Ejercicio físico para el tratamiento de la insuficiencia venosa crónica no ulcerada
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010637.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Diego Araujo (DA): Developed the first draft of the protocol and the review; approved the final version prior to submission and made an intellectual contribution to the review.
Cibele Ribeiro (CR): Conceived the review question; advised and developed the first draft of the protocol and the full review; approved the final version prior to submission and made an intellectual contribution to the review.
Álvaro Maciel (AM): Made an intellectual contribution to the protocol and approved the final version of the review prior to submission.
Selma Bruno (SB): Made an intellectual contribution to the protocol and approved the final version of the review prior to submission.
Guilherme Fregonezi (GF): Made an intellectual contribution to the protocol and approved the final version of the review prior to submission.
Fernando Dias (FD): Conceived the review question; advised, developed and coordinated the first draft of the protocol and the review; approved the final version prior to submission and made an intellectual contribution to the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
-
FAPERN and CT‐INFRA intermediated by MCT/CNPq ‐ Grant PPP 2009, Brazil.
Financial support
-
Ministry of Science and Technology and National Council for Scientific and Techological Development (CNPq)‐ UNIVERSAL 2010, Brazil.
Financial support
Declarations of interest
The review authors report that this work was partially supported by a grant from the Ministry of Science and Technology and National Council for Scientific and Techological Development (CNPq)‐ UNIVERSAL 2010, Brazil, and by a grant from FAPERN and CT‐INFRA intermediated by MCT/CNPq ‐ Grant PPP 2009, Brazil.
DA: none known.
CR: none known.
AM: none known.
SB: none known.
GF: none known.
FD: none known.
Acknowledgements
The review authors would like to thank Dr Marlene Stewart (Managing Editor) and Dr Cathryn Broderick (Assistant Managing Editor) for their assistance in the development of the protocol for this review and the full review. We thank Thiago Nunes for assistance in the initial draft of the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Jun 14 | Physical exercise for the treatment of non‐ulcerated chronic venous insufficiency | Review | Diego N Araujo, Cibele TD Ribeiro, Alvaro CC Maciel, Selma S Bruno, Guilherme AF Fregonezi, Fernando AL Dias | |
| 2016 Dec 03 | Physical exercise for the treatment of non‐ulcerated chronic venous insufficiency | Review | Diego N Araujo, Cibele TD Ribeiro, Alvaro CC Maciel, Selma S Bruno, Guilherme AF Fregonezi, Fernando AL Dias | |
| 2013 Jul 13 | Physical exercise for the treatment of non‐ulcerated chronic venous insufficiency | Protocol | Diego N Araujo, Cibele TD Ribeiro, Alvaro CC Maciel, Selma S Bruno, Guilherme AF Fregonezi, Fernando AL Dias | |
Differences between protocol and review
We considered venous refilling time to be a relevant variable and have added this as a primary outcome. We amended the review to allow for both ejection fraction and venous refilling time to be obtained using the same evaluation procedure (plethysmography).
We considered including studies investigating individuals with CVI and venous ulcers if data for the two ulcerated and non‐ulcerated groups were not analysed and presented separately provided participants with leg ulcers formed less than 25% of the total study population.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
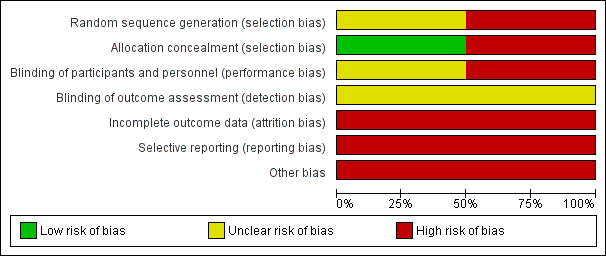
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Ejection fraction, outcome: 1.1 Ejection fraction [%].](/es/cdsr/doi/10.1002/14651858.CD010637.pub2/media/CDSR/CD010637/rel0002/CD010637/image_n/nCD010637-AFig-FIG04.png)
Forest plot of comparison: 1 Ejection fraction, outcome: 1.1 Ejection fraction [%].
![Forest plot of comparison: 2 Venous refilling time, outcome: 2.1 Half refilling time [seconds].](/es/cdsr/doi/10.1002/14651858.CD010637.pub2/media/CDSR/CD010637/rel0002/CD010637/image_n/nCD010637-AFig-FIG05.png)
Forest plot of comparison: 2 Venous refilling time, outcome: 2.1 Half refilling time [seconds].
![Forest plot of comparison: 2 Venous refilling time, outcome: 2.2 Total refilling time [seconds].](/es/cdsr/doi/10.1002/14651858.CD010637.pub2/media/CDSR/CD010637/rel0002/CD010637/image_n/nCD010637-AFig-FIG06.png)
Forest plot of comparison: 2 Venous refilling time, outcome: 2.2 Total refilling time [seconds].
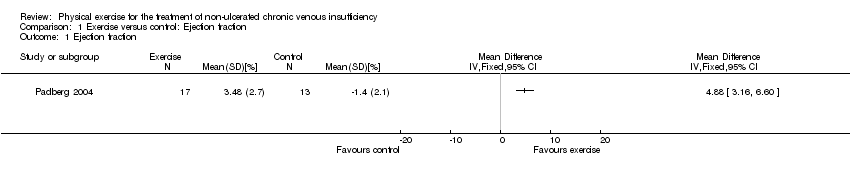
Comparison 1 Exercise versus control: Ejection fraction, Outcome 1 Ejection fraction.
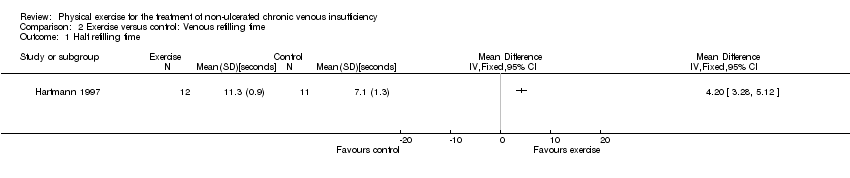
Comparison 2 Exercise versus control: Venous refilling time, Outcome 1 Half refilling time.

Comparison 2 Exercise versus control: Venous refilling time, Outcome 2 Total refilling time.
| Physical exercise compared with no treatment for non‐ulcerated chronic venous insufficiency | |||||
| Population: People with non‐ulcerated chronic venous insufficiency | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no exercise | Risk with physical exercise | ||||
| Intensity of disease signs and symptoms1 | see footnotes1 | ||||
| Ejection fraction Follow up: 24 weeks | The mean change in ejection fraction from baseline was ‐1.4% | The mean change in ejection fraction from baseline in the intervention group was 4.88% more (3.16 more to 6.6 more) | 30 | ⊕⊖⊖⊖ | |
| Half refilling time Follow up: 24 weeks | The mean half refilling time was 7.1 seconds | The mean half refilling time in the intervention group was 4.20 seconds more (3.28 more to 5.12 more) | 23 | ⊕⊖⊖⊖ | |
| Total refilling time Follow up: 24 weeks | The mean total refilling time was 16.3 seconds | The mean total refilling time in the intervention group was 9.40 seconds more (7.77 more to 11.03 more) | 23 | ⊕⊖⊖⊖ | |
| Incidence of venous leg ulcer3 | ‐ | ‐ | ‐ | see footnote3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| 1 unable to pool data because data were collected using different tools | |||||
| Classification | Description/Definition |
| Clinical | |
| 0 | no visible or palpable signs of venous disease |
| 1 | telangiectases or reticular veins |
| 2 | varicose veins |
| 3 | oedema |
| 4a | pigmentation or eczema |
| 4b | lipodermatosclerosis or atrophie blanchie |
| 5 | healed venous ulcer |
| 6 | active venous ulcer |
| S | symptomatic, including ache, pain, tightness, skin irritation, heaviness, muscle cramp and other complaints attributable to venous dysfunction |
| A | asymptomatic |
| Etiologyl | |
| Ec | congenital (present since birth) |
| Ep | primary |
| Es | secondary (post‐thrombotic, traumatic) |
| En | no venous cause identified |
| Anatomy distribution | |
| As | superficial (great and short saphenous veins) |
| Ap | perforator (thigh and leg perforating veins) |
| Ad | deep (cava, iliac, gonadal, femoral, profunda, popliteal, tibial, and muscular veins) |
| An | no venous location identified |
| Pathophysiology | |
| Pr | reflux (axial and perforating veins) |
| Po | obstruction (acute and chronic) |
| Pr,o | combination of both reflux and obstruction (valvular dysfunction and thrombus) |
| Pn | no venous pathophysiology identified |
| CEAP classification: classification of chronic venous disease according to clinical manifestation, etiologic factors, anatomic distribution of disease, and underlying pathophysiologic findings See Eklof 2004 for further details about CEAP | |
| Clinical descriptor | Absent (0) | Mild (1) | Moderate (2) | Severe (3) |
| Pain | None | Occasional | Daily not limiting | Daily limiting |
| Varicose veins | None | Few | Calf or thigh | Calf and thigh |
| Venous oedema | None | Foot and ankle | Below knee | Knee and above |
| Skin pigmentation | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Inflammation | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Induration | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Number of active ulcers | None | 1 | 2 | 3 or more |
| Ulcer duration | None | < 3 month | 3 ‐ 12 month | > 1 year |
| Active ulcer size | None | < 2 cm | 2 ‐ 6 cm | > 6 cm |
| Compression therapy | None | Intermittent | Most days | Fully comply |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ejection fraction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Half refilling time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Total refilling time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

