Therapie mit Eisen bei anämischen Erwachsenen ohne chronische Nierenerkrankung
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial | |
| Participants | Country: Norway Sample size: 30 Postrandomisation dropout(s): 0 (0%) Revised sample size: 30 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: Further details: iron sorbitol citric acid complex 100 mg/d until calculated dose was reached Further details: ferrous fumarate 300 mg in divided doses Further details: no intervention | |
| Outcomes | Outcomes reported were mortality, haemoglobin levels and serious adverse events Time of measurements: 3 weeks from start of treatment | |
| Notes | Attempts were made to contact study authors in August 2012. They replied in September 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Thirty envelopes were used. In ten were written oral iron, in ten parenteral iron, and in ten no iron. The envelopes were mixed, and one envelope was opened for each patient" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "Thirty envelopes were used. In ten were written oral iron, in ten parenteral iron, and in ten no iron. The envelopes were mixed, and one envelope was opened for each patient" (study author replies) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The patient and persons who administrated the treatment were informed. The laboratory results were opened at the end of the study" (study author replies) |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The patient and persons who administrated the treatment were informed. The laboratory results were opened at the end of the study" (study author replies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | Low risk | Quote: "Not funded" (study author replies) |
| Methods | Randomised clinical trial | |
| Participants | Country: multi‐centric (Europe) Sample size: 158 Postrandomisation dropout(s): 0 (0%) Revised sample size: 158 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, proportion of patients requiring blood transfusion, haemoglobin levels, quality of life measurements and serious adverse events (in the subgroup of anaemic participants) Time of measurement: 24 weeks from start of treatment | |
| Notes | Attempts were made to contact the study author in August 2012. They provided additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by IVRS (Interactive Voice Response System), stratified by region in a 2:1 ratio (FCM:placebo). Blocks of 6 (4FCM, 2 placebo) were generated per sites" Comment: The IVRS utilises a dynamic randomisation system with an adaptive minimisation technique for prespecified stratification variables (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a central interactive voice‐response system, we randomly assigned eligible patients, in a 2:1 ratio, to receive either ferric carboxymaltose (provided by Vifor Pharma) or placebo (normal saline)" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Using a central interactive voice‐response system, we randomly assigned eligible patients, in a 2:1 ratio, to receive either ferric carboxymaltose (provided by Vifor Pharma) or placebo (normal saline)" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Using a central interactive voice‐response system, we randomly assigned eligible patients, in a 2:1 ratio, to receive either ferric carboxymaltose (provided by Vifor Pharma) or placebo (normal saline)" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No anaemic patients were excluded from the trial (study author replies) |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Source of funding bias | High risk | Quote: "Sponsored by Vifor Pharma" |
| Methods | Randomised clinical trial | |
| Participants | Country: multi‐centric (worldwide) Sample size: 243 Postrandomisation dropout(s): 5 (2.1%) Revised sample size: 238 Females: 158 (66.4%) Mean age: 63 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements, change in Hb and serious adverse events Time of measurement: end of treatment | |
| Notes | Reason for postrandomisation dropout(s): did not receive drug Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomization list was created and maintained by an independent randomization group at the study sponsor using permuted blocks" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study was blinded to the dose of darbepoetin alfa administered and open‐label for IV iron administration" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes were reported |
| Source of funding bias | Unclear risk | Comment: This information was not available |
| Methods | Randomised clinical trial | |
| Participants | Country: international multi‐centric trial in Europe Sample size: 398 Postrandomisation dropout(s): 2 (0.5%) Revised sample size: 396 Females: 240 (60.6%) Mean age: 61 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements, quality of life and serious adverse events Time of measurements: not stated | |
| Notes | Reason for postrandomisation dropout(s): did not receive darbopoetin Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization, assigned using an interactive voice response system, was stratified by tumor type…" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Important clinical outcomes were reported |
| Source of funding bias | Unclear risk | Comment: This information was not available |
| Methods | Randomised clinical trial | |
| Participants | Country: Brazil Number randomly assigned: 23 Postrandomisation dropouts: 0 (0%) Revised sample size: 23 Average age: 66 years Females: 7 (30.4%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups: Group 3: control (n = 6) Further details: placebo | |
| Outcomes | Outcomes reported were mortality, serious adverse events and haemoglobin | |
| Notes | Time of measurement: 13 weeks from start of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization system will be based on a computerized table of random numbers and performed in blocks of 3 per participating center" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each of the 8 participating centers will randomize patients by telephone contact with the randomization center at Hospital de Clınicas de Porto Alegre" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were randomized in a double‐blind method to receive…Each participating center will elect a third‐party blind individual (usually a registered nurse) who will open the allocated medication box, prepare iron sucrose infusions or saline, and administer the preparations to patients using opaque devices. Both patient and attending physicians or nurses will be blind to allocated therapy. Oral medications and oral placebo will be identical in all aspects" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were randomized in a double‐blind method to receive…Each participating center will elect a third‐party blind individual (usually a registered nurse) who will open the allocated medication box, prepare iron sucrose infusions or saline, and administer the preparations to patients using opaque devices. Both patient and attending physicians or nurses will be blind to allocated therapy. Oral medications and oral placebo will be identical in all aspects" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Mortality and serious adverse events were reported |
| Source of funding bias | High risk | Quote: "The sponsors provided the intravenous iron formulation, the placebo formulations for oral and intravenous administration as well as most of the study costs" |
| Methods | Randomised clinical trial | |
| Participants | Country: Thailand Sample size: 44 Postrandomisation dropout(s): 0 (0%) Revised sample size: 44 Females: 44 (100%) Mean age: 51 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements, quality of life, haemoglobin levels and serious adverse events Time of measurements: not stated | |
| Notes | Attempts were made to contact study authors in August 2012. They replied promptly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using a random table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the first investigation knew the allocation number"; "We had the other persons enrol the patients. But the first author run the allocation number according to the stratification" (study author replies) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patient and drug administrator were not blinded, however the outcome assessors was blinded" (study author replies) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patient and drug administrator were not blinded, however the outcome assessors was blinded" (study author replies) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "There were no post‐randomisation drop‐outs" (study author replies) |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | Low risk | Quote: "We did not have source of funding. However, the company (DKSH Limited, Bangkok, Thailand) supported Venofer. But the company did not involve in research protocol and manuscript writing" (study author replies) |
| Methods | Randomised controlled trial | |
| Participants | Country: UK Sample size: 18 Postrandomisation dropout(s): not stated Revised sample size: 18 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were blood transfusion requirements and haemoglobin levels Time of measurements: blood transfusion requirements postoperatively and haemoglobin levels preoperatively | |
| Notes | This study included anaemic and non‐anaemic participants. Only anaemic participants were included in this review Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were allocated to either the treatment (iron) group or a placebo group, based on a computer‐generated randomization sequence provided by the Research and Development Support Unit" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation codes were sealed in sequentially numbered opaque envelopes which were secured within a locked store room in a dedicated research unit (this was remote from the clinical areas of the hospital where participants were to undergo outpatient, ward and operative treatment). Only after recruitment was an envelope opened by the investigator administering the infusion, following the inscribed strict numerical order and for the relevant subset appropriate to the Hb status of the participant" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Although the investigator administering the infusion was not blinded to the treatment group, this was concealed from the patient by using an opaque sheath to cover the drug giving set. The chief investigator and clinicians involved in perioperative care also remained blinded to the treatment group for the duration of the trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The chief investigator and clinicians involved in perioperative care also remained blinded to the treatment group for the duration of the trial" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2 postrandomisation dropouts were reported. Whether these participants were anaemic was not clear |
| Selective reporting (reporting bias) | High risk | Comment: Important clinical outcomes were not reported |
| Source of funding bias | High risk | Comment: The review authors thank Syner‐Med Pharmaceutical Products Limited for providing Venofer and for funding the blood tests |
| Methods | Randomised clinical trial | |
| Participants | Country: multi‐centric (Europe) Sample size: 485 Postrandomisation dropout(s): 2 (0.4%) Revised sample size: 483 Females: 284 (58.8%) Mean age: 39 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were serious adverse events and haemoglobin levels | |
| Notes | Reason for postrandomisation dropout(s): did not receive treatment Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized 1:1 to each of the treatment arms according to a predefined, computer‐generated list and stratified by gender and disease (CD/UC) as provided via sequentially numbered randomization envelopes by data management, PAREXEL International GmbH" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized 1:1 to each of the treatment arms according to a predefined, computer‐generated list and stratified by gender and disease (CD/UC) as provided via sequentially numbered randomization envelopes by data management, PAREXEL International GmbH" Comment: Allocation concealment probably achieved |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Both participants and physicians were aware of which treatment was being administered" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Both participants and physicians were aware of which treatment was being administered" |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported |
| Source of funding bias | High risk | Quote: "The authors thank all investigators at all study sites (see Appendix), the support in funding and conducting the study (Barbara von Eisenhart Rothe, responsible Medical Director; Janne Harjunpää, statistical analysis; Vifor Pharma, Switzerland), and medical writing support (Walter Fürst; SFL Regulatory Affairs & Scientific Communication, Switzerland)" |
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden Sample size: 67 Postrandomisation dropout(s): 0 (0%) Revised sample size: 67 Females: 42 (62.7%) Mean age: 76 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements and haemoglobin levels Time of measurements: end of treatment | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central randomization procedure was used (fax formular) and patients received numbers in a consecutive order according to a predetermined scheme and randomly allocated to either receive IV iron or no iron" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "A central randomization procedure was used (fax formular) and patients received numbers in a consecutive order according to a predetermined scheme and randomly allocated to either receive IV iron or no iron" (study author replies) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was a prospective, open‐label, randomized, multicenter study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was a prospective, open‐label, randomized, multicenter study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | High risk | Comment: This work was supported by grants from Roche AB, Sweden, and the Research and Development Centre, Sundsvall Hospital, Sundsvall, Sweden |
| Methods | Randomised clinical trial | |
| Participants | Country: Australia Number randomly assigned: 605 Postrandomisation dropouts: 0 (0%) Revised sample size: 605 Average age: not stated Females: not stated Inclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups: | |
| Outcomes | Outcomes reported were mortality and serious adverse events | |
| Notes | Time of measurement: 5 weeks after start of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization scheme and codes were computer‐generated" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "An interactive voice response system (IVRS) was used to assign unique randomization numbers for each subject. This is a telephone based system that was accessible at all times. Randomization numbers were not reused for any use" (study author replies) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Open‐label" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Mortality and morbidity were reported |
| Source of funding bias | High risk | Comment: sponsored by AMAG Pharmaceuticals Inc |
| Methods | Randomised clinical trial | |
| Participants | Country: Canada Sample size: 26 Postrandomisation dropout(s): 5 (19.2%) Revised sample size: 21 Females: 5 (23.8%) Mean age: 62 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were blood transfusion requirements and haemoglobin levels Time of measurements: 42nd postoperative day | |
| Notes | Study authors used arbitrary weights to adjust haemoglobin levels based on transfusion Attempts were made to contact study authors in August 2012. They replied promptly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In each stratum, patients were randomized in randomly permuted blocks of three patients according to a computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignments were placed into opaque sequentially numbered envelopes with pharmacy" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "For all three groups the IV solution was draped with an opaque cover and the IV tubing was covered with a translucent tape. Patients in the vontrol group and the iron group received SC and IV injections of normal saline" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "For all three groups the IV solution was draped with an opaque cover and the IV tubing was covered with a translucent tape. Patients in the vontrol group and the iron group received SC and IV injections of normal saline" |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | High risk | Quote: "K. Karkouti is supported in part by the Canadian Institutes of Health Research and the Canadian Blood Services. T.M. Yau is supported in part by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario. K. Karkouti and S.A. McCluskey have received research funding and speakers’ fees from Ortho Biotech" |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Sample size: 20 Postrandomisation dropout(s): not stated Revised sample size: 20 Females: 9 (45%) Mean age: not stated Inclusion criteria:
(only anaemic patients were included in this review) | |
| Interventions | Participants were randomly assigned to the following groups:C | |
| Outcomes | Outcome reported was blood transfusion requirements | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised (by telephone to a distant centre) to…" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "It was not possible to use a placebo and blind the patient, as oral iron alters stool colour" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical team (surgeons, nurses, anaesthetists) were blinded to treatment allocation. The collection of data was performed by a research fellow not involved in the direct |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported |
| Source of funding bias | Unclear risk | Comment: This information was not available |
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden Sample size: 91 Postrandomisation dropout(s): not stated Revised sample size: 91 Females: 63 (69.2%) Mean age: 42 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were haemoglobin levels and serious adverse events Time of measurements: end of treatment | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to any of the treatments over the Internet, by applying the minimization method to ensure a balance within the patient factors age, B‐Hb and S‐ferritin" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated to any of the treatments over the Internet, by applying the minimization method to ensure a balance within the patient factors age, B‐Hb and S‐ferritin" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Venofer is a dark‐brown, non‐transparent, aqueous solution to be administered intravenously. There is no placebo solution available" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Although the trial states that observer blinding was performed, further details were not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | High risk | Comment: Important clinical outcomes were not reported |
| Source of funding bias | High risk | Comment: The study was supported by Renapharma AB, Uppsala, Sweden |
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Sample size: 148 Postrandomisation dropout(s): 0 (0%) Revised sample size: 148 Females: 59 (39.9%) Mean age: 68 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were haemoglobin levels Time of measurements: end of treatment | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized using a computer‐generated list of random numbers elaborated by an external data manager" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation concealment incorporates the involvement of an external data manager, not a physician involved in patient management, who assigned the randomization sequence in order to protect the randomization sequence until allocation" (study author replies) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an open‐label, randomized, controlled, prospective study comparing the efficacy and safety of rHuEPO combined with oral lactoferrin (Lattoglobina; Grunenthal‐ Formenti, Milan, Italy) or IV iron supplementation in advanced cancer patients with anemia undergoing chemotherapy" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was an open‐label, randomized, controlled, prospective study comparing the efficacy and safety of rHuEPO combined with oral lactoferrin (Lattoglobina; Grunenthal‐ Formenti, Milan, Italy) or IV iron supplementation in advanced cancer patients with anemia undergoing chemotherapy" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | Low risk | Quote: "This work was supported by the Associazione Sarda per la ricerca nell’Oncologia Ginecologica‐ONLUS"; "No funding has been received from the drug manufacturer. The study was not sponsored nor funded by any manufacturers" (study author replies) |
| Methods | Randomised clinical trial | |
| Participants | Country: Lebanon Sample size: 80 Postrandomisation dropout(s): 0 (0%) Revised sample size: 80 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements and haemoglobin levels Time of measurements: 30 days from start of treatment | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Drug manipulation and assignment were handled by the central pharmacy to assure double blinding" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Although a placebo was used, further details of placebo such as colour were not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts for the main clinical outcomes were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Important clinical outcomes were reported |
| Source of funding bias | Unclear risk | Comment: This information was not available |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomly assigned: 300 Postrandomisation dropouts: 0 (0%) Revised sample size: 300 Average age: 82 years Females: 245 (81.7%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups Group 2: control (n = 150) | |
| Outcomes | Outcomes reported were mortality, serious adverse events and haemoglobin levels | |
| Notes | Attempts were made to contact study authors in November 2012. They replied promptly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The envelopes for this study were prepared by me, sealed, mixed up and then numbered" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was accomplished by opening a sealed opaque numbered envelope for each patient" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Potential weaknesses of the study include the lack of a placebo" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Also, there was no blinding of the outcome assessors for the present study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Source of funding bias | Low risk | Quote: "There was no external funding for this study" (study author replies) |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Sample size: 200 Postrandomisation dropout(s): 0 (0%) Revised sample size: 200 Females: 103 (51.5%) Mean age: 57 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were blood transfusion requirements | |
| Notes | Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was accomplished by the investigational pharmacy (SB) using a block pattern stratified by ICU type" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All involved parties with the exception of the investigational pharmacist were blinded to the identity of the study drug, including patients and their families, those administering the intervention, and those assessing the outcomes..." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All involved parties with the exception of the investigational pharmacist were blinded to the identity of the study drug, including patients and their families, those administering the intervention, and those assessing the outcomes..." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported |
| Source of funding bias | Unclear risk | Comment: This information was not available |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Sample size: 502 Postrandomisation dropout(s): 12 (2.4%) Revised sample size: 490 Females: 320 (65.3%) Mean age: 64 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: Further details: sodium ferric gluconate complex in sucrose IV 187.5 mg over 90 minutes once every 3 weeks for 5 doses Further details: ferrous sulphate oral 325 mg once daily Further details: oral placebo | |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements, quality of life and serious adverse events Time of measurements: 16 weeks from start of treatment | |
| Notes | Reason for postrandomisation dropout(s): did not have haemoglobin measurements Attempts were made to contact study authors in August 2012. They replied promptly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "It was computer generated" (study author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment was done by calling the central randomization center by telephone" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients and investigators were blinded to assignment of oral iron or oral placebo, but, for practical reasons, assignment to IV iron versus an oral product was not blinded" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Patients and investigators were blinded to assignment of oral iron or oral placebo, but, for practical reasons, assignment to IV iron versus an oral product was not blinded" |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Important clinical outcomes were reported |
| Source of funding bias | Unclear risk | Quote: "Supported in part by Public Health Service Grant No. CA‐124477 and grant from Amgen (Thousand Oaks, CA) to the Mayo Clinic Cancer Research Consortium" |
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomly assigned: 72 Postrandomisation dropouts: not stated Revised sample size: 72 Average age: 70 years Females: 30 (41.7%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups: Further details: ferrous sulphate 200 mg thrice daily orally for 6 weeks after surgery | |
| Outcomes | Outcomes reported were haemoglobin levels | |
| Notes | Attempts were made to contact study authors in November 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Those patients who entered into the study were randomly assigned by the hospital pharmacy into two groups using computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Those patients who entered into the study were randomly assigned by the hospital pharmacy into two groups using computer generated random numbers" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "One group received ferrous sulphate in 200 mg capsules and the second (37 patients) were given a gelatine placebo. Both groups were prescribed their capsules three times daily for six weeks after discharge from hospital. Both patients and investigators were blind to the treatment allocated until the end of the trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "One group received ferrous sulphate in 200 mg capsules and the second (37 patients) were given a gelatine placebo. Both groups were prescribed their capsules three times daily for six weeks after discharge from hospital. Both patients and investigators were blind to the treatment allocated until the end of the trial" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported |
| Source of funding bias | Low risk | Quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" |
| Methods | Randomised controlled trial | |
| Participants | Country: worldwide Number randomly assigned: 812 Postrandomisation dropouts: 4 (0.5%) Revised sample size: 808 Average age: 45 years Females: 720 (89.1%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups | |
| Outcomes | Outcomes reported were mortality, morbidity, quality of life and haemoglobin levels | |
| Notes | Time of measurement: 5 weeks after start of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized using an Interactive Voice Randomization System" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Blinding was accomplished by having both ferumoxytol and normal saline administered in a shrouded manner by a unblinded Test Article Administrator, while both study participants and all other study staff including the investigator were blinded to what was administered" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinding was accomplished by having both ferumoxytol and normal saline administered in a shrouded manner by a unblinded Test Article Administrator, while both study participants and all other study staff including the investigator were blinded to what was administered" |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: Mortality and morbidity were reported |
| Source of funding bias | Unclear risk | Quote: "All data were analyzed by representatives of the clinical and biostatistical groups of AMAG Pharmaceuticals" |
| Methods | Randomised clinical trial | |
| Participants | Country: USA, Mexico Sample size: 477 Postrandomisation dropout(s): 24 (5%) Revised sample size: 453 Females: 453 (100%) Mean age: 39 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to the following groups: | |
| Outcomes | Outcomes reported were mortality, quality of life, haemoglobin levels and serious adverse events Time of measurements: 6 weeks from start of treatment | |
| Notes | Reason for postrandomisation dropout(s): did not complete study or haemoglobin levels were not available Attempts were made to contact study authors in August 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence for the VIT02/03 studies were generated by using Proc Plan in SAS Version 8 by the CRO we used for these trials" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment group and subject number were assigned by blocked randomization using an interactive voice response system" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an open‐label, Phase 3, randomized, active control clinical trial with two parallel treatment groups" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "This was an open‐label, Phase 3, randomized, active control clinical trial with two parallel treatment groups" |
| Incomplete outcome data (attrition bias) | High risk | Comment: Postrandomisation dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Comment: All important clinical outcomes were reported |
| Source of funding bias | Unclear risk | Quote: "Support for this study was provided by American Regent, Inc., the human drug division of Luitpold Pharmaceuticals, Shirley, NY" |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No separate data for anaemic and non‐anaemic participants | |
| Protocol for a trial | |
| Not a randomised controlled trial | |
| Includes pregnant participants | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Significant proportion of participants had chronic kidney disease; no separate data were available for participants without chronic kidney disease | |
| Not a randomised clinical trial | |
| No separate data for anaemic and non‐anaemic patients | |
| Not a randomised clinical trial | |
| Protocol for a trial | |
| Not a randomised clinical trial | |
| It was not clear whether this was a randomised controlled trial. As the study author does not work at the institution any longer and no forwarding details were provided, we were unable to contact the study author to ascertain whether this was a randomised controlled trial | |
| Not a randomised controlled trial | |
| No separate data for anaemic and non‐anaemic participants | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not in anaemic participants | |
| Includes pregnant and postpartum participants | |
| Not in anaemic participants | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Includes non‐anaemic participants | |
| Not assessing the role of iron | |
| The age group of participants is not stated; the study was conducted in the Paediatric Department of the hospital, suggesting that a significant proportion of (if not all) participants were children | |
| Intervention in non‐anaemic participants | |
| Not a randomised controlled trial | |
| Includes participants with chronic kidney disease and postpartum anaemia | |
| Not a randomised controlled trial | |
| Participants received different co‐interventions | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Comparison between intravenous iron and oral iron in combination with blood transfusion | |
| Includes pregnant participants | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Participants received different co‐interventions | |
| Anaemic participants were excluded | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Includes pregnant participants | |
| Includes postpartal participants | |
| Not clear whether the study was a randomised controlled trial. Study authors were contacted and replied but were unable to provide information on the method of randomisation | |
| Not clear whether participants were anaemic preoperatively | |
| Includes non‐anaemic participants | |
| Includes non‐anaemic participants | |
| No separate data for anaemic and non‐anaemic participants | |
| Includes participants with chronic kidney disease | |
| Includes participants with chronic kidney disease | |
| Participants were undergoing haemodialysis for kidney failure | |
| No separate data for anaemic and non‐anaemic participants | |
| No separate data for anaemic and non‐anaemic participants |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Country: India Sample size: 60 Postrandomisation dropout(s): 0 (0%) Revised sample size: 60 Females: 41 (68.3%) Mean age: 36 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Attempts were made to contact study authors in Augusut 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 229 Postrandomisation dropout(s): 72 (31.4%) Revised sample size: 157 Females: 101 (64.3%) Mean age: 62 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were changes in haemoglobin levels. In addition, the trial provided information on adverse events, but this information was reported in the 'as treated analysis.' As a result, a proportion of participants with serious adverse events in the control group actually belonged to the intervention group (who had discontinuation of treatment). We did not obtain this information because of the significant bias that this would introduce Time of measurement: maximum haemoglobin rise during study period |
| Notes | Reason for postrandomisation dropout(s): required interventions (27); adverse events (18); participant requests (28); investigator decisions (7); intolerable signs and symptoms (11); other (17) Attempts were made to contact study author in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 157 Postrandomisation dropout(s): 2 (1.3%) Revised sample size: 155 Females: 65 (41.9%) Mean age: 65 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: Further details: iron dextran by total dose infusion (intravenous) diluted in 500 mL normal saline administered at a rate of 175 mL/h Further details: iron dextran 100 mg intravenously in divided doses until chemotherapy Further details: ferrous sulphate 325 mg twice daily until chemotherapy Further details: no intervention |
| Outcomes | Outcomes reported were haemoglobin levels Time of measurements: 6 weeks from start of treatment or end of chemotherapy treatment |
| Notes | Reason for postrandomisation dropout(s): no postbaseline haemoglobin value Attempts were made to contact study authors in August 2012. Although they replied, they did not provide information on allocation concealment. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised clinical trial |
| Participants | Country: Spain Number randomly assigned: 65 Postrandomisation dropouts: not stated Revised sample size: 65 Average age: not stated Females: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to 2 groups |
| Outcomes | Outcomes reported were haemoglobin and quality of life |
| Notes | Time of measurement: 30 days from discharge |
| Methods | Randomised controlled trial |
| Participants | Country: China Number randomly assigned: 105 Postrandomisation dropouts: not stated Revised sample size: 105 Average age: 41 years Females: 94 (89.5%) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to 2 groups: Group 2: preparation 2 (n = 36) Further details: ferrous sulphate controlled release tablets 500 mg per day orally for 8 weeks Group 3: preparation 3 (n = 38) |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Attempts were made to contact study authors in November 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 49 Postrandomisation dropout(s): 3 (6.1%) Revised sample size: 46 Females: 46 (100%) Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): withdrew from study Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Norway Sample size: 19 Postrandomisation dropout(s): 2 (10.5%) Revised sample size: 17 Females: 13 (76.5%) Mean age: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | None of the outcomes of interest for the review were reported in this trial |
| Notes | Reason for postrandomisation dropout(s): Treatment was stopped after 6 days because of side effects of oral iron Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Italy Sample size: 24 Postrandomisation dropout(s): not stated Revised sample size: 24 Females: 14 (58.3%) Mean age: 61 years Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were serious adverse events and haemoglobin levels |
| Notes | Attempts were made to contact study authors in Augusut 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Italy Sample size: 24 Postrandomisation dropout(s): not stated Revised sample size: 24 Females: not stated Mean age: 68 years Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | None of the outcomes of interest were reported |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 50 Postrandomisation dropout(s): 14 (28%) Revised sample size: 36 Females: 36 (100%) Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): side effects (6), non‐compliance (3), moved (2), discovery of pregnancy (1) and normal serum ferritin concentrations (2) Attempts were made to contact study authors in Augusut 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 189 Postrandomisation dropout(s): 60 (31.7%) Revised sample size: 129 Females: 89 (69%) Mean age: 65 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: Further details: ferrous gluconate 125 mg IV weekly for 8 weeks Further details: ferrous sulphate 325 mg oral daily for 8 weeks Further details: no treatment |
| Outcomes | Outcomes reported were mortality, blood transfusion requirements, haemoglobin levels and serious adverse events Time of measurements: 4 weeks after start of treatment |
| Notes | Reason for postrandomisation dropout(s): Did not receive drugs as planned for various reasons including discontinuation of treatment. Information was obtained for as many participants as possible Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Awaiting full text |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | This reference obtained from The Cochrane Library appears to be incorrect, and the correct article could not be obtained |
| Methods | Randomised controlled trial |
| Participants | Country: South Africa Sample size: 173 Postrandomisation dropout(s): 82 (47.4%) Revised sample size: 91 Females: not stated Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): not stated Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Germany Number randomly assigned: 81 Postrandomisation dropouts: not stated Revised sample size: 81 Average age: not stated Females: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to 2 groups: |
| Outcomes | Outcomes reported were serious adverse events and haemoglobin |
| Notes | Time of measurement: not stated Numerical details of serious adverse events and haemoglobin were not reported |
| Methods | Randomised controlled trial |
| Participants | Country: India Sample size: 50 Postrandomisation dropout(s): not stated Revised sample size: 50 Females: 17 (34%) Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcome reported was haemoglobin rise Time of measurements: 3 weeks after start of treatment |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Awaiting full text |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | This appears to be the same as another included reference (Kim 2009), but we could not confirm this, as this article was not available |
| Methods | Randomised controlled trial |
| Participants | Country: South Korea Sample size: 75 Postrandomisation dropout(s): not stated Revised sample size: 75 Females: 75 (100%) Mean age: 52 years Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were blood transfusion requirements, haemoglobin levels and serious adverse events Time of measurements: end of chemotherapy cycle |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: South Korea Sample size: 76 Postrandomisation dropout(s): 20 (26.3%) Revised sample size: 56 Females: 56 (100%) Mean age: 42 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels and serious adverse events Reasons for postrandomisation dropouts: < 80% compliance Time of measurements: not stated |
| Notes | Reason for postrandomisation dropout(s): less than 80% compliance Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: international multi‐centric trial Sample size: 200 Postrandomisation dropout(s): 4 (2%) Revised sample size: 196 Females: 117 (59.7%) Mean age: 42 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were mortality, quality of life, haemoglobin levels and serious adverse events Time of measurements: not stated |
| Notes | Reason for postrandomisation dropout(s): no efficacy data available (these participants could be included for safety analyses) Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: UK Sample size: 126 Postrandomisation dropout(s): 22 (17.5%) Revised sample size: 104 Females: 90 (86.5%) Mean age: 51 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were mortality, haemoglobin levels and serious adverse events |
| Notes | Reason for postrandomisation dropout(s): unreliable data (5); did not return for follow‐up (12); did not undergo tests (5) Attempts were made to contact study authors in Augusut 2012. Although authors replied, they could not provide information on allocation concealment. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: China Sample size: 140 Postrandomisation dropout(s): 9 (6.4%) Revised sample size: 131 Females: 119 (90.8%) Mean age: 39 years Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): unclear Attempts were made to contact study author in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Greece Sample size: 64 Postrandomisation dropout(s): not stated Revised sample size: 64 Females: not stated Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels Time of measurements: not stated |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Brazil Number randomly assigned: 18 Postrandomisation dropouts: not stated Revised sample size: 18 Average age: 59 years Females: 8 (44.4%) Inclusion criteria:
|
| Interventions | Participants were randomly assigned to 2 groups: Group 2: preparation 2 (n = 9) |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Attempts were made to contact study authors in November 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and surgically resectable colorectal neoplasm |
| Interventions | Iron sucrose (intravenous) vs ferrous sulphate (oral) |
| Outcomes | Mortality, blood transfusion requirements and haemoglobin levels |
| Notes | Recruitment status of this study is not known |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and with ongoing or planned chemotherapy |
| Interventions | Iron sucrose (intravenous) vs no intervention |
| Outcomes | Adverse events, quality of life and change in haemoglobin levels |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and non‐myeloid malignancies |
| Interventions | Iron dextran complex (intravenous) or iron sucrose injection vs no intervention |
| Outcomes | None of the outcomes of interest for this review were measured in this trial |
| Notes | Study active, not recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia |
| Interventions | Ferric carboxymaltose (intravenous) vs iron dextran complex (intravenous) |
| Outcomes | Safety |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and undergoing orthopaedic surgery |
| Interventions | Ferric carboxymaltose (intravenous) vs placebo |
| Outcomes | Change in haemoglobin |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and inflammatory bowel disease |
| Interventions | Ferric carboxymaltose (intravenous) vs iron sucrose (intravenous) |
| Outcomes | Adverse events and quality of life |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and acute upper gastrointestinal bleeding |
| Interventions | Ferric carboxymaltose (intravenous) vs ferrous sulphate (oral) vs placebo (after discharge) |
| Outcomes | Haemoglobin and quality of life |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia |
| Interventions | Ferric carboxymaltose (intravenous) vs other intravenous iron (standard of care) vs ferrous sulphate (oral) |
| Outcomes | Change in haemoglobin |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and inflammatory bowel disease |
| Interventions | Iron oligosaccharide (intravenous) vs iron sulphate |
| Outcomes | Change in haemoglobin |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and multiple myeloma |
| Interventions | Ferric carboxymaltose (intravenous) vs standard of care |
| Outcomes | Change in haemoglobin |
| Notes | Study completed |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and non‐Hodgkin's lymphoma, multiple myeloma, leukaemia or any chemotherapy excluding anthracycline‐containing chemotherapy |
| Interventions | Ferric carboxymaltose (intravenous) vs standard of care |
| Outcomes | Change in haemoglobin |
| Notes | Study ongoing but not recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia |
| Interventions | Ferumoxytol (intravenous) vs placebo |
| Outcomes | Change in haemoglobin |
| Notes | Study has been completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia |
| Interventions | Ferumoxytol (intravenous) vs iron sucrose (intravenous) |
| Outcomes | Change in haemoglobin |
| Notes | Study has been completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and non‐myeloid malignancies who are going to receive at least 2 more chemotherapy cycles |
| Interventions | Iron isomaltoside (intravenous) vs iron sulphate |
| Outcomes | Drug‐related serious adverse events and change in haemoglobin |
| Notes | Study currently ongoing but not recruiting |
| Methods | Randomised controlled trial |
| Participants | Adult Indian participants with iron deficiency anaemia |
| Interventions | Ferrous bisglycinate chelate 120 mg (oral) vs ferrous bisglycinate chelate 60 mg (oral) vs ferrous ascorbate (oral) |
| Outcomes | Change in haemoglobin |
| Notes | Study has been completed |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and requiring intensive care for trauma |
| Interventions | Iron sucrose (intravenous) vs placebo |
| Outcomes | Mortality and blood transfusion |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and heavy menstrual bleeding |
| Interventions | Ferric carboxymaltose (intravenous) vs iron dextran injection (intravenous) |
| Outcomes | None of the outcomes of interest for this review were included in this trial |
| Notes | Study ongoing but not recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with unexplained anaemia |
| Interventions | Iron sucrose (intravenous) started immediately vs iron sucrose (intravenous) started after 12 weeks |
| Outcomes | Change in haemoglobin, clinical events, adverse events, quality of life |
| Notes | Study terminated because of lack of recruitment |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and inflammatory bowel disease |
| Interventions | Ferric trimaltol (oral) vs placebo |
| Outcomes | Adverse events, quality of life, change in haemoglobin |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and inflammatory bowel disease |
| Interventions | Ferric trimaltol (oral) vs placebo |
| Outcomes | Adverse events, change in haemoglobin, quality of life |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adult Chinese participants with iron deficiency anaemia |
| Interventions | Ferrous (II) glycine sulphate complex (oral) vs polyferose (oral) |
| Outcomes | Change in haemoglobin from baseline |
| Notes | Study active, not recruiting; awaiting follow‐up of participants |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and inflammatory bowel disease |
| Interventions | Ferric oxide, saccharated (intravenous) vs placebo |
| Outcomes | Haemoglobin and quality of life |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and lower gastrointestinal bleeding not requiring surgical treatment |
| Interventions | Ferric carboxymaltose (intravenous) vs placebo |
| Outcomes | Adverse events and haemoglobin |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and colorectal adenocarcinoma |
| Interventions | Ferric carboxymaltose (intravenous) vs ferrous sulphate |
| Outcomes | Mortality, morbidity, haemoglobin, quality of life, length of hospital stay |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia after gastrectomy for cancer |
| Interventions | Ferric carboxymaltose (intravenous) vs placebo |
| Outcomes | Adverse events and haemoglobin |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia |
| Interventions | Heme‐iron polypeptide (oral) vs heme‐iron (oral) vs organic iron (oral) vs placebo |
| Outcomes | Change in haemoglobin |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia and congestive cardiac failure |
| Interventions | Ferric carboxymaltose (intravenous) vs placebo |
| Outcomes | None of the outcomes of interest for this review were included as outcomes in this trial |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia after bariatric abdominoplasty |
| Interventions | Iron sucrose (intravenous) vs iron(III)‐hydroxide polymaltose complex (oral) |
| Outcomes | Haemoglobin levels |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with anaemia and suitable for palliative chemotherapy for oesophageal cancer |
| Interventions | Iron isomaltoside (intravenous) vs standard of care |
| Outcomes | Blood transfusion requirements, haemoglobin, quality of life |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Adults with iron deficiency anaemia |
| Interventions | Ferric carboxymaltose (intravenous) vs intravenous iron (standard of care) |
| Outcomes | Quality of life |
| Notes | Study currently recruiting |
| Methods | Randomised controlled trial |
| Participants | Country: international multi‐centric trial in Europe Sample size: 18 Postrandomisation dropout(s): 0 (0%) Revised sample size: 18 Females: 7 (38.9%) Mean age: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were mortality and haemoglobin levels Time of measurements: 2 weeks after end of treatment |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised clinical trial |
| Participants | Country: Europe (multi‐centric trial) Number randomly assigned: 110 Postrandomisation dropouts: 3 (2.7%) Revised sample size: 107 Average age: 66 years Females: 99 (92.5%) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were mortality, serious adverse events and haemoglobin levels |
| Notes | Time of measurement: 14 days after start of treatment Attempts were made to contact study authors in September 2013. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Spain Sample size: 165 Postrandomisation dropout(s): 102 (61.8%) Revised sample size: 63 Females: 9 (14.3%) Mean age: 63 years Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were blood transfusion requirements and haemoglobin levels Time of measurements: 30 days from start of treatment |
| Notes | Reason for postrandomisation dropout(s): not reported Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Italy Sample size: 40 Postrandomisation dropout(s): 2 (5%) Revised sample size: 38 Females: 33 (86.8%) Mean age: 41 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): severe side effects Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Italy Sample size: 149 Postrandomisation dropout(s): 0 (0%) Revised sample size: 149 Females: 104 (69.8%) Mean age: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were mortality and serious adverse events Time of measurements: 12 weeks after end of treatment |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: UK Sample size: 68 Postrandomisation dropout(s): 2 (2.9%) Revised sample size: 66 Females: 55 (83.3%) Mean age: 82 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels |
| Notes | Reason for postrandomisation dropout(s): lost to follow‐up Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Germany Sample size: 36 Postrandomisation dropout(s): not stated Revised sample size: 36 Females: 15 (41.7%) Mean age: 66 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: Further details: ferric gluconate 125 mg over 30 minutes from day 3 to day 8 Further details: ferrous glycine sulphate 200 mg/d starting from day 3 to day 8 Further details: no intervention |
| Outcomes | Outcomes reported were haemoglobin levels Time of measurements: end of treatment |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Uganda Sample size: 120 Postrandomisation dropout(s): not stated Revised sample size: 120 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to 6 groups of 20 participants each with total dose infusion at 5%, 7.5%, 10%, 15%, 20% and 25% |
| Outcomes | The only outcome reported was death in 1 participant in the 20% total dose infusion group Time of measurements: not stated |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: Germany Sample size: 46 Postrandomisation dropout(s): 11 (23.9%) Revised sample size: 35 Females: not stated Mean age: not stated Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels Time of measurements: 6 weeks after start of treatment |
| Notes | Reason for postrandomisation dropout(s): did not complete the study Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: USA Sample size: 584 Postrandomisation dropout(s): not stated Revised sample size: 584 Females: not stated Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were serious adverse events Time of measurements: not reported |
| Notes | Number of participants belonging to each group was not reported. No serious adverse events were reported in either group Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
| Methods | Randomised controlled trial |
| Participants | Country: India Sample size: 254 Postrandomisation dropout(s): not stated Revised sample size: 254 Females: not stated Mean age: not stated Inclusion criteria:
|
| Interventions | Participants were randomly assigned to the following groups: |
| Outcomes | Outcomes reported were haemoglobin levels and severe adverse events Time of measurements: 45 days from start of treatment |
| Notes | Attempts were made to contact study authors in August 2012. No replies were received. So, we were unable to determine whether allocation concealment was adequate |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Preoperative intravenous iron to treat anaemia in major surgery |
| Methods | Randomised controlled trial |
| Participants | Adults undergoing major abdominal surgery and anaemia |
| Interventions | Ferric carboxymaltose vs placebo |
| Outcomes | Mortality, blood transfusion, haemoglobin levels, quality of life, adverse events, length of hospital stay |
| Starting date | 2013 |
| Contact information | Mr Toby Richards (University College London) |
| Notes | The study has not started recruiting |
Accessed as up‐to‐date November 2014.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.68, 1.61] |
| Analysis 1.1  Comparison 1 Oral iron vs inactive control, Outcome 1 Mortality. | ||||
| 2 Proportion requiring blood transfusion Show forest plot | 3 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| Analysis 1.2  Comparison 1 Oral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion. | ||||
| 3 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3 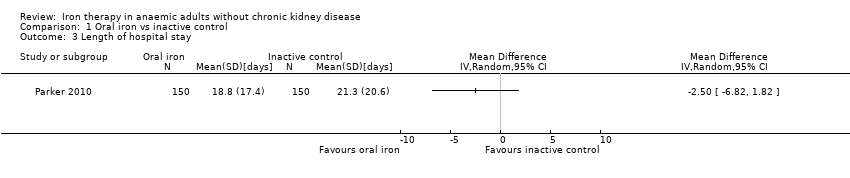 Comparison 1 Oral iron vs inactive control, Outcome 3 Length of hospital stay. | ||||
| 4 Haemoglobin Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 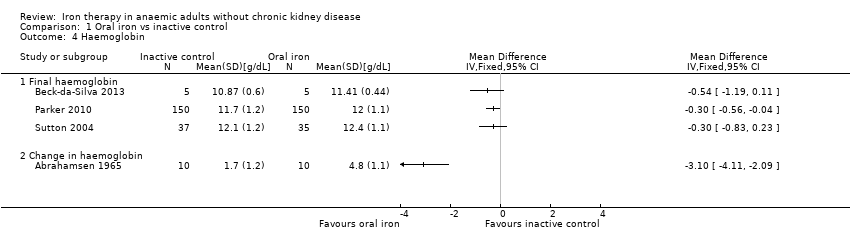 Comparison 1 Oral iron vs inactive control, Outcome 4 Haemoglobin. | ||||
| 4.1 Final haemoglobin | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Change in haemoglobin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.5 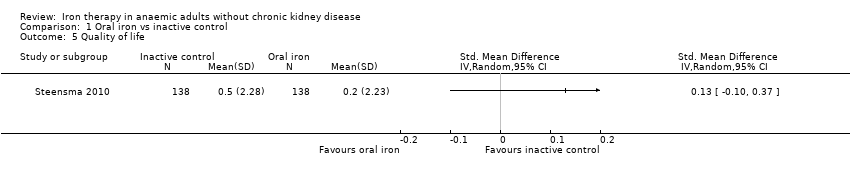 Comparison 1 Oral iron vs inactive control, Outcome 5 Quality of life. | ||||
| 6 Serious adverse events Show forest plot | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| Analysis 1.6  Comparison 1 Oral iron vs inactive control, Outcome 6 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| Analysis 2.1  Comparison 2 Parenteral iron vs inactive control, Outcome 1 Mortality. | ||||
| 2 Proportion requiring blood transfusion Show forest plot | 8 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.06] |
| Analysis 2.2  Comparison 2 Parenteral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion. | ||||
| 3 Haemoglobin Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Parenteral iron vs inactive control, Outcome 3 Haemoglobin. | ||||
| 3.1 Final haemoglobin | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change in haemoglobin | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4 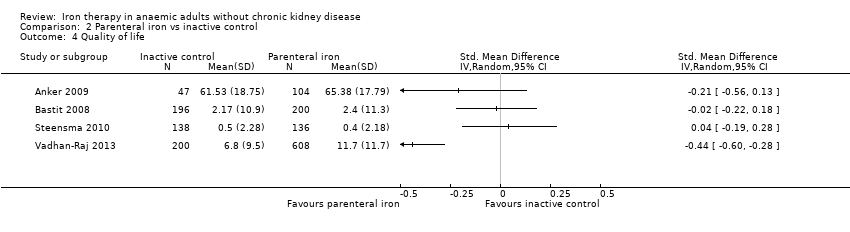 Comparison 2 Parenteral iron vs inactive control, Outcome 4 Quality of life. | ||||
| 5 Serious adverse events Show forest plot | 7 | 1802 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| Analysis 2.5  Comparison 2 Parenteral iron vs inactive control, Outcome 5 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| Analysis 3.1 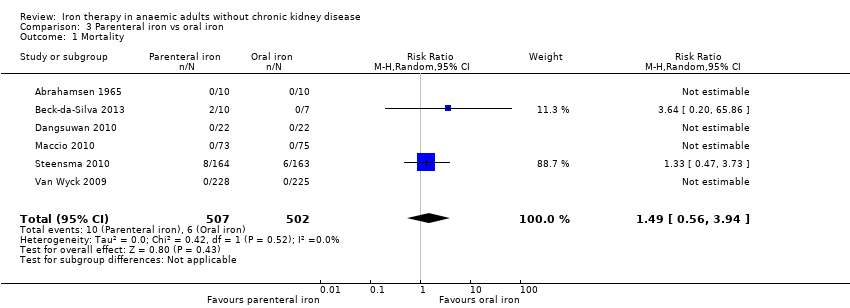 Comparison 3 Parenteral iron vs oral iron, Outcome 1 Mortality. | ||||
| 2 Proportion requiring blood transfusion Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| Analysis 3.2 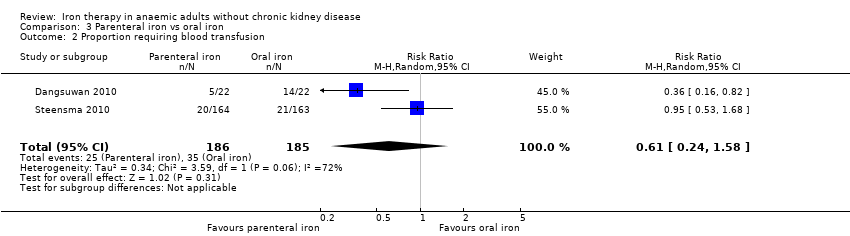 Comparison 3 Parenteral iron vs oral iron, Outcome 2 Proportion requiring blood transfusion. | ||||
| 3 Mean blood transfused Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Parenteral iron vs oral iron, Outcome 3 Mean blood transfused. | ||||
| 4 Haemoglobin Show forest plot | 6 | 769 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.73, ‐0.27] |
| Analysis 3.4  Comparison 3 Parenteral iron vs oral iron, Outcome 4 Haemoglobin. | ||||
| 4.1 Final haemoglobin | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.74, ‐0.21] |
| 4.2 Change in haemoglobin | 3 | 621 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.29, 0.46] |
| 5 Quality of life Show forest plot | 3 | 771 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.13] |
| Analysis 3.5  Comparison 3 Parenteral iron vs oral iron, Outcome 5 Quality of life. | ||||
| 6 Serious adverse events Show forest plot | 7 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.54] |
| Analysis 3.6  Comparison 3 Parenteral iron vs oral iron, Outcome 6 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1 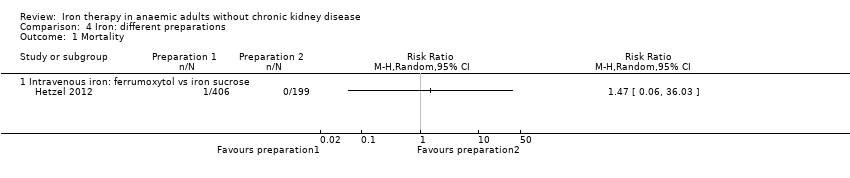 Comparison 4 Iron: different preparations, Outcome 1 Mortality. | ||||
| 1.1 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haemoglobin Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2 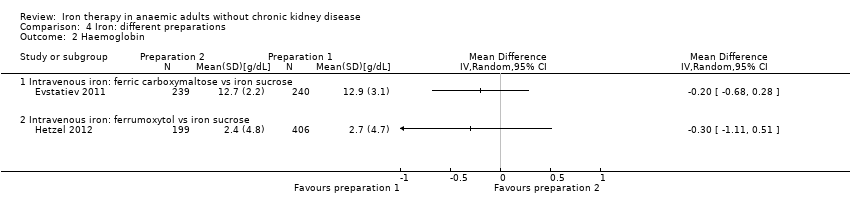 Comparison 4 Iron: different preparations, Outcome 2 Haemoglobin. | ||||
| 2.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Iron: different preparations, Outcome 3 Serious adverse events. | ||||
| 3.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (parenteral iron vs inactive control stratified by clinical setting) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| Analysis 5.1 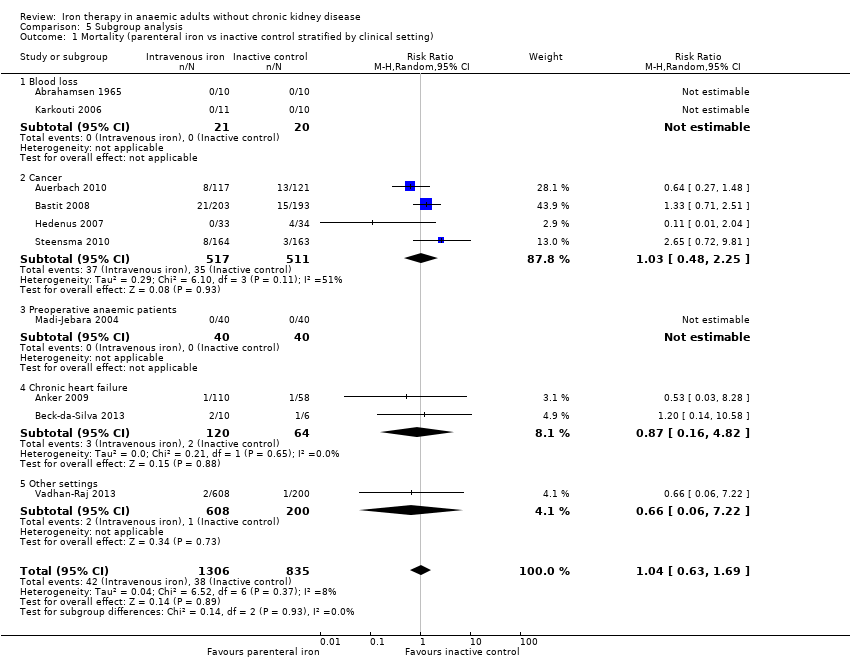 Comparison 5 Subgroup analysis, Outcome 1 Mortality (parenteral iron vs inactive control stratified by clinical setting). | ||||
| 1.1 Blood loss | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Cancer | 4 | 1028 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.25] |
| 1.3 Preoperative anaemic patients | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Chronic heart failure | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.16, 4.82] |
| 1.5 Other settings | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.06, 7.22] |
| 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| Analysis 5.2  Comparison 5 Subgroup analysis, Outcome 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use). | ||||
| 2.1 Supplementary erythropoietin | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.91] |
| 2.2 No supplementary erythropoietin | 7 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.58, 3.90] |
| 3 Mortality (parenteral iron vs oral iron stratified by clinical setting) Show forest plot | 5 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| Analysis 5.3 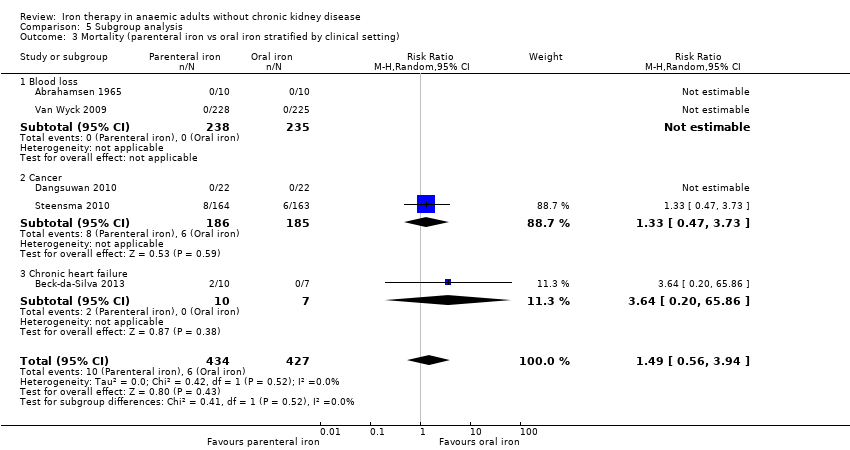 Comparison 5 Subgroup analysis, Outcome 3 Mortality (parenteral iron vs oral iron stratified by clinical setting). | ||||
| 3.1 Blood loss | 2 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cancer | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.73] |
| 3.3 Chronic heart failure | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 3.64 [0.20, 65.86] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Twenty‐one studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
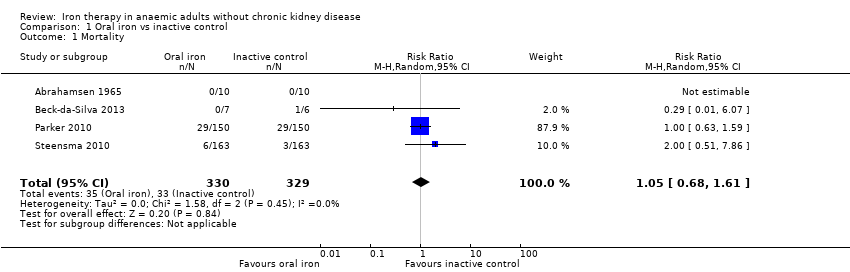
Comparison 1 Oral iron vs inactive control, Outcome 1 Mortality.

Comparison 1 Oral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion.
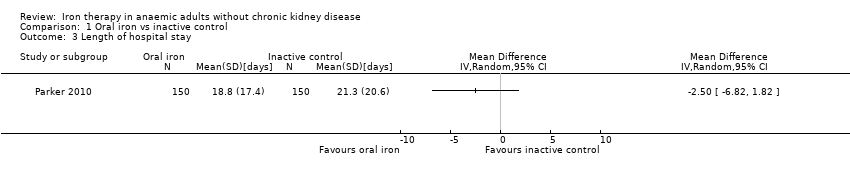
Comparison 1 Oral iron vs inactive control, Outcome 3 Length of hospital stay.

Comparison 1 Oral iron vs inactive control, Outcome 4 Haemoglobin.
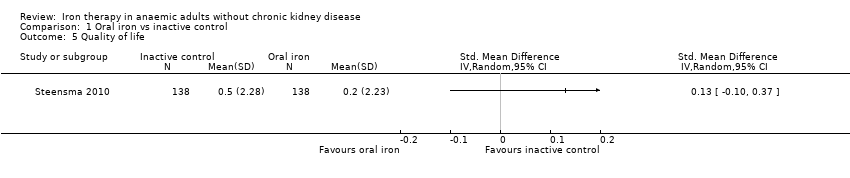
Comparison 1 Oral iron vs inactive control, Outcome 5 Quality of life.

Comparison 1 Oral iron vs inactive control, Outcome 6 Serious adverse events.

Comparison 2 Parenteral iron vs inactive control, Outcome 1 Mortality.
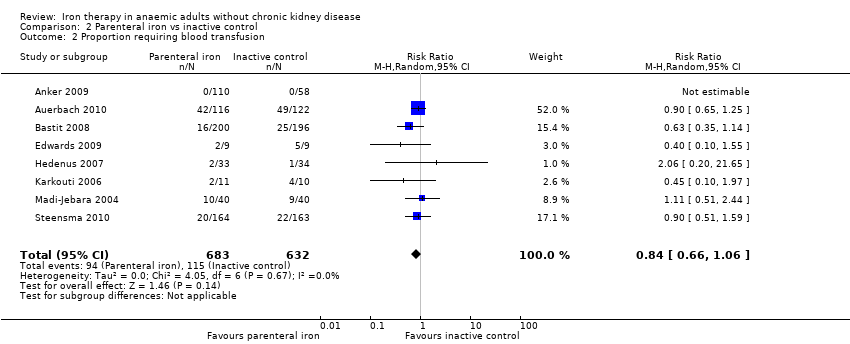
Comparison 2 Parenteral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion.
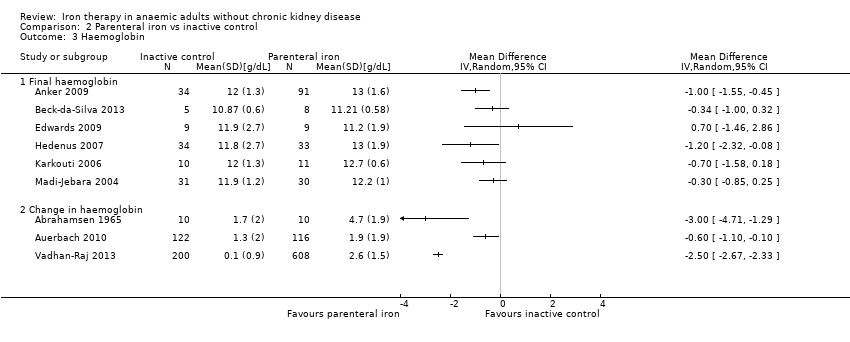
Comparison 2 Parenteral iron vs inactive control, Outcome 3 Haemoglobin.

Comparison 2 Parenteral iron vs inactive control, Outcome 4 Quality of life.

Comparison 2 Parenteral iron vs inactive control, Outcome 5 Serious adverse events.

Comparison 3 Parenteral iron vs oral iron, Outcome 1 Mortality.

Comparison 3 Parenteral iron vs oral iron, Outcome 2 Proportion requiring blood transfusion.
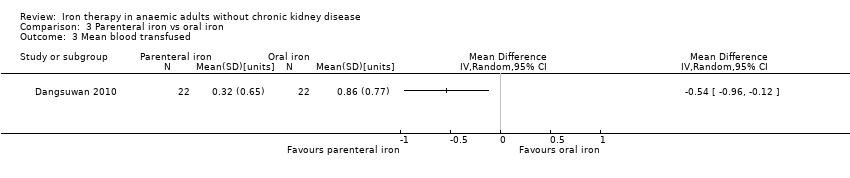
Comparison 3 Parenteral iron vs oral iron, Outcome 3 Mean blood transfused.
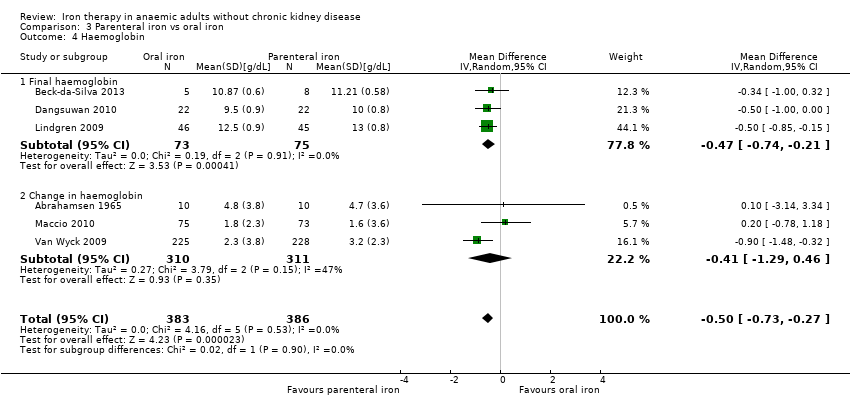
Comparison 3 Parenteral iron vs oral iron, Outcome 4 Haemoglobin.
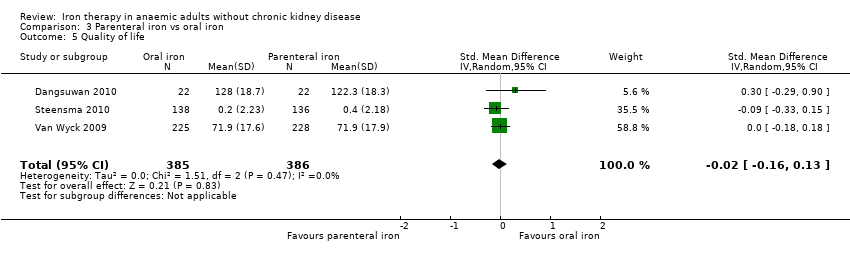
Comparison 3 Parenteral iron vs oral iron, Outcome 5 Quality of life.
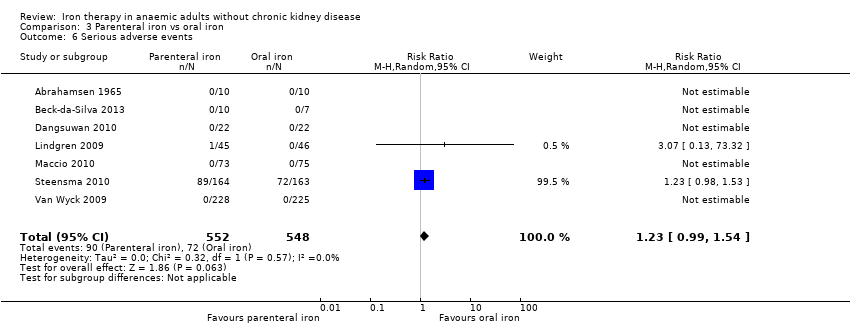
Comparison 3 Parenteral iron vs oral iron, Outcome 6 Serious adverse events.
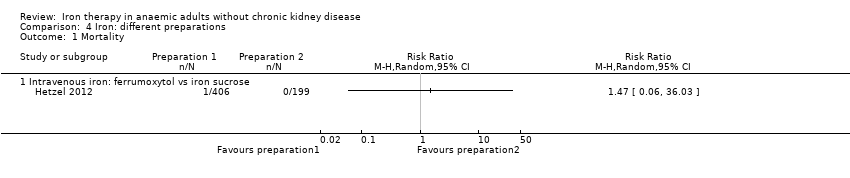
Comparison 4 Iron: different preparations, Outcome 1 Mortality.
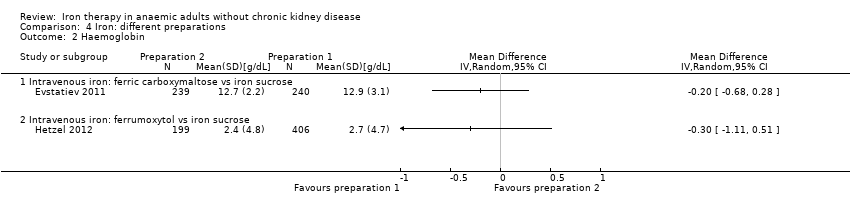
Comparison 4 Iron: different preparations, Outcome 2 Haemoglobin.
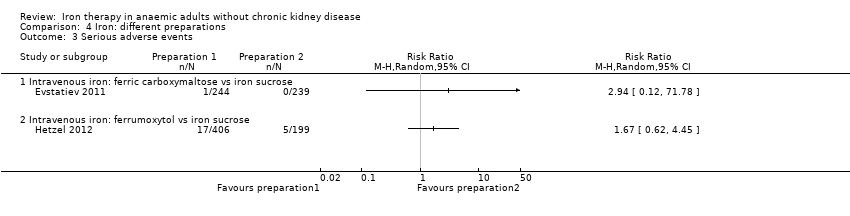
Comparison 4 Iron: different preparations, Outcome 3 Serious adverse events.
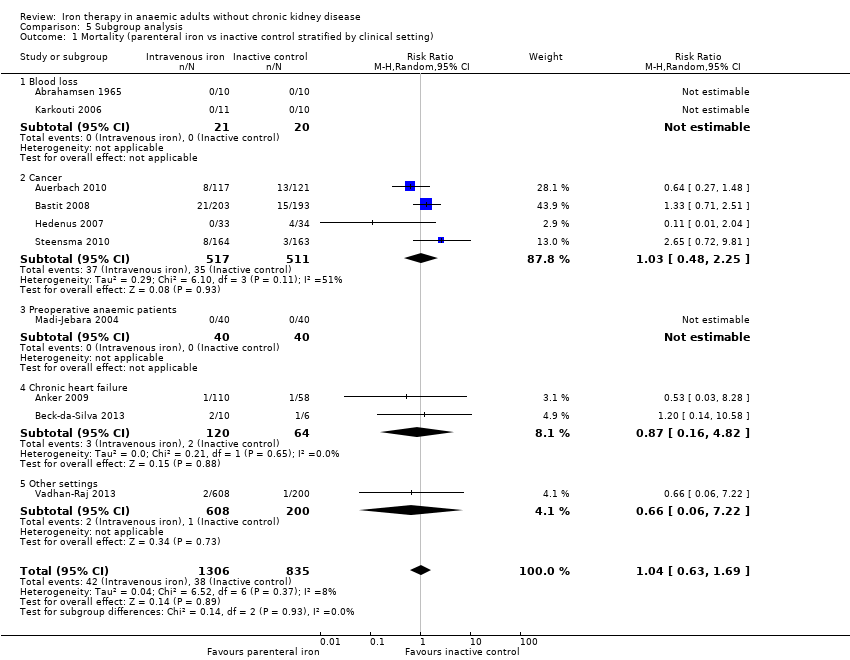
Comparison 5 Subgroup analysis, Outcome 1 Mortality (parenteral iron vs inactive control stratified by clinical setting).
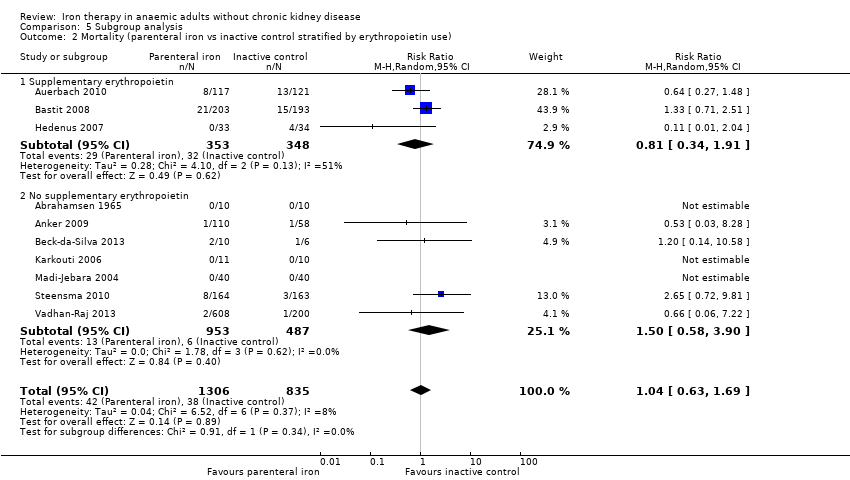
Comparison 5 Subgroup analysis, Outcome 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use).
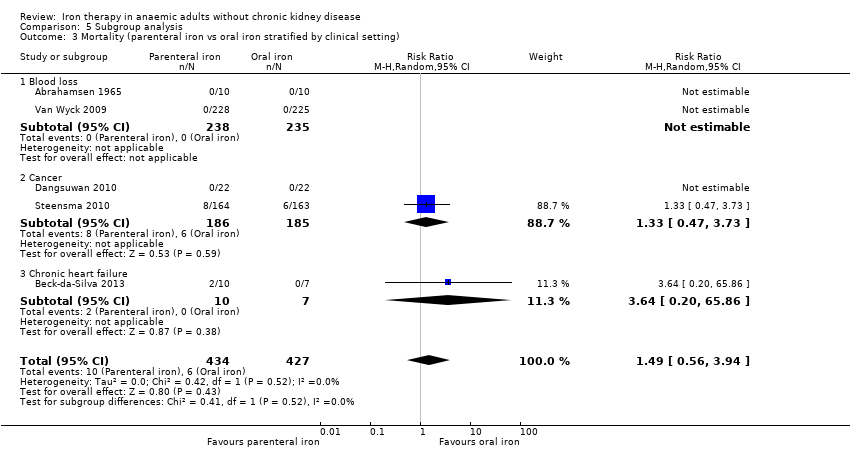
Comparison 5 Subgroup analysis, Outcome 3 Mortality (parenteral iron vs oral iron stratified by clinical setting).
| Oral iron vs inactive control for anaemic patients | |||||
| Patient or population: patients with anaemia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Inactive control | Oral iron | ||||
| Mortality | 100 per 1000 | 105 per 1000 (68 to 161) | RR 1.05 (0.68 to 1.61) | 659 | ⊕⊝⊝⊝ |
| Proportion requiring blood transfusion | 279 per 1000 | 206 per 1000 | RR 0.74 | 546 | ⊕⊝⊝⊝ |
| Length of hospital stay | Mean hospital stay in control groups was | Mean hospital stay in intervention groups was | 300 | ⊕⊝⊝⊝ | |
| Haemoglobin | Mean haemoglobin in control groups ranged between 11.4 g/dL and 12.4 g/dL | Point estimate of haemoglobin in intervention groups in the individual studies was 0.30 to 3.10 higher | 402 | ⊕⊝⊝⊝ | |
| Quality of life | ‐ | Mean quality of life in intervention groups was | 276 | ⊕⊝⊝⊝ | |
| Serious adverse events | 205 per 1000 | 197 per 1000 | RR 0.96 (0.76 to 1.22) | 731 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is the average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | |||||
| Parenteral iron vs inactive control for anaemic patients | |||||
| Patient or population: patients with anaemia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Inactive control | Parenteral iron | ||||
| Mortality | 46 per 1000 | 47 per 1000 | RR 1.04 (0.63 to 1.69) | 2141 | ⊕⊝⊝⊝ |
| Proportion requiring blood transfusion | 182 per 1000 | 153 per 1000 | RR 0.84 (0.66 to 1.06) | 1315 | ⊕⊝⊝⊝ |
| Haemoglobin | Mean haemoglobin in control groups ranged between 11.2 g/dL and 13.0 g/dL | The point estimate of haemoglobin in intervention groups in the individual studies was from 0.30 to 3.00 higher | 1371 | ⊕⊝⊝⊝ | |
| Quality of life | ‐ | The point estimate of haemoglobin in intervention groups in the individual studies was between 0.04 standard deviations lower and 0.44 standard deviations higher | 1629 | ⊕⊝⊝⊝ | |
| Serious adverse events | 184 per 1000 | 184 per 1000 | RR 1 (0.74 to 1.34) | 1802 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is the average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | |||||
| Parenteral iron vs oral iron for anaemic patients | ||||||
| Patient or population: patients with anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Parenteral iron | |||||
| Mortality | 12 per 1000 | 18 per 1000 | RR 1.49 (0.56 to 3.94) | 1009 | ⊕⊝⊝⊝ | |
| Proportion requiring blood transfusion | 189 per 1000 | 115 per 1000 | RR 0.61 (0.24 to 1.58) | 371 | ⊕⊝⊝⊝ | |
| Mean blood transfused | Mean mean blood transfused in control groups was | Mean blood transfused in intervention groups was | 44 | ⊕⊝⊝⊝ | ||
| Haemoglobin | Mean haemoglobin in control groups was between | Mean haemoglobin in intervention groups was | 769 | ⊕⊕⊝⊝ | ||
| Quality of life | ‐ | Mean quality of life in intervention groups was | 771 | ⊕⊝⊝⊝ | SMD 0.09 (‐0.04 to 0.22) | |
| Serious adverse events | 131 per 1000 | 162 per 1000 | RR 1.23 (0.99 to 1.54) | 1100 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk is average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | ||||||
| Group characteristics | Haemoglobin | Haematocrit | Haematocrit |
| Children (6 months to 59 months) | 110 | 6.83 | 0.33 |
| Children (5 to 11 years) | 115 | 7.13 | 0.34 |
| Children (12 to 14 years) | 120 | 7.45 | 0.36 |
| Non‐pregnant women (over 15 years of age) | 120 | 7.45 | 0.36 |
| Pregnant women | 110 | 6.83 | 0.33 |
| Men (over 15 years of age) | 130 | 8.07 | 0.39 |
| aHaematocrit is the volume of packed red blood cells expressed in terms of fraction or percentages in a whole blood specimen (NCBI‐Hematocrit). | |||
|
Study name | Participant characteristics | Clinical setting | Co‐interventions | Routes and methods of administration of parenteral iron |
Comparison | |||||||||||
| Sample size (after postrandomisation dropouts) | Mean age (years) | Females | Postrandomisation dropouts | Blood loss conditions | Cancer | Preoperative | Chronic heart failure | Autoimmune | Miscellaneous | Added erythropoietin | Added oral iron | Parenteral iron—intravenous | Parenteral iron—intramuscular | Total dose infusion | ||
| 30 | Not stated | Not stated | 0 (0%) | Yes | No | No | No | No | No | No | Not applicable | No | Yes | No | Parenteral iron vs oral iron vs inactive control | |
| 158 | Not stated | Not stated | 0 (0%) | No | No | No | Yes | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 238 | 63 | 158 (66.4%) | 5 (2.1%) | No | Yes | No | No | No | No | Yes | Oral iron was allowed as part of standard treatment | Yes | No | No | Parenteral iron vs inactive control | |
| 396 | 61 | 240 (60.6%) | 2 (0.5%) | No | Yes | No | No | No | No | Yes | Oral iron was allowed as part of standard treatment | Yes | No | No | Parenteral iron vs inactive control | |
| 23 | 66 | 7 | Not stated | No | No | No | Yes | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron vs inactive control | |
| 44 | 51 | 44 (100%) | 0 (0%) | No | Yes | No | No | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 18 | Not stated | Not stated | Not stated | No | No | Yes | No | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 483 | 39 | 284 (58.8%) | 2 (0.4%) | No | No | No | No | Yes | No | No | No | Yes | No | Yes | Different preparations | |
| 67 | 76 | 42 (62.7%) | 0 (0%) | No | Yes | No | No | No | No | Yes | No | Yes | No | No | Parenteral iron vs inactive control | |
| 605 | Not stated | Not stated | Not stated | No | No | No | No | No | Yes | No | No | Yes | No | No | Different preparations | |
| 21 | 62 | 5 (23.8%) | 5 (19.2%) | Yes | No | No | No | No | No | No | Yes | Yes | No | No | Parenteral iron vs inactive control | |
| 20 | Not stated | 9 (45%) | Not stated | No | No | Yes | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 91 | 42 | 63 (69.2%) | Not stated | No | No | No | No | Yes | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 148 | 68 | 59 (39.9%) | 0 (0%) | No | Yes | No | No | No | No | Yes | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 80 | Not stated | Not stated | 0 (0%) | No | No | Yes | No | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 300 | 82 | 245 (81.7%) | 0 (0%) | Yes | No | No | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 200 | 57 | 103 (51.5%) | 0 (0%) | No | No | No | No | No | Yes | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 490 | 64 | 320 (65.3%) | 12 (2.4%) | No | Yes | No | No | No | No | Yes | Not applicable | Yes | No | No | Parenteral iron vs oral iron vs inactive control | |
| 72 | 70 | 30 (41.7%) | Not stated | Yes | No | No | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 808 | 45 | 720 | 4 (0.5%) | No | No | No | No | No | Yes | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 453 | 39 | 453 (100%) | 24 (5%) | Yes | No | No | No | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.68, 1.61] |
| 2 Proportion requiring blood transfusion Show forest plot | 3 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 3 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Haemoglobin Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Final haemoglobin | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Change in haemoglobin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Serious adverse events Show forest plot | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 2 Proportion requiring blood transfusion Show forest plot | 8 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.06] |
| 3 Haemoglobin Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Final haemoglobin | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change in haemoglobin | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Serious adverse events Show forest plot | 7 | 1802 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| 2 Proportion requiring blood transfusion Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 3 Mean blood transfused Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Haemoglobin Show forest plot | 6 | 769 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.73, ‐0.27] |
| 4.1 Final haemoglobin | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.74, ‐0.21] |
| 4.2 Change in haemoglobin | 3 | 621 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.29, 0.46] |
| 5 Quality of life Show forest plot | 3 | 771 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.13] |
| 6 Serious adverse events Show forest plot | 7 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haemoglobin Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (parenteral iron vs inactive control stratified by clinical setting) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 1.1 Blood loss | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Cancer | 4 | 1028 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.25] |
| 1.3 Preoperative anaemic patients | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Chronic heart failure | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.16, 4.82] |
| 1.5 Other settings | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.06, 7.22] |
| 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 2.1 Supplementary erythropoietin | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.91] |
| 2.2 No supplementary erythropoietin | 7 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.58, 3.90] |
| 3 Mortality (parenteral iron vs oral iron stratified by clinical setting) Show forest plot | 5 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| 3.1 Blood loss | 2 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cancer | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.73] |
| 3.3 Chronic heart failure | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 3.64 [0.20, 65.86] |

