نقش درمانهای روانشناسی در مدیریت افسردگی مقاوم به درمان در بزرگسالان
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, parallel groups | |
| Participants | Recruited from outpatient clinic of a hospital Location: USA Criteria for depression: Structured Clinical Interview for DSM‐IV diagnoses (SCID‐I) Age: range 18‐65 years (mean 41.8 years) 24 participants in total (n = 18 (75%) female) Baseline HAMD score: DBT + TAU = 16.9; waitlist/TAU = 18.9 Baseline BDI score: DBT + TAU = 27.9; waitlist/TAU = 27.4 | |
| Interventions | Group I: dialectical behavioural therapy plus usual care DBT ‐ 16 weekly sessions, each lasting 1 hour 30 minutes, with weekly between‐session homework assignments. The group was co‐led by 2 clinical psychologists trained and experienced in group DBT. Group II: waitlist and usual care Both groups continued antidepressant treatment as part of usual care. | |
| Outcomes | Continuous measure of depressive symptoms (Hamilton Depression Scale (HAMD) and Beck Depression Inventory (BDI) scores) Follow‐up at 16 weeks | |
| Definition of TRD | Non‐response to at least 6 weeks of an adequate dose of an antidepressant Standard effective doses were predefined by consensus of 2 senior psychiatrists with expertise in depression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were "block randomised" by gender and age. |
| Allocation concealment (selection bias) | Unclear risk | No information was given regarding concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and therapists were aware of the treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Participants and therapists were aware of the treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The independent assessors were blind to the condition each patient had been assigned when they conducted week 0 and week 16 assessments." |
| Incomplete outcome data (attrition bias) | High risk | 23% and 18% dropout in intervention and control groups, respectively. Study authors did not report any approach to dealing with missing data and, when contacted, confirmed that no such analyses were undertaken. |
| Selective reporting (reporting bias) | Low risk | No protocol is available. Outcomes listed in methods were all reported in results. Study author was contacted for any additional outcomes or analyses conducted, and they provided a later publication of the study with additional (post hoc) analyses on 1 of the outcomes. |
| Other bias | Low risk | No differential attrition, baseline differences, or selective follow‐up is apparent. |
| Methods | RCT, parallel group | |
| Participants | Recruited from outpatient departments of 2 hospitals Location: Tokyo, Japan Criteria for depression: DMS‐IV and HAMD ≥ 16 Age: mean (SD) 39.5 (9.2) years for CBT group, 41.7 (10.7) for usual care group; range 20 to 65 years 80 participants (n = 29 (36%) female) Baseline HAMD‐GRID score: CBT + TAU = 20.9; TAU = 20.8 Baseline BDI score: CBT + TAU = 27; TAU = 27.2 | |
| Interventions | Group I: cognitive‐behavioural therapy in addition to usual care CBT was delivered by 4 psychiatrists, 1 clinical psychologist, and 1 psychiatric nurse, all of whom were trained in delivering CBT and were supervised and independently rated by a specialist on adherence to CBT protocols. Participants could receive between 16 and 20 sessions. Group II: usual care Investigators imposed no restrictions on the treatment that participants in the usual care group could receive, except CBT. Both groups continued antidepressant therapy as part of usual care. | |
| Outcomes | Change in clinician‐rated 17‐item GRID‐Hamilton Depression Rating Scale (GRID‐HAMD) score at 16 weeks Severity and change in scores of subjective depression symptoms (BDI)‐II Dropout Proportions of responders (> 50% reduction in GRID‐HAMD from baseline) and remitters (< 7 GRID‐HAMD) Safety (numbers of serious adverse events) Quality of life (SF‐36 mental; SF‐36 physical) | |
| Definition of TRD | Non‐response to at least 8 weeks of adequate therapeutic dosage of antidepressant medication as part of usual care | |
| Notes | Closed recruitment in August 2013. Data collection to be completed in December 2014 but no publication yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central computerised registration system designed for this study (which) automatically randomises patients and generates a message noting their assigned treatment." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation will be concealed and stratified by site (n = 2) with the minimisation method to balance the age and baseline HAMD score. The cutoff for age and depression level used for minimisation will not be disclosed until study completion." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Due to the nature of the intervention, neither the patients, the treating psychiatrists, [n]or the study therapists can be completely blinded, but the two latter groups are strongly instructed not to disclose the randomisation status to patients at assessments." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Due to the nature of the intervention, neither the patients, the treating psychiatrists, [n]or the study therapists can be completely blinded, but the two latter groups are strongly instructed not to disclose the randomisation status to patients at assessments." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Study design uses a standardised psychiatric interview to assess depression symptomatology by blind raters; the assessors were not involved with treatment delivery and study coordination and were prohibited from accessing any information that could confer participant allocation. The success of assessor masking was tested...percent agreement was 52 and kappa coefficient was 0.00, indicating masking was successful." |
| Incomplete outcome data (attrition bias) | Low risk | Primary analysis was ITT with all randomised participants included. Missing values were not imputed but were tested in sensitivity analyses using imputations for departures from missing at random. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Some outcomes are expected in future publications, so we cannot judge yet. |
| Other bias | Low risk | No differential attrition, baseline differences, [n]or selective follow‐up apparent |
| Methods | RCT, parallel group | |
| Participants | Recruited from outpatient clinic of a hospital Location: Brazil Criteria for depression: DMS‐IV Age: mean (SD) 49.3 (12.3) years for IPT group, 49.18 (12.5) for usual care group 40 participants (n = 34 (85%) female) Baseline BDI score: CBT + TAU = 31.4; TAU = 28.8 Baseline HAMD score: CBT + TAU = 19.8; TAU = 18.4 | |
| Interventions | Group I: interpersonal psychotherapy (IPT) plus usual care IPT performed according to treatment guidelines; 16 individual 40‐minute weekly sessions administered by third year psychiatric residents and 1 psychiatrist Group II: Usual care ‐ pharmacotherapy and clinical management. Clinicians were free to choose medication(s) plus other treatments that followed standard clinical guidelines. Both groups continued antidepressant therapy as part of usual care. | |
| Outcomes | Depressive symptoms: HAMD 17 as continuous score and dichotomous outcome (response ‐ defined as 50% reduction; remission < 7); BDI as continuous score; CGI‐S as continuous score QOL: WHOQOL continuous score Dropout | |
| Definition of TRD | Non‐response to at least 1 trial of antidepressant medication in adequate dose and duration. Adequate dose was defined as the equivalent of at least 75 mg of amitriptyline. Adequate duration ‐ at least 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation sequence generated by computer prior to the recruitment" |
| Allocation concealment (selection bias) | Low risk | Quote: "Single randomisation was carried out by means of sequentially numbered brown sealed envelopes containing the randomisation sequence." |
| Blinding of participants and personnel (performance bias) | High risk | No information is available, but it is likely that participants and personnel provided/received psychotherapy and knew about it; no placebo was used. It is possible that the clinicians who delivered this were blind to treatment allocation, but no information is provided to indicate whether this was the case. |
| Blinding of outcome assessment (detection bias) | High risk | No information is available, but it is unlikely that participants were blind to treatment; no placebo was used. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Investigators responsible for the outcome assessments were blinded to the treatment assignment." |
| Incomplete outcome data (attrition bias) | High risk | 27.5% dropout; IPT group (n = 6), usual care (n = 5); although authors stated using ITT, not all randomised participants were analysed as per their CONSORT figure Quote: "We performed analyses using the full data set including all patients randomly assigned to any of the two interventions; Considering the intention‐to‐treat sample the differences in response rates and remission were not significant." Comment: awaiting trial author response on ITT queries |
| Selective reporting (reporting bias) | Unclear risk | The paper additionally reports response and remission, which were not stated in the protocol. Unclear how this may affect trial findings |
| Other bias | Low risk | No differential attrition, baseline differences, nor selective follow‐up apparent |
| Methods | RCT, parallel group | |
| Participants | Recruited from secondary care outpatient departments Location: Halifax, Canada Criteria for depression: DMS‐IV and HAMD ≥ 16 Age: range 18 to 65 years (mean age 38.9 years for ISTDP group, 44.2 years for usual care group) 60 participants in total (n = 38 (63.3%) female) Baseline score HAMD: ISTDP = 23.5; TAU = 24.03 | |
| Interventions | Intensive short‐term dynamic psychotherapy with treatment as usual vs standard care/treatment as usual | |
| Outcomes | Change in depression severity (HAMD; PHQ‐9) Dropout Quality of life (SF‐12) Cost‐effectiveness | |
| Definition of TRD | Participants with non‐remitting depression following at least 1 course of antidepressants. Participants will have had at least 1 treatment trial of antidepressants at an acceptable therapeutic dose (length ≥ 6 weeks) for the current depressive episode without adequate response (score on the Hamilton Rating Scale for Depression ≥ 16 ) at the time of screening interview. | |
| Notes | The published paper presented only the depression outcome and dropout at 6 months and stated that other outcomes will be presented in a following paper. Request made to study author for unpublished data; no response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For purposes of randomisation, a researcher external to the study team generated a permuted block randomisation sequence using a digital random number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "Screening assessments and enrolment were conducted by the study research assistant who remained blind throughout the randomisation and allocation process. Allocation was conducted at the end of enrolment by an administrative assistant." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Therapists and patients could not be blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Therapists and patients could not be blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The study research assistant was blind to treatment condition; and to maintain concealment, patients were instructed to refrain from discussing their treatment during assessments; the study (included) use of blinded outcome ratings." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The 60 randomised participants were included in our primary ‘intention to treat’ analysis sample; the missing data [were] distributed equally between the two groups." |
| Selective reporting (reporting bias) | Unclear risk | Comment: some outcomes expected in future publications, so we cannot judge yet |
| Other bias | Low risk | No differential attrition, baseline differences, nor selective follow‐up apparent |
| Methods | RCT, parallel groups | |
| Participants | Recruited from GP practices Location: UK Criteria for depression: ICD‐10 and at least 15 on the Beck Depression Inventory (BDI‐II) Age: range 18 to 65 years (mean age 45.5 years for CBT group, 45.1 years for usual care group) 25 participants in total (n = 21 (84%) female) Baseline BDI score: CBT + TAU = 31.3; TAU = 26.6 | |
| Interventions | Group I: cognitive‐behavioural therapy in addition to usual care CBT was delivered by 2 therapists who received weekly supervision of a specialist. Participants could receive between 12 and 20 sessions. Group II: usual care Investigators applied no restrictions on the treatment that participants in the usual care group could receive. Both groups continued antidepressant therapy as part of usual care. | |
| Outcomes | Depressive symptoms (BDI‐II) as continuous score and dichotomous outcome (response ‐ defined as at least a 50% reduction in BDI score) | |
| Definition of TRD | Non‐response to at least 6 weeks of antidepressant treatment at British National Formulary (BNF) recommended doses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted by an individual independent of the recruitment process |
| Blinding of participants and personnel (performance bias) | High risk | Participants and therapists were aware of the treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Participants were aware of the treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable ‐ no observer‐rated scales |
| Incomplete outcome data (attrition bias) | Low risk | Last observation carried forward approach used for participants with missing data (n = 2) |
| Selective reporting (reporting bias) | Low risk | Trial protocol available. Only BDI results were reported in the 2007 publication, but data on unpublished outcomes (QOL, final mean, SD values for BDI for each group, reasons for dropout by group) were obtained from the study author. |
| Other bias | Low risk | No differential attrition, baseline differences, nor selective follow‐up apparent |
| Methods | RCT, parallel group (CoBalT trial) | |
| Participants | Recruited from GP practices Location: UK Criteria for depression: ICD‐10 criteria for depression and at least 14 on the Beck Depression Inventory (BDI‐II) Age: range 18 to 75 years (mean age 49.2 years for CBT group, 50.0 years for usual care group) 469 participants in total (n = 339 (72%) female) Baseline BDI score: CBT + TAU = 31.8; TAU = 31.8 | |
| Interventions | Group I: cognitive‐behavioural therapy plus usual care Participants received a course of 12 sessions of CBT, with (up to) 6 additional sessions if deemed necessary by the therapist. Eleven trained and supervised therapists were representative of NHS psychological therapy services. At 12 months, the median number of sessions received was 12 (IQR 6 to 17). Group II: usual care Investigators applied no restrictions on treatment options for participants randomised to be managed as usual by their GP. Participants could be referred for counselling or for secondary care (including for CBT). Both groups continued antidepressant medication as part of usual care. | |
| Outcomes | Depressive symptoms were measured by (1) Beck Depression Inventory (BDI‐II) ‐ mean scores, response (reduction in BDI of at least 50% compared with baseline), and remission (BDI‐II score < 10); and (2) Patient Health Questionnaire (PHQ‐9). Measures of anxiety (Generalised Anxiety Disorder Assessment (GAD‐7)) and panic (Brief PHQ) Quality of life (QOL): SF mental and SF physical scales of the SF‐12 version 2 and the EuroQOL (EQ‐5D) Economic outcomes: primary and secondary care resource use, direct costs to NHS and Personal Social Services, and participants' out‐of‐pocket personal expenses and indirect costs such as travel | |
| Definition of TRD | Non‐response to at least 6 weeks of antidepressant treatment at British National Formulary (BNF) recommended doses | |
| Notes | A predefined analysis plan was agreed upon with the Trial Steering Committee. The primary outcome for the main trial (measured at 6 months post randomisation) was a dichotomous outcome of response (defined as at least a 50% reduction in depressive symptoms compared with baseline), but for long‐term follow‐up (on average, 46 months post randomisation), the primary outcome was specified as a continuous outcome (BDI‐II score) to maximise power. This change was made at the time the request for additional funding was submitted to the funder (6 November 2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was by means of a computer‐generated code....Allocation was stratified by centre and minimised (with a probability weighting of 0.80) according to baseline BDI score (14–19, 20–28, ≥ 29); whether the general practice had a counsellor (yes or no); previous treatment with antidepressants (yes or no); and duration of present episode of depression (< 1 year, 1–2 years, ≥ 2 years)." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation...from a remote automated telephone randomisation service, which thus ensured that the treatment allocation was concealed from the recruiting researcher" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Because of the nature of the intervention, it was not possible to mask participants, general practitioners, CBT therapists, or researchers to the treatment allocation." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Because of the nature of the intervention, it was not possible to mask participants, general practitioners, CBT therapists, or researchers to the treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable ‐ no observer‐rated scales |
| Incomplete outcome data (attrition bias) | Low risk | ITT done with and without imputation for missing data with similar results Quote: "Trial dealt with any missing data at an individual item level by adopting the following rule. If > 10% of the items were incomplete, then the data collected on that measure for that participant were disregarded. However, if < 10% of items on a particular measure were missing, missing item(s) were imputed using the mean of the remaining items (rounded to an integer). Sensitivity analyses were conducted using the method of multiple imputation by chained equation (MICE) to examine the impact of missing data on the main findings." |
| Selective reporting (reporting bias) | Low risk | Protocol is available. Depression outcomes and main QOL measures (SF‐12) are reported fully for all time points described. Additional QOL measures collected for the economic analyses (EQ‐5D‐3L, SF‐6D) are not reported separately but were used to derive QALYs. |
| Other bias | Low risk | No differential attrition, baseline differences, nor selective follow‐up apparent |
BDI: Beck Depression Inventory.
BNF: British National Formulary.
CBT: cognitive‐behavioural therapy.
CGI‐S: Clinical Global Impressions Scale.
DBT: dialectical behaviour therapy.
DSM: Diagnostic and Statistical Manual of Mental Disorders.
EQ‐5D: EuroQOL Group Quality of Life Questionnaire based on five dimensions.
EQ‐5D‐3L: EuroQOL Group Quality of Life Questionnaire based on a three‐level scale.
EuroQOL: EuroQOL Group Quality of Life Questionnaire.
GAD: generalised anxiety disorder.
GP: general practice.
HAMD: Hamilton Depression Rating Scale.
ICD: International Classification of Diseases.
IPT: interpersonal therapy.
IQR: interquartile range.
ISTDP: intensive short‐term dynamic psychotherapy.
ITT: intention‐to‐treat.
MICE: multiple imputation by chained equation.
mg: milligram.
NHS: National Health Service.
PHQ: Patient Health Questionnaire.
QALY: quality‐adjusted life‐year.
QOL: quality of life.
RCT: randomised controlled trial.
SCID: Structured Clinical Interview for DSM‐IV diagnoses.
SD: standard deviation.
SF: Short Form.
TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| All participants did not meet diagnostic criteria at randomisation | |
| Participants did not meet age criteria | |
| All participants did not meet diagnostic criteria at randomisation | |
| Not an RCT | |
| All participants did not meet diagnostic criteria at randomisation | |
| Did not meet intervention criteria; not TRD: participants currently recovered from depression | |
| Not TRD: no dose or duration of prior treatment provided as criteria for TRD | |
| Not TRD: no prior antidepressant treatment | |
| Not an RCT | |
| Did not meet intervention criteria | |
| Not TRD: not all participants on prior antidepressant treatment | |
| Not TRD: major depressive disorder | |
| All participants did not meet diagnostic criteria at randomisation | |
| Not TRD: not all participants on prior antidepressant treatment | |
| Participants did not meet diagnostic criteria at randomisation; not TRD: recurrent mood disorder | |
| Participants did not meet diagnostic criteria at randomisation | |
| Irrelevant comparison | |
| Did not meet intervention criteria; not TRD: no prior antidepressant treatment | |
| Not an RCT | |
| Not TRD: depression secondary to type 2 diabetes | |
| Participants did not meet age criteria and did not meet diagnostic criteria at randomisation | |
| Did not meet intervention criteria; not TRD: comorbid depression with substance abuse | |
| Did not meet intervention criteria. All participants did not meet diagnostic criteria at point of randomisation; not TRD: 'partly remitted depression' ‐ no further detail | |
| Not TRD: recurrent or chronic depression | |
| Not TRD: remitted people only | |
| Not TRD: recurrent depression | |
| Not TRD: MDD (recurrent) or bipolar disorder; participants in remission | |
| Participants did not meet age criteria; not TRD: relapse prevention/currently remitted | |
| All participants did not meet diagnostic criteria at the point of randomisation | |
| All participants did not meet diagnostic criteria at randomisation: REVAMP | |
| Did not meet intervention criteria; not TRD: MDD comorbid with chronic medical illness | |
| Not TRD: relapse prevention | |
| Did not meet intervention criteria | |
| Not RCT; not TRD: MDD | |
| Not TRD: MDD or MDD with personality disorder | |
| Not TRD: chronic depression with comorbid personality disorder | |
| Not an RCT | |
| Not TRD: included participants with history of antidepressant medication | |
| Participants did not meet age criteria | |
| Not TRD: included participants without antidepressant treatment; dose and duration of treatment not a consideration for inclusion | |
| Not TRD: relapsing depression; no other details | |
| Not TRD: included participants without antidepressant treatment | |
| Not TRD: recurrent major depression | |
| Did not meet intervention criteria | |
| Did not meet intervention criteria | |
| Did not meet intervention criteria; all patients did not meet diagnostic criteria at randomisation | |
| Not an RCT | |
| Not an RCT | |
| Not TRD: prevention of relapse in partly remitted participants with residual depressive symptoms | |
| Did not meet intervention criteria | |
| Participants did not meet age criteria. | |
| Not TRD: chronic depression; no inclusion criteria for dose and duration of antidepressant treatment | |
| Not TRD: chronic depression; no inclusion criteria for dose and duration of antidepressant treatment | |
| Not TRD: chronic depression; no inclusion criteria for dose and duration of antidepressant treatment | |
| No TRD: chronic depression comorbid with chronic medical illness | |
| Participants did not meet diagnostic criteria; not TRD: recurrent depressive disorder with or without a current episode | |
| Not TRD: included patients with residual depressive symptoms and with psychotic depression | |
| All patients did not meet diagnostic criteria at randomisation; not TRD: partly remitted recent MDD | |
| Participants did not meet diagnostic criteria. | |
| Not TRD: included patients without antidepressant treatement | |
| Not TRD: included those who could not tolerate antidepressant medication; diagnostic criteria not applied at randomisation | |
| Not an RCT | |
| Not TRD: MDD within past 18 months, but not in previous two months; duration of antidepressant less than 4 weeks |
MDD: major depressive disorder.
RCT: randomized controlled trial.
TRD: treatment‐resistant depression.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT ‐ parallel group |
| Participants | Unclear if TRD |
| Interventions | Psychotherapy added to usual care with AD |
| Outcomes | Depression |
| Notes | No full text |
| Methods | RCT ‐ parallel group |
| Participants | Unclear if TRD |
| Interventions | Psychotherapy added to usual care with AD |
| Outcomes | Depression |
| Notes | No full text found |
| Methods | RCT ‐ parallel group |
| Participants | Unclear if TRD |
| Interventions | Unclear |
| Outcomes | Depression |
| Notes | No full text found |
| Methods | RCT ‐ parallel group |
| Participants | Unclear if TRD |
| Interventions | Psychotherapy added to usual care with AD |
| Outcomes | Depression |
| Notes | No full text found |
AD: antidepressant.
RCT: randomised controlled trial.
TRD: treatment‐resistant depression.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ISRCTN85784627 (REFRAMED) |
| Methods | RCT |
| Participants | 18 years or older; TRD defined as 2 or more previous episodes of depression or chronic depression; in current episode, participants must have taken an adequate dose of antidepressant medication for at least 6 weeks without relief |
| Interventions | Radically Open Dialectical Behaviour Therapy (RO‐DBT) vs Treatment as Usual |
| Outcomes | Primary outcomes: Depression (HAMD, LIFE‐RIFT) at 6 and 12 month after treatment Health‐related quality of life (EQ‐5D‐3 L) Health services use/costs (AD‐SUS) Secondary outcomes: Suicide (MSSI, SBQ) Depression and affect (PHQ‐9, PANAS) |
| Starting date | 01/01/2012 |
| Contact information | Dr Roelie Hempel University Road |
| Notes | http://www.isrctn.com/ISRCTN85784627 |
AD‐SUS: Adult Service Use Schedule.
EQ: EuroQOL.
EQ‐5D‐3L: EuroQoL Group Quality of Life Questionnaire based on three‐level scale.
HAMD: Hamilton Depression Scale.
LIFE‐RIFT: Range of Impaired Functioning Tool.
MSSI: Modified Scale for Suicide Ideation.
PANAS: Positive and Negative Affect Schedule.
PHQ: Patient Health Questionnaire.
RCT: randomised controlled trial.
RO‐DBT: radically open dialectical behaviour therapy.
SBQ: Sedentary Behavior Questionnaire.
TRD: treatment‐resistant depression.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported depressive symptoms short term (up to 6 months) ‐ BDI Show forest plot | 5 | 575 | Mean Difference (IV, Random, 95% CI) | ‐4.07 [‐7.07, ‐1.07] |
| Analysis 1.1  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 1 Self‐reported depressive symptoms short term (up to 6 months) ‐ BDI. | ||||
| 1.1 CBT with usual care vs usual care alone | 3 | 522 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐7.49, ‐1.63] |
| 1.2 DBT with usual care vs usual care alone | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐10.79 [‐23.83, 2.25] |
| 1.3 IPT with usual care vs usual care alone | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐6.70, 8.30] |
| 2 Self‐reported depressive symptoms short term (up to 6 months) ‐ PHQ‐9 Show forest plot | 2 | 482 | Mean Difference (IV, Random, 95% CI) | ‐4.66 [‐8.72, ‐0.59] |
| Analysis 1.2  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 2 Self‐reported depressive symptoms short term (up to 6 months) ‐ PHQ‐9. | ||||
| 2.1 ISTDP with usual care vs usual care alone | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐7.25 [‐11.37, ‐3.13] |
| 2.2 CBT with usual care vs usual care alone | 1 | 422 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐4.27, ‐1.73] |
| 3 Self‐reported depressive symptoms short term (up to 6 months) ‐ SMD (BDI & PHQ‐9) Show forest plot | 6 | 635 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.65, ‐0.14] |
| Analysis 1.3  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 3 Self‐reported depressive symptoms short term (up to 6 months) ‐ SMD (BDI & PHQ‐9). | ||||
| 3.1 CBT with usual care vs usual care alone | 3 | 522 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.56, ‐0.13] |
| 3.2 DBT with usual care vs usual care alone | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.66, 0.21] |
| 3.3 IPT with usual care vs usual care alone | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.60, 0.74] |
| 3.4 ISTDP with usual care vs usual care alone | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.41, ‐0.35] |
| 4 Clinician‐rated depressive symptoms short term (up to 6 months) ‐ HAMD Show forest plot | 4 | 193 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐5.71, ‐0.85] |
| Analysis 1.4  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 4 Clinician‐rated depressive symptoms short term (up to 6 months) ‐ HAMD. | ||||
| 4.1 DBT with usual care vs usual care alone | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.81 [‐11.04, ‐0.58] |
| 4.2 IPT with usual care vs usual care alone | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.05, 4.25] |
| 4.3 ISTDP with usual care vs usual care | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐11.22, ‐0.46] |
| 4.4 CBT with usual care vs usual care alone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐5.75, ‐0.65] |
| 5 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ BDI Show forest plot | 2 | 475 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.21, 0.40] |
| Analysis 1.5  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 5 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ BDI. | ||||
| 5.1 CBT with usual care vs usual care alone | 2 | 475 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.21, 0.40] |
| 6 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ PHQ‐9 Show forest plot | 1 | 395 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.22, ‐0.58] |
| Analysis 1.6  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 6 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ PHQ‐9. | ||||
| 7 Clinician‐rated depressive symptoms medium term (7 to 12 months) ‐ HAMD Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐7.88, ‐1.52] |
| Analysis 1.7  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 7 Clinician‐rated depressive symptoms medium term (7 to 12 months) ‐ HAMD. | ||||
| 7.1 CBT with usual care vs usual care alone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐7.88, ‐1.52] |
| 8 Self‐reported depressive symptoms long term (longer than 12 months) ‐ BDI Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐7.57, ‐0.83] |
| Analysis 1.8  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 8 Self‐reported depressive symptoms long term (longer than 12 months) ‐ BDI. | ||||
| 9 Self‐reported depressive symptoms long term (longer than 12 months) ‐ PHQ‐9 Show forest plot | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.26, 0.06] |
| Analysis 1.9  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 9 Self‐reported depressive symptoms long term (longer than 12 months) ‐ PHQ‐9. | ||||
| 10 Dropout short term (up to 6 months) Show forest plot | 6 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.24] |
| Analysis 1.10  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 10 Dropout short term (up to 6 months). | ||||
| 10.1 CBT with usual care vs usual care alone | 3 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.16] |
| 10.2 IPT with usual care vs usual care alone | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.33] |
| 10.3 DBT with usual care vs usual care alone | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.26, 6.28] |
| 10.4 ISTDP with usual care vs usual care alone | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.67, 8.18] |
| 11 Dropout medium term (7 to 12 months) Show forest plot | 2 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.47] |
| Analysis 1.11  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 11 Dropout medium term (7 to 12 months). | ||||
| 11.1 CBT with usual care vs usual care alone | 2 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.47] |
| 12 Dropout long term (longer than 12 months) Show forest plot | 1 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.97] |
| Analysis 1.12  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 12 Dropout long term (longer than 12 months). | ||||
| 13 Response (50% reduction in depressive symptoms from baseline) short term (up to 6 months) Show forest plot | 4 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.20, 2.69] |
| Analysis 1.13  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 13 Response (50% reduction in depressive symptoms from baseline) short term (up to 6 months). | ||||
| 14 Response (50% reduction in depressive symptoms from baseline)medium term (7 to 12 months) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.42, 2.10] |
| Analysis 1.14  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 14 Response (50% reduction in depressive symptoms from baseline)medium term (7 to 12 months). | ||||
| 15 Response (50% reduction in depressive symptoms from baseline) long term (longer than 12 months) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.13, 2.32] |
| Analysis 1.15  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 15 Response (50% reduction in depressive symptoms from baseline) long term (longer than 12 months). | ||||
| 16 Remission (< 7 on HAMD or < 10 on BDI) short term (up to 6 months) Show forest plot | 6 | 635 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.46, 2.52] |
| Analysis 1.16  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 16 Remission (< 7 on HAMD or < 10 on BDI) short term (up to 6 months). | ||||
| 17 Remission (< 7 on HAMD or < 10 on BDI) medium term (7 to 12 months) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.51, 2.56] |
| Analysis 1.17  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 17 Remission (< 7 on HAMD or < 10 on BDI) medium term (7 to 12 months). | ||||
| 18 Remission (< 7 on HAMD or < 10 on BDI) long term (longer than 12 months) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.97, 2.53] |
| Analysis 1.18  Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 18 Remission (< 7 on HAMD or < 10 on BDI) long term (longer than 12 months). | ||||
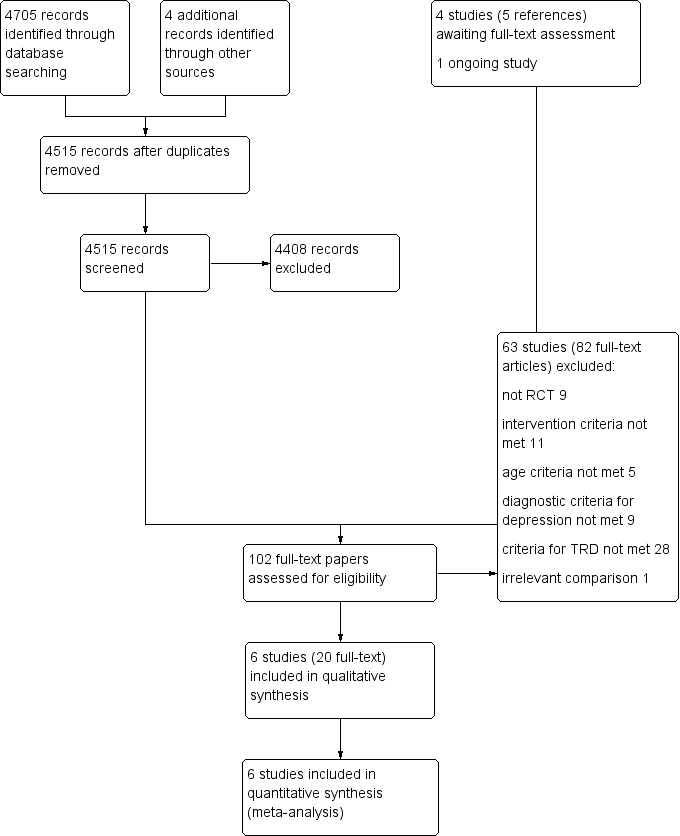
Study flow diagram.
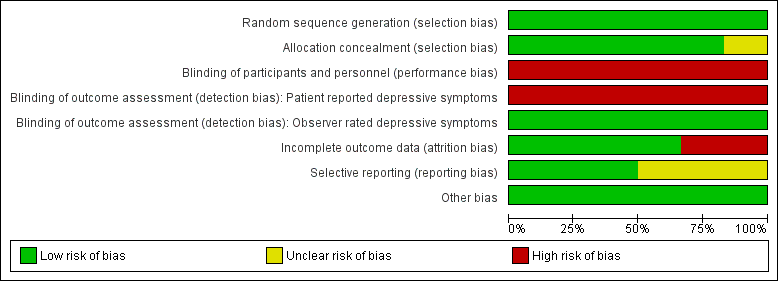
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
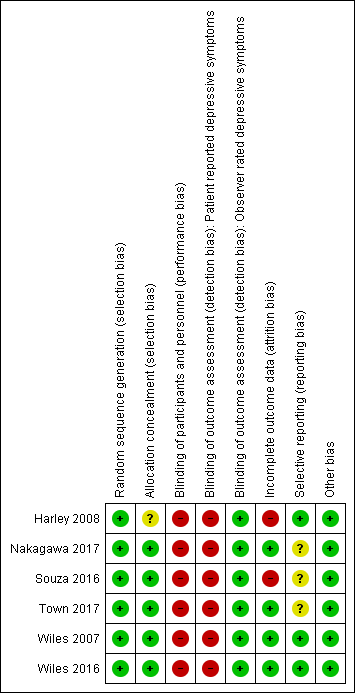
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 1 Self‐reported depressive symptoms short term (up to 6 months) ‐ BDI.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 2 Self‐reported depressive symptoms short term (up to 6 months) ‐ PHQ‐9.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 3 Self‐reported depressive symptoms short term (up to 6 months) ‐ SMD (BDI & PHQ‐9).
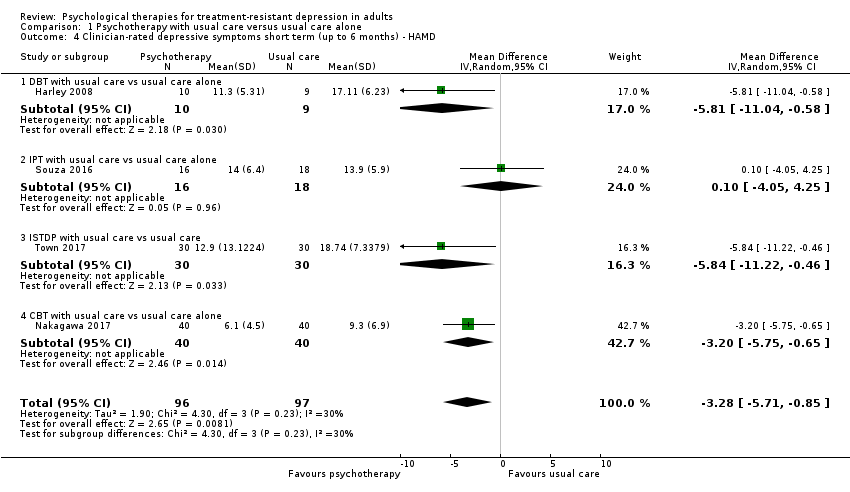
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 4 Clinician‐rated depressive symptoms short term (up to 6 months) ‐ HAMD.
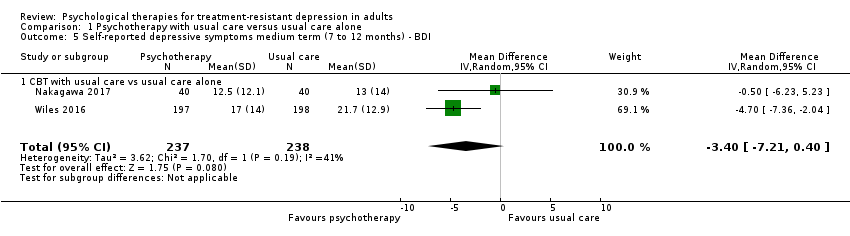
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 5 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ BDI.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 6 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ PHQ‐9.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 7 Clinician‐rated depressive symptoms medium term (7 to 12 months) ‐ HAMD.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 8 Self‐reported depressive symptoms long term (longer than 12 months) ‐ BDI.
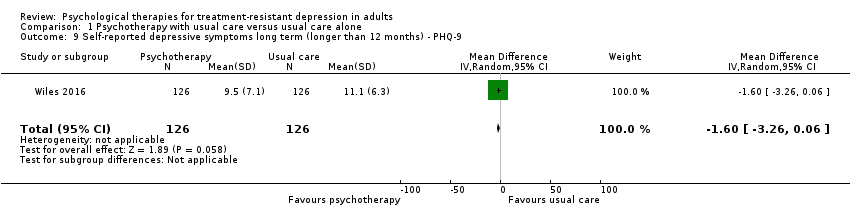
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 9 Self‐reported depressive symptoms long term (longer than 12 months) ‐ PHQ‐9.

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 10 Dropout short term (up to 6 months).
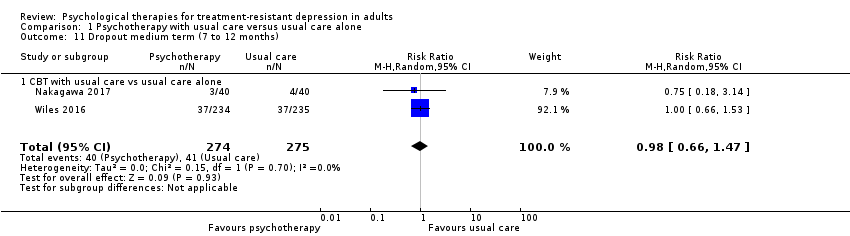
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 11 Dropout medium term (7 to 12 months).

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 12 Dropout long term (longer than 12 months).

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 13 Response (50% reduction in depressive symptoms from baseline) short term (up to 6 months).
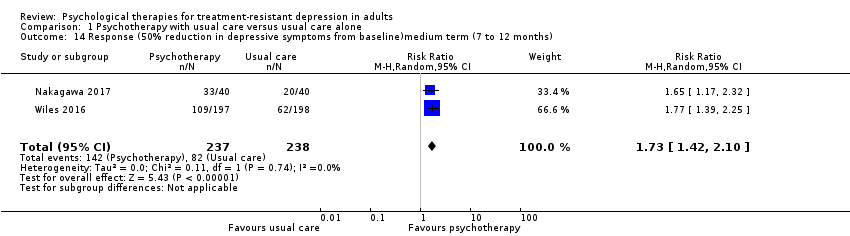
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 14 Response (50% reduction in depressive symptoms from baseline)medium term (7 to 12 months).

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 15 Response (50% reduction in depressive symptoms from baseline) long term (longer than 12 months).
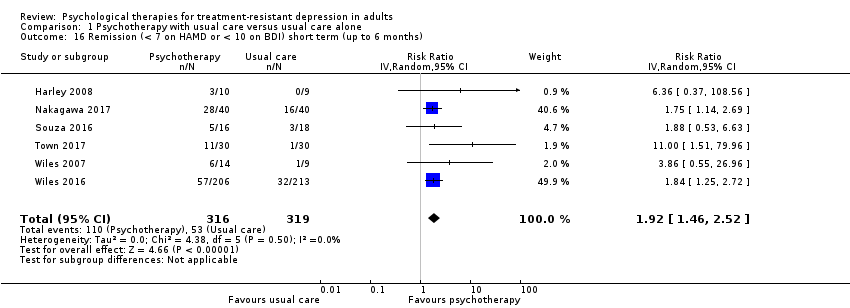
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 16 Remission (< 7 on HAMD or < 10 on BDI) short term (up to 6 months).

Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 17 Remission (< 7 on HAMD or < 10 on BDI) medium term (7 to 12 months).
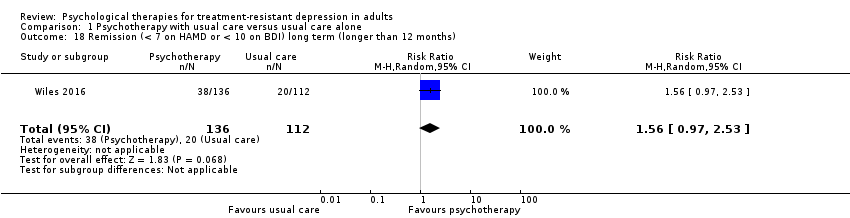
Comparison 1 Psychotherapy with usual care versus usual care alone, Outcome 18 Remission (< 7 on HAMD or < 10 on BDI) long term (longer than 12 months).
| Psychotherapy as an adjunct to usual care compared with usual care alone for treatment‐resistant depression in adults | ||||||
| Patient or population: adults with treatment‐resistant depression | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care alone | Risk with psychotherapy as an adjunct to usual care | |||||
| Self‐reported depressive symptoms short term (up to 6 months) ‐ BDI (BDI) | Mean depressive symptoms short term (up to 6 months) ‐ BDI was 21.1 | MD 4.07 lower | MD ‐4.07 (‐7.01 to ‐1.07) | 575 | ⊕⊕⊕⊝ MODERATEa,b | One large and 4 small studies comprising mainly women. Third‐wave cognitive/behavioural therapies given (individual CBT in 3 studies, group DBT in 1, and individual IPT in 1) |
| Self‐reported depressive symptoms short term (up to 6 months) ‐ SMD (BDI & PHQ9) | Mean depressive symptoms short term (up to 6 months) ‐ BDI was 21.1, and PHQ9 was 14.79 | SMD 0.4 SD lower | SMD ‐0.4 (‐0.65 to ‐0.14) | 635 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | All 6 studies combined |
| Observer‐rated depressive symptoms short term (up to 6 months) ‐ PHQ‐9 | Mean depressive symptoms short term (up to 6 months) ‐ PHQ‐9 was 14.8 | MD 4.66 lower | MD ‐4.66 (‐8.72 to ‐0.59) | 482 | ⊕⊕⊕⊝ MODERATEa | One large study from UK and one relatively small one from Canada |
| Observer‐rated depressive symptoms short term (up to 6 months) ‐ HAMD | Mean depressive symptoms short term (up to 6 months) ‐ HAMD was 14.76 | MD 3.28 lower | MD ‐3.28 (‐5.71 to ‐0.85) | 193 | ⊕⊕⊝⊝ c,d | Although blinded outcome assessment, 4 small studies each using a different type of psychotherapy: group DBT; ISTDP; CBT; IPT |
| Dropout short term (up to 6 months) | Study population | RR 0.85 | 698 | ⊕⊕⊕⊕ | Objective outcome; data reported in all studies; Although a proxy for acceptability, it suggests that intervention may be as acceptable as usual care | |
| 149 per 1000 (14%) | 126 per 1000 (12.6%) | |||||
| Response (50% reduction in depressive symptoms from baseline) short term (up to 6 months) | Study population | RR 1.80 | 556 | ⊕⊕⊝⊝ LOW a,e | ‐ | |
| 264 per 1000 | 476 per 1000 | |||||
| Remission (< 7 on HAMD or < 10 on BDI) short term (up to 6 months) | Study population | RR 1.92 | 635 | ⊕⊕⊕⊝ MODERATEa,b | One large and 5 small studies comprising mainly women. Third‐wave cognitive/behavioural therapies given (individual CBT in 3 studies; individual IPT, ISTDP, and group DBT in 1 study each) | |
| 166 per 1000 | 319 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aOutcome assessment not blind. bAllocation concealment unclear for one of the two smaller studies. cRisk of bias due to incomplete outcome data in two of the studies. dStudies are small. Effects not in the same direction for IPT study (n = 30). eReporting bias likely as less frequently reported than remission or mean scores. | ||||||
| Psychotherapy as an adjunct to usual care compared with usual care alone for treatment‐resistant depression in adults | ||||||
| Patient or population: adults with treatment‐resistant depression | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care alone | Risk with psychotherapy as an adjunct to usual care | |||||
| Self‐reported depressive symptoms medium term (7 to 12 months) ‐ BDI | Mean depressive symptoms score at medium term ‐ BDI was 17.5 | MD 3.4 lower | ‐ | 475 | ⊕⊕⊝⊝ | Two studies (CBT): outcome assessment not blind as participants aware; wide confidence intervals |
| Observer‐rated depressive symptoms medium term (7 to 12 months) ‐ PHQ‐9 | Mean depressive symptoms score at medium term ‐ BDI was 13 | MD 1.9 lower | ‐ | 395 | ⊕⊕⊝⊝ | Single study (CBT) |
| Self (patient)‐reported depressive symptoms long term (longer than 12 months) ‐ BDI | Mean depressive symptoms score at long term ‐ BDI was 23.4 | MD 4.2 lower | ‐ | 248 | ⊕⊕⊝⊝ | 46‐Month results |
| Observer‐rated depressive symptoms long term (longer than 12 months) ‐ PHQ‐9 | Mean depressive symptoms score at long term ‐ BDI was 11.1 | MD 1.6 lower | ‐ | 252 | ⊕⊕⊝⊝ | 46‐Month results |
| Dropout medium term (7 to 12 months)* | Study population | RR 0.98 | 549 | ⊕⊕⊕⊝ | Two studies (CBT) | |
| 149 per 1000 | 146 per 1000 | |||||
| Dropout long term (longer than 12 months)* | Study population | RR 0.80 | 469 | ⊕⊕⊝⊝ | 46‐Month results | |
| 523 per 1000 | 419 per 1000 | |||||
| Response (50% reduction in BDI) medium term (7 to 12 months)* | Study population | RR 1.73 | 475 | ⊕⊕⊝⊝ | Two studies (CBT) | |
| 345 per 1000 | 434 per 1000 | |||||
| Response (50% reduction in BDI) long term (longer than 12 months)* | Study population | RR 1.62 | 248 | ⊕⊕⊝⊝ | 46‐Month results | |
| 268 per 1000 | 434 per 1000 | |||||
| Remission (< 7 on HAMD or < 10 on BDI) medium term (7 to 12 months)* | Study population | RR 1.97 | 475 | ⊕⊕⊕⊝ MODERATEa | Two studies (CBT) | |
| 223 per 1000 | 439 per 1000 | |||||
| Remission (< 7 on HAMD or < 10 on BDI) long term (longer than 12 months)* | Study population | RR 1.56 | 248 | ⊕⊕⊝⊝ | 46‐Month results | |
| 179 per 1000 | 279 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aOutcome assessment not blind. bWide confidence intervals. cSingle study data. dResults at 46 months favour psychotherapy intervention when earlier results (6‐month and 12‐month) showed no difference. eReporting bias likely as less frequently reported than remission or mean scores. | ||||||
| Study ID | Total N randomised | Follow‐up time point, months | Reason for dropout given in Intervention group (psychotherapy as an adjunct to usual care) | Reason for dropout given in control group (usual care alone) |
| 24 | 4 | 1 difficulty finding child care; 1 work schedule conflict; 1 decided group was not a good fit | 1 moved; 1 medical problem | |
| 469 | 6 | 25 not followed up | 22 not followed up | |
| 12 | 36 not followed up | 37 not followed up | ||
| 25 | 4 | NA | 2 lost to follow‐up | |
| 80 | 6 | 1 not contactable; 1 patient discontinued because of lumbago | 1 not contactable; patient discontinued owing to family health problem | |
| 12 | 1 not contactable | 1 not contactable; 1 died | ||
| 60 | 6 | 2 did not start therapy; 3 not contactable; 2 withdrew | 3 not contactable | |
| N: number NA: not available | ||||
| Study ID | Measure | N psychotherapy + usual care | Final mean psych + usual care | SD psych + usual care | N usual care | Final mean usual care | SD usual care | Effect size (Cohen's D)a | Significance (as reported in the study) |
| SAS workb | 10 | 65.7 | 19.27 | 9 | 69.56 | 17.66 | 1.60 | P < 0.05 | |
| LIFE workb | 10 | 2.7 | 1.34 | 9 | 3.11 | 1.69 | 0.56 | Not significant | |
| SAS social or leisureb | 10 | 64.30 | 12.91 | 9 | 72.56 | 16.21 | 0.77 | Not significant | |
| LIFE recreationb | 10 | 2.7 | 1.06 | 9 | 3 | 1.19 | 0.49 | Not significant | |
| LIFE satisfactionb | 10 | 2.7 | 0.95 | 9 | 3.33 | 1.19 | 1.12 | P < 0.05 | |
| SOS‐10c | 10 | 35.3 | 13.12 | 9 | 21.56 | 11.09 | 1.18 | P < 0.05 | |
| N: number P: P value SD: standard deviation aCohen's D > 0.5 is moderate effect and > 0.8 is large effect. bSAS‐SR and LIFE‐RIFT (SAS work/social recreational, LIFE work/recreation/satisfaction): Lower scores are healthier. cSchwartz Outcome Scale‐10 (SOS‐10): Higher scores are healthier. | |||||||||
| Study ID | Measure | Time point (months) | N psychotherapy + usual care | N usual care | Mean difference | 95% CI lower | 95% CI upper |
| Unpublished toola | 4 | 14 | 9 | 1.20 | ‐1.61 | 4.01 | |
| SF‐12 mentalb | 6 | 201 | 209 | 6 | 3.5 | 8.2 | |
| SF‐12 mentalb | 12 | 194 | 195 | 4.1 | 1.6 | 6.7 | |
| SF‐12 mentalb | 46 | 132 | 110 | 3.5 | 0.7 | 6.3 | |
| SF‐12 physicalb | 6 | 201 | 209 | −1.7 | −3.4 | 0.02 | |
| SF‐12 physicalb | 12 | 194 | 195 | 0.3 | −1.4 | 2 | |
| SF‐12 physicalb | 46 | 132 | 110 | 0.9 | ‐2 | 3.7 | |
| WHOQOL overall QOLc | 6 | 16 | 18 | 0.80 | ‐2.67 | 4.27 | |
| WHOQOL physicalc | 6 | 16 | 18 | 7.10 | ‐3.04 | 17.24 | |
| WHOQOL psychologicalc | 6 | 16 | 18 | 3.00 | ‐8.51 | 14.51 | |
| WHOQOL socialc | 6 | 16 | 18 | 6.50 | ‐6.71 | 19.71 | |
| SF‐36 mentalb | 6 | 40 | 40 | ‐2.32 | ‐7.25 | 2.6 | |
| SF‐36 mentalb | 12 | 40 | 40 | ‐1.27 | ‐6.26 | 3.71 | |
| SF‐36 physicalb | 6 | 40 | 40 | ‐1.17 | ‐6.46 | 3.81 | |
| SF‐36 physicalb | 12 | 40 | 40 | 0.95 | ‐4.4 | 6.82 | |
| CI: confidence interval N: number aA 6‐item instrument (unpublished) on which one could score between zero and 12: lower scores denote poorer QOL bSF physical/mental: Higher score denotes better quality of life cWHOQOL: Higher scores denote higher quality of life. | |||||||
| Study ID | Outcome | Measure | Time point, months | N psychotherapy + usual care | N psychotherapy + usual care with outcome | % | N usual care | N usual care with outcome | % |
| Serious adverse event | Hospitalisation due to depression exacerbation | 12 | 40 | 0 | 0 | 40 | 2 | 5 | |
| Serious adverse event | Suicide | 6 | 40 | 0 | 0 | 40 | 1 | 2.5 | |
| Adverse event | Increases in depressive symptoms | 6 | 30 | 0 | 0 | 30 | 2 | 6 | |
| N: number | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Self‐reported depressive symptoms short term (up to 6 months) ‐ BDI Show forest plot | 5 | 575 | Mean Difference (IV, Random, 95% CI) | ‐4.07 [‐7.07, ‐1.07] |
| 1.1 CBT with usual care vs usual care alone | 3 | 522 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐7.49, ‐1.63] |
| 1.2 DBT with usual care vs usual care alone | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐10.79 [‐23.83, 2.25] |
| 1.3 IPT with usual care vs usual care alone | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐6.70, 8.30] |
| 2 Self‐reported depressive symptoms short term (up to 6 months) ‐ PHQ‐9 Show forest plot | 2 | 482 | Mean Difference (IV, Random, 95% CI) | ‐4.66 [‐8.72, ‐0.59] |
| 2.1 ISTDP with usual care vs usual care alone | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐7.25 [‐11.37, ‐3.13] |
| 2.2 CBT with usual care vs usual care alone | 1 | 422 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐4.27, ‐1.73] |
| 3 Self‐reported depressive symptoms short term (up to 6 months) ‐ SMD (BDI & PHQ‐9) Show forest plot | 6 | 635 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.65, ‐0.14] |
| 3.1 CBT with usual care vs usual care alone | 3 | 522 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.56, ‐0.13] |
| 3.2 DBT with usual care vs usual care alone | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.66, 0.21] |
| 3.3 IPT with usual care vs usual care alone | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.60, 0.74] |
| 3.4 ISTDP with usual care vs usual care alone | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.41, ‐0.35] |
| 4 Clinician‐rated depressive symptoms short term (up to 6 months) ‐ HAMD Show forest plot | 4 | 193 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐5.71, ‐0.85] |
| 4.1 DBT with usual care vs usual care alone | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.81 [‐11.04, ‐0.58] |
| 4.2 IPT with usual care vs usual care alone | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.05, 4.25] |
| 4.3 ISTDP with usual care vs usual care | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐11.22, ‐0.46] |
| 4.4 CBT with usual care vs usual care alone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐5.75, ‐0.65] |
| 5 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ BDI Show forest plot | 2 | 475 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.21, 0.40] |
| 5.1 CBT with usual care vs usual care alone | 2 | 475 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.21, 0.40] |
| 6 Self‐reported depressive symptoms medium term (7 to 12 months) ‐ PHQ‐9 Show forest plot | 1 | 395 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.22, ‐0.58] |
| 7 Clinician‐rated depressive symptoms medium term (7 to 12 months) ‐ HAMD Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐7.88, ‐1.52] |
| 7.1 CBT with usual care vs usual care alone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐7.88, ‐1.52] |
| 8 Self‐reported depressive symptoms long term (longer than 12 months) ‐ BDI Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐7.57, ‐0.83] |
| 9 Self‐reported depressive symptoms long term (longer than 12 months) ‐ PHQ‐9 Show forest plot | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.26, 0.06] |
| 10 Dropout short term (up to 6 months) Show forest plot | 6 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.24] |
| 10.1 CBT with usual care vs usual care alone | 3 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.16] |
| 10.2 IPT with usual care vs usual care alone | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.33] |
| 10.3 DBT with usual care vs usual care alone | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.26, 6.28] |
| 10.4 ISTDP with usual care vs usual care alone | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.67, 8.18] |
| 11 Dropout medium term (7 to 12 months) Show forest plot | 2 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.47] |
| 11.1 CBT with usual care vs usual care alone | 2 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.47] |
| 12 Dropout long term (longer than 12 months) Show forest plot | 1 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.97] |
| 13 Response (50% reduction in depressive symptoms from baseline) short term (up to 6 months) Show forest plot | 4 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.20, 2.69] |
| 14 Response (50% reduction in depressive symptoms from baseline)medium term (7 to 12 months) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.42, 2.10] |
| 15 Response (50% reduction in depressive symptoms from baseline) long term (longer than 12 months) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.13, 2.32] |
| 16 Remission (< 7 on HAMD or < 10 on BDI) short term (up to 6 months) Show forest plot | 6 | 635 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.46, 2.52] |
| 17 Remission (< 7 on HAMD or < 10 on BDI) medium term (7 to 12 months) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.51, 2.56] |
| 18 Remission (< 7 on HAMD or < 10 on BDI) long term (longer than 12 months) Show forest plot | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.97, 2.53] |

