Zuclopentixol versus placebo para la esquizofrenia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: participants allocated into three arms, but randomisation not described and appears to be implied. Blinding: double. Duration: four weeks. Design: single centre, parallel, three arms. Setting: inpatient. Country: Greece. | |
| Participants | Diagnosis: schizophrenia described in hospital records of at least six years' duration. No criteria defined. N: total number randomised is 54. Sex: 29 males, 25 females. Age: ˜ 26 to 64 years, average age 42.8 years. History: the participants included were those who were hospitalised with chronic schizophrenia and whose symptoms had appeared at least six years before the study initiation. Exclusions: not described. | |
| Interventions | 1. Zuclopenthixol dihydrochloride (Clopenthixol) oral, dose 50 mg three times a day. N = 18. 2. Chlorpromazine oral, dose 100 mg three times a day. N = 18. 3. Placebo. N = 18. Each tablet was identical in appearance. | |
| Outcomes | Adverse events: extrapyramidal side effects, oculogyric crisis. Unable to use: Behavioural and inferential scales: no means or standard deviations (SD). F scores were described, but unable to convert them to mean and SD. | |
| Notes | Allocation ‐ "The patients were subsequently allocated into three groups". Assumed to imply randomisation but would suggest a high risk of bias. There were some data reported on the behavioural and inferential scales, only F scores, however in the absence of detailed reporting by the intervention groups, we were unable to use this data. Data regarding extrapyramidal side effects were reported only for the zuclopenthixol arm and implied that there were no adverse events in the other arms and this is the assumption we have made. No data on loss to follow up or efficacy reported. An attempt was made to contact the hospital where this study took place. The contact email facility on the web site was used but no response has been received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No statement that it is randomised, stated participants were allocated to three arms, randomisation implied. |
| Allocation concealment (selection bias) | High risk | Implied randomisation and no description of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | States patients and raters blinded. Medication distributed by attending physician, unclear what involvement they had. |
| Blinding of outcome assessment (detection bias) | Low risk | Raters blinded to medication. |
| Incomplete outcome data (attrition bias) | High risk | Have presented variance charts but no breakdown by groups and no description of losses in groups. |
| Selective reporting (reporting bias) | High risk | No evidence of pre‐agreed protocol before trial. Only limited data available and no clear description of objectives in the methods of the paper. |
| Other bias | Unclear risk | None identified. |
| Methods | Allocation: randomised. Blinding: double. Duration: 12 weeks. Design: single centre, three arms. Setting: inpatient. Country: United States of America. | |
| Participants | Diagnosis: people with chronic schizophrenia of at least two years since diagnosis. No Critera defined. N: total randomised = 57. Sex: 25 males, 32 females. Age: between 21 to 61years. History: describes participants with chronic schizophrenia but no diagnostic criteria or minimum duration. Exclusions: people with organic disease. | |
| Interventions | 1. Zuclopenthixol dihydrochloride (Clopenthixol) oral average dose 205 mg> N = 15 2. Chlorpromazine oral average dose 830 mg, N = 14. 3. Haloperidol average dose 12.3 mg, N = 14. 4. Placebo N = 14. | |
| Outcomes | Global state: CGI improvement (This was presented as continuous scale data so was converted to binary for inclusion in the review. A score of 'better' was considered an outcome event and scores indicating 'no change or worse' were not.), CGI severity. Adverse effects: extrapyramidal side effects, sedation, laboratory values, and weight loss and weight gain. Leaving the study early. Unable to use: (as no standard deviations available) Mental state: BPRS (no SD and unable to calculate from available data). Behaviour: Nurses' observation scale for inpatient evaluation, Oklahoma behaviour rating scale, Venables‐O'connor scale (no SD and unable to calculate from available data). | |
| Notes | An attempt was made to contact the author at the institution but the author had moved on. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomly assigned to treatment but no details on randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Does state that participants were blinded but no evidence that personnel were blind. Personnel were able to adjust the dose of medication or add in other treatments for side effects, or both, hence high risk. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No attempt to address this is made. |
| Incomplete outcome data (attrition bias) | High risk | Although four subjects left early, three from chlorpromazine arm and only one from placebo, there is no mention of any from zuclopenthixol arm. However, when describing side effects they report that one patient taking zuclopenthixol experienced persistent liver abnormalities which "resulted in withholding medication". The patient subsequently recovered. They do not describe whether this patient was included in the final assessments or not. |
| Selective reporting (reporting bias) | High risk | No evidence that a pre‐selected protocol was agreed and adhered to. Did not state in the methods what would be reported. |
| Other bias | Unclear risk | No evidence of other bias. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised. Intervention: molindone versus chlorpromazine. | |
| Allocation: randomised. Participants: people with schizophrenia. Intervention: chlorpromazine versus placebo, measuring cholesterol levels. | |
| Allocation: randomised. Participants: people with schizophrenia. Intervention: D‐fenfluramine versus placebo. | |
| Allocation: randomised. Participants: people with schizophrenia. Intervention: risperidone versus clopenthixol. | |
| Allocation: randomised. Participants: people with schizophrenia. Intervention: chlorpromazine versus placebo. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinically significant response: Improvement (CGI) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 1 Clinically significant response: Improvement (CGI) ‐ short term. | ||||
| 1.1 as rated by psychiatrist | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 1.2 as rated by nurse | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.06, 6.20] |
| 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.07] |
| Analysis 1.2 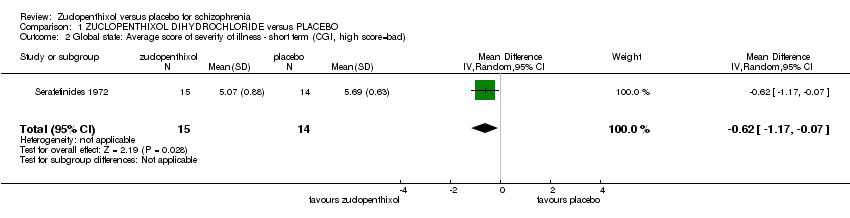 Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad). | ||||
| 3 Adverse effects: 1. Various ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 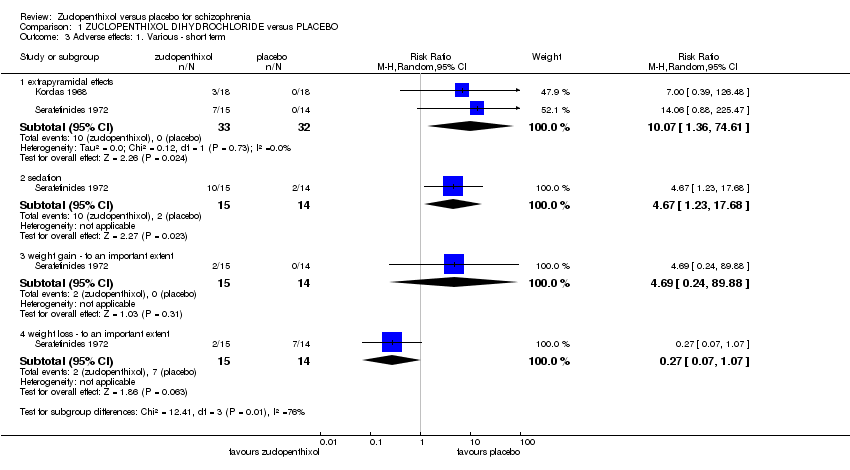 Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 3 Adverse effects: 1. Various ‐ short term. | ||||
| 3.1 extrapyramidal effects | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 10.07 [1.36, 74.61] |
| 3.2 sedation | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [1.23, 17.68] |
| 3.3 weight gain ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.24, 89.88] |
| 3.4 weight loss ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.07, 1.07] |
| 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 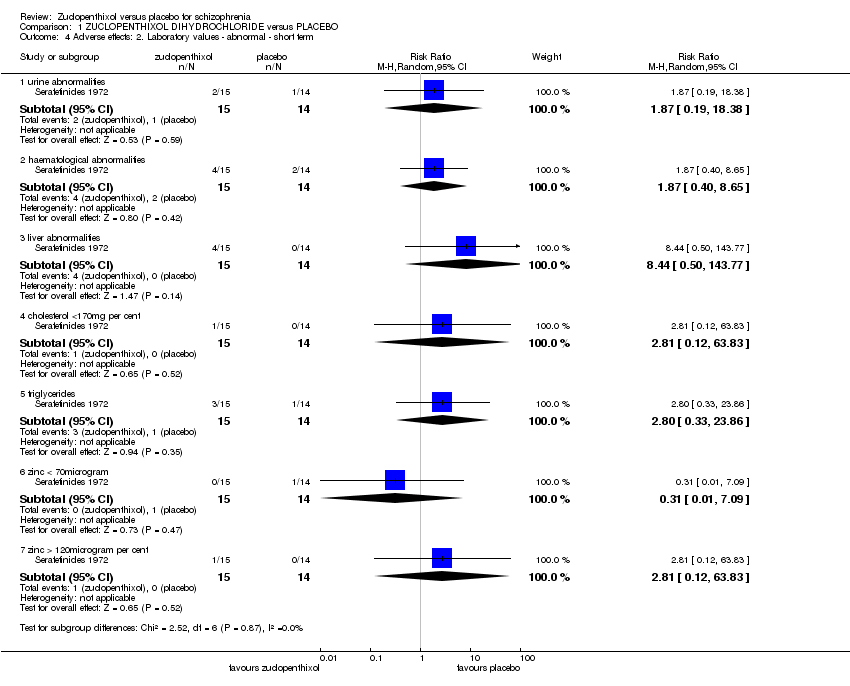 Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term. | ||||
| 4.1 urine abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.19, 18.38] |
| 4.2 haematological abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.40, 8.65] |
| 4.3 liver abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 4.4 cholesterol <170mg per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 4.5 triglycerides | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.8 [0.33, 23.86] |
| 4.6 zinc < 70microgram | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.09] |
| 4.7 zinc > 120microgram per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 5 Leaving the study early Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.06, 13.54] |
| Analysis 1.5  Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 5 Leaving the study early. | ||||

Zuclopenthixol structure
Processing search results

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
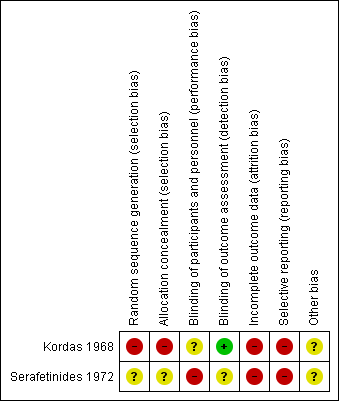
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
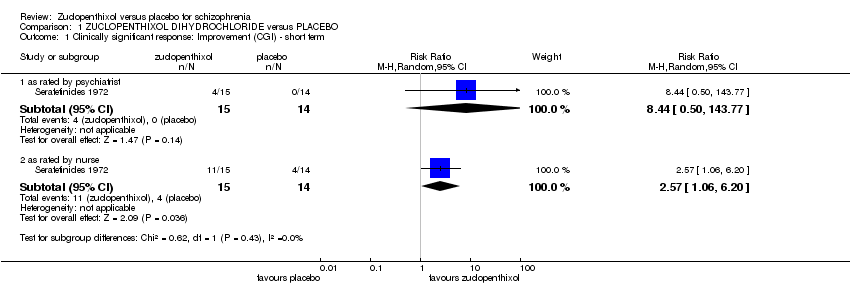
Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 1 Clinically significant response: Improvement (CGI) ‐ short term.

Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad).
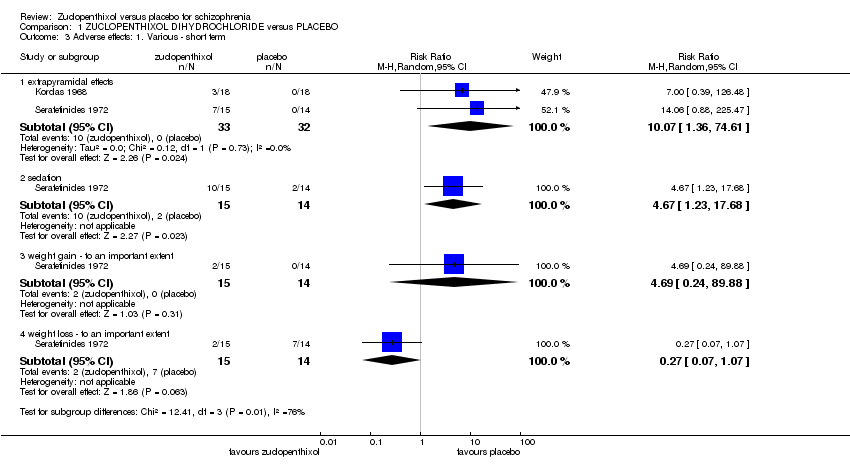
Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 3 Adverse effects: 1. Various ‐ short term.

Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term.

Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 5 Leaving the study early.
| Excluded Study | Comparison | Existing review |
| Chlorpromazine versus molindone | ||
| Chlorpromazine versus placebo | ||
| Chlorpromazine versus placebo | ||
| Clopenthixol versus risperidone | ||
| D‐fenfluramine versus placebo |
| Methods | Allocation: randomised, full description of methods of randomisation and allocation concealment. |
| Participants | Diagnosis: people with schizophrenia (according to a diagnostic criteria). |
| Interventions | 1. Zuclopenthixol dihydrochloride. N = 150. 2. Placebo. N = 150. |
| Outcomes | Global state: clinically important response to treatment, average score/change of the global state. Mental state: general measurement and specific domains (depressive symptoms, positive symptoms, negative symptoms). Leaving the study early, due to any reason, due to inefficacy of treatment, and due to adverse events. Adverse events: any serious adverse event recorded. Service use: number of hospitalisations, days in hospital. |
| Methods | Allocation: randomised, fully explicit description of methods of randomisation and allocation concealment. |
| Participants | Diagnosis: people with schizophrenia (according to a diagnostic criteria). Acutely disturbed behaviour as described by the study |
| Interventions | 1. Zuclopenthixol acetate either alone or in combination with other medications. n = 150 2. Placebo. n = 150 |
| Outcomes | Global state: clinically important response to treatment, average score/change of the global state. Mental state: general measurement and specific domains (such as depressive symptoms, positive symptoms, negative symptoms) Leaving the study early, due to any reason, due to inefficacy of treatment, and due to adverse events. Adverse events: any serious adverse event recorded. Pharmacological interactions. |
| ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO for schizophrenia | ||||||
| Patient or population: people with with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO | |||||
| Clinically significant response on global state ‐ as defined by each of the studies, Improvement (CGI) ‐ short term ‐ as rated by nurse | Study population | RR 2.57 | 29 | ⊕⊝⊝⊝ | For this SOF table outcome CGI data as rated by a nurse and a psychiatrist were both available. The nurse data were chosen as it includes both control event and | |
| 286 per 1000 | 734 per 1000 | |||||
| Relapse as defined by the studies | No studies reported these important outcomes | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies. | ||||||
| Adverse effects: Sedation | Low | RR 4.67 | 29 | ⊕⊝⊝⊝ | No use of formal rating scales.Several other adverse events were recorded but sedation considered important. | |
| 50 per 1000 | 234 per 1000 | |||||
| Moderate | ||||||
| 150 per 1000 | 701 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 1000 per 1000 | |||||
| Leaving the study early | Study population | RR 0.93 | 29 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 93 per 1000 | |||||
| Average change in quality of life/satisfaction | No studies reported these important outcomes | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ randomisation method unclear as describes "randomly assigned". Incomplete outcome data and does not accurately describe losses. Patients blinded but unclear if raters and clinicians are blinded | ||||||
| ZUCLOPENTHIXOL ACETATE versus PLACEBO for schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | ZUCLOPENTHIXOL ACETATE versus PLACEBO | ||||
| Clinically significant response on global state | No studies reported any of these important outcomes | ||||
| Relapse as defined by the studies | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies. | |||||
| Other adverse effects, general and specific | |||||
| Leaving the study early | |||||
| Average change in quality of life/satisfaction | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | |||||
| ZUCLOPENTHIXOL DECANOATE versus PLACEBO for schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | ZUCLOPENTHIXOL DECANOATE versus PLACEBO | ||||
| Clinically significant response on global state | No studies reported any of these important outcomes | ||||
| Relapse as defined by the studies | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies | |||||
| Other adverse effects, general and specific | |||||
| Leaving the study early | |||||
| Average change in quality of life/satisfaction | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | |||||
| Focus of the review | Participants | Reference |
| Zuclopenthixol acetate | acutely ill people with schizophrenia | |
| Zuclopenthixol decanoate | people with schizophrenia | |
| Zuclopenthixol dihydrochloride | people with schizophrenia |
| Individual data | Mean | Sum of mean squares | SD | |||||||||||||||
| CGI severity score clopenthixol | 3 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 5.07 | 396 | 0.88 |
| Individual data | Mean | Sum of mean squares | SD | |||||||||||||
| CGI Scores Placebo | 4 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5.69 | 426 | 0.63 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinically significant response: Improvement (CGI) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 as rated by psychiatrist | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 1.2 as rated by nurse | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.06, 6.20] |
| 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.07] |
| 3 Adverse effects: 1. Various ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 extrapyramidal effects | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 10.07 [1.36, 74.61] |
| 3.2 sedation | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [1.23, 17.68] |
| 3.3 weight gain ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.24, 89.88] |
| 3.4 weight loss ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.07, 1.07] |
| 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 urine abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.19, 18.38] |
| 4.2 haematological abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.40, 8.65] |
| 4.3 liver abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 4.4 cholesterol <170mg per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 4.5 triglycerides | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.8 [0.33, 23.86] |
| 4.6 zinc < 70microgram | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.09] |
| 4.7 zinc > 120microgram per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 5 Leaving the study early Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.06, 13.54] |

