Stosowanie sulodeksydu w leczeniu żylnych owrzodzeń podudzi
Appendices
Appendix 1. CENTRAL search strategy
| CENTRAL search strategy |
| #1 MeSH descriptor Leg Ulcer explode all trees |
Appendix 2. Ovid MEDLINE search strategy
| Ovid MEDLINE search strategy |
| #1 exp Leg Ulcer/ |
Appendix 3. Ovid EMBASE search strategy
| Ovid EMBASE search strategy |
| #1 exp Leg Ulcer/ |
Appendix 4. EBSCO CINAHL search strategy
| EBSCO CINAHL search strategy |
| S1 (MH "Leg Ulcer+") |
Appendix 5. Chinese Biomedical Literature Database (CBM) search strategy
| Chinese Biomedical Literature Database (CBM) search strategy |
| #1 静脉曲张溃疡/全部树/全部副主题词 [主题词] #2 (ulcer or 溃疡) [缺省] #3 #1 or #2 #4 (sulodexide or 舒洛地特 or 伟素) [缺省] #5 #3 and #4 |
Appendix 6. China National Knowledge Infrastructure Database (CNKI) search strategy
| China National Knowledge Infrastructure Database (CNKI) search strategy |
| #1 (ulcer or 溃疡) [主题] #2 (sulodexide or 舒洛地特 or 伟素) [主题] #3 #1 and #2 |
Appendix 7. Wan Fang Database search strategy
| Wan Fang Database search strategy |
| #1 (ulcer or 溃疡) [题名或关键词] #2 (sulodexide or 舒洛地特 or 伟素) [题名或关键词] #3 #1 and #2 |
Appendix 8. VIP Database search strategy
| VIP Database search strategy |
| #1 (ulcer or 溃疡) [题名或关键词] #2 (sulodexide or 舒洛地特 or 伟素) [题名或关键词] #3 #1 and #2 |
Appendix 9. Criteria for judging risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low risk or high risk to be made. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following:
-
Insufficient information available to permit judgement of low risk or high risk.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes is not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
-
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
-
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
-
Insufficient reporting of attrition/exclusions to permit judgement of low risk or high risk (e.g. number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
-
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
-
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
-
Not all of the study's pre‐specified primary outcomes have been reported.
-
One or more, primary outcome is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
-
One or more, reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more, outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low risk or high risk. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
-
had a potential source of bias related to the specific study design used;
-
has been claimed to have been fraudulent;
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists;
-
insufficient rationale or evidence that an identified problem will introduce bias.

PRISMA flow diagram of literature screening
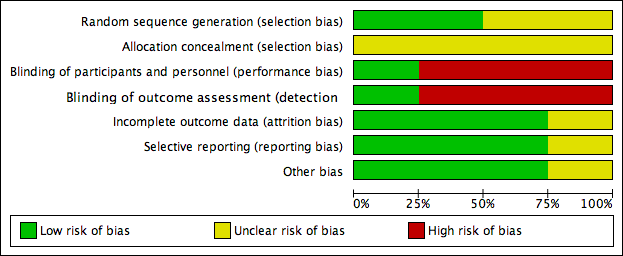
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
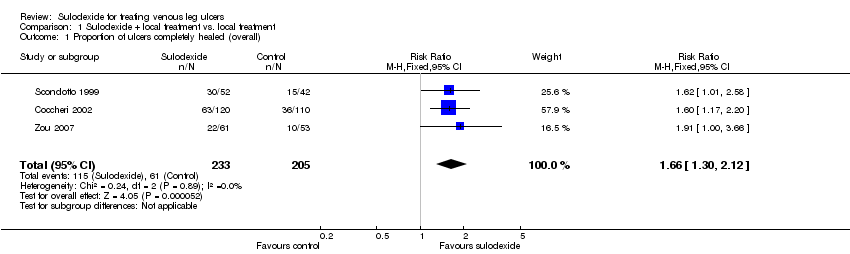
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 1 Proportion of ulcers completely healed (overall).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 2 Proportion of ulcers completely healed (sensitivity analysis).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 3 Proportion of ulcers completely healed at 30 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 4 Proportion of ulcers completely healed at 60 days.
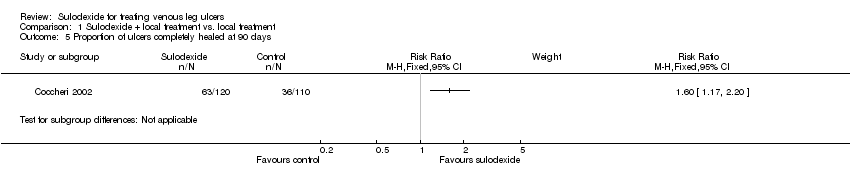
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 5 Proportion of ulcers completely healed at 90 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 6 Adverse effects.
| Sulodexide + local treatment compared to local treatment for treating venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| local treatment | Sulodexide + local treatment | |||||
| Proportion of ulcers completely healed (overall) | 298 per 1000 | 494 per 1000 | RR 1.66 | 438 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 30 days | 189 per 1000 | 360 per 1000 | RR 1.91 | 114 | ⊕⊝⊝⊝ | |
| Proportion of ulcers completely healed at 60 days | 250 per 1000 | 412 per 1000 | RR 1.65 | 324 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 90 days | 327 per 1000 | 524 per 1000 | RR 1.6 | 230 | ⊕⊕⊝⊝ | |
| Time to complete ulcer healing | Available data were limited and not analysed | |||||
| Change in absolute ulcer size | Available data were limited and not analysed | |||||
| Ulcer recurrence | Not reported | |||||
| Adverse effects | 31 per 1000 | 44 per 1000 | RR 1.44 | 344 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | Not reported | |||||
| Direct costs | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors). 2 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (single study with very wide confidence intervals). 3 Downgraded one level for risk of bias (lack of allocation concealment) and one level for imprecision (single study with very wide confidence intervals). 4 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed (overall) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.12] |
| 2 Proportion of ulcers completely healed (sensitivity analysis) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3 Proportion of ulcers completely healed at 30 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Proportion of ulcers completely healed at 60 days Show forest plot | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.20, 2.28] |
| 5 Proportion of ulcers completely healed at 90 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Adverse effects Show forest plot | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.48, 4.34] |

