مدیریت محافظهکارانه در مقابل مدیریت مداخلهای برای پنوموتوراکس خودبهخودی اولیه در بزرگسالان
Appendices
Appendix 1. CENTRAL (The Cochrane Library) search strategy
#1 MeSH descriptor: [Pneumothorax] explode all trees
#2 pneumothorax
#3 #1 or #2
#4 MeSH descriptor: [Thoracotomy] explode all trees
#5 MeSH descriptor: [Thoracostomy] explode all trees
#6 MeSH descriptor: [Chest Tubes] explode all trees
#7 MeSH descriptor: [Drainage] explode all trees
#8 MeSH descriptor: [Suction] explode all trees
#9 (aspiration or thoraco?tom* or suction):ti,ab or chest tube* or (drain* near/3 (chest or intercostal or thoracic))
#10 #4 or #5 or #6 or #7 or #8 or #9
#11 #3 and #10
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Pneumothorax/ or pneumothorax.af.
2. exp Thoracotomy/ or exp Thoracostomy/ or exp Chest Tubes/ or Drainage/ or Suction/ or (aspiration or thoraco?tom* or suction).ti,ab. or chest tube*.mp. or (drain* adj3 (chest or intercostal or thoracic)).mp.
3. 1 and 2
4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp pneumothorax/ or pneumothorax.af.
2. thoracotomy/ or thorax drainage/ or chest tube/ or suction/ or (aspiration or thoraco?tom* or suction).ti,ab. or chest tube*.ti,ab. or drain* adj3 (chest or intercostal or thoracic)).ti,ab.
3. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 (MM "Pneumothorax") OR "Pneumothorax"
S2 ((MM "Thoracotomy") OR (MM "Chest Tubes") OR (MH "Drainage") OR (MH "Suction")) OR AB (aspiration or thoraco?tom* or suction) OR TI (aspiration or thoraco?tom* or suction) OR TX chest tube* OR ((drain* N3 (chest or intercostal or thoracic)))
S3 ((MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (MM "Prospective Studies+") OR (MM "Clinical Trial Registry") OR (MM "Double‐Blind Studies") OR (MM "Single‐Blind Studies") OR (MM "Triple‐Blind Studies") OR (MM "Multicenter Studies") OR (MM "Placebos")) OR (random* or placebo*)
S4 S1 AND S2 AND S3
Appendix 5. ISI Web of Science search strategy
#1 TS=pneumothorax
#2 TI=(aspiration or thoraco?tom* or suction) or TS=chest tube* or TS=(drain* SAME (chest or intercostal or thoracic))
#3 TS=(random* or ((controlled or clinical) SAME trial*) or placebo* or multicenter or prospective) or TS=((blind* or mask*) SAME (single or double or triple or treble))
#4 #3 AND #2 AND #1
Appendix 6. Screening form
| Author | Title | Journal | Date vol page | Controlled/RCT | Pneumothorax | Conservative treatment | Exclude | Get full reference |
|
|
|
|
| 2 author consensus | 2 author consensus | 2 author consensus |
|
|
Appendix 7. Data Extraction Form
CARG
Data collection form
Intervention review
Notes on using a data extraction form:
-
Be consistent in the order and style you use to describe the information for each report
-
Record any missing information as unclear or not described, to make it clear that the information was not found in the study report(s), not that you forgot to extract it.
-
Include any instructions and decision rules on the data collection form, or in an accompanying document. It is important to practice using the form and give training to any other authors using the form.
| Review title or ID |
|
|
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
|
|
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
|
| Notes:
|
1. General Information
| Date form completed(dd/mm/yyyy) |
|
| Name/ID of person extracting data |
|
| Report title (title of paper/ abstract/ report that data are extracted from) |
|
| Report ID (ID for this paper/ abstract/ report) |
|
| Reference details
|
|
| Report author contact details |
|
| Publication type (e.g. full report, abstract, letter) |
|
| Study funding sources (including role of funders) |
|
| Possible conflicts of interest (for study authors) |
|
| Notes:
| |
2. Study Eligibility
| Study Characteristics | Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) | Yes | No | Unclear | Location in text (pg & ¶/fig/table) | |
| Type of study | Randomized Controlled Trial |
| ||||
| Controlled Clinical Trial (quasi‐randomized trial) |
| |||||
| Participants
|
|
| ||||
| Types of intervention |
|
| ||||
| Types of outcome measures |
|
| ||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion
|
| |||||
| Notes:
| ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
| Description Include comparative information for each group (i.e. intervention and controls) if available | Location in text (pg & ¶/fig/table) | |
| Population description (from which study participants are drawn) |
|
| |
| Setting (including location and social context) |
|
| |
| Inclusion criteria 1st episode of spontaneous pneumothorax Radiological evidence |
|
| |
| Exclusion criteria Trauma Other secondary cause Recurrence |
|
| |
| Informed consent obtained
|
Yes No Unclear |
|
|
| Notes:
| |||
4. Methods
|
| Descriptions as stated in report/paper
| Location in text (pg & ¶/fig/table) | |
| Aim of study
|
|
| |
| Design(e.g. parallel, crossover, cluster) |
|
| |
| Unit of allocation (by individuals, cluster/ groups or body parts) |
|
| |
| Start date
|
|
| |
| End date
|
|
| |
| Total study duration
|
|
| |
| Ethical approval needed/ obtained for study |
Yes No Unclear |
|
|
| Notes:
| |||
5. Risk of Bias assessment
See Chapter 8 of theHiggins 2011
| Domain | Risk of bias
| Support for judgement
| Location in text (pg & ¶/fig/table) | ||
| Low risk | High risk | Unclear | |||
| Random sequence generation (selection bias) |
|
| |||
| Allocation concealment (selection bias)
|
|
| |||
| Blinding of participants and personnel (performance bias) | Outcome group: All/
|
| |||
| (if required) | Outcome group:
|
| |||
| Blinding of outcome assessment (detection bias) | Outcome group: All/
|
| |||
| (if required) | Outcome group:
|
| |||
| Incomplete outcome data (attrition bias)
|
|
| |||
| Selective outcome reporting? (reporting bias) |
|
| |||
| Other bias
|
|
| |||
| Notes:
| |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Total no. randomized (or total pop. at start of study for NRCTs) |
|
|
| Clusters (if applicable, no., type, no. people per cluster) |
|
|
| Baseline imbalances |
|
|
| Withdrawals and exclusions (if not provided below by outcome) |
|
|
| Age |
|
|
| Sex |
|
|
| Race/Ethnicity |
|
|
| Size of pneumothorax |
|
|
| Co‐morbidities (should be excluded if co‐existent lung disease
|
|
|
| Other treatment received(additional to study intervention) |
|
|
| Other relevant sociodemographics
|
|
|
| Subgroups measured Smoking Other
|
|
|
| Subgroups reported Smoking Other |
|
|
| Notes:
| ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Group name Conservative or Interventional
|
|
|
| No. randomized to group (specify whether no. people or clusters) |
|
|
| Theoretical basis(include key references)
|
|
|
| Description of intervention(include sufficient detail for replication, e.g. Type of supportive therapy ‐ oxygen, analgesia etc; or type of intervention – chest tube, needle aspiration etc) |
|
|
| Duration of treatment period |
|
|
| Timing(e.g. frequency, duration of each episode) |
|
|
| Delivery(e.g. mechanism, medium, intensity, fidelity) |
|
|
| Providers (e.g. type of hospital, specialty, training etc) |
|
|
| Co‐interventions
|
|
|
| Economic variables |
|
|
| Notes:
| ||
8. Outcomes
Copy and paste table for each outcome.
Early recurrence
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Outcome name Recurrence ‐ early |
|
|
| Time points measured |
|
|
| Time points reported |
|
|
| Outcome definition(with diagnostic criteria if relevant) |
|
|
| Person measuring/reporting |
|
|
| Imputation of missing data |
|
|
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|
|
| Power No. with data in conservative mx group N. with data in interventional group |
|
|
| Notes:
| ||
Repeat for late recurrence
Mortality
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Outcome name Mortality
|
|
|
| Time points measured |
|
|
| Time points reported |
|
|
| Person measuring/reporting |
|
|
| Imputation of missing data |
|
|
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|
|
| Power No. with data conservative No. with data interventional |
|
|
| Notes:
| ||
Length of hospital stay
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Outcome name Length of hospital stay |
|
|
| Outcome definition |
|
|
| Person measuring/reporting |
|
|
| Unit of measurement
|
|
|
| Imputation of missing data |
|
|
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|
|
| Power Number of participants with Data: Conservative group Interventional group |
|
|
| Notes:
| ||
Pleurectomy or pleurodesis
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Outcome name Pleurectomy or pleurodesis
|
|
|
| Time points measured |
|
|
| Time points reported |
|
|
| Outcome definition |
|
|
| Person measuring/reporting |
|
|
| Imputation of missing data |
|
|
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|
|
| Power No. with data conservative No. with data interventional |
|
|
| Notes:
| ||
Complications
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Outcome name Complications
|
|
|
| Time points measured |
|
|
| Time points reported |
|
|
| Outcome definition |
|
|
| Person measuring/reporting |
|
|
| Imputation of missing data |
|
|
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|
|
| Power No. with data conservative No. with data interventional |
|
|
| Notes:
| ||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome – Early recurrence
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) | ||||
| Comparison |
|
| ||||
| Outcome |
|
| ||||
| Subgroup |
|
| ||||
| Timepoint |
|
| ||||
| Results | Conservative treatment | Interventional treatment |
| |||
| No. events | No. participants | No. events | No. participants | |||
|
|
|
|
| |||
| No. missing participants and reasons |
|
|
| |||
| No. participants moved from other group and reasons |
|
|
| |||
| Any other results reported |
|
| ||||
| Unit of analysis(by individuals, cluster/groups or body parts)
|
|
| ||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) |
|
| ||||
| Reanalysis required?(specify) |
Yes No Unclear |
|
| |||
| Reanalysis possible? |
Yes No Unclear |
|
| |||
| Reanalysed results |
|
| ||||
| Notes:
| ||||||
Repeat for late recurrence
Mortality
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) | ||||
| Comparison |
|
| ||||
| Outcome |
|
| ||||
| Subgroup |
|
| ||||
| Timepoint |
|
| ||||
| Results | Intervention | Comparison |
| |||
| No. events | No. participants | No. events | No. participants | |||
|
|
|
|
| |||
| No. missing participants and reasons |
|
|
| |||
| No. participants moved from other group and reasons |
|
|
| |||
| Any other results reported |
|
| ||||
| Unit of analysis(by individuals, cluster/groups or body parts)
|
|
| ||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) |
|
| ||||
| Reanalysis required?(specify) |
Yes No Unclear |
|
| |||
| Reanalysis possible? |
Yes No Unclear |
|
| |||
| Reanalysed results |
|
| ||||
| Notes:
| ||||||
Pleurectomy or pleurodesis
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) | ||||
| Comparison |
|
| ||||
| Outcome |
|
| ||||
| Subgroup |
|
| ||||
| Timepoint |
|
| ||||
| Results | Intervention | Comparison |
| |||
| No. events | No. participants | No. events | No. participants | |||
|
|
|
|
| |||
| No. missing participants and reasons |
|
|
| |||
| No. participants moved from other group and reasons |
|
|
| |||
| Any other results reported |
|
| ||||
| Unit of analysis(by individuals, cluster/groups or body parts)
|
|
| ||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) |
|
| ||||
| Reanalysis required?(specify) |
Yes No Unclear |
|
| |||
| Reanalysis possible? |
Yes No Unclear |
|
| |||
| Reanalysed results |
|
| ||||
| Notes:
| ||||||
Complications
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) | ||||
| Comparison |
|
| ||||
| Outcome |
|
| ||||
| Subgroup |
|
| ||||
| Timepoint |
|
| ||||
| Results | Intervention | Comparison |
| |||
| No. events | No. participants | No. events | No. participants | |||
|
|
|
|
| |||
| No. missing participants and reasons |
|
|
| |||
| No. participants moved from other group and reasons |
|
|
| |||
| Any other results reported |
|
| ||||
| Unit of analysis(by individuals, cluster/groups or body parts)
|
|
| ||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) |
|
| ||||
| Reanalysis required?(specify) |
Yes No Unclear |
|
| |||
| Reanalysis possible? |
Yes No Unclear |
|
| |||
| Reanalysed results |
|
| ||||
| Notes:
| ||||||
Length of hospital stay
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) | ||||||||
| Comparison |
|
| ||||||||
| Outcome |
|
| ||||||||
| Subgroup |
|
| ||||||||
| Timepoint |
|
| ||||||||
| Post‐intervention or change from baseline? |
|
| ||||||||
| Results | Intervention | Comparison |
| |||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants |
| ||||
|
|
|
|
|
|
| |||||
| No. missing participants and reasons |
|
|
| |||||||
| No. participants moved from other group and reasons |
|
|
| |||||||
| Any other results reported
|
|
| ||||||||
| Unit of analysis (individuals, cluster/ groups or body parts) |
|
| ||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) |
|
| ||||||||
| Reanalysis required?(specify) |
Yes No Unclear |
|
| |||||||
| Reanalysis possible? |
Yes No Unclear |
|
| |||||||
| Reanalysed results |
|
| ||||||||
| Notes:
|
| |||||||||
10. Applicability
| Have important populations been excluded from the study?(e.g. smokers) |
Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) |
Yes No Unclear |
|
| Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
| Notes:
| ||
11. Other information
|
| Description as stated in report/paper
| Location in text (pg & ¶/fig/table) |
| Key conclusions of study authors
|
|
|
| References to other relevant studies
|
|
|
| Correspondence required for further study information(from whom, what and when) |
| |
| Notes:
| ||
Risk of Bias Table
| Unique ID
| ISDN | First author | Journal / conference | Year | Reviewer |
|
|
|
|
|
|
|
| Domain | Description of what was done | Risk of bias judgement |
| Sequence generation (How were patients allocated into intervention groups) |
| Low High Unclear |
| Allocation concealment (Was allocation list concealed prior to allocation) |
| Low High Unclear |
| Blinding of participants and personnel (Bias due to knowledge of allocation by participant or personnel). |
| Low High Unclear |
| Blinding of outcome assessment ‐ objective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Blinding of outcome assessment – subjective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Incomplete outcome data (attrition due to amount, nature or handling of incomplete outcome data) |
| Low High Unclear |
| Selective reporting (Are all pre‐specified outcomes reported) |
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
Risk of Bias Table
| Unique ID
| ISDN | First author | Journal / conference | Year | Reviewer |
|
|
|
|
|
|
|
| Domain | Description of what was done | Risk of bias judgement |
| Sequence generation (How were patients allocated into intervention groups) |
| Low High Unclear |
| Allocation concealment (Was allocation list concealed prior to allocation) |
| Low High Unclear |
| Blinding of participants and personnel (Bias due to knowledge of allocation by participant or personnel). |
| Low High Unclear |
| Blinding of outcome assessment ‐ objective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Blinding of outcome assessment – subjective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Incomplete outcome data (attrition due to amount, nature or handling of incomplete outcome data) |
| Low High Unclear |
| Selective reporting (Are all pre‐specified outcomes reported) |
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
Appendix 8. Risk of Bias Tool
Risk of Bias Table
| Unique ID
| ISDN | First author | Journal / conference | Year | Reviewer |
|
|
|
|
|
|
|
| Domain | Description of what was done | Risk of bias judgement |
| Sequence generation (How were patients allocated into intervention groups) |
| Low High Unclear |
| Allocation concealment (Was allocation list concealed prior to allocation) |
| Low High Unclear |
| Blinding of participants and personnel (Bias due to knowledge of allocation by participant or personnel). |
| Low High Unclear |
| Blinding of outcome assessment ‐ objective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Blinding of outcome assessment – subjective outcomes (knowledge of the allocation by outcome assessor) |
| Low High Unclear |
| Incomplete outcome data (attrition due to amount, nature or handling of incomplete outcome data) |
| Low High Unclear |
| Selective reporting (Are all pre‐specified outcomes reported) |
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
| Other potential source of bias
|
| Low High Unclear |
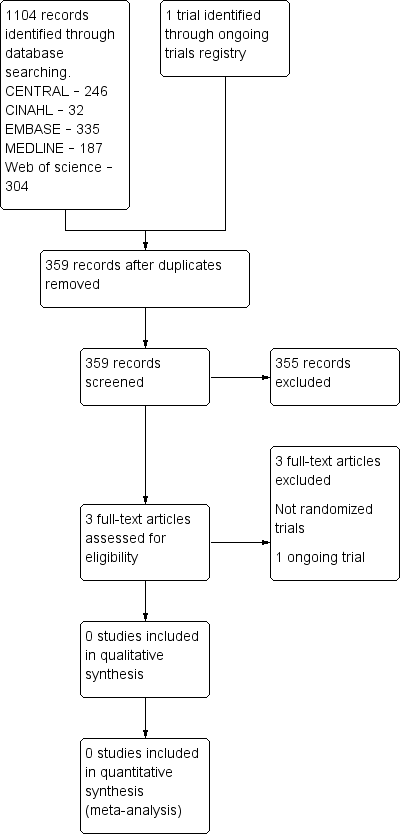
Study flow diagram.

