تاثیر توپیرامات در پیشگیری از بروز میگرن اپیزودیک در بزرگسالان
چکیده
پیشینه
برخی از داروهای ضد صرع، اما نه همه آنها، در عملکرد بالینی برای پروفیلاکسی از بروز میگرن، قابل استفاده هستند. این امر ممکن است با تنوع عملکردهای این داروها در سیستم اعصاب مرکزی توضیح داده شود. مرور حاضر، بخشی از نسخه بهروز شده از یک مرور کاکرین است که نخستینبار در سال 2004 منتشر، و پیش از آن در سال 2007 (بدون هیچ تغییری در نتیجهگیری) بهروز شد.
اهداف
توصیف و ارزیابی شواهد حاصل از کارآزماییهای کنترل شده در مورد اثربخشی و تحملپذیری توپیرامات (topiramate) در پیشگیری از بروز حملات میگرنی در بیماران بزرگسال مبتلا به میگرن اپیزودیک.
روشهای جستوجو
پایگاه مرکزی ثبت کارآزماییهای کنترل شده کاکرین (CENTRAL؛ کتابخانه کاکرین ، 2012، شماره 12)؛ PubMed/MEDLINE (1966 تا 15 ژانویه 2013)؛ MEDLINE In‐Process (هفته جاری، 15 ژانویه 2013)، و EMBASE (1974 تا 15 ژانویه 2013) را جستوجو کرده و تا ژانویه 2013، مجلات Headache و Cephalalgia را به صورت دستی جستوجو کردیم.
معیارهای انتخاب
مطالعات میبایست در قالب کارآزماییهایی کنترل شده و آیندهنگر و با بررسی توپیرامات انجام شده باشند که اغلب بهطور منظم برای پیشگیری از وقوع حملات میگرن، به منظور بهبود کیفیت زندگی مرتبط با میگرن، یا هر دو، تجویز شد.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم مطالعات را انتخاب و دادهها را استخراج کردند. برای دادههای مربوط به فراوانی سردرد، تفاوتهای میانگین (MDs) را میان توپیرامات و مقایسه کننده (دارونما (placebo)، کنترل فعال، یا توپیرامات با دوزی متفاوت) برای مطالعات منفرد محاسبه کرده و اینها را از کل مطالعات تجمیع کردیم. برای دادههای دو حالتی (dichotomous) در پاسخدهندگان به درمان (بیماران با کاهش ≥ 50% در فراوانی سردرد)، نسبتهای شانس (ORs) و در موارد منتخب، خطرات نسبی (RRs) را محاسبه کردیم؛ تعداد افراد مورد نیاز برای درمان (numbers needed to treat; NNT) را نیز محاسبه کردیم. MDها را برای ابزار انتخاب شده ارزیابی کیفیت زندگی محاسبه کردیم. در نهایت، دادههای مربوط به عوارض جانبی را از کارآزماییهای کنترل شده با دارونما خلاصه کرده و تفاوت خطر (risk difference; RD) و تعداد افراد مورد نیاز برای آسیب (NNHs) را محاسبه کردیم.
نتایج اصلی
بیست مقاله که به توصیف 17 کارآزمایی منحصربهفرد پرداختند، معیارهای ورود را داشتند. آنالیز دادههای نه کارآزمایی (1737 شرکتکننده) نشان داد که توپیرامات در مقایسه با دارونما، فراوانی سردرد را تا تقریبا 1.2 حمله در هر 28 روز کاهش داد (MD: ‐1.20؛ 95% فاصله اطمینان (CI): 1.59‐ تا 0.80‐). دادههای نه کارآزمایی (1190 شرکتکننده) نشان میدهند که توپیرامات نسبت پاسخدهندگان به درمان را در مقایسه با دارونما تقریبا دو برابر کرد (RR: 2.02؛ 95% CI؛ 1.57 تا 2.60؛ NNT: 4؛ 95% CI؛ 3 تا 6). تجزیهوتحلیل جداگانه دوزهای مختلف توپیرامات، MDهای مشابهی را در مقابل دارونما در دوز 50 میلیگرم (0.95‐؛ 95% CI؛ 1.95‐ تا 0.04؛ سه مطالعه؛ 520 شرکتکننده)، 100 میلیگرم (1.15‐؛ 95% CI؛ 1.58‐ تا 0.71‐؛ شش مطالعه؛ 1620 شرکتکننده) و 200 میلیگرم (0.94‐؛ 95% CI؛ 1.53‐ تا 0.36‐؛ پنج مطالعه؛ 804 شرکتکننده) ایجاد کرد. هر سه دوز، نسبت پاسخدهندگان به درمان را نسبت به دارونما بهطور قابلتوجهی افزایش دادند؛ ORها به شرح زیر بودند: برای 50 میلیگرم، 2.35 (95% CI؛ 1.60 تا 3.44؛ سه مطالعه؛ 519 شرکتکننده)؛ برای 100 میلیگرم، 3.49 (95% CI؛ 2.23 تا 5.45؛ پنج مطالعه؛ 852 شرکتکننده)؛ و برای 200 میلیگرم، 2.49 (95% CI؛ 1.61 تا 3.87؛ شش مطالعه؛ 1025 شرکتکننده). هر سه دوز نیز سه حوزه یا بیشتر از کیفیت زندگی را در مقایسه با دارونما بهطور قابلتوجهی بهبود بخشیدند. متاآنالیز سه مطالعه که شامل بیش از یک دوز توپیرامات بود، نشان میدهد که 200 میلیگرم موثرتر از 100 میلیگرم نیست. با توجه به میانگین فراوانی سردرد و/یا نرخ پاسخدهی، هفت کارآزمایی با استفاده از مقایسهکنندههای فعال نشان دادند که (الف) تفاوت معنیداری میان توپیرامات و آمیتریپتیلین وجود ندارد (یک مطالعه، 330 شرکتکننده)؛ (ب) تفاوت معنیداری میان توپیرامات و فلوناریزین وجود ندارد (یک مطالعه، 83 شرکتکننده)؛ (ج) تفاوت معنیداری میان توپیرامات و پروپرانولول وجود ندارد (دو مطالعه، 342 شرکتکننده)؛ (د) تفاوت معنیداری میان توپیرامات و ریلکس کردن وجود ندارد (یک مطالعه، 61 شرکتکننده)؛ اما (ه) یک مزیت قابلتوجه جزئی از توپیرامات نسبت به والپروات دیده شد (دو مطالعه، 120 شرکتکننده). ریلکس کردن کیفیت زندگی خاص میگرن را بهطور قابلتوجهی بیشتر از توپیرامات بهبود بخشید. در کارآزماییهای توپیرامات در مقابل دارونما، هفت عارضه جانبی (AEs) توسط حداقل سه مطالعه گزارش شدند. اینها معمولا خفیف و ماهیت غیر جدی داشتند. به جز اختلال چشایی و کاهش وزن، تفاوت معنیداری در فراوانی AEs بهطور کلی، یا از هفت AE خاص، میان دارونما و توپیرامات 50 میلیگرم وجود نداشت. AEها بهطور کلی و همه AEهای خاص به جز حالت تهوع در توپیرامات 100 میلیگرمی بهطور قابلتوجهی بیشتر از دارونما رخ دادند، با NNHs که از 3 تا 25 متغیر بوده، و RDها در مقابل دارونما برای توپیرامات 200 میلیگرم حتی بیشتر بود، با NNH از 2 تا 17.
نتیجهگیریهای نویسندگان
متاآنالیز نشان میدهد که توپیرامات در دوز 100 میلیگرم در روز در کاهش فراوانی سردرد موثر است و در بیماران بزرگسال مبتلا به میگرن اپیزودیک به خوبی تحمل میشود. این یافتهها، شواهد خوبی را برای حمایت از استفاده از آن در مدیریت معمول بالین ارائه میدهد. انجام مطالعات بیشتری که بهطور خاص برای مقایسه اثربخشی یا بیخطری (safety) توپیرامات در مقابل دیگر مداخلات با اثربخشی ثابت شده در پیشگیری از میگرن طراحی شوند، مورد نیاز است.
خلاصه به زبان ساده
نقش توپیرامات در پیشگیری از بروز حملات میگرن در بزرگسالان
برای درمان صرع، داروهای مختلفی، که در مجموع «ضد صرع» نامیده میشوند، مورد استفاده قرار میگیرند. چندین سال است که برخی از این داروها برای پیشگیری از بروز حملات میگرنی نیز تجویز میشوند. برای مرور حاضر، محققان در سازمان همکاری کاکرین شواهدی را در مورد تاثیرات توپیرامات در بیماران بزرگسال (≥ 16 سال) مبتلا به میگرن «اپیزودیک» (سردرد < 15 روز در ماه) بررسی کردند. آنها پژوهشهای منتشر شده را تا 15 ژانویه 2013 بررسی کرده و 17 مطالعه مرتبط را یافتند. توپیرامات در مقایسه با دارونما، فراوانی سردردهای میگرنی را تقریبا تا 1.2 مورد در ماه کاهش داد (نه مطالعه، 1737 شرکتکننده). همچنین احتمال کاهش سردردهای میگرنی در بیماران به میزان 50% یا بیشتر با توپیرامات بیش از دو برابر بیشتر از دارونما گزارش شد (نه مطالعه، 1190 شرکتکننده). بروز عوارض جانبی مرتبط با توپیرامات شایع اما عموما خفیف بودند؛ با این حال، توپیرامات میتواند باعث نقص مادرزادی شود، بنابراین باید در زنان در سنین باروری با احتیاط مصرف شود. انجام پژوهشهای بیشتری برای مقایسه توپیرامات با دیگر داروهای فعال مورد استفاده در پیشگیری از بروز حملات میگرنی مورد نیاز است.
Authors' conclusions
Background
Description of the condition
Migraine is a common and disabling health problem among children and predominantly young and middle‐aged adults. Surveys from the main regions of the world suggest that the global prevalence of migraine is 14.7% (18.8% among women and 10.7% among men) (GBD 2010 Study). This disorder results in significant disability and work loss, and several studies have addressed the issue of the costs of migraine. In one of the most recent publications, aggregate direct and indirect costs to society due to migraine among adults in the European Union were estimated to amount to 50 billion Euros (67 billion US dollars) annually, or about 1222 Euros (1634 US dollars) annually per sufferer (Linde 2012).
Description of the intervention
Drug therapy for migraine falls into two categories: acute and preventive. Acute therapy aims at the symptomatic treatment of the head pain and other symptoms associated with an acute attack of migraine. The primary goals of preventive treatment are to reduce attack frequency, severity, and duration. Moreover, such therapy is commonly employed in an attempt to improve responsiveness to acute treatment, enhance functional status, and reduce disability. Evidence‐based guidelines on the drug treatment of migraine have been developed and published by the European Federation of Neurological Societies (EFNS; Evers 2009). These guidelines suggest that prophylactic therapy should be considered for patients with migraine when quality of life, business duties, or school attendance are severely impaired; when the frequency of attacks is two or more per month; when there is a lack of response to acute drug treatment; and when frequent, very long, or uncomfortable auras occur.
This review considers the evidence for the efficacy and tolerability of topiramate for preventing episodic migraine in adults. The prophylactic treatment of migraine in children is the subject of a separate Cochrane review (Victor 2003).
Topiramate is a sulphamate‐substituted monosaccharide (2,3:4,5‐Bis‐O‐(1‐methylethylidene)‐b‐D‐fructo‐pyranose sulfamate) derived from the naturally occurring sugar D‐fructose (Edvinsson 2010). The absolute bioavailability, or oral bioavailability, of topiramate is 81% to 95% and is not affected by food. The distribution volume for women is approximately 50% that of men. Topiramate is metabolised to a moderate degree (circa 20%). Six metabolites have been identified. Total clearance is low (20 to 30 mL/min) after oral intake. This occurs predominantly via renal excretion (renal clearance 10 to 20 mL/min). Topiramate has a long half‐life (19 to 25 hours). Patients with normal renal function may need four to eight days to reach steady‐state plasma concentrations. The typical dose range of topiramate used in migraine is 50 to 200 mg.
How the intervention might work
We use the term 'antiepileptics' here to refer generally to those drugs in common use for the treatment of epilepsy. The pharmacological treatment of epilepsy can be traced back as far as 1857, but the period of greatest development of antiepileptics was between 1935 and 1960, when 13 drugs were developed and marketed (Porter 1992). In recent decades, renewed interest has led to the development of several novel antiepileptics which may confer advantages in tolerability (Dalkara 2012), and these are beginning to be used in migraine also.
The use of antiepileptics for the prophylactic treatment of migraine is theoretically warranted by several known modes of action which relate either to the general modulation of pain systems or more specifically to systems involved in the pathophysiology of migraine (Silberstein 2008; Wiffen 2010). It is necessary to point out, however, that it is not currently possible to state with any certainty which particular mode or modes of action of topiramate are relevant to the prophylaxis of migraine (Edvinsson 2010). The efficacy of topiramate in migraine seems to be mediated by the interaction with multiple sites of action. It decreases the frequency of action potentials elicited by depolarising electric current, which gives expression to a blockade of voltage‐dependent Na+ channels. Topiramate modulates cortical excitability in migraineurs, but it does not appear that this alone explains the efficacy in migraine prophylaxis. Topiramate inhibits the excitatory activity of glutamate at the receptor subtype for kainate/AMPA. It has been shown to inhibit neurons of the trigeminocervical complex via a GABA‐mediated mechanism. Furthermore, topiramate inhibits the release of CGRP from prejunctional trigeminal neurons. An inhibitory effect on high‐voltage‐dependent (HVA) Ca2+ channels especially in periaqueductal grey is a possible mechanism to explain the therapeutic effect in migraine. A differential sensitivity to topiramate has been observed for HVA Ca2+ channels located on cortical neurons and those in the periaqueductal grey region (PAG). Topiramate inhibits N‐, P‐, and L‐type channels in PAG neurons, whereas in cortical neurons it modulates only P‐ and L‐type channels. Chronic, but not acute, treatment suppresses cortical spreading depression in rats. Long‐term effects on gene regulation have to be considered. Collectively this points towards a reduction in excitatory transmission and increase in inhibitory neurotransmission.
Why it is important to do this review
Some antiepileptic drugs are marketed specifically for migraine prophylaxis. The EFNS (Evers 2009) and the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society (Silberstein 2012) list topiramate among first‐line migraine prophylactics. There is a fairly substantial body of evidence from controlled trials supporting the efficacy of many of the agents used for preventing migraine, yet such therapies are used by only a small percentage of patients with migraine — 3% to 12% in various studies (Clarke 1996; Edmeads 1993; Mehuys 2012). It is hoped that this review and others like it will increase awareness of migraine prophylactic treatment options and help to provide a systematic basis for making the best possible choice of such therapy in those individuals in need of it.
The present review is part of a series of reviews which, taken together, represent an update of a Cochrane review on 'Anticonvulsant drugs for migraine prophylaxis' (Chronicle 2004; Mulleners 2008; first published in 2004, and previously updated (conclusions not changed) in 2007). The old review has been split into four separate reviews for updating:
-
Topiramate for the prophylaxis of episodic migraine in adults (the present review; Linde 2013a)
-
Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults (Linde 2013b)
-
Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults (Linde 2013c)
-
Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults (Linde 2013d)
Objectives
To describe and assess the evidence from controlled trials on the efficacy and tolerability of topiramate for preventing migraine attacks in adult patients with episodic migraine.
Methods
Criteria for considering studies for this review
Types of studies
The International Headache Society (IHS) has provided a useful document setting out guidelines for the conduct of clinical trials in migraine, to which current investigators are encouraged to adhere (Tfelt‐Hansen 2012). This document was not used as the sole basis for considering studies in this review, as too many potentially informative past studies would likely have been excluded on methodological grounds. However, many of its recommendations have been used as a basis for what follows.
Included studies were required to be prospective, controlled trials of self administered topiramate taken regularly to prevent the occurrence of migraine attacks, to improve migraine‐related quality of life, or both. We included trials only if allocation to treatment groups was randomised or pseudo‐randomised (based on some non‐random process unrelated to the treatment selection or expected response). Blinding was not required. We excluded concurrent cohort comparisons and other non‐experimental designs.
Types of participants
Study participants were required to be adults (at least 16 years of age) and to meet reasonable criteria designed to distinguish migraine from tension‐type headache. If patients with both types of headache were included in a trial, results were required to be stratified by headache diagnosis. We did not require the use of a specific set of diagnostic criteria (eg, Ad Hoc Cttee 1962; IHS Cttee 1988; ICHD‐II 2004), but migraine diagnoses had to be based on at least some of the distinctive features of migraine, eg, nausea/vomiting, severe head pain, throbbing character, unilateral location, phono/photophobia, or aura. Secondary headache disorders had to be excluded using reasonable criteria.
We anticipated that some of the trials identified would include patients described as having mixed migraine and tension‐type headaches or combination headaches, and the protocol for this review described detailed procedures for dealing with such trials. In the end, no such precautions were necessary. We excluded studies evaluating treatments for chronic daily headache, chronic migraine, and transformed migraine. The reasons for this are: (a) the definition of chronic migraine is still heavily debated, and a revision of the 2004 IHS criteria for this condition has been proposed (Olesen 2006); (b) transformed migraine and chronic daily headache, although commonly used terms, are insufficiently validated diagnoses; (c) the separation of these conditions from headache due to medication overuse is not always clear in many studies; and (d) there is some evidence that suggests that chronic migraine may be more refractory to standard prophylactic treatment than episodic migraine. We explicitly excluded trials and treatment groups including only patients with tension‐type headache.
Types of interventions
Included studies were required to have at least one arm in which topiramate (without concomitant use of other migraine prophylactic treatment) was given regularly during headache‐free intervals with the aim of preventing the occurrence of migraine attacks, improving migraine‐related quality of life, or both. Acceptable comparator groups included placebo, no intervention, active drug treatment (ie, with proven efficacy, not experimental), the same drug treatment with a clinically relevant different dose, and non‐pharmacological therapies with proven efficacy in migraine. The analysis included only drugs and dosages that are commercially available.
We recorded any data reported on treatment compliance in the Characteristics of included studies table. After examination of these data, it did not seem necessary to stratify the analysis by compliance.
We anticipated that most trials would permit the use of medication for acute migraine attacks experienced during the trial period. We therefore recorded descriptions of trial rules concerning the use of acute medication in the Characteristics of included studies table whenever such information was provided. We did not otherwise model or adjust for this factor in our analysis.
Types of outcome measures
We collected and analysed trial data on headache frequency, responders (patients with ≥ 50% reduction in headache frequency), quality of life, and adverse events.
Search methods for identification of studies
Search strategies used in our earlier review (Chronicle 2004; Mulleners 2008) are detailed in Appendix 1 (last search date 31 December 2005). For the present update, trained information specialists developed detailed search strategies for each database searched (Appendix 2). The new searches overlapped the old searches by a full year to ensure complete coverage. The last search date for all updated searches was 15 January 2013.
Databases searched for this update were:
-
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012, Issue 12; years searched = 2005 to 2012);
-
MEDLINE (via OVID), 2005 to 15 January 2013;
-
MEDLINE In‐Process (via OVID), current week, 15 January 2013;
-
EMBASE (via OVID), 2005 to 15 January 2013.
Additional strategies for identifying trials included searching the reference lists of review articles and included studies, searching books related to headache, and consulting experts in the field. We attempted to identify all relevant published trials, irrespective of language. We handsearched two journals, Headache and Cephalalgia, in their entirety through January 2013.
Data collection and analysis
Selection of studies
Two of us independently screened titles and abstracts of studies identified by the literature search for eligibility. Papers that could not be excluded with certainty on the basis of information contained in the title and/or abstract were retrieved in full for screening. Disagreements were resolved through discussion. We retrieved papers passing this initial screening process, and two of us independently reviewed the full texts. Disagreements at the full‐text stage were resolved through internal discussion and, in a few cases, through correspondence with members of the editorial staff of the Cochrane Pain, Palliative and Supportive Care Review Group. We were not blinded to study investigators' names and institutions, journal of publication, or study results at any stage of the review.
The search strategy described above identified a large number of short conference and journal abstracts. The majority of these either (a) reported partial results of ongoing trials; (b) provided insufficient information on trial design or results; (c) were early reports of included studies; or (d) were reproductions of abstracts of papers published in full (for example, the journal Headache reproduces abstracts of interest to readers, and these are found by PubMed). We agreed that short abstracts of this kind would be excluded from consideration.
Data extraction and management
Two of us independently abstracted information on patients, methods, interventions, efficacy outcomes, and adverse events from the original reports onto specially designed, pre‐tested paper forms. Disagreements were again resolved through discussion.
We anticipated that trials would vary in length, that outcomes would be measured over various units of time (eg, number of attacks per two weeks versus number of attacks per four weeks), and that results would be reported for numerous different time points (eg, four‐week headache frequency at two months versus at four months). We attempted to standardise the unit of time over which headache frequency was measured at 28 days (four weeks) wherever possible. We recorded outcomes beginning four weeks after the start of treatment and continued through all later assessment periods. We made decisions about which time points to include in the final analysis once the data had been collected.
We anticipated that outcomes measured on a continuous scale (eg, headache frequency) would be reported in a variety of ways, eg, as mean pre‐treatment, post‐treatment, and/or change scores. Among change scores, we preferred the mean of within‐patient changes (from baseline to on‐treatment in a parallel‐group trial) over the change in group means because the first both results in a lower variance (taking into account the correlation between baseline and post‐treatment scores in each patient) and adjusts for imbalances in baseline headache frequencies, while the latter has only the second advantage. When neither type of change score was reported, we compared post–treatment means between groups, assuming that baseline data would be balanced due to randomisation. We anticipated that many trials would report group means, without reporting data on the variance associated with these means. In such cases, we attempted to calculate or estimate variances based on primary data, test statistics, and/or error bars in graphs.
When efficacy outcomes were reported in dichotomous form (success/failure), we required that the threshold for distinguishing between treatment success and failure be clinically significant; for example, we interpreted a ≥ 50% reduction in headache frequency as meeting this criterion. In such cases, we recorded, for each treatment arm, the number of patients included in the analysis and the number with each outcome.
The protocol for this review specified rules for dealing with outcome data reported on an ordinal scale (eg, for reduction in headache frequency: 0%, 1% to 24%, 25% to 49%, 50% to 74%, 75% to 99%, 100%) but, in fact, none of the included trials reported ordinal data for outcomes of interest.
We envisaged that the preferred methods of collecting and presenting data on quality of life would most likely be the Migraine‐Specific Questionnaire (MSQ) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36). However, other instruments and other types of outcomes related to quality of life (eg, work absenteeism) were not excluded a priori, and these data were kept under review before specifying rules for analysing outcome data in this domain.
We recorded the proportion of patients reporting adverse events for each treatment arm wherever possible. The identity and rates of specific adverse events were also recorded. We anticipated that reporting of adverse events would vary greatly across trials with regard to the terminology used, method of ascertainment, and classification of adverse events as drug‐related or not and as severe or not.
Assessment of risk of bias in included studies
We completed a 'Risk of bias' table for each study, using assessments of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). For new studies identified in the present update, two of us completed this assessment independently; for older studies, one of us performed the assessment and a second author reviewed and commented on it. Disagreements were resolved through discussion.
We also assessed the methodological quality of individual trials using the scale devised by Jadad and colleagues (Jadad 1996), operationalised as follows:
-
Was the study described as randomised? (1 = yes; 0 = no)
-
Was the method of randomisation well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate)
-
Was the study described as double‐blind? (1 = yes; 0 = no)
-
Was the method of double‐blinding well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate)
-
Was there a description of withdrawals and dropouts sufficient to determine the number of patients in each treatment group entering and completing the trial? (1 = yes; 0 = no)
Each trial thus received a score of 0 to 5 points, with higher scores indicating higher quality in the conduct or reporting of the trial. Two review authors scored the studies independently, and a consensus score was then arrived at through discussion. The consensus score is reported for each study in the Characteristics of included studies table and was not used as a weighting in statistical analyses.
Measures of treatment effect
The primary outcome considered for the efficacy analysis was headache frequency. Among headache frequency measures, we preferred number of migraine attacks to number of days with migraine. The latter measure confusingly incorporates attack duration into the measure of headache frequency. Moreover, attack duration is affected by the use of symptomatic medication, which is permitted in most trials. We also analysed headache frequency in terms of a responder rate, or the proportion of patients with a ≥ 50% reduction in headache frequency from pre‐ to post‐treatment.
As noted above (Data extraction and management), we kept patient‐reported quality of life data under review as studies were selected. For comparisons with placebo and direct dose comparisons, the data chosen for a rigorous analysis were measured by the Migraine‐Specific Questionnaire (MSQ) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36). For active treatment comparisons, we analysed data measured by the Migraine Disability Assessment (MIDAS) and the Migraine‐Specific Quality of Life Questionnaire (MSQoL). We decided not to include data measured by the less commonly used Headache Impact Test (HIT‐6), Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form (Q‐LES‐Q‐SF), and Medical Outcomes Study 12‐item Short‐Form Health Survey (SF‐12).
The analysis considered only outcome data obtained directly from the patient and not those judged by the treating physician or study personnel. Efficacy data based on contemporaneous and timed (usually daily) recording of headache symptoms were preferred to those based on global or retrospective assessments.
In addition, we tabulated adverse events for each included study.
Unit of analysis issues
In the case of cross‐over trial designs, we anticipated that the data reported would normally not permit analysis of paired within‐patient data. We therefore analysed cross‐over trials as if they were parallel‐group trials, combining data from all treatment periods. If a carry‐over effect was found and data were reported by period, then the analysis was restricted to period‐one data only. In no trial were complete within‐patient data reported, so within‐patient improvement scores were not calculated.
Dealing with missing data
Where data were missing or inadequate, we attempted to obtain these data by correspondence with study authors.
Assessment of heterogeneity
We tested estimates of efficacy (both mean differences (MDs) and odds ratios (ORs)) for homogeneity. When significant heterogeneity was present, we made an attempt to explain the differences based on the clinical characteristics of the included studies. We did not statistically combine studies that were clinically dissimilar. However, when a group of studies with statistically heterogeneous results appeared to be clinically similar, we did combine study estimates. We performed all pooled analyses using a random‐effects model.
As a sensitivity analysis, we also planned to calculate a pooled effect estimate using a fixed‐effect model for major outcomes (headache frequency, responder rate, and any adverse event) when the random‐effects result was near‐significant (0.05 ≤ P ≤ 0.15) and the pooled studies were homogeneous (heterogeneity statistics: P > 0.15/I2 < 30%). Such a sensitivity analysis would evaluate whether conclusions might differ based on the statistical model used for pooling in situations where a fixed‐effect model might reasonably be considered instead of a random‐effects model. In fact, however, no such sensitivity analyses were warranted in the present review.
Data synthesis
We anticipated that continuous outcome measures of headache frequency would be reported on different and often incompatible scales. Although we attempted to standardise the extraction of headache frequency data to a 28‐day (four‐week) period, this was not possible in every case. In our previous review (Chronicle 2004; Mulleners 2008), we therefore analysed these data using the standardised mean difference (SMD, with 95% confidence intervals (CIs)) rather than the mean difference (MD). The introduction of change scores in the newly included studies for some of the reviews in this series necessitated a change in the analysis plan from SMDs to MDs. The latter also has the advantage of giving a result in clinically meaningful units (ie, x fewer migraines per 28 days).
We used dichotomous data meeting our definition of a clinically significant threshold to calculate odds ratios (ORs), with 95% CIs. Although we prefer ORs because of their statistical properties, some readers may find it simpler to interpret the clinical significance of our findings using risk ratios (RRs); we have therefore calculated RRs where appropriate. We additionally computed numbers needed to treat (NNTs), with 95% CIs, as the reciprocal of the risk difference (RD) versus placebo (McQuay 1998).
In the same way, we used data on the proportion of patients reporting adverse events to calculate RDs and numbers needed to harm (NNHs).
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses by dose where possible. We considered further subgroup analyses by method of randomisation and by completeness of blinding, but did not undertake them because of insufficient data.
Results
Description of studies
Results of the search
The PubMed search strategy for our previous review (Chronicle 2004; Mulleners 2008) yielded 1089 potentially eligible citations, while the EMBASE and CENTRAL searches yielded 290 and 6952 citations, respectively. No additional citations were retrieved from the Cochrane Pain, Palliative & Supportive Care Trials Register or from other sources. After title and abstract screening, we obtained 58 published papers on antiepileptics for full‐text scrutiny. Of these, 16 (six included, 10 excluded) investigated topiramate.
The MEDLINE search strategy for the present update (from 2005 on) yielded 188 citations as possible candidates for the current series of reviews on antiepileptic drugs for migraine prophylaxis; the search of MEDLINE In‐Process identified an additional 20 citations. The EMBASE and CENTRAL updates identified 484 and 85 citations, respectively. Three additional study reports (all unpublished and all pertaining to gabapentin) were identified from other sources. After title and abstract screening, we obtained 37 published and three unpublished papers on antiepileptics for full‐text scrutiny. Of these, 30 (14 included, 16 excluded) investigated topiramate.
Thus, for the present update, we reviewed a total of 46 papers on topiramate at the full‐text screening stage. Of these, we included 20 papers and excluded 26.
Included studies
The 20 included papers reported data from 17 unique studies. Of these, 10 compared topiramate with placebo (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Edwards 2000; Gupta 2007; Lipton 2011; Mei 2004; Silberstein 2004; Storey 2001), three directly compared different doses of topiramate (Brandes 2004; Diener 2004; Silberstein 2004), and seven compared topiramate to another active intervention (Afshari 2012; Ashtari 2008; Diener 2004; Dodick 2009; Luo 2012; Shaygannejad 2006; Varkey 2011).
Two trials (Gupta 2007; Shaygannejad 2006) had a cross‐over design; the remaining 15 trials had a parallel‐group design.
The doses of topiramate investigated ranged from 50 to 200 mg/day. This can be compared to the range of doses used in epilepsy, which is 200 to 400 mg.
The duration of the treatment phase of the included trials varied from 4 to 52 weeks, with a mean of 19 weeks.
See the Characteristics of included studies for further details.
Excluded studies
Of the 46 papers on topiramate obtained for full‐text scrutiny, 26 were excluded for reasons given in the Characteristics of excluded studies table. The most common reasons for exclusion were: review article/meta‐analysis (three papers), conference abstract only (three papers), and chronic migraine only (two papers).
Risk of bias in included studies
We scored methodological quality using the Jadad scale as indicated in the Assessment of risk of bias in included studies section, with a maximum attainable score of 5. The median quality score was 3 (mean 3.6; range 2 to 5).
Of 102 risk of bias items scored for the 17 studies, the majority of ratings were either 'unclear' (52 (51%)) or 'low' (38 (37%)) (Figure 1; Figure 2); we judged nine studies (Afshari 2012; Edwards 2000; Gupta 2007; Lipton 2011; Luo 2012; Mei 2004; Shaygannejad 2006; Silberstein 2006; Varkey 2011) as having a 'high' risk of bias for at least one item (Figure 2).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fewer than half of the studies (Figure 2) provided an adequate methodological description of how allocation sequences were generated. Most commonly this was achieved by a computer‐generated randomisation schedule, balanced by using permuted blocks and stratification by centre (see the Characteristics of included studies table). Likewise, fewer than half of the studies (Figure 2) provided an adequate methodological description of attempts to conceal allocation of intervention assignment. One common method was to keep sealed envelopes containing preprinted medication code labels in a limited access area until subjects qualified for participation, although interactive voice response systems were also used (see the Characteristics of included studies table). A high risk of selection bias was valued for Varkey 2011 only. Given the open nature of that study, comparing topiramate to non‐pharmacological treatments, the high number of withdrawals in the topiramate arm suggests a predetermined treatment preference among randomised subjects and a 'refusal to start' using a prophylactic drug.
Blinding
Participants and clinicians were blinded during the conduct of the majority of studies, but details of the methodology were reported for only five of them (see Figure 2 and the Characteristics of included studies table). Double‐blinding was typically achieved by packaging and labelling identical appearing tablets according to the randomisation codes. We judged four studies to have a high risk of performance bias. In Afshari 2012 it is suspected that standard medications with different appearances were provided by a third party according to allocation label. In Gupta 2007, placebo was identical in appearance and packaging to the active drug, but the lamotrigine and topiramate tablets were different in appearance, and therefore two different placebos were used. For effective blinding a double‐dummy design should have been used. Luo 2012 had an open‐label design. As Varkey 2011 compared pharmacological and non‐pharmacological treatments, blindness to treatment was not possible to achieve. Remarkably, that was the only paper clearly stating that the analyst was effectively blinded. The risk of detection bias in most studies (16 of 17; Figure 2) is unclear.
Incomplete outcome data
Completeness of data was adequately reported for nine of the 17 studies (Figure 2). Usually an intention‐to‐treat (ITT) analysis was applied (see the Characteristics of included studies table). The review authors were particularly concerned over incomplete outcome data in Luo 2012 and Mei 2004, which considered complete cases only. Furthermore, Mei 2004 presented safety data for a vaguely defined subgroup of participants only.
Selective reporting
We judged the risk of reporting bias as low in eight and unclear in five of the 17 studies (Figure 2). A major obstacle to meta‐analysis was the lack of variance measures, as in both Dodick 2009 and Silberstein 2006 (see the Characteristics of included studies table). There were other concerns over the selective availability of data, including in: Edwards 2000, which has never been published as a full report; Lipton 2011, where ≥ 50% reduction in migraine days was investigated but only reported as "higher in the topiramate group compared with the placebo treatment group"; and Shaygannejad 2006, where only two types of adverse events were reported for the topiramate‐treated participants.
Other potential sources of bias
Statistically significant results are more likely to be published than trials affirming a null result. This tendency for negative or inconclusive results to remain unpublished is inherently problematic also in the context of this review. Also, of eight corresponding authors whom we contacted and asked to provide supplementary unpublished information, only four responded with the requested information. Although it is unlikely that the requested input would have changed the conclusions of this review, the authors' support in clarifying reporting issues would undoubtedly have increased the quality and robustness of this review.
Effects of interventions
Topiramate versus placebo
Methodological considerations
Significant statistical heterogeneity was evident across trials for the efficacy outcomes. The clinical similarity of trials was therefore examined to determine whether studies should be combined for statistical meta‐analysis. Although there was methodological variation as described above (Risk of bias in included studies), the included trials were fundamentally similar with regard to basic design, patients, and measures. Note that three trials compared different doses of topiramate with placebo (50 mg, 100 mg, and 200 mg/day in Brandes 2004 and Silberstein 2004, and 100 mg and 200 mg/day in Diener 2004). In the combined analyses, these trials have contributed data only for the dose judged by trial investigators to be most clinically advisable (100 mg/day in each case). Complete data for all three doses are considered below in separate analyses.
All doses reported below are given in terms of mg/day.
Headache frequency
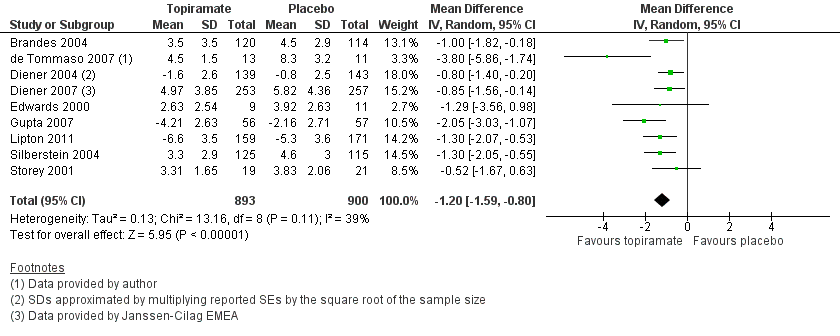
Nine trials of topiramate (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Edwards 2000; Gupta 2007; Lipton 2011; Silberstein 2004; Storey 2001; 1737 patients (one study had 56 cross‐over patients)) showed a significant reduction in headache frequency (per 28‐day period) in the active group compared to the placebo group in the combined analysis (mean difference (MD) ‐1.20; 95% confidence interval (CI) ‐1.59 to ‐0.80; Figure 3). It should be noted that two of these trials (Edwards 2000; Storey 2001) reported significant reductions in migraine frequency in the active treatment group (topiramate 200 mg) compared to placebo, using analysis of covariance to control for baseline differences in frequency; by contrast, our analysis of the post‐treatment mean headache frequencies showed no statistically significant differences (Edwards 2000: MD ‐1.29; 95% CI ‐3.56 to 0.98; Storey 2001: MD ‐0.52; 95% CI ‐1.67 to 0.63). Separate analyses of all the data on the three topiramate doses studied (Figure 4) suggest similar MDs versus placebo for 50 mg (‐0.95; 95% CI ‐1.95 to 0.04; three studies; 520 participants (one study had 56 cross‐over patients)), 100 mg (‐1.15; 95% CI ‐1.58 to ‐0.71; six studies; 1620 participants), and 200 mg doses (‐0.94; 95% CI ‐1.53 to ‐0.36; five studies; 804 participants).

Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Forest plot of comparison: 2 Topiramate (separate analyses of various doses) versus placebo, outcome: 2.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
In clinical terms, the difference in effect between topiramate and placebo observed in the combined analysis (Figure 3) corresponds to a reduction in headache frequency of a little more than one headache per 28 days. The median baseline headache frequency in the topiramate groups of the placebo‐controlled trials was 5.6 attacks per 28 days (mean 7.0; range: 4.8 to 11.6).
Responders (patients with ≥ 50% reduction in headache frequency)
In the combined analysis of nine trials (Brandes 2004; de Tommaso 2007; Diener 2004; Edwards 2000; Gupta 2007; Mei 2004; Silberstein 2004; Silberstein 2006; Storey 2001; 1190 participants (one study had 56 cross‐over patients)) topiramate demonstrated overall superiority of treatment to placebo in the proportion of responders (odds ratio (OR) 3.18; 95% CI 2.10 to 4.82; Analysis 1.2), although there was noticeable variability in the ORs across studies. Separate analysis of all the data on the various topiramate doses studied (Analysis 2.2) showed that all three (50, 100, and 200 mg) significantly increased the proportion of responders. ORs were as follows: for 50 mg, 2.35 (95% CI 1.60 to 3.44; three studies; 520 participants (one study had 56 cross‐over participants)); for 100 mg, 3.49 (95% CI 2.23 to 5.45; five studies; 852 participants); and for 200 mg, 2.49 (95% CI 1.61 to 3.87; six studies; 1025 participants).
In clinical terms, the effect observed in the combined analysis suggests that patients are twice as likely to experience a ≥ 50% reduction in frequency with topiramate as with placebo. Details are as follows:
-
The proportion of responders with topiramate was 47% (310/660; range: 26% to 63%);
-
The proportion of responders with placebo was 23% (136/586; range 0% to 34%);
-
The risk ratio (RR) for topiramate versus placebo was 2.02 (95% CI 1.57 to 2.60) (Figure 5);
-
The number needed to treat (NNT) for topiramate versus placebo was 4 (95% CI 3 to 6).

Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.3 RRs for responders (patients with ≥ 50% reduction in headache frequency).
Quality of life
Two studies (Brandes 2004; Silberstein 2004) reported data on patient‐reported quality of life as measured by the Migraine‐Specific Questionnaire (MSQ) (three domains) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36) (eight domains). In the combined analyses of topiramate 50 mg versus placebo (463 participants), a significant difference was found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐emotional function (Analysis 2.13), and SF‐36 bodily pain (Analysis 2.17), all favouring topiramate 50 mg. In the combined analyses of topiramate 100 mg versus placebo (474 participants), a significant difference was again found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐role function prevention (Analysis 2.12), and MSQ‐emotional function (Analysis 2.13), all favouring topiramate 100 mg. In the combined analyses of topiramate 200 mg versus placebo (458 participants), a significant difference was found for 4 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐role function prevention (Analysis 2.12), MSQ‐emotional function (Analysis 2.13), and SF‐36 role physical (Analysis 2.14), all favouring topiramate 200 mg.
In summary, the disease‐specific MSQ found better quality of life on topiramate than on placebo, but the generic SF‐36 was more equivocal (only 2 of 24 analyses pointed in this direction).
Direct dose comparisons
Three studies directly compared different doses of topiramate and reported data on headache frequency (Analysis 3.1) and responders (Analysis 3.2). Data from Brandes 2004 and Silberstein 2004 were used to compare all three doses of topiramate, with additional data from Diener 2004 contributing to the comparison between 200 mg and 100 mg. The 200 mg dose was significantly superior to 50 mg both in terms of reducing headache frequency (MD ‐0.96; 95% CI ‐1.53 to ‐0.40; 463 participants) and increasing the proportion of responders (OR 1.66; 95% CI 1.15 to 2.41; 462 participants). Likewise, 100 mg was superior to 50 mg (MD ‐0.71; 95% CI ‐1.32 to ‐0.10 (479 participants); and OR 1.80; 95% CI 1.25 to 2.60 (478 participants)). The 200 mg dose was not significantly superior to 100 mg for either outcome measure (756 participants).
Two studies (Brandes 2004 and Silberstein 2004) directly comparing different doses of topiramate reported data on patient‐reported quality of life as measured by the MSQ (three domains) and SF‐36 (eight domains). In the combined analyses of topiramate 50 mg versus 100 mg (479 participants), a significant difference was found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 3.3), MSQ‐role function prevention (Analysis 3.4), and MSQ‐emotional function (Analysis 3.5), all favouring the higher dose. In the combined analyses of topiramate 50 mg versus 200 mg (463 participants), a significant difference was found for only 1 of 11 domains: MSQ‐role function restrictive (Analysis 3.3), favouring the higher dose. In the combined analyses of topiramate 100 mg versus 200 mg (474 participants), a significant difference was found for only 1 of 11 domains: SF‐36 physical functioning (Analysis 3.8), favouring the lower dose.
It is important to note, however, that none of these studies was designed to have the statistical power to make comparisons between doses.
Topiramate versus active comparators
Seven trials examined topiramate versus active comparators, including:
-
amitriptyline (one study, 330 participants);
-
flunarizine (one study, 83 participants);
-
propranolol (two studies, 342 participants);
-
sodium valproate (two studies, 120 participants);
-
relaxation (one study, 61 participants).
Dodick 2009 compared topiramate to amitriptyline (both drugs titrated to maximum tolerated dose between 50 and 100 mg). Data were insufficient for us to calculate MDs for headache frequency, our preferred outcome measure. There was no significant difference between treatments in the proportion of responders (OR 0.68; 95% CI 0.44 to 1.05; 330 participants; Analysis 4.1) or in the change from baseline in Migraine Disability Assessment (MIDAS) scores (MD 2.10; 95% CI ‐2.93 to 7.13; 295 participants; Analysis 4.2).
One small, open study (Luo 2012) compared topiramate titrated to 100 mg (or lower if lack of tolerance) to flunarizine 5 mg. There was no significant difference in mean headache frequency during treatment (MD 0.30; 95% CI ‐0.37 to 0.97; 83 participants; Analysis 5.1) or in the proportion of responders (OR 0.83; 95% CI 0.34 to 2.03; 83 participants; Analysis 5.2). It should be noted that the flunarizine dose used in this study (5 mg) is in the lower range of doses used in routine clinical practice (5 to 10 mg).
In a comparison between topiramate 50 mg and propranolol 80 mg, Ashtari 2008 did not demonstrate a significant difference in mean headache frequency during treatment (MD ‐0.37; 95% CI ‐1.15 to 0.41; 60 participants; Analysis 6.1). In a larger study, Diener 2004 included an additional arm (propranolol 160 mg) in a trial of topiramate (200 mg and 100 mg) versus placebo. A comparison of propranolol with topiramate 100 mg (the dose judged by trial investigators to be most clinically advisable) showed no significant difference in the change in headache frequency from baseline (MD 0.00; 95% CI ‐0.60 to 0.60; 282 participants; Analysis 6.1) or in the proportion of responders (OR 1.32; 95% CI 0.82 to 2.13; 282 participants; Analysis 6.2). The pooled results of these two studies do not indicate a significant difference between topiramate and propranolol with regard to headache frequency (MD ‐0.14; 95% CI ‐0.61 to 0.34; 342 participants; Analysis 6.1).
Two fairly small studies compared topiramate 50 mg with sodium valproate 400 mg. Afshari 2012 did not demonstrate a significant difference in mean headache frequency during treatment (MD ‐0.60; 95% CI ‐1.57 to 0.37; 56 participants; Analysis 7.1) or in MIDAS score during the treatment phase (MD ‐3.90; 95% CI ‐8.72 to 0.92; 56 participants; Analysis 7.2). On the basis of their statistical analysis, the authors of Shaygannejad 2006 found no significant differences in efficacy between the two drugs. However, our analysis of post‐treatment mean headache frequencies demonstrated a slight but significant advantage for topiramate over valproate (MD ‐1.20; 95% CI ‐2.16 to ‐0.24; 32 (cross‐over) participants; Analysis 7.1). The pooled results of these two studies indicate a significant difference between topiramate and sodium valproate, in favour of topiramate, for this outcome (MD ‐0.90; 95% CI ‐1.58 to ‐0.22; Analysis 7.1). In clinical terms, the observed effect corresponds to a reduction in headache frequency of approximately one headache per 28 days with topiramate versus sodium valproate. The median baseline headache frequency in the topiramate groups of the two trials was 6.1 headaches per 28 days (mean 6.1; range: 5.4 to 6.8). It should be noted that the doses used in these two studies are not those used in routine clinical practice for the management of migraine.
In a small, open study, Varkey 2011 compared topiramate (200 mg or maximum tolerated dose < 200 mg) to relaxation and found no significant difference in the change in headache frequency from baseline (MD ‐0.14; 95% CI ‐0.30 to 0.02; 61 participants; Analysis 8.1) or in the proportion of responders (OR 0.88; 95% CI 0.27 to 2.81; 61 participants; Analysis 8.2). There was a significant difference in the change of quality of life (Migraine‐Specific Quality of Life Questionnaire (MSQoL), 0 to 100 points) from baseline (MD ‐1.50; 95% CI ‐2.45 to ‐0.55; 61 participants; Analysis 8.3), favouring relaxation. As discussed above (Blinding (performance bias and detection bias)), there was a high risk of selection bias in this trial.
Safety
During the process of extracting safety data, it became clear that the range of adverse events (AEs) and the method of their reporting varied very considerably from trial to trial. In the nine trials of topiramate versus placebo, seven specific adverse events were reported by at least three trials. We calculated risk differences (RDs) separately for the various doses of topiramate (50 mg, 100 mg, and 200 mg) versus placebo for any adverse event (Analysis 2.3), anorexia (Analysis 2.4), fatigue (Analysis 2.5), memory problems (Analysis 2.6), nausea (Analysis 2.7), paresthesia (Analysis 2.8), taste disturbance (Analysis 2.9), and weight loss (Analysis 2.10). We then calculated numbers needed to harm (NNHs) and 95% CIs where appropriate, and these are reported in Table 1. Except for taste disturbance (Analysis 2.9) and weight loss (Analysis 2.10), there were no significant differences in the frequency of AEs in general or of the individual AEs between placebo and topiramate 50 mg. All AEs except nausea were significantly more common with topiramate 100 mg than with placebo, with NNHs varying from 3 to 25, and the RDs were even higher for topiramate 200 mg versus placebo, with NNHs varying from 2 to 17 (Table 1). RDs were generally smaller in Diener 2007, which is logical considering its design, with an initial open‐label phase (with a possibility to withdraw or adjust dosage due to AEs), followed by randomisation to the double‐blind, parallel‐group phase of prolonged topiramate treatment at individualised dose or placebo.
| Type of AE | 50 mg/day | 100 mg/day | 200 mg/day |
| Any AE | NNH not defined* | 11 (7 to 33) | 5 (3 to 12) |
| Anorexia | NNH not defined* | 17 (10 to 50) | 12 (8 to 20) |
| Fatigue | NNH not defined* | 25 (17 to 100) | 12 (8 to 25) |
| Memory problems | NNH not defined* | 25 (17 to 100) | 12 (9 to 17) |
| Nausea | NNH not defined* | NNH not defined* | 17 (9 to 50) |
| Paresthesia | NNH not defined* | 3 (2 to 6) | 2 (2 to 3) |
| Taste disturbance | 7 (5 to 14) | 14 (8 to 100) | 7 (5 to 11) |
| Weight loss | 25 (14 to 100) | 17 (11 to 33) | 11 (8 to 14) |
| Percentage of patients in active group withdrawing because of AEs | Afshari 2012: 5%; Ashtari 2008: 3%; Brandes 2004: 17%; Gupta 2007: 2%; Silberstein 2004: 17% | Brandes 2004: 26%; de Tommaso 2007: 8%; Diener 2004: 28%; Dodick 2009: 20%; Lipton 2011: 12%; Mei 2004: 29%; Silberstein 2004: 19% | Brandes 2004: 21%; Diener 2004: 44%; Edwards 2000: 27%; Silberstein 2004: 32%; Silberstein 2006: 15%; Storey 2001: 11%; Varkey 2011: 12% |
* The 95% CI of the difference in AE rates between treatment and placebo arms (the risk difference, RD) crosses zero.
Abbreviations: AE = adverse event; CI = confidence interval; NNH = number needed to harm
Table 1 also reports, by dose, the percentages of patients in active treatment groups who withdrew because of AEs in each trial. The mean percentage withdrawing because of AEs at 100 mg was 20%.
Discussion
Summary of main results
Placebo‐controlled trials
Meta‐analysis of the studies included in this review demonstrates clearly that topiramate is efficacious for the prophylaxis of migraine. Mean headache frequency was significantly reduced (by approximately 1.2 headaches per month) with topiramate as compared to placebo (nine studies with a median baseline headache frequency of 5.6 attacks per 28 days contributed to this analysis). Furthermore, and perhaps of greater clinical relevance (though less informative scientifically), patients were approximately twice as likely to have a ≥ 50% reduction in headache frequency with topiramate than with placebo (nine studies contributed to this analysis).
According to two large trials (Brandes 2004; Silberstein 2004), topiramate 50 mg gives rise to a significant increase in the proportion of responders (patients with ≥ 50% reduction in headache frequency), but not to a significant overall decrease in monthly headache frequency. In contrast, one small trial (Gupta 2007) found superiority over placebo of topiramate 50 mg both with regard to reduction of headache frequency and responder rate.
All included trials comparing topiramate 100 mg to placebo (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Lipton 2011; Mei 2004; Silberstein 2004) showed unambiguous statistically significant superiority of topiramate, although one study did not report data enabling a comparison of reduction of headache frequency (Mei 2004), and two studies (Diener 2007; Lipton 2011) did not report data enabling a comparison of the proportion of responders.
The four large trials comparing topiramate 200 mg to placebo were equivocal in that all showed a statistically significant superiority for topiramate with regard to the responder rate, whereas only two studies (Brandes 2004; Silberstein 2004) also showed a statistically significant difference in reduction of headache frequency, while two do not (Diener 2004; Silberstein 2006). In two additional small trials of topiramate 200 mg (Edwards 2000; Storey 2001), the analysis for headache frequency did not reveal a significant difference versus placebo in either study, although one trial reported topiramate 200 mg to be significantly more efficacious for responder rates (Edwards 2000).
Based on the combined analyses of two studies (Brandes 2004; Silberstein 2004), all three doses of topiramate significantly improved three or more domains of quality of life as compared to placebo.
Dose comparisons
The studies including more than one dose of topiramate suggest that 200 mg is no more effective than 100 mg.
Trials with active comparators
With regard to reduction of mean headache frequency and/or responder rate, the six trials using active comparators found (a) no significant difference in efficacy between topiramate and amitriptyline (Dodick 2009); (b) no significant difference in efficacy between topiramate and flunarizine; (c) no significant difference in efficacy between topiramate and propranolol (Ashtari 2008; Diener 2004); (d) no significant difference in efficacy between topiramate and relaxation (Varkey 2011); (e) a slight significant advantage of topiramate over valproate (pooled results of Afshari 2012 and Shaygannejad 2006). Furthermore, relaxation increased migraine‐specific quality of life significantly more than topiramate (Varkey 2011). However, only three of these seven studies (Diener 2007, 95%; Dodick 2009, 85%; Varkey 2011, 80%) reported adequate power; the others were likely to have been underpowered.
Safety
Topiramate does not appear to give rise to an unexpectedly high rate of adverse events when used for migraine prophylaxis, although a large percentage of patients taking topiramate report paresthesia. Withdrawals due to adverse events were somewhat higher in trials of topiramate than would generally be expected on the basis of trials of other antiepileptic drugs, particularly sodium valproate or divalproex sodium.
Overall completeness and applicability of evidence
The studies identified were sufficient to address all of the objectives of the review. Our analysis demonstrates that topiramate is efficacious for preventing attacks in adult patients with episodic migraine, and these results fit into the context of current practice. Since the comparisons with flunarizine and valproate were in all probability underpowered, the evidence from these is incomplete. The trials comparing topiramate with amitriptyline and propranolol are of relevance since both these drugs have proven efficacy in the prophylaxis of migraine. The trial comparing topiramate with relaxation is also interesting. Further well‐designed trials of topiramate against other drug categories and non‐pharmacological interventions are desirable.
Several important issues need to be taken into account in any assessment of the efficacy of a drug for migraine prophylaxis. Diagnostic criteria, baseline headache frequency, washout periods for previous medication, rules for rescue medication, and the statistical power of the comparison were handled very variably in the 17 included studies. As investigations of the efficacy of various agents become more commonplace, it seems increasingly important that scientists and clinicians are at least aware of the trial guidelines suggested by the International Headache Society (Tfelt‐Hansen 2012). Even if these guidelines cannot — for operational or scientific reasons — be adhered to in their entirety, they provide a useful consultative framework at the early stages of trial design.
Quality of the evidence
The identified body of evidence allows a robust conclusion of an overall superiority of topiramate over placebo with regard to reduction of mean headache frequency (nine trials with 1737 participants) and the proportion of responders (nine trials with 1190 participants). The separate analyses of doses should, however, be viewed with some caution. Several of the included trials were almost certainly underpowered (de Tommaso 2007; Edwards 2000; Storey 2001). The studies including more than one dose of topiramate were generally not designed to enable direct dose comparisons, and the results of the dose comparisons reported here should therefore be viewed with some caution. It should be noted that all seven trials with active comparators are potentially problematic for reasons including lack of blinding, insufficient statistical power, and possibly incomplete statistical analysis. As usual in the context of clinical trials research, there was considerable heterogeneity in both headline results and general levels of analytical and statistical sophistication. It is fair to say that we faced several difficulties in deriving adequate information from the results of the 17 included studies. First, means and standard deviations were not always fully reported for each phase of trials. In tandem with this problem, reported measures of variability — either appearing in the text, tabulated, or as error bars in graphs — were not always adequately described or labelled. Second, methods of statistical analysis were generally under‐specified, leading in some cases to a lack of clarity as to which comparisons were significant and which were not. Third, there was considerable variability in how intention‐to‐treat analyses were performed. In a few cases, this gave rise to uncertainty about the numbers of patients continuing to each phase of the trial.
Potential biases in the review process
Of 102 risk of bias items scored for the 17 studies, the majority of ratings were either 'unclear' (52 (51%)) or 'low' (38 (37%)) (Figure 1; Figure 2). As described in detail above (Risk of bias in included studies), we judged nine trials as having a 'high' risk of bias for at least one item, as follows: allocation concealment (Varkey 2011), blinding of participants and personnel (Afshari 2012; Gupta 2007; Luo 2012; Varkey 2011), incomplete outcome data (Edwards 2000; Luo 2012; Mei 2004), and/or selective reporting (Edwards 2000; Lipton 2011; Shaygannejad 2006; Silberstein 2006). A strength of this review is that the methods used for searching and study selection make it highly likely that the absolute majority of relevant trial results in the public domain were identified. There is nevertheless an obvious risk that the reports of some trials may have been classified and thus remain unobtainable.
Agreements and disagreements with other studies or reviews
The overall conclusion in this review, that topiramate is efficacious for preventing attacks in adult patients with episodic migraine, is well in line with guideline recommendations of the European Federation of Neurological Societies (EFNS) (Evers 2009) and the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society (Silberstein 2012).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Forest plot of comparison: 2 Topiramate (separate analyses of various doses) versus placebo, outcome: 2.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.3 RRs for responders (patients with ≥ 50% reduction in headache frequency).

Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 2 ORs for responders (patients with ≥ 50% reduction in headache frequency).
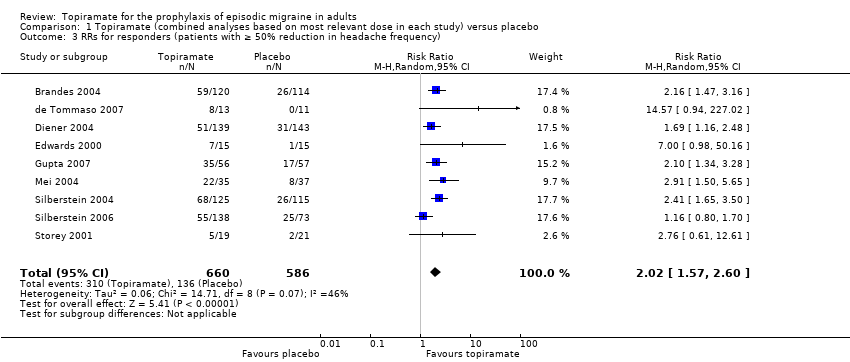
Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 3 RRs for responders (patients with ≥ 50% reduction in headache frequency).

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
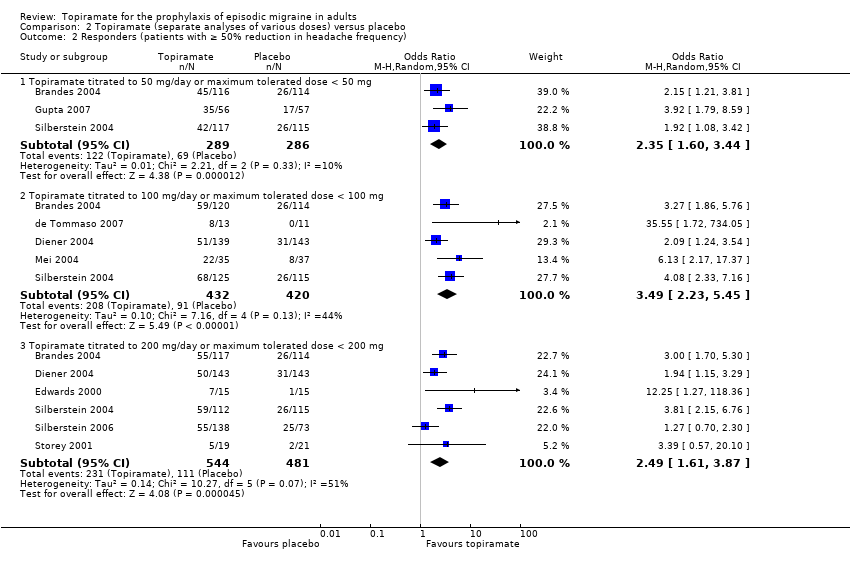
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
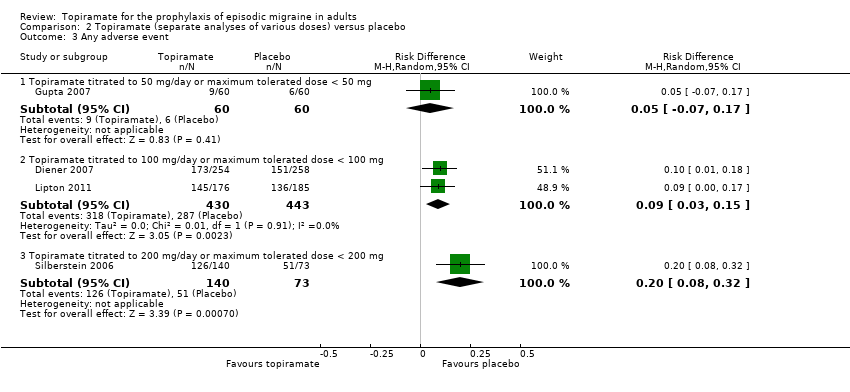
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 3 Any adverse event.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 4 Anorexia.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 5 Fatigue.
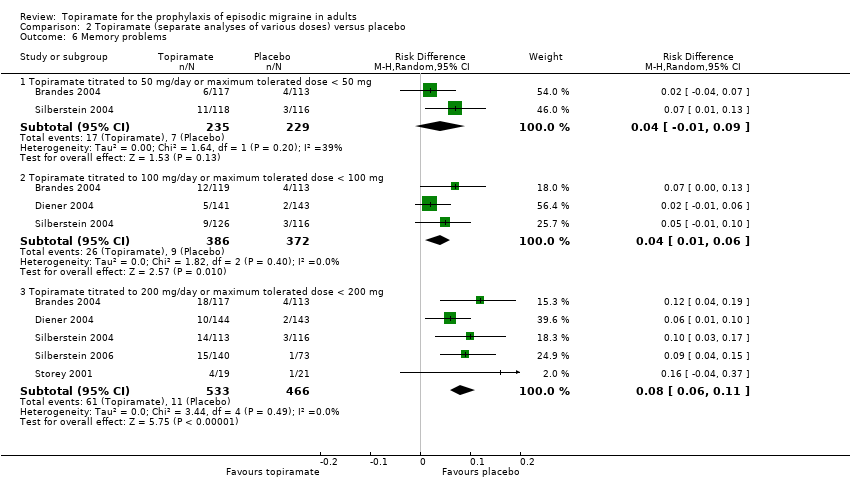
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 6 Memory problems.
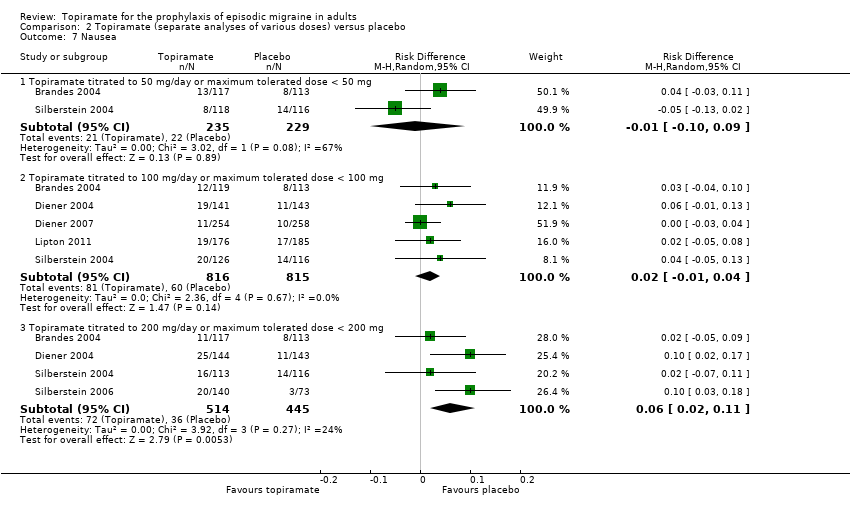
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 7 Nausea.
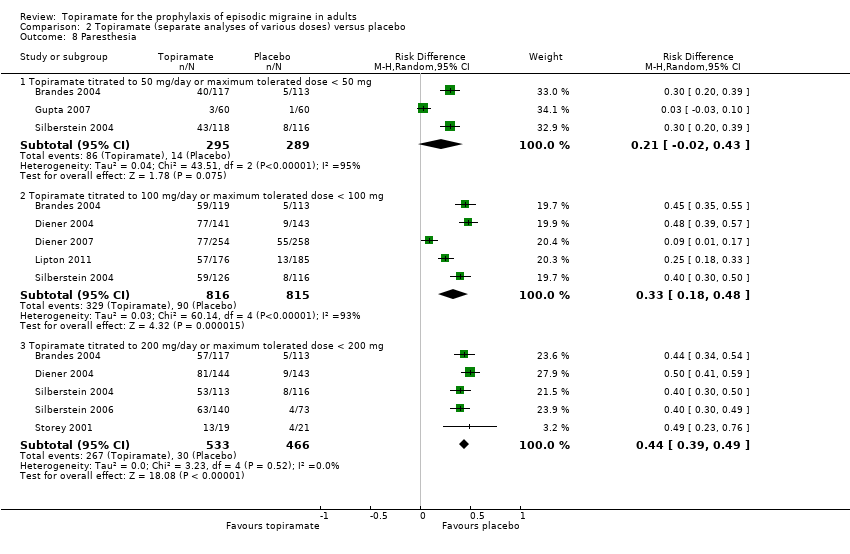
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 8 Paresthesia.
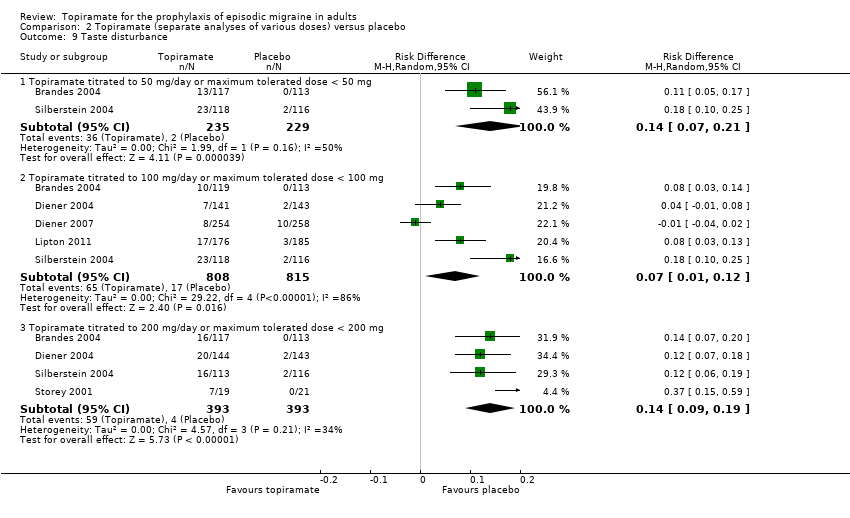
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 9 Taste disturbance.
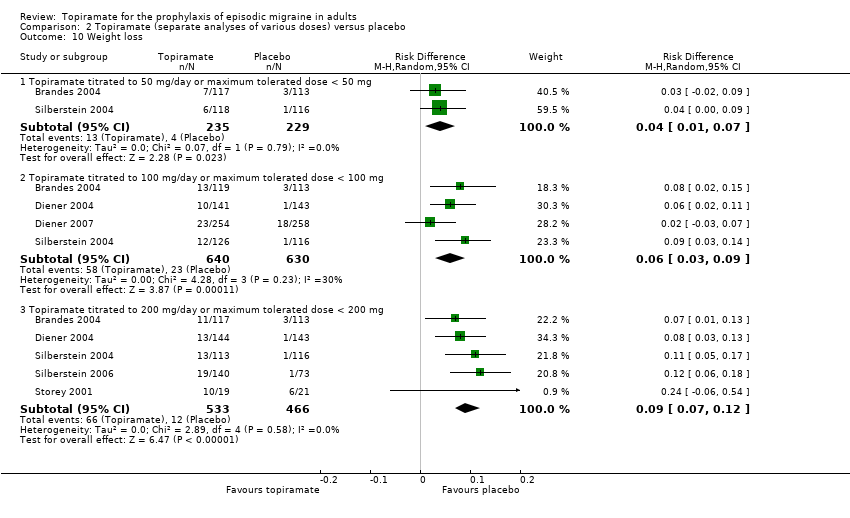
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 10 Weight loss.
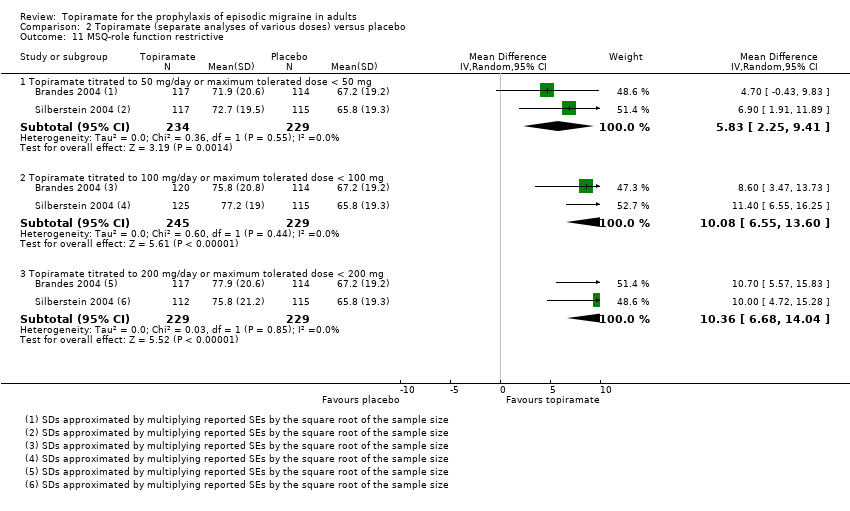
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 11 MSQ‐role function restrictive.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 12 MSQ‐role function prevention.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 13 MSQ‐emotional function.
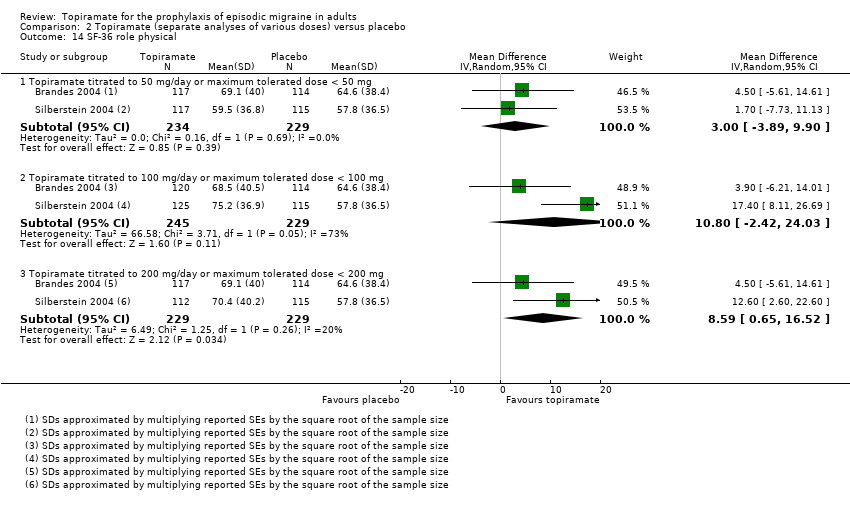
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 14 SF‐36 role physical.
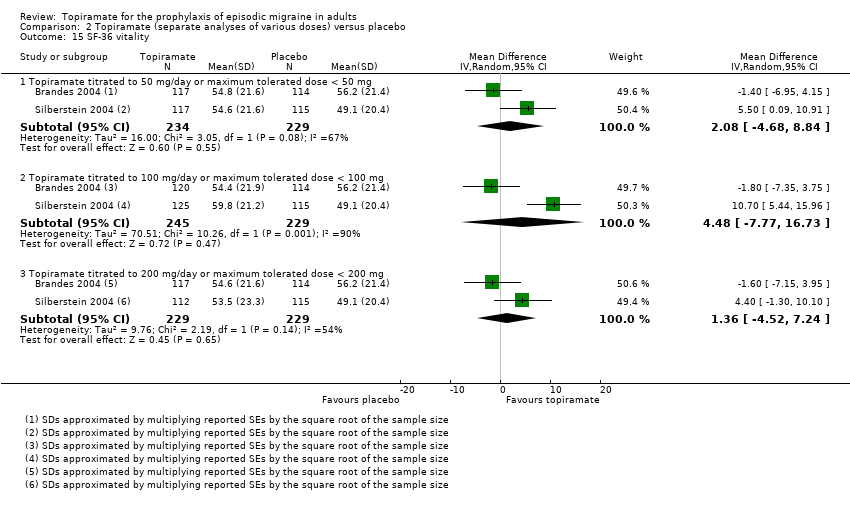
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 15 SF‐36 vitality.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 16 SF‐36 physical functioning.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 17 SF‐36 bodily pain.
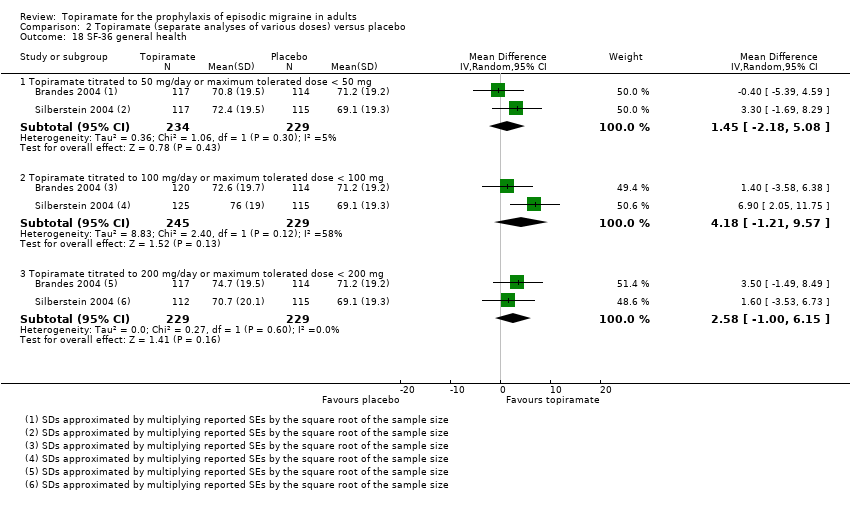
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 18 SF‐36 general health.
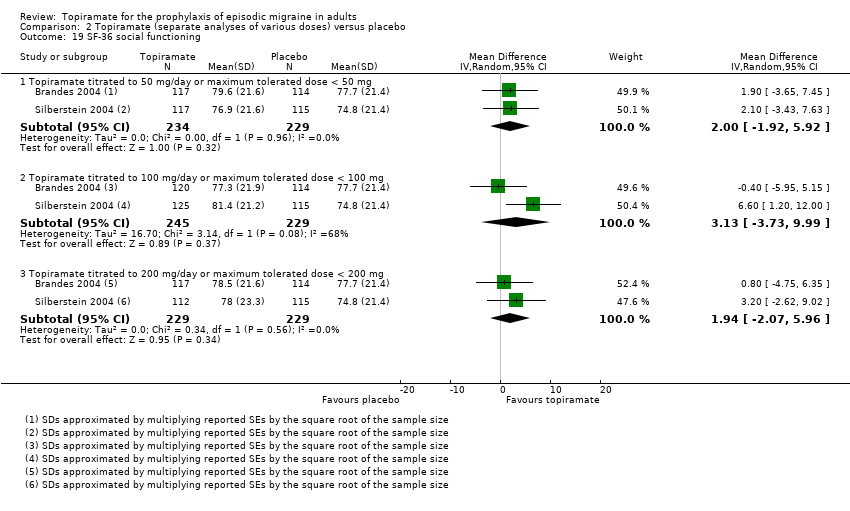
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 19 SF‐36 social functioning.
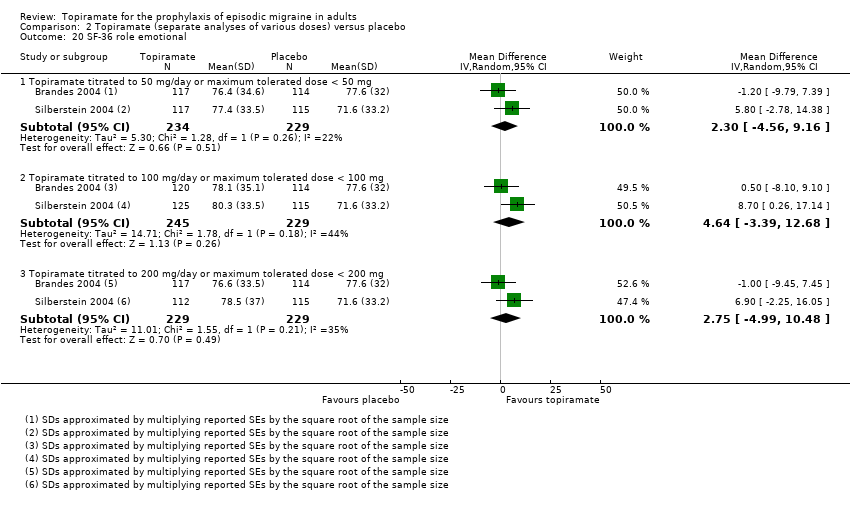
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 20 SF‐36 role emotional.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 21 SF‐36 mental health.

Comparison 3 Topiramate direct dose comparisons, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Comparison 3 Topiramate direct dose comparisons, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 3 Topiramate direct dose comparisons, Outcome 3 MSQ‐role function restrictive.

Comparison 3 Topiramate direct dose comparisons, Outcome 4 MSQ‐role function prevention.

Comparison 3 Topiramate direct dose comparisons, Outcome 5 MSQ‐emotional function.

Comparison 3 Topiramate direct dose comparisons, Outcome 6 SF‐36 role physical.

Comparison 3 Topiramate direct dose comparisons, Outcome 7 SF‐36 vitality.

Comparison 3 Topiramate direct dose comparisons, Outcome 8 SF‐36 physical functioning.
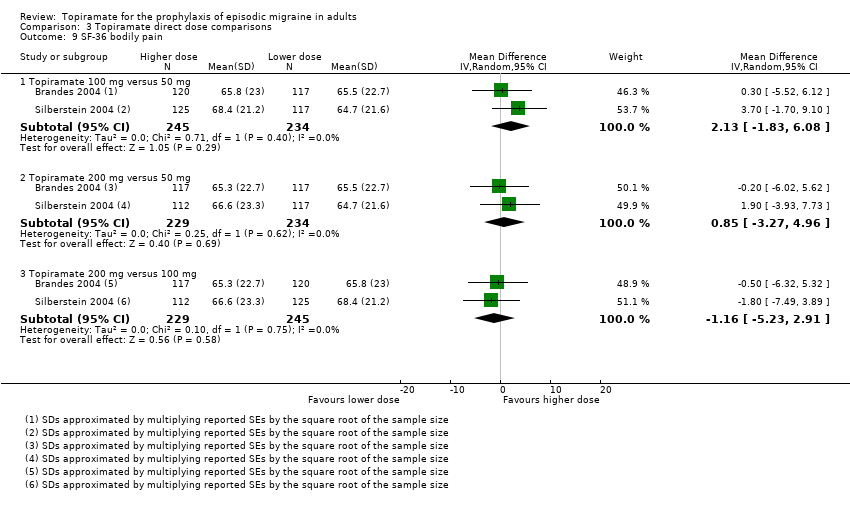
Comparison 3 Topiramate direct dose comparisons, Outcome 9 SF‐36 bodily pain.

Comparison 3 Topiramate direct dose comparisons, Outcome 10 SF‐36 general health.
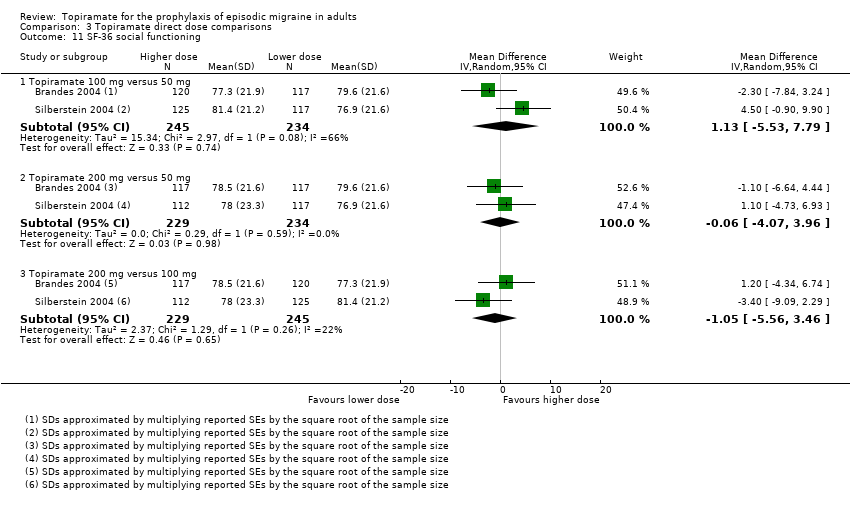
Comparison 3 Topiramate direct dose comparisons, Outcome 11 SF‐36 social functioning.

Comparison 3 Topiramate direct dose comparisons, Outcome 12 SF‐36 role emotional.

Comparison 3 Topiramate direct dose comparisons, Outcome 13 SF‐36 mental health.

Comparison 4 Topiramate versus amitriptyline, Outcome 1 Responders (patients with ≥ 50% reduction in headache frequency).
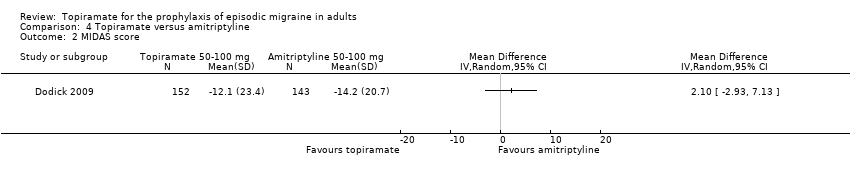
Comparison 4 Topiramate versus amitriptyline, Outcome 2 MIDAS score.
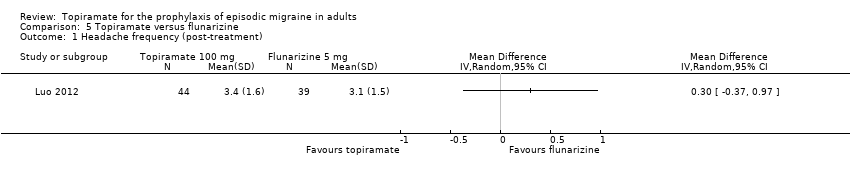
Comparison 5 Topiramate versus flunarizine, Outcome 1 Headache frequency (post‐treatment).

Comparison 5 Topiramate versus flunarizine, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 6 Topiramate versus propranolol, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
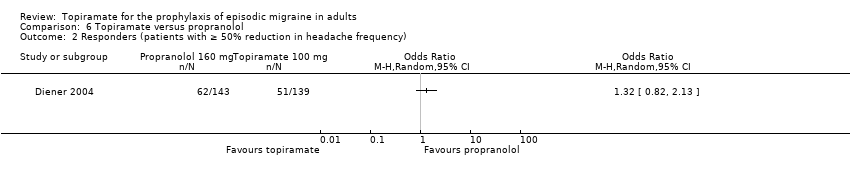
Comparison 6 Topiramate versus propranolol, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 7 Topiramate versus sodium valproate, Outcome 1 Headache frequency (post‐treatment).
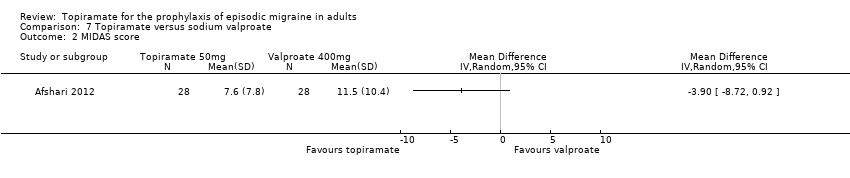
Comparison 7 Topiramate versus sodium valproate, Outcome 2 MIDAS score.

Comparison 8 Topiramate versus relaxation, Outcome 1 Headache frequency (change from baseline to post‐treatment).

Comparison 8 Topiramate versus relaxation, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
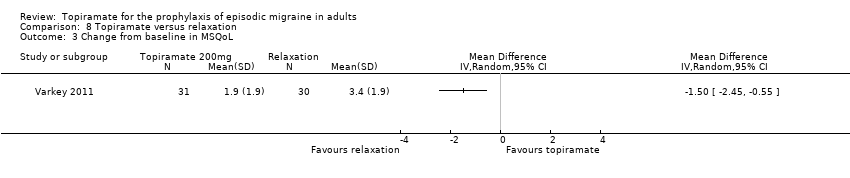
Comparison 8 Topiramate versus relaxation, Outcome 3 Change from baseline in MSQoL.
| Type of AE | 50 mg/day | 100 mg/day | 200 mg/day |
| Any AE | NNH not defined* | 11 (7 to 33) | 5 (3 to 12) |
| Anorexia | NNH not defined* | 17 (10 to 50) | 12 (8 to 20) |
| Fatigue | NNH not defined* | 25 (17 to 100) | 12 (8 to 25) |
| Memory problems | NNH not defined* | 25 (17 to 100) | 12 (9 to 17) |
| Nausea | NNH not defined* | NNH not defined* | 17 (9 to 50) |
| Paresthesia | NNH not defined* | 3 (2 to 6) | 2 (2 to 3) |
| Taste disturbance | 7 (5 to 14) | 14 (8 to 100) | 7 (5 to 11) |
| Weight loss | 25 (14 to 100) | 17 (11 to 33) | 11 (8 to 14) |
| Percentage of patients in active group withdrawing because of AEs | Afshari 2012: 5%; Ashtari 2008: 3%; Brandes 2004: 17%; Gupta 2007: 2%; Silberstein 2004: 17% | Brandes 2004: 26%; de Tommaso 2007: 8%; Diener 2004: 28%; Dodick 2009: 20%; Lipton 2011: 12%; Mei 2004: 29%; Silberstein 2004: 19% | Brandes 2004: 21%; Diener 2004: 44%; Edwards 2000: 27%; Silberstein 2004: 32%; Silberstein 2006: 15%; Storey 2001: 11%; Varkey 2011: 12% |
| * The 95% CI of the difference in AE rates between treatment and placebo arms (the risk difference, RD) crosses zero. Abbreviations: AE = adverse event; CI = confidence interval; NNH = number needed to harm | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | 1793 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.59, ‐0.80] |
| 2 ORs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [2.10, 4.82] |
| 3 RRs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.57, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 576 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.95, 0.04] |
| 1.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 6 | 1620 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.58, ‐0.71] |
| 1.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 804 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.53, ‐0.36] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.60, 3.44] |
| 2.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 852 | Odds Ratio (M‐H, Random, 95% CI) | 3.49 [2.23, 5.45] |
| 2.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 6 | 1025 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [1.61, 3.87] |
| 3 Any adverse event Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 3.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 873 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 3.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 1 | 213 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 4 Anorexia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 4.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 4.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5 Fatigue Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 5.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 5.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.04, 0.13] |
| 6 Memory problems Show forest plot | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 3 | 758 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 6.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.06, 0.11] |
| 7 Nausea Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 7.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 7.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 8 Paresthesia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [‐0.02, 0.43] |
| 8.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.18, 0.48] |
| 8.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [0.39, 0.49] |
| 9 Taste disturbance Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.21] |
| 9.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1623 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.12] |
| 9.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 786 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.09, 0.19] |
| 10 Weight loss Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 10.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 4 | 1270 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 10.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.07, 0.12] |
| 11 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.83 [2.25, 9.41] |
| 11.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.08 [6.55, 13.60] |
| 11.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 10.36 [6.68, 14.04] |
| 12 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.84 [‐0.24, 5.92] |
| 12.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.39 [3.37, 9.41] |
| 12.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.06 [1.87, 8.25] |
| 13 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.58 [0.61, 8.54] |
| 13.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.22 [6.31, 14.14] |
| 13.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.45 [4.38, 12.52] |
| 14 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐3.89, 9.90] |
| 14.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.80 [‐2.42, 24.03] |
| 14.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.59 [0.65, 16.52] |
| 15 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.08 [‐4.68, 8.84] |
| 15.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐7.77, 16.73] |
| 15.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐4.52, 7.24] |
| 16 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐3.28, 4.36] |
| 16.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐3.29, 8.86] |
| 16.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐5.27, 3.35] |
| 17 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.35 [0.04, 8.66] |
| 17.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐1.29, 14.00] |
| 17.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.12 [‐1.25, 11.49] |
| 18 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐2.18, 5.08] |
| 18.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐1.21, 9.57] |
| 18.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.00, 6.15] |
| 19 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐1.92, 5.92] |
| 19.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐3.73, 9.99] |
| 19.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐2.07, 5.96] |
| 20 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐4.56, 9.16] |
| 20.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.64 [‐3.39, 12.68] |
| 20.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐4.99, 10.48] |
| 21 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐4.59, 6.98] |
| 21.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐5.65, 10.81] |
| 21.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.57 [‐4.21, 7.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.32, ‐0.10] |
| 1.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.53, ‐0.40] |
| 1.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.55, 0.61] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate 100 mg versus 50 mg | 2 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.25, 2.60] |
| 2.2 Topiramate 200 mg versus 50 mg | 2 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.41] |
| 2.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.24] |
| 3 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 4.22 [0.65, 7.80] |
| 3.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.82, 8.28] |
| 3.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐3.37, 3.99] |
| 4 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 3.54 [0.48, 6.60] |
| 4.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐2.13, 6.69] |
| 4.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐6.67, 4.30] |
| 5 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.67, 9.58] |
| 5.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐0.21, 8.02] |
| 5.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐5.80, 2.34] |
| 6 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 7.70 [‐8.27, 23.67] |
| 6.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.51 [‐5.17, 16.19] |
| 6.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.31, 4.90] |
| 7 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐3.05, 7.92] |
| 7.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐4.64, 3.39] |
| 7.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐9.38, 3.35] |
| 8 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.02, 5.72] |
| 8.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐4.92, 2.04] |
| 8.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐7.18, ‐0.32] |
| 9 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐1.83, 6.08] |
| 9.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐3.27, 4.96] |
| 9.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐5.23, 2.91] |
| 10 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐0.76, 6.21] |
| 10.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐4.36, 6.62] |
| 10.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.85, 5.65] |
| 11 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐5.53, 7.79] |
| 11.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐4.07, 3.96] |
| 11.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.56, 3.46] |
| 12 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐3.79, 8.45] |
| 12.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐5.69, 6.94] |
| 12.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐7.92, 4.63] |
| 13 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.87, 4.49] |
| 13.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.87, 3.67] |
| 13.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 2 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.34] |
| 1.1 Topiramate 50 mg versus propranolol 80 mg | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.15, 0.41] |
| 1.2 Topiramate 100 mg versus propranolol 160 mg | 1 | 282 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.60, 0.60] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Change from baseline in MSQoL Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

