Sulodexida para el tratamiento de la úlcera venosa de la pierna
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010694.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 junio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Bin Wu: conceived the review question, developed the protocol and full review, completed the first draft, performed part of the writing and editing of the review, advised on the review and approved final version prior to submission.
Jing Lu: developed the protocol, selected studies and extracted data, performed part of the writing and editing of the review, advised on the review and approved the final version prior to submission.
Ming Yang: developed the protocol, participated in study selection and data extraction, assessed risk of bias, made an intellectual contribution, advised on part of the review and approved the final version prior to submission.
Ting Xu: conceived the review question, made an intellectual contribution, advised on the review and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum (Editor): edited the protocol and the review; advised on methodology, interpretation and protocol content. Approved the final protocol and review prior to submission.
Joan Webster (Editor): edited the review; advised on methodology, interpretation and review content. Approved the final review prior to submission.
Sally Bell‐Syer and Gill Rizzello: co‐ordinated the editorial process. Advised on interpretation and content.
Ruth Foxlee: designed the search strategy and edited the search methods section. Reetu Child updated the search and edited the search methods section.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Bin Wu: none known
Jing Lu: none known
Ming Yang: none known
Ting Xu: none known.
Acknowledgements
We are grateful to Rachel Richardson for her comments on the protocol. We would also like to acknowledge the contribution of peer referees Susan O' Meara, Gill Norman, Gill Worthy, Audrey Demetriouk Janet Whale, Morag Heirs, Giovanni Casazza, Patricia Davies, Madhu Periasamy and Shirley Manknell and copy editors Elizabeth Royle and Denise Mitchell.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jun 02 | Sulodexide for treating venous leg ulcers | Review | Bin Wu, Jing Lu, Ming Yang, Ting Xu | |
| 2013 Aug 10 | Sulodexide for treating venous leg ulcers | Protocol | Bin Wu, Jing Lu, Ming Yang, Ting Xu | |
Differences between protocol and review
-
EndNote X3 was updated to EndNote X7.
-
RevMan 5.1 software was updated to RevMan 5.3.
-
We decided to add a 'Summary of findings' table to evaluate the overall quality of evidence for each outcome.
-
For assessing reporting biases, we added comparison between the study protocols and the final study reports, or between 'Methods' section of the published studies and the 'Results' section.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Aged, 80 and over; Humans; Middle Aged;
PICO

PRISMA flow diagram of literature screening
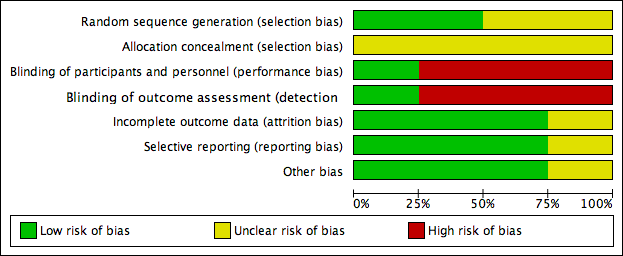
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
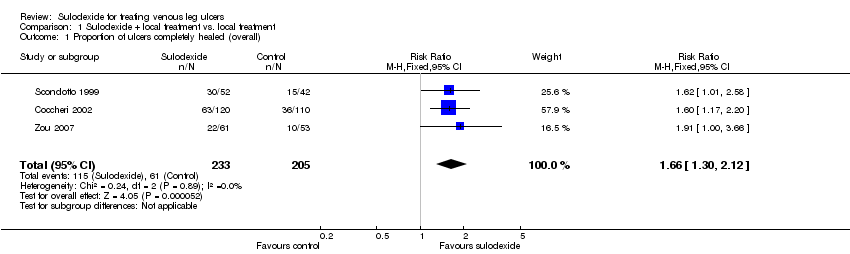
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 1 Proportion of ulcers completely healed (overall).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 2 Proportion of ulcers completely healed (sensitivity analysis).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 3 Proportion of ulcers completely healed at 30 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 4 Proportion of ulcers completely healed at 60 days.
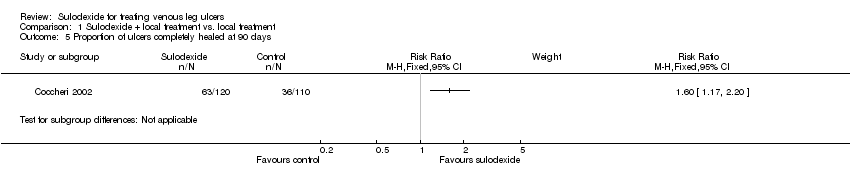
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 5 Proportion of ulcers completely healed at 90 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 6 Adverse effects.
| Sulodexide + local treatment compared to local treatment for treating venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| local treatment | Sulodexide + local treatment | |||||
| Proportion of ulcers completely healed (overall) | 298 per 1000 | 494 per 1000 | RR 1.66 | 438 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 30 days | 189 per 1000 | 360 per 1000 | RR 1.91 | 114 | ⊕⊝⊝⊝ | |
| Proportion of ulcers completely healed at 60 days | 250 per 1000 | 412 per 1000 | RR 1.65 | 324 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 90 days | 327 per 1000 | 524 per 1000 | RR 1.6 | 230 | ⊕⊕⊝⊝ | |
| Time to complete ulcer healing | Available data were limited and not analysed | |||||
| Change in absolute ulcer size | Available data were limited and not analysed | |||||
| Ulcer recurrence | Not reported | |||||
| Adverse effects | 31 per 1000 | 44 per 1000 | RR 1.44 | 344 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | Not reported | |||||
| Direct costs | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors). 2 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (single study with very wide confidence intervals). 3 Downgraded one level for risk of bias (lack of allocation concealment) and one level for imprecision (single study with very wide confidence intervals). 4 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed (overall) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.12] |
| 2 Proportion of ulcers completely healed (sensitivity analysis) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3 Proportion of ulcers completely healed at 30 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Proportion of ulcers completely healed at 60 days Show forest plot | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.20, 2.28] |
| 5 Proportion of ulcers completely healed at 90 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Adverse effects Show forest plot | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.48, 4.34] |

