Intervenciones farmacológicas para los trastornos somatoformes en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010628.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 07 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos mentales comunes
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Preparation of the protocol:
Maria Kleinstäuber: developed and drafted the protocol.
Michael Witthöft, Andrés Steffanowski, Michael Lambert, Günter Meinhardt, Klaus Lieb, Wolfgang Hiller: supervised preparation of the protocol and gave feedback on the draft version of the protocol.
Preparation of the review:
Maria Kleinstäuber and Michael Witthöft independently reviewed titles and abstracts in the first step and screened full‐texts regarding eligibility of studies for the review in the second step.
Maria Kleinstäuber and Michael Witthöft independently appraised the quality of the included studies.
Maria Kleinstäuber and a research assistant trained in the data extraction procedure independently extracted data of the included studies.
Andrés Steffanowski, Wolfgang Hiller, Harm van Marwijk, and Michael Lambert supervised the preparation of the review process and arbitrated in the case of disagreements.
Sources of support
Internal sources
-
Johannes Gutenberg‐University of Mainz, Germany.
External sources
-
National Institute for Health Research, UK.
Funding for this review was provided by the NIHR Cochrane Incentive Award Scheme 2014
Declarations of interest
Maria Kleinstäuber: none known.
Michael Witthöft: none known.
Andrés Steffanowski: none known.
Harm van Marwijk: none known.
Wolfgang Hiller: none known.
Michael Lambert: none known.
Acknowledgements
Special thanks to CCDAN's Trials Search Coordinator for assistance in developing search strategies for our review; to Kim Jones for proofreading; to Yoko Tsui, Li Ping Claire Su, Sean Woodland, and Norio Watanabe for translations; to Changlin Ai and Zheng Gang Bai for providing us with important information about included Chinese articles and Chinese classifications of mental disorders; to Maruschka Heil for double coding the studies; and to Harm van Marwijk for supporting us with managing the antidepressant classes. Furthermore, we thank all experts in somatoform disorders who provided us with information about ongoing trials, who supported our literature search, or who provided us with missing data (Carlo Altamura, Massimiliano Aragona, Brian Fallon, Harald Freyberger, Fumiyuki Goto, Peter Henningsen, Jeffrey L. Jackson, Thomas Müller, Russell Noyes, Patrick Onghena, Judith Rosmalen, Andreas Schröder, and Amarendra N. Singh).
Funding for this review was provided by the National Institute for Health Research (NIHR) Cochrane Incentive Award Scheme 2014.
CRG Funding Acknowledgement:
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Review Group.
Disclaimer:
the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 07 | Pharmacological interventions for somatoform disorders in adults | Review | Maria Kleinstäuber, Michael Witthöft, Andrés Steffanowski, Harm van Marwijk, Wolfgang Hiller, Michael J Lambert | |
| 2013 Jul 04 | Pharmacological interventions for somatoform disorders in adults | Protocol | Maria Kleinstäuber, Michael Witthöft, Andrés Steffanowski, Michael Lambert, Günter Meinhardt, Klaus Lieb, Wolfgang Hiller | |
Differences between protocol and review
1) For literature search the following search terms were added: "somatoform disorder*", somatoform and "autonomic dysfunction", "somatic symptom disorder*","pain disorder".
Rationale: the former search strategy did not include these important terms although they are part of the title of the review and the inclusion criteria. Therefore, it was necessary to add them.
2) The syntax for the PsycINFO search was adapted.
Rationale: according to the changes in the search strategy (see the first difference between protocol and review), the syntax for the PsycINFO search had to be adapted.
3) We made changes to the searches of electronic databases: instead of the search in PubMed and EMBASE, we conducted an additional search in CENTRAL.
Rationale: we decided to run one search in CENTRAL because this database included all records from PubMed, indexed as publication type=RCT as well as records from EMBASE, obtained from a Cochrane RCT screening project.
4) In the section 'Types of interventions', we added the comparator intervention 'combination of medications'. Correspondingly, we added the comparison 'medication versus a combination of medications' to the section 'Data extraction and management'.
Rationale: the literature search obtained three studies where the efficacy of a combination of medications was compared with a single medication. In order to avoid excluding these studies and because they also fit the objectives of our review, we decided to add another comparison.
5) In the section 'Types of participants' under 'Participants characteristics', we modified the age criterion. We added the following sentence: "where a trial has defined adults to include those older than 65 years but most participants were under 65 years of age we included the trial; however, we excluded any trial that focused on older adults or where the mean age of participants was greater than 65 years'.
Rationale: we modified the inclusion criteria of people regarding age in order to prevent the exclusion of good‐quality studies only because they slightly exceed our originally defined age range.
6) In the section 'Types of participants' under 'Diagnosis', we deleted the originally required structured clinical interview for making the diagnoses.
Rationale: with screening the full texts of the potentially eligible studies for this review it became clear that requiring a structured clinical interview was too strict and inappropriate a criterion. There were several studies where a unstructured psychiatric interview was conducted in order to determine the diagnosis(es) of a participant.
7) In the section 'Types of participants' under 'Diagnosis', we added the DSM‐5 diagnosis 'somatic symptom disorder' and the Chinese Classification of Mental Disorders (CCMD‐III).
Rationale: the DSM‐5 was published in 2014 shortly after the protocol of this review had been published. In the protocol, we had already announced that the diagnostic criteria would be adapted as soon as the DSM‐5 was published. The criteria of somatoform disorders in the CCMD‐III correspond to the criteria of somatoform disorders in DSM‐IV or ‐III and in ICD‐10 or ‐9.
8) In the section 'Types of outcome measures', we made the following changes:
-
under 'Primary outcomes', we noted that the acceptability rate was also presented as risk ratio.
Rationale: the calculation of the risk ratio gives the opportunity to compare the treatment and comparator group for the number of drop‐outs.
-
under 'Secondary outcomes', we divided the originally combined outcome 'depression and anxiety' into two separate outcomes.
Rationale: during coding the characteristics and statistical values of the included studies we realised that in many studies qualitatively equivalent measures of depression and anxiety were available (e.g. the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS)). Therefore, it was impossible to decide which of these measures should be included. Furthermore, we decided against excluding one or both outcomes from the review because anxiety as well as depressive symptoms are import co‐morbid complaints in people with somatoform disorders.
-
under 'Secondary outcomes', we added that for the outcomes anxiety and depression the subscales of validated questionnaires assessing general psychopathology and VAS were acceptable.
Rationale: subscales of validated questionnaires assessing general psychopathology or VAS were accepted measures for the 'severity/intensity of MUPS'. We decided afterwards to accept them as eligible measures of depression and anxiety.
-
under 'Secondary outcomes', we noted that the drop‐out rate due to adverse effects was also presented as an RR.
Rationale: the calculation of the RR gives the opportunity to compare the treatment and comparator group for number of drop‐outs due to adverse effects.
11) In the section 'Types of outcome measures' under 'Secondary outcomes' we clarified that the treatment response referred to the primary outcome 'severity of MUPS'.
Rationale: during the coding of the statistical values of the included studies it became clear that the section about treatment response as outcome was not formulated clearly enough. Therefore, we tried to clarify this section.
12) In the section 'Subgroup analysis and investigation of heterogeneity', we changed the following aspects: Subgroup analyses were conducted only for the primary outcomes
Rationale: subgroup analyses have to be treated with caution, as they are hypothesis‐forming rather than hypothesis‐testing and should be reduced to a reasonable minimum.
13) In the section 'Sensitivity analysis', we changed the following analysis:
-
we changed exclusion of results based on mixed‐effects models or on intention‐to‐treat approach (last observation carried forward (LOCF)) into exclusion of results based on complete‐case analyses
Rationale: we changed this sensitivity analysis because we realised that a large number of studies dealt with missing values by applying the LOCF approach and only in a small number of trials conducted complete‐case analyses.
We added further sensitivity analyses:
-
exclusion of Chinese studies.
Rationale: the CCDANCTR retrieved many Chinese studies. While several of these were identified through routine searches of MEDLINE, EMBASE, and PsycINFO (used to inform the register), the provenance of some of the other Chinese studies in the CCDANCTR was less clear and probably dated back to an opportunistic search of Wang Fang Data (c/o The British Library) in 2007. Therefore, we decided to add a sensitivity analysis of excluding studies that were conducted in China. Unfortunately, none of the comparisons had enough studies available in order to conduct this sensitivity analysis.
We excluded the following sensitivity analyses that were originally proposed in the protocol from the review:
-
exclusion of CRCTs;
-
exclusion of CRCTs for which intracluster correlation coefficients were used;
-
for dichotomous outcomes only: exclusion of results based on available/observed cases.
Rationale: we excluded these sensitivity analyses because no or insufficient studies were available.
-
Sensitivity analyses were conducted only when at least 10 studies were available and only for the primary outcomes.
Rationale: results of sensitivity analyses with a very small number of studies are difficult to interpret. Furthermore, sensitivity analyses have to be treated with caution, as they are hypothesis‐forming rather than hypothesis‐testing and should be reduced to a reasonable minimum.
14) In the section 'Summary of findings tables', we deleted the sentence on reporting Summary of findings tables only for the medication versus placebo comparisons.
Rationale: several peer reviewers suggested that a 'Summary of findings' table for the tricyclic antidepressants versus new‐generation antidepressants and antidepressants alone versus antidepressants plus antipsychotic comparison should be added.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans; Middle Aged;
PICO

PRISMA study flow diagram.
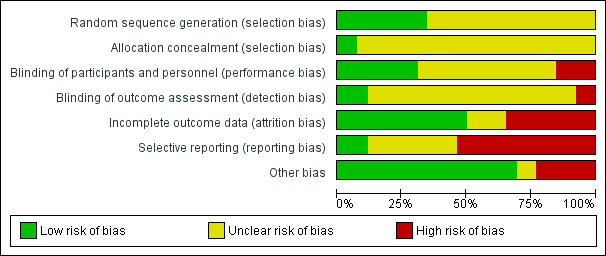
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Pharmacotherapy versus placebo, outcome: 1.1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Forest plot of comparison: 2 Tricyclic antidepressants versus new‐generation antidepressants, outcome: 2.1 Reduction of the level of severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Forest plot of comparison: 3 New‐generation antidepressants (serotonin antagonist and reuptake inhibitors (SARI), noradrenergic and specific serotonergic antidepressant (NaSSA), selective serotonin reuptake inhibitor (SSRI)) versus other new‐generation antidepressants, outcome: 3.1 Reduction of the level of severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Comparison 1 Pharmacotherapy versus placebo, Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).
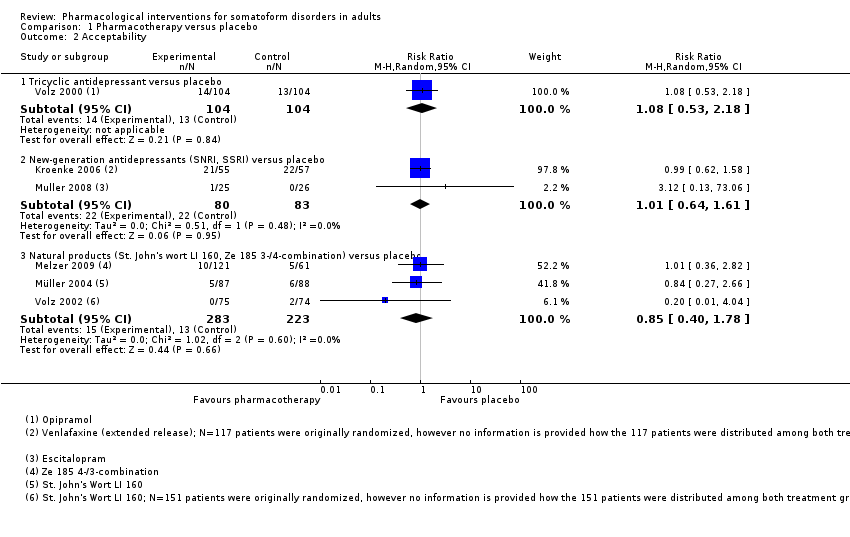
Comparison 1 Pharmacotherapy versus placebo, Outcome 2 Acceptability.

Comparison 1 Pharmacotherapy versus placebo, Outcome 3 Anxiety (post‐treatment score on self report and clinician‐rated scales).
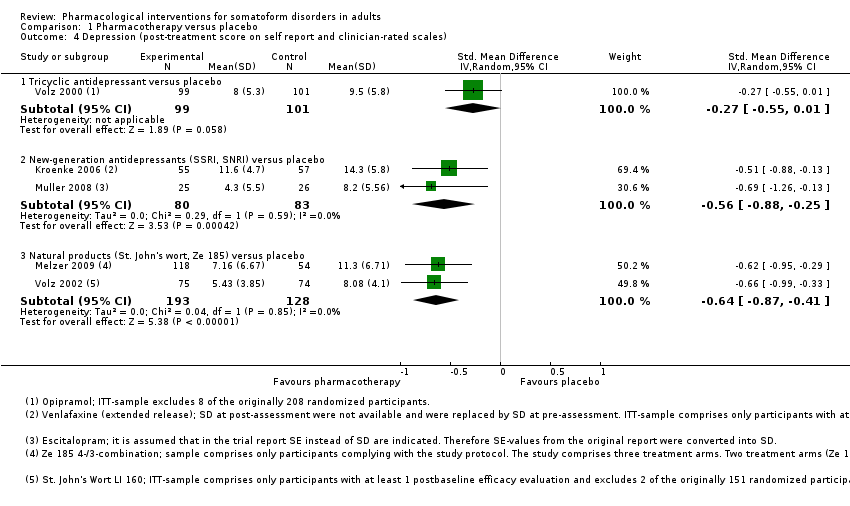
Comparison 1 Pharmacotherapy versus placebo, Outcome 4 Depression (post‐treatment score on self report and clinician‐rated scales).
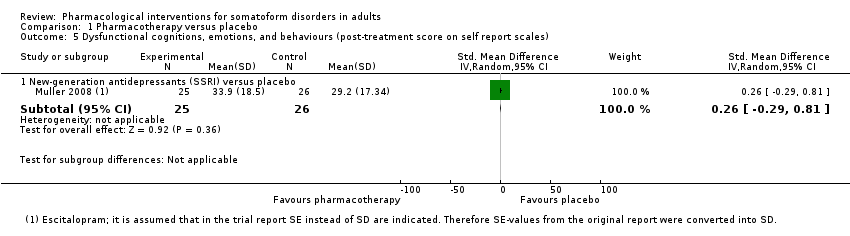
Comparison 1 Pharmacotherapy versus placebo, Outcome 5 Dysfunctional cognitions, emotions, and behaviours (post‐treatment score on self report scales).
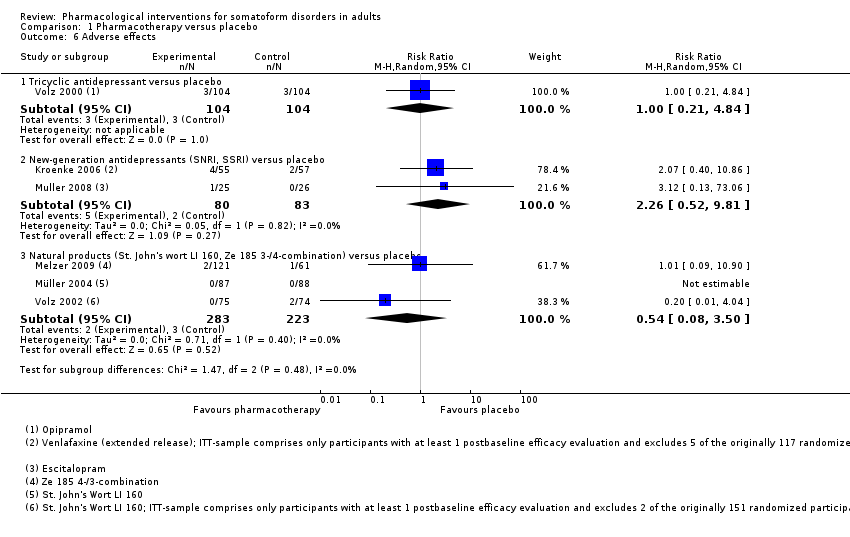
Comparison 1 Pharmacotherapy versus placebo, Outcome 6 Adverse effects.

Comparison 1 Pharmacotherapy versus placebo, Outcome 7 Treatment response (post‐treatment score on self report and clinician‐rated scales).

Comparison 1 Pharmacotherapy versus placebo, Outcome 8 Functional disability and quality of life (post‐treatment score on self report scales).

Comparison 2 Tricyclic antidepressants versus another medication, Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Comparison 2 Tricyclic antidepressants versus another medication, Outcome 2 Acceptability.

Comparison 2 Tricyclic antidepressants versus another medication, Outcome 3 Anxiety (post‐treatment score on self report and clinician‐rated scales).
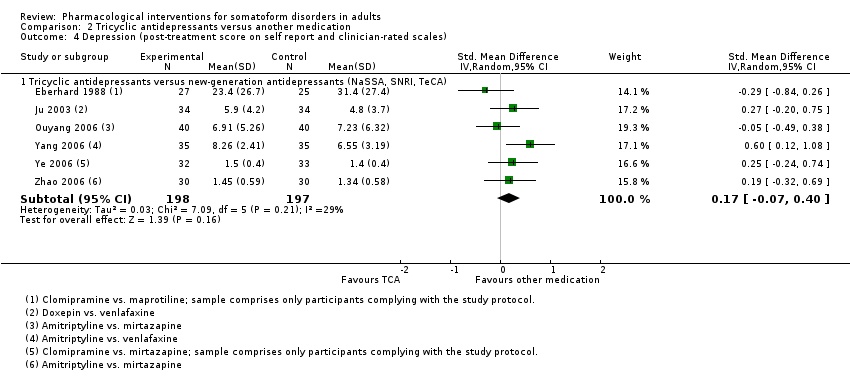
Comparison 2 Tricyclic antidepressants versus another medication, Outcome 4 Depression (post‐treatment score on self report and clinician‐rated scales).

Comparison 2 Tricyclic antidepressants versus another medication, Outcome 5 Adverse effects.

Comparison 2 Tricyclic antidepressants versus another medication, Outcome 6 Treatment response (post‐treatment score on self report and clinician‐rated scales).
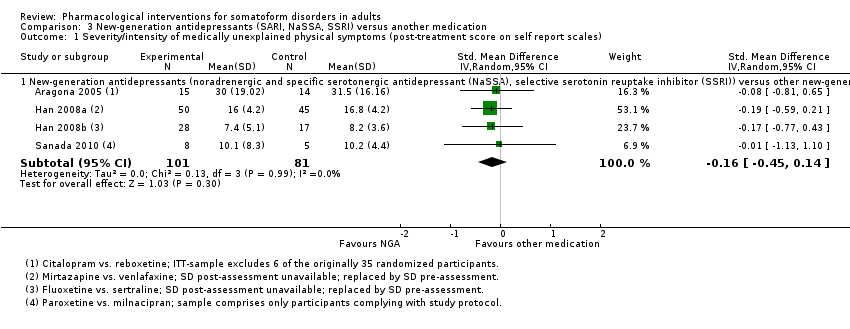
Comparison 3 New‐generation antidepressants (SARI, NaSSA, SSRI) versus another medication, Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).
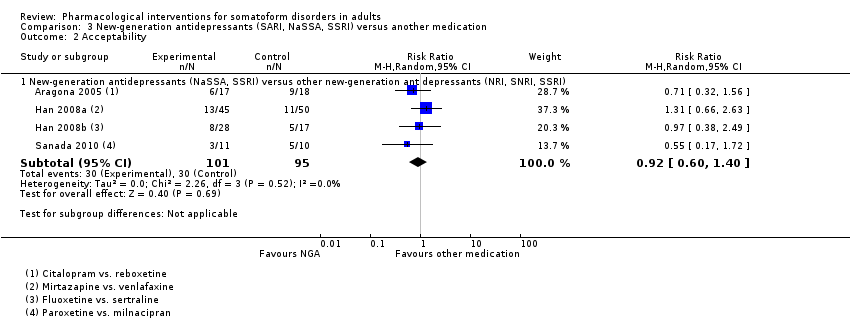
Comparison 3 New‐generation antidepressants (SARI, NaSSA, SSRI) versus another medication, Outcome 2 Acceptability.
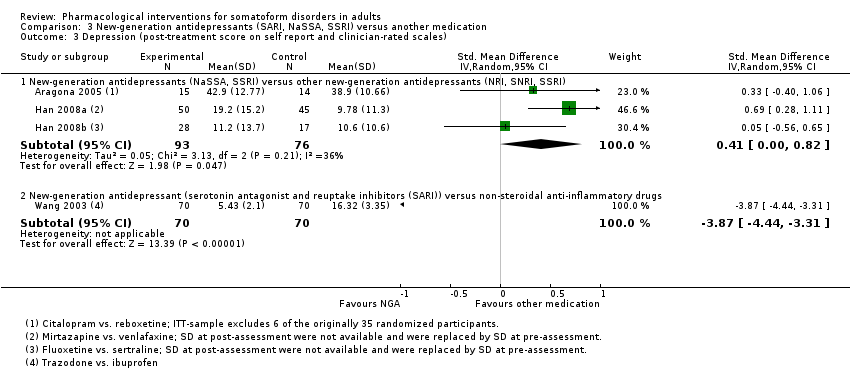
Comparison 3 New‐generation antidepressants (SARI, NaSSA, SSRI) versus another medication, Outcome 3 Depression (post‐treatment score on self report and clinician‐rated scales).
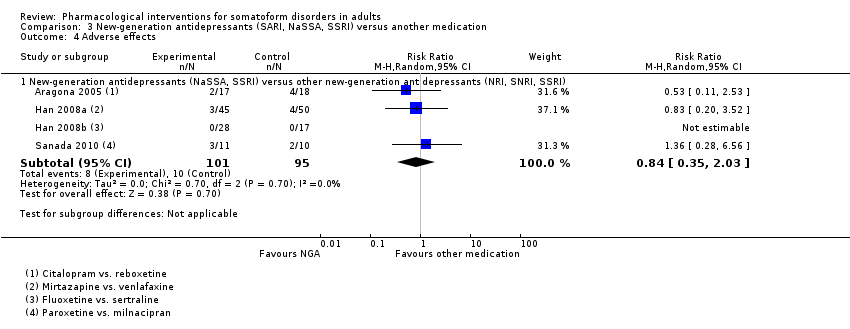
Comparison 3 New‐generation antidepressants (SARI, NaSSA, SSRI) versus another medication, Outcome 4 Adverse effects.
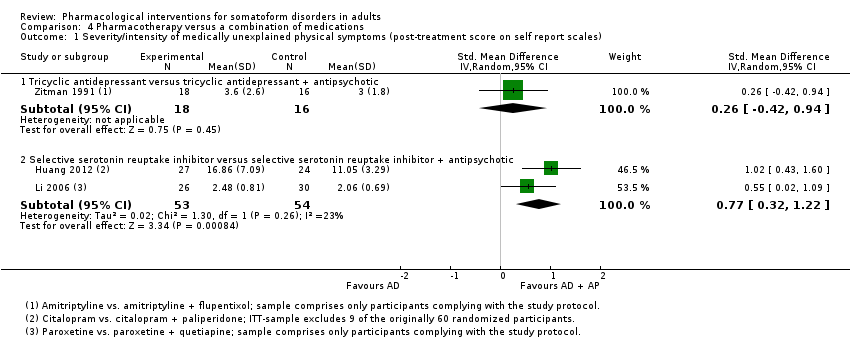
Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).
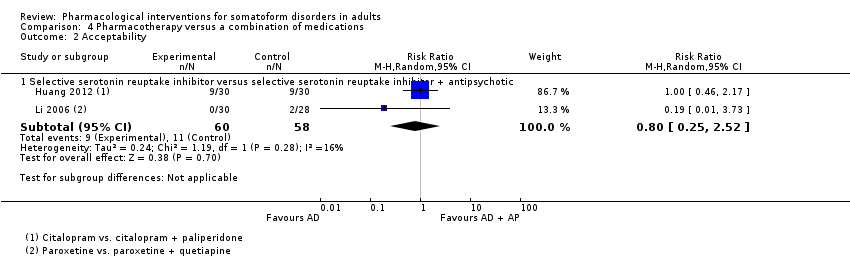
Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 2 Acceptability.

Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 3 Anxiety (post‐treatment score on self report and clinician‐rated scales).

Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 4 Depression (post‐treatment score on self report and clinician‐rated scales).
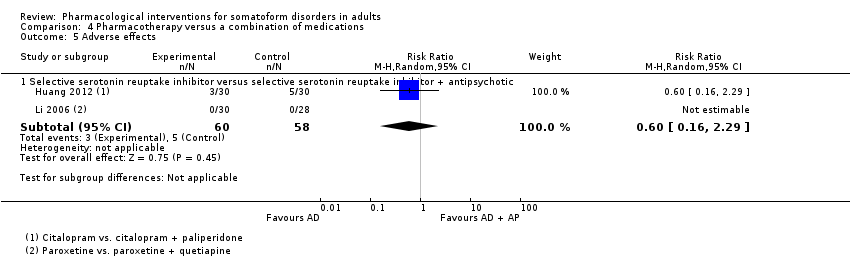
Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 5 Adverse effects.

Comparison 4 Pharmacotherapy versus a combination of medications, Outcome 6 Treatment response (post‐treatment score on self report and clinician‐rated scales).
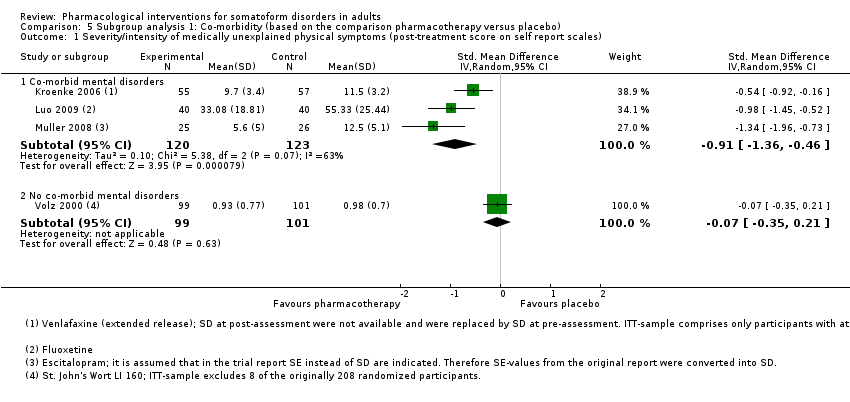
Comparison 5 Subgroup analysis 1: Co‐morbidity (based on the comparison pharmacotherapy versus placebo), Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Comparison 5 Subgroup analysis 1: Co‐morbidity (based on the comparison pharmacotherapy versus placebo), Outcome 2 Acceptability.

Comparison 6 Subgroup analysis 2: Source of funding (based on the comparison pharmacotherapy versus placebo), Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales).

Comparison 6 Subgroup analysis 2: Source of funding (based on the comparison pharmacotherapy versus placebo), Outcome 2 Acceptability.

Comparison 7 Subgroup analysis 3: Source outcome rating (based on the comparison pharmacotherapy versus placebo), Outcome 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score).

Comparison 8 Best‐case/worst‐case analysis (based on comparison 'new‐generation antidepressants versus placebo'), Outcome 1 Treatment response (post‐treatment score on self report and clinician‐rated scales).

Comparison 9 Best‐case/worst‐case analysis (based on comparison 'natural products versus placebo'), Outcome 1 Treatment response (post‐treatment score on self report and clinician‐rated scales).
| Tricyclic antidepressants versus placebo for somatoform disorders in adults | ||||||
| Patient or population: somatoform disorders in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tricyclic antidepressants versus placebo | |||||
| Severity/intensity of MUPS (post‐treatment score on self report scales) | ‐ | The mean severity/intensity of MUPS (post‐treatment score on self report scales) in the intervention groups was | ‐ | 239 | ⊕⊕⊝⊝ | SMD ‐0.13 (95% CI ‐0.39 to 0.13) |
| Acceptability (all‐cause drop‐outs) | No data available | |||||
| Anxiety (post‐treatment score on self report and clinician‐rated scales) | No data available | |||||
| Depression (post‐treatment score on self report and clinician‐rated scales) | No data available | |||||
| Adverse effects (drop‐outs due to adverse effects) | No data available | |||||
| Treatment response (post‐treatment score on self report and clinician‐rated scales) | No data available | |||||
| Functional disability and quality of life (post‐treatment score on self report and clinician‐rated scales) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 SCL‐90‐R and VAS: high scale scores correspond to a negative outcome. | ||||||
| New‐generation antidepressants versus placebo for somatoform disorders in adults | ||||||
| Patient or population: somatoform disorders in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | New‐generation antidepressants versus placebo | |||||
| Severity/intensity of MUPS (post‐treatment score on self report scales) | ‐ | The mean severity/intensity of MUPS in the intervention groups was | ‐ | 243 | ⊕⊝⊝⊝ | SMD ‐0.91 (95% CI ‐1.36 to ‐0.46) |
| Acceptability (all‐cause drop‐outs)5 | Study population | RR 1.01 | 163 | ⊕⊕⊝⊝ | ‐ | |
| 265 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 193 per 1000 | 195 per 1000 | |||||
| Anxiety (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean anxiety score in the intervention groups was | ‐ | 163 | ⊕⊝⊝⊝ | SMD ‐0.88 (95% CI ‐1.81 to 0.05) |
| Depression (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean depression score in the intervention groups was | ‐ | 163 | ⊕⊝⊝⊝ | SMD ‐0.56 (95% CI ‐0.88 to ‐0.25) |
| Adverse effects (drop‐outs due to adverse effects)5 | Study population | RR 2.26 | 163 | ⊕⊕⊝⊝ | ‐ | |
| 24 per 1000 | 54 per 1000 | |||||
| Moderate | ||||||
| 18 per 1000 | 41 per 1000 | |||||
| Treatment response (post‐treatment score on self report and clinician‐rated scales) | Study population | RR 2 | 163 | ⊕⊝⊝⊝ | ‐ | |
| 337 per 1000 | 675 per 1000 | |||||
| Moderate | ||||||
| 319 per 1000 | 638 per 1000 | |||||
| Functional disability and quality of life (post‐treatment score on self report scales) | ‐ | The mean functional disability score/quality of life score in the intervention groups was | ‐ | 163 | ⊕⊝⊝⊝ | SMD ‐0.52 (95% CI ‐1 to ‐0.04) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 PHQ‐15 and MOSPM: high scale scores correspond to a negative outcome. | ||||||
| Natural products versus placebo for somatoform disorders in adults | ||||||
| Patient or population: somatoform disorders in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Natural products versus placebo | |||||
| Severity/intensity of MUPS (post‐treatment score on self report scales) | ‐ | The mean severity/intensity of MUPS in the intervention groups was | ‐ | 322 | ⊕⊕⊝⊝ | SMD ‐0.74 (95% CI ‐0.97 to ‐0.51) |
| Acceptability (all‐cause drop‐outs4) | Study population | RR 0.85 | 506 | ⊕⊕⊝⊝ | ‐ | |
| 58 per 1000 | 50 per 1000 | |||||
| Moderate | ||||||
| 68 per 1000 | 58 per 1000 | |||||
| Anxiety (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean anxiety score in the intervention groups was | ‐ | 321 | ⊕⊕⊝⊝ | SMD ‐0.83 (95% CI ‐1.13 to ‐0.52) |
| Depression (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean depression score in the intervention groups was | ‐ | 321 | ⊕⊕⊝⊝ | SMD ‐0.64 (95% CI ‐0.87 to ‐0.41) |
| Adverse effects (drop‐outs due to adverse effects4) | Study population | RR 0.54 | 506 | ⊕⊕⊝⊝ | ‐ | |
| 13 per 1000 | 7 per 1000 | |||||
| Moderate | ||||||
| 16 per 1000 | 9 per 1000 | |||||
| Treatment response (post‐treatment score on self report and clinician‐rated scales) | Study population | RR 1.77 | 324 | ⊕⊝⊝⊝ | ‐ | |
| 340 per 1000 | 601 per 1000 | |||||
| Moderate | ||||||
| 352 per 1000 | 623 per 1000 | |||||
| Functional disability and quality of life (post‐treatment score on self report scales) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 SOMS‐7 and SCL‐90‐R: high scale scores correspond to a negative outcome. | ||||||
| Tricyclic antidepressants versus another medication for somatoform disorders in adults | ||||||
| Patient or population: somatoform disorders in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tricyclic antidepressants versus another medication | |||||
| Severity/intensity of MUPS (post‐treatment score on self report scales) | ‐ | The mean severity/intensity of MUPS in the intervention groups was | ‐ | 177 | ⊕⊕⊝⊝ | SMD ‐0.16 (95% CI ‐0.55 to 0.23) |
| Acceptability (all‐cause drop‐outs4) | Study population | RR 1.48 | 556 | ⊕⊕⊝⊝ | ‐ | |
| 28 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Anxiety (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean anxiety score in the intervention groups was | ‐ | 255 | ⊕⊝⊝⊝ | SMD 0.37 (95% CI ‐0.21 to 0.95) |
| Depression (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean depression score in the intervention groups was | ‐ | 395 | ⊕⊕⊝⊝ | SMD 0.17 (95% CI ‐0.07 to 0.4) |
| Adverse effects (drop‐outs due to adverse effects4) | Study population | RR 2.37 | 556 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment response (post‐treatment score on self report and clinician‐rated scales) | Study population | RR 0.93 | 130 | ⊕⊕⊝⊝ | ‐ | |
| 677 per 1000 | 630 per 1000 | |||||
| Moderate | ||||||
| 681 per 1000 | 633 per 1000 | |||||
| Functional disability and quality of life | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 VAS ‐ Pain and SCL‐90‐R: high scale scores correspond to a negative outcome. | ||||||
| Antidepressants versus a combination of medications for somatoform disorders in adults | ||||||
| Patient or population: somatoform disorders in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pharmacotherapy versus a combination of medications | |||||
| Severity/intensity of MUPS (post‐treatment score on self report scales) | ‐ | The mean severity/intensity of MUPS in the intervention groups was | ‐ | 107 | ⊕⊕⊝⊝ | SMD 0.77 (95% CI 0.32 to 1.22) |
| Acceptability (all‐cause drop‐outs4) | Study population | RR 0.8 | 118 | ⊕⊕⊝⊝ | ‐ | |
| 190 per 1000 | 152 per 1000 | |||||
| Moderate | ||||||
| 186 per 1000 | 149 per 1000 | |||||
| Anxiety (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean anxiety in the intervention groups was | ‐ | 107 | ⊕⊝⊝⊝ | SMD 0.95 (95% CI ‐0.91 to 2.82) |
| Depression (post‐treatment score on self report and clinician‐rated scales) | ‐ | The mean depression in the intervention groups was | ‐ | 107 | ⊕⊝⊝⊝ | SMD 0.58 (95% CI ‐0.33 to 1.48) |
| Adverse effects (drop‐outs due to adverse effects) | No data available | |||||
| Treatment response (post‐treatment score on self report and clinician‐rated scales) | No data available | |||||
| Functional disability and quality of life | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 SOMS‐7 and SCL‐90 Somatisation Score: high scale scores correspond to a negative outcome. | ||||||
| Diagnostic category | Eligible for the current review? | ||
| DSM‐IV | ICD‐10 | yes | no |
| Somatisation disorder (300.81) | Somatisation disorder (F45.0) | x | ‐ |
| Undifferentiated somatoform disorder (300.82) | Undifferentiated somatoform disorder (F45.1) | x | ‐ |
| ‐ | Somatoform autonomic dysfunction (F45.3) | x | ‐ |
| Pain disorder (307.8) | Persistent somatoform pain disorder (F45.4) | x | ‐ |
| Hypochondriasis (300.7) | Hypochondriacal disorder (F45.2) | ‐ | x |
| ‐ | Other somatoform disorders (F45.8) | ‐ | x |
| Somatoform disorders, unspecified (300.82) | Somatoform disorders, unspecified (F45.9) | ‐ | x |
| Body dysmorphic disorder (300.7) | Body dysmorphic disorder (F45.2) | ‐ | x |
| Conversion disorder (300.11) | Dissociative and conversion disorders (F44)* | ‐ | x |
| DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders; ICD‐10: International Classification of Diseases. Note. *Conversion disorder is not classified as a somatoform disorder in ICD‐10 but is a separate diagnostic category. | |||
| Trial ID | Experimental treatment group ‐ medication class | Control treatment group ‐ medication class | Co‐morbid mental disorders? Yes/no/ns | Detailed information about co‐morbid diagnoses in participants or exclusion of participants with co‐morbid diagnoses (for studies that do not provide details about co‐morbid conditions) | Detailed information about the exclusion of participants with co‐morbid diagnoses |
| SNRI | ‐ | Yes | Major depression, generalised anxiety disorder, social anxiety disorder | People with current/past history of mania, bipolar disorder, schizophrenia, or other psychotic disorder; history of serious or clinically unstable psychiatric condition; known or suspected alcohol or drug abuse within 6 months of screening | |
| SSRI | ‐ | Yes | 38/80 participants had depression (17 item‐HDRS ≥ 17) | People with co‐exist depressive symptoms occurred prior to pain with HDRS ‐ Total Score ≥ 17 | |
| NP | ‐ | No | ‐ | People with historically known or clinical indication of a psychiatric disorder | |
| NP | ‐ | ns | ‐ | People with co‐morbid depression, drug/alcohol abuse, schizophrenia, or schizo‐affective disorder | |
| SSRI | ‐ | Yes | Dysthymia (27.5%); major depressive episode (2.0%); anxiety disorder (panic disorder/agoraphobia/social anxiety disorder/generalised anxiety disorder) (52.9%) | People with somatic symptoms that were judged to be secondary to a psychiatric disorder other than MDS; current or past psychotic disorder; significant suicidal risk | |
| TCA | ‐ | ns | ‐ | People with psychotic illness (including MDS), organic brain syndrome, or alcohol dependence | |
| TCA | ‐ | No | ‐ | People with other significant Axis I diagnoses (e.g. panic disorder, major depressive disorder, substance abuse) | |
| NP | ‐ | ns | ‐ | People with an additional diagnosis of depression, schizophrenia, schizo‐affective disorder, or dementia | |
| AP | AP | Yes | Dysthymia (n = 27), anxiety disorder NOS (n = 6) | ns | |
| SSRI | NRI | No | ‐ | People with a diagnosis of another mental disorder | |
| TeCA | TCA | ns | ‐ | People with major depressive disorder, abuse of drugs, and other psychiatric illnesses | |
| SSRI | SSRI | ns | ‐ | People with history of or current (or both) psychotic disorders (such as schizophrenia, schizoaffective disorder, and bipolar disorder); current DSM Axis I disorders that could possibly account for the somatic symptoms (e.g. MDD, anxiety disorders, factitious disorder, malingering, or another somatoform disorder such as somatisation disorder); substance abuse of dependence in the previous 12 months; effect of co‐morbid psychiatric disorders on the effects of the antidepressants cannot be excluded because of the absence of a structured clinical interview, although participants were rigorously evaluated according to DSM‐IV criteria | |
| SSRI + AP | SSRI | no | ‐ | People with a diagnosis of another mental disorder (e.g. panic disorder, MDD, or substance abuse) | |
| SNRI | TCA | ns | ‐ | People with drug dependence, severe psychosis, or paranoia | |
| SNRI | TCA | ns | ‐ | People with psychotic symptoms, severe brain injury, or substance abuse | |
| SSRI | TCA | ns | ‐ | ns | |
| SSRI + AP | SSRI | ns | ‐ | ns | |
| NaSSA | TCA | Yes | 38/80 participants had depression (17 item‐HDRS ≥ 17). No information about other co‐morbidities in the sample was provided | People whose pain was caused by depression, anxiety, or schizophrenia | |
| SSRI | SNRI | ns | ‐ | ns | |
| SARI | NSAID | Yes | Based on diagnostic criteria in CCMD‐3: depression (n = 46), dysthymia (n = 50), hypochondriasis (n = 20) | Severely depressed, suicidal people | |
| SNRI | TCA | ns | ‐ | ns | |
| NaSSA | TCA | ns | ns | People with any type of drug dependence | |
| SNRI | TCA | ns | ‐ | ns | |
| NaSSA | TCA | no | ‐ | People with other mental disorders | |
| TCA + AP | TCA | yes | Atypical depression (n = 1), dysthymic disorder (n = 1), panic disorder (n = 1), nicotine abuse (n = 1), tea abuse (n = 1), benzodiazepine abuse (n = 1) | People with a serious psychiatric disease necessitating immediate treatment; or who were alcohol or illicit drug dependent | |
| Anxiety disorder NOS: anxiety disorder not otherwise specified; AP: antipsychotic; CCMD: Chinese Classification of Mental Disorders; SARI: serotonin antagonist and reuptake inhibitor; DSM: Diagnostic and Statistical Manual of Mental Disorders; HDRS: Hamilton Depression Rating Scale; ID: identification; ns: not specified; MDD: major depressive disorder; MDS: major depressive syndrome; n: number; NaSSA: noradrenergic specific serotonergic antidepressant; NP: natural product; NRI: noradrenaline reuptake inhibitor; NSAID: non‐steroidal anti‐inflammatory drug; SNRI: serotonin noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; TeCA: tetracyclic antidepressant. | |||||
| Trial ID | Experimental treatment group ‐ medication class | Control treatment group ‐ medication class | Detailed information about the exclusion of or permission of concomitant treatments | Detailed information about concomitant treatments |
| SNRI | ‐ | ‐ Exclusion: use of triptans, psychoactive herbal medications, or any other psychoactive drugs or having a positive urine drug test at screening ‐ Permitted: zaleplon, zopiclone (1 dose nightly) as needed for insomnia for up to 6 nights during the 14 days immediately following randomisation and short‐term treatments for symptoms of allergies, colds, or influenza (without psychotropic effects) | ‐ Proportion of participants who took at least 1 concomitant medication in venlafaxine ER groups vs. placebo were 63.6% vs. 63.3%; for NSAIDs 10% vs. 13%; for paracetamol (acetaminophen) 7% vs. 9%; for COX‐2 inhibitors 8% vs. 3%; and for salicylates 4% vs. 4% | |
| SSRI | ‐ | ‐ Exclusion: use of antidepressants for the treatment of pain or depression | ‐ | |
| NP | ‐ | ‐ Exclusion: psychotherapy, physiotherapy, acupuncture, or using psychoactive drugs including central stimulants and α‐/β‐blockers were excluded ‐ Permitted: in case of sleeping disorders, chloral hydrate was allowed up to 3 g/day | ‐ | |
| NP | ‐ | ‐ Exclusion: use of psychotropic drugs 4 weeks before and during the study, concomitant psychotherapy, and concomitant treatment with phenprocoumon or cyclosporin (or both) | ‐ | |
| SSRI | ‐ | ‐ Exclusion: use of psychotropics or cognitive‐behavioural therapy | ‐ | |
| TCA | ‐ | ‐ | ‐ | |
| TCA | ‐ | ‐ | ‐ | |
| NP | ‐ | ‐ Exclusion: concomitant treatment with psychopharmacological active compounds | ‐ | |
| AP | AP | ‐ | ‐ | |
| SSRI | NRI | ‐ Exclusion: psychotropic drugs endowed with an analgesic action (e.g. amitriptyline) ‐ Permitted: benzodiazepines at low doses for people with sleep disturbances ‐ Because people with other mental disorders were excluded, participants did not take any other type of psychotropic drugs | ‐ | |
| TeCA | TCA | ‐ Exclusion: other central nervous system‐active drugs | ‐ | |
| NaSSA | ‐ | ‐ Exclusion: other psychotropic medications; use of prescription analgesics, muscle relaxants, and corticosteroids ‐ Permitted: hypnosedatives and benzodiazepines only for temporary control of insomnia or anxiety; concomitant medications such as non‐prescription paracetamol were allowed only on an as‐needed basis | ‐ Mirtazapine group: 46% lorazepam, 10% alprazolam ‐ Venlafaxine group: 32% lorazepam, 29% alprazolam | |
| SSRI | SSRI | ‐ Exclusion: other psychotropic medications; use of prescription analgesics, muscle relaxants, and corticosteroids ‐ Permitted: hypnosedatives and benzodiazepines only for temporary control of insomnia or anxiety; concomitant medications such as non‐prescription paracetamol were allowed only on an as‐needed basis | ‐ Fluoxetine group: 32.1% lorazepam, 7.1% alprazolam ‐ Sertraline group: 35.3% lorazepam, 17.6% alprazolam | |
| SSRI + AP | SSRI | ‐ Exclusion: other psychotropic medications ‐ Permitted: benzodiazepines for insomnia, only for temporary control of the symptoms | ‐ | |
| SNRI | TCA | ‐ Permitted: treatment of adverse effects insomnia/anxiety with benzodiazepines and nausea with vitamin B6 | ‐ | |
| SNRI | TCA | ‐ Exclusion: antidepressant and AP medication ‐ Permitted: sleep medication alprazolam (0.4‐0.8 mg/day) | ‐ | |
| SSRI | TCA | ‐ Permitted: alprazolam of a maximum dose of 0.8 mg/day | ‐ | |
| SSRI + AP | SSRI | ‐ | ‐ | |
| NaSSA | TCA | ‐ Exclusion: any other medication ‐ Permitted: exception of benzodiazepines for participants with sleep difficulties | ‐ | |
| SSRI | SNRI | ‐ | ‐ | |
| DAS | NSAID | ‐ Exclusion: any other medication | ‐ | |
| SNRI | TCA | ‐ | ‐ | |
| SNRI | TCA | ‐ Permitted: alprazolam (0.4‐0.8 mg/day) for participants with sleep difficulties | ‐ | |
| NaSSA | TCA | ‐ Permitted: zolpidem for participants with sleep difficulties | ‐ | |
| NaSSA | TCA | ‐ Exclusion: use of MAOI or other antidepressants during the first 2 weeks of treatment; use of other medication such as antidepressants, mood stabiliser, antipsychotic medication, or electroconvulsive therapy during the 8 treatment weeks ‐ Permitted: benzodiazepines were allowed for participants with insomnia. Benzhexol was allowed for participants with extrapyramidal adverse effects | ‐ | |
| TCA + AP | TCA | ‐ Permitted: benzodiazepines and non‐narcotic analgesics could be continued during the trial, but the participants were asked to keep the dose as low as possible | ‐ | |
| AP: antipsychotic; COX: cyclo‐oxygenase; DAS: ; ER: extended release; ID: identification; MAOI: monoamine oxidase inhibitors; NaSSA: noradrenergic specific serotonergic antidepressant; NP: natural product; NRI: noradrenaline reuptake inhibitor; NSAID: non‐steroidal anti‐inflammatory drug; SNRI: serotonin noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; TeCA: tetracyclic antidepressant. | ||||
| Trial ID | Experimental treatment | Placebo | Control treatment | ||||||||||
| Medication class | Chemical agent | Attrition | Total | % attrition | Attrition | Total | % attrition | Medication class | Chemical agent | Attrition | Total | % attrition | |
| TCA | Amitriptyline | ns | 26 | 25.0 | ns | 24 | 31.0 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| TCA | Amitriptyline | 0 | 15 | 0 | ‐ | ‐ | ‐ | SSRI | Paroxetinea | 0 | 45 | 0 | |
| TCA | Amitriptyline | 0 | 32 | 0 | ‐ | ‐ | ‐ | SNRI | Venlafaxine | 0 | 36 | 0 | |
| TCA | Amitriptyline | 0 | 35 | 0 | ‐ | ‐ | ‐ | SNRI | Venlafaxine | 0 | 35 | 0 | |
| TCA | Amitriptyline | 0 | 35 | 0 | ‐ | ‐ | ‐ | SNRI | Venlafaxine | 0 | 35 | 0 | |
| TCA | Amitriptyline | 0 | 40 | 0 | ‐ | ‐ | ‐ | NaSSA | Mirtazapine | 0 | 40 | 0 | |
| TCA | Amitriptyline | ns | 30 | ne | ‐ | ‐ | ‐ | NaSSA | Mirtazapine | ns | 30 | ne | |
| TCA | Amitriptyline | ns | ns | ne | ‐ | ‐ | ‐ | TCA + AP | Amitriptyline + flupentixol | ns | ns | ne | |
| TCA | Clomipramine | 2 | 35 | 5.7 | ‐ | ‐ | ‐ | NaSSA | Mirtazapine | 2 | 35 | 5.7 | |
| TCA | Clomipramine | 13 | 40 | 32.5 | ‐ | ‐ | ‐ | TeCA | Maprotiline | 5 | 30 | 16.7 | |
| TCA | Doxepin | 0 | 34 | 0 | ‐ | ‐ | ‐ | SNRI | Venlafaxine | 0 | 34 | 0 | |
| TCA | Opipramol | 14 | 104 | 13.5 | 13 | 104 | 12.4 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| TCA + AP | Amitriptyline + flupentixol | ns | ns | ne | ‐ | ‐ | ‐ | TCA | Amitriptyline | ns | ns | ne | |
| SSRI | Paroxetinea | 0 | 45 | 0 | ‐ | ‐ | ‐ | TCA | Amitriptyline | 0 | 15 | 0 | |
| SSRI | Paroxetine | 0 | 30 | 0 | ‐ | ‐ | ‐ | SSRI + AP | Paroxetine + quetiapine | 2 | 28 | 7.1 | |
| SSRI | Paroxetine | 3 | 11 | 27.3 | ‐ | ‐ | ‐ | SNRI | Milnacipran | 5 | 10 | 50.0 | |
| SSRI | Fluoxetine | ns | 40 | ne | ns | 40 | ne | ‐ | ‐ | ‐ | ‐ | ‐ | |
| SSRI | Fluoxetine | 8 | 28 | 28.6 | ‐ | ‐ | ‐ | SSRI | Sertraline | 5 | 17 | 29.4 | |
| SSRI | Citalopram | 9 | 30 | 30.0 | ‐ | ‐ | ‐ | SSRI + AP | Citalopram + paliperidone | 9 | 30 | 30.0 | |
| SSRI | Citalopram | 6 | 17 | 35.3 | ‐ | ‐ | ‐ | NRI | Reboxetine | 9 | 18 | 50.0 | |
| SSRI | Escitalopram | 1 | 25 | 4.0 | 0 | 26 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| SSRI | Sertraline | 5 | 17 | 29.41 | ‐ | ‐ | ‐ | SSRI | Fluoxetine | 8 | 28 | 28.6 | |
| SSRI + AP | Citalopram + paliperidone | 9 | 30 | 30.0 | ‐ | ‐ | ‐ | SSRI | Citalopram | 9 | 30 | 30.00 | |
| SSRI + AP | Paroxetine + quetiapine | 0 | 30 | 0 | ‐ | ‐ | ‐ | SSRI | Paroxetine | 2 | 28 | 7.1 | |
| SNRI | Venlafaxine (extended release) | 21 | 55 | 38.2 | 22 | 57 | 38.6 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| SNRI | Venlafaxine | 0 | 36 | 0 | ‐ | ‐ | ‐ | TCA | Amitriptyline | 0 | 32 | 0 | |
| SNRI | Venlafaxine | 0 | 35 | 0 | ‐ | ‐ | ‐ | TCA | Amitriptyline | 0 | 35 | 0 | |
| SNRI | Venlafaxine | 0 | 35 | 0 | ‐ | ‐ | ‐ | TCA | Amitriptyline | 0 | 35 | 0 | |
| SNRI | Venlafaxine | 0 | 34 | 0 | ‐ | ‐ | ‐ | TCA | Doxepin | 0 | 34 | 0 | |
| SNRI | Venlafaxine | 13 | 45 | 28.9 | ‐ | ‐ | ‐ | NaSSA | Mirtazapine | 11 | 50 | 22.0 | |
| SNRI | Milnacipran | 5 | 10 | 50.0 | ‐ | ‐ | ‐ | SSRI | Paroxetine | 3 | 11 | 27.3 | |
| NaSSA | Mirtazapine | 0 | 40 | 0 | ‐ | ‐ | ‐ | TCA | Amitriptyline | 0 | 40 | 0 | |
| NaSSA | Mirtazapine | 2 | 35 | 5.7 | ‐ | ‐ | ‐ | TCA | Clomipramine | 2 | 35 | 5.7 | |
| NaSSA | Mirtazapine | ns | 30 | ne | ‐ | ‐ | ‐ | TCA | Amitriptyline | ns | 30 | ne | |
| NaSSA | Mirtazapine | 11 | 50 | 22.0 | ‐ | ‐ | ‐ | SNRI | Venlafaxine | 13 | 45 | 28.9 | |
| TeCA | Maprotiline | 5 | 30 | 16.7 | ‐ | ‐ | ‐ | TCA | Clomipramine | 13 | 40 | 32.5 | |
| NRI | Reboxetine | 9 | 18 | 50.0 | ‐ | ‐ | ‐ | SSRI | Citalopram | 6 | 17 | 35.29 | |
| SARI | Traxodone | 0 | 70 | 0 | ‐ | ‐ | ‐ | NSAID | Ibuprofen | 0 | 70 | 0 | |
| AP | Levosulpride | 2 | 15 | 13.3 | ‐ | ‐ | ‐ | AP | Racemic sulpiride | 2 | 15 | 13.3 | |
| AP | Racemic sulpiride | 2 | 15 | 13.3 | ‐ | ‐ | ‐ | AP | Levosulpride | 2 | 15 | 13.3 | |
| NP | St. John's wort LI 160 | 5 | 87 | 5.8 | 6 | 88 | 6.8 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| NP | St. John's wort LI 160 | 0 | 75 | 0.0 | 2 | 74 | 2.7 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| NP | Ze 185 3‐/4‐combinationc | 10 | 121 | 8.3 | 5 | 61 | 8.2 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| AP: antipsychotic; ID: identification; NaSSA: noradrenergic specific serotonergic antidepressant; ns: not specified; ne: not estimable; NP: natural product; NRI: noradrenaline reuptake inhibitor, SARI: serotonin antagonist and reuptake inhibitor; SNRI: serotonin noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; TeCA: tetracyclic antidepressant. Total sample sizes and attrition rates refer to the total number of randomised participants. a Trial included 3 arms: paroxetine, open paroxetine, and placebo; we combined the attrition rate of paroxetine and open paroxetine. b 117 participants were originally randomised but information about sample sizes of treatment and control group were missing. However, study authors stated that in the intention‐to‐treat (ITT) population, they only included participants who had at least 1 post‐baseline efficacy evaluation yielding in an ITT sample of 112. No information was provided about how the 117 participants were distributed among treatment groups. Therefore, we calculated drop‐out rate based on the 112 sample. c Trial included 3 arms: Ze 185 4‐combination, Ze 185 3‐combination, and placebo; we combined the attrition rate of the Ze 185 3‐combination and 4‐combination. d Originally 151 participants were randomised. No participants were excluded after the placebo run‐in phase. However, study authors defined ITT population as a sample of all randomised participants with at least 1 assessment under trial medication. 2 participants had to be excluded from the analysis due to missing values for the primary efficacy variable after baseline. Therefore, the ITT sample included 149 participants. No information was provided how the original 151 participants were distributed among groups. Therefore, we calculated drop‐out rate based on the sample. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Tricyclic antidepressants versus placebo | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.39, 0.13] |
| 1.2 New‐generation antidepressants (serotonin noradrenaline reuptake inhibitor (SNRI), selective serotonin reuptake inhibitor (SSRI)) versus placebo | 3 | 243 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.36, ‐0.46] |
| 1.3 Natural products (St. John's wort LI 160) versus placebo | 2 | 322 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.97, ‐0.51] |
| 2 Acceptability Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Tricyclic antidepressant versus placebo | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.53, 2.18] |
| 2.2 New‐generation antidepressants (SNRI, SSRI) versus placebo | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.64, 1.61] |
| 2.3 Natural products (St. John's wort LI 160, Ze 185 3‐/4‐combination) versus placebo | 3 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.40, 1.78] |
| 3 Anxiety (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Tricyclic antidepressants versus placebo | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.29, 0.26] |
| 3.2 New‐generation antidepressants (SNRI, SSRI) versus placebo | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.81, 0.05] |
| 3.3 Natural products (St. John's wort LI 160, Ze 185 3‐/4‐combination) versus placebo | 2 | 321 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.13, ‐0.52] |
| 4 Depression (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Tricyclic antidepressant versus placebo | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.55, 0.01] |
| 4.2 New‐generation antidepressants (SSRI, SNRI) versus placebo | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.88, ‐0.25] |
| 4.3 Natural products (St. John's wort, Ze 185) versus placebo | 2 | 321 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.87, ‐0.41] |
| 5 Dysfunctional cognitions, emotions, and behaviours (post‐treatment score on self report scales) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 New‐generation antidepressants (SSRI) versus placebo | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.29, 0.81] |
| 6 Adverse effects Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Tricyclic antidepressant versus placebo | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.84] |
| 6.2 New‐generation antidepressants (SNRI, SSRI) versus placebo | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.52, 9.81] |
| 6.3 Natural products (St. John's wort LI 160, Ze 185 3‐/4‐combination) versus placebo | 3 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.08, 3.50] |
| 7 Treatment response (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Tricyclic antidepressant versus placebo | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.95, 1.73] |
| 7.2 New‐generation antidepressants (SNRI, SSRI) versus placebo | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.90, 4.43] |
| 7.3 Natural products (St. John's wort LI 160) versus placebo | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.34, 2.34] |
| 8 Functional disability and quality of life (post‐treatment score on self report scales) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Tricyclic antidepressant versus placebo | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.58, 0.60] |
| 8.2 New‐generation antidepressants (SNRI, SSRI) versus placebo | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [1.00, ‐0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Tricyclic antidepressants versus new‐generation antidepressants (noradrenergic and specific serotonergic antidepressant (NaSSA), tetracyclic antidepressant (TeCA)) | 3 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.55, 0.23] |
| 2 Acceptability Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Tricyclic antidepressants versus new‐generation antidepressants (NaSSA, serotonin noradrenaline reuptake inhibitor (SNRI), TeCA) | 8 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.59, 3.72] |
| 3 Anxiety (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Tricyclic antidepressants versus new‐generation antidepressants (NaSSA, SNRI, SSRI) | 4 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.21, 0.95] |
| 4 Depression (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Tricyclic antidepressants versus new‐generation antidepressants (NaSSA, SNRI, TeCA) | 6 | 395 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.40] |
| 5 Adverse effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Tricyclic antidepressant versus new‐generation antidepressants (NaSSA, SNRI, TeCA) | 8 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.39, 14.28] |
| 6 Treatment response (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Tricyclic antidepressant versus new‐generation antidepressants (NaSSA) | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 New‐generation antidepressants (noradrenergic and specific serotonergic antidepressant (NaSSA), selective serotonin reuptake inhibitor (SSRI)) versus other new‐generation antidepressants (noradrenaline reuptake inhibitor (NRI), serotonin noradrenaline reuptake inhibitor (SNRI), SSRI) | 4 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.45, 0.14] |
| 2 Acceptability Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 New‐generation antidepressants (NaSSA, SSRI) versus other new‐generation antidepressants (NRI, SNRI, SSRI) | 4 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.60, 1.40] |
| 3 Depression (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 New‐generation antidepressants (NaSSA, SSRI) versus other new‐generation antidepressants (NRI, SNRI, SSRI) | 3 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.00, 0.82] |
| 3.2 New‐generation antidepressant (serotonin antagonist and reuptake inhibitors (SARI)) versus non‐steroidal anti‐inflammatory drugs | 1 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐4.44, ‐3.31] |
| 4 Adverse effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 New‐generation antidepressants (NaSSA, SSRI) versus other new‐generation antidepressants (NRI, SNRI, SSRI) | 4 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Tricyclic antidepressant versus tricyclic antidepressant + antipsychotic | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.42, 0.94] |
| 1.2 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [0.32, 1.22] |
| 2 Acceptability Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.25, 2.52] |
| 3 Anxiety (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.91, 2.82] |
| 4 Depression (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Tricyclic antidepressant versus tricyclic antidepressant + antipsychotic | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.09, 1.49] |
| 4.2 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.33, 1.48] |
| 5 Adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.16, 2.29] |
| 6 Treatment response (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Selective serotonin reuptake inhibitor versus selective serotonin reuptake inhibitor + antipsychotic | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.7 [0.94, 3.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Co‐morbid mental disorders | 3 | 243 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.36, ‐0.46] |
| 1.2 No co‐morbid mental disorders | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 2 Acceptability Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Co‐morbid mental disorder | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.64, 1.61] |
| 2.2 No co‐morbid mental disorder | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score on self report scales) Show forest plot | 7 | 804 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.97, ‐0.36] |
| 1.1 Funding by pharmaceutical industry | 5 | 685 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.02, ‐0.27] |
| 1.2 No funding by pharmaceutical industry | 2 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.29, ‐0.21] |
| 2 Acceptability Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Funding by pharmaceutical industry | 6 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.70, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity/intensity of medically unexplained physical symptoms (post‐treatment score) Show forest plot | 7 | 1377 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.00, ‐0.49] |
| 1.1 Self report scales | 7 | 804 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.97, ‐0.36] |
| 1.2 Clinician‐rated scales | 4 | 573 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.42, ‐0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment response (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete‐case analysis | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.82, 4.97] |
| 1.2 Best‐case analysis | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.90, 3.53] |
| 1.3 Worst‐case analysis | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.30, 6.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment response (post‐treatment score on self report and clinician‐rated scales) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete‐case analysis | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.29, 2.36] |
| 1.2 Best‐case analysis | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.26, 2.94] |
| 1.3 Worst‐case analysis | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.27, 1.93] |

