Interventions for the management of malignant pleural effusions: a network meta‐analysis
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single centre RCT comparing the efficacy of cosmetic talc with iodopovidone for pleurodesis (India) | |
| Participants | Inclusion: recurrent symptomatic pleural effusion with improvement of breathlessness with thoracentesis; or primary or secondary pneumothorax Exclusion: allergy to iodine; thyroid disorder; trapped lung; air leak; advanced malignancy with expected survival < 30 days 36 participants randomised | |
| Interventions | 28 Fr intercostal drain to completely drain effusion or treat pneumothorax. Pleurodesis agent given when < 150ml/day drainage and complete lung re‐expansion on chest x‐ray. All participants received intrapleural lignocaine (2 mg/kg) and IV tramadol prior to pleurodesis. Iodopovidone: 20 ml 10% iodopovidone in 80 ml saline Cosmetic talc: 5 g sterilised 'baby powder' After agent administered, chest tube clamped for four hours. Repeat administration of agent if > 250 ml/day drainage. Drain removed when < 100 ml/day output Followed up at 1 week, 1 month, 3 months and 6 months and then every 3 months thereafter | |
| Outcomes | Pleurodesis success according to need for thoracentesis (complete success = relief of symptoms related to the effusion and no re‐accumulation on CXR at 30 days; partial success = reduced dyspnoea related to the effusion with only partial re‐accumulation of fluid on chest x‐ray and no requirement for therapeutic thoracentesis; failure = lack of success as defined above) Chest pain (measured by visual analogue scale score) Complications Time to pleurodesis | |
| Notes | People with trapped lung excluded. Unpublished data obtained from authors relating to subgroup of participants in the study with malignant pleural effusion‐ only this data was included for the purposes of this review Included in network meta‐analysis for pleurodesis efficacy and fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | "Blinding of the allocation to treatments was not possible". Agents have different appearances |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence, visucal analogue scale scores and complications would all be biased by lack of patient blinding. Mortality would not be effected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up. Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | Cosmetic talc used rather than medicinal talc, but sterilised and comparable particle size by electron microscopy. No external funding for the study |
| Methods | Single centre RCT of povidone‐iodine and bleomycin pleurodesis for malignant pleural effusion (Iran) | |
| Participants | Inclusion: biopsy or cytologically proven malignant pleural effusion (all tumour types); recurrent and symptomatic effusion; chest radiograph confirming lung expansion of 90% after thoracentesis; Karnofsky Performance Score > 70 Exclusion: co‐morbidities that preclude general anaesthesia; bleeding disorders; massive thoracic skin infiltration; active infectious disease 39 participants randomised | |
| Interventions | All participants underwent a 28 Fr intercostal drain under local anaesthetic (+/‐ IV opiates if required). Study agent administered intrapleurally the next day with 5 ml 2% lidocaine Bleomycin group: 1 mg/kg bleomycin in 60 ml saline. 1 dose Povidone‐iodine group: 5% (volume unclear). 1 dose After administration of the study agent, the drain was clamped for one hour and removed when < 200ml fluid output/day. If the fluid output remained high after 10 days, they were discharged home with a Heimlich valve in place | |
| Outcomes | Effusion recurrence on chest x‐ray at 30 days Pain (measured by numeric scale) at discharge and day 30 Dysponea (measured by numeric scale) at discharge and day 30 | |
| Notes | Minimal raw data in results section ‐ tables quoted in text but not available on line. Attempted to contact study authors by e mails ‐ no response People with trapped lung excluded from trial entry Pleurodesis success measured only using chest x‐ray criteria Included in network meta‐analysis for pleurodesis efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Block randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Differing appearances of bleomycin and iodine make blinding not possible (although not stated explicitly in paper) |
| Blinding of outcome assessment (detection bias) | High risk | Pain and dyspnoea may be biased by lack of blinding. Not stated whether CXRs were evaluated by a blinded clinician. No response from study authors regarding this |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to see the tables. Response rates only given as % (no actual numbers), so unclear whether there was LTFU |
| Selective reporting (reporting bias) | Unclear risk | Raw data not provided for many of the outcomes. Tables missing |
| Other bias | Low risk | No other biases identified |
| Methods | Two‐centre RCT of intrapleural quinacrine (mepacrine) vs tetracycline via tube thoracostomy for malignant pleural effusion (USA) | |
| Participants | Inclusion: (1) documented cancer with pleural effusion (2) pleural fluid cytology or pleural biopsy confirming malignancy or exudate effusion presumed to be malignant (3) symptomatic from the effusion or rapidly re‐accumulating effusion > 500 ml All cell types. No exclusion criteria 20 participants randomised. | |
| Interventions | Both groups had a closed tube thoracostomy, drained overnight prior to the installation Quinacrine group: intrapleural quinacrine (100 mg in 30 ml normal saline) once daily for four days Tetracycline group: one dose of intrapleural tetracycline (500 mg in 30 ml N saline) The drains were clamped for six hours post installation with patient rotation. Drain removed when < 60 ml/24 hour drainage | |
| Outcomes | Pleurodesis success (defined on chest x‐ray criteria only at 30 days as 'Complete response' (complete lack of re‐accumulation of pleural fluid); 'Partial response' (re‐accumulation of pleural fluid < 50% of the volume present before the sclerosis); 'Failure' (re‐accumulation of fluid to > 50% of the volume present before the attempted sclerosis)) Side effects of treatment (pain, fever) | |
| Notes | People with trapped lung not excluded. CR and PR counted as a pleurodesis success for purposes of analysis One participant allocated to quinacrine arm having had treatment failure with tetracycline not included in the analysis Included in network meta‐analysis for pleurodesis efficacy and pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified and unable to contact study authors |
| Allocation concealment (selection bias) | Unclear risk | Not specified and unable to contact study authors |
| Blinding of participants and personnel (performance bias) | Unclear risk | No comment on whether study was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether CXR evaluation was blinded. Pain and fever outcomes may have been affected if patients were unblinded to treatment allocation, however not stated in the paper whether this was the case |
| Incomplete outcome data (attrition bias) | Low risk | Two of 14 randomised to tetracycline excluded from analysis (one died and one LTFU). No LTFU in mepacrine arm (overall LTFU 13%) |
| Selective reporting (reporting bias) | Low risk | All specified endpoints reported |
| Other bias | High risk | Eight of 22 participants included in the study did not have proven pleural malignancy |
| Methods | RCT of mitoxantrone versus mepacrine via an intercostal drain (Sweden ‐ number of centres not specified) | |
| Participants | Cytologically proven, symptomatic MPE with an expected survival of greater than three months (Karnofsky Performance Score > 60). Excluded if cytotoxic chemotherapy in the preceding month All cell types included 30 participants randomised | |
| Interventions | Both groups had a 12‐14 Fr chest tube inserted and effusion drained. Pleurodesis agent was given through the chest tube and patient's position changed for two hours after administration Group 1: 1 dose of intrapleural mitoxantrone 30 mg in 50 ml N saline was given; the drain was closed for 48 hours and removed after the 'pleural cavity was emptied' Group 2: 2 doses of intrapleural mepacrine chloride 200 mg in 20 ml N saline were given on consecutive days and the drain removed when < 150 ml fluid production/day | |
| Outcomes | Pleural fluid re‐accumulation at 4 and 12 weeks (defined as 'Complete response' (CR), 'Partial response' (PR) (if recurrence of pleural fluid but thoracocentesis not considered to be indicated) or 'Progressive disease'. Side effects/toxicity (visual analogue scale pain and fever scores) Symptom questionnaires (participant grades symptom on a numeric scale for four key symptoms‐ pain, shortness of breath, nausea and tiredness) Pharmacokinetics of mitoxantrone | |
| Notes | People with trapped lung not excluded from the study CR and PR counted as pleurodesis success for analysis Included in network meta‐analysis for pleurodesis efficacy and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified and unable to find contact details for study authors |
| Allocation concealment (selection bias) | Unclear risk | Not specified and unable to find contact details for study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Study personnel not blinded as drugs are of different colours. However, participants were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Participants blind to treatment allocation, therefore fever, pain and symptom scores unbiased. "Radiological evaluation was made by an independent radiologist' |
| Incomplete outcome data (attrition bias) | Low risk | One participant in each study arm did not receive treatment due to "unexpected medical emergencies", therefore deemed non‐evaluable. Follow‐up data clearly documented for the remaining patients |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | Drain suction use was imbalanced between the treatment arms (10/14 received suction in mepacrine group vs 1/14 in mitoxantrone group) |
| Methods | Single centre RCT of tetracycline pleurodesis using a small percutaneous catheter (CH10), compared to a large‐bore chest tube (CH24) inserted after thoracoscopy (Denmark) | |
| Participants | Symptomatic, recurrent MPE, proven on pleural fluid cytology. Expected survival of > 3 months (all tumour types included) 21 participants randomised | |
| Interventions | Group 1: small percutaneous catheter (CH10 65 cm) inserted under local anaesthesia Group 2: medical thoracoscopy, followed by insertion of a large‐bore chest tube (CH24) Both groups received pleurodesis with 500 mg tetracycline and 100 mg bupivicaine intrapleurally. The drain was clamped for six hours after instillation after which suction was applied. Drain removed when fluid output < 200 ml in 24 hours | |
| Outcomes | Treatment response at 3, 6 and 9 weeks defined by roentgenographic response ('Complete response' ‐ no recurrence of pleural fluid; 'Partial response' ‐ slight re‐accumulation with blunted costophrenic angle; 'No response' ‐ complete recurrence of pleural fluid) and clinical response (by the need for new thoracentesis) Questionnaire evaluating discomfort in connection with the tube and the pleurodesis | |
| Notes | Trapped lung not accounted for in inclusion/exclusion criteria, but one patient excluded as they had hydropneumothorax at time of instillation CR and PR included as pleurodesis successes for analysis Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation by lot" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind, as different drain sizes used (although not stated explicitly in the paper) |
| Blinding of outcome assessment (detection bias) | High risk | "All data were evaluated by the same physician, who was without knowledge of the result of the randomisation". However, symptom‐based adverse events and symptomatic need for repeat pleural intervention may be biased by lack of patient blinding |
| Incomplete outcome data (attrition bias) | Low risk | All data reported and justified. Missing outcome data balanced between the two treatment arms (two excluded from group 1 (one died of cancer soon after drain insertion and one developed hydropneumothorax necessitating large‐bore drain), one excluded from group 2 (patient withdrew consent for study participation) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT comparing thoracoscopic mechanical pleurodesis (TMP) with talc slurry (Slovenia) | |
| Participants | Inclusion: breast carcinoma and a resulting morphologically confirmed MPE Exclusion: unfit for general anaesthetic (GA) 87 participants randomised | |
| Interventions | TMP arm: thoracoscopy (under GA) with adhesiolysis, pleural biopsy and scarification of the visceral and parietal pleura to induce bleeding. Chest tube inserted at the end of procedure Talc slurry arm: chest tube inserted under local anaesthetic. 5 g talc in 100 ml saline insufflated through chest tube Participants in both arms had the drain removed when < 100ml/24hour drainage | |
| Outcomes | Recurrence of effusion on chest x‐ray (CXR) at 1 day, 1 week, 1 month, 3 months and 6 months Duration of chest tube drainage Duration of hospitalisation Complications Mortality (30 days and 6 months) | |
| Notes | People with trapped lung not excluded. Pleurodesis success defined using CXR criteria alone Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible to blind the study as comparing talc slurry with thoracoscopy |
| Blinding of outcome assessment (detection bias) | High risk | Not stated whether radiological assessments were done in a blinded fashion. Complication reporting, time of tube drainage may be effected by lack of patient and personnel blinding. Mortality outcome not effected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed. Minimal missing data. 6/45 patients died within six months in TMP group vs 8/42 in talc slurry arm |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No documentation of patient experience (e.g. QOL or degree of discomfort), relative costs or need for repeat pleural intervention Pleurodesis success defined using radiology only. Participants who did not have evidence of recurrence at death were classified as pleurodesis successes |
| Methods | Unblinded, multi‐centre RCT comparing indwelling pleural catheter (IPC) with talc slurry pleurodesis (UK)‐ TIME‐2 Trial. | |
| Participants | Inclusion criteria: clinically confident diagnosis of MPE requiring pleurodesis Exclusion criteria: age < 18, expected survival of < 3 months, chylothorax, previous ipsilateral lobectomy or pneumonectomy, previous attempted pleurodesis, pleural infection, WCC < 1000/microlitre, hypercapnic ventilatory failure, pregnancy, lactating mothers, irreversible bleeding diathesis, irreversible visual impairment 106 participants randomised | |
| Interventions | Group 1: IPC inserted with drainage three times a week (or as required to relieve dyspnoea) Group 2: 12 F Seldinger chest tube and 4 g talc slurry as an inpatient All patients had standard oncological management for the primary tumour | |
| Outcomes | Primary outcome: mean daily dyspnoea visual analogue score (VAS) over the first 42 days Secondary outcomes: proportion achieving clinically significant decrease in mean VAS dyspnoea; mean VAS dyspnoea at 6 weeks, 3 months and 6 months; mean daily chest pain VAS over the first 42 days; mean VAS chest pain at 6 weeks, 3 months and 6 months; nights spent in hospital; self‐reported quality of life; frequency of adverse events | |
| Notes | Participants with trapped lung in group 2 did not receive talc pleurodesis, but remained in trial follow‐up Pleurodesis in the IPC group was defined as removal of IPC following spontaneous cessation of drainage with no significant fluid recurrence on chest x‐ray (CXR) or ultrasound scan (USS) and no further ipsilateral pleural intervention. In the talc group, pleurodesis failure defined as the need for further ipsilateral pleural intervention If participants died during follow up, included as a pleurodesis success if no intervention prior to death Included in network meta‐analysis for pleurodesis efficacy and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel due to nature of interventions (IPC vs talc slurry) |
| Blinding of outcome assessment (detection bias) | High risk | VAS scores, QOL and symptom recurrence (which informs assessment of pleurodesis efficacy) could be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | LTFU clearly documented with reasons given |
| Selective reporting (reporting bias) | Low risk | All predefined endpoints reported |
| Other bias | Low risk | No other biases identified |
| Methods | Multi‐centre RCT comparing bedside talc pleurodesis and daily tunnelled catheter drainage for management of malignant pleural effusion (USA) | |
| Participants | Inclusion: symptomatic patients with histo/cytologically proven malignancy and a previously untreated, unilateral pleural effusion requiring management; ECOG performance score 0‐2 Exclusion: active pleural infection; talc allergy; contraindications to talc use; trapped lung; survival < 60 days; severe comorbid medical conditions 68 participants randomised | |
| Interventions | Talc pleurodesis group: 4 g to 5 g sterile talc slurry in 100 ml saline infused into pleural space via > 24 Fr chest drain. Tube clamped for two hours. Drain removed when < 150 ml drainage/24hours Indwelling pleural catheter (IPC) group: PleurX catheter inserted and drained daily (output volumes recorded). Removed when < 30 ml output on three consecutive days | |
| Outcomes | Primary: compare the proportion of maintained successful treatments 30 days after the intervention (success defined as being (1) alive (2) no effusion recurrence (3) > 90% lung re‐expansion after complete drainage (4) completion of the intervention by two weeks ie drain removed or IPC functioning normally) Secondary: Quality of life (QOL); dyspnoea; patient satisfaction and acceptability; lung expansion; pleurodesis success; fluid drainage volume; days device in place; removal of device before death; survival | |
| Notes | Pleurodesis success measured at 30 days according to chest x‐ray (CXR) and need for repeat pleural intervention People with known trapped lung excluded from trial entry Included in network meta‐analysis for pleurodesis efficacy and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation via a web‐based randomisation service |
| Allocation concealment (selection bias) | Low risk | Permuted block randomisation via a web‐based randomisation service |
| Blinding of participants and personnel (performance bias) | High risk | Due to nature of interventions, not possible to blind participants or personnel (IPC vs talc slurry) |
| Blinding of outcome assessment (detection bias) | High risk | "Pleurodesis success was classified by an unblinded local investigator" (personal communication). QOL, symptom recurrence and patient satisfaction questionnaires may be biased by lack of patient blinding |
| Incomplete outcome data (attrition bias) | Low risk | Five excluded from analysis in each arm, but justifications given |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | Target recruitment numbers not reached |
| Methods | Prospective, single centre RCT of thoracoscopic talc poudrage versus bedside bleomycin pleurodesis via a small‐bore chest tube (Switzerland) | |
| Participants | Inclusion criteria: documented MPE (all cell types); complete lung expansion on post drainage chest x‐ray (CXR); improvement in symptoms after drainage; expected survival of > 1 month; capable of undergoing medical thoracoscopy Exclusion criteria: loculated effusion; previous drainages or previous pleurodesis; adverse reaction to the study medication; severe coagulation disorder 36 participants randomised | |
| Interventions | Group 1: bedside pleurodesis via small‐bore chest tube (OD = 2.7 mm) of 60 IU bleomycin. Tube unclamped after two hours and left on suction until removal at least 48 hours later Group 2: thoracoscopy with induced pneumothorax under sedation. 5 g talc sprayed into pleural cavity under direct vision after drainage of effusion and disruption of adhesions. Drain kept under suction for at least 48 hours | |
| Outcomes | Recurrence of effusion (defined as a newly detected effusion needing drainage or occupying > 33% of the pleural space on CXR as compared with the first CXR after drain removal, or death from any cause) at 30, 90 and 180 days Medication use Volume of fluid drained Duration of hospital stay Cost Symptom VAS Scores (pain, shortness of breath, cough and general well‐being) | |
| Notes | People with trapped lung excluded from study enrolment Included in network meta‐analysis for pleurodesis efficacy, mortality, fever and pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants or personnel due to nature of interventions (talc poudrage vs bleomycin via chest tube) |
| Blinding of outcome assessment (detection bias) | High risk | Not stated if radiology was interpreted by a blinded physician. However length of stay, VAS scores and symptom recurrence may be biased by lack of participant blinding. Mortality would not be affected by unblinded nature of the study |
| Incomplete outcome data (attrition bias) | Low risk | Five withdrawals in total, but a similar number in each group |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No external funding source |
| Methods | Multi‐centre RCT comparing talc poudrage with talc slurry pleurodesis in MPE. Both groups received 4 g ‐ 5 g sterile talc intrapleurally (USA) | |
| Participants | Inclusion criteria: history of malignancy (all tumour types), pleural effusion requiring sclerosis, ECOG performance status 0‐2, life expectancy > 2 months, ability to undergo general anaesthesia Exclusion criteria: pregnancy, previous intrapleural therapy or radiation therapy encompassing the entire hemithorax, changes in systemic therapy within two weeks, chylous or bilateral effusions requiring therapy 501 participants randomised | |
| Interventions | TS Group: talc administered as a slurry in 100 ml saline through a chest tube at the bedside TTI Group: talc insufflated during thoracoscopy in the operating room | |
| Outcomes | Primary endpoint: the percentage of patients whose lung initially re‐expanded > 90% and who had a successful pleurodesis at 30 days after treatment (defined according to cvhest x‐ray (CXR) criteria) Secondary endpoint: time to recurrence of effusion; frequency of complications and toxicities; ability to re‐expand the lung as assessed by CXR; oain; patient satisfaction; quality of life (QOL). | |
| Notes | People with trapped lung excluded from analysis Included in network meta‐analysis for pleurodesis efficacy, mortality, pain and fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation lists |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation lists |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind the study due to the nature of the interventions (talc poudrage vs talc slurry) |
| Blinding of outcome assessment (detection bias) | High risk | Not stated if radiological assessment was blinded. QOL and complications may be affected by lack of patient and personnel blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing data accounted for and balanced between the treatment arms (10 in slurry group and 9 in thoracoscopy group excluded as ineligible or participant withdrew consent; 33/163 slurry participants and 25/177 thoracoscopy participants died within 30 days of randomisation) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | Trapped lung defined by different means in the two treatment arms, which may have effected their primary endpoint. However, this does not have an impact on the pleurodesis success rates |
| Methods | Single centre RCT of intrapleural cisplatin +/‐ bevacizumab in MPE due to non‐small cell lung cancer (NSCLC) (China) | |
| Participants | Inclusion: Advanced NSCLC; large uni‐ or bilateral pleural effusion; positive pleural fluid cytology; no intrapleural therapy in previous month; Karnofsky performance score > 60%; age > 18; predicted survival > 3 months; no major organ disfunction; no previous chemotherapy in previous six weeks Exclusion: squamous cell carcinoma; allergy to biological agents; no detectable lesions; uncontrolled central nervous system metastasis; pregnancy or breastfeeding; infected wound; refractory psychiatric illness 72 participants randomised | |
| Interventions | Participants underwent pleural fluid drainage by thoracentesis. Treatment given intrapleurally. Rest for two hours. Then rotate every 15 mins. Given every two weeks for 3 cycles Cisplatin: 30 mg cisplatin intrapleurally Cisplatin and bevacizumab: 30 mg cisplatin and 300 mg bevacizumab intrapleurally | |
| Outcomes | Treatment response ('Complete remission (CR)' = accumulated fluid disappeared and stable for at least four weeks; 'Partial remission (PR)' = > 50% of the accumulated fluid had disappeared, symptoms had improved and the remaining fluid had not increased for at least four weeks; 'Remission not obvious (NC)' = < 50% of the accumulated fluid had disappeared; 'Progression (PD)' = accumulated fluid had increased). Treatment success defined as CR + PR Progression‐free survival Overall survival Adverse reactions Quality of life (QOL) Pleural fluid VEGF levels | |
| Notes | People with trapped lung eligible for trial involvement Pleurodesis defined clinically and using radiology Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not stated and no response from study authors to clarify |
| Allocation concealment (selection bias) | Unclear risk | Methods not stated and no response from study authors to clarify |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if blinded and no response from study authors to clarify |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded. If not blinded, QOL, performance status, side effects and symptom recurrence could be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing data accounted for. ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other biases identified |
| Methods | Three‐arm, single centre RCT comparing intrapleural bleomycin, tetracycline and combination treatment for pleurodesis of MPE (Iran) | |
| Participants | Inclusion: histologically or cytologically proven, symptomatic MPE (all cell types) Exclusion: none 60 participants randomised | |
| Interventions | All participants had 28 Fr intercostal drain inserted into 6th intercostal space. Complete drainage of the effusion was confirmed on chest x‐ray (CXR). All participants given 10‐15ml 1% lignocaine intrapleurally Tetracycline arm: 20 mg/kg tetracycline (max 2 g) in 50 ml saline given intrapleurally. 1 dose Bleomycin arm: 1 u/kg (max 60 units) in 50 ml saline given intrapleurally. 1 dose Combination arm: 20 mg/kg tetracycline in 40 ml saline and 1 u/kg bleomycin in 50 ml saline, given intrapleurally, one after the other (tube clamped for five mins between instillations) Drain clamped for two hours post instillation with patient rotation. Suction connected after 24 hours. Drain removed when < 50 ml/8 hours drainage and complete lung expansion on CXR | |
| Outcomes | Pleurodesis success (defined as 'complete response' (no accumulation of effusion on CXR), 'partial response' (effusion recurred but did not require aspiration) or 'failure' (participant required repeat thoracentesis for re‐accumulation of the effusion) at 30 days (also at 60 days, 90 days and 6 months) Side effects | |
| Notes | All participants in the study were receiving chemotherapy or tamoxifen, or both People with trapped lung not excluded from participation in the study Participants who died prior to the analysed time point were excluded from the analysis Combination of clinical need for repeat intervention and radiological re‐accumulation of effusion used to define pleurodesis failure Included in network meta‐analysis for pleurodesis efficacy, mortality and fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "'...simple randomised manner". No further details given |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly and unable to contact authors. However, different volumes and regimes were used for the two groups |
| Blinding of outcome assessment (detection bias) | High risk | Not stated if radiology reported blindly. Complication‐reporting may have been affected by lack of participant blinding |
| Incomplete outcome data (attrition bias) | Low risk | Minimal data on baseline patient characteristics, but all outcome data reported and withdrawals justified. Six participants died within six months of randomisation (2 in tetracycline arm; 1 in bleomycin arm and 3 in combination arm) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of medical vs surgical pleurodesis with tetracycline (UK) | |
| Participants | Inclusion criteria: cytology‐proven MPE and histological or cytological evidence of metastatic breast cancer Exclusion criteria: unsuitable for general anaesthetic (GA); > 75 years old; severe non‐metastatic lung disease; evidence of life‐threatening metastatic disease at other sites 34 participants randomised | |
| Interventions | Medical group: intercostal cannula inserted into mid‐axillary line 7th/8th intercostal space and fluid aspirated. When drainage complete, 500 mg tetracycline in 100 ml N saline inserted IP. Drain removed after 24 hours Surgical group: under GA, bronchoscopy then thoracoscopy performed. 500 ml tetracycline in 100 ml saline inserted after fluid removed. Drain removed at 24 hours | |
| Outcomes | Fluid re‐accumulation on chest x‐ray (CXR) Need for repeat pleural aspirations Mortality | |
| Notes | Pleurodesis failure defined as need for repeat aspiration. Trapped lung not accounted for Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given regarding randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details given regarding randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to nature of the interventions (surgery vs chest tube) |
| Blinding of outcome assessment (detection bias) | High risk | Need for repeat aspirations and other treatments given for cancer after pleurodesis may have been biased by lack of blinding of personnel and participants. Not stated if CXRs were reported by a blinded person |
| Incomplete outcome data (attrition bias) | Low risk | Reasons given for withdrawals (5/34 excluded (15%) ‐ 3 never received the treatment; 1 was randomised in error; 1 participant's records were lost) |
| Selective reporting (reporting bias) | High risk | No data on safety or side effects |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of talc poudrage and mustine (via chest tube) in patients with breast cancer. All patients underwent VATS procedure under general anaesthetic. (UK) | |
| Participants | Inclusion criteria: histologically confirmed breast cancer and radiologically verified pleural effusion Exclusion criteria: no previous local treatment; non‐malignant cause for the effusion 46 participants randomised | |
| Interventions | Talc group: talc poudrage performed during VATS (dose of talc not stated), two chest drains in place for five days (with or without suction) Mustine group: after VATS and once lung fully re‐expanded on CXR, 15 mg mustine solution instilled via intercostal drain. Clamped for two hours. Drain removed when drainage stopped | |
| Outcomes | Success of pleurodesis (defined by lack of re‐accumulation of effusion on CXR) at one month; complications | |
| Notes | If died prior to one‐month follow up, excluded from analysis of pleurodesis success Participants with trapped lung eligible for enrolment Included in network meta‐analysis for pleurodesis efficacy and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified for metastatic disease requiring treatment. "balanced randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind patients or personnel due to the nature of the procedures |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether radiographic interpretation of CXRs were performed by a blinded person. Reporting of complications could be biased by lack of participant and personnel blinding |
| Incomplete outcome data (attrition bias) | Low risk | 3/23 non‐evaluable in talc group; 6/23 non‐evaluable in mustine group. All non‐evaluable patients died prior to one‐month follow up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | High risk | Different number of intercostal drains in the two groups. Different duration of drainage for two groups |
| Methods | Single centre RCT of intrapleural talc and tetracycline in MPE secondary to breast cancer (UK) | |
| Participants | Inclusion criteria: histologically confirmed breast cancer and a symptomatic pleural effusion on radiology Exclusion criteria: previous treatment for effusion, other than simple needle aspiration; non‐malignant cause for effusion; unsuitable for general anaesthetic; history of sensitivity to tetracycline 41 participants randomised | |
| Interventions | Talc group: thoracoscopy, talc insufflated (dose not stated). Intercostal drain remained in situ for five days Tetracycline group: thoracoscopy. Tetracycline 500 mg in 40 ml N saline inserted 16 ‐ 24 hours later via chest tube. Intercostal drain left in place for 3 ‐ 5 days | |
| Outcomes | Pleurodesis success (defined by lack of re‐accumulation on CXR); complications; mortality | |
| Notes | Pleurodesis success defined according to CXR only Participants with trapped lung eligible for trial entry Included in network meta‐analysis of pleurodesis efficacy and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised with stratification for metastatic disease" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind patients or personnel due to the nature of the procedures |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether radiographic interpretation of CXRs were performed by a blinded person. Reporting of complications could be biased by lack of participant and personnel blinding |
| Incomplete outcome data (attrition bias) | High risk | Participants were excluded from the primary analysis if they died within the first month. Higher proportion of deaths in the talc group (6/18 = 33%) compared to the tetracycline group (2/23 = 9%) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre, prospective RCT comparing intrapleural administration of mistletoe preparation (viscum fraxini‐2) with bleomycin in patients with MPE (Egypt) | |
| Participants | Inclusion: histologically confirmed, recurrent, symptomatic MPE (all cell types); > 18 years old; ECOG performance score ≤ 2; adequate bone marrow, liver and kidney function; written consent; ability to comply with the follow up Exclusion: chronic air leak; known hypersensitivity to mistletoe; uncorrectable bleeding tendency; encysted pleural effusion; pregnancy/breastfeeding; currently active second malignancy; co‐enrolment in another clinical trial; previous unsuccessful pleurodesis; pleural infection 23 participants randomised | |
| Interventions | Participants underwent effusion drainage using a chest tube or needle drainage (depending on effusion size). Agent injected through the needle or chest tube viscum group: 5 ampoules in 10 ml 5% glucose instilled intrapleurally Bleomycin group: 60 units delivered intrapleurally | |
| Outcomes | Pleurodesis efficacy (assessed at six weeks) Toxicity (measured using NCI common terminology for adverse events) | |
| Notes | People with trapped lung not excluded from participation Pleurodesis defined using radiology and symptomatic effusion recurrence Included in network meta‐analysis for pleurodesis efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised". No other details given and no response from study authors |
| Allocation concealment (selection bias) | Unclear risk | "randomised". No other details given and no response from study authors |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly but drugs were of different formulations |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | High risk | Two patients in viscum arm excluded from analysis as treatment was discontinued due to an allergic reaction |
| Selective reporting (reporting bias) | Low risk | Data available although minimal data on side effects |
| Other bias | Low risk | No other risks of bias identified |
| Methods | Single centre RCT evaluating duration of chest tube drainage after a talc slurry pleurodesis (UK) | |
| Participants | Inclusion criteria: confirmed MPE requiring palliation of breathlessness due to the effusion (all cell types) Exclusion criteria: expected survival < 3 months; Karnofsky score < 40; previous unsuccessful pleurodesis; ipsilateral endobronchial obstruction; evidence of trapped lung 41 participants randomised | |
| Interventions | All participants had 8 ‐ 14 Fr intercostal drain inserted under ultrasound guidance. 4 g talc slurry when effusion fully drained and trapped lung excluded on CXR In one group, drain removed after 24 hours. In the other group, drain removed at 72 hours. Drains removed regardless of fluid drainage | |
| Outcomes | Pleurodesis failure at one month (defined according to fluid recurrence requiring repeat aspiration). Length of hospital stay. Mortality | |
| Notes | People with trapped lung excluded from the study. Study didn't complete recruitment numbers required by the power calculation Participants who died in first month after randomisation excluded from the analysis Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes in random blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes in random blocks of 10 |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind due to nature of interventions (drain removal after 24 or 48 hours) |
| Blinding of outcome assessment (detection bias) | High risk | Need for repeat pleural interventions, length of stay may be biased by lack of blinding. Mortality data not biased |
| Incomplete outcome data (attrition bias) | Low risk | Deaths within the first month well matched between the two arms (3 patients in each arm). No other LTFU |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported. Unpublished data on complications provided by the authors |
| Other bias | Low risk | No other biases identified |
| Methods | RCT comparing intrapleural mitoxantrone with normal saline after thoracoscopy in patients with MPE (Germany) | |
| Participants | Inclusion: complete resolution of the effusion after thoracoscopy; malignancy on pleural biopsy Exclusion: No chemotherapy within four weeks of pleurodesis 103 participants randomised | |
| Interventions | All participants underwent thoracoscopy. After 24 hours participants were randomised Mitoxantrone arm: 30 mg mitoxantrone given intrapleurally Control arm: isotonic saline instilled intrapleurally Drain clamped for 48 hours and if > 300 ml effusion after 48 hours, a second dose was given; if not the drain was removed. If a second dose was given, the drain was removed 48 hours later | |
| Outcomes | Pleural fluid re‐accumulation at two months (defined as a complete response (complete disappearance of all pleural effusion), partial response (half of the effusion or doubling of the time for thoracocentesis) no change (the same volume of effusion) or progressive disease (uncontrollable effusion) Toxicity Remission duration Survival | |
| Notes | Treatment response definitions somewhat unclear People with trapped lung eligible for trial involvement Included in network meta‐analysis for pleurodesis efficacy and fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding or whether drugs were of similar appearances or volumes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether CXR interpretation was blinded to treatment allocation. Side effects and performance status reporting could be biased if participants and personnel were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 8/103 participants excluded from the analysis (7 died within four weeks of randomisation due to tumour progression; 1 was lost to follow up) |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | High risk | Ambiguous definitions of pleurodesis success |
| Methods | Single centre RCT comparing talc slurry and bleomycin pleurodesis (Brazil) | |
| Participants | Inclusion: documented recurrent symptomatic MPE (with positive cytology or confirmed metastatic disease elsewhere with no other cause found for the effusion); symptomatic relief by therapeutic aspiration; complete lung re‐expansion after therapeutic aspiration Exclusion: previous unsuccessful pleurodesis; pleural infection; chronic air leak; karnofsky performance score < 30% 71 participants randomised | |
| Interventions | 28 ‐ 36 Fr chest tube inserted under local anaesthetic. Lung re‐expansion confirmed prior to randomisation Talc group: 4 g talc in 100 ml saline intrapleurally Bleomycin group: 60 units of bleomycin in 100 ml saline intrapleurally After instillation, drain clamped for four hours, then put on suction for 24 hours. Drain removed when < 200 ml/24hours drained | |
| Outcomes | Pleurodesis success (defined as no recurrence of effusion on clinical and radiologic follow‐up or patient symptom‐free with small residual effusion not requiring thoracentesis) at 1, 3 and 6 months Length of hospital stay Cost analysis Complications | |
| Notes | People with trapped lung excluded from trial entry Included in network meta‐analysis for pleurodesis efficacy and mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Computer randomisation |
| Blinding of participants and personnel (performance bias) | High risk | "Study not blinded" (personal communication with authors) |
| Blinding of outcome assessment (detection bias) | High risk | "study not blinded" (personal communication with authors). Not stated if radiology reported blindly but pleurodesis efficacy also based on symptom recurrence, so could be biased by lack of participant blinding |
| Incomplete outcome data (attrition bias) | Low risk | All reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported and further clarification received from authors regarding complications and mortality |
| Other bias | High risk | High levels of steroid use in participants, which may have effected pleurodesis success rates. Steroid use not well balanced between the treatment arms (4/37 in talc group, 8/34 in beomycin group) |
| Methods | Prospective, single centre RCT of bleomycin and talc in MPE secondary to breast cancer (UK) | |
| Participants | Inclusion criteria: breast carcinoma with radiographically confirmed pleural effusion Exclusion criteria: previous local treatment (apart from simple aspiration); evidence of a non‐malignant cause for the effusion 29 participants randomised | |
| Interventions | All participants had effusion drained to dryness under general anaesthetic Talc group: talc pleurodesis (dose and mode of administration not specified, but assumed to be poudrage from text) Bleomycin group: chest tube inserted. Bleomycin 1 mg/kg in 50 ml normal saline instilled after a CXR confirming lung re‐expansion | |
| Outcomes | Success of pleurodesis (defined as continued absence of re‐accumulation of pleural fluid on all follow‐up radiographs) | |
| Notes | Different modes of administration of talc and bleomycin Contacted study authors for more information, but no reply People with trapped lung eligible for study entry Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind due to the nature of the interventions (talc poudrage vs bleomycin) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether radiology reporting was blinded |
| Incomplete outcome data (attrition bias) | Low risk | A number of participants not included in the primary analysis, but balanced numbers between the two treatment arms (4/13 in talc group, 3/16 in bleomycin group) |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | No other biases identified |
| Methods | Multi‐centre RCT of pleurodesis using Corynbacterium parvum vs bleomycin (Sweden) | |
| Participants | Inclusion criteria: pleural effusion due to metastases from cytologically‐ or histologically‐proven bronchogenic carcinoma or adenocarcinoma; at least two previous aspirations of effusion 40 participants randomised | |
| Interventions | Corynebacterium parvum 7 mg in 10 ‐ 20 ml saline IP or bleomycin 60 mg in 100 ml saline intrapleurally A second dose of the allocated agent was given if the first was ineffective No details given about method of drainage prior to instillation of pleurodesis agent or how long the drain remained in place | |
| Outcomes | Pleurodesis success ("Success" = no recurrence of fluid within six weeks; "Partial success" = 2 instillations required within six weeks, with no recurring effusion within six weeks of the second instillation) | |
| Notes | People with trapped lung eligible for trial entry For the purposes of this review, if participants required more than one treatment due to effusion recurrence within six weeks, they were counted as a failure Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | No specific mention of blinding but drugs reconstituted in different volumes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Definition of pleurodesis efficacy quite vague and not stated if blinded. Side effect reporting may be influenced by lack of blinding of participants and personnel |
| Incomplete outcome data (attrition bias) | High risk | No data on mortality. Numbers don't add up for side effects data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of intrapleural cisplatin vsOK‐432 vs combination (Japan) | |
| Participants | Inclusion criteria: symptomatic, histocytologically confirmed pleural malignancy secondary to Non‐small cell lung cancer(NSCLC), ECOG performance score 0‐3, adequate renal, haematological and cardiac function Exclusion Criteria: previous intrapleural therapy, trapped lung or atelectasis after chest tube inserted 49 participants randomised | |
| Interventions | All participants underwent pleural fluid drainage via a 20 Fr chest tube. After administration of the allocated treatment, chest drain was clamped for six hours and then connected to 20 cm H2O suction. Drain removed when < 100 ml/day Cisplatin group: 50 mg cisplatin via chest tube on day 1 and 4 ok‐432 group: one dose of 5 KEOK‐432 via chest tube Combination group: 50 mg cisplatin on day 1 and 4, followed by 5 KEOK‐432 on day 7 | |
| Outcomes | Effusion recurrence (as defined by a newly detected effusion needing drainage or occupying > 33% of pleural space on CXR); mortality; adverse events | |
| Notes | people with trapped lung excluded from the study Study authors contacted for further information, but no response Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding but participants received different dosing regimes depending on study arm |
| Blinding of outcome assessment (detection bias) | High risk | Adverse event reporting could be affected by knowledge of treatment allocation. Not stated whether CXR interpretation was performed in a blinded fashion for definition of pleurodesis efficacy |
| Incomplete outcome data (attrition bias) | Low risk | Number of deaths clearly stated. If participants died, still included in analysis for pleurodesis success prior to death |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes reported |
| Other bias | High risk | Drain left in for different duration in the three groups. Steroids were given to participants who received cisplatin |
| Methods | Multicentre phase 2 trial ofOK‐432, evaluating two different doses of intrapleural (IP) OK‐432 (Japan) | |
| Participants | Inclusion criteria: histological or cytological proof of MPE with non‐small cell lung cancer (NSCLC); no previous therapy for MPE; age > 20; ECOG performance score 0‐3; life expectancy > 12weeks; adequate organ and bone marrow function; daily chest tube drainage < 200 ml Exclusion criteria: previous TB pleuritis; unstable heart disease or diabetes; active double cancer; pregnancy; lactation; allergy to OK‐432 or benzylpenicillin 38 participants randomised | |
| Interventions | All participants underwent chest tube drainage. Two doses ofOK‐432 given (on days 1 and 3) Arm A: IPOK‐432 at a dose of 10 KE in 100 ml saline Arm B: IPOK‐432 at a dose of 1 KE in 100 ml saline | |
| Outcomes | MPE control on day 28 (defined as a complete response (the effusion disappeared completely and no further treatment required), partial response (the effusion persisted but local treatment was not needed) or no change (further local treatment was needed or the residual effusion volume was > 100 ml) MPE control rate Duration of drainage Fluid volume drained Time to progression Drug adverse events Overall survival | |
| Notes | People with trapped lung included in the study For purposes of this review, complete and partial responses were counted as pleurodesis successes Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated whether blinded. Drugs diluted in same volume in both study arms |
| Blinding of outcome assessment (detection bias) | Unclear risk | Need for repeat intervention and side effects could be biased if patients and personnel unblinded, but not stated if this was the case |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | High risk | In arm B, if low dose ineffective, patients given a high dose of OK‐432 anyway (prior to measurement of primary outcome) Paper does not state whether patients were symptomatic from MPE at enrolment |
| Methods | Single centre RCT of intrapleural Adriamycin, nitrogen mustard and rolitetracycline (Australia) | |
| Participants | Histocytologically confirmed malignant effusions (pleural or pericardial or peritoneal); no previous intracavitary chemotherapy; no concurrent radiotherapy or systemic treatment 38 participants reported as being randomised in total (26 of whom had malignant pleural effusion). However in the discussion it refers to 90 participants being randomised originally | |
| Interventions | All participants had a needle thoracentesis to dryness. The drug was diluted in 20 ml saline and injected through needle as a bolus Adriamycin group: 30 mg intrapleurally Nitrogen mustard group: 20 mg intrapleurally Rolitetracycline group: 500 mg intrapleurally | |
| Outcomes | Pleurodesis success at eight weeks (defined as complete response (CR) (absence of significant effusion on CXR), partial response (reduction in frequency of aspiration with improvement in exercise tolerance and CXR) or no response) Complications | |
| Notes | People with trapped lung eligible for the trial For the purposes of this review, only data on participants with pleural effusions included in our analysis and only CR counted as a pleurodesis success Included in network meta‐analysis for pleurodesis efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of whether anyone was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if CXR interpretation was done blind to treatment allocation |
| Incomplete outcome data (attrition bias) | High risk | "More than half of the original 90 patients randomised were ineligible for assessment because of subsequent systemic therapy... or... early death". Although in the results, it states 38 participants were randomised |
| Selective reporting (reporting bias) | Low risk | Only a brief report and side effects data for the pleural and peritoneal effusions combined. However, generally all predefined outcomes reported |
| Other bias | Unclear risk | Six participants received more than one of the treatments, but not clear whether re‐randomised separately each time |
| Methods | Single centre RCT comparing intrapleural (IP) bleomycin and tetracycline in MPE (USA) | |
| Participants | Inclusion: histologically proven malignancy; symptomatic pleural effusion with either > 3 g/dl protein or malignant cells on cytology Exclusion: allergy to either study drug 42 procedures randomised in 34 participants | |
| Interventions | All participants underwent chest tube drainage Tetracycline arm: 500 mg tetracycline in 50 ml saline IP. 1 dose Bleomycin arm: 89 units in 50 ml saline IP. 1 dose For both arms, drain clamped for eight hours after instillation and participant moved positions. Thereafter, tube opened and suction applied. Drain removed when < 40 ml/24hours drained (or on day 7 if ongoing high output) | |
| Outcomes | Treatment response at one month ('Complete response' (no re‐accumulation of the effusion); 'Partial response' (asymptomatic re‐accumulation of the effusion developed that was < 50% of its original volume); 'no response') Side effects Length of time chest tube in place following pleurodesis | |
| Notes | Bilateral disease included. Some participants randomised to the trial more than once People with trapped lung eligible for trial entry Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Toss of coin" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding. Both drugs administered in 50 ml saline |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. No mention of whether CXR interpretation was performed by a blinded individual |
| Incomplete outcome data (attrition bias) | High risk | 11/34 (32%) participants non‐evaluable for pleurodesis outcome (3 in bleomycin group and 8 in tetracycline group) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | High risk | Unclear whether participants who were given both agents because the first agent failed were included in the analysis |
| Methods | Single centre, prospective RCT of mepacrine versus bleomycin as pleurodesis agent in malignant pleural effusion (Norway) | |
| Participants | Inclusion: malignant pleural effusion; previous treatment with a therapeutic tap; life expectancy of > 1 month Exclusion: previous pleurodesis; renal failure; participantrequiring continuous oxygen 40 patients randomised. | |
| Interventions | 28 or 32 Fr chest tube inserted under local anaesthetic. Suction applied until fluid production about 100 ml/day and no effusion on CXR. Tube clamped and sclerosing agent injected. Patient rotation for two hours after instillation. Drain removed when < 100 ml/day output Mepacrine group: 800 mg mepacrine in 20 ml saline Bleomycin group: 60 mg bleomycin in 100 ml saline | |
| Outcomes | Pleurodesis success (classified as (1) no re‐accumulation (2) small amounts of fluid re‐accumulation with no or mild symptoms (3) re‐accumulation of fluid with severe dyspnoea needing thoracocentesis) Median survival Side effects | |
| Notes | People with trapped lung not excluded from trial entry For purposes of this review, participants with no re‐accumulation or small amount of re‐accumulation with no or mild symptoms were counted as pleurodesis successes Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not stated specifically but drugs reconstituted in different volumes |
| Blinding of outcome assessment (detection bias) | High risk | Participant reporting of symptoms may be effected by lack of blinding. Not stated whether CXR interpretation was blind to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | High mortality in first three months, therefore data only analysed at month 1 |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre, prospective RCT of talc vs doxycyline in the control of MPE (Poland) | |
| Participants | Inclusion criteria: pleural effusion with clinical suspicion of malignant origin Exclusion criteria: failure to confirm malignancy by pleural biopsy; mesothelioma; failure to achieve full re‐expansion of the lung 33 participants randomised | |
| Interventions | All participants all VATS under general anaesthetic and pleural biopsy. First dose of sclerosant given at end of procedure. Tube removed when full re‐expansion, no air leak and < 150 ml/day drainage. Rotation after procedure Talc: single 10 g dose intrapleurally by insufflation Doxycycline: 500 mg in 25 ml solution given intrapleurally. Up to 3 doses (if daily drainage > 150 ml/day) | |
| Outcomes | 'Long term' and 'short term' pleurodesis outcome (defined by need for repeat thoracentesis as 'Excellent' (no fluid re‐accumulation), 'Good' (limited residual fluid, not increasing, no indications for thoracentesis) or 'Poor' (fluid re‐accumulation requiring thoracentesis) Complications | |
| Notes | For purposes of this review, 'Excellent' and 'Good' pleurodesis outcomes included as pleurodesis successes for analysis Study authors emailed for further information, but no response Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to the nature of the interventions, although not stated explicitly |
| Blinding of outcome assessment (detection bias) | High risk | Pleurodesis efficacy defined by symptom recurrence and hence could be biased by lack of blinding. Not stated whether assessment of fluid re‐accumulation was performed by a blinded individual |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of participants randomised not clear from paper |
| Selective reporting (reporting bias) | High risk | Treatment complications and survival not reported |
| Other bias | High risk | Number of doses for the two arms, therefore potential for confounding |
| Methods | RCT (two recruiting centres) of intrapleural Corynebacterium parvum and tetracycline for pleurodesis of malignant pleural effusion (UK) | |
| Participants | Inclusion: histologically or cytologically proven MPE Exclusion: participants on chemotherapy; participants receiving treatment with steroids 36 patients randomised. | |
| Interventions | Effusion aspirated to dryness prior to administering study agent. After agent instilled, the participants moved from side to side for six hours. If the participant had symptomatic recurrence of the effusion within a month, the allocated treatment was repeated Tetracycline group: 500 mg in 20 ml saline given intrapleurally. The tetracycline was administered via an intercostal tube at one centre and with needle drainage at the other centre C. parvum group: 7 mg in 20 ml saline intrapleurally through a needle, after the effusion was drained to dryness | |
| Outcomes | Symptomatic recurrence of pleural effusion one month after the last dose Side effects (pain, fever, nausea and vomiting, rash) | |
| Notes | People with trapped lung eligible for trial entry The side effects were reported per procedure rather than per patient For this review, if participants had a successful pleurodesis after the second dose of study agent, these were included in the analysis as a success. For the tetracycline group, the results from the two administration methods were combined for the purposes of analysis Included in network meta‐analysis for pleurodesis efficacy, fever, pain and mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not mentioned in the paper. Both drugs reconstituted in 20 ml saline |
| Blinding of outcome assessment (detection bias) | Unclear risk | If study was unblinded, reporting of side effects, symptomatic pleural fluid re‐accumulation could be biased |
| Incomplete outcome data (attrition bias) | Low risk | Participants excluded from analysis if died prior to one month, but the numbers were small and fairly well balanced between the groups (1/17 in C. parvum group; 3/19 in tetracycline group ie 11% LTFU in total) |
| Selective reporting (reporting bias) | Low risk | Thorough reporting of toxicity |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of tetracycline and mechlorethamine (mustine) for pleurodesis of malignant pleural effusions (Greece) | |
| Participants | Inclusion: documented MPE (all tumour types); respiratory distress was the main problem of the participants Exclusion: other therapy given simultaneously (chemotherapy or radiation therapy) 40 participants randomised | |
| Interventions | All participants had a 32 Fr intercostal drain inserted with local anaesthetic and effusion drained overnight. Complete drainage confirmed on CXR After pleurodesis, drain flushed with 20 ml saline. Participants rotated and drain unclamped after two hours and put onto ‐20 cm H2O suction. Drain removed when < 50 ml/day drainage Tetracycline group: 500 mg tetracycline in 20 ml 2% lignocaine intrapleurally. 1 dose Mechlorethamine group: 0.2 mg/kg of mechlorethamine in 20 ml saline intrapleurally. 1 dose | |
| Outcomes | Response to therapy at 60 days ('complete response' (CR) (complete lack of re‐accumulation of pleural fluid for at least 60 days), 'partial response' (PR) (small pleural effusion, asymptomatic, not requiring further treatment), 'failure' (all other cases)) Side effects | |
| Notes | Minimal data provided on baseline participantcharacteristics of the two groups Pleurodesis defined according to symptomatic effusion recurrence For the purposes of this review, CR and PR included as a successful pleurodesis People with trapped lung included in the study Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding in the paper. Drugs given in the same volume but not stated whether their appearances were similar |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if CXR interpretation was blinded for assessment of pleurodesis efficacy |
| Incomplete outcome data (attrition bias) | Low risk | All participants followed up until the primary endpoint at 60 days |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of OK‐432 and mitomycin C pleurodesis in lung cancer patients with MPE (Taiwan) | |
| Participants | Inclusion criteria: histo/cyto proven MPE due to lung cancer; effusion requiring repeated thoracentesis; ECOG performance score 0‐3 Exclusion criteria: previous anticancer chemotherapy within four weeks; previous radiation therapy to the ipsilateral chest within four weeks; concomitant systemic chemo or radio‐therapy; history or evidence of penicillin allergy 55 participants randomised | |
| Interventions | All participants hospitalised and a chest drain or pigtail catheter inserted into effusion. Drainage until < 200 ml/day. Tube clamped for one hour after drug administration. Drug administration repeated weekly for four weeks or until effusion resolved ok‐432 group: 1 KE intrapleurally Mitomycin C: 8 mg in 30 ml water intrapleurally | |
| Outcomes | Pleurodesis success at four weeks (defined as 'complete response' (CR) (no fluid accumulation and participants free of symptoms), 'partial response' (PR) (recurrence of effusion < 50% of original effusion volume, not symptomatic and no need for thoracentesis for symptom relief) or 'failure' (recurrence of effusion > 50% of the original volume, symptomatic and need for thoracentesis to relieve symptoms)) Survival Effusion‐free period | |
| Notes | People with trapped lung included in the study For this review, PR & CR counted as pleurodesis successes Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of whether the study was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Two participants excluded due to early death, both in OK‐432 arm |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | RCT of bleomycin, tetracycline and talc for pleurodesis of malignant pleural effusion | |
| Participants | Inclusion: MPE (either cytology positive or an exudative effusion attributed to a histologically confirmed malignancy elsewhere) (all cell types); life expectancy > 2 months Exclusion: contraindication to placement of a chest tube; allergy to bleomycin, talc or tetracycline 50 participants randomised | |
| Interventions | Chest tube placed using blunt dissection and allowed to drain for at least 24 hours until < 150 ml/day output. Sclerosing agent instilled intrapleurally. Participants repositioned every seven minutes after agent instilled. Then, tube unclamped and suction applied, until < 150 ml/24hours drainage when the drain was removed. If the drainage remained high, a second instillation was attempted Bleomycin group: 60 units bleomycin in 50 ml 5% dextrose Tetracycline group: 750 mg tetracycline in 100 ml saline, with 100 mg lidocaine Talc group: 5 g talc in 250 ml saline, with 100 mg lidocaine | |
| Outcomes | Successs of sclerosis at 30 days (defined as a lack of significant re‐accumulation on CXR with control of symptoms due to the effusion) Survival Median length of hospitalisation from date of sclerosis to discharge Side effects | |
| Notes | Participantswho died within 30 days of the sclerosis were included as treatment failures in the study Small difference in median age and cell types between the treatment arms Trapped lung not accounted for Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Random number generator |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly if the study was blinded, but the different drugs were given as different volumes |
| Blinding of outcome assessment (detection bias) | High risk | Symptom and side effect reporting would be affected by lack of blinding. Not stated if CXR interpretation was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 4/50 (8%) loss to follow up for primary outcome but balanced between the treatment arms |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT evaluating the distribution of talc during a talc slurry pleurodesis ‐ comparing rotation with non‐rotation of participants after instillation of talc slurry (Netherlands) | |
| Participants | Inclusion: symptomatic MPE confirmed by cytology or histology (all cell types) Exclusion: haemorrhagic disease; trapped lung; previous pleurodesis on ipsilateral side; other disease which would interfere with the study; participants on systemic treatment or expected to be within four weeks of pleurodesis; expected survival < 1month 20 participants randomised | |
| Interventions | Chest drain inserted and pleurodesis performed when drainage < 150 ml/24 hours and lung fully re‐expanded. Talc suspension was radiolabeled. Dynamic scintigraphy performed during, immediately after and one hour after instillation Rotation arm: sequence of four positions changing every 10 mins after instillation of talc for one hour Non‐rotation arm: strict bed rest in supine position after instillation Tube removed when < 100 ml/24hour fluid drained | |
| Outcomes | Distribution of talc in the thoracic cavity, measured on scintigram immediately after instillation of talc and after one hour Success rate of pleurodesis (defined on CXR) at four weeks | |
| Notes | People with trapped lung excluded Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes (10 allocating participant to rotation and 10 to non‐rotation) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if CXR reporting was performed by a blinded individual |
| Incomplete outcome data (attrition bias) | Low risk | Small numbers but no LTFU. Minimal data on baseline participant characteristics |
| Selective reporting (reporting bias) | Low risk | No serious side effects. Some discomfort in rotation group (not quantified). All study participants alive at one months' follow up. (Personal communication) |
| Other bias | Low risk | CXR only used to define pleurodesis. Small numbers in the study |
| Methods | Single centre RCT of tetracycline vs bleomycin pleurodesis in MPE (Spain) | |
| Participants | Inclusion: MPE (all cell types) causing respiratory symptoms, proved by cytological examination or pleural biopsy and an expected survival of at least one month, with a KPS ≥ 50 Exclusion: prior intrapleural instillation therapy; chest radiotherapy during the preceding two weeks; previously received systemic bleomycin; trapped lung; allergy to study drugs 70 participants randomised | |
| Interventions | All participants underwent tube thoracostomy with suction drainage until < 100 ml/day output Tetracycline group: 1.5 g in 100 ml saline intrapleurally, with 9 ml 5% lignocaine Bleomycin group: 60 mg in 100 ml saline intrapleurally Tube clamped for four hours after instillation, then suction drainage. Drain removed when < 100‐150 ml/day output | |
| Outcomes | Response to pleurodesis (defined as 'complete response' (CR) (no clinical or radiological recurrence of effusion), 'partial response' (PR) (small amount of fluid re‐accumulation on CXR but no symptoms), 'failure' (re‐accumulation of fluid causing symptoms or needing thoracocentesis)) Adverse effects of the procedure | |
| Notes | People with trapped lung excluded from trial entry For this review, CRs and PRs included as pleurodesis successes Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding in the paper. Agents given in the same volume but no comment on whether appearances were similar |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if CXR interpretation was blinded. Other symptom and side effect outcomes could be biased if participants and personnel not blind to treatment allocation, but not stated if this was the case |
| Incomplete outcome data (attrition bias) | Low risk | 8/70 (11%) excluded from analysis due to death (5) or LTFU (3) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT comparing pleurodesis using mixed particle Talc (>50% of particles are <20μm) vs graded Talc (<50% of particles are <20μm) (UK) | |
| Participants | Inclusion: Symptomatic pleural effusion, proven to be malignant by cytology or pleural biopsy (all cell types). Exclusion: Expected survival <6 weeks; bleeding diathesis contraindicating intercostal drain insertion; extensive trapped lung; previous ipsilateral pleurodesis; Age <18; Inability to give informed consent. 48 patients randomised. | |
| Interventions | 12Fr intercostal drain inserted. Drainage until <150ml/day output. Agent instilled and left in for 2 hours, before suction being applied. Drain removed after 48 hours. Mixed particle talc group: >50% of talc particles are <20μm. Single 4g intrapleural dose. Graded talc group: <50% of talc particles are <20μm. Single 4g intrapleural dose. | |
| Outcomes | Change in Aa gradient 48hours post pleurodesis breathing air Change in PaO2 at 48hours post pleurodesis Clinical efficacy of pleurodesis at 3 months Presence/absence of fever at 48hours Change in CRP Change in IL8 | |
| Notes | Patients with trapped lung excluded. Pleurodesis success defined as no re‐accumulation of pleural fluid sufficient to require drainage. Paper presented 2 trials and only trial 2 was relevant to this review (trial 1 was RCT of mixed talc vs tetracycline, but pleurodesis success data was not collected) Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | pre‐sealed numbered, opaque, sealed envelopes with stratification |
| Allocation concealment (selection bias) | Low risk | pre‐sealed numbered, opaque, sealed envelopes with stratification |
| Blinding of participants and personnel (performance bias) | Low risk | 'investigators and patients blind to treatment allocation' (personal communication with authors) |
| Blinding of outcome assessment (detection bias) | Low risk | 'investigators and patients blind to treatment allocation' (personal communication with authors) |
| Incomplete outcome data (attrition bias) | Low risk | Missing data justified and balanced between the 2 groups (3 patients LTFU) |
| Selective reporting (reporting bias) | Unclear risk | The study comprised of two sections and pleurodesis success only reported for the particle size section. The RCT of talc/tetracycline did not report pleurodesis success but this was not one of the pre‐defined outcome measures. |
| Other bias | Low risk | No other biases identified |
| Methods | Multicentre RCT of LC9018 plus doxorubicin vs doxorubicin alone in MPE secondary to lung cancer (Japan) LC9018 is a biologic response modifier prepared from heat‐killed, freeze‐dried Lactobacillus casei YIT 9018 | |
| Participants | Inclusion: positive histology for primary lung cancer; unilateral pleural effusion; expected survival > 8 weeks; no treatment within four weeks; performance score 0‐3; no concurrent cancer; no severe hepatic/renal/bone marrow failure; age ≤ 75 Exclusion: previous intrapleural (IP) treatment with a biologic response modifier; pregnant women and women of child‐bearing potential; history of allergy 95 participants randomised | |
| Interventions | Effusion completely drained. Both treatment arms received a maximum of two intrapleural doses, 1 week apart Control group: doxorubicin 40 mg in 20‐50 ml saline LC9018 group: as control group, then LC9018 0.2 mg in 20‐50 ml saline | |
| Outcomes | Efficacy of effusion control at four weeks (defined as 'complete response' (CR) (negative cytologic findings with no re‐accumulation of fluid), 'partial response' (PR) (negative cytologic findings with asymptomatic minimal fluid accumulation, not requiring additional aspiration) or 'failure' (detectable intrapleural fluid even after tube drainage with no improvement or exacerbation on radiology compared with before treatment, or failure to confirm conversion to negative cytology)) Side effects Change in performance status | |
| Notes | People with trapped lung excluded post randomisation For this review, CR and PR counted as pleurodesis success Not included in network meta‐analysis NB: doxorubicin is the generic name for Adriamycin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation system |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation system |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not clear |
| Blinding of outcome assessment (detection bias) | Low risk | "Blinded committee assessed data regarding safety and efficacy" |
| Incomplete outcome data (attrition bias) | High risk | 19/95 participants excluded from final analysis, for a variety of reasons, including five participants with protocol violations |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Primary outcome measure included CXR resolution and conversion to cytology negative effusion. Not clear from methodology whether some participants who were asymptomatic had effusion drained to evaluate cytology status and were then classified as 'failures' |
| Methods | Single centre RCT of mepacrine hydrochloride, triethylenethiophosphoramide and pleurocentesis alone in the treatment of MPE (Denmark) | |
| Participants | Inclusion: unilateral MPE (positive cytology, > 200 IU/L LDH and > 30 g/L protein) (all cell types); one previous pleurocentesis of > 500 ml Exclusion: participant receiving chemotherapy or radiotherapy 41 participants randomised | |
| Interventions | Pleurocenteis with intrapleural instillation of the study agent, three times a week for one week Mepacrine group: 100 mg for first dose, 200 mg for second dose, 200 mg for third dose (ie 500 mg in total) Triethylenethiophosphoromide group: 20 mg at each instillation (ie 60 mg total) Pleurocentesis group: 10 ml saline at each instillation All participants were followed up at 3 weeks, 6 weeks, 2 months and 3 months, when a pleurocentesis was performed | |
| Outcomes | Treatment effect (a beneficial effect was defined as < 500 ml fluid aspirated at each pleurocentesis performed up to three months) Side effects | |
| Notes | People with trapped lung not excluded from trial entry Minimal data presented on whether the treatment groups were well balanced at baseline Included in network meta‐analysis for pleurodesis efficacy, pain and fever | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding in the paper |
| Incomplete outcome data (attrition bias) | Low risk | Minimal early deaths (3/25) and numbers well matched between the groups |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | High risk | Unsure if groups well balanced at baseline. Pleurodesis success defined by aspirating fluid on all participants and not by clinical need for pleural intervention |
| Methods | RCT of intrapleural Corynebacterium parvum vs mustine in recurrent MPE (UK) | |
| Participants | Recurrent effusion associated with histologically proved malignant disease (all cell types); at least two previous pleural aspirations; symptoms of dyspnoea, cough or local pain 21 participants randomised | |
| Interventions | Effusion completely aspirated using an Abrams pleural biopsy needle Group A: intrapleural mustine 20 mg (max 2 doses) Group B: intrapleural C. parvum 7 mg (max 2 doses) | |
| Outcomes | Response to pleurodesis (defined by fluid re‐accumulation on CXR and need for repeat aspiration ‐ success/partial success/failure) at four weeks Symptoms (nausea, vomiting, pain) | |
| Notes | Trapped lung not accounted for Only 'success' counted as a pleurodesis success for analysis (not partial successes as these participants required a further aspiration of effusion) Included in network meta‐analysis for pleurodesis efficacy and mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding in the paper. If unblinded, symptom and side effect reporting could have been biased |
| Incomplete outcome data (attrition bias) | Low risk | Three participants excluded from analysis as died before primary outcome measure |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Unclear who provided C. parvum and their study involvement |
| Methods | Single Centre RCT of thoracoscopic talc poudrage versus povidone‐iodine pleurodesis through an intercostal drain (Egypt) | |
| Participants | Inclusion: MPE as a complication of breast carcinoma Exclusion: performance status > 3; allergy to iodine; trapped lung; no change in MRC dyspnoea scale after thoracentesis; pleural fluid pH < 7.2; pleural fluid glucose < 60 mg/dl; extrathoracic metastasis 42 participants randomised | |
| Interventions | All participants underwent a VATS drainage and adhesiolysis Talc poudrage group: 4 g talc insufflation under thoracoscopic guidance at the end of the VATS procedure Iodine group: recovered from VATS. Then later that day, 20 ml 10% povidone‐iodine in 30 ml saline injected through the chest drain at the bedside. Drain clamped for four hours after instillation | |
| Outcomes | Efficacy of pleurodesis at two months (response defined as 'complete response' (CR) (absence of fluid re‐accumulation), 'partial response' (PR) (residual pleural fluid or re‐accumulation, which did not require further thoracocentesis or remained asymptomatic) or 'failure' (additional pleural procedures were necessary) Complications Length of hospital stay (in days) Survival Change in MRC dyspnoea score | |
| Notes | People with trapped lung excluded from trial entry CR + PR counted as pleurodesis success for analysis Included in network meta‐analysis for pleurodesis efficacy, mortality and fever | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation software used |
| Allocation concealment (selection bias) | Low risk | Computer randomisation software used |
| Blinding of participants and personnel (performance bias) | High risk | Not able to blind given the nature of the interventions |
| Blinding of outcome assessment (detection bias) | High risk | Symptom and side effect reporting would be effected by lack of blinding. Not stated if radiology was interpreted blindly. Mortality would not be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Minimal missing data (primary outcome data available for all patients at two months) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of talc vs bleomycin in MPE (Belgium) | |
| Participants | Inclusion criteria: hist/cytologically proven, symptomatic MPE; karnofsky performance score ≥ 50; expected survival of one year or less. Exclusion criteria: previous pleurodesis attempt 26 participants randomised | |
| Interventions | 14 Fr chest drain with suction drainage until completely drained. Intrapleural lignocaine and subcutaneous morphine given prior to instillation of study drug. After instillation of drug, drain clamped for 30 mins and then left on suction drainage until output < 150 ml/24hours Bleomycin group: 1 mg/kg bleomycin in 50 ml saline intrapleurally. 1 dose Talc group: 5 g in 50 ml saline intrapleurally. 1 dose | |
| Outcomes | Response to therapy (defined by re‐accumulation on CXR and need for repeat procedure). Time point unclear Side effects Survival | |
| Notes | People with trapped lung were included in the study Included in network meta‐analysis for pleurodesis efficacy and fever | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numerical table |
| Allocation concealment (selection bias) | Low risk | Computer‐generated numerical table |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly but drugs have different appearances |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence and side effects could be biased by lack of blinding. Not stated if CXR interpretation was blinded |
| Incomplete outcome data (attrition bias) | Low risk | No LTFU. Outcome data provided on all participants |
| Selective reporting (reporting bias) | Low risk | Time point used to define pleurodesis not specified |
| Other bias | Unclear risk | No fixed endpoint for follow up |
| Methods | Single centre RCT of intrapleural streptokinase in MPE undergoing chest drainage (Turkey) | |
| Participants | Inclusion: definitive diagnosis of MPE with dyspnoea Exclusion: mesothelioma; endobronchial tumour causing obstruction; anticoagulant medication 48 participants randomised between Jan 2007 and Dec 2008 | |
| Interventions | All participants had 10 Fr pleural catheter inserted under local anaesthetic. Pleurodesis (5 g talc in 50 ml saline) given only in those patients with complete lung re‐expansion and < 250 ml drain output per day. Drain removed when output < 150 ml/day or after three days Those randomised to streptokinase received 3 doses of 250000 IU in 100 ml N saline at 12‐hourly intervals intrapleurally prior to pleurodesis | |
| Outcomes | Primary: lung expansion on chest X‐ray Secondary: success of pleurodesis at one month; Volume of 24‐hour pleural drainage before and after fibrinolytic | |
| Notes | Pleurodesis defined as "no accumulation of moderate to massive pleural fluid or any accumulation which causes dyspnoea" Didn't pleurodese those with trapped lung. Degree of loculation or septation on imaging at baseline was not recorded. Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based random‐number generator |
| Allocation concealment (selection bias) | Low risk | Web‐based random‐number generator |
| Blinding of participants and personnel (performance bias) | High risk | Nature of interventions precluded blinding (one group got 3 doses of drug and other group got nothing) |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding and side effects and symptom reporting could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | LTFU for pleurodesis success (1/17 in control group; 4/23 in streptokinase group ‐ 1 died; 1 in intensive care; 3 LTFU). Only those with full lung re‐expansion were given pleurodesis and this could have been affected by giving streptokinase, which might effect pleurodesis success rate, although this was not the study's primary outcome measure |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of talc vs bleomycin in MPE (Singapore) | |
| Participants | Inclusion: symptomatic, unilateral MPE confirmed by cytology or pleural biopsy (all cell types) Exclusion: trapped lung or loculated effusion; incomplete drainage (e.g. > 100 ml/day for 10 days); previously treated effusions; life expectancy < 1month 50 participants randomised | |
| Interventions | 20 ‐ 24 Fr tube thoracostomy until complete lung re‐expansion on CXR and < 100 ml/day for two days. Both drugs diluted in 50 ml saline and 10 ml 1% lignocaine. After study drug inserted, drain clamped for six hours with patient rotation. Then suction applied. Drain removed when < 200 ml/day drainage Talc group: 1 dose. 5 g talc intrapleurally Bleomycin group: 1 dose. 1 unit/kg bleomycin intrapleurally | |
| Outcomes | Treatment response at one month (according to recurrence of effusion on CXR. Scoring system 0‐3 used for size of effusion) Hospital stay (days) Side effects within 48 hours of pleurodesis | |
| Notes | People with trapped lung excluded from trial entry Pleurodesis success based only on radiology Included in network meta‐analysis for pluerodesis efficacy, pain, fever and mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly, however drugs have differing appearances |
| Blinding of outcome assessment (detection bias) | Low risk | "A single investigator who was blinded to treatment allocation scored all the follow up chest x rays" |
| Incomplete outcome data (attrition bias) | Low risk | 12/50 patients excluded due to death or LTFU in first month, but balanced between treatment arms |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Multi‐centre RCT bleomycin vs Corynebacterium parvum in MPE (UK) | |
| Participants | Inclusion criteria: histocytologically proven malignancy with effusion (all cell types); life expectancy of > 30 days Exclusion criteria: previous intrapleural drug administration; change in cancer treatment in previous 30 days 58 participants randomised | |
| Interventions | Aspiration of effusion with a cannula. Study drug instilled through the cannula. After cannula removed, participantrepositioned every five minutes Bleomycin group: 60 mg bleomycin in 100 ml saline. Single dose intrapleurally C. parvum group: 7 mg in 20 ml saline. Single dose intrapleurally | |
| Outcomes | Efficacy of pleurodesis agent at 30 days (defined as 'complete response' (CR) (no re‐accumulation of fluid confirmed by CXR), 'partial response' (PR) (minimal fluid re‐accumulation not sufficient to produce symptoms &/or need for a further aspiration) or 'failure') Duration of treatment response Toxicity Efficacy of pleurodesis at 2, 3, 6, 9 and 12 months | |
| Notes | People with trapped lung included in the study For this review, CR and PR counted as pleurodesis success Included in network meta‐analysis for pleurodesis efficacy, mortality, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially labelled sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sequentially labelled sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly, but agents given as different volumes |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence and side effect reporting would be influenced by lack of blinding. Not stated if CXR assessment was blinded. Mortality data would not be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 14/58 (24%) excluded from primary analysis due to death or not receiving drug. But, balanced numbers between the groups |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre, prospective RCT comparing rapid and standard drainage prior to talc slurry pleurodesis (Turkey) | |
| Participants | Inclusion: potentially recurrent histologically &/or cytologically proven malignant pleural effusion (all cell types) Exclusion: participants whose lung did not expand; endobronchial lesion; suitable for curative therapy 79 participants randomised | |
| Interventions | All participants underwent insertion of a 12 Fr chest drain in the posterior axillary lune with local anaesthetic (bupivacaine) and IM ketorolac Rapid group: 1 litre drained every eight hours until complete drainage. Then talc slurry administered once CXR showed complete fluid evacuation and no trapped lung Standard group: drainage of a maximum of 1.5 litre/day. Talc slurry administered once CXR showed complete fluid evaluation and no trapped lung and pleural fluid drainage < 300 ml/day | |
| Outcomes | Primary outcome: efficacy of pleurodesis assessed at 1, 2, 3, and 6 months Secondary outcome: hospital length of stay | |
| Notes | people with trapped lung excluded from study entry Pleurodesis efficacy defined using a combination of radiology and symptomatic effusion re‐accumulation Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Not stated and no response from study authors |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind given nature of two treatment groups with such different drainage regimes |
| Blinding of outcome assessment (detection bias) | Low risk | "The assessment of success was performed by an investigator blinded to allocation" |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if any LTFU‐ not stated in paper and no response from study authors |
| Selective reporting (reporting bias) | High risk | Minimal data provided on side effect and mortality data. Not all time points reported as stated in methods |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Two‐centre, prospective RCT of silver nitrate vs talc slurry in MPE (Brazil) | |
| Participants | Inclusion: documented MPE (positive pleural biopsy or cytology ‐ all cell types); karnofsky performance score > 60; life expectancy > 1 month Exclusion: loculated or trapped lungs after drainage 60 participants randomised | |
| Interventions | 26/28 Fr chest tube. After study drug instilled, clamped for one hour with patient rotation. Then suction applied. Drain removed when < 100 ml drained Talc group: 5 g talc in 50 ml saline. 1 dose intrapleurally Silver nitrate group: 20 ml of 0.5 ml silver nitrate. 1 dose intrapleurally | |
| Outcomes | Radiological resolution of effusion on CXR (monthly for four months) Pain before and after treatment (measured on a 0‐10 linear scale) | |
| Notes | People with trapped lung excluded from study entry Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Picking paper from a box |
| Allocation concealment (selection bias) | Low risk | Picking paper from a box |
| Blinding of participants and personnel (performance bias) | High risk | Not stated if blinded but agents have different appearances |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if CXR interpretation was blinded. Pain scores may be biased if participants not blinded |
| Incomplete outcome data (attrition bias) | Low risk | High rate of LTFU (11/60 (18%)) but reasons explored in the discussion |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Prospective RCT of bleomycin vs doxycycline in MPE (USA) | |
| Participants | Inclusion: symptomatic effusion; proven or strongly suspected that malignancy is the cause for the effusion Exclusion: previous pleurodesis; allergy to bleomycin or doxycycline; chemotherapy in the previous 30 days 106 participants randomised | |
| Interventions | All participants underwent a 14 Fr chest drain insertion. When drainage < 200 ml/day and lung fully re‐expanded on CXR, participant randomised Bleomycin group: 60 units bleomycin in 50 ml saline intrapleurally Doxycycline group: 500 mg doxycycline in 50 ml saline + 10 ml lignocaine After 18 ‐ 24 hours, if drainage < 200 ml, drain removed. If > 200 ml, second dose of the same agent given and drain then removed | |
| Outcomes | Radiographic response at 30 days (classified as: complete response, partial response, progressive disease, expired with no re‐accumulation, expired with re‐accumulation, lost to follow up) Mortality Side effects | |
| Notes | Trapped lung not accounted for If participants died prior to day 30, included in analysis according to their outcome at the time of their death For this review, complete response, partial response and expired with no re‐accumulation counted as pleurodesis success Included in network meta‐analysis for pleurodesis efficacy, mortality, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation |
| Blinding of participants and personnel (performance bias) | High risk | "Study investigators and participants not blinded to treatment allocation" (personal communication with study authors) |
| Blinding of outcome assessment (detection bias) | High risk | "Study investigators and participants not blinded to treatment allocation" (personal communication with authors) |
| Incomplete outcome data (attrition bias) | High risk | Significant LTFU rate (26/106 (ie 25%)) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Radiological outcome on CXR used to define pleurodesis success |
| Methods | Single centre RCT comparing the pleurodesis success of doxycycline and bleomycin in MPE (Iran) | |
| Participants | Inclusion: symptomatic, cytologically proven MPE Exclusion: allergy to doxycycline or bleomycin; past history of sclerotherapy; systemic chemotherapy immediately prior to or in the next two months after sclerotherapy 42 participants randomised | |
| Interventions | All participants underwent 'fluid evacuation'. Agent then instilled through the tube, which was clamped for one hour. Then suction applied and drain removed when < 100 ml/24 hr drainage Bleomycin group: 45 mg bleomycin intrapleurally Doxycyclline group: 600 mg doxycycline in 50 ml saline and 10 ml 1% lignocaine intrapleurally | |
| Outcomes | CXR appearances of the effusion size at two months (mild, moderate or severe) Need for repeat pleural fluid drainage Dyspnoea (mild, moderate or severe) Complications | |
| Notes | People with trapped lung not excluded Pleurodesis success primarily defined radiologically, but data presented at three months for need for repeat pleural intervention For this review, need for repeat pleural drainage was used as measure of pleurodesis success Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated and no response from study authors to clarify |
| Allocation concealment (selection bias) | Unclear risk | Not stated and no response from study authors to clarify |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if anyone was blinded. No response from study authors |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded. No response from study authors |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Open label, multicentre parallel group RCT of VATS pleurectomy and talc pleurodesis (either slurry or poudrage) in mesothelioma (UK) | |
| Participants | Inclusion: age > 18; confirmed or suspected MPM with pleural effusion; fit enough for VATS pleurectomy Exclusion: previous pleurodesis; previous primary treatment for MPM; history of previous malignancy and suspected MPM Those with suspected MPM who were found to have a different cause after randomisation were excluded from analysis 196 participants randomised | |
| Interventions | VATS pleurectomy group: thoracoscopic debulking pleurectomy‐decortication under GA, according to agreed protocol Pleurodesis group: 4 g talc pleurodesis (either slurry or poudrage) | |
| Outcomes | Primary outcome: survival at one year post randomisation Secondary outcomes: presence or absence of effusion on CXR, QOL (EQ5D and QLQ‐LC13, QLQ‐C30), lung function and exercise tolerance, complications, healthcare utilisation costs | |
| Notes | People with trapped lung included. No data available on whether participants in the pleurodesis arm who had poudrage may have had trapped lung released at the same time Pleurodesis success defined according to CXR (as assessed by reporting radiologist, unblinded to treatment allocation) Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator in blocks of 10. 1:1. stratified by EORTC score (low or high) |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation line operated by staff independent to the study |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind due to nature of the interventions |
| Blinding of outcome assessment (detection bias) | High risk | Participants and investigators not blinded, leading to potential bias in reporting of quality of life, exercise tolerance and complications. CXRs not interpreted blindly (personal communication with authors) |
| Incomplete outcome data (attrition bias) | Low risk | Participants excluded after randomisation if MPM not confirmed, but this was stated a‐priori. Missing data well balanced between the treatment arms |
| Selective reporting (reporting bias) | Low risk | Very thorough reporting of all stated outcomes |
| Other bias | Low risk | No other biases identified |
| Methods | Multicentre RCT of intrapleural bleomycin and tetracycline in MPE (USA) | |
| Participants | Inclusion: exudative MPE (proven on cytology or pleural biopsy); ECOG performance score (PS) 0‐2 Exclusion: previous intrapleural therapy; prior systemic therapy with bleomycin; severe congestive heart failure; radiotherapy to the chest in the previous two weeks 115 participants randomised | |
| Interventions | All participants had a chest tube placed and had evidence of lung re‐expansion on CXR. After the study drug was inserted the tube was clamed and the participant's position rotated. After several hours the chest tube was removed Group 1: tetracycline 1 g intrapleurally in 100 ml saline Group 2: bleomycin 120 units intrapleurally in 100 ml saline (due to slow accrual, this group was dropped after accruing 15 participants) Group 3: bleomycin 60 units intrapleurally in 100 ml saline | |
| Outcomes | Recurrance of effusion at 30 days and 90 days (defined according to CXR) Time to effusion recurrence within 90 days Time to maximum change in ECOG PS Change from initial PS to the best PS (worsened/no change/improved) Adverse events | |
| Notes | People with trapped lung excluded Group 2 dropped due to slow accrual and data on the 15 participants assigned to this group not provided Included in network meta‐analysis for pleurodesis efficacy, mortality, pain and fever | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation, with stratification |
| Allocation concealment (selection bias) | Low risk | Computer randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if anyone was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded |
| Incomplete outcome data (attrition bias) | High risk | Lots of patients excluded from analysis (41/115 'non‐evaluable'). Reasons given |
| Selective reporting (reporting bias) | High risk | Data on 15 patients randomised to high dose bleomycin group not reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of pleurodesis with doxycycline and C. parvum in MPE (Finland) | |
| Participants | Inclusion: pleural effusion refractory to repeat aspirations; pleural malignancy ‐ all cell types (histocytologically proven or confirmed malignancy elsewhere) Exclusion: none 41 participants randomised | |
| Interventions | 16 Fr Argyll drain inserted under local anaesthetic and drained with suction until output < 100 ml/day. CXR to confirm lung re‐expansion prior to pleurodesis D100 group: doxycycline 100 mg given intrapleurally. 1 dose D600 group: doxycycline 600 mg given intrapleurally. 1 dose C1 group: C. parvum 1 mg intrapleurally. 1 dose C7 group: C. parvum 7 mg intrapleurally. 1 dose All drugs diluted in 20 ml saline and a 50 ml flush was administered after the dose. Chest tube removed immediately after sclerosant given | |
| Outcomes | Pleurodesis success (defined using CXR and need for repeat thoracentesis at 30 days) Mortality Side effects Blood/pleural fluid IL‐6 Daily CRP for seven days | |
| Notes | For the purposes of our analysis, we have decided to combine the two doses of each agent to allow comparison between the agents themselves People with trapped lung excluded from study Included in network meta‐analysis for pleurodesis efficacy, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if anyone was blinded. Unable to contact study authors |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded. Unable to contact study authors |
| Incomplete outcome data (attrition bias) | Low risk | 6/41 (15%) patients LTFU |
| Selective reporting (reporting bias) | High risk | Minimal data provided on survival or biochemical markers. Minimal data on baseline participant characteristics and whether the treatment groups were well matched |
| Other bias | Low risk | Underpowered |
| Methods | Single centre RCT evaluating intrapleural bleomycin vs interferon alfa‐2b in the palliative treatment of malignant effusion (Italy) | |
| Participants | Inclusion: cytologically proven MPE requiring at least two thoracenteses in preceding four weeks; at least 3 L drained in the preceding four weeks; adequate pulmonary re‐expansion on CXR after thoracentesis; last systemic treatment administered at least six weeks prior to enrolment; no further chemotherapy options; Karnofsky performance score > 40 Exclusion: none 160 participants randomised | |
| Interventions | All patients underwent a 9 Fr intercostal drain insertion under ultrasound scan (USS) guidance. Fluid was drained via a 3‐way‐tap until USS revealed no residual effusion. Study drug administered IP via the chest tube. Tube was then clamped for two hours and participants changed position every 15 minutes. Tube removed 24 ‐ 48 hours after the last dose Bleomycin group: 0.75 mg/kg bleomycin in 50 ml saline. A repeated dose was given if > 100 ml/day output three days after the first dose IFN alfa‐2b group: 1 million units/10 kg in 200 ml saline. Six doses given every four days | |
| Outcomes | Treatment response at 30 days (complete response (no fluid re‐accumulation), partial response (asymptomatic fluid recurrence < 50% of the original effusion, not requiring thoracentesis), no response (fluid recurrence > 50% of the original effusion, requiring thoracentesis)) Time to progression Number of thoracenteses until death | |
| Notes | Deaths included in the analysis as failures (as presented in the paper as ITT analysis) People with trapped lung excluded from trial entry Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly but two drugs were given as different volumes |
| Blinding of outcome assessment (detection bias) | High risk | Synptom recurrence and adverse event reporting may be biased by lack of blinding. Mortality not biased by lack of blinding. Not stated if CXR interpretation was blinded |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Multi‐centre RCT comparing pleurodesis using bleomycin with mitoxantrone (Germany). Paper in German | |
| Participants | Inclusion: symptomatic, cytologically proven MPE; life expectancy > 3 months; WHO performance score 0‐2 Exclusion: prior chemotherapy or pleurodesis in previous four weeks; contraindication to bleomycin or mitoxantrone; persistent pneumothorax; leucopenia; thrombocytopaenia; incomplete pleural fluid drainage 102 participants randomised | |
| Interventions | All participants had a 24 Fr chest drain inserted and left in situ for 48 hours Bleomycin group: single dose of 60 mg bleomycin in 100 ml saline intrapleurally Mitoxantrone group: single dose of 30 mg mitoxantrone in 100 ml saline intrapleurally Drains clamped for six hours after instillation and left in place for 24 ‐ 48 hours with or without suction | |
| Outcomes | Pleurodesis success rate at four weeks (defined by recurrence of effusion requiring repeat pleural procedure) Toxicity/adverse events Length of hospital stay Time to repeat pleural intervention | |
| Notes | Translated from German People with trapped lung excluded from participation Included in network meta‐analysis for pleurodesis efficacy, mortality, fever and pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if anyone was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded. If unblinded, symptom recurrence, adverse event reporting and length of stay could have been biased |
| Incomplete outcome data (attrition bias) | Low risk | Six participants excluded from analysis, but reasons given and balanced numbers in the two treatment arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT comparing talc instillation with pleural drainage only in the treatment of MPE (Denmark) | |
| Participants | Inclusion: histologically proven MPE (all cell types) causing respiratory distress, which is progressive and resistant to conventional therapy Exclusion: failure of the underlying lung to totally re‐expand within 72 hours of the thoracoscopy 31 participants randomised | |
| Interventions | All participants underwent thoracoscopy, during which multiple biopsies were taken and a drain inserted. Suction applied until complete lung re‐expansion Drainage alone group: constant suction for 72 hours after complete lung re‐expansion. Then, drain removed Talc and drainage group: 10 g sterile talc in 250 ml saline instilled through chest tube and clamped for two hours. Then suction applied for 72 hours and the drain was removed | |
| Outcomes | Fluid re‐accumulation on CXR every month for three months | |
| Notes | People with trapped lung excluded from trial entry No data provided on whether treatment arms well matched at baseline Power calculation performed Unclear if adverse events reported for all participants or only those who completed the follow up Pleurodesis defined using radiology only Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | "Closed envelope system" |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to nature of the interventions (pleural drainage alone, or with talc administration) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Adverse event reporting could be biased by lack of blinding. Not stated if CXR interpretation was blinded |
| Incomplete outcome data (attrition bias) | High risk | 10/31 (32%) excluded from primary analysis (but well balanced between the two treatment arms) |
| Selective reporting (reporting bias) | Low risk | No comment on mortality or survival, but an old study and not stated as an outcome measure in the paper |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT evaluating VATS talc poudrage and talc slurry in malignant pleural effusion (Brazil) | |
| Participants | Inclusion: biopsy or cytology proven MPE (all cell types); recurrent and symptomatic effusion; chest radiograph confirming lung expansion of > 90% after thoracentesis; Karnofsky PS ≥ 70 Exclusion: comorbidities that preclude GA; bleeding disorders; massive thoracic skin infiltration; active infection; refusal to sign informed consent 60 participants randomised | |
| Interventions | One dose of 5 g non‐calibrated talc given intrapleurally to both trial groups. Post procedure care and analgesia the same for the two groups. No suction used in either group. Drain removed when < 200 ml/24 hr drainage, or after 10 days if drain volume too high, participants were discharged with the drain in situ and a Heimlich valve VATS group: VATS performed under general anaesthesia, followed by talc poudrage. 28 Fr chest drain inserted at end of procedure Talc slurry group: 28 Fr chest drain inserted under local anaesthetic. Following day, talc suspended in 60 ml saline with 5 ml 2% lignocaine and instilled through chest drain. Clamped for one hour post procedure | |
| Outcomes | Lung expansion on CT measured on a 3‐point scale at baseline, 1, 3 and 6 months Clinical efficacy (success defined as no need for a new pleural procedure due to symptoms and radiological effusion recurrence) Quality of Life Safety Survival Chest drain duration Length of hospital stay Perioperative complications | |
| Notes | Raw data for survival, pleurodesis rates at certain time points, intervention rates at certain time points and QOL data not presented People with trapped lung excluded from trial entry Pleurodesis success rate defined using symptoms and radiology Study authors contacted for further information, but no reply Included in network meta‐analysis for pleurodesis efficacy and fever | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to nature of the interventions (talc poudrage vs talc slurry) |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence, quality of life, inpatient stay and adverse event reporting could be biased by lack of blinding. Interpretation of CTs was done by two blinded observers, however pleurodesis efficacy was defined by need for repeat intervention |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT evaluating three different doses of silver nitrate for pleurodesis in malignant pleural effusion (Brazil) | |
| Participants | Inclusion criteria: recurrent and symptomatic MPE (with pleural histological or cytological confirmation); previous CXR showing full lung expansion (> 90%) after chest drainage; Karnofsky performance score > 40; written consent Exclusion criteria: trapped lung after pleural catheter insertion; haemorrhagic diathesis (PT < 50% or platelets < 80,000); active pleural or systemic infection; neoplastic infiltration of the skin at the site of pleural catheter insertion; inability to understand QoL questionnaires; contralateral pleurodesis < 30 days before study entry 60 participants randomised | |
| Interventions | All participants were admitted for five days and had baseline assessment. All had a 14 Fr chest drain inserted under USS guidance prior to randomisation. The randomised interventions were given via the chest tube, which was then clamped for one hour. Drain removed on day 5. The silver nitrate was dissolved in 100 ml distilled water, which was passed through a 0.22 micrometer filter to ensure sterility within six hours of instillation Group 1: 30 ml of 0.3% silver nitrate (90 mg) given as a single dose intrapleurally Group 2: 30 ml of 0.5% silver nitrate (150 mg) given as a single dose intrapleurally Group 3: 60 ml of 0.3% silver nitrate (180 mg) given as a single dose intrapleurally | |
| Outcomes | Primary outcome: occurrence of serious or severe adverse event during follow up Secondary outcomes: systemic inflammation (measured using CRP); chest pain (measured using VAS score); effusion recurrence (defined as need for additional pleural procedures during trial follow up); residual pleural cavity volume (calculated using difference between day 5 and day 30 on CT) | |
| Notes | People with trapped lung excluded from study entry Pleurodesis failure defined as need for additional pleural procedure during follow up Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Pharmacy employees and clinicians who instilled the sclerosant were aware of treatment allocation, but these clinicians were not involved in patient follow up. Participants, investigators that followed participants up and rated their complications were blinded to group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Pharmacy employees and clinicians who instilled the sclerosant were aware of treatment allocation, but these clinicians were not involved in patient follow up. Participants, investigators that followed participants up and rated their complications were blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | LTFU well balanced and justified |
| Selective reporting (reporting bias) | Low risk | No data provided for MRC dyspnoea score. Otherwise all predefined outcome measures reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT comparing intrapleural talc and mepacrine given via a chest tube after thoracoscopy (Sweden) | |
| Participants | Inclusion criteria: recurrant, symptomatic pleural effusions, known or suspected to be due to malignancy; eligible for thoracoscopy and pleurodesis Exclusion criteria: incomplete lung re‐expansion after thoracoscopy 89 participants with confirmed malignant pleural effusions were randomised (110 participants randomised in total, but some had benign causes) | |
| Interventions | All participants underwent a local anaesthetic thoracoscopy, with biopsies and a 20 Fr drain was inserted at the end of the procedure. A chest X‐ray was performed to ensure lung re‐expansion before randomisation Mepacrine group: 500 mg mepacrine in 200 ml saline intrapleurally Talc group: 5 g talc in 200 ml saline intrapleurally In both groups, a second dose was given if > 50 ml/day drainage on day 3. Drains removed when < 50 ml/24hour drainage | |
| Outcomes | Primary: pleurodesis success (using clinical and radiological definition). Reported at day 6, 2 weeks, 2 months, 4 months and 6 months Secondary: analgesia use; side effects; mortality | |
| Notes | People with trapped lung excluded. Note that two doses may have been given Included in network meta‐analysis for pleurodesis efficacy and mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind as drugs different appearances |
| Blinding of outcome assessment (detection bias) | Low risk | Radiologists reporting CXRs were blind to treatment allocation. Symptom recurrence and adverse event reporting may be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for loss to follow up and exclusions reported and well matched between the groups |
| Selective reporting (reporting bias) | Low risk | Data for those with proven MPE obtained from authors |
| Other bias | Low risk | No other biases identified |
| Methods | Single Centre RCT of short‐term vs long‐term drainage before tetracycline pleurodesis of MPE (USA) | |
| Participants | Inclusion: moderate to large MPE, proved by cytology or pleural biopsy, causing respiratory symptoms; expected survival of > 1 month; KPS > 40% Exclusion: previous chemical pleurodesis on the ipsilateral side; ipsilateral atelectasis due to complete airway obstruction by tumour 25 participants randomised | |
| Interventions | 28 Fr chest drain inserted. Tetracycline 1.5 g in 100 ‐ 150 ml pleurodesis Standard Care (long‐term drainage): tube suction drainage until lung re‐expansion and < 150 ml/day drainage, then tetracycline pleurodesis and drain removed the following day Short‐term drainage: tube suction drainage until lung re‐expansion, then tetracycline pleurodesis and drain removed the following day. | |
| Outcomes | Pleurodesis success at one month (defined using CXR and need for repeat procedure) Duration of tube drainage Patient outcome (dead/alive ‐ ? time point) | |
| Notes | Lung re‐expansion confirmed on CXR prior to instillation of tetracycline Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind as different timings of interventions |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence could be biased by lack of blinding. Not stated if radiology was reported blindly |
| Incomplete outcome data (attrition bias) | Low risk | 1/25 patients LTFU |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported; minimal information on safety/complications |
| Other bias | Low risk | None |
| Methods | Single centre RCT of rapid vs standard pleurodesis with oxytetracycline (Turkey) | |
| Participants | Symptomatic MPE, confirmed on cytology or pleural biopsy 27 participants randomised | |
| Interventions | 12 Fr drain inserted. Pleurodesis agent: oxytetracycline 35 mg/kg Standard protocol: drainage until lung re‐expansion & fluid drainage < 150 ml/day. Then pleurodesis as a single dose. Drain clamped for six hours and removed when < 150 ml/day drainage Rapid protocol: pleurodesis given as 4 divided doses, every six hours after aspiration through the drain | |
| Outcomes | Response to pleurodesis (CR/PR/Failure) as defined by radiological recurrence and need for thoracentesis | |
| Notes | People with trapped lung not excluded Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Random number table |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind as different durations of drainage and aspiration schedules |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence and duration of hospital stay may be biased by lack of blinding. Mortality not biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing data well balanced between the groups. At one month 2/27 had died and were therefore non‐evaluable |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of talc insufflation versus talc slurry for symptomatic MPE (Hong Kong) | |
| Participants | Inclusion: established, symptomatic MPE (all cell types); dyspnoea improved after tube thoracostomy or large volume thoracentesis Exclusion: Karnofsky score < 30%; FEV1 < 0.5 L; trapped lung; chemotherapy or radiotherapy within six months 57 participants randomised | |
| Interventions | Talc insufflation group: all participants underwent a GA with one lung ventilation in the lateral decubitus position. 10 mm port inserted. Adehsions and loculations broken down. 5 g talc insufflated into the chest. 28 Fr tube at end of procedure, connected to suction. Drain removed when < 50 ml/24 hours drainage Talc slurry group: chest tube. 5 g talc in 50 ml saline and 10 ml 2% lidocaine instilled through the drain. Drain clamped for two hours and participant turned Drain reconnected to suction and removed when output < 50 ml/24hours | |
| Outcomes | Radiological recurrence of effusion Complications of the procedure Post‐procedure chest drain duration Length of hospital stay Parenteral meperidine use | |
| Notes | People with trapped lung excluded from trial entry Included in network meta‐analysis for pleurodesis efficacy and mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to nature of the interventions |
| Blinding of outcome assessment (detection bias) | High risk | Adverse event reporting and length of stay may be biased by lack of blinding. Not stated whether radiology was reported blindly |
| Incomplete outcome data (attrition bias) | Low risk | All data reported. Survial data not entirely clear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | Unclear how many patients in the poudrage arm had a drain in situ at the time of trial entry. Pleurodesis success only defined using radiology |
| Methods | Multicentre RCT of bleomycin,OK‐432 and cisplatin plus etoposide (PE) pleurodesis in MPE (Japan) | |
| Participants | Inclusion: cyto or histo proven MPE associated with newly diagnosed NSCLC; age ≤ 75; ECOG performance score 0 ‐ 2; full lung re‐expansion after chest drainage; adequate bone marrow reserve, liver and renal functions Exclusion: prior chemotherapy, thoracic RT or thoracic surgery; bilateral pleural effusion or pericardial effusion; symptomatic brain metastases; active synchronous cancer; interstitial pneumonitis; pulmonary fibrosis; uncontrolled angina/MI in preceding three months; uncontrolled diabetes or hypertension; pregnancy or breastfeeding; penicillin allergy 102 participants randomised | |
| Interventions | Large‐ or small‐bore chest tube inserted. After instillation of the study agent, participant rotated position for three hours Bleomycin group: 1 dose, 1 mg/kg (max 60 mg) of intrapleural bleomycin in 100 ml saline ok‐432 group: 1 dose of 0.2 KE units/kg (max 10 KE) of intrapleuralOK‐432 in 100 ml saline PE group: 1 dose cisplatin 80 mg/m2 and etoposide 80 mg/m2 intrapleurally in 100 ml saline | |
| Outcomes | Pleural progression‐free survival at 4, 8, 12 and 24 weeks (defined on CXR and need for local treatment) Overall survival Toxicity | |
| Notes | People with trapped lung not eligible for inclusion Study authors emailed for more information, but no response Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated if anyone was blinded. Same volume of instillate in both arms |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if anyone was blinded. If unblinded, reporting of symptom recurrence and toxicity could have been biased. Not stated if radiology was reported blindly but the definition of pleurodesis also incorporated symptom recurrence |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Radiology may be difficult to assess as population has underlying lung cancer |
| Methods | RCT of tetracycline pleurodesis versus placebo of the same pH as tetracycline (USA) | |
| Participants | Inclusion: biopsy proven malignancy; recurrent pleural effusion; expected survival > 1 month; Karnofsky performance score ≥ 40% 30 participants randomised | |
| Interventions | Chest tube inserted and in place for at least 24 hours. After pleurodesis agent instilled, tube clamped for two hours and participant's position changed. Then left in place for 12 ‐ 24 hours until minimal drainage Tetracycline group: 500 mg tetracycline in 50 ml saline intrapleurally. 1 dose Control group: 0.6 ml multivitamins, 5 ml of 0.1 N HCl and 50 ml saline intrapleurally. 1 dose | |
| Outcomes | Reaccumulation of effusion on CXR at 1 month and 3 months (CR/PR/Stabilisation/progression) Side effects | |
| Notes | CR, PR & stable disease counted as pleurodesis success for purposes of analysis Some participants with bilateral effusions entered into the study, but not clear whether both sides were randomised. Therefore for purposes of analysis, only the first side has been included Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐Blind" (no further details given) |
| Blinding of outcome assessment (detection bias) | Low risk | "Double‐Blind" (no further details given) |
| Incomplete outcome data (attrition bias) | High risk | Time point at which primary outcome measured not clear. Minimal data on baseline participant characteristics. Participants who died within one month excluded from analysis (11/30 not evaluable) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other biases identified |
| Methods | Single centre RCT of intrapleural Ad‐p53 and cisplatin, compared with cisplatin alone in MPE due to lung cancer (China) | |
| Participants | Inclusion: MPE due to lung cancer confirmed by CT, thoracic ultrasound and cytohistological examination; expected survival of > 3 months; Karnofsky PS > 60 Exclusion: abnormal ECT, liver function, kidney function and routine blood examination; previous chemotherapy, radiotherapy or biological therapy 35 participants randomised | |
| Interventions | All participants had chest drain inserted and effusion drained completely. All received systemic vinorelbine. All received 10 mg intrapleural (IP) dexamethasone after instillation of trial drugs. Drug administration was repeated weekly for four weeks or until pleural effusion resolved Combination group: Ad‐p53 (1 x 1012VP) in 100 ml saline IP. Then cisplatin 40 mg/m2 in 100 ml saline IP Single agent group: cisplatin 40 mg/m2 in 100 ml saline IP | |
| Outcomes | Therapeutic efficacy (CR/PR/SD/PD) ‐ as defined by extent of effusion and radiology and symptoms, at four weeks Change in Karnofsky performance status from baseline to four weeks Adverse events | |
| Notes | People with trapped lung not excluded from the study CR and PR counted as a successful pleurodesis for the purposes of analysis Study authors emailed for further information, but no response Not included in network meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not stated explicitly but combination group received two intrapleural treatments, while other arm only received one |
| Blinding of outcome assessment (detection bias) | High risk | Symptoms, quality of life and adverse events could be biased by lack of blinding. Not stated if radiology was reported blindly |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other biases identified |
| Methods | Prospective RCT of talc vs bleomycin pleurodesis for symptomatic MPE (USA) | |
| Participants | Inclusion: MPE (all cell types); life expectancy > 1 month Exclusions: significant loculated effusions; trapped lung 40 procedures randomised in 35 participants | |
| Interventions | All participants underwent tube thoracostomy (either at the end of a limited thoracotomy (two participants) or inserted at bedside (33 participants)). Tube remained on suction. After sclerosant injected intrapleurally, tube clamped for two hours and participant rotated Talc group: 5 g talc in 50 ml saline, with 20 ml 1% lignocaine. 1 dose Bleomycin group: 60 U bleomycin in 50 ml saline, with 20 ml 1% lignocaine. 1 dose | |
| Outcomes | Effusion control on CXR (at a minimum of two weeks) Dyspnoea (according to functional class 1 ‐ 4) Pain (according to scale 0 ‐ 10) Cost Length of hospital stay | |
| Notes | People with trapped lung excluded Participants only included in primary analysis if out of hospital and able to attend follow up at two weeks Study authors emailed for more information, but no response Included in network meta‐analysis for pleurodesis efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not explicitly stated but drugs had different appearances |
| Blinding of outcome assessment (detection bias) | High risk | Symptom recurrence, pain, breathlessness, duration of stay and adverse events could all be biased by lack of blinding. Not stated radiology reported blindly |
| Incomplete outcome data (attrition bias) | High risk | No clear time point when follow up performed. Only those available for follow up included in the analysis. Unclear how many randomised to each arm (only data on numbers analysed by treatment arm) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other biases identified |
ECOG: electrocochleography
IV: intravenous
LTFU: loss to follow up
QOL: quality of life
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study not truly randomised. Participants allocated to treatment groups using alternation. | |
| Unable to differentiate between participants with benign and malignant disease in the results section. Also high risk of bias from randomisation method (allocated to treatment groups based on the last digit of their hospital number). | |
| Unclear from text if truly randomised ‐ participants given number on entering study ‐ allocated to bleomycin if number was odd and allocated to povidone group if number was even. Study authors emailed for clarification but no response. | |
| Study not truly randomised. Participants allocated to treatment groups based on the day of the calendar month. | |
| Pilot data (not randomised) and randomised data presented grouped together. Unable to split out the pilot group. | |
| Participants with pleural effusions and ascites included, but unable to differentiate between them in the results section. | |
| Unable to differentiate between pleural, pericardial and peritoneal effusions in the results. No response from study authors. | |
| Study not truly randomised. Participants allocated to bleomycin group if met a list of criteria, otherwise given mitoxantrone. | |
| Study not truly randomised. Participants allocated to treatment groups based on the month of their diagnosis with MPE. | |
| Participants with pleural and peritoneal effusions included in the study and unable to differentiate them in the results. | |
| Insufficient information in paper to extract required data (e.g. unclear how pleurodesis success was defined and at what time point). |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised study comparing highly agglutinative staphylococcin plus cisplatin with cisplatin alone. |
| Participants | 74 participants with malignant pleural effusion and ascites |
| Interventions | Unclear from abstract how agents were delivered |
| Outcomes | Reduction in effusion/ascites volume Karnofsky score |
| Notes | Full text only available in Chinese and unable to translate. Need to confirm if pleural and ascites data is presented separately and how the agents were delivered |
| Methods | RCT of pleural perfusion of nedaplatin and cisplatin in MPE due to non‐small cell lung cancer |
| Participants | 68 participants with lung cancer associated with malignant pleural effusion |
| Interventions | Participants randomised into two groups:
Agents given weekly for 2 ‐ 4 weeks |
| Outcomes | Treatment response Side effects Karnofsky performance status Survival |
| Notes | Full text only available in Chinese and unable to translate |
| Methods | RCT of intrapleural Adriamycin and Nocardia rubra cell wall skeleton, compared with Adriamycin alone |
| Participants | 55 participants with MPE due to lung cancer |
| Interventions | Agents given via tube thoracostomy. No other details available |
| Outcomes | Treatment response |
| Notes | In Japanese. Unable to translate |
| Methods | Trial comparing bleomycin,OK‐432 and cisplatin plus etoposide in MPE due to non‐small cell lung cancer |
| Participants | Malignant pleural effusion due to previously untreated non‐small cell lung cancer |
| Interventions | Intrapleural bleomycin,OK‐432 and cisplatin plus etoposide |
| Outcomes | Progression‐free survival |
| Notes | In Japanese. Unable to translate. No details in abstract as to whether it is randomised or the number of participants in the study |
| Methods | RCT comparing intrapleural pseudomonas aeruginosa, with cisplatin and interleukin‐2 |
| Participants | 90 participants with malignant pleural effusion |
| Interventions | Agents administered through intrathoracic infusion. No other information available |
| Outcomes | Clinical efficacy and adverse reactions |
| Notes | Written in Chinese and unable to obtain a translation. Only abstract available in English |
| Methods | RCT of intrapleural Ya‐Dan‐Zhi's grease (YDZ) and cisplatin in MPE |
| Participants | 72 participants with MPE |
| Interventions | Randomly divided between three groups:
|
| Outcomes | Treatment effect Side effects |
| Notes | In Chinese and unable to obtain a translation. Unclear from abstract if study would be eligible for inclusion in the review |
| Methods | RCT comparing intrapleural doxycycline and bleomycin |
| Participants | 34 patients with MPE requiring repeated thoracentesis |
| Interventions | Participants received either intrapleural doxycycline or bleomycin |
| Outcomes | Fluid volume Side effects Response to treatment (on CXR and clinical examination) Survival |
| Notes | In Korean. Only abstract available in English. Unable to obtain a translation |
| Methods | RCT evaluating the effect of intrapleural highly agglutinative staphylococcin (HAS) combined with nedaplatin, compared to nedaplatin alone |
| Participants | 58 participants with MPE |
| Interventions | Participants randomised to two groups:
|
| Outcomes | Treatment response Adverse effects Quality of life |
| Notes | In Chinese. Only abstract available in English and unclear from it whether the study is eligible. Unable to obtain translation of the full text |
| Methods | RCT comparing cisplatin and lentinan in malignant pleural effusion |
| Participants | 64 participants with MPE |
| Interventions | Randomised into two groups:
|
| Outcomes | Response rates |
| Notes | In Chinese. Only abstract available in English and unclear from it whether the study is eligible. Unable to obtain translation of the full text |
| Methods | RCT comparing matrine injection (yanshu) combined with intrapleural cisplatin for treatment of haematologic malignancies complicated by pleural effusion |
| Participants | 46 participants with haematological malignancy complicated by pleural effusion |
| Interventions | Participants randomly divided into two groups:
|
| Outcomes | Efficacy Adverse effects |
| Notes | In Chinese. Only abstract available in English and unclear from it whether the study is eligible. Unable to obtain translation of the full text |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Australasian Malignant Pleural Effusion (AMPLE) Trial |
| Methods | Multicentre, international RCT comparing IPC to talc pleurodesis |
| Participants | Aiming to recruit 146 participants to the study Inclusion: symptomatic MPE requiring intervention and written informed consent Exclusion: age < 18; effusion < 2 cm max depth; expected survival < 3 months; chylothorax; previous ipsilateral lobectomy or pneumonectomy; previous pleurodesis; pleural infection; white cell count < 1 x109/L; hypercapnic ventilatory failure; pregnancy or lactation; irreversible bleeding diathesis; irreversible visual impairment; inability to give informed consent or comply with the protocol |
| Interventions | Participants randomised 1:1 to IPC or talc pleurodesis |
| Outcomes | Primary: number of days spent in hospital for any cause following intervention until death or end of study follow‐up Secondary: admissions for pleural effusion‐associated causes; survival and adverse events; breathlessness and QOL; health cost assessment |
| Starting date | 1 June 2012 |
| Contact information | |
| Notes |
| Trial name or title | The efficacy of indwelling pleural catheter placement versus IPC placement plus sclerosant (talc) in patients with malignant pleural effusions managed exclusively as outpatients |
| Methods | Multi‐centre, single‐blind RCT of talc slurry or placebo administered via an indwelling pleural catheter |
| Participants | Aiming to recruit 154 participants to the study Inclusion criteria: Exclusion criteria: |
| Interventions | Participants in both arms undergo IPC insertion. At day 10 post insertion, participants randomised to 4 g intrapleural talc or placebo. Followed up for 10 weeks. Participants blinded to treatment allocation |
| Outcomes | Primary: pleurodesis success at five weeks post randomisation Secondary: QOL; pain and breathlessness VAS Scores; volume of pleural fluid drained; mortality; hospital inpatient bed days; degree of loculation on ultrasound; pleurodesis success at 10 weeks post randomisation; number of pleural procedures required to relieve pleural fluid |
| Starting date | 11 July 2012 |
| Contact information | |
| Notes |
| Trial name or title | Effectiveness of doxycycline for treating pleural effusions related to cancer in an outpatient population (OPUS) |
| Methods | RCT of doxycycline versus placebo administration via a PleurX catheter in MPE |
| Participants | Malignant pleural effusion; fully expanded lung post drainage of the pleural effusion Target recruitment of 50 participants |
| Interventions | Participants have a PleurX catheter inserted and are then randomised to intrapleural doxycycline or placebo |
| Outcomes | Primary: pleurodesis rate at 90 days Secondary: time to pleurodesis |
| Starting date | 2009 |
| Contact information | |
| Notes | NCT01411202 |
| Trial name or title | Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry: a randomised trial to determine the most effective method for the management of malignant pleural effusions in patients with a good performance status (The TAPPS Study) |
| Methods | The TAPPS trial is a multi‐centre randomised controlled study which compares the efficacy of chest drain insertion and talc slurry instillation with local anaesthetic thoracoscopy and talc poudrage, in the management of malignant pleural effusions |
| Participants | Inclusion: clinically confident diagnosis of malignant pleural effusion requiring pleurodesis; fit enough to undergo local anaesthetic thoracoscopy; expected survival > 3 months Exclusion: patients requiring a thoracoscopy to make a diagnosis; age < 18 years; pregnancy or lactation; evidence of extensive lung entrapment; insufficient pleural fluid to safely perform local anaesthetic thoracoscopy; adverse reaction to talc; contraindication to thoracoscopy or chest tube insertion Aiming to recruit 330 participants. |
| Interventions | Control arm: 12 ‐ 14 Fr seldinger drain, then 4 g talc slurry pleurodesis Intervention arm: medical thoracoscopy, with 4 g talc poudrage at end of the procedure |
| Outcomes | Primary endpoint: the number of participants who experience pleurodesis failure up to three months (90 days) post randomisation Secondary endpoints: requirement for further pleural procedures up to six months post‐randomisation; percentage radiographic (chest X‐ray) pleural opacification at 1, 3 and 6 months post randomisation; quality of life; thoracic pain; breathlessness; pleurodesis failure at 1 and 6 months; mortality |
| Starting date | 26 September 2012 |
| Contact information | |
| Notes |
| Trial name or title | The first therapeutic interventions in malignant effusion trial (TIME‐1) |
| Methods | 2 x 2 randomised factorial trial to assess whether non‐steroidal analgesia and the use of small‐bore chest tubes will reduce pain during pleurodesis for MPE, compared to standard care |
| Participants | 320 target recruitment (interim analysis after 120 participants) Inclusion: clinically confident diagnosis of MPE requiring pleurodesis; written informed consent; expected survival > 1 month Exclusion: age < 18; primary lymphoma or small cell lung carcinoma; pregnancy or lactation; history of gastro intestinal (GI) bleeding or untreated peptic ulceration; known sensitivity to non‐steroidal anti‐inflammatory drugs (NSAIDs) or opiates; hypercapnic ventilatory failure; intravenous drug use; severe renal or liver disease; bleeding diathesis; warfarin therapy which must be continued; current or recent corticosteroid therapy |
| Interventions | Participants will be randomised to one of the following arms:
|
| Outcomes | Primary: pain score over 72 hours post pleurodesis Secondary: success of pleurodesis at 6 weeks and 3 months; presence of ipsilateral, chronic chest pain at 6 weeks and 3 months |
| Starting date | 1 September 2006 |
| Contact information | |
| Notes |
| Trial name or title | Adjuvant urokinase in the treatment of malignant pleural effusion: the third therapeutic intervention in malignant effusion trial (TIME3‐UK) |
| Methods | A double blind, randomised controlled trial to evaluate whether use of intrapleural urokinase aids the drainage of multi‐septated pleural effusion compared to placebo |
| Participants | Inclusion: clinically confident diagnosis of pleural malignancy; significant multi‐loculated pleural effusion despite the presence of a patent in‐situ chest tube; MPE requiring drainage and pleurodesis for symptom control Exclusion: age < 18; expected survival < 28 days; previous ipsilateral pneumonectomy; previous IP fibrinolytics; ipsilateral pleural infection; sensitivity to urokinase; coincidental stroke, major haemorrhage or trauma; major surgery in past five days; chylothorax; white cell count < 1 x 109; pregnancy or lactation; irreversible bleeding diathesis; platelets < 100 x 109; irreversible visual impairment |
| Interventions | Participants randomised to three doses of 100,000 IU urokinase 12 hourly intrapleurally or placebo through an intercostal drain. All participants then undergo a talc pleurodesis. Followed up for 12 months |
| Outcomes | Primary: mean daily breathlessness score over 28 days from randomisation; time to pleurodesis failure Secondary: improvement of effusion on radiology; volume of pleural fluid drained; QOL; healthcare costs |
| Starting date | 2008 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 21 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Bleomycin, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Bleomycin vs iodine | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.18, 3.57] |
| 1.2 Bleomycin vs talc slurry | 5 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.55, 2.70] |
| 1.3 Bleomycin vs tetracycline | 5 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.93] |
| 1.4 Bleomycin vs talc poudrage | 2 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 9.70 [2.10, 44.78] |
| 1.5 Bleomycin vs C. parvum | 2 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [0.02, 189.25] |
| 1.6 Bleomycin vs doxycycline | 2 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.54, 4.20] |
| 1.7 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.15, 0.65] |
| 1.8 Bleomycin vs mitoxantrone | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.86] |
| 1.9 Bleomycin vs mepacrine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 6.40 [1.12, 36.44] |
| 1.10 Bleomycin vs combined tetracycline and bleomycin | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 5.57 [0.25, 124.19] |
| 1.11 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 1.1 [0.39, 3.07] |
| 1.12 Bleomycin vs OK‐432 | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.49, 4.17] |
| 1.13 Bleomycin vs viscum | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 5.33 [0.62, 45.99] |
| 2 Pain Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Bleomycin, Outcome 2 Pain. | ||||
| 2.1 Bleomycin vs talc slurry | 2 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.41, 6.80] |
| 2.2 Bleomycin vs tetracycline | 4 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.27] |
| 2.3 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.31] |
| 2.4 Bleomycin vs C. parvum | 2 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.27, 1.85] |
| 2.5 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 32.34 [1.89, 552.23] |
| 2.6 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.56] |
| 2.7 Bleomycin vs mepacrine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.11, 1.94] |
| 2.8 Bleomycin vs doxycycline | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.26, 2.70] |
| 2.9 Bleomycin vs OK‐432 | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.12] |
| 2.10 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.32, 2.16] |
| 3 Mortality Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Bleomycin, Outcome 3 Mortality. | ||||
| 3.1 Bleomycin vs combined tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.18] |
| 3.2 Bleomycin vs talc slurry | 2 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.29, 2.75] |
| 3.3 Bleomycin vs tetracycline | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.44] |
| 3.4 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.20, 3.43] |
| 3.5 Bleomycin vs C. parvum | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.94] |
| 3.6 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.25, 0.87] |
| 3.7 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.95, 4.86] |
| 3.8 Bleomycin vs OK‐432 | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [0.98, 7.23] |
| 3.9 Bleomycin vs doxycycline | 2 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.53, 3.90] |
| 3.10 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.82, 6.01] |
| 4 Fever Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Bleomycin, Outcome 4 Fever. | ||||
| 4.1 Bleomycin vs talc Slurry | 3 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.31, 2.56] |
| 4.2 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.11, 7.05] |
| 4.3 Bleomycin vs tetracycline | 5 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.67, 6.34] |
| 4.4 Tetracycline vs C. parvum | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.12] |
| 4.5 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 151.35 [9.08, 2522.62] |
| 4.6 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.37, 3.36] |
| 4.7 Bleomycin vs mepacrine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.14, 1.92] |
| 4.8 Bleomycin vs doxycycline | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 2.69 [0.08, 89.51] |
| 4.9 Bleomycin vs combined tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.69] |
| 4.10 Bleomycin vs OK432 | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.13] |
| 4.11 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.82, 6.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Talc slurry, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Talc slurry vs talc poudrage | 3 | 599 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.92, 1.85] |
| 1.2 Talc slurry vs bleomycin | 5 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.82] |
| 1.3 Talc slurry vs IPC | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.61] |
| 1.4 Talc slurry vs mepacrine | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.14, 1.60] |
| 1.5 Talc slurry vs placebo | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.51] |
| 1.6 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.18, 25.78] |
| 1.7 Talc slurry vs tetracycline | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.32, 5.17] |
| 1.8 Talc slurry vs silver nitrate | 1 | 25 | Odds Ratio (M‐H, Random, 95% CI) | 5.82 [0.21, 158.82] |
| 1.9 Talc slurry vs TMP | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 2.31 [0.77, 6.93] |
| 2 Mortality Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Talc slurry, Outcome 2 Mortality. | ||||
| 2.1 Talc slurry vs talc poudrage | 2 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.33, 2.85] |
| 2.2 Talc slurry vs bleomycin | 2 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.36, 3.46] |
| 2.3 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Talc slurry vs IPC | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.10] |
| 2.5 Talc slurry vs mepacrine | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.70, 5.02] |
| 2.6 Talc slurry vs TMP | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 10.64 [0.55, 203.85] |
| 3 Pain Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Talc slurry, Outcome 3 Pain. | ||||
| 3.1 Talc slurry vs bleomycin | 3 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.46] |
| 3.2 Talc slurry vs talc poudrage | 1 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.04, 4.36] |
| 3.3 Talc slurry vs tetracycline | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.07, 1.36] |
| 3.4 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Talc slurry vs IPC | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.95] |
| 3.6 Talc slurry vs placebo | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fever Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Talc slurry, Outcome 4 Fever. | ||||
| 4.1 Talc slurry vs talc poudrage | 2 | 479 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.42, 6.48] |
| 4.2 Talc slurry vs bleomycin | 3 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.36, 2.51] |
| 4.3 Talc slurry vs tetracycline | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.28, 4.32] |
| 4.4 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.23, 10.94] |
| 4.5 Talc slurry vs silver nitrate | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.7 [0.15, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Talc poudrage, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Talc poudrage vs talc slurry | 3 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.09] |
| 1.2 Talc poudrage vs bleomycin | 2 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.48] |
| 1.3 Talc poudrage vs tetracycline | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.76] |
| 1.4 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.08, 3.80] |
| 1.5 Talc poudrage vs mustine | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.71] |
| 1.6 Talc poudrage vs doxycycline | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.47] |
| 2 Mortality Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2 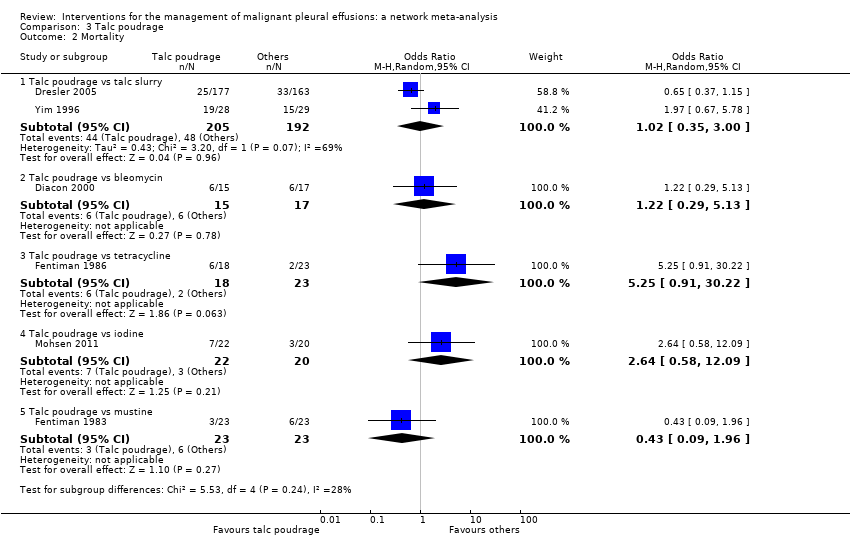 Comparison 3 Talc poudrage, Outcome 2 Mortality. | ||||
| 2.1 Talc poudrage vs talc slurry | 2 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.35, 3.00] |
| 2.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.29, 5.13] |
| 2.3 Talc poudrage vs tetracycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 5.25 [0.91, 30.22] |
| 2.4 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.58, 12.09] |
| 2.5 Talc poudrage vs mustine | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.09, 1.96] |
| 3 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Talc poudrage, Outcome 3 Pain. | ||||
| 3.1 Talc poudrage vs talc slurry | 1 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.96] |
| 3.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.14, 95.78] |
| 3.3 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 9.97 [0.50, 198.04] |
| 4 Fever Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Talc poudrage, Outcome 4 Fever. | ||||
| 4.1 Talc poudrage vs talc slurry | 2 | 479 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.37] |
| 4.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.14, 9.38] |
| 4.3 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.43, 41.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Tetracycline, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Tetracycline vs C. parvum | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.52, 19.64] |
| 1.2 Tetracycline vs talc slurry | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.13] |
| 1.3 Tetracycline vs Adriamycin | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.9 [0.05, 16.59] |
| 1.4 Tetracyclines vs placebo | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.94] |
| 1.5 Tetracycline vs talc poudrage | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 12.10 [1.32, 111.30] |
| 1.6 Tetracycline vs mustine | 2 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.35] |
| 1.7 Tetracycline vs combined tetracycline and bleomycin | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 8.27 [0.40, 172.05] |
| 1.8 Tetracycline vs bleomycin | 5 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 2.00 [1.07, 3.75] |
| 1.9 Tetracycline vs mepacrine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.12, 20.99] |
| 2 Pain Show forest plot | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Tetracycline, Outcome 2 Pain. | ||||
| 2.1 Tetracycline vs talc slurry | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 3.28 [0.73, 14.68] |
| 2.2 Tetracycline vs bleomycin | 4 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.79, 3.43] |
| 2.3 Tetracycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.45] |
| 2.4 Tetracycline vs mustine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 33.87 [1.80, 636.88] |
| 2.5 Tetracycline vs mepacrine | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.23] |
| 2.6 Tetracycline vs placebo | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fever Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Tetracycline, Outcome 3 Fever. | ||||
| 3.1 Tetracycline vs talc slurry | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.63] |
| 3.2 Tetracycline vs bleomycin | 5 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.50] |
| 3.3 Tetracycline vs C. parvum | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.06] |
| 3.4 Tetracycline vs mepacrine | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.89] |
| 3.5 Tetracycline vs combination tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.69] |
| 3.6 Tetracycline vs placebo | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Tetracycline vs mustine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality Show forest plot | 4 | 202 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.26] |
| Analysis 4.4  Comparison 4 Tetracycline, Outcome 4 Mortality. | ||||
| 4.1 Tetracycline vs talc poudrage | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.10] |
| 4.2 Tetracycline vs bleomycin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.69, 3.69] |
| 4.3 Tetracycline vs C. parvum | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.28, 31.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 C. parvum, Outcome 1 Pleurodesis failure. | ||||
| 1.1 C. parvum vs bleomycin | 2 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.01, 57.48] |
| 1.2 C. parvum vs tetracycline | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 1.94] |
| 1.3 C. parvum vs doxycycline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.12, 2.33] |
| 1.4 C. parvum vs mustine | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.52] |
| 2 Pain Show forest plot | 4 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.10, 5.75] |
| Analysis 5.2  Comparison 5 C. parvum, Outcome 2 Pain. | ||||
| 2.1 C. parvum vs bleomycin | 2 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.54, 3.75] |
| 2.2 C . parvum vs tetracycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [0.69, 8.66] |
| 2.3 C. parvum vs doxycycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [1.84, 29.55] |
| 3 Fever Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 C. parvum, Outcome 3 Fever. | ||||
| 3.1 C. parvum vs bleomycin | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [0.90, 5.92] |
| 3.2 C. parvum vs tetracycline | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 288.00 [16.62, 4991.05] |
| 3.3 C. parvum vs mustine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 4.41 [0.16, 121.68] |
| 3.4 C. parvum vs doxycycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [1.84, 29.55] |
| 4 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 C. parvum, Outcome 4 Mortality. | ||||
| 4.1 C. parvum vs bleomycin | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.51, 5.38] |
| 4.2 C. parvum vs tetracycline | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.55] |
| 4.3 C. parvum vs mustine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.07, 2.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Indwelling pleural catheter (IPC), Outcome 1 Pleurodesis failure. | ||||
| 1.1 IPC vs talc slurry | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 3.35 [1.64, 6.83] |
| 2 Mortality Show forest plot | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.23] |
| Analysis 6.2  Comparison 6 Indwelling pleural catheter (IPC), Outcome 2 Mortality. | ||||
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Indwelling pleural catheter (IPC), Outcome 3 Pain. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Iodine, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.26, 11.83] |
| 1.2 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.71] |
| 1.3 Iodine vs bleomycin | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.28, 5.59] |
| 2 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 Iodine, Outcome 2 Fever. | ||||
| 2.1 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.28] |
| 2.2 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.02, 2.33] |
| 3 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.3  Comparison 7 Iodine, Outcome 3 Mortality. | ||||
| 3.1 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.73] |
| 4 Pain Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.4 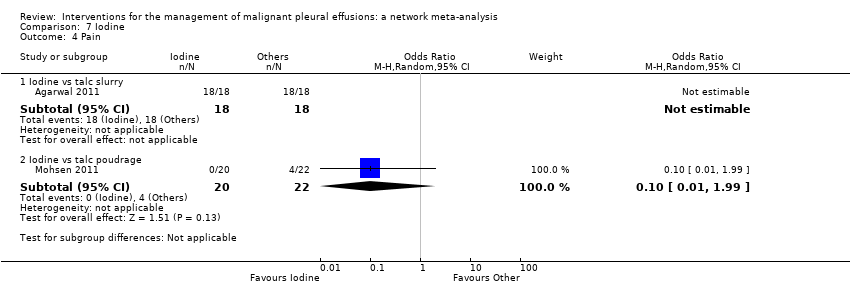 Comparison 7 Iodine, Outcome 4 Pain. | ||||
| 4.1 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Doxycycline, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Doxycycline vs talc poudrage | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 42.69 [2.13, 856.61] |
| 1.2 Doxycycline vs bleomycin | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 1.3 Doxycycline vs C. parvum | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.43, 8.48] |
| 2 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.2  Comparison 8 Doxycycline, Outcome 2 Pain. | ||||
| 2.1 Doxycycline vs bleomycin | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.80] |
| 2.2 Doxycycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.96] |
| 3 Fever Show forest plot | 3 | 189 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 2.16] |
| Analysis 8.3  Comparison 8 Doxycycline, Outcome 3 Fever. | ||||
| 3.1 Doxycycline vs bleomycin | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.01, 12.35] |
| 3.2 Doxycycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.4 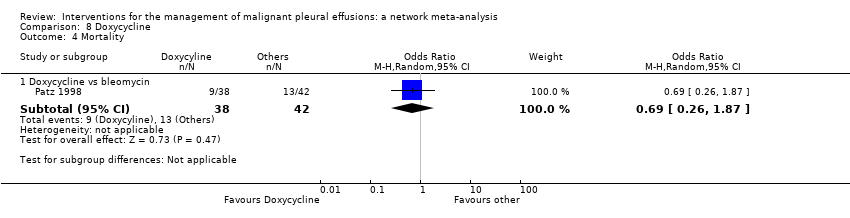 Comparison 8 Doxycycline, Outcome 4 Mortality. | ||||
| 4.1 Doxycycline vs bleomycin | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.26, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | 628 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.04] |
| Analysis 9.1  Comparison 9 Mode of administration, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Talc | 3 | 599 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.09] |
| 1.2 Tetracycline | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Duration of drainage after pleurodesis administration, Outcome 1 Pleurodesis failure. | ||||
| 2 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.2  Comparison 10 Duration of drainage after pleurodesis administration, Outcome 2 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 11.1  Comparison 11 OK‐432, Outcome 1 Pleurodesis failure. | ||||
| 1.1 OK‐432 and mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.11] |
| 1.2 OK‐432 vs cisplatin and etoposide | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.26, 2.27] |
| 1.3 OK‐432 and cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.92] |
| 1.4 High dose vs low dose | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.38, 9.44] |
| 1.5 OK‐432 vs bleomycin | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.03] |
| 1.6 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 12.44 [1.32, 117.03] |
| 2 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 11.2  Comparison 11 OK‐432, Outcome 2 Pain. | ||||
| 2.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 6.67 [1.15, 38.60] |
| 2.2 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.43] |
| 2.3 OK‐432 vs mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 8.00] |
| 2.4 OK‐432 vs bleomycin | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 2.53 [0.89, 7.15] |
| 2.5 OK‐432 vs cisplatin and etoposide | 1 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 2.1 [0.73, 6.01] |
| 3 Fever Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 11.3  Comparison 11 OK‐432, Outcome 3 Fever. | ||||
| 3.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 256.00 [14.70, 4457.27] |
| 3.2 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 14.00 [1.46, 134.25] |
| 3.3 OK‐432 vs mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 26.67 [5.91, 120.42] |
| 3.4 OK‐432 vs bleomycin | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.47, 4.35] |
| 3.5 OK‐432 vs cisplatin and etoposide | 1 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [1.08, 9.30] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 11.4  Comparison 11 OK‐432, Outcome 4 Mortality. | ||||
| 4.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.31, 5.53] |
| 4.2 OK‐432 vs combined OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.44, 10.91] |
| 4.3 OK‐432 vs bleomycin | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.03] |
| 4.4 OK‐432 vs cisplatin and etoposide | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.32, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 3 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 4.56 [1.66, 12.52] |
| Analysis 12.1  Comparison 12 Mepacrine, Outcome 1 Pain. | ||||
| 1.1 Mepacrine vs bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.52, 9.00] |
| 1.2 Mepacrine vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 5.6 [0.81, 38.51] |
| 1.3 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 14.53 [0.71, 298.21] |
| 1.4 Mepacrine vs triethylenethiophosphoramide | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 23.71 [1.19, 474.06] |
| 2 Fever Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 12.2  Comparison 12 Mepacrine, Outcome 2 Fever. | ||||
| 2.1 Mepacrine vs bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.52, 7.01] |
| 2.2 Mepacrine vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.13, 56.79] |
| 2.3 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 62.43 [2.85, 1365.52] |
| 2.4 Mepacrine vs triethylene... | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 23.83 [3.35, 169.39] |
| 3 Pleurodesis failure Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 12.3  Comparison 12 Mepacrine, Outcome 3 Pleurodesis failure. | ||||
| 3.1 Mepacrine vs talc slurry | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.62, 6.96] |
| 3.2 Mepacrine vs bleomycin | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.89] |
| 3.3 Mepacrine vs tetracycline | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.05, 8.20] |
| 3.4 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.73] |
| 3.5 Mepacrine vs mitoxantrone | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 7.61 [0.35, 163.82] |
| 3.6 Mepacrine vs triethylene... | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 0.98] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 12.4  Comparison 12 Mepacrine, Outcome 4 Mortality. | ||||
| 4.1 Mepacrine vs talc slurry | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.43] |
| 4.2 Mepacrine vs mitoxantrone | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.23, 11.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 13.1  Comparison 13 Interferon (IFN), Outcome 1 Pleurodesis failure. | ||||
| 1.1 IFN vs bleomycin | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 3.25 [1.54, 6.89] |
| 2 Pain Show forest plot | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| Analysis 13.2  Comparison 13 Interferon (IFN), Outcome 2 Pain. | ||||
| 3 Fever Show forest plot | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.11] |
| Analysis 13.3  Comparison 13 Interferon (IFN), Outcome 3 Fever. | ||||
| 4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 13.4  Comparison 13 Interferon (IFN), Outcome 4 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 14.1  Comparison 14 Triethylenethiophophoramide, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.03, 3.69] |
| 1.2 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 4.95 [1.02, 24.10] |
| 2 Pain Show forest plot | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.10, 20.15] |
| Analysis 14.2  Comparison 14 Triethylenethiophophoramide, Outcome 2 Pain. | ||||
| 2.1 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.30] |
| 2.2 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 7.43 [0.35, 156.28] |
| 3 Fever Show forest plot | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.00, 26.74] |
| Analysis 14.3  Comparison 14 Triethylenethiophophoramide, Outcome 3 Fever. | ||||
| 3.1 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [0.15, 81.92] |
| 3.2 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 15.1  Comparison 15 Adriamycin, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Adriamycin vs mustine | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.01, 10.18] |
| 1.2 Adriamycin vs tetracycline | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.06, 20.49] |
| 1.3 Adriamycin vs LC9018 and Adriamycin | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 4.29 [1.62, 11.35] |
| 2 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 15.2  Comparison 15 Adriamycin, Outcome 2 Fever. | ||||
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 15.3  Comparison 15 Adriamycin, Outcome 3 Pain. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 16.1  Comparison 16 Placebo, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Placebo vs mepacrine | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 14.40 [1.37, 150.81] |
| 1.2 Placebo vs mitoxantrone | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.56, 3.17] |
| 1.3 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 2.91 [0.27, 31.21] |
| 1.4 Placebo vs talc slurry | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 13.93 [0.66, 293.99] |
| 1.5 Placebo vs tetracycline | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 3.33 [0.51, 21.58] |
| 2 Pain Show forest plot | 3 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.82] |
| Analysis 16.2  Comparison 16 Placebo, Outcome 2 Pain. | ||||
| 2.1 Placebo vs talc slurry | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Placebo vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Placebo vs mepacrine | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.41] |
| 2.4 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.83] |
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 16.3  Comparison 16 Placebo, Outcome 3 Fever. | ||||
| 3.1 Placebo vs mepacrine | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.79] |
| 3.2 Placebo vs mitoxantone | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.35] |
| 3.3 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.1  Comparison 17 Mustine, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Mustine vs tetracycline | 2 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 2.72 [0.74, 9.98] |
| 1.2 Mustine vs talc poudrage | 1 | 37 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.40, 45.76] |
| 1.3 Mustine vs C. parvum | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 10.8 [1.64, 70.93] |
| 1.4 Mustine vs Adriamycin | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 2.71 [0.10, 74.98] |
| 2 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.2  Comparison 17 Mustine, Outcome 2 Fever. | ||||
| 2.1 Mustine vs tetracycline | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mustine vs C. parvum | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 6.25] |
| 3 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.3  Comparison 17 Mustine, Outcome 3 Mortality. | ||||
| 3.1 Mustine vs talc poudrage | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [0.51, 10.86] |
| 3.2 Mustine vs C. parvum | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 2.4 [0.38, 15.32] |
| 4 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.4  Comparison 17 Mustine, Outcome 4 Pain. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 18.1  Comparison 18 Mitoxantrone, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Mitoxantrone vs placebo | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.79] |
| 1.2 Mitoxantrone vs mepacrine | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 7.61 [0.35, 163.82] |
| 1.3 Mitoxantrone vs bleomycin | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [1.17, 8.65] |
| 2 Pain Show forest plot | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.64, 6.76] |
| Analysis 18.2 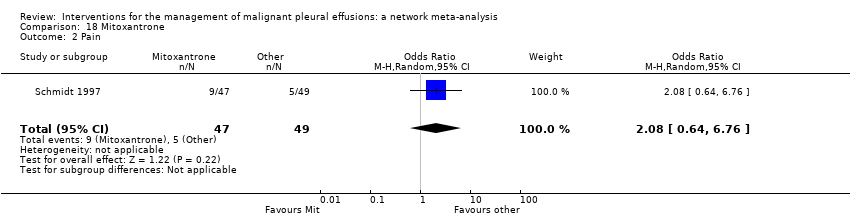 Comparison 18 Mitoxantrone, Outcome 2 Pain. | ||||
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 18.3  Comparison 18 Mitoxantrone, Outcome 3 Fever. | ||||
| 3.1 Mitoxantrone vs bleomycin | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.71] |
| 3.2 Mitoxantrone vs placebo | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 3.28 [1.26, 8.49] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 18.4  Comparison 18 Mitoxantrone, Outcome 4 Mortality. | ||||
| 4.1 Mitoxantrone vs bleomycin | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.05] |
| 4.2 Mitoxantrone vs mepacrine | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.09, 4.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.07, 4.64] |
| Analysis 19.1  Comparison 19 Drain size, Outcome 1 Pleurodesis failure. | ||||
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 19.2  Comparison 19 Drain size, Outcome 2 Pain. | ||||
| 3 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 19.3  Comparison 19 Drain size, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.30] |
| Analysis 20.1  Comparison 20 Thoracoscopic mechanical pleurodesis (TMP), Outcome 1 Pleurodesis failure. | ||||
| 2 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 20.2  Comparison 20 Thoracoscopic mechanical pleurodesis (TMP), Outcome 2 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.70, 2.30] |
| Analysis 21.1  Comparison 21 Other, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Rotation vs no rotation | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 2.25 [0.17, 29.77] |
| 1.2 Streptokinase vs no streptokinase | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.53, 9.02] |
| 1.3 Mixed particle talc vs graded talc | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.23, 11.70] |
| 1.4 Talc pleurodesis vs VATS parietal pleurectomy | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.49, 2.09] |
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.2  Comparison 21 Other, Outcome 2 Pain. | ||||
| 2.1 Streptokinase vs control | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 77.47] |
| 3 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.3  Comparison 21 Other, Outcome 3 Fever. | ||||
| 3.1 Mixed particle talc vs graded talc | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 15.92 [1.81, 140.16] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.4 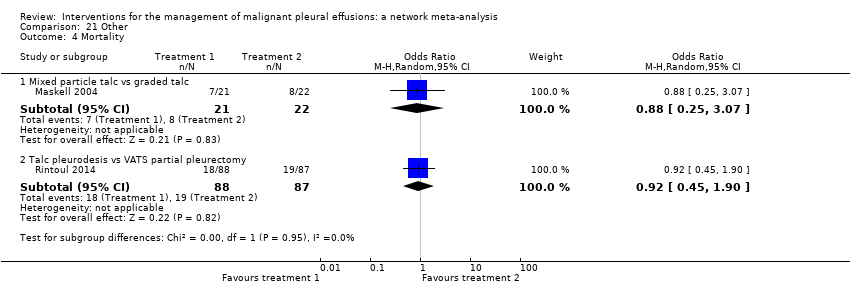 Comparison 21 Other, Outcome 4 Mortality. | ||||
| 4.1 Mixed particle talc vs graded talc | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.25, 3.07] |
| 4.2 Talc pleurodesis vs VATS partial pleurectomy | 1 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 22.1  Comparison 22 Silver nitrate, Outcome 1 Pleurodesis failure. | ||||
| 2 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 22.2  Comparison 22 Silver nitrate, Outcome 2 Fever. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 23.1  Comparison 23 Cisplatin, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Cisplatin vs cisplatin and bevacizumab | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 5.0 [1.66, 15.09] |
| 1.2 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.52, 8.17] |
| 1.3 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 25.67 [2.68, 245.84] |
| 1.4 Cisplatin vs rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 4.67 [0.99, 22.03] |
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 23.2  Comparison 23 Cisplatin, Outcome 2 Pain. | ||||
| 2.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.87] |
| 2.2 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.21] |
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 23.3  Comparison 23 Cisplatin, Outcome 3 Fever. | ||||
| 3.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.07] |
| 3.2 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.52] |
| 3.3 Cisplatin vs rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.51] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 23.4  Comparison 23 Cisplatin, Outcome 4 Mortality. | ||||
| 4.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.18, 3.23] |
| 4.2 Cisplatin vs combination OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.32, 8.59] |
| 4.3 Cisplatin vs combination rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.82] |
| Analysis 24.1  Comparison 24 Duration of drainage prior to administration of sclerosant, Outcome 1 Pleurodesis failure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.1  Comparison 25 Dose of silver nitrate, Outcome 1 Pleurodesis failure. | ||||
| 1.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 1.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 2 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.2 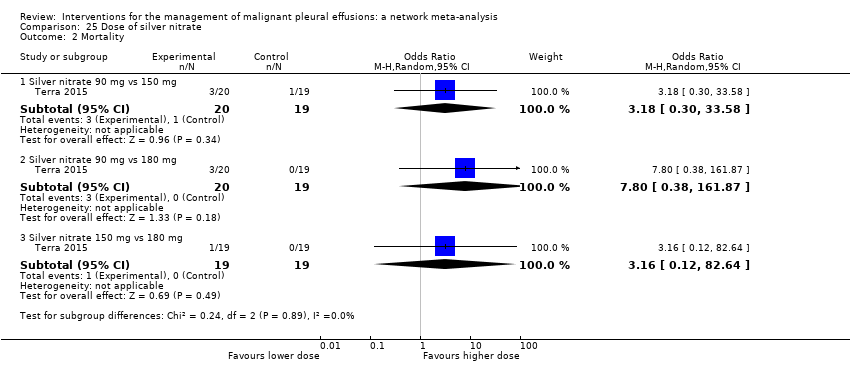 Comparison 25 Dose of silver nitrate, Outcome 2 Mortality. | ||||
| 2.1 Silver nitrate 90 mg vs 150 mg | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.30, 33.58] |
| 2.2 Silver nitrate 90 mg vs 180 mg | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 7.80 [0.38, 161.87] |
| 2.3 Silver nitrate 150 mg vs 180 mg | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [0.12, 82.64] |
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.3  Comparison 25 Dose of silver nitrate, Outcome 3 Pain. | ||||
| 3.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 4 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.4  Comparison 25 Dose of silver nitrate, Outcome 4 Fever. | ||||
| 4.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.24] |
| 4.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 4.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.24, 10.70] |

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Network plot of the pleurodesis efficacy network. The nodes are weighted according to the number of participants randomised to the intervention. The edges (line thicknesses) are weighted according to the number of studies included in each comparison.

Estimated (95% Cr‐I) ranks for each of the pleurodesis methods from the main network

Inconsistency plot for the main network. Treatment codes: 01 = Talc slurry; 02 = Talc poudrage; 03 = Bleomycin; 04 = Tetracycline; 05 = C. parvum; 06 = Interferon; 07 = Iodine; 08 = Indwelling pleural catheter; 09 = Placebo; 10 = Mustine; 11 = Mitoxantrone; 12 = Mepacrine; 13 = Doxycyline; 14 = Triethylenethiophosphoramide; 15 = Adriamycin. Abbreviations: ROR = Ratio of Odds Ratios; 95% CI = 95% Confidence interval. Heterogeneity variance was set at 0.8847 (reflecting the estimation of Tau from the network)
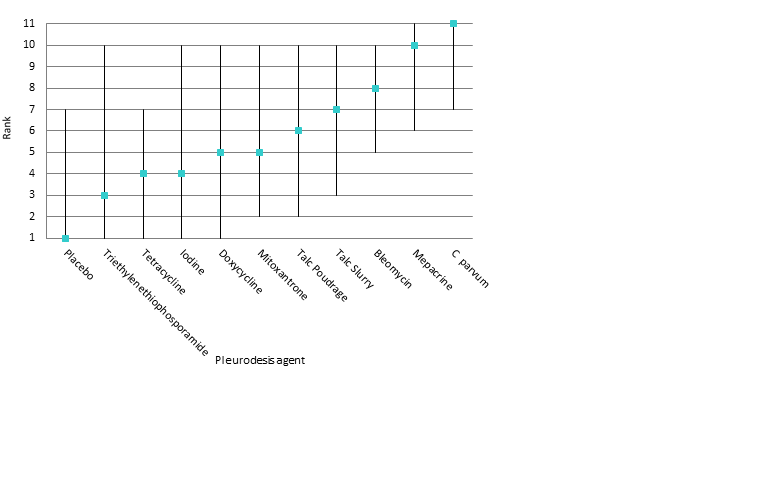
Estimated rank (95% Cr‐I) for causing fever (a low rank suggests less fever)

Comparison 1 Bleomycin, Outcome 1 Pleurodesis failure.
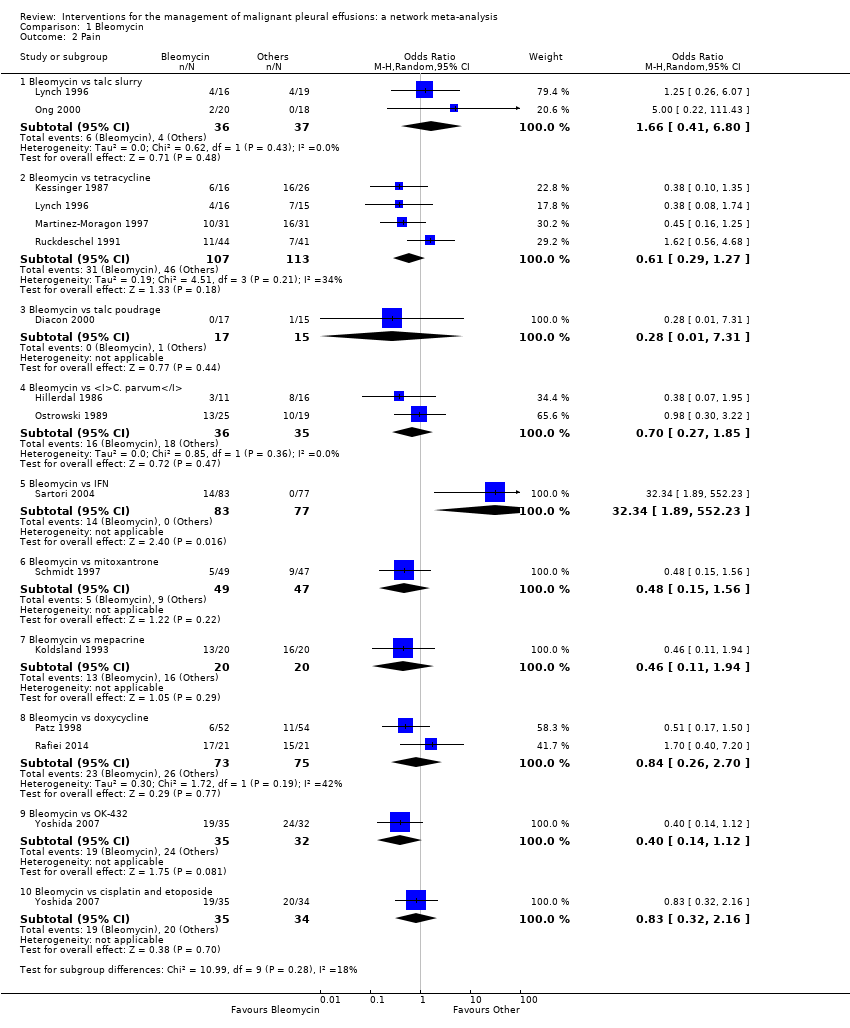
Comparison 1 Bleomycin, Outcome 2 Pain.

Comparison 1 Bleomycin, Outcome 3 Mortality.

Comparison 1 Bleomycin, Outcome 4 Fever.

Comparison 2 Talc slurry, Outcome 1 Pleurodesis failure.

Comparison 2 Talc slurry, Outcome 2 Mortality.

Comparison 2 Talc slurry, Outcome 3 Pain.

Comparison 2 Talc slurry, Outcome 4 Fever.

Comparison 3 Talc poudrage, Outcome 1 Pleurodesis failure.

Comparison 3 Talc poudrage, Outcome 2 Mortality.
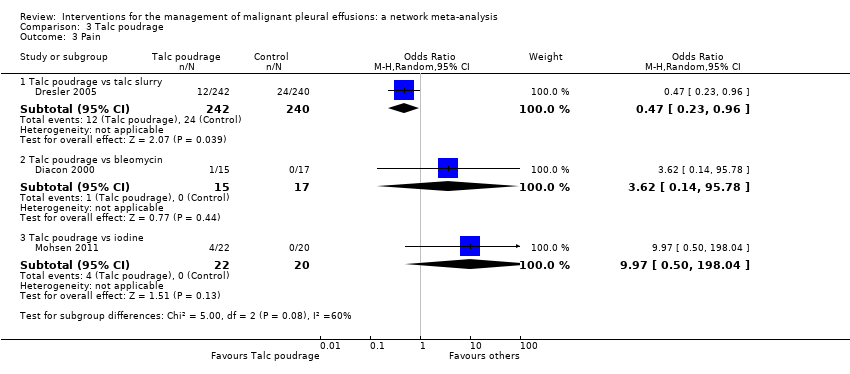
Comparison 3 Talc poudrage, Outcome 3 Pain.

Comparison 3 Talc poudrage, Outcome 4 Fever.
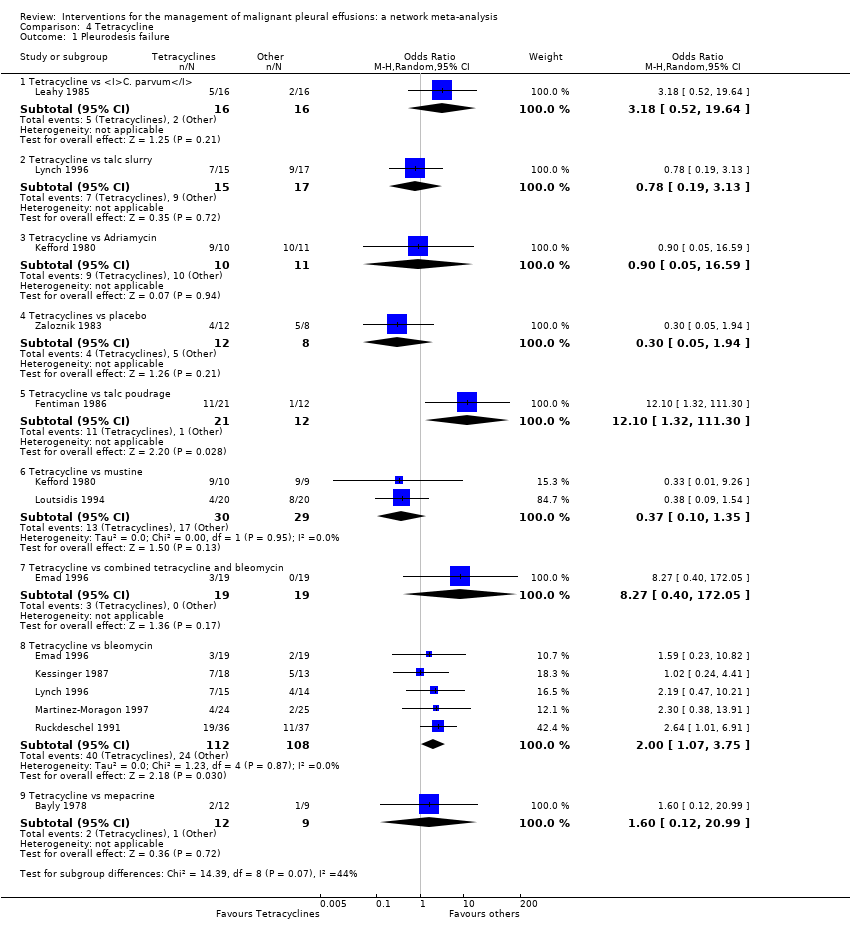
Comparison 4 Tetracycline, Outcome 1 Pleurodesis failure.
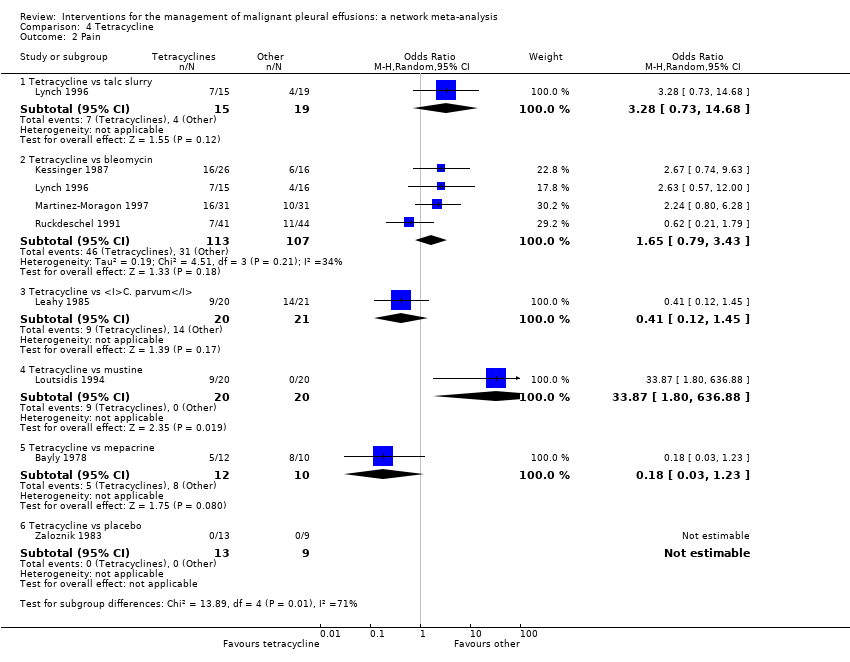
Comparison 4 Tetracycline, Outcome 2 Pain.

Comparison 4 Tetracycline, Outcome 3 Fever.
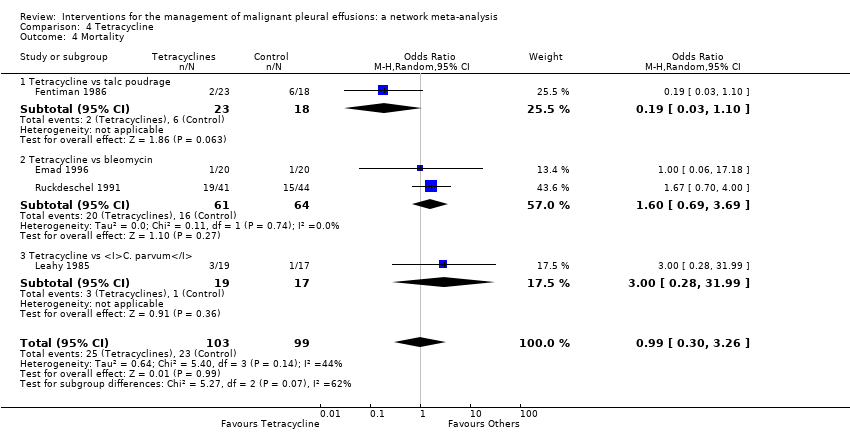
Comparison 4 Tetracycline, Outcome 4 Mortality.

Comparison 5 C. parvum, Outcome 1 Pleurodesis failure.

Comparison 5 C. parvum, Outcome 2 Pain.

Comparison 5 C. parvum, Outcome 3 Fever.

Comparison 5 C. parvum, Outcome 4 Mortality.

Comparison 6 Indwelling pleural catheter (IPC), Outcome 1 Pleurodesis failure.

Comparison 6 Indwelling pleural catheter (IPC), Outcome 2 Mortality.

Comparison 6 Indwelling pleural catheter (IPC), Outcome 3 Pain.

Comparison 7 Iodine, Outcome 1 Pleurodesis failure.

Comparison 7 Iodine, Outcome 2 Fever.

Comparison 7 Iodine, Outcome 3 Mortality.

Comparison 7 Iodine, Outcome 4 Pain.

Comparison 8 Doxycycline, Outcome 1 Pleurodesis failure.

Comparison 8 Doxycycline, Outcome 2 Pain.

Comparison 8 Doxycycline, Outcome 3 Fever.

Comparison 8 Doxycycline, Outcome 4 Mortality.

Comparison 9 Mode of administration, Outcome 1 Pleurodesis failure.

Comparison 10 Duration of drainage after pleurodesis administration, Outcome 1 Pleurodesis failure.

Comparison 10 Duration of drainage after pleurodesis administration, Outcome 2 Mortality.

Comparison 11 OK‐432, Outcome 1 Pleurodesis failure.

Comparison 11 OK‐432, Outcome 2 Pain.

Comparison 11 OK‐432, Outcome 3 Fever.

Comparison 11 OK‐432, Outcome 4 Mortality.

Comparison 12 Mepacrine, Outcome 1 Pain.

Comparison 12 Mepacrine, Outcome 2 Fever.

Comparison 12 Mepacrine, Outcome 3 Pleurodesis failure.

Comparison 12 Mepacrine, Outcome 4 Mortality.

Comparison 13 Interferon (IFN), Outcome 1 Pleurodesis failure.

Comparison 13 Interferon (IFN), Outcome 2 Pain.

Comparison 13 Interferon (IFN), Outcome 3 Fever.

Comparison 13 Interferon (IFN), Outcome 4 Mortality.

Comparison 14 Triethylenethiophophoramide, Outcome 1 Pleurodesis failure.

Comparison 14 Triethylenethiophophoramide, Outcome 2 Pain.
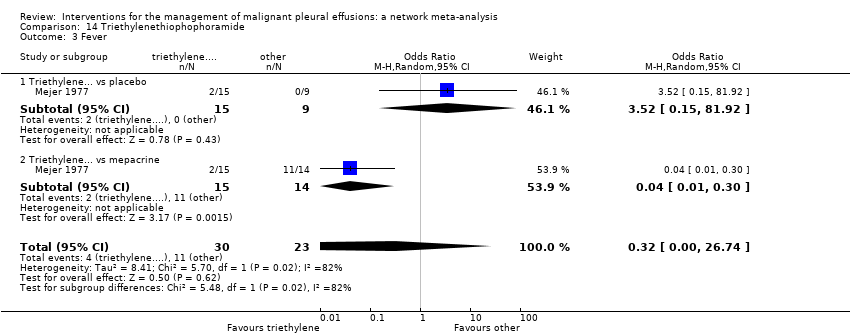
Comparison 14 Triethylenethiophophoramide, Outcome 3 Fever.

Comparison 15 Adriamycin, Outcome 1 Pleurodesis failure.

Comparison 15 Adriamycin, Outcome 2 Fever.
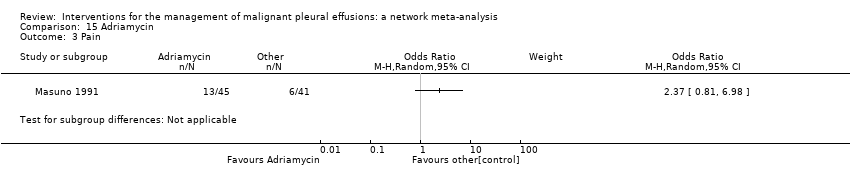
Comparison 15 Adriamycin, Outcome 3 Pain.

Comparison 16 Placebo, Outcome 1 Pleurodesis failure.

Comparison 16 Placebo, Outcome 2 Pain.

Comparison 16 Placebo, Outcome 3 Fever.
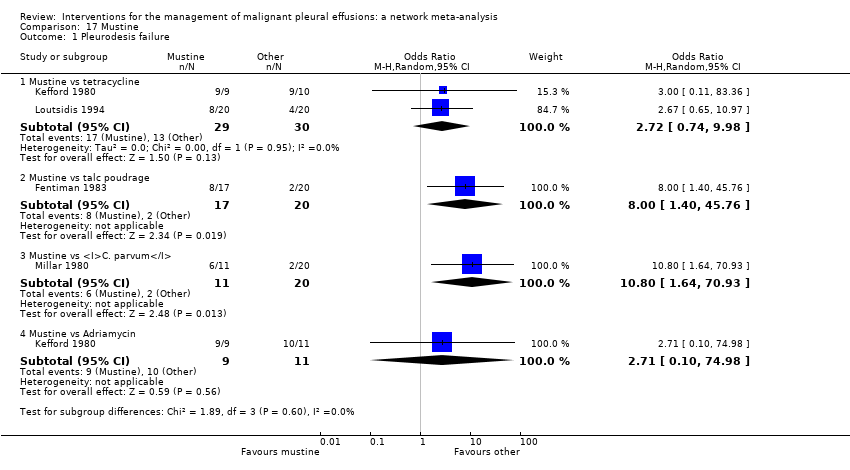
Comparison 17 Mustine, Outcome 1 Pleurodesis failure.

Comparison 17 Mustine, Outcome 2 Fever.

Comparison 17 Mustine, Outcome 3 Mortality.

Comparison 17 Mustine, Outcome 4 Pain.

Comparison 18 Mitoxantrone, Outcome 1 Pleurodesis failure.

Comparison 18 Mitoxantrone, Outcome 2 Pain.

Comparison 18 Mitoxantrone, Outcome 3 Fever.
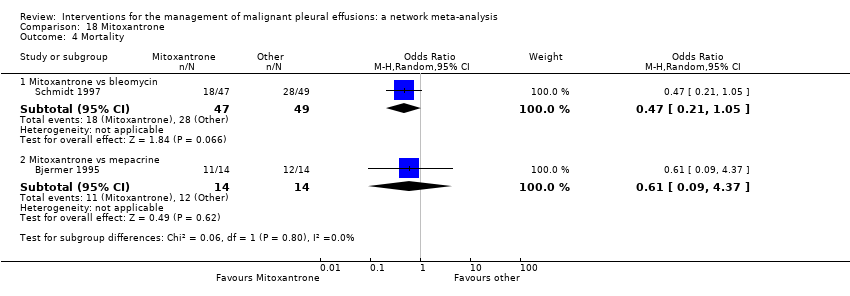
Comparison 18 Mitoxantrone, Outcome 4 Mortality.

Comparison 19 Drain size, Outcome 1 Pleurodesis failure.

Comparison 19 Drain size, Outcome 2 Pain.

Comparison 19 Drain size, Outcome 3 Mortality.

Comparison 20 Thoracoscopic mechanical pleurodesis (TMP), Outcome 1 Pleurodesis failure.

Comparison 20 Thoracoscopic mechanical pleurodesis (TMP), Outcome 2 Mortality.

Comparison 21 Other, Outcome 1 Pleurodesis failure.
Comparison 21 Other, Outcome 2 Pain.

Comparison 21 Other, Outcome 3 Fever.

Comparison 21 Other, Outcome 4 Mortality.

Comparison 22 Silver nitrate, Outcome 1 Pleurodesis failure.

Comparison 22 Silver nitrate, Outcome 2 Fever.

Comparison 23 Cisplatin, Outcome 1 Pleurodesis failure.

Comparison 23 Cisplatin, Outcome 2 Pain.

Comparison 23 Cisplatin, Outcome 3 Fever.

Comparison 23 Cisplatin, Outcome 4 Mortality.

Comparison 24 Duration of drainage prior to administration of sclerosant, Outcome 1 Pleurodesis failure.

Comparison 25 Dose of silver nitrate, Outcome 1 Pleurodesis failure.

Comparison 25 Dose of silver nitrate, Outcome 2 Mortality.

Comparison 25 Dose of silver nitrate, Outcome 3 Pain.

Comparison 25 Dose of silver nitrate, Outcome 4 Fever.
| Treatment | Talc slurry | Talc poudrage | Bleomycin | Tetracycline | C. parvum | Placebo | Mustine | Mitoxantrone | Mepacrine |
| Talc poudrage | 0.76 (0.54, 1.09); n = 3; Tau2 = 0; I2 = 0% | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bleomycin | 1.22 (0.55, 2.70); n = 5*; Tau2 = 0.1; I2 = 12% | 9.70 (2.10, 44.78); n = 2; Tau2 = 0; I2= 0% | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tetracycline | 0.78 (0.19, 3.13); n = 1* | 12.10 (1.32, 111.30); n = 1 | 2.00 (1.07, 3.75); n = 5*; Tau2 = 0; I2 = 0% | NA | ‐ | ‐ | ‐ | ‐ | ‐ |
| C. parvum | NA | NA | 0.55 (0.01, 57.48); n = 2; Tau2 = 11; I2 = 94% | 0.31 (0.05, 1.94); n = 1 | NA | ‐ | ‐ | ‐ | ‐ |
| Interferon | NA | NA | 3.25 (1.54, 6.89); n = 1 | NA | NA | ‐ | ‐ | ‐ | ‐ |
| Iodine | 0.47 (0.04, 5.71); n = 1 | 1.76 (0.26, 11.83); n = 1 | 1.25 (0.28, 5.59); n = 1 | NA | NA | ‐ | ‐ | ‐ | ‐ |
| Indwelling pleural catheter | 3.35 (1.64, 6.83); n = 2 Tau2 = 0; I2 = 0% | NA | NA | NA | NA | ‐ | ‐ | ‐ | ‐ |
| Placebo | 13.93 (0.66, 293.99); n = 1 | NA | NA | 3.33 (0.51, 21.58); n = 1 | NA | NA | ‐ | ‐ | ‐ |
| Mustine | NA | 8.00 (1.40, 45.76); n = 1 | NA | 2.72 (0.74, 9.98) n = 2*; Tau2= 0; I2= 0% | 3.00 (0.40, 22.71); n = 1 | NA | NA | ‐ | ‐ |
| Mitoxantrone | NA | NA | 3.18 (1.17, 8.65); n = 1 | NA | NA | 0.75 (0.32, 1.79); n = 1 | NA | NA | ‐ |
| Mepacrine | 2.08 (0.62, 6.96); n = 1 | NA | 0.16 (0.03, 0.89); n = 1 | 0.63 (0.05, 8.20); n = 1 | NA | 0.15 (0.03, 0.64); n = 1* | NA | 7.61 (0.35, 163.82); n = 1 | NA |
| Doxycycline | NA | 42.69 (2.13, 856.61); n = 1 | 0.67 (0.24, 1.86); n = 2; Tau2= 0; I2= 0% | NA | 1.91 (0.43, 8.48); n = 1 | NA | NA | NA | NA |
| Triethylenethiophosphoramide | NA | NA | NA | NA | NA | 2.06 (0.43, 9.80); n = 1* | NA | NA | 4.95 (1.02, 24.10); n = 1* |
| Adriamycin | NA | NA | NA | 1.11 (0.06, 20.49); n = 1* | NA | NA | 0.37 (0.01, 10.18); n = 1* | NA | NA |
| n = the number of studies included in the pair‐wise comparison. * Indicates that the comparison included a three‐arm study. NA = no direct pair‐wise comparison available. Results that are statistically significant at the conventional level of P < 0.05 are shaded in grey. ‐ indicates the odds ratio is already expressed elsewhere in the table comparing the interventions the other way around. | |||||||||
| Talc slurry | Talc poudrage | Bleomycin | Tetracycline | C. parvum | Interferon | Iodine | Indwelling pleural catheter | Placebo | Mustine | Mitoxantrone | Mepacrine | Doxycyline | Triethylenethiophosphoramide | viscum | |
| Talc poudrage | 0.42 (0.13, 1.19) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bleomycin | 2.56 (1.05, 6.67) | 6.03 (2.19, 20.46) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tetracycline | 3.71 (1.22, 11.67) | 8.77 (2.74, 33.01) | 1.45 (0.59, 3.46) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C. parvum | 1.48 (0.34, 6.57) | 3.49 (0.79, 17.64) | 0.58 (0.16, 1.95) | 0.40 (0.10, 1.52) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Interferon | 8.49 (0.94, 82.98) | 19.96 (2.22, 229.60) | 3.33 (0.43, 25.66) | 2.29 (0.26, 21.65) | 5.75 (0.55, 64.16) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iodine | 1.25 (0.22, 6.77) | 2.97 (0.55, 17.21) | 0.49 (0.09, 2.49) | 0.34 (0.05, 2.04) | 0.85 (0.11, 6.35) | 0.15 (0.01, 1.90) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Indwelling pleural catheter | 3.47 (0.75, 16.46) | 8.19 (1.32, 59.02) | 1.36 (0.22, 8.01) | 0.94 (0.14, 6.27) | 2.36 (0.28, 19.88) | 0.41 (0.03, 5.96) | 2.76 (0.29, 28.48) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Placebo | 19.50 (3.73, 128.50) | 46.51 (7.86, 375.90) | 7.64 (1.55, 44.22) | 5.29 (1.04, 31.95) | 13.28 (1.91, 110.80) | 2.29 (0.18, 34.14) | 15.63 (1.72, 179.10) | 5.61 (0.59, 65.18) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mustine | 7.50 (1.35, 43.86) | 17.75 (3.59, 105.70) | 2.94 (0.58, 14.84) | 2.02 (0.43, 9.79) | 5.07 (0.91, 29.81) | 0.88 (0.06, 11.71) | 5.98 (0.68, 58.17) | 2.16 (0.22, 22.76) | 0.38 (0.04, 3.32) | NA | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mitoxantrone | 12.87 (2.36, 89.02) | 30.53 (5.11, 259.50) | 5.04 (1.04, 28.67) | 3.48 (0.64, 22.72) | 8.76 (1.24, 73.66) | 1.51 (0.12, 22.89) | 10.28 (1.12, 119.70) | 3.71 (0.38, 44.85) | 0.66 (0.13, 3.52) | 1.73 (0.19, 17.80 | NA | ‐ | ‐ | ‐ | ‐ |
| Mepacrine | 0.98 (0.22, 4.15) | 2.32 (0.45, 12.99) | 0.38 (0.09, 1.52) | 0.27 (0.05, 1.17) | 0.67 (0.10, 4.06) | 0.12 (0.01, 1.31) | 0.78 (0.09, 6.55) | 0.28 (0.03, 2.32) | 0.05 (0.01, 0.28) | 0.13 (0.02, 0.99) | 0.08 (0.01, 0.47) | NA | ‐ | ‐ | ‐ |
| Doxycycline | 3.49 (0.68, 19.56) | 8.23 (1.70, 50.18) | 1.37 (0.31, 6.09) | 0.94 (0.18, 5.09) | 2.36 (0.46, 13.09) | 0.41 (0.03, 5.14) | 2.78 (0.33, 26.50) | 1.00 (0.11, 10.23) | 0.18 (0.02, 1.53) | 0.47 (0.06, 3.77) | 0.27 (0.03, 2.31) | 3.56 (0.50, 28.59) | NA | ‐ | ‐ |
| Triethylenethiophosphoramide | 5.53 (0.40, 80.97) | 13.07 (0.89, 227.30) | 2.16 (0.16, 29.77) | 1.50 (0.10, 21.61) | 3.74 (0.21, 66.99) | 0.65 (0.02, 17.63) | 4.40 (0.22, 98.58) | 1.59 (0.08, 35.28) | 0.28 (0.02, 3.62) | 0.74 (0.04, 15.00) | 0.43 (0.02, 6.80) | 5.60 (0.55, 63.81) | 1.59 (0.08, 31.05) | NA | ‐ |
| Adriamycin | 2.31 (0.03, 165.40) | 5.53 (0.08, 403.50) | 0.90 (0.01, 59.43) | 0.62 (0.01, 38.58) | 1.57 (0.02, 114.20) | 0.27 (0.00, 27.43) | 1.85 (0.02, 162.70) | 0.67 (0.01, 62.01) | 0.12 (0.00, 9.46) | 0.31 (0.00, 20.50) | 0.18 (0.00, 14.59) | 2.36 (0.03, 191.30) | 0.66 (0.01, 52.71) | 0.42 (0.00, 54.35) | NA |
| Viscum | 0.39 (0.01, 8.23) | 0.92 (0.03, 21.77) | 0.15 (0.01, 2.73) | 0.10 (0.00, 2.17) | 0.26 (0.01, 6.21) | 0.04 (0.00, 1.55) | 0.31 (0.01, 9.07) | 0.11 (0.00, 3.44) | 0.02 (0.00, 0.53) | 0.05 (0.00, 1.41) | 0.03 (0.00, 0.79) | 0.39 (0.01, 10.28) | 0.11 (0.00, 2.83) | 0.07 (0.00, 3.48) | 0.16 (0.00, 26.60) |
| Results that are statistically significant at the conventional level of P < 0.05 are shaded in grey. ‐ indicates the odds ratio is already expressed elsewhere in the table comparing the interventions the other way around. NA= not applicable. | |||||||||||||||
| Study | Reason study excluded from network | Intrapleural agent or intervention 1 | Pleurodesis failure rate for agent 1 | Intrapleural agent or intervention 2 | Pleurodesis failure rate for agent 2 | OR (95% CI) of agent 1 compared with agent 2*** |
| Du 2013 | Lung cancer specific therapy | Cisplatin and bevacizumab | 6/36 | Cisplatin | 17/34 | 0.20 (0.07, 0.60) |
| Emad 1996* | No pleurodesis failures in the Combined group | Tetracycline** | 3/19 | Combined tetracycline and bleomycin | 0/19 | 8.27 (0.40, 172.05) |
| Bleomycin** | 2/19 | Combined tetracycline and bleomycin | 0/19 | 5.57 (0.25, 124.19) | ||
| Ishida 2006* | Lung cancer specific therapy | OK‐432 | 8/17 | Cisplatin | 11/17 | 0.48 (0.12, 1.92) |
| OK‐432 | 8/17 | OK‐432 and cisplatin | 1/15 | 12.44 (1.32, 117.03) | ||
| Cisplatin | 11/17 | OK‐432 and cisplatin | 1/15 | 25.67 (2.68, 245.84) | ||
| Kasahara 2006 | Lung cancer specific therapy | High dose OK‐432 | 5/19 | Low dose OK‐432 | 3/19 | 1.90 (0.38, 9.44) |
| Luh 1992 | Lung cancer specific therapy | OK‐432 | 3/26 | Mitomycin C | 9/27 | 0.26 (0.06, 1.11) |
| Maskell 2004 | Two Talc slurry preparations | Mixed particle talc | 3/14 | Graded talc (particles >20µm) | 2/14 | 1.64 (0.23, 11.70)) |
| Masuno 1991 | Lung cancer specific therapy | LC9018 and Adriamycin | 10/38 | Adriamycin | 23/38 | 0.23 (0.09, 0.62) |
| Paschoalini 2005 | No pleurodesis failures in Silver Nitrate group | Talc slurry | 1/9 | Silver nitrate | 0/16 | 5.85 (0.21, 158.82) |
| Rintoul 2014 | MPM specific surgical technique | Talc pleurodesis (slurry or poudrage) | 25/62 | VATS pleurectomy | 24/60 | 0.88 (0.43, 1.82) |
| Terra 2015* | Comparison of different doses of Silver Nitrate | 90 mg silver nitrate | 0/20 | 150 mg silver nitrate | 0/20 | not estimable |
| 90 mg silver nitrate | 0/20 | 180 mg silver nitrate | 2/20 | 0.18 (0.01, 4.01) | ||
| 150 mg silver nitrate | 0/20 | 180 mg silver nitrate | 2/20 | 0.19 (0.01, 4.01) | ||
| Yoshida 2007* | Lung cancer specific therapy | OK‐432 | 8/33 | Bleomycin | 11/35 | 0.70 (0.24, 2.03) |
| OK‐432 | 8/33 | Cisplatin and etoposide | 10/34 | 0.77 (0.26, 2.27) | ||
| Bleomycin | 11/35 | Cisplatin and etoposide | 10/34 | 1.10 (0.39, 3.07) | ||
| Zhao 2009 | Lung cancer specific therapy | rAd‐p53 and cisplatin | 3/17 | Cisplatin | 9/18 | 0.21 (0.05, 1.01) |
| *Three‐arm study. **The results for the pair‐wise comparison between tetracycline and bleomycin are included in the network meta‐analysis. ***Results that are statistically significant at the conventional level of P < 0.05 are shaded in grey | ||||||
| Type of method to optimise pleurodesis | Study | Intervention 1 | Pleurodesis failure rate for intervention 1 | Intervention 2 | Pleurodesis failure rate for intervention 2 | OR (95% CI) of intervention 1 compared with intervention 2* |
| Mode of administration | Evans 1993 | Tetracycline pleurodesis at the end of thoracoscopy | 2/15 | Tetracycline pleurodesis through an intercostal cannula | 5/14 | 0.28 (0.04, 1.76) |
| Chest tube size | Clementsen 1998 | Small‐bore chest drain | 2/9 | Large‐bore chest drain | 3/9 | 0.57 (0.07, 4.64) |
| Patient rotation | Mager 2002 | Rotation after instillation of talc | 2/10 | No rotation after instillation of talc | 1/10 | 2.25 (0.17, 29.77) |
| Duration of drainage after administration of the sclerosant | Goodman 2006 | Drain removed 24 hours after pleurodesis | 2/16 | Drain removed 72 hours after pleurodesis | 4/19 | 0.54 (0.08, 3.40) |
| Villanueva 1994 | Drain removal the day after pleurodesis | 2/9 | Drain removal when < 150 ml/day output | 3/15 | 1.14 (0.15, 8.59) | |
| Yildirim 2005 | Fractionated dose oxytetracycline (4 divided doses at 6‐hourly intervals) | 0/12 | Single bedside instillation of oxytetracycline | 2/8 | 0.10 (0.00, 2.50) | |
| Duration of drainage prior to administration of the sclerosant | Ozkul 2014 | Early instillation of talc slurry after drain insertion | 5/40 | Instillation of talc slurry when daily drainage from chest tube < 300 ml/day | 6/39 | 0.79 (0.22, 2.82) |
| Intrapleural fibrinolytics | Okur 2011 | Intrapleural streptokinase | 14/19 | No intrapleural streptokinase | 9/16 | 2.18 (0.53, 9.02) |
| Pleural abrasion at thoracoscopy | Crnjac 2004 | Talc slurry | 11/42 | Thoracoscopic mechanical pleurodesis | 6/45 | 2.31 (0.77, 6.93) |
| * Results that are statistically significant at the conventional level of P < 0.05 are shaded in grey | ||||||
| Talc slurry | Talc poudrage | Bleomycin | Tetracycline | C. parvum | Iodine | Mepacrine | Placebo | Mitoxantrone | Doxycycline | |
| Talc poudrage | 0.66 (0.09, 3.98) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bleomycin | 1.26 (0.24, 6.82) | 1.93 (0.22, 19.42) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tetracycline | 0.29 (0.04, 2.09) | 0.45 (0.04, 5.74) | 0.23 (0.06, 0.88) | NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C. parvum | 6.31 (0.61, 70.69) | 9.71 (0.65, 176.70) | 5.01 (0.92, 29.12) | 21.46 (3.10, 175.70) | NA | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iodine | 0.27 (0.02, 3.69) | 0.42 (0.03, 6.09) | 0.21 (0.01, 4.25) | 0.93 (0.03, 23.41) | 0.04 (0.00, 1.29) | NA | ‐ | ‐ | ‐ | ‐ |
| Mepacrine | 4.52 (0.30, 76.00) | 6.95 (0.34, 182.20) | 3.58 (0.40, 36.59) | 15.41 (1.62, 178.80) | 0.71 (0.05, 11.99) | 16.72 (0.43, 831.10) | NA | ‐ | ‐ | ‐ |
| Placebo | 0.06 (0.00, 2.00) | 0.10 (0.00, 4.27) | 0.05 (0.00, 1.08) | 0.22 (0.00, 5.71) | 0.01 (0.00, 0.32) | 0.23 (0.00, 17.55) | 0.01 (0.00, 0.30) | NA | ‐ | ‐ |
| Mitoxantrone | 0.48 (0.02, 10.24) | 0.73 (0.02, 22.95) | 0.38 (0.02, 5.02) | 1.64 (0.07, 29.71) | 0.08 (0.00, 1.60) | 1.75 (0.03, 99.74) | 0.11 (0.00, 2.16) | 7.57 (0.59, 138.80) | NA | ‐ |
| Doxycycline | 0.49 (0.03, 6.13) | 0.75 (0.04, 14.68) | 0.39 (0.05, 2.66) | 1.67 (0.14, 17.22) | 0.08 (0.01, 0.63) | 1.81 (0.05, 69.03) | 0.11 (0.00, 1.93) | 7.69 (0.19, 539.10) | 1.02 (0.04, 33.23) | NA |
| Triethylenephosphoramide | 0.24 (0.00, 17.04) | 0.37 (0.00, 35.93) | 0.19 (0.00, 9.80) | 0.81 (0.02, 47.08) | 0.04 (0.00, 2.63) | 0.88 (0.01, 139.50) | 0.05 (0.00, 1.49) | 3.62 (0.07, 529.40) | 0.49 (0.01, 49.44 | 0.49 (0.01, 45.90) |
| Results that are statistically significant at the conventional level of P < 0.05 are shaded in grey. ‐ indicates the odds ratio is already expressed elsewhere in the table comparing the interventions the other way around. NA= not applicable | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 21 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Bleomycin vs iodine | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.18, 3.57] |
| 1.2 Bleomycin vs talc slurry | 5 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.55, 2.70] |
| 1.3 Bleomycin vs tetracycline | 5 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.93] |
| 1.4 Bleomycin vs talc poudrage | 2 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 9.70 [2.10, 44.78] |
| 1.5 Bleomycin vs C. parvum | 2 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [0.02, 189.25] |
| 1.6 Bleomycin vs doxycycline | 2 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.54, 4.20] |
| 1.7 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.15, 0.65] |
| 1.8 Bleomycin vs mitoxantrone | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.86] |
| 1.9 Bleomycin vs mepacrine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 6.40 [1.12, 36.44] |
| 1.10 Bleomycin vs combined tetracycline and bleomycin | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 5.57 [0.25, 124.19] |
| 1.11 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 1.1 [0.39, 3.07] |
| 1.12 Bleomycin vs OK‐432 | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.49, 4.17] |
| 1.13 Bleomycin vs viscum | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 5.33 [0.62, 45.99] |
| 2 Pain Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Bleomycin vs talc slurry | 2 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.41, 6.80] |
| 2.2 Bleomycin vs tetracycline | 4 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.27] |
| 2.3 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.31] |
| 2.4 Bleomycin vs C. parvum | 2 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.27, 1.85] |
| 2.5 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 32.34 [1.89, 552.23] |
| 2.6 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.56] |
| 2.7 Bleomycin vs mepacrine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.11, 1.94] |
| 2.8 Bleomycin vs doxycycline | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.26, 2.70] |
| 2.9 Bleomycin vs OK‐432 | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.12] |
| 2.10 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.32, 2.16] |
| 3 Mortality Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bleomycin vs combined tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.18] |
| 3.2 Bleomycin vs talc slurry | 2 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.29, 2.75] |
| 3.3 Bleomycin vs tetracycline | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.44] |
| 3.4 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.20, 3.43] |
| 3.5 Bleomycin vs C. parvum | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.94] |
| 3.6 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.25, 0.87] |
| 3.7 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.95, 4.86] |
| 3.8 Bleomycin vs OK‐432 | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [0.98, 7.23] |
| 3.9 Bleomycin vs doxycycline | 2 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.53, 3.90] |
| 3.10 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.82, 6.01] |
| 4 Fever Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Bleomycin vs talc Slurry | 3 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.31, 2.56] |
| 4.2 Bleomycin vs talc poudrage | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.11, 7.05] |
| 4.3 Bleomycin vs tetracycline | 5 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.67, 6.34] |
| 4.4 Tetracycline vs C. parvum | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.12] |
| 4.5 Bleomycin vs IFN | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 151.35 [9.08, 2522.62] |
| 4.6 Bleomycin vs mitoxantrone | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.37, 3.36] |
| 4.7 Bleomycin vs mepacrine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.14, 1.92] |
| 4.8 Bleomycin vs doxycycline | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 2.69 [0.08, 89.51] |
| 4.9 Bleomycin vs combined tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.69] |
| 4.10 Bleomycin vs OK432 | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.13] |
| 4.11 Bleomycin vs cisplatin and etoposide | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.82, 6.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Talc slurry vs talc poudrage | 3 | 599 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.92, 1.85] |
| 1.2 Talc slurry vs bleomycin | 5 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.82] |
| 1.3 Talc slurry vs IPC | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.61] |
| 1.4 Talc slurry vs mepacrine | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.14, 1.60] |
| 1.5 Talc slurry vs placebo | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.51] |
| 1.6 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.18, 25.78] |
| 1.7 Talc slurry vs tetracycline | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.32, 5.17] |
| 1.8 Talc slurry vs silver nitrate | 1 | 25 | Odds Ratio (M‐H, Random, 95% CI) | 5.82 [0.21, 158.82] |
| 1.9 Talc slurry vs TMP | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 2.31 [0.77, 6.93] |
| 2 Mortality Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Talc slurry vs talc poudrage | 2 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.33, 2.85] |
| 2.2 Talc slurry vs bleomycin | 2 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.36, 3.46] |
| 2.3 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Talc slurry vs IPC | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.10] |
| 2.5 Talc slurry vs mepacrine | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.70, 5.02] |
| 2.6 Talc slurry vs TMP | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 10.64 [0.55, 203.85] |
| 3 Pain Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Talc slurry vs bleomycin | 3 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.46] |
| 3.2 Talc slurry vs talc poudrage | 1 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.04, 4.36] |
| 3.3 Talc slurry vs tetracycline | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.07, 1.36] |
| 3.4 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Talc slurry vs IPC | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.95] |
| 3.6 Talc slurry vs placebo | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fever Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Talc slurry vs talc poudrage | 2 | 479 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.42, 6.48] |
| 4.2 Talc slurry vs bleomycin | 3 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.36, 2.51] |
| 4.3 Talc slurry vs tetracycline | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.28, 4.32] |
| 4.4 Talc slurry vs iodine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.23, 10.94] |
| 4.5 Talc slurry vs silver nitrate | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.7 [0.15, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Talc poudrage vs talc slurry | 3 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.09] |
| 1.2 Talc poudrage vs bleomycin | 2 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.48] |
| 1.3 Talc poudrage vs tetracycline | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.76] |
| 1.4 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.08, 3.80] |
| 1.5 Talc poudrage vs mustine | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.71] |
| 1.6 Talc poudrage vs doxycycline | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.47] |
| 2 Mortality Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Talc poudrage vs talc slurry | 2 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.35, 3.00] |
| 2.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.29, 5.13] |
| 2.3 Talc poudrage vs tetracycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 5.25 [0.91, 30.22] |
| 2.4 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.58, 12.09] |
| 2.5 Talc poudrage vs mustine | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.09, 1.96] |
| 3 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Talc poudrage vs talc slurry | 1 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.96] |
| 3.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.14, 95.78] |
| 3.3 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 9.97 [0.50, 198.04] |
| 4 Fever Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Talc poudrage vs talc slurry | 2 | 479 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.37] |
| 4.2 Talc poudrage vs bleomycin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.14, 9.38] |
| 4.3 Talc poudrage vs iodine | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.43, 41.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tetracycline vs C. parvum | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.52, 19.64] |
| 1.2 Tetracycline vs talc slurry | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.13] |
| 1.3 Tetracycline vs Adriamycin | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.9 [0.05, 16.59] |
| 1.4 Tetracyclines vs placebo | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.94] |
| 1.5 Tetracycline vs talc poudrage | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 12.10 [1.32, 111.30] |
| 1.6 Tetracycline vs mustine | 2 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.35] |
| 1.7 Tetracycline vs combined tetracycline and bleomycin | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 8.27 [0.40, 172.05] |
| 1.8 Tetracycline vs bleomycin | 5 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 2.00 [1.07, 3.75] |
| 1.9 Tetracycline vs mepacrine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.12, 20.99] |
| 2 Pain Show forest plot | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Tetracycline vs talc slurry | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 3.28 [0.73, 14.68] |
| 2.2 Tetracycline vs bleomycin | 4 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.79, 3.43] |
| 2.3 Tetracycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.45] |
| 2.4 Tetracycline vs mustine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 33.87 [1.80, 636.88] |
| 2.5 Tetracycline vs mepacrine | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.23] |
| 2.6 Tetracycline vs placebo | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fever Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tetracycline vs talc slurry | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.63] |
| 3.2 Tetracycline vs bleomycin | 5 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.50] |
| 3.3 Tetracycline vs C. parvum | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.06] |
| 3.4 Tetracycline vs mepacrine | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.89] |
| 3.5 Tetracycline vs combination tetracycline and bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.69] |
| 3.6 Tetracycline vs placebo | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Tetracycline vs mustine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality Show forest plot | 4 | 202 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.26] |
| 4.1 Tetracycline vs talc poudrage | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.10] |
| 4.2 Tetracycline vs bleomycin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.69, 3.69] |
| 4.3 Tetracycline vs C. parvum | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.28, 31.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 C. parvum vs bleomycin | 2 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.01, 57.48] |
| 1.2 C. parvum vs tetracycline | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 1.94] |
| 1.3 C. parvum vs doxycycline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.12, 2.33] |
| 1.4 C. parvum vs mustine | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.52] |
| 2 Pain Show forest plot | 4 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.10, 5.75] |
| 2.1 C. parvum vs bleomycin | 2 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.54, 3.75] |
| 2.2 C . parvum vs tetracycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [0.69, 8.66] |
| 2.3 C. parvum vs doxycycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [1.84, 29.55] |
| 3 Fever Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 C. parvum vs bleomycin | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [0.90, 5.92] |
| 3.2 C. parvum vs tetracycline | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 288.00 [16.62, 4991.05] |
| 3.3 C. parvum vs mustine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 4.41 [0.16, 121.68] |
| 3.4 C. parvum vs doxycycline | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [1.84, 29.55] |
| 4 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 C. parvum vs bleomycin | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.51, 5.38] |
| 4.2 C. parvum vs tetracycline | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.55] |
| 4.3 C. parvum vs mustine | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.07, 2.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 IPC vs talc slurry | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 3.35 [1.64, 6.83] |
| 2 Mortality Show forest plot | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.23] |
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.26, 11.83] |
| 1.2 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.71] |
| 1.3 Iodine vs bleomycin | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.28, 5.59] |
| 2 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.28] |
| 2.2 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.02, 2.33] |
| 3 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.73] |
| 4 Pain Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Iodine vs talc slurry | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Iodine vs talc poudrage | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Doxycycline vs talc poudrage | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 42.69 [2.13, 856.61] |
| 1.2 Doxycycline vs bleomycin | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 1.3 Doxycycline vs C. parvum | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.43, 8.48] |
| 2 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Doxycycline vs bleomycin | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.80] |
| 2.2 Doxycycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.96] |
| 3 Fever Show forest plot | 3 | 189 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 2.16] |
| 3.1 Doxycycline vs bleomycin | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.01, 12.35] |
| 3.2 Doxycycline vs C. parvum | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Doxycycline vs bleomycin | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.26, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | 628 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.04] |
| 1.1 Talc | 3 | 599 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.09] |
| 1.2 Tetracycline | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 OK‐432 and mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.11] |
| 1.2 OK‐432 vs cisplatin and etoposide | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.26, 2.27] |
| 1.3 OK‐432 and cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.92] |
| 1.4 High dose vs low dose | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.38, 9.44] |
| 1.5 OK‐432 vs bleomycin | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.03] |
| 1.6 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 12.44 [1.32, 117.03] |
| 2 Pain Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 6.67 [1.15, 38.60] |
| 2.2 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.43] |
| 2.3 OK‐432 vs mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 8.00] |
| 2.4 OK‐432 vs bleomycin | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 2.53 [0.89, 7.15] |
| 2.5 OK‐432 vs cisplatin and etoposide | 1 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 2.1 [0.73, 6.01] |
| 3 Fever Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 256.00 [14.70, 4457.27] |
| 3.2 OK‐432 vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 14.00 [1.46, 134.25] |
| 3.3 OK‐432 vs mitomycin C | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 26.67 [5.91, 120.42] |
| 3.4 OK‐432 vs bleomycin | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.47, 4.35] |
| 3.5 OK‐432 vs cisplatin and etoposide | 1 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [1.08, 9.30] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 OK‐432 vs cisplatin | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.31, 5.53] |
| 4.2 OK‐432 vs combined OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.44, 10.91] |
| 4.3 OK‐432 vs bleomycin | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.03] |
| 4.4 OK‐432 vs cisplatin and etoposide | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.32, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 3 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 4.56 [1.66, 12.52] |
| 1.1 Mepacrine vs bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.52, 9.00] |
| 1.2 Mepacrine vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 5.6 [0.81, 38.51] |
| 1.3 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 14.53 [0.71, 298.21] |
| 1.4 Mepacrine vs triethylenethiophosphoramide | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 23.71 [1.19, 474.06] |
| 2 Fever Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mepacrine vs bleomycin | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.52, 7.01] |
| 2.2 Mepacrine vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.13, 56.79] |
| 2.3 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 62.43 [2.85, 1365.52] |
| 2.4 Mepacrine vs triethylene... | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 23.83 [3.35, 169.39] |
| 3 Pleurodesis failure Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mepacrine vs talc slurry | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.62, 6.96] |
| 3.2 Mepacrine vs bleomycin | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.89] |
| 3.3 Mepacrine vs tetracycline | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.05, 8.20] |
| 3.4 Mepacrine vs placebo | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.73] |
| 3.5 Mepacrine vs mitoxantrone | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 7.61 [0.35, 163.82] |
| 3.6 Mepacrine vs triethylene... | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 0.98] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Mepacrine vs talc slurry | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.43] |
| 4.2 Mepacrine vs mitoxantrone | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.23, 11.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 IFN vs bleomycin | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 3.25 [1.54, 6.89] |
| 2 Pain Show forest plot | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| 3 Fever Show forest plot | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.11] |
| 4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.03, 3.69] |
| 1.2 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 4.95 [1.02, 24.10] |
| 2 Pain Show forest plot | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.10, 20.15] |
| 2.1 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.30] |
| 2.2 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 7.43 [0.35, 156.28] |
| 3 Fever Show forest plot | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.00, 26.74] |
| 3.1 Triethylene... vs placebo | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [0.15, 81.92] |
| 3.2 Triethylene... vs mepacrine | 1 | 29 | Odds Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Adriamycin vs mustine | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.01, 10.18] |
| 1.2 Adriamycin vs tetracycline | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.06, 20.49] |
| 1.3 Adriamycin vs LC9018 and Adriamycin | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 4.29 [1.62, 11.35] |
| 2 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Placebo vs mepacrine | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 14.40 [1.37, 150.81] |
| 1.2 Placebo vs mitoxantrone | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.56, 3.17] |
| 1.3 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 2.91 [0.27, 31.21] |
| 1.4 Placebo vs talc slurry | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 13.93 [0.66, 293.99] |
| 1.5 Placebo vs tetracycline | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 3.33 [0.51, 21.58] |
| 2 Pain Show forest plot | 3 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.82] |
| 2.1 Placebo vs talc slurry | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Placebo vs tetracycline | 1 | 22 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Placebo vs mepacrine | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.41] |
| 2.4 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.83] |
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Placebo vs mepacrine | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.79] |
| 3.2 Placebo vs mitoxantone | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.35] |
| 3.3 Placebo vs triethylene... | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mustine vs tetracycline | 2 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 2.72 [0.74, 9.98] |
| 1.2 Mustine vs talc poudrage | 1 | 37 | Odds Ratio (M‐H, Random, 95% CI) | 8.00 [1.40, 45.76] |
| 1.3 Mustine vs C. parvum | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 10.8 [1.64, 70.93] |
| 1.4 Mustine vs Adriamycin | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 2.71 [0.10, 74.98] |
| 2 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mustine vs tetracycline | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mustine vs C. parvum | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 6.25] |
| 3 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mustine vs talc poudrage | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [0.51, 10.86] |
| 3.2 Mustine vs C. parvum | 1 | 21 | Odds Ratio (M‐H, Random, 95% CI) | 2.4 [0.38, 15.32] |
| 4 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mitoxantrone vs placebo | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.79] |
| 1.2 Mitoxantrone vs mepacrine | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 7.61 [0.35, 163.82] |
| 1.3 Mitoxantrone vs bleomycin | 1 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [1.17, 8.65] |
| 2 Pain Show forest plot | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.64, 6.76] |
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mitoxantrone vs bleomycin | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.71] |
| 3.2 Mitoxantrone vs placebo | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 3.28 [1.26, 8.49] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Mitoxantrone vs bleomycin | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.05] |
| 4.2 Mitoxantrone vs mepacrine | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.09, 4.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.07, 4.64] |
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.30] |
| 2 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 4 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.70, 2.30] |
| 1.1 Rotation vs no rotation | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 2.25 [0.17, 29.77] |
| 1.2 Streptokinase vs no streptokinase | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.53, 9.02] |
| 1.3 Mixed particle talc vs graded talc | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.23, 11.70] |
| 1.4 Talc pleurodesis vs VATS parietal pleurectomy | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.49, 2.09] |
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Streptokinase vs control | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 77.47] |
| 3 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Mixed particle talc vs graded talc | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 15.92 [1.81, 140.16] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Mixed particle talc vs graded talc | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.25, 3.07] |
| 4.2 Talc pleurodesis vs VATS partial pleurectomy | 1 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cisplatin vs cisplatin and bevacizumab | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 5.0 [1.66, 15.09] |
| 1.2 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.52, 8.17] |
| 1.3 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 25.67 [2.68, 245.84] |
| 1.4 Cisplatin vs rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 4.67 [0.99, 22.03] |
| 2 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.87] |
| 2.2 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.21] |
| 3 Fever Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.07] |
| 3.2 Cisplatin vs OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.52] |
| 3.3 Cisplatin vs rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.51] |
| 4 Mortality Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cisplatin vs OK‐432 | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.18, 3.23] |
| 4.2 Cisplatin vs combination OK‐432 and cisplatin | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.32, 8.59] |
| 4.3 Cisplatin vs combination rAd‐p53 and cisplatin | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pleurodesis failure Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 1.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 2 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Silver nitrate 90 mg vs 150 mg | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.30, 33.58] |
| 2.2 Silver nitrate 90 mg vs 180 mg | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 7.80 [0.38, 161.87] |
| 2.3 Silver nitrate 150 mg vs 180 mg | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [0.12, 82.64] |
| 3 Pain Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 4 Fever Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Silver nitrate 90 mg vs 150 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.24] |
| 4.2 Silver nitrate 90 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 4.3 Silver nitrate 150 mg vs 180 mg | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.24, 10.70] |

