درناژ پروفیلاکتیک شکمی برای جراحی پانکراس
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial | |
| Participants | Country: Italy Number randomized: 114 Postrandomization dropout: 0 (0%) Mean age: 56.6 years Females: 55 (48.2%) Pancreatic cancer: 56 (49.1%) Biliary cancer: 2 (1.8%) Ampullary cancer: 7 (6.1%) Chronic pancreatitis: 3 (2.6%) Other: 46 (40.4%) Pancreaticoduodenectomy: 75 (65.8%) Distal pancreatectomy: 39 (34.2%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 114) were randomly assigned to 1 of 2 groups Group 1: early drain removal (postoperative day 3; N = 57) Group 2: late drain removal (postoperative day 5 or later; N = 57) | |
| Outcomes | Pancreatic fistula, abdominal complications, pulmonary complications, reoperation, length of hospital stay, hospital readmission, postoperative mortality, morbidity, and hospital costs | |
| Notes | Two drainage tubes (Penrose drains) were placed in relation to the pancreatic and biliary anastomoses through separate skin incisions after pancreaticoduodenectomy. One drainage tube was placed in relation to the pancreatic stump through separate skin incisions after distal pancreatectomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients were randomized by a computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Masking: Open Label" in the protocol |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT00931554). All of the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
| Methods | Randomized controlled trial | |
| Participants | Country: USA Number randomized: 179 Postrandomization dropout: 0 (0%) Mean age: 65.4 years Females: 90 (50.3%) Pancreatic cancer: 142 (79.3%) Biliary cancer: 3 (1.7%) Duodenal cancer: 10 (5.6%) Ampullary cancer: 24 (13.4%) Chronic pancreatitis: 0 (0%) Pancreaticoduodenectomy: 139 (77.7%) Distal pancreatectomy: 40 (22.3%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 179) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 91) Group 2: no drainage (N = 88) | |
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, additional radiological intervention, and length of hospital stay | |
| Notes | Two drainage tubes (Jackson‐Pratt closed suction drains) were placed in relation to the pancreatic and biliary anastomoses through separate skin incisions after pancreaticoduodenectomy. One drainage tube was placed in relation to the pancreatic stump through separate skin incisions after distal pancreatectomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomized during surgery by the envelope method" Comment: no information was provided whether the envelope was opaque or not |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's prespecified outcomes in the method section were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
| Methods | Randomized controlled trial | |
| Participants | Country: China Number randomized: 160 Postrandomization dropout: 0 (0%) Mean age: 59.6 years Females: 42 (26.3%) Pancreatic cancer: 53 (33.1%) Biliary cancer: 36 (22.5%) Duodenal cancer: 28 (17.5%) Ampullary cancer: 33 (20.6%) Chronic pancreatitis: 5 (3.1%) Pancreaticoduodenectomy: 160 (100%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 160) were randomly assigned to 1 of 2 groups Group 1: active drain (N = 82) Group 2: passive drain (N = 78) | |
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiological intervention, and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we randomized our patients using a computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were prospectively assigned a code and data were recorded in a database by two nurses" Comment: no information provided whether the 2 nurses were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's prespecified outcomes in the method section were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
| Methods | Randomized controlled trial | |
| Participants | Country: USA Number randomized: 137 Postrandomization dropout: 3 (2.2%) Mean age: 63.2 years Females: 62 (45.3%) Pancreatic cancer: 67 (48.9%) Biliary cancer: not mentioned Duodenal cancer: not mentioned Ampullary cancer: 17 (12.4%) Chronic pancreatitis: 15 (10.9%) Pancreaticoduodenectomy: 137 (100%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 137) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 68) Group 2: no drainage (N = 69) | |
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiologic intervention, and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computerized randomization system at the coordinating center" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Masking: open label" |
| Incomplete outcome data (attrition bias) | High risk | Quote: "There were 3 cases for which the randomization group assignment was inadvertently not followed" |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01441492). All of the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
| Methods | Randomized controlled trial | |
| Participants | Country: USA and Canada Number randomized: 399 Postrandomization dropout: 55 (13.8%) Mean age: 61.0 years Females: 205 (60.0%) Pancreatic cancer: 171 (49.7%) Biliary cancer: not mentioned Duodenal cancer: not mentioned Ampullary cancer: not mentioned Chronic pancreatitis: 33 (9.6%) Pancreaticoduodenectomy: 0 (0%) Distal pancreatectomy: 344 (100%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 399) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 202) Group 2: no drainage (N = 197) | |
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiologic intervention, length of hospital stay, and quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized preoperatively to DP with or without intraperitoneal drain placement using a computerized randomization system at the coordinating center" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Masking: open label" |
| Incomplete outcome data (attrition bias) | High risk | Quote: "There were 55 patients who were excluded from the study after randomization and were not followed. Four patients, 2 in each group, were lost to follow‐up and excluded from the analysis". |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01441492). All of the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
| Methods | Randomized controlled trial | |
| Participants | Country: Germany Number randomized: 438 Postrandomization dropout: 43 (2.2%) Mean age: 63.4 years Females: 139 (35.2%) Pancreatic cancer: 159 (40.3%) Biliary cancer: 23 (5.8%) Duodenal cancer: 5 (1.3%) Ampullary cancer: 19 (4.8%) Chronic pancreatitis: 101 (25.6%) Pancreaticoduodenectomy: 328 (83.0%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 67 (17.0%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants (N = 395) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 202) Group 2: no drainage (N = 193) | |
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, operation time, and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random list was created by GWT‐TUD Ltd" |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation sequence was implemented by the use of sequentially numbered opaque envelopes" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) | High risk | Quote: "A total of 438 patients were randomized. Forty‐three patients (9.8%) were excluded because no pancreatic resection with consecutive pancreaticojejunal anastomosis was performed. Thus, the intention‐to‐treat population consisted of 395 patients". Comment: there are 43 postrandomization dropouts. The study did not perform an intention‐to‐treat analysis which included the 43 dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (ISRCTN04937707). All of the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
DP: distal pancreatectomy; IU: international unit; N: number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A non‐randomized study | |
| A non‐randomized study | |
| A non‐randomized study | |
| A non‐randomized study | |
| A non‐randomized study | |
| A non‐randomized study | |
| Case series | |
| A non‐randomized study | |
| A non‐randomized study | |
| Randomized controlled trial about pancreatic duct drainage | |
| A non‐randomized study | |
| A non‐randomized study | |
| A non‐randomized study |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | DRAPA Trial ‐ Closed‐suction drains versus closed gravity drains in pancreatic surgery: study protocol for a randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | Country: Czech Republic
Exclusion criteria:
|
| Interventions | Participants are randomly assigned to 1 or 2 groups Group 1: closed suction drain (active drain) Group 2: closed gravity drain (passive drain) |
| Outcomes | Primary outcome: rate of postoperative pancreatic fistula Secondary outcomes: postoperative morbidity, including wound infection, intra‐abdominal collections, delayed gastric emptying, postoperative hemorrhage, pneumonia, abdominal rupture, cardiac events, and neurological complications |
| Starting date | October 2013 |
| Contact information | Principal investigator: Filip Čečka, Department of Surgery, University Hospital Hradec Kralove, Hradec Kralove, Czech Republic, 50005 Tel: +42049583 ext 4272 Email: [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (30 days) Show forest plot | 3 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.99] |
| Analysis 1.1 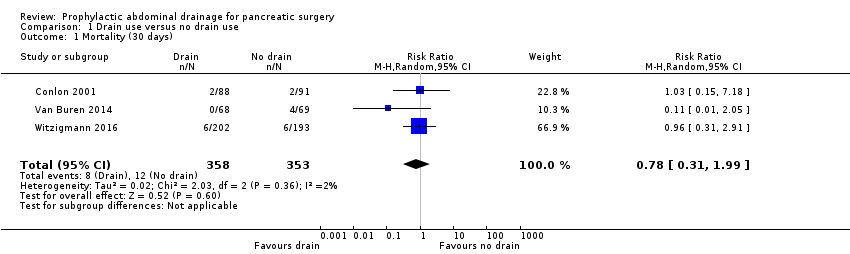 Comparison 1 Drain use versus no drain use, Outcome 1 Mortality (30 days). | ||||
| 2 Mortality (90 days) Show forest plot | 2 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.90] |
| Analysis 1.2  Comparison 1 Drain use versus no drain use, Outcome 2 Mortality (90 days). | ||||
| 3 Intra‐abdominal infection Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.80] |
| Analysis 1.3 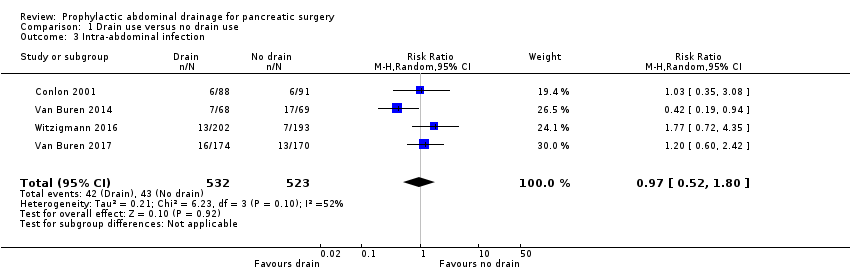 Comparison 1 Drain use versus no drain use, Outcome 3 Intra‐abdominal infection. | ||||
| 4 Wound infection Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| Analysis 1.4 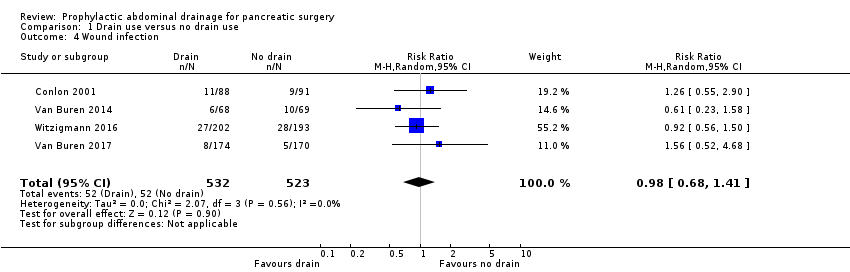 Comparison 1 Drain use versus no drain use, Outcome 4 Wound infection. | ||||
| 5 Morbidity Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| Analysis 1.5 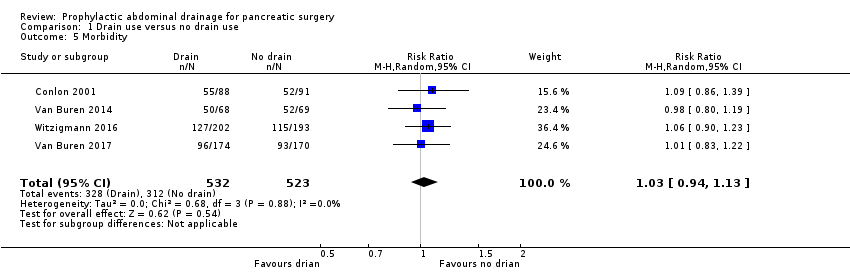 Comparison 1 Drain use versus no drain use, Outcome 5 Morbidity. | ||||
| 6 Length of hospital stay (days) Show forest plot | 3 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.60, 0.29] |
| Analysis 1.6 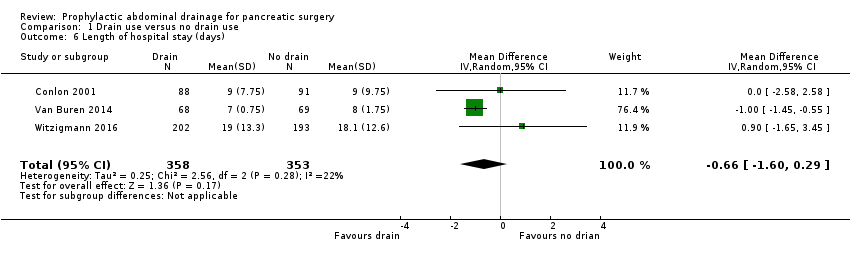 Comparison 1 Drain use versus no drain use, Outcome 6 Length of hospital stay (days). | ||||
| 7 Additional open procedures for postoperative complications Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.79, 2.23] |
| Analysis 1.7 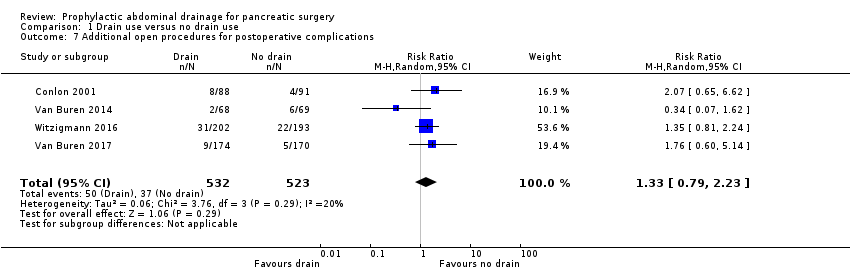 Comparison 1 Drain use versus no drain use, Outcome 7 Additional open procedures for postoperative complications. | ||||
| 8 Additional radiological interventions for postoperative complications Show forest plot | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.87] |
| Analysis 1.8  Comparison 1 Drain use versus no drain use, Outcome 8 Additional radiological interventions for postoperative complications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (30 days) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.1 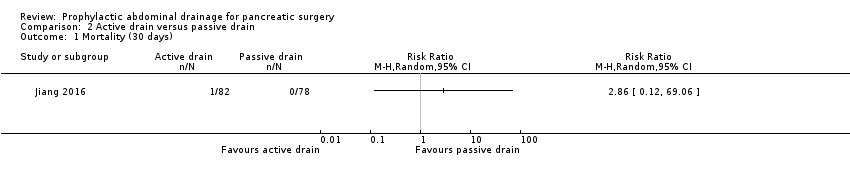 Comparison 2 Active drain versus passive drain, Outcome 1 Mortality (30 days). | ||||
| 2 Intra‐abdominal infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Active drain versus passive drain, Outcome 2 Intra‐abdominal infection. | ||||
| 3 Wound infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Active drain versus passive drain, Outcome 3 Wound infection. | ||||
| 4 Morbidity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Active drain versus passive drain, Outcome 4 Morbidity. | ||||
| 5 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Active drain versus passive drain, Outcome 5 Length of hospital stay (days). | ||||
| 6 Additional open procedures for postoperative complications Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Active drain versus passive drain, Outcome 6 Additional open procedures for postoperative complications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Morbidity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Early versus late drain removal, Outcome 1 Morbidity. | ||||
| 2 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Early versus late drain removal, Outcome 2 Length of hospital stay (days). | ||||
| 3 Hospital costs (EUR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3 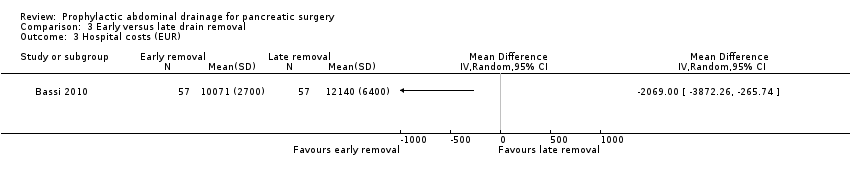 Comparison 3 Early versus late drain removal, Outcome 3 Hospital costs (EUR). | ||||
| 4 Additional open procedures for postoperative complications Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Early versus late drain removal, Outcome 4 Additional open procedures for postoperative complications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (90 days) ‐ worst‐case scenario Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.05, 89.11] |
| Analysis 4.1 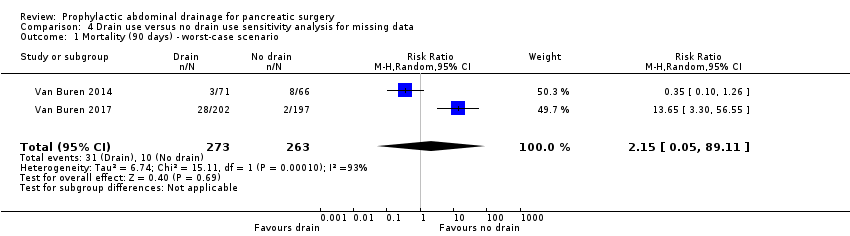 Comparison 4 Drain use versus no drain use sensitivity analysis for missing data, Outcome 1 Mortality (90 days) ‐ worst‐case scenario. | ||||
| 2 Mortality (90 days) ‐ best‐case scenario Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.01] |
| Analysis 4.2  Comparison 4 Drain use versus no drain use sensitivity analysis for missing data, Outcome 2 Mortality (90 days) ‐ best‐case scenario. | ||||
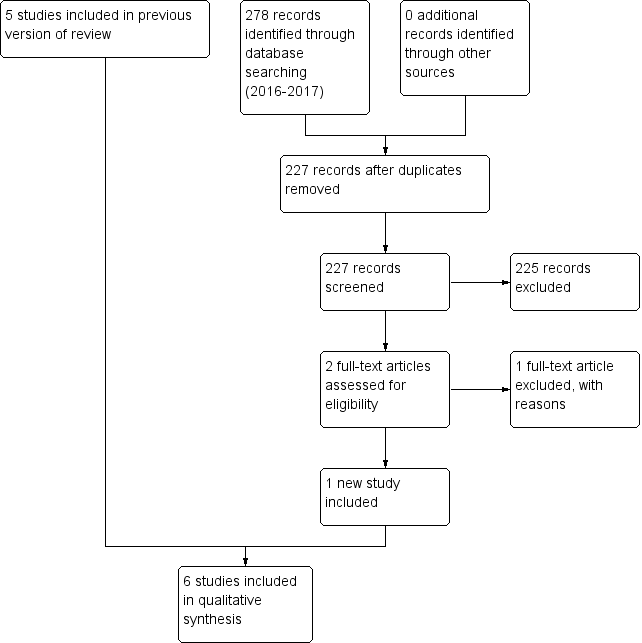
Study flow diagram: 2018 review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Drain use versus no drain use, Outcome 1 Mortality (30 days).

Comparison 1 Drain use versus no drain use, Outcome 2 Mortality (90 days).
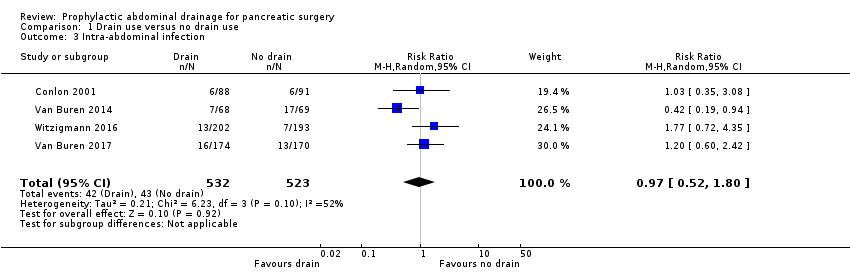
Comparison 1 Drain use versus no drain use, Outcome 3 Intra‐abdominal infection.
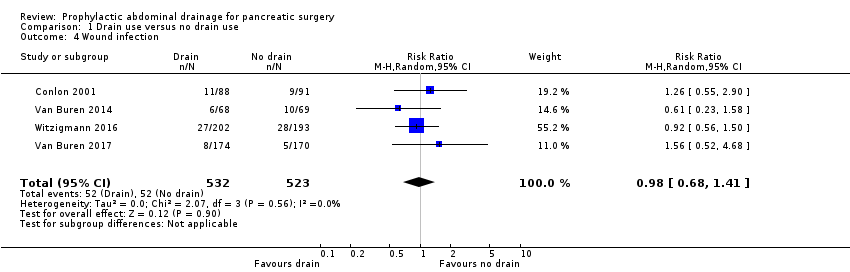
Comparison 1 Drain use versus no drain use, Outcome 4 Wound infection.
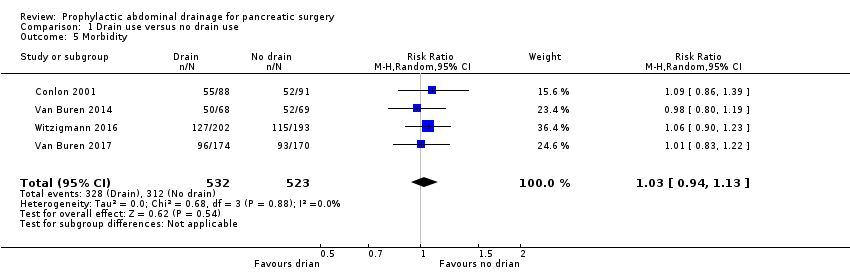
Comparison 1 Drain use versus no drain use, Outcome 5 Morbidity.

Comparison 1 Drain use versus no drain use, Outcome 6 Length of hospital stay (days).
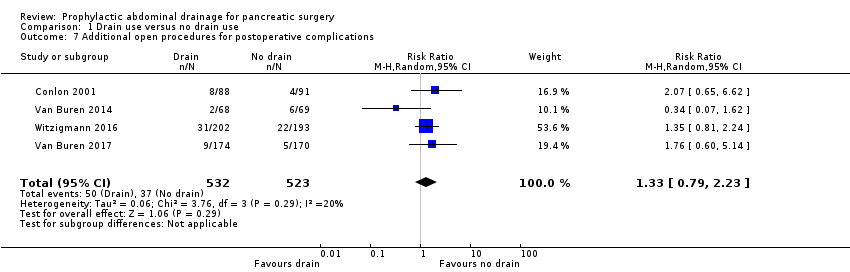
Comparison 1 Drain use versus no drain use, Outcome 7 Additional open procedures for postoperative complications.

Comparison 1 Drain use versus no drain use, Outcome 8 Additional radiological interventions for postoperative complications.
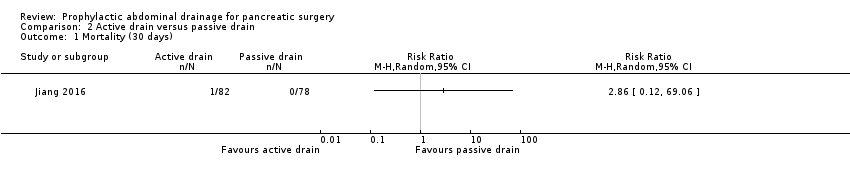
Comparison 2 Active drain versus passive drain, Outcome 1 Mortality (30 days).
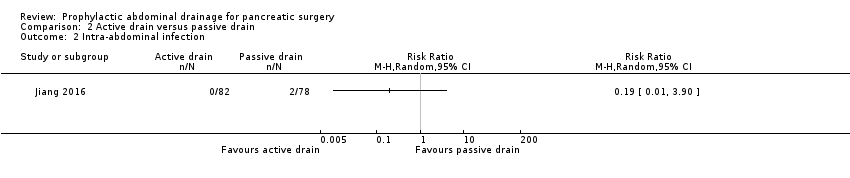
Comparison 2 Active drain versus passive drain, Outcome 2 Intra‐abdominal infection.

Comparison 2 Active drain versus passive drain, Outcome 3 Wound infection.

Comparison 2 Active drain versus passive drain, Outcome 4 Morbidity.
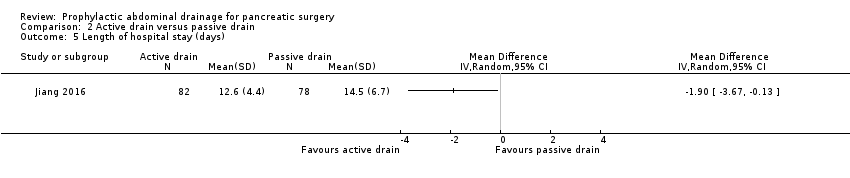
Comparison 2 Active drain versus passive drain, Outcome 5 Length of hospital stay (days).
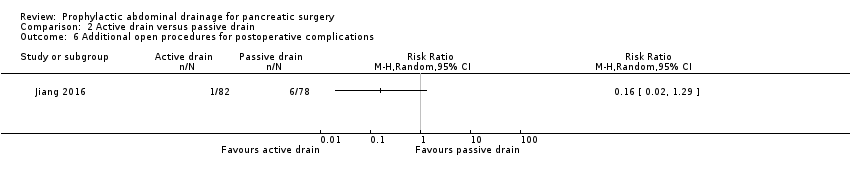
Comparison 2 Active drain versus passive drain, Outcome 6 Additional open procedures for postoperative complications.
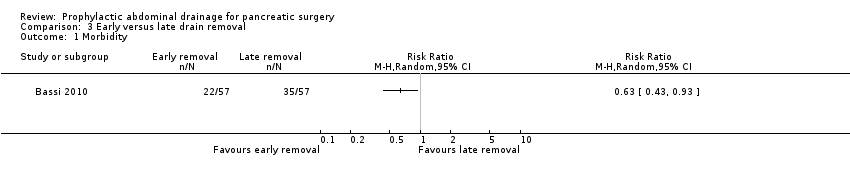
Comparison 3 Early versus late drain removal, Outcome 1 Morbidity.
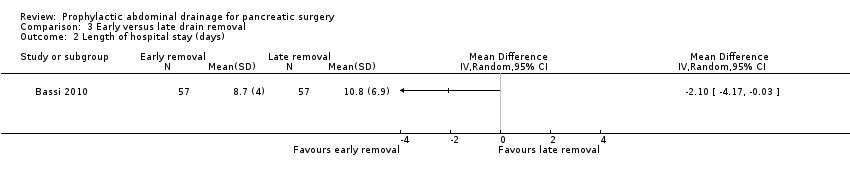
Comparison 3 Early versus late drain removal, Outcome 2 Length of hospital stay (days).

Comparison 3 Early versus late drain removal, Outcome 3 Hospital costs (EUR).

Comparison 3 Early versus late drain removal, Outcome 4 Additional open procedures for postoperative complications.

Comparison 4 Drain use versus no drain use sensitivity analysis for missing data, Outcome 1 Mortality (90 days) ‐ worst‐case scenario.
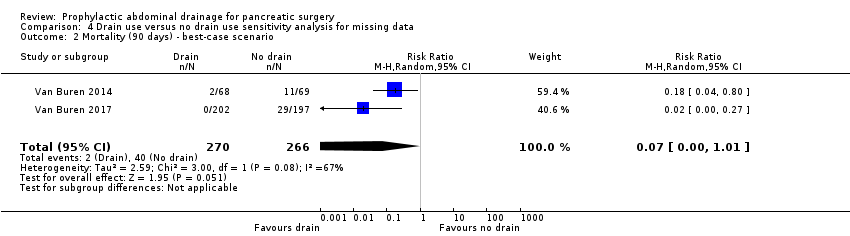
Comparison 4 Drain use versus no drain use sensitivity analysis for missing data, Outcome 2 Mortality (90 days) ‐ best‐case scenario.
| Drain use versus no drain use for pancreatic surgery | ||||||
| Patient or population: people undergoing elective open pancreatic resections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with no drain use | Risk with drain use | |||||
| Mortality Follow‐up: 30 days | 23 per 1000 | 18 per 1000 (7 to 46) | RR 0.78 | 1055 | ⊕⊕⊕⊝ | |
| Mortality Follow‐up: 90 days | 42 per 1000 | 10 per 1000 (3 to 38) | RR 0.23 | 478 | ⊕⊕⊕⊝ | |
| Intra‐abdominal infection Follow‐up: 30 days | 82 per 1000 | 80 per 1000 (43 to 148) | RR 0.97 | 1055 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 99 per 1000 | 97 per 1000 (68 to 140) | RR 0.98 | 1055 | ⊕⊕⊝⊝ | |
| Drain‐related complications Follow‐up: 30 days | See comment | See comment | Not estimable | 179 | ⊕⊕⊝⊝ | There was 1 drain‐related complication in the drainage group. The drainage tube was broken. |
| Morbidity Follow‐up: 30 days | 597 per 1000 | 614 per 1000 (561 to 674) | RR 1.03 | 1055 | ⊕⊕⊕⊝ | |
| Length of hospital stay Follow‐up: 30 days | The mean length of hospital stay in the no drain groups was 13.8 days | The mean length of hospital stay in the drain groups was | MD ‐0.66 (‐1.60 to 0.29) | 711 | ⊕⊕⊕⊝ | |
| Hospital costs Follow‐up: 30 days | Not reported | |||||
| Additional open procedures for postoperative complications Follow‐up: 30 days | 71 per 1000 | 94 per 1000 (56 to 158) | RR 1.33 | 1055 | ⊕⊕⊝⊝ | |
| Additional radiological interventions for postoperative complications Follow‐up: 30 days | 121 per 1000 | 105 per 1000 (48 to 227) | RR 0.87 | 660 | ⊕⊝⊝⊝ | |
| Pain Follow‐up: 30 days | Not reported | |||||
| Quality of life Follow‐up: 30 days FACT‐PA questionnaire: scale 0 to 144, where higher values indicate better quality of life | The mean quality of life score in the no drain group was 104 points | The mean quality of life score in the drain groups was | Not estimable (see comment) | 399 | ⊕⊕⊝⊝ | The study reported the mean quality of life score, without mentioning the standard deviation. |
| * The basis for the assumed risk was the control group proportion in the study. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious imprecision (very few events, confidence interval of risk ratio overlapped 0.75 and 1.25). | ||||||
| Active drain versus passive drain for pancreatic surgery | ||||||
| Patient or population: people undergoing elective open pancreatic resections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with passive drain | Risk with active drain | |||||
| Mortality Follow‐up: 30 days | 1 per 1000 | 3 per 1000 (0 to 69) | RR 2.86 | 160 | ⊕⊕⊝⊝ | There were no events in the control arm. A risk of "1 per 1000" was chosen for illustration. RR was calculated using a correction factor and should be interpreted with caution. |
| Mortality Follow‐up: 90 days | Not reported | |||||
| Intra‐abdominal infection Follow‐up: 30 days | 26 per 1000 | 5 per 1000 (0 to 100) | RR 0.19 | 160 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 90 per 1000 | 61 per 1000 (21 to 184) | RR 0.68 | 160 | ⊕⊝⊝⊝ | |
| Drain‐related complications Follow‐up: 30 days | Not reported | |||||
| Morbidity Follow‐up: 30 days | 321 per 1000 | 218 per 1000 (131 to 369) | RR 0.68 | 160 | ⊕⊕⊝⊝ | |
| Length of hospital stay Follow‐up: 30 days | The mean length of hospital stay in the passive drain group was 14.5 days | The mean length of hospital stay in the active drain group was | MD ‐1.90 (‐3.67 to ‐0.13) | 160 | ⊕⊕⊝⊝ | |
| Hospital costs Follow‐up: 30 days | Not reported | |||||
| Additional open procedures for postoperative complications Follow‐up: 30 days | 77 per 1000 | 12 per 1000 (2 to 99) | RR 0.16 | 160 | ⊕⊝⊝⊝ | |
| Additional radiological interventions for postoperative complications Follow‐up: 30 days | Not reported | |||||
| Pain Follow‐up: 30 days | Not reported | |||||
| Quality of life Follow‐up: 30 days | Not reported | |||||
| * The basis for the assumed risk was the control group proportion in the study. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for very serious imprecision (small sample sizes, very few events, confidence intervals of risk ratios overlapped 0.75 and 1.25). | ||||||
| Early versus late drain removal for pancreatic surgery | ||||||
| Patient or population: people undergoing elective open pancreatic resections Setting: hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with late drain removal | Risk with early drain removal | |||||
| Mortality Follow‐up: 30 days | There was no mortality in either group. | 114 | ⊕⊕⊕⊝ | |||
| Mortality Follow‐up: 90 days | Not reported | |||||
| Intra‐abdominal infection Follow‐up: 30 days | Not reported | |||||
| Wound infection Follow‐up: 30 days | Not reported | |||||
| Drain‐related complications Follow‐up: 30 days | Not reported | |||||
| Morbidity Follow‐up: 30 days | 614 per 1000 | 387 per 1000 (264 to 571) | RR 0.63 | 114 | ⊕⊕⊝⊝ | |
| Length of hospital stay Follow‐up: 30 days | The mean length of hospital stay in the late removal group was 10.8 days | The mean length of hospital stay in the early removal group was | MD ‐2.10 (‐4.17 to ‐0.03) | 114 | ⊕⊕⊝⊝ | |
| Hospital costs Follow‐up: 30 days | The mean hospital costs in the late removal group was EUR 12140.00 | The mean hospital costs in the early removal group was EUR 2069 lower | MD ‐2069.00 (‐3872.26 to ‐265.74) | 114 | ⊕⊕⊝⊝ | |
| Additional open procedures for postoperative complications Follow‐up: 30 days | 18 per 1000 | 6 per 1000 | RR 0.33 | 114 | ⊕⊝⊝⊝ | |
| Additional radiological interventions for postoperative complications Follow‐up: 30 days | Not reported | |||||
| Pain Follow‐up: 30 days | Not reported | |||||
| Quality of life Follow‐up: 30 days | Not reported | |||||
| * The basis for the assumed risk was the control group proportion in the study. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Publication bias could not be assessed because of the few number of studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (30 days) Show forest plot | 3 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.99] |
| 2 Mortality (90 days) Show forest plot | 2 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.90] |
| 3 Intra‐abdominal infection Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.80] |
| 4 Wound infection Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 5 Morbidity Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 6 Length of hospital stay (days) Show forest plot | 3 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.60, 0.29] |
| 7 Additional open procedures for postoperative complications Show forest plot | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.79, 2.23] |
| 8 Additional radiological interventions for postoperative complications Show forest plot | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (30 days) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Intra‐abdominal infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Wound infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Morbidity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Additional open procedures for postoperative complications Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Morbidity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Hospital costs (EUR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Additional open procedures for postoperative complications Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (90 days) ‐ worst‐case scenario Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.05, 89.11] |
| 2 Mortality (90 days) ‐ best‐case scenario Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.01] |

