مداخلات تغییر سیستم برای ترک سیگار
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Cluster‐randomized clinical trial Setting: 176 Primary Care Centres (PCCs) in Spain Intervention providers: Physicians and nurses Data collection: Baseline survey at the clinic and then telephone follow‐up interviews Study duration: 24 months follow‐up (study conducted between 2003 and 2005) | |
| Participants | 2827 participants, age (mean ± SD) 42.8 ± 13.6 years, 50.0% men, cigarettes per day (mean ± SD) 20.4 ± 10.8 Intervention group: 1345 people from 82 PCCs; control group: 1482 people from 94 PCCs | |
| Interventions | 1) Intervention
2) Control: Usual care that included brief quitting advice for disease related to smoking. Some control group smokers used cessation medications | |
| Outcomes | 1‐year continuous abstinence at 2‐year follow‐up 6 months continuous abstinence at 2‐year follow‐up 6 months continuous abstinence at 1‐year follow‐up Point prevalence abstinence at 2‐year follow‐up Point prevalence abstinence at 1‐year follow‐up | |
| Notes | The study was conducted with financial help from the Spanish Preventive Services Network granted by the Carlos III Health Institute. The authors state that they have no conflict of interest to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician blinded to the sites' identities generated random sequence using a computer programme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization, PCCs were informed about their allocation only after giving final consent |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible with the study design |
| Blinding of outcome assessment (detection bias) | Unclear risk | The clinical part of the questionnaire was administered by the clinicians involved in the study and non‐clinical part was administered by a blinded interviewer |
| Incomplete outcome data (attrition bias) | Low risk | 43.3% in the intervention and 44.8% in the control group lost to follow‐up, included as smokers. Similar dropout rate in both groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | High risk | Only 37.3% ex‐smokers confirmed their smoking status biochemically |
| Methods | Design: Cluster‐randomized clinical trial Setting: 14 community health centre dental clinics in 3 states in the USA Intervention providers: Dentists, dental hygienists and dental assistants Data collection: Baseline survey at the clinic and then mailed follow‐up survey Study duration: 7.5 months follow‐up (study conducted between 2005 and 2008) | |
| Participants | 2549 participants, aged (mean ± SD) 40.5 ± 12.6 years, 42.8% men, average cigarettes per day (mean ± SD) 16.1 ± 10.4. | |
| Interventions | 1) Intervention (based on 5As)
2) Control: Practitioners in the control group continued to provide usual care | |
| Outcomes | 7‐day point prevalence abstinence and prolonged abstinence at 6 weeks and 7½ months follow‐up | |
| Notes | The study was supported by the National Institutes of Health, National Cancer Institute. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not specified |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding of outcome assessment, but the authors have judged that the outcome assessment is not likely to be biased, as a mail survey was used to collect follow‐up data |
| Incomplete outcome data (attrition bias) | High risk | Higher dropout rate in intervention group (30.7% vs 26.1%; P < 0.01). Multiple imputation to replace missing data |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | High risk | No biochemical verification of smoking status |
| Methods | Design: Cluster‐randomized clinical trial Setting: 20 Veterans Affairs medical centres (VAMCs) in the USA Intervention providers: Physicians, nurses, psychologists and pharmacists Data collection: Telephone survey among 3 cohorts of participants; 1) baseline cross‐sectional survey; 2) a second cross‐sectional survey 1 year after initiation of the intervention; and 3) follow‐up survey of smokers identified in the baseline survey; data were also collected from medical records Study duration: 12 months follow‐up (the study dates are not reported in the manuscript) | |
| Participants |
| |
| Interventions | 1) Intervention
2) Control: provided 5 copies of AHCPR smoking cessation guideline to each control clinic | |
| Outcomes | Cessation outcomes: Self‐reported smoking status at 1‐year follow‐up Process outcomes: Improvement in documentation of tobacco use, delivery of treatment to all smokers, and use of pharmacotherapy collected by participant surveys and from medical records | |
| Notes | This study was supported by a grant from the Veterans Administration Health Services Research and Development Service. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study sites (n=20) were randomly assigned to the intervention or control group using simple (not stratified or block) randomisation" |
| Allocation concealment (selection bias) | Low risk | "The remaining 20 sites were randomised." Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded interviewer |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout rate by group not given |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | High risk | No biochemical verification of smoking status |
| Methods | Design: Cluster‐randomized clinical trial Setting: 14 dental clinics in the USA Intervention providers: Dentist, office staff and hygienist Data collection: From electronic health records Study duration: No follow‐up, 14 months data collection (study conducted between 2006 and 2007) | |
| Participants | Electronic health record‐generated data (asking, advising and referral) included all people visiting the participating clinics which included 66,516 patients (32,802 intervention and 33,714 control) | |
| Interventions | 1) Intervention
2) Control: Standard care | |
| Outcomes | Number of participants counselled or referred, or both | |
| Notes | Only secondary outcomes were evaluated. This study was supported by the National Institute of Drug Abuse. No conflict of interest declaration was included in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Matched pairs of facilities were then randomly assigned to intervention or control." Presumably did the randomization |
| Allocation concealment (selection bias) | Low risk | Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | Relevant data were generated from electronic health records (objective assessment) |
| Incomplete outcome data (attrition bias) | Low risk | No follow‐up involved |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
| Methods | Design: Cluster‐randomized clinical trial Setting: 16 community chain pharmacies in the USA Intervention providers: Pharmacists and technicians Data collection: from pharmacy staff using feedback form. Staff were instructed to check off relevant items (asked, advised, provided quitline cards) for the activity performed. Referral data were obtained from quitline reports Study duration: No follow‐up; 1 month data collection (study conducted between 2008 and 2009) | |
| Participants | 32 pharmacists and 48 technicians (No data collected from pharmacy clients; all data collected from pharmacy staff). | |
| Interventions | 1) Intervention
2) Control: Control group pharmacies received an informal presentation on quitline services, quitline cards and enrolment in Fax to Quit (FTQ) services, a free service | |
| Outcomes | Number of participants asked about tobacco use Number of tobacco users advised to Quit Number of tobacco users enrolled for quitline service Number of quitline cards given | |
| Notes | Data collected form the pharmacy staff. The study was supported by the Wisconsin department of Health Services; the Sonderegger Research Center, School of Pharmacy, University of Wisconsin–Madison; and Clinical and Translational Science Award program, National Center for Research Resources, National Institutes of Health. The authors state that they had no conflicts of interest to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sixteen pharmacies were randomly assigned to a control group or experimental group using block randomisation, which involved random assignment into groups after matching on a block covariate" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was carried out by research assistants blinded to the study goal. The authors were not involved in the assignment process" |
| Blinding of participants and personnel (performance bias) | Low risk | Pharmacy staff were not aware of the existence of 2 groups in the study. The primary author who conducted staff training was blinded to pharmacists’ self‐efficacy scores |
| Blinding of outcome assessment (detection bias) | High risk | The outcome data were directly obtained from the providers using a self‐filled documentation form |
| Incomplete outcome data (attrition bias) | Low risk | No follow‐up in the study |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
| Methods | Design: Cluster‐randomized clinical trial Setting: 16 primary care practices in the USA Intervention providers: Physicians, nurses and medical assistants Data collection: Participant self‐report via an exit survey Study duration: No follow‐up; 9 months data collection (study conducted between 2005 and 2006) | |
| Participants | All persons visiting the participating clinics which included 10,395 patients (5669 intervention and 4726 control). Tobacco user population: 1815 adult smokers (857 intervention and 958 control) | |
| Interventions | 1) Intervention
2) Control: Traditional tobacco use vital sign stamp (only smoking status recorded) | |
| Outcomes | Number of participants asked about tobacco use Number of participants advised to quit Number of participants received in‐office cessation support (primary end point) Number of participants discussing ideas and plans to quit smoking Number of participants referred to quitline | |
| Notes | 36% of potentially eligible people did not participate in the survey, but the participation proportion did not differ between study groups. This study was funded through a grant from the Agency for Healthcare Research and Quality. One author declares stock in a quitline service provider. No other disclosures were made. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number generator was used to randomise practices within the strata to intervention and control arms" |
| Allocation concealment (selection bias) | Low risk | "From the potential pool of 51 sites, 29 practices were targeted for recruitment and 16 were enrolled." Presumably randomized all clusters at once |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible with study design |
| Blinding of outcome assessment (detection bias) | High risk | No blinded outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | No follow‐up in the study |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
| Methods | Design: Cluster‐randomized clinical trial Setting: 20 paediatric practices in the USA Intervention providers: Paediatricians, nurses and medical assistants Data collection: Telephone survey Study duration: 12 months follow‐up (study conducted between 2010 and 2012) | |
| Participants | 1980 parent smokers; | |
| Interventions | 1) Intervention
2) Control: Usual care | |
| Outcomes | Biochemically validated parental smoking cessation rates Number of parents asked about tobacco use Number of parents advised and counselled to quit Number of parents prescribed cessation medication Number of parents referred to the state quitline | |
| Notes | This study was supported the National Institute of Health. One author declares work as an unpaid consultant for a pharmaceutical company. No other disclosures to report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random generator was used to generate a sequence of group assignments within each of the 6 blocks created by combining the 2 strata" |
| Allocation concealment (selection bias) | Low risk | "The first 22 practices that responded were enrolled and randomly assigned to intervention or control groups." Presumably randomised all clusters at once |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | High risk | Although not statistically significant, the follow‐up rate was marginally higher in the control group (64.5% vs 72.4%; P = 0.11) and hence considered as high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Only midwives are involved in the program | |
| 3‐arm study comparing 2 types of interventions (education only vs education plus fee‐for‐service) with a control group; No evidence of organization‐wide involvement, and no identification or treatment components. The remuneration was provided only during the 6‐month study period | |
| Only non‐urgent emergency department patients were targeted | |
| Evaluating the effect of electronic health record‐generated provider feedback to improve 5As delivery (only feedback introduced; no other components). Only GPs are targeted | |
| Comparing 2 methods of disseminating a smoking cessation programme; Not a system initiated change; no effort to improve identification or treatment component | |
| Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs | |
| Comparing 2 methods of disseminating a smoking cessation programme; Not a system initiated change; no effort to improve identification or treatment component | |
| Only training implemented | |
| Included hospitalized African‐American smokers only | |
| Only smokers interested in remaining abstinent after discharge and living within 50 miles of the participating hospital were included in the study | |
| Only Medicaid‐enrolled smokers were included in the study; research assistant identified participants, not clinic staff | |
| Controlled before‐and‐after study, not meeting study design requirements | |
| Controlled before‐and‐after study, not meeting study design requirements | |
| Research assistants identified smokers | |
| Telephone follow‐up counselling was done by a researcher | |
| No evidence of effort to identify and treat all smokers accessing the system. Interventions were different at various sites | |
| Only those randomized to the intervention arm received assistance and follow‐up support, which were provided by a researcher; tailored only for Korean participants | |
| Research staff provided the intervention | |
| Before‐and‐after study, not meeting study design requirements | |
| Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff | |
| Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff | |
| Research staff provided adjunct interventions (motivational call and reminder letter); only brief advice provided by the clinic staff | |
| Research staff provided telephone counselling service | |
| Not all smokers included in the intervention; RCT design | |
| Research team was involved in the delivery of the intervention. Not all smokers were identified and treated | |
| Not all smokers included in the study; women receiving prenatal care and WIC services and planning to receive paediatric care at 1 of the CHCs were eligible. Those at less than 2 months to the due date and non‐pregnant excluded | |
| The intervention was provided only during recall dental visits. No training was provided | |
| Quasi‐experimental research design. No evidence that all smokers accessing the system were identified and treated | |
| Intervention mainly consisted of referring to an onsite counselling programme; clinicians were not involved in the intervention. | |
| Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs | |
| No random selection of sites (controlled before‐and‐after study), not meeting study design requirements | |
| Implemented only the reimbursement (pay‐for‐performance) component of the system change intervention and targeted only GPs | |
| Not all smokers received intervention; RCT design; smokers were identified by a researcher | |
| Research staff identified smokers. Not all smokers received intervention | |
| Research team was involved in delivering the intervention | |
| Research team was involved in delivering the intervention | |
| Not all smokers received intervention; RCT design | |
| Not all smokers received intervention; RCT design ; smokers were identified by a researcher | |
| No evidence of effort to identify and treat all smokers accessing the system |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | An organizational change intervention for increasing the delivery of smoking cessation support in addiction treatment centres: study protocol for a randomized controlled trial |
| Methods | Design: Cluster‐randomized clinical trial Setting: 33 drug and alcohol treatment services in Australia Intervention providers: all staff Data collection: Participant surveys |
| Participants | People attending drug and alcohol treatment services |
| Interventions | Intervention includes assistance in developing smoke‐free policies, identifying all smokers, nomination of champions, staff training and educational participant and service resources, and free nicotine replacement therapy in order to integrate smoking cessation support as part of usual participant care |
| Outcomes | Biochemically‐verified 7‐day point prevalence abstinence at 6 weeks will be the primary outcome measure. |
| Starting date | 02/02/15 |
| Contact information | A/Prof Billie Bonevski, Centre for Translational Neuroscience and Mental Health, University of Newcastle, NSW, Australia |
| Notes | This study is a potentially included study. |
| Trial name or title | Dentists United to Extinguish Tobacco (DUET): a study protocol for a cluster randomized, controlled trial for enhancing implementation of clinical practice guidelines for treating tobacco dependence in dental care settings. |
| Methods | Design: Cluster‐randomized clinical trial Setting: 18 dental care clinics in the USA Intervention providers: dentist, dental hygienist, and dental assistants Data collection: patient exit interviews, provider surveys, site observations, chart audits |
| Participants | All people attending the clinic |
| Interventions | Intervention 1: Staff training and current best practice (CBP) |
| Outcomes | Provider adherence to PHS guidelines, 7‐day point prevalence abstinence, 24‐hour quit attempts, provider attitudes and cost per quit |
| Starting date | |
| Contact information | |
| Notes | This study is ongoing and has a published protocol. The arm 3 may be eligible to include in this review if they make sufficient effort to identify all smokers |

Study flow diagram.
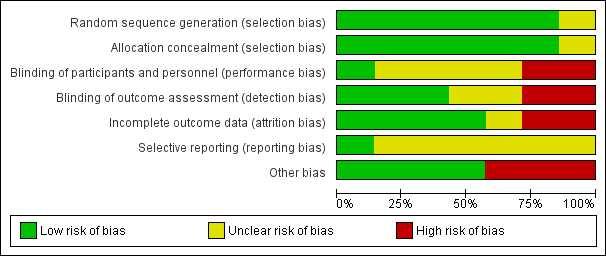
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
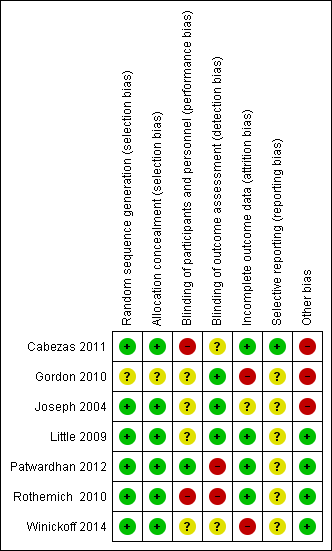
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| System change interventions for tobacco control (primary cessation outcome) | |||
| Patient or population: Smokers | |||
| Outcomes* | No of Participants | Quality of the evidence | Comments |
| Cessation outcome | 7142 | ⊕⊝⊝⊝ | 2 of the 4 studies significantly favoured the intervention. Mixed effect and low quality evidence preclude drawing conclusions |
| NB. Illustrative comparative risks and relative effects columns have been removed, as only narrative syntheses were conducted due to the presence of significant heterogeneity among studies. | |||
| GRADE Working Group grades of evidence | |||
| 1Self‐reported abstinence was verified only in one study, and one study reported higher dropout rate in one group. | |||
| System change interventions for tobacco control (secondary outcomes) | |||
| Patient or population: Smokers | |||
| Outcomes* | No of Participants | Quality of the evidence | Comments |
| Provision of cessation counselling | 10,949 | ⊕⊕⊝⊝ | 3 of the 4 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Asking about tobacco use | 2615 | ⊕⊕⊝⊝ | 2 of the 3 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Provision of cessation advice | 3003 | ⊕⊕⊝⊝ | 2 of the 3 studies significantly favoured the intervention. The low quality of the evidence precludes drawing conclusions |
| Quitline referral | 3006 | ⊕⊝⊝⊝ | All 3 studies significantly favoured the intervention. However, the low quality of the evidence precludes drawing conclusions |
| Quitline enrolment | 1191 | ⊕⊝⊝⊝ | Both studies significantly favoured the intervention. However, the low quality of the evidence precludes drawing conclusions |
| Prescription of NRT or other pharmacotherapy | 2615 | ⊕⊕⊝⊝ | Of the 2 studies, 1 significantly favoured the intervention. Mixed effect and low‐quality evidence preclude drawing conclusions |
| NB. Illustrative comparative risks and relative effects columns have been removed, as only narrative syntheses were conducted due to the presence of significant heterogeneity among studies *We have not included data from one study as the data were collected as counts (no denominator) | |||
| GRADE Working Group grades of evidence | |||
| 1Included studies had high risk of detection bias. | |||
| Identification/ documentation of smoking status | Smoking cessation Training/ resources/feedback to providers | Dedicated staff to support cessation activities | Policies to improve access to cessation interventions | Free smoking cessation treatment from the organization | Reimburse clinicians for providing smoking cessation support | |
| Cabezas 2011 | Yes | Yes | No | No | Yes | No |
| Gordon 2010 | Yes | Yes | No | No | Yes | No |
| Joseph 2004 | Yes | Yes | No | Yes | Yes | No |
| Little 2009 | Yes | Yes | No | Yes | Yes | No |
| Patwardhan 2012 | Yes | Yes | No | Yes | Yes | No |
| Rothemich 2010 | Yes | Yes | No | Yes | Yes | No |
| Winickoff 2013 | Yes | Yes | No | Yes | Yes | No |
| Study | Asking about tobacco use | Documentation of smoking status | Advice to quit | Counselling to quit | Initiation of NRT or other pharmacotherapy | Quitline referral | Quitline enrolment |
| Cabezas 2011 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Gordon 2010 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Joseph 2004 | No difference between groups (76.0% vs 74.3%; P = 0.71) | Favoured intervention (60.7% vs 67.0%; P < 0.001) | Not assessed | No difference between groups (73.9% vs 71.8%; P = 0.60) | No difference between groups (14.7% vs 18.0%; P = 0.38) | Not assessed | Not assessed |
| Little 2009 | Not assessed | Not assessed | Not assessed | Favoured intervention (69% vs 3%; P < 0.01) | Not assessed | Not assessed | Not assessed |
| Patwardhan 2012* | Favoured intervention (636 vs 5; P < 0.001) | Not assessed | Favoured intervention (25 vs 3; P < 0.01) | Not assessed | Not assessed | Favoured intervention (240 vs 85; P = 0.02) | Favoured intervention (81 vs 8; P < 0.001) |
| Rothemich 2010 | Not assessed | Not assessed | No difference between groups (58.2% vs 55.3%; P = 0.39). | Favoured intervention (34.4% vs 27.7%; P = 0.001) | Not assessed | Favoured intervention (21.4% vs 8.7%; P < 0.001) | Not assessed |
| Winickoff 2013 | Favoured intervention (59.4% vs 32.6%; P < 0.001) | Not assessed | Favoured intervention (50.5% vs 26.9%; P < 0.001). | Favoured intervention (54.7% vs 19.2%; P < 0.001) | Favoured intervention (18.5% vs 2.4%; P < 0.001) | Favoured intervention (37.2% vs 9.3%; P < 0.001) | Favoured intervention (4.1% vs 1.1%; P < 0.01) |
| *data collected as counts (no denominator) | |||||||

