Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL)
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "cerebrovascular trauma"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vasospasm, intracranial"]
#2 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 ((transi* near/3 isch*m* near/3 attack*) or TIA or TIAs):ti,ab
#6 #1 or #2 or #3 or #4 or #5
#7 [mh ^"PPAR gamma"/AG,DE,ME,PD,TU]
#8 [mh ^"Peroxisome Proliferator‐Activated Receptors"/AG,DE,ME,PD,TU] or [mh ^"Transcription Factors"/AG,DE,ME,PD,TU]
#9 [mh ^Thiazolidinediones]
#10 (("peroxisome proliferator‐activated receptor gamma" or "PPAR gamma" or "PPAR‐gamma" or "PPARgamma" or PPARG or NR1C3) near/5 (agonist* or modulator* or stimulat* or stimulant* or activat*)):ti,ab
#11 (thiazolidinedione* or glitazone* or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone):ti,ab
#12 #7 or #8 or #9 or #10 or #11
#13 #6 and #12
Appendix 2. MEDLINE search strategy (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. ((transi$ adj3 isch?em$ adj3 attack$) or TIA$1).tw.
6. 1 or 2 or 3 or 4 or 5
7. PPAR gamma/ag, de, me, pd, tu
8. Peroxisome Proliferator‐Activated Receptors/ag, de, me, pd, tu or Transcription Factors/ag, de, me, pd, tu
9. Thiazolidinediones/
10. ((peroxisome proliferator‐activated receptor gamma or PPAR gamma or PPAR‐gamma or PPARgamma or PPARG or NR1C3) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$ or activat$)).tw.
11. (thiazolidinedione$ or glitazone$ or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone).tw,nm.
12. 7 or 8 or 9 or 10 or 11
13. Randomised Controlled Trials as Topic/
14. random allocation/
15. Controlled Clinical Trials as Topic/
16. control groups/
17. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
18. double‐blind method/
19. single‐blind method/
20. Placebos/
21. placebo effect/
22. Drug Evaluation/
23. Research Design/
24. randomised controlled trial.pt.
25. controlled clinical trial.pt.
26. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
27. random$.tw.
28. (controlled adj5 (trial$ or stud$)).tw.
29. (clinical$ adj5 trial$).tw.
30. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
31. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
33. placebo$.tw.
34. controls.tw.
35. (RCT or RCTs).tw. or trial.ti.
36. or/13‐35
37. 6 and 12 and 36
38. exp animals/ not humans.sh.
39. 37 not 38
Appendix 3. Embase search strategy (Ovid)
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or vertebrobasilar insufficiency/ or stroke/ or stroke patient/ or stroke unit/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. ((transi$ adj3 isch?em$ adj3 attack$) or TIA$1).tw.
6. 1 or 2 or 3 or 4 or 5
7. peroxisome proliferator activated receptor gamma agonist/
8. peroxisome proliferator activated receptor gamma/ct, ad, dt [Clinical Trial, Drug Administration, Drug Therapy]
9. peroxisome proliferator activated receptor gamma 2/dt [Drug Therapy]
10. peroxisome proliferator activated receptor/ct, ad, dt [Clinical Trial, Drug Administration, Drug Therapy]
11. transcription factor/ct, ad, dt [Clinical Trial, Drug Administration, Drug Therapy]
12. peroxisome proliferator activated receptor gamma coactivator 1alpha/ct, dt [Clinical Trial, Drug Therapy]
13. peroxisome proliferator activated receptor gamma coactivator 1beta/dt [Drug Therapy]
14. exp glitazone derivative/
15. ((peroxisome proliferator‐activated receptor gamma or PPAR gamma or PPAR‐gamma or PPARgamma or PPARG or NR1C3) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$ or activat$)).tw.
16. (thiazolidinedione$ or glitazone$ or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone).tw.
17. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18. 6 and 17
19. Randomized Controlled Trial/
20. Randomization/
21. Controlled Study/
22. control group/
23. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
24. Double Blind Procedure/
25. Single Blind Procedure/ or triple blind procedure/
26. placebo/
27. "types of study"/
28. random$.tw.
29. (controlled adj5 (trial$ or stud$)).tw.
30. (clinical$ adj5 trial$).tw.
31. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
32. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
33. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
34. placebo$.tw.
35. (RCT or RCTs).tw. or trial.ti.
36. or/19‐35
37. 18 and 36
38. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
39. 37 not 38
Appendix 4. CINAHL search strategy (EBSCO)
S16. S11 AND S15
S15. S12 OR S13 OR S14
S14. TI thiazolidinedione* or glitazone* or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone or AB thiazolidinedione* or glitazone* or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone
S13. ( TI peroxisome proliferator‐activated receptor gamma or PPAR gamma or PPAR‐gamma or PPARgamma or PPARG or NR1C3 or AB peroxisome proliferator‐activated receptor gamma or PPAR gamma or PPAR‐gamma or PPARgamma or PPARG or NR1C3 ) AND ( TI agonist* or modulator* or stimulat* or stimulant* or activat* or AB agonist* or modulator* or stimulat* or stimulant* or activat* )
S12. (MH "Thiazolidinediones") OR (MH "Troglitazone") OR (MH "Rosiglitazone") OR (MH "Pioglitazone")
S11. S1 OR S2 OR S3 OR S6 OR S9 OR S10
S10. TI transient ischaemic attack* or TI transient ischemic attack* or AB transient ischaemic attack* or AB transient ischemic attack* or TI TIA or TI TIA s or AB TIA or AB TIAs
S9. S7 and S8
S8. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S7. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S6. S4 and S5
S5. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S4. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S3. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S2. (MH "Stroke Patients") OR (MH "Stroke Units")
S1. (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
Appendix 5. AMED search strategy (Ovid)
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. ((transi$ adj3 isch?em$ adj3 attack$) or TIA$1).tw.
6. 1 or 2 or 3 or 4 or 5
7. ((peroxisome proliferator‐activated receptor gamma or PPAR gamma or PPAR‐gamma or PPARgamma or PPARG or NR1C3) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$ or activat$)).tw.
8. (thiazolidinedione$ or glitazone$ or pioglitazone or rosiglitazone or troglitazone or netoglitazone or rivoglitazone or ciglitazone or balaglitazone or darglitazone or edaglitazone or englitazone or lobeglitazone).tw.
9. 7 or 8
10. 6 and 9
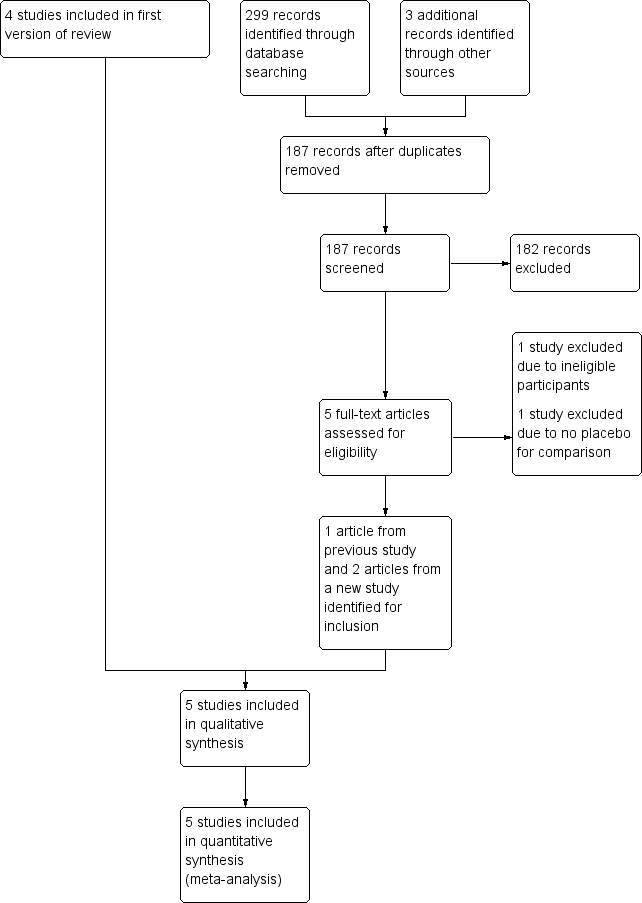
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
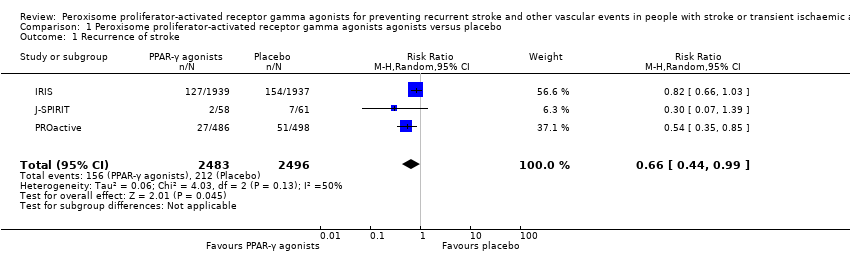
Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1 Recurrence of stroke.
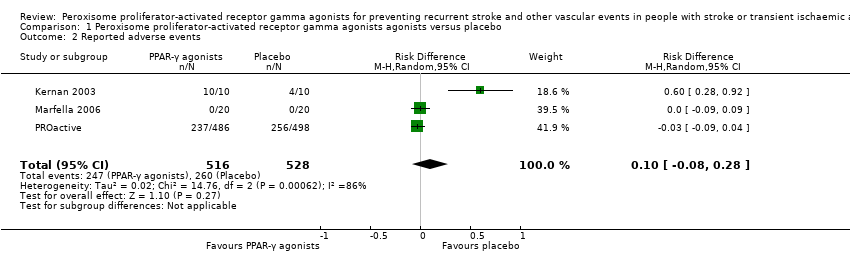
Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2 Reported adverse events.
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ | |
| Reported adverse events Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝ | |
| Serious vascular events | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 126 of 498 (25%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 98 of 498 (20%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98 of 486 (20%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76 of 486 (16%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation (RR 0.80, 95% CI 0.63 to 1.01). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.79, 95% CI 0.61 to 1.04). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99). | 984 | ⊕⊕⊝⊝ | Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — | |
| Disability due to vascular events | Not reported | Not reported | — | — | — | |
| Improvement in quality of life | Not reported | Not reported | — | — | — | |
| Insulin sensitivity Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ | |
| Ubiquitin‐proteasome activity in carotid plaques | Ubiquitin 468.7 ± 89 ng/mg; proteasome 20S 79.8 ± 25 pmol/mg; nitrotyrosine 3.5 ± 0.42 nmol/pg; superoxide anion production 6.26 ± 1.4 pmol/L | Ubiquitin 322 ± 79 ng/mg; proteasome 20S 46.8 ± 10 pmol/mg; nitrotyrosine 2.2 ± 0.21 nmol/pg; superoxide anion production 3.57 ± 1.1 pmol/L | Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01) with less ubiquitin (P < 0.01), proteasome 20S (P < 0.01), nuclear factor kappa B (P < 0.01), nitrotyrosine (P < 0.01), superoxide anion production (P < 0.01), and more collagen content (P < 0.01). | 40 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| 2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |

