Pengurusan surgikal berbanding bukan surgikal untuk empiema pleural
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Country: Turkey Number of study centres: 1 | |
| Participants | N recruited = 70 N randomised = 70 N reported outcomes = 70 Mean age = 47.35 years Gender (m/f) = 59/11 N tube thoracostomy = 35 N VATS = 35 Inclusion criteria: Diagnosis of empyema by:
Required values for pleural fluid measurements not given. Exclusion criteria: not reported | |
| Interventions | Treatment before study: not reported Titration period and treatment:
| |
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): not reported | |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "We studied whether insertion of a chest tube by initially using video‐assisted thoracoscopy (VAT) results in shortening of hospitalisation time and reduces the necessity of open decortication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Low risk | Closed‐envelope method |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Superiority design Country: Turkey Number of study centres: 1 | |
| Participants | N recruited = 54 N randomised = 54 (Stage II = 23, Stage III = 31) N reported outcomes = 54 Mean age = 8.02 years Gender (m/f) = 32/22 N fibrinolytics = 27 N VATS = 27 Inclusion criteria: (Children's study, however maximum age not stated.)
Exclusion criteria:
| |
| Interventions | Treatment before study: Pre‐hospital: 76% nil, remainder had irregular antibiotics After diagnosis: All received empiric treatment of ampicillin sulbactam plus cefotaxime, and an 18 to 24 Fr chest tube was inserted in all cases and connected to a closed drainage system with negative suction. Titration period and treatment: After diagnosis, participants received either STK or VATS VATS: After procedure, chest tube was left in place. 6 participants required conversion to mini‐thoracotomy. STK: Normal saline with 250,000 U/100 mL STK was administered into the pleural cavity through the chest tube in 70‐ to 120‐mL volume once a day, and the tube was held by a clamp for 4 to 6 hours. The period of fibrinolytic treatment was determined as 4.45 days (3 to 5 days). 8 participants required further VATS. Common: Chest tube removed when daily drainage less than 50 mL and radiological healing and full expansion detected. | |
| Outcomes | Primary outcome(s): Success was based on whether further intervention was required. Lung was fully expanded and the procedure was considered successful: STK: 19 of 27 cases (70.37%) versus VATS: 21 cases of 27 (77.77%) (P = 0.533) Secondary outcome(s):
| |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "The aim of this study was to prospectively compare thoracoscopic debridement and fibrinolytic treatment in cases with stage II and III empyema and to present a perspective for treatment options." Funding: not disclosed Conflicts of interest: not disclosed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | The following statement might indicate potential selection bias, however, based on data in the paper, it is unclear if any participants were excluded from the fibrinolytics group after randomisation: "Patients with hemorrhagic diathesis, stroke or manifest haemorrhage having occurred in the previous six months and those who had used fibrinolytic agents for any reason in the previous two years were excluded from the fibrinolytic treatment group." Funding source not reported. |
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Number of study centres: 1 | |
| Participants | N recruited = 30 N randomised = 30 N reported outcomes = 30 Mean age = 3.9 years (study in children) Gender m/f = 15/15 N tube thoracostomy = 15 N open thoracotomy = 15 Inclusion criteria: Patients with pleural empyema confirmed by:
Required values for pleural fluid measurements not given. Exclusion criteria:
| |
| Interventions | Treatment before study: 80% of participants had been treated with antibiotics for periods ranging from 2 to 20 days prior to admission (mean 4.7 days). 4 of these participants were treated in another centre for pneumonia and were given 2nd‐ or 3rd‐generation cephalosporins and aminoglycosides. The other participants were treated with beta‐lactams. Titration period and treatment:
| |
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): In both groups, duration of chest tube drainage was found to be longer in participants whose pleural fluid pH was < 7.2. | |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "The aim of this study is to compare the effectiveness of closed‐tube thoracostomy and open thoracotomy procedures in the management of empyema in children." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: VATS:thoracostomy = 10:8 Country: USA Number of study centres: 1 | |
| Participants | N recruited = 18 N randomised = 18 N reported outcomes = 18 Mean age = 64.5 months (study in children) Gender m/f = 10/8 N tube thoracostomy = 8 N VATS = 10 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Treatment before study: All participants had received antibiotic therapy that was appropriate for the suspected micro‐organisms prior to enrolment. Titration period and treatment:
| |
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s):
Other outcome(s):
| |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "This trial of paediatric patients with community‐acquired pneumonia and associated parapneumonic processes compared primary video‐assisted thoracoscopic surgery with conventional thoracostomy drainage." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random number method in groups of 10 generated by a Spectrum Health research nurse" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Number of study centres: 6 | |
| Participants | N recruited = 149 N randomised = 105 N reported outcomes = 103 Mean age = VATS 4.14 years; urokinase 4.63 years Gender m/f = 61/42 N Urokinase = 50 N VATS = 53 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to either the VATS debridement group or the urokinase instillation group. VATS: The procedure was carried out by or under direct supervision of a senior surgeon. 1 or 2 chest tubes were left in place after the procedure. Urokinase: 12Fr to 14Fr chest tubes were used with insertion site selected using sonography. The pleural fluid was first drained, then urokinase was instilled into the pleural cavity through the tube. 10 mL of a 1000 IU/mL solution of urokinase in children aged 1 year and 40 mL in older children was administered every 12 hours for 3 days. After instillation, the chest tube was clamped for 4 hours. It was then unclamped and connected to a suction system at –20 cm H₂O for 8 hours. Common:
| |
| Outcomes | Primary outcome(s):
Secondary outcome(s):
Other outcome(s): At 3 months: n = VATS (36 (67.9%)) versus urokinase (42 (84%)) Of those, normal X‐ray or only small changes: VATS (66.7%) versus urokinase (59.5%); P = 0.4 | |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "The aim of this study was to compare the efficacy of drainage plus urokinase versus video‐assisted thoracoscopic surgery in the treatment of PPE in childhood." Funding: Institute of Health Carlos III, under the Spanish Ministry of Science and Innovation Conflicts of interest: 2 of the authors (Moreno‐Galdo, Perez‐Yarza) are advisory board members of AbbVie Inc, and have received funding from several pharmaceutical companies for travel to conferences and fees for speaking. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence, stratified according to centre and with varying block sizes to ensure balance in both groups |
| Allocation concealment (selection bias) | Low risk | "The attending physician accessed a Web platform and obtained the treatment assigned to each new patient," after consent had been signed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Loss of participants to 3‐month follow‐up, but this would not have affected any of the primary or secondary outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes published in protocol on ClinicalTrials.gov were included in the results. |
| Other bias | Unclear risk | 2 authors (Moreno‐Galdo, Perez‐Yarza) are advisory board members of AbbVie Inc, and have received funding from several pharmaceutical companies for travel to conferences and fees for speaking. |
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Superiority design Number of study centres: 1 Country: USA | |
| Participants | N recruited = 36 N randomised = 36 N reported outcomes = 36 Mean age = 5 years Gender = not reported N tPA = 18 N VATS = 18 Inclusion criteria: Only patients under 18 years of age were included. Diagnosis of empyema:
Exclusion criteria:
| |
| Interventions | Titration period and treatment: VATS: Performed by 1 of 5 staff surgeons. Chest drain left in place post procedure. tPA: 12Fr chest tube inserted and drained with suction. Fibrinolysis was performed by mixing 4 mg of tPA into 40 mL of sterile normal saline. Tube clamped for 1 hr before continuing suction. This was done once on tube insertion and 2 additional doses each at 24 hrs. Common: Clindamycin (10 mg/kg/dose) every 6 hours and ceftriaxone (25 mg/kg per dose) every 12 hours. If haemodynamic instability existed (hypotension, need for vasoactive medications, or persistent tachycardia), then vancomycin (15 mg/kg per dose) every 6 hours was added. Antibiotic therapy was tailored toward positive culture results and the individual patient’s course. Tubes removed when no air leakage and drainage output was < 1 mL/kg per day calculated for the previous 12 hours. | |
| Outcomes | At diagnosis, there were no differences between groups in age, weight, degree of oxygen support, white blood cell count, or days of symptoms. No difference in LOS after intervention, days of oxygen requirement, days until afebrile, or analgesic requirements VATS higher cost. 3 in tPA group required VATS. 1 in VATS group required ventilator, 1 required ventilator and temporary dialysis Primary outcome(s):
| |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "we conducted a prospective, randomised trial comparing VATS to fibrinolytic therapy in children with empyema." Funding: not disclosed Conflicts of interest: not disclosed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated using an individual unit of randomization in an unstratified sequence in blocks of 4" |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was accessed to identify the next allotment after the consent form was signed" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design Number of study centres: 1 Country: UK | |
| Participants | N recruited = 60 N randomised = 60 N reported outcomes = 60 Median age = VATS 3.57 years; urokinase 3.07 years Gender m/f = 33/27 N Urokinase = 30 N VATS = 30 Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Treatment before study: none reported Titration period and treatment: VATS: After the procedure, 1 or 2 chest drains were placed in the portholes on negative suction pressure. 4 children required conversion to mini‐thoracotomy. Urokinase: Pleural fluid was allowed to drain out first, after which intrapleural urokinase was instilled every 12 hours for 3 days, in a dose of 10,000 U in 10 mL normal saline in children under 1 yr of age and 40,000 U in 40 mL normal saline in children above 1 yr of age. 5 children required conversion to VATS. Common: Chest drains were removed when there was minimal drainage (40 to 60 mL/24 hours), and children were discharged home if they remained afebrile for 24 hours after drain removal and at the attending paediatrician's discretion. | |
| Outcomes | Ultrasound staging at entry: Stage I: VATS (6) versus Uro (10) Stage II: VATS (13) versus Uro (8) Stage III: VATS (11) versus Uro (12) Primary outcome(s):
Secondary outcome(s):
At 6 months: 16 lost to follow‐up Chest X‐ray on 24 VATS and 20 Uro "Abnormal chest x‐ray" VATS 21 versus Uro 18 Stage I: VATS had a reduced hospital stay of borderline statistical significance: VATS (5 (3 to 5) days) versus Uro (6 (4 to 25) days) (P = 0.056). However, there was an outlier of 25 days in the urokinase group. No difference in Stage II or III | |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "Our study is the first randomised prospective trial to compare chest drain with intrapleural urokinase against primary VATS for the treatment of empyema in children." Funding: not disclosed Conflicts of interest:"None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization scheme was generated by using the internet web site http://www.randomization.com" |
| Allocation concealment (selection bias) | Low risk | "The trial coordinator was contacted by telephone to reveal treatment allocation" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Loss of patients to 6‐month chest radiograph would not influence any other outcome measure. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: VATS:thoracostomy = 11:9 Number of study centres: 1 | |
| Participants | N recruited = 20 N randomised = 20 N reported outcomes = 20 Mean age = 42.45 years Gender m/f = 15/5 N VATS = 11 Inclusion criteria:
Exclusion criteria: not reported | |
| Interventions | Treatment before study: not reported Titration period and treatment:
| |
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): not reported | |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "To determine the optimal treatment of empyema thoracis (within the fibrinopurulent phase of illness) comparing pleural drainage and fibrinolytic therapy versus video‐assisted thoracoscopic surgery (VATS), with regard to efficacy and duration of hospitalisation." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned. However, outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
cm H₂O: centimetre of water (measurement of pressure)
CT: computed tomography
ICU: intensive care unit
IQR: interquartile range
IU: international unit
Fr: French (catheter‐sizing scale)
LDH: lactate dehydrogenase
LOS: length of stay
N: number
STK: streptokinase
tPA: tissue plasminogen activator
Uro: urokinase
VATS: video‐assisted thoracoscopic surgery
WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Compared 2 surgical treatments | |
| Patients that failed to recover after 2 weeks of thoracostomy were randomised to receive either VATS or ongoing medical management. | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Paper was a commentary of a RCT. | |
| Compared 2 surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Trial was not randomised. | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments | |
| Compared 2 non‐surgical treatments |
RCT: randomised controlled trial
VATS: video‐assisted thoracoscopic surgery
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT Randomisation ratio: open thoracotomy:fibrinolytics = 43:35 Number of study centres: 1 |
| Participants | N recruited = 78 N randomised =78 N reported outcomes = 78 Mean age = thoracotomy 55.53 years; fibrinolytics 56.42 years Gender m/f = 67/11 N fibrinolysis = 35 N thoracotomy = 43 Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to receive either open thoracotomy or chest tube with fibrinolysis. Open thoracotomy: Postero‐lateral thoracotomy with complete decortication of parietal and visceral pleura. 2 large‐bore chest tubes (32Fr) were left in place following the procedure. Fibrinolysis: 14Fr chest tube inserted using Seldinger technique. 250,000 units of streptokinase in 100 mL normal saline was injected and left in place for 4 hours prior to draining. This was repeated every 24 hours for a maximum of 14 days. If drainage continued after 14 days, open drainage was performed. Common: For both procedures, chest tubes were removed when the drainage volume < 50 mL per day. |
| Outcomes | Duration of treatment, mean (SD) days: thoracotomy 13.95 (1.02) versus fibrinolysis 12.91 (1.01); P = 0.66 Treatment success, number of participants (%): thoracotomy 42 (97.7) versus fibrinolysis 30 (85.7); P = 0.04 Duration of hospitalisation, mean (SD) days: thoracotomy 12.09 (2.18) versus fibrinolysis 17.6 (1.95); P < 0.0001 Survival, number of participants (%): thoracotomy 42 (97.7) versus fibrinolysis 33 (94.3); P = 0.43 |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "To confirm that either Fibrinolytic therapy or open decortication which of the two is an effective First line treatment of pleural empyema." Funding: no funding Conflicts of interest: no conflicts of interest |
| Methods | RCT Randomisation ratio: VATS:thoracostomy = 26:28 Number of study centres: not stated |
| Participants | N recruited = 54 N randomised =54 N reported outcomes = 54 Mean age = not stated Gender m/f = 31/23 N thoracostomy = 28 N VATS = 26 Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to receive either VATS or thoracostomy. VATS: No further details provided. Thoracostomy: No further details provided. |
| Outcomes | Duration of chest tube drainage (days): VATS 4.46 ± 1.79; thoracostomy 10.36 ± 5.16; P < 0.001 |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "To analyse cases of parapneumonic pleural empyema in children undergoing chest tube drainage alone or early video‐thoracoscopy in our hospital in order to determine the factors associated with a favourable treatment outcome." Funding: not stated Conflicts of interest: no significant relationships |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria:
Exclusion criteria:
Conducted RCT in 100 patients with complicated parapneumonic effusions with septa or empyema with frank pus |
| Interventions | Participants will be randomised to receive either simple chest tube drainage or early medical thoracoscopy. The latter will be performed in local anaesthesia and analgosedation according to the standards set by the European Study on Medical Video‐Assisted Thoracoscopy (ESMEVAT) group. Fibrinolysis will be used routinely. A nested study on the intrapleural pharmacokinetics of linezolid as antibiotic agent will be performed in 20 participants. Follow‐up will be structured on day 1, day 7, before discharge, and after 3 months including chest radiographs and clinical and laboratory evaluations. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes | Responsible party: University Hospital Basel, Switzerland |
| Methods | Patients with empyema who failed to recover with antibiotics and closed‐tube drainage were divided into 2 groups. Group A underwent VATS. Group B were treated with continued drainage and the addition of fibrinolytic therapy. No further details are available. |
| Participants | 68 patients Group A = 32 patients Group B = 36 patients Patient demographics not available. |
| Interventions | Group A underwent VATS debridement and decortication. Group B were treated with continued thoracostomy drainage and the addition of fibrinolytic therapy. No further details are available. |
| Outcomes | Length of hospital stay Chest tube drainage time Fever duration Conversion to thoracotomy Complications |
| Notes | Study start date: October 2005 Completed: January 2007 |
RCT: randomised controlled trial
SD: standard deviation
VATS: video‐assisted thoracoscopic surgery
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.1 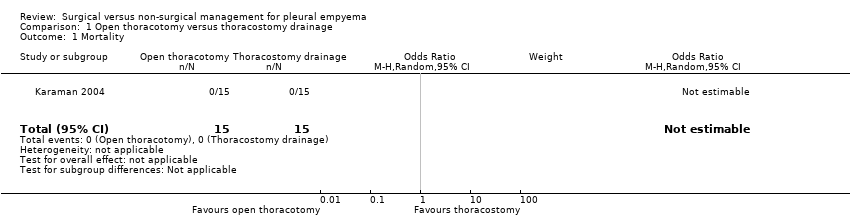 Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 1 Mortality. | ||||
| 2 Length of hospital stay (days) Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐5.9 [‐7.29, ‐4.51] |
| Analysis 1.2  Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 2 Length of hospital stay (days). | ||||
| 3 Procedural complications Show forest plot | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.02, 0.63] |
| Analysis 1.3  Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 3 Procedural complications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 7 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| Analysis 2.1 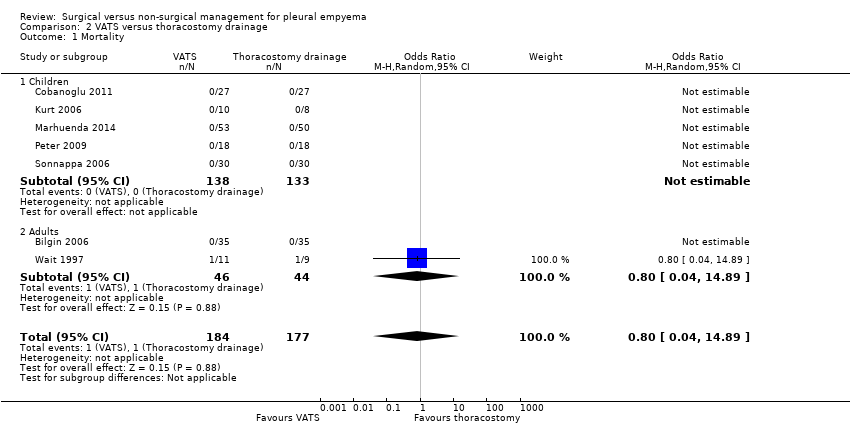 Comparison 2 VATS versus thoracostomy drainage, Outcome 1 Mortality. | ||||
| 1.1 Children | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| 2 Length of hospital stay (days) Show forest plot | 5 | 231 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐4.26, ‐0.77] |
| Analysis 2.2 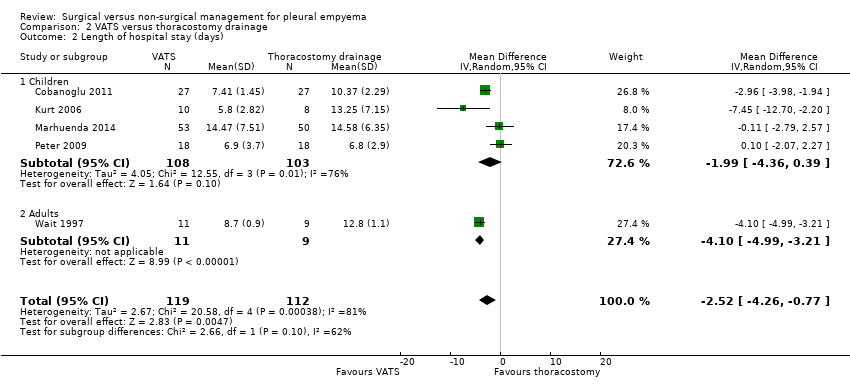 Comparison 2 VATS versus thoracostomy drainage, Outcome 2 Length of hospital stay (days). | ||||
| 2.1 Children | 4 | 211 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐4.36, 0.39] |
| 2.2 Adults | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐4.99, ‐3.21] |
| 3 Procedural complications Show forest plot | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.75] |
| Analysis 2.3 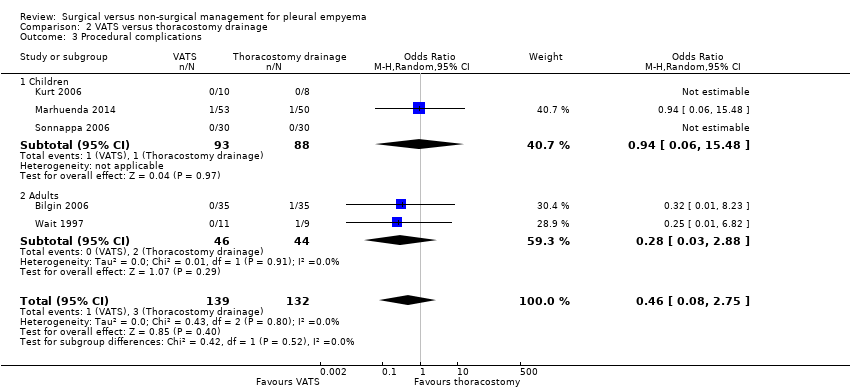 Comparison 2 VATS versus thoracostomy drainage, Outcome 3 Procedural complications. | ||||
| 3.1 Children | 3 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.48] |
| 3.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.88] |

Study flow diagram.
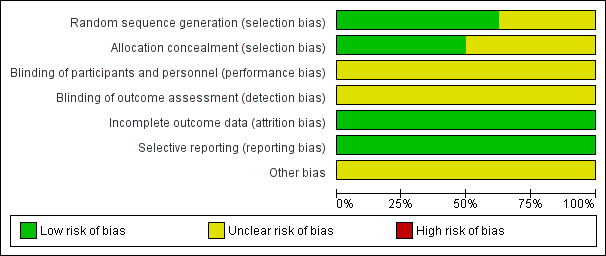
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
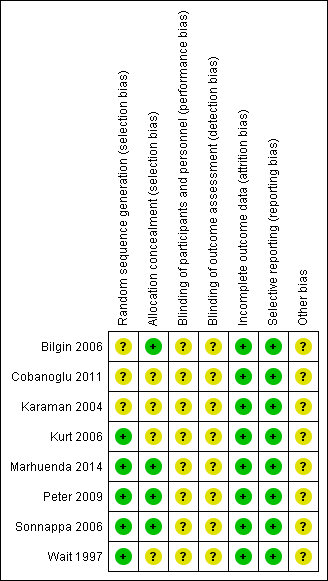
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
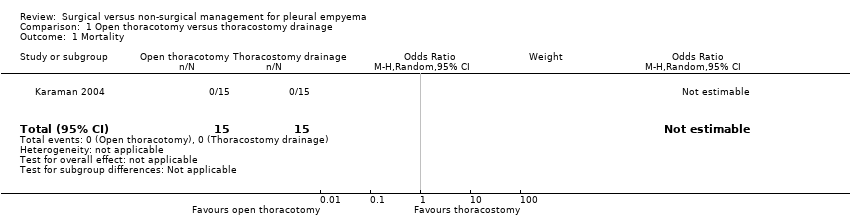
Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 1 Mortality.

Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 2 Length of hospital stay (days).

Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 3 Procedural complications.

Comparison 2 VATS versus thoracostomy drainage, Outcome 1 Mortality.

Comparison 2 VATS versus thoracostomy drainage, Outcome 2 Length of hospital stay (days).

Comparison 2 VATS versus thoracostomy drainage, Outcome 3 Procedural complications.
| Open thoracotomy compared to thoracostomy drainage for pleural empyema | ||||||
| Patient or population: children with pleural empyema | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Thoracostomy drainage | Open thoracotomy | |||||
| Mortality Follow‐up: up to 3 months after discharge | Risk in study population | Not estimable | 30 | ⊕⊕⊕⊝ | No deaths occurred in either group. | |
| — | — | |||||
| Length of hospital stay (days) Follow‐up: up to 3 months after discharge | The mean length of hospital stay in the control group was 15.4 days. | The mean length of hospital stay in the intervention group was 5.9 days fewer (7.29 fewer to 4.51 fewer). | — | 30 | ⊕⊕⊕⊝ | |
| Procedural complications Follow‐up: up to 3 months after discharge | Risk in study population | OR 0.10 | 30 | ⊕⊕⊕⊝ | ||
| 600 per 1000 | 130 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to small sample size and only one study. | ||||||
| VATS compared to thoracostomy drainage for pleural empyema | ||||||
| Patient or population: children and adults with pleural empyema | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Thoracostomy drainage | VATS | |||||
| Mortality | Risk in study population | OR 0.80 | 361 | ⊕⊕⊝⊝ | Data only for adults | |
| 6 per 1000 | 5 per 1000 | |||||
| Mortality: children | Risk in study population | Not estimable | 271 | ⊕⊕⊕⊝ | No deaths occurred in either group. | |
| Not pooled | Not pooled | |||||
| Mortality: adults Follow‐up: not reported | Risk in study population | OR 0.80 | 90 | ⊕⊕⊕⊝ | No deaths occurred in Bilgin 2006. Data based on Wait 1997 | |
| 23 per 1000 | 18 per 1000 | |||||
| Length of hospital stay (days) Follow‐up: 1 year in Cobanoglu 2011 and 3 months in Marhuenda 2014 | Control group | The mean length of hospital stay in the intervention group was 2.52 days fewer (4.26 fewer to 0.77 fewer). | — | 231 | ⊕⊕⊕⊝ | Note: Follow‐up period not reported in Kurt 2006; Peter 2009; Wait 1997. |
| Procedural complications Follow‐up: 6 months in Bilgin 2006 and not reported in Wait 1997 | Risk in study population | OR 0.46 | 271 | ⊕⊕⊕⊝ | ||
| 23 per 1000 | 11 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to wide confidence intervals (data from only one study) and indirectness (data only available for adults). | ||||||
| Trial | Total treatment cost (USD): | Total treatment cost (USD): | P value |
| Mean 386.672 ± 72.06 | Mean 957.487 ± 137.238 | < 0.001 | |
| Median (IQR) 21,947 (17,895 to 37,458) | Median (IQR) 19,714 (17,325 to 23,000) | 0.315 | |
| Mean 9127 | Mean 11,379 | < 0.001 | |
| Mean 7600 ± 5400 | Mean 11,700 ± 2900 | 0.02 | |
| Mean 24,052 ± 3466 | Mean 16,642 ± 2841 | 0.11 | |
| IQR: interquartile range | |||
| Trial | Duration of chest tube | Duration of chest tube drainage: | P value |
| Mean 9.48 ± 2.50 | Mean 6.56 ± 1.55 | < 0.001 | |
| Mean 13.8 ± 2.3 | Mean 7.5 ± 1.1 | < 0.05 | |
| Mean 9.63 ± 5.45 | Mean 2.80 ± 0.63 | < 0.001 | |
| Median (IQR) 5 (4 to 6) | Median (IQR) 4 (3 to 5) | < 0.001 | |
| Mean 9.8 ± 1.3 | Mean 5.8 ± 1.1 | 0.03 | |
| IQR: interquartile range | |||
| Trial | Postintervention fever duration: | Postintervention fever | P value |
| Mean 3.9 ± 2.1 | Mean 3.4 ± 2.4 | 0.782 | |
| Mean 6.25 ± 4.10 | Mean 3.60 ± 2.95 | 0.146 | |
| Median (IQR) 6 (3 to 7) | Median (IQR) 4 (2 to 7) | 0.62 | |
| Median (range) 2.5 (0 to 25) | Median (range) 2.5 (0 to 10) | 0.635 | |
| Mean 3.8 ± 2.9 | Mean 3.1 ± 2.7 | 0.46 | |
| IQR: interquartile range | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Length of hospital stay (days) Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐5.9 [‐7.29, ‐4.51] |
| 3 Procedural complications Show forest plot | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.02, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 7 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| 1.1 Children | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| 2 Length of hospital stay (days) Show forest plot | 5 | 231 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐4.26, ‐0.77] |
| 2.1 Children | 4 | 211 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐4.36, 0.39] |
| 2.2 Adults | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐4.99, ‐3.21] |
| 3 Procedural complications Show forest plot | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.75] |
| 3.1 Children | 3 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.48] |
| 3.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.88] |

