below under 'Other bias') | |
| Interventions | Comparison: xylitol lozenges versus placebo lozenges Gp A (n = 344): one peppermint flavoured lozenge (1 g xylitol) dissolved in the mouth five times per day (total dose = 5 g xylitol per day) Gp B (n = 347): as above but containing sucralose (considered to be inert, i.e. neither causes nor prevents caries) instead of xylitol Duration of treatment: 33 months | |
| Outcomes |
| |
| Notes | Sample size calculation: "80% power to detect a 20% reduction in the D₂FS increment assuming a two‐tailed test with a type I error rate of 5%. The target sample size of 750 allowed a 10% attrition rate per year" The information and quotes used in this table and the risk of bias table below are from both the papers listed under Bader 2013 in the reference section (one paper is dedicated to design/methods) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out using a web‐based randomization application process. Allocation assignments were stratified by site and age group (≥ 50, < 50 yrs.) in permuted blocks of varying sizes within each stratum" Comment: this is an appropriate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "permuted blocks of varying sizes within each stratum" Comment: the use of varying sizes of random permuted blocks implies that a reasonable attempt was made to prevent those admitting participants from knowing upcoming assignments. We feel that this was probably done properly in this study |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The placebo lozenge was identical in size and color to the active lozenge...Both the active and placebo lozenges were peppermint flavored" and "Staff and participants were blinded to treatment assignment" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The placebo lozenge was identical in size and color to the active lozenge...Both the active and placebo lozenges were peppermint flavored" and "Staff and participants were blinded to treatment assignment" Comment: participants and personnel would not know to which group a participant was assigned |
| Incomplete outcome data (attrition bias) | Low risk | Only 3% of randomised participants were not included in the final analysis (Gp A: 4%; Gp B: 3%) Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | All raw data is publicly available on the study website (www.xactstudy.org). The authors also provided us with the mean and SD for the 33 month increment |
| Other bias | Low risk |
|
| Methods | Trial design: cluster (3 arms) Location: interventions given in schools in Tartu, Estonia; clinical examinations took place in standard dental units of the Department of Stomatology, University of Tartu Number of centres: 10 schools/4 dental units Recruitment period: January to February 2008 | |
| Participants | Inclusion criteria: first and second grade primary school children Exclusion criteria: children who were not at school on the day of the baseline clinical examination Baseline caries: (d₄‐₆mfs + D₄‐₆MFS) Gp A: mean 11.22 (SE 0.74); Gp B: mean 12.71 (SE 0.8) Age at baseline (years): Gp A: mean 8.2 (SD 0.5); Gp B: mean 8.1 (SD 0.6) Any other details of important prognostic factors: "fluoride content in drinking water is low" Number randomised: 320 (Gp A: 156; Gp B: 164) (numbers only including the two relevant arms) Number evaluated: 252 (Gp A: 126; Gp B: 126) | |
| Interventions | Comparison: the 3 arms were as follows: 1) erythritol candy (excluded: erythritol is considered, like xylitol, to be caries‐preventive so cannot be used as a control group) 2) xylitol candy 3) sorbitol candy Gp A (n = 156): four candies (90% xylitol) three times per day (total dose = 7.5 g xylitol per day) Gp B (n = 164): as above but with sorbitol instead of xylitol Duration of treatment: 3 years * Sorbitol is considered to be inert (i.e. neither causes nor prevents caries) and therefore is commonly used as a control in xylitol studies | |
| Outcomes |
| |
| Notes | Sample size calculation: based on previous study and target was achieved | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At enrolment...school classes were randomly divided into three groups...The list of all classes from all participating schools...was used as a sample frame. The statistician...allocated the classrooms according to computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealment of allocation was maintained by the Cargill company up to the main statistical analyses and fixing of the data" Comment: it appears that allocation was carried out remotely (third‐party allocation). We feel that this was probably done properly in this study |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" and "Each child consumed four candies three times per school day, not knowing which group they belonged to" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind clinical examinations of the children in all groups were completed four times" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | High risk | 21% of randomised participants were not included in the final analysis (Gp A: 19%; Gp B: 23%). If the missing participants had higher mean caries increments in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | High risk | Inappropriate combining of deciduous and permanent teeth in the results (dmfs and DMFS) |
| Other bias | Low risk | Quote: "clinical examinations of the children in all groups were completed...by four trained and calibrated investigators" and "Consistency of the ICDAS codes by each of the examiners and between the examiners was very high (K > 0.9)" Comment: we consider that the risk of differential diagnostic activity was low |
| Methods | Trial design: cluster (5 arms) Location: interventions given in schools in Kotka, Finland; clinical examinations took place in local dental clinics Number of centres: 21 schools Recruitment period: not stated | |
| Participants | Inclusion criteria: not stated but all children were in grade 4 (10 years old) Exclusion criteria: not stated but from the study flow diagram (figure 1) systemic disease appears to be an exclusion criterion Baseline caries: (D₃MFS) Gp A: mean 0.35 (SD 0.8); Gp B: mean 0.27 (SD 0.7) Age at baseline (months): Gp A: mean 123.4 (SD 4.2); Gp B: mean 122.6 (SD 3.6) Any other details of important prognostic factors: "all subjects would have accessed water with a fluoride content not exceeding 1.5 mg/mL"; the study was conducted in a low‐caries prevalence population (i.e. average DMFT of 12‐year‐olds approximately 0.8 for this area, compared to the Finnish average of 1.2) Number randomised: 228 (Gp A: 110; Gp B: 118) (numbers only including the two relevant arms) Number evaluated: 200 (Gp A: 99; Gp B: 101) | |
| Interventions | Comparison: the 5 arms were as follows: 1) xylitol/maltitol* lozenges for 1 year (excluded: we felt it was more appropriate to use the arm with longer‐term use) 2) xylitol/maltitol lozenges for 2 years (included) 3) erythritol/maltitol lozenges for 1 year (excluded: erythritol is considered, like xylitol, to be caries‐preventive so cannot be used as a control group) 4) erythritol/maltitol lozenges for 2 years (excluded: see arm 3) 5) control (included: no lozenges and no additional prevention) Gp A (n = 110): eight xylitol/maltitol lozenges per day (two in the morning, three after lunch, and three before the child went home) (total dose = 4.7 g xylitol plus 4.6 g maltitol per day) Gp B (n = 118): children received the same comprehensive routine caries prevention as those in the xylitol group, but no lozenges Duration of treatment: children in Gp A received the lozenges for 2 years | |
| Outcomes |
| |
| Notes | Sample size calculation: "It was estimated that a 20% difference between the control and each study group...would be clinically relevant...To avoid the risk of a false‐negative result (the type II error) and for the test to have a 90% power to detect a statistically significant difference, even when taking attrition of 10% or less per year into account, one hundred subjects per group were required" Adverse effects: mentioned but no usable data Funding source: CSM Leaf (Turku, Finland) provided the lozenges | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 21 participating schools were assigned as clusters by means of restricted randomization and blocking into five different groups of approximately the same size" and "After the determination of the groups, they were randomly given a role as one of the four groups receiving colour‐coded lozenges...or a nonlozenge control group, by Comment: this is an appropriate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The generation of the random allocation sequence, enrolling the participants, ascertaining treatment assignment and administering the intervention was performed by the Chief Dental Officer of Kotka who did not take part in the clinical examinations. The allocation sequence was concealed until all analyses completed" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Although the study is described as double‐blinded, we are only interested in the 2‐year xylitol arm and the control group who received no lozenges. Therefore we cannot discount that this would have an effect on the behaviour/motivation (in terms of oral care) of the participants which may affect the results (for example, control group participants may overcompensate by taking extra care of their oral health, or conversely, the xylitol group may feel they do not need to take as much care of their oral health as they usually would due to an expected effect of the xylitol) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the examining dentist...the dentists interpreting the radiographs...were all completely blinded and not aware of the group the child belonged to until all the data analyses had been carried out" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | Low risk | 12% of randomised participants were not included in the final analysis (Gp A: 10%; Gp B: 14%). This amount of attrition may be considered low for a 4‐year study. Also, one school of 20 participants in Gp A discontinued the intervention during the first year, but they were included in the analyses on an intention‐to‐treat basis Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Unclear risk | There were no usable data reported for adverse events. This should be considered an important outcome in xylitol trials |
| Other bias | High risk |
|
| Methods | Trial design: parallel (3 arms) Location: communities in the Republic of the Marshall Islands (in the northern Pacific Ocean) Number of centres: unclear Recruitment period: April to August 2006 | |
| Participants | Inclusion criteria: children aged 9 to 15 months Exclusion criteria: in the lower 10th percentile of US standard weight and height; history of oesophageal or digestive disease; congenital craniofacial malformation; history of adenoidectomy, tympanostomy tubes, or tympanic membrane perforations (due to secondary outcome of reduction of acute otitis media) Baseline caries: not stated Age at baseline (months): Gp A: mean 15.9 (SD 2.6); Gp B: mean 13.7 (SD 2.4); Gp C: mean 15.6 (SD 2.7) Gender: Gp A: 57.6% female; Gp B: 56.3% female; Gp C: 48.3% female Any other details of important prognostic factors: high rate of early childhood caries on the islands; drinking water contains no appreciable fluoride; generally poor diets Number randomised: 100 (Gp A: 35; Gp B: 33; Gp C: 32) Number evaluated: 94 (Gp A: 33; Gp B: 32; Gp C: 29) | |
| Interventions | Comparison: xylitol topical oral syrup (A) versus xylitol topical oral syrup (B) versus xylitol topical oral syrup (C) Gp A (n = 35): three doses of syrup per day: two doses contained 4 g xylitol each, plus one dose with 2 g sorbitol (total dose = 8 g xylitol plus 2 g sorbitol per day) Gp B (n = 33): three doses of syrup per day: each dose contained 2.67 g xylitol (total dose = 8 g xylitol per day) Gp C (n = 32): three doses of syrup per day: one dose contained 2.67 g xylitol, plus two doses contained 2 g sorbitol each (total dose = 2.67 g xylitol plus 4 g sorbitol per day) Duration of treatment: 1 year
| |
| Outcomes |
| |
| Notes | Sample size calculation: "We estimated that the rate of decayed cavitated lesions for children at 24 months of age was 60% in the control group and 30% in the xylitol‐treated groups. Based on 80% power to detect a significant difference (2‐sided P=.05) between the xylitol‐treated and control groups, 32 children were required for each study group" Adverse effects: percentage experiencing loose stools or diarrhoea is reported per group. Appears to be at participant level (i.e. not counting multiple events experienced by the same child) but the percentages stated do not equate to whole persons (e.g. 11.7% of 33 participants is 3.861 persons) Funding source: grants from: a) the Health Resources and Services Administration Maternal and Child Health Bureau; and b) the National Institute of Dental and Craniofacial Research. Danisco USA donated the raw materials for making the syrups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were given identification numbers that had been randomly assigned to study groups by a statistician using block randomization and the sample function of commercially available statistical software...Block sizes of 30 and 15 were used for the Laura district, and block sizes of 36 and 18 were used for the Delap district. Except for the statistician, all study team members were blinded until study completion" Comment: this is an appropriate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "subjects were given identification numbers that had been randomly assigned to study groups by a statistician using block randomization and the sample function of commercially available statistical software...Block sizes of 30 and 15 were used for the Laura district, and block sizes of 36 and 18 were used for the Delap district. Except for the statistician, all study team members were blinded until study completion" Comment: use of different block sizes administered by a statistician implies that a reasonable attempt was made to prevent those admitting participants from knowing upcoming assignments. We feel that this was probably done properly in this study |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" and "The syrups were matched for color, taste, and viscosity" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The examiner was always blinded to study group assignment" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only 6% of randomised participants were not included in the final analysis (Gp A: 6%; Gp B: 3%; Gp C: 9%). Attrition was actually 16% but most of these had at least an interim examination and were included in the intention‐to‐treat analysis. The reasons for drop‐out in each group were the same and in similar proportions (either moved off island or parent stopped giving syrup and withdrew) Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Unclear risk | There were no usable data reported for adverse events. This should be considered an important outcome in xylitol trials |
| Other bias | Low risk | Quote: "A single dental examiner" and "Compared with another examiner (P.M.), the study examiner demonstrated excellent reliability for caries diagnosis (interrater correlation coefficient, 1.00 at the prestudy dental examination and 0.96 at the midstudy examination)" |
| Methods | Trial design: parallel (2 arms) Location: public dental clinic in Lycksele, Sweden Number of centres: 1 Recruitment period: not stated | |
| Participants | Inclusion criteria: all healthy 2‐year‐old children, born in 2000 and the first quarter of 2001, attending the public dental clinic in Lyycksele Exclusion criteria: children with severe disabilities; children that did not co‐operate for an oral inspection Baseline caries: not stated Age at baseline: all children were 2 years old Gender: Gp A: 49% female; Gp B: 46% female Any other details of important prognostic factors: not stated Number randomised: 132 (Gp A: 66; Gp B: 66) Number evaluated: 118 (Gp A: 55; Gp B: 63) | |
| Interventions | Comparison: xylitol sucking tablets versus control (no tablets and no additional prevention) Gp A (n = 66): one tablet (0.48 g xylitol) slowly dissolved in the mouth per day, at bedtime after toothbrushing, for the first 6 months, then two tablets (one in the morning and one in the evening) for another year (total dose = 1 g xylitol per day) Gp B (n = 66): children received the same routine prevention and restorative care and advice as those in the xylitol group, but no tablets Duration of treatment: children in Gp A received the tablets for 1.5 years | |
| Outcomes |
| |
| Notes | Sample size calculation: only a post‐investigation sample size analysis was performed Adverse effects: not reported Funding source: grants from: a) the County of Västerbotten; b) the Patent Revenue Fund for Dental Prophylaxis; and c) the Swedish Dental Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the children were randomly assigned" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the children were randomly assigned" Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | It was not possible to blind participants and personnel because the control group had no tablets. Therefore we cannot discount that this would have an effect on the behaviour/motivation (in terms of oral care) of the participants/carers which may affect the results (for example, control group participants/carers may overcompensate by taking extra care of their oral health, or conversely, the xylitol group may feel they do not need to take as much care of their oral health as they usually would due to an expected effect of the xylitol) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "single‐blind" and "Caries was registered by tactile and visual examination in a dental chair by two blinded calibrated examiners" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | High risk | 11% of randomised participants were not included in the final analysis, but this was unbalanced with appreciably higher attrition in the xylitol group (Gp A: 17%; Gp B: 5%). If the higher rate of attrition was related to the intervention, then this could be considered a risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Adverse events should be considered an important outcome in xylitol trials, but were not considered in this study |
| Other bias | High risk |
|
| Methods | Trial design: parallel (4 arms) Location: public dental clinic in Hyltebruk, Sweden Number of centres: 1 Recruitment period: not stated | |
| Participants | Inclusion criteria: healthy 12‐ to 13‐year‐olds Exclusion criteria: not stated Baseline caries: not clearly stated (presented graphically) but there was a statistically significant difference between Gp A and Gp B Age at baseline: all children were 12 to 13 years old Gender: not stated Any other details of important prognostic factors: the study site was chosen due to a relatively high caries frequency and stable population; the water supply had a fluoride concentration of about 0.1 ppm Number randomised: 322 (Gp A: 78; Gp B: 83; Gp C: 78; Gp D: 83) Number evaluated: 284 (Gp A: 67; Gp B: 74; Gp C: 68; Gp D: 75) | |
| Interventions | Comparison: the 4 arms were as follows: 1) xylitol toothpaste with sorbitol and normal level of fluoride 2) xylitol toothpaste with sorbitol and low level of fluoride 3) toothpaste with sorbitol and normal level of fluoride 4) toothpaste with sorbitol and low level of fluoride Gp A (n = 78): twice daily brushing with toothpaste containing 0.8% sodium monofluorophosphate, 3% xylitol, 6% sorbitol Gp B (n = 83): twice daily brushing with toothpaste containing 0.03% sodium fluoride, 3% xylitol, 6% sorbitol Gp C (n = 78): twice daily brushing with toothpaste containing 0.8% sodium monofluorophosphate, 9% sorbitol Gp D (n = 83): twice daily brushing with toothpaste containing 0.03% sodium fluoride, 9% sorbitol Duration of treatment: 3 years
| |
| Outcomes |
| |
| Notes | Sample size calculation: 65 to 75 participants per group to allow 95% power to detect a true difference between group means of approximately 4 DFS (theoretical calculations made via a pilot study) Adverse effects: not reported Funding source: Kema Nobel Consumer Goods Division (Stockholm, Sweden) provided toothpastes (also states they provided "economical support" but this may just be the toothpastes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All children taking part were randomly distributed into four experimental groups" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All children taking part were randomly distributed into four experimental groups" Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "They were produced and delivered in 100‐gram tubes, marked with the name 'Toothpaste' in different colours...corresponding to groups 1‐4 respectively" and "The study was carried out double‐blind for subjects as well as for examiners" Comment: participants and personnel would not know which group a participant was assigned to |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The study was carried out double‐blind for subjects as well as for examiners" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | Low risk | 12% of randomised participants were not included in the final analysis (Gp A: 14%; Gp B: 11%; Gp C: 13%; Gp D: 10%). This amount of attrition may be considered as low for a 3‐year study and the reasons were mainly due to them moving away from the area Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | High risk | The caries data have not been reported adequately (on a small graph) or in a way that would allow them to be included in a meta‐analysis (i.e. there is no measure of variance (SD, SE or 95% CIs) reported for the mean DFS scores). Adverse events should be considered an important outcome in xylitol trials, but were not considered in this study |
| Other bias | Low risk | Quote: "The children were clinically examined...by one of the authors" and "A pilot study...was performed to train the examiner and to standardize the clinical and radiographic registrations" Comment: we consider that the risk of differential diagnostic activity was low |
| Methods | Trial design: parallel (2 arms) Location: schools in the metropolitan area of San José, Costa Rica Number of centres: 17 schools Recruitment period: not stated | |
| Participants | Inclusion criteria: 8‐ to 10‐year‐olds (however, children younger than 8 years yet at the scholastic third grade level, or older than 10 years yet still at the scholastic fifth grade level, were accepted if they met the following criterion); minimum of one DFS Exclusion criteria: not stated Baseline caries: (DFS) Gp A: mean 5.6 (SD 2.9); Gp B: mean 5.5 (SD 3.0) Age at baseline: unclear due to first inclusion criterion above but stratified by age Gender: not stated but stratified by gender Any other details of important prognostic factors: the water supply had a fluoride concentration of less than 0.1 ppm; fluoridated table salt was introduced in Costa Rica around the time the study began, but availability was equal for all participants Number randomised: 2630 (not reported by group) Number evaluated: 1677 (Gp A: 840; Gp B: 837) | |
| Interventions | Comparison: xylitol plus fluoride toothpaste versus fluoride toothpaste Gp A (n = 840 evaluated): twice daily brushing for 1 minute with toothpaste containing 10% xylitol plus 0.243% sodium fluoride (1100 ppm fluoride); participants were instructed to spit out the slurry after brushing and rinse thoroughly with water; eating/drinking was discouraged for at least 30 minutes following brushing; application of toothpaste to toothbrush was supervised Gp B (n = 837 evaluated): as above but without xylitol Duration of treatment: 3 years | |
| Outcomes |
| |
| Notes | Sample size calculation: the authors state that the study met the sample size requirements of the American Dental Association (ADA) guidelines for clinical trials of anticaries toothpaste efficacy (minimum 80% power to detect 10% difference) Adverse effects: none observed Funding source: several authors employed by Colgate‐Palmolive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each child was randomly assigned to use either..." Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each child was randomly assigned to use either..." Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Product was provided in plain white tubes (identified only by a solid colour‐coded label) in order to maintain the double‐blindness" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Product was provided in plain white tubes (identified only by a solid colour‐coded label) in order to maintain the double‐blindness" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | High risk | 36% of randomised participants were not included in the final analysis (not reported by group). If the missing participants had higher mean caries increments in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Caries evaluations were carried out by a single dentist who was calibrated before and during the study. We consider that the risk of differential diagnostic activity was low |
| Methods | Trial design: parallel (2 arms) Location: schools in the central plateau of Costa Rica (including San José, Cartago, Alajuela, and Heredia) Number of centres: 28 schools Recruitment period: not stated | |
| Participants | Inclusion criteria: 7‐ to 12‐year‐olds; minimum of one decayed/filled surface (DFS) Exclusion criteria: not stated Baseline caries: (DFS) Gp A: mean 3.69 (SD 2.2); Gp B: mean 3.7 (SD 2.19) Age at baseline: all children were 7 to 12 years old; stratified by age Gender: not stated but stratified by gender Any other details of important prognostic factors: the water supply had a fluoride concentration of less than 0.1 ppm Number randomised: 3394 (not reported by group) Number evaluated: 2539 (Gp A: 1280; Gp B: 1259) | |
| Interventions | Comparison: xylitol plus fluoride toothpaste versus fluoride toothpaste Gp A (n = 1280 evaluated): twice daily brushing for 1 minute with toothpaste containing 10% xylitol plus 0.836% sodium monofluorophosphate (1100 ppm fluoride); participants were instructed to spit out the slurry after brushing and rinse thoroughly with water; eating/drinking was discouraged for at least 30 minutes following brushing; application of toothpaste to toothbrush was supervised Gp B (n = 1259 evaluated): as above but without xylitol Duration of treatment: 30 months | |
| Outcomes |
| |
| Notes | Sample size calculation: not stated Adverse effects: none observed Funding source: several authors employed (or formerly employed) by Colgate‐Palmolive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children accepted for participation were stratified into two balanced groups within the participating schools on the basis of age and sex" Comment: although the word 'random' is not used, and we were unable to obtain a response from the corresponding author, we are confident that participants would have been randomised on the basis that: a) the Sintes 1995 study with three of the same authors was randomised; and b) we have information from another Cochrane review that toothpaste trials by Colgate‐Palmolive are normally randomised. However, there is insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Each subject was provided the assigned study dentifrice in tubes with colour‐coded labels, to ensure that the use of the assigned dentifrice was maintained throughout the study" Comment: the use of colour‐coded labels implies that blinding was carried out. We feel that this was probably done properly in this study |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" Comment: we assumed that this refers to blinding of participants and outcome assessors |
| Incomplete outcome data (attrition bias) | High risk | 25% of randomised participants were not included in the final analysis (not reported by group). If the missing participants had higher mean caries increments in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Caries evaluations were carried out by a single dentist who was calibrated before and during the study. We consider that the risk of differential diagnostic activity was low |
| Methods | Trial design: parallel (3 arms) Location: healthcare centres in Muurame and Korpilahti, Finland Number of centres: not stated Recruitment period: September 2004 to February 2007 | |
| Participants | Inclusion criteria: healthy child; parents willing to use the novel slow‐release pacifier and the tablet; child started receiving tablet before age of 2 months (if they did not, but the parents were motivated to remain in the study, they were offered the possibility of administering the crushed up tablet on a spoon) Exclusion criteria: not stated Baseline caries: children were aged 2 months so had no teeth and no caries Age at baseline: 1 to 2 months Gender: Gp A: 57% male; Gp B: 46% male Any other details of important prognostic factors: not stated Number randomised: 108 (Gp A: 54; Gp B: 54) Number evaluated: 62 (Gp A: 33; Gp B: 29) | |
| Interventions | Comparison: the 3 arms were as follows: 1) probiotic bacteria (BB‐12) plus xylitol tablet via a slow‐release pacifier or a spoon (excluded) 2) xylitol tablet via a slow‐release pacifier or a spoon 3) sorbitol tablet via a slow‐release pacifier or a spoon Gp A (n = 54): one tablet (100 mg or 300 mg xylitol ‐ depending on size of pacifier) twice per day via a novel slow‐release pacifier or crushed on a spoon (total dose = 200 mg to 600 mg xylitol per day) Gp B (n = 54): as above but with sorbitol instead of xylitol Duration of treatment: tablets were given from the age of 1 to 2 months until the child was 2 years of age * Sorbitol is considered to be inert (i.e. neither causes nor prevents caries) and therefore is commonly used as a control in xylitol studies | |
| Outcomes |
| |
| Notes | Sample size calculation: based on microbial colonisation percentages rather than on caries Adverse effects: not reported Funding source: personal grants from: a) the Emil Aaltonen and Sohlberg Foundations; b) the Finnish Dental Society Apollonia and the Finnish Dental Association. All study materials were donated by industry but they did not provide any financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The infants were assigned to 1 of 3 study groups by one of the authors...according to a randomization list which had been previously computer‐generated in blocks of 3" Comment: this is an appropriate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The infants were assigned to 1 of 3 study groups by one of the authors...according to a randomization list which had been previously computer‐generated in blocks of 3. The block randomization was prepared by a statistician with no clinical involvement in the trial. All the study personnel and participants were blinded to treatment assignment" Comment: blocks of three would mean that it was difficult to conceal the random sequence if not done properly. However, it sounds as if the statistician carried this out remotely so that there could be no foreknowledge of intervention assignment by the study personnel |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" and "Test tablets of a similar size and colour were administered" and "All the study personnel and participants were blinded to treatment assignment as well as the colour code of the tablet bottles for the duration of the study" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" and "All the study personnel and participants were blinded to treatment assignment as well as the colour code of the tablet bottles for the duration of the study. Only one of the authors...had the code...However, she did not participate in producing or analysing the data at any stage of the trial and had no contact with the study participants" Comment: outcome assessment appears to have been adequately blinded |
| Incomplete outcome data (attrition bias) | High risk | 43% of randomised participants were not included in the final analysis (Gp A: 38%; Gp B: 46%). If the missing participants had a higher risk of caries in one group than the other, as the attrition rate increased, so would over/understatement of the risk ratio |
| Selective reporting (reporting bias) | Unclear risk | Adverse events should be considered an important outcome in xylitol trials, but were not considered in this study |
| Other bias | Low risk | Quote: "All children were examined by a dentist...trained specifically for the study examination" and "The intraexaminer agreement percentage at the surface level was 97.3%" Comment: we consider that the risk of differential diagnostic activity was low |
| Methods | Trial design: parallel (2 arms) Location: paediatric dental clinic in San Francisco, USA Number of centres: 1 Recruitment period: January 2007 to January 2008 | |
| Participants | Inclusion criteria: healthy children aged 6 to 35 months; mothers using the wipes were primary caregivers (> 8 hours per day); minimum of one active caries lesion within a year Exclusion criteria: oral or systemic diseases; mother or child had taken antibiotics (or other medication which may potentially affect oral flora) in the previous 3 months Baseline caries: (dmfs > 0) Gp A: 2; Gp B: 1 Age at baseline (months): Gp A: mean 16.7 (SD 8.6); Gp B: mean 17.9 (SD 8.6) Gender: Gp A: 64% male; Gp B: 59% male Any other details of important prognostic factors: 80% of the population attending this clinic were of low socioeconomic status Number randomised: 44 (Gp A: 22; Gp B: 22) Number evaluated: 44 (Gp A: 22; Gp B: 22) (there were 2 drop‐outs in Gp A and 5 in Gp B but all participants were analysed on an intention‐to‐treat basis, and imputation procedure is clearly stated) | |
| Interventions | Comparison: xylitol wipes versus placebo wipes Gp A (n = 22): mothers used two wipes to clean the teeth and gums three times per day (in addition to their normal toothbrushing) (total dose = 4.2 g xylitol per day) Gp B (n = 22): as above but without xylitol Duration of treatment: 1 year | |
| Outcomes |
| |
| Notes | Sample size calculation: based on previous study and on maternal microbial transmission rather than on caries Adverse effects: none observed Funding source: "This research project was supported by the California Society of Pediatric Dentistry Foundation, a Graduate Scientific Research Award from American Academy of Pediatric Dentistry, and NIH/NIDCR grant U54 DE019285. Xylitol and placebo wipes were provided free of charge from DR Products Inc" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were then randomized...using a pre‐set computer‐generated random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "The groups were blinded as Groups A and B" and "Only one investigator...who was not involved in any patient contact, dental examinations, and microbiological assays, knew the group assignment" Comment: it appears that allocation was carried out remotely by an investigator who did not know into which group they were allocating participants |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blinded" and "The placebo wipes were custom synthesized...for the study and were identical in appearance and composition" Comment: participants and personnel would not know to which group a participant was assigned |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blinded" and "Only one investigator...who was not involved in any patient contact, dental examinations, and microbiological assays, knew the group assignment. All the other investigators involved in participant contact, microbiological assays, and statistical analysis were blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | 16% dropped out between baseline and final examination (Gp A: 9%; Gp B: 23%). Although all participants were included in the analysis on an intention‐to‐treat basis and imputation rules were clearly stated, we cannot be certain that the study is free of attrition bias |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Quote: "The two dental examiners were trained by one investigator (LZ) to score caries lesions in a standard pediatric dental setting. Cross calibration on inter‐examiner reliability was performed on seven children (15% of the study population). The two examiners showed 100% agreement on caries scoring, with Kappa = 1 (P < 0.01)" Comment: we consider that the risk of differential diagnostic activity was low |
CIs = confidence intervals; DFS = decayed filled surfaces; DMFS/dmfs = decayed missing filled surfaces; DMFT = decayed missing filled teeth; Gp = group; ppm = parts per million; SD = standard deviation; SE = standard error
In the above tables, where a number appears after the 'D', this indicates the severity of the carious lesion progressing from enamel (1 to 3) to dentin (4 to 6)
Characteristics of excluded studies [ordered by year of study]
Ir a:
| Study | Reason for exclusion |
| Xylitol (plus sorbitol and 'other polyols') gum versus sucrose (plus sorbitol and 'other polyols') gum. No appropriate control group as sucrose is cariogenic (causes caries) | |
| Cluster randomised controlled trial but with serious problems with the randomisation procedure (some of the clusters were self selecting i.e. some schools did not allow chewing of gum in the classroom and therefore they were the controls and were not randomly assigned) | |
| Sealants versus 2‐year use of xylitol gum versus 3‐year use of xylitol gum. No appropriate comparison of interest | |
| Cluster randomised controlled trial but with serious problems with the randomisation procedure (some of the clusters were selectively rather than randomly assigned) | |
| Cluster randomised controlled trial but with one cluster per arm, and therefore of inappropriate design | |
| Children using xylitol gum but not brushing teeth during daycare hours versus children brushing their teeth with fluoride toothpaste during daycare hours (after lunch) but not using xylitol gum. No appropriate control group | |
| Cluster randomised controlled trial but with two clusters per arm, and therefore of inappropriate design | |
| The study authors kindly provided us with a prepublication copy of the study and we were able to see that the intervention was only given for 9 months. Our inclusion criterion states that the intervention must be given for at least 1 year |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 33 months follow‐up (DFS) Show forest plot | 1 | 669 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.58, 0.30] |
| Analysis 1.1  Comparison 1 Adults: xylitol lozenges versus control lozenges, Outcome 1 Caries increment at 33 months follow‐up (DFS). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 4 years follow‐up (DMFS) Show forest plot | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.99, 1.55] |
| Analysis 2.1  Comparison 2 Children: xylitol lozenges versus no treatment, Outcome 1 Caries increment at 4 years follow‐up (DMFS). | ||||
| 2 Number with caries increment at 4 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.26] |
| Analysis 2.2 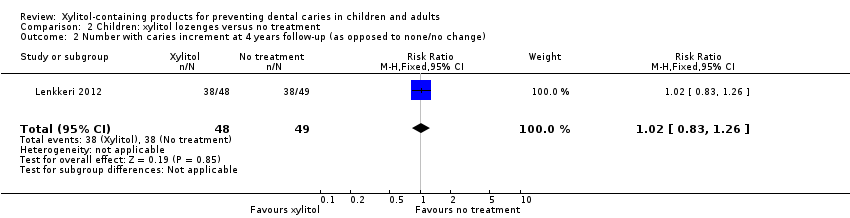 Comparison 2 Children: xylitol lozenges versus no treatment, Outcome 2 Number with caries increment at 4 years follow‐up (as opposed to none/no change). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of decayed primary teeth at 1 year follow‐up Show forest plot | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.03, ‐0.18] |
| Analysis 3.1 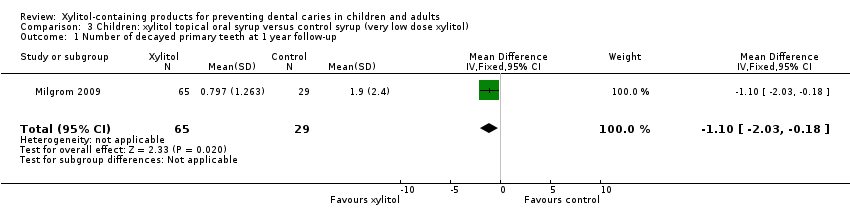 Comparison 3 Children: xylitol topical oral syrup versus control syrup (very low dose xylitol), Outcome 1 Number of decayed primary teeth at 1 year follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 2 years follow‐up (dmfs) Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.12, 0.28] |
| Analysis 4.1  Comparison 4 Children: xylitol sucking tablets versus no treatment, Outcome 1 Caries increment at 2 years follow‐up (dmfs). | ||||
| 2 Number with caries increment at 2 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.45] |
| Analysis 4.2  Comparison 4 Children: xylitol sucking tablets versus no treatment, Outcome 2 Number with caries increment at 2 years follow‐up (as opposed to none/no change). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 2.5 to 3 years follow‐up (Prevented Fraction) Show forest plot | 2 | Prevented Fraction (Fixed, 95% CI) | 0.13 [0.08, 0.18] | |
| Analysis 5.1  Comparison 5 Children: xylitol plus fluoride toothpaste versus fluoride toothpaste, Outcome 1 Caries increment at 2.5 to 3 years follow‐up (Prevented Fraction). | ||||
| 2 Caries increment at 2.5 to 3 years follow‐up (DFS) Show forest plot | 2 | 4216 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.42, ‐0.14] |
| Analysis 5.2 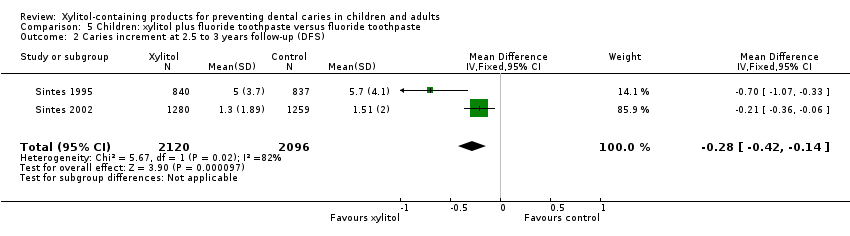 Comparison 5 Children: xylitol plus fluoride toothpaste versus fluoride toothpaste, Outcome 2 Caries increment at 2.5 to 3 years follow‐up (DFS). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with caries increment at 4 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.69, 13.65] |
| Analysis 6.1  Comparison 6 Children: xylitol tablets versus control (sorbitol) tablets, Outcome 1 Number with caries increment at 4 years follow‐up (as opposed to none/no change). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with caries increment at 1 year follow‐up (as opposed to none/no change) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |
| Analysis 7.1 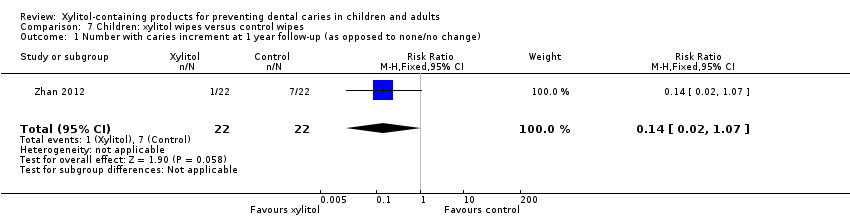 Comparison 7 Children: xylitol wipes versus control wipes, Outcome 1 Number with caries increment at 1 year follow‐up (as opposed to none/no change). | ||||

Study flow diagram.
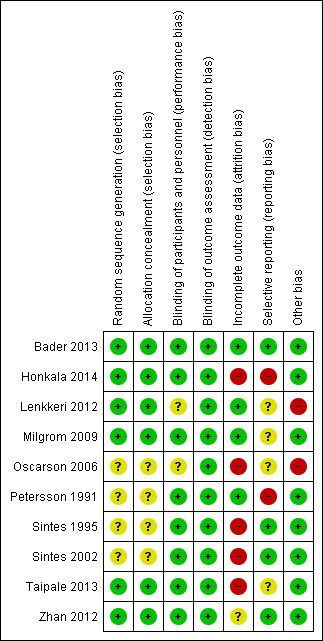
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Adults: xylitol lozenges versus control lozenges, Outcome 1 Caries increment at 33 months follow‐up (DFS).

Comparison 2 Children: xylitol lozenges versus no treatment, Outcome 1 Caries increment at 4 years follow‐up (DMFS).

Comparison 2 Children: xylitol lozenges versus no treatment, Outcome 2 Number with caries increment at 4 years follow‐up (as opposed to none/no change).

Comparison 3 Children: xylitol topical oral syrup versus control syrup (very low dose xylitol), Outcome 1 Number of decayed primary teeth at 1 year follow‐up.

Comparison 4 Children: xylitol sucking tablets versus no treatment, Outcome 1 Caries increment at 2 years follow‐up (dmfs).

Comparison 4 Children: xylitol sucking tablets versus no treatment, Outcome 2 Number with caries increment at 2 years follow‐up (as opposed to none/no change).

Comparison 5 Children: xylitol plus fluoride toothpaste versus fluoride toothpaste, Outcome 1 Caries increment at 2.5 to 3 years follow‐up (Prevented Fraction).

Comparison 5 Children: xylitol plus fluoride toothpaste versus fluoride toothpaste, Outcome 2 Caries increment at 2.5 to 3 years follow‐up (DFS).

Comparison 6 Children: xylitol tablets versus control (sorbitol) tablets, Outcome 1 Number with caries increment at 4 years follow‐up (as opposed to none/no change).

Comparison 7 Children: xylitol wipes versus control wipes, Outcome 1 Number with caries increment at 1 year follow‐up (as opposed to none/no change).
| Xylitol toothpaste compared with control toothpaste for preventing dental caries | ||||||
| Patient or population: children with permanent teeth Settings: schools Intervention: fluoride toothpaste containing 10% xylitol Comparison: fluoride toothpaste | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Xylitol | |||||
| Caries: increment (DFS) prevented fraction (PF) at 2.5 to 3 years follow‐up (higher DFS score indicates worse caries) | The (weighted) mean caries increment for control groups was | The mean caries increment in the xylitol groups was 0.28 lower (0.42 to 0.14 lower) | PF¹ = 0.13 (0.08 to 0.18) | 4216 | ⊕⊕⊝⊝ | The PF of 0.13 means that there was a 13% reduction in caries in the xylitol group There is no compelling evidence, from other comparisons in this systematic review, to support the use of xylitol products. The body of evidence for all other comparisons and caries outcomes is rated as being low to very low quality. This is because they are single studies with imprecision mostly due to very small sample sizes, and most of which have a high risk of bias |
| Adverse effects | Both studies reported that there were no adverse effects in either the xylitol or control group | |||||
| CI: Confidence interval; DFS: decayed filled surfaces; PF: prevented fraction | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ The prevented fraction (PF) is calculated as follows: the mean increment in the controls minus the mean increment in the treated group divided by the mean increment in the controls ² Downgraded due to high risk of bias in the included studies (due to high attrition) and both studies were conducted by the same authors in the same population | ||||||
| Comparison (number) | Increment (Study) | PF (95% CI) | Notes |
| Adults | |||
| Xylitol lozenges versus control lozenges (1.1) | 33‐month caries increment (Bader 2013) | 0.08 (–0.03 to 0.20) | 8% reduction in caries in test group |
| Children | |||
| Xylitol lozenges versus no treatment (2.1) | 4 year caries increment (Lenkkeri 2012)
| ‐0.10 (‐0.59 to 0.39) | 10% increase in caries in test group compared to control |
|
| |||
| Xylitol topical oral syrup versus control syrup (3.1) | Caries in primary teeth over 1 year follow‐up (Milgrom 2009) | 0.58 (0.33 to 0.83) | 58% reduction in caries in test group |
|
| |||
| Xylitol sucking tablets versus no treatment (4.1) | 2 year caries increment (Oscarson 2006) | 0.53 (0.001 to 1.04) | 53% reduction in caries in test group |
|
| |||
| Xylitol plus fluoride toothpaste versus fluoride toothpaste (5.1) | 2.5 to 3 year caries increment (Sintes 1995) | 0.12 (0.06 to 0.18) | 12% reduction in caries in test group |
| 2.5 to 3 year caries increment (Sintes 2002) | 0.14 (0.05 to 0.23) | 14% reduction in caries in test group | |
| CI = confidence interval | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 33 months follow‐up (DFS) Show forest plot | 1 | 669 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.58, 0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 4 years follow‐up (DMFS) Show forest plot | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.99, 1.55] |
| 2 Number with caries increment at 4 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of decayed primary teeth at 1 year follow‐up Show forest plot | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.03, ‐0.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 2 years follow‐up (dmfs) Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.12, 0.28] |
| 2 Number with caries increment at 2 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries increment at 2.5 to 3 years follow‐up (Prevented Fraction) Show forest plot | 2 | Prevented Fraction (Fixed, 95% CI) | 0.13 [0.08, 0.18] | |
| 2 Caries increment at 2.5 to 3 years follow‐up (DFS) Show forest plot | 2 | 4216 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.42, ‐0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with caries increment at 4 years follow‐up (as opposed to none/no change) Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.69, 13.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with caries increment at 1 year follow‐up (as opposed to none/no change) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |

