Primary prophylaxis for venous thromboembolism in people undergoing major amputation of the lower extremity
Abstract
Background
Patients undergoing major amputation of the lower limb are at increased risk of venous thromboembolism (VTE). Risk factors for VTE in amputees include advanced age, sedentary lifestyle, longstanding arterial disease and an identifiable hypercoagulable condition. Evidence suggests that pharmacological prophylaxis (for example heparin, factor Xa inhibitors, vitamin K antagonists, direct thrombin inhibitors, antiplatelets) is effective in preventing deep vein thrombosis (DVT) but it is associated with an increased risk of bleeding. Mechanical prophylaxis (for example antiembolism stockings, intermittent pneumatic compression and foot impulse devices), on the other hand, is non‐invasive and has no side effects. However, it is not always appropriate in patients with contraindications such as peripheral arterial disease (PAD), arteriosclerosis or bilateral lower limb amputations. It is important to determine the most effective thromboprophylaxis and whether this is one treatment alone or in combination with another. To date, no systematic review has been conducted examining the effectiveness of thromboprophylaxis in preventing VTE in people undergoing amputation.
Objectives
To determine the effectiveness of thromboprophylaxis in preventing VTE in people undergoing major amputation of the lower extremity.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched May 2013) and CENTRAL (2013, Issue 4). Clinical trials databases were searched (May 2013). No date or language restrictions were applied. Non‐English trials were translated where required and reference lists of identified studies were handsearched.
Selection criteria
Randomised controlled trials and quasi‐randomised controlled trials in which people undergoing a major unilateral or bilateral amputation (for example hip disarticulation, transfemoral, knee disarticulation and transtibial) of the lower extremity were allocated to different types or regimens of thromboprophylaxis (including pharmacological or mechanical prophylaxis) or placebo.
Data collection and analysis
Selection of studies, data extraction and risk of bias were completed independently by two review authors. Any disagreements were resolved by discussion. Meta‐analysis could not be completed as the two included studies compared different treatments and therefore the data could not be pooled.
Main results
Two studies with a combined total of 288 participants met the inclusion criteria for this review. One study compared low molecular weight heparin with unfractionated heparin and found no difference between the treatments in the prevention of DVT (odds ratio (OR) 1.23, 95% confidence interval (CI) 0.28 to 5.35). No bleeding events or deaths occurred. This study was open label and therefore at a high risk of performance bias. Additionally, the method of randomisation was not reported and therefore the risk of selection bias was unclear. In the second study heparin did not significantly improve prevention of pulmonary embolism (OR 1.02, 95% CI 0.44 to 2.37) when compared to placebo. Furthermore, when the level of amputation was considered, the incidence of pulmonary embolism was similar between the two treatment groups: above knee amputation (OR 0.79, 95% CI 0.31 to 1.97) and below knee amputation (OR 1.53, 95% CI 0.09 to 26.43). Ten participants died during the study; five underwent a post‐mortem and three were found to have had a recent pulmonary embolism, all of whom had been on placebo. Bleeding events were reported in less than 10% of participants in both treatment groups but specific data were not presented. This study did not report the methods used to conceal allocation of treatment and therefore it was unclear if selection bias occurred. However, this study appeared to be free from all other sources of bias. No study looked at mechanical prophylaxis.
Authors' conclusions
As only two studies were included in this review, each comparing different interventions, there is insufficient evidence to make any conclusions regarding the most effective thromboprophylaxis regimen in patients undergoing lower limb amputation. Further large‐scale studies that are of good quality are required.
PICO
Plain language summary
Venous blood clot prevention in people undergoing lower limb amputation
Amputation of the leg is most often performed to remove dead tissue (gangrene), painful ulcers, tumours, or tissue with an inadequate blood supply. One of the most common causes of an inadequate blood supply is a narrowing of the arteries of the legs, which accounts for approximately 70% of amputations. In patients with this condition, blood clots are more likely to cause problems such as venous thromboembolism (VTE). This comprises two conditions, a blood clot in the legs (deep vein thrombosis (DVT)) or a blood clot in the arteries of the lungs (pulmonary embolism (PE)). The risk of these events occurring is higher in people undergoing amputations. There are two forms of preventive measures for VTE, drugs or compression devices. Drugs are proven to be effective in preventing VTE but are also associated with adverse side effects. Compression stockings or devices do not cause side effects but are not suitable for everyone. Current guidelines recommend that any person undergoing an amputation of the lower limb should be offered drugs to prevent a blood clot. However, in people with amputations it is not clear which method is best. This review aimed to establish the best method.
After searching for relevant studies, we found two studies with a combined total of 288 participants to be included in this review of evidence. One study compared two forms of the anticoagulant heparin. Low molecular weight heparin offered no significant improvement over unfractionated heparin in the prevention of DVT. Furthermore neither drug caused bleeding. However, in this study both the participants and study personnel were aware of which treatment was being administered. This may have biased the results. It is unclear if other bias was introduced in the study because the process of randomly allocating treatment was not adequately described. The second study concluded that heparin was not more effective in preventing a PE than placebo whether the amputation was above or below the knee. Bleeding occurred in less than 10% of each treatment group but the study authors did not report specific numbers and therefore this could not be analysed. This study did not report the methods used to conceal how treatment was allocated but it was judged to be free from other sources of bias.
This review found that there are too few trials to determine the most effective strategy in preventing VTE in people undergoing amputation of the lower limb. No study looked at mechanical forms of preventing VTE, such as compression devices, and therefore it is not possible to make any conclusions about these. Further good quality and large‐scale studies are required.
Authors' conclusions
Background
Description of the condition
Lower extremity amputation is performed to remove ischaemic (tissue with a restricted blood supply), infected or necrotic tissue, or in tumour involvement of the surrounding bone or tissue. Dysvascularities (loss of or defective blood supply in a limb), including those related to peripheral vascular disease (PVD) and diabetes mellitus, account for 72% of lower limb amputations in the UK (NASDAB 2009). Trauma, infection, malignancy and neurological disorders account for the remainder of cases (Dillingham 2002; Heikkinen 2007; Ziegler‐Graham 2008). PVD includes arterial, venous and lymphatic disease. The prevalence of PVD in the general population is 12% to 14%, and increases to 20% in those aged over 70 years (Shammas 2007). Furthermore, the prevalence is higher still in people with diabetes, an estimated 29% (American Diabetes Association 2003). The observed increase in life expectancy in the UK (Office for National Statistics), coupled with the 50% increase in the incidence of diabetes since 1997 (Kanavos 2012), means that the prevalence of PVD is likely to rise. As a consequence, more people are likely to require amputation of the lower extremity.
People undergoing major lower extremity amputation are at high risk of venous thromboembolism (VTE) (Burke 2000; Harper 1973; Huang 2005; Libertiny 1999; Yeager 1995). Major lower limb amputations are classed as hip disarticulation, transfemoral (above knee), knee disarticulation and transtibial (below knee). Studies have shown the incidence of VTE in people undergoing major lower limb amputation to be between 9.4% (Bandeira 2008) and 13.2% (Struijk‐Mulder 2010a). Risk factors for VTE that are common to people undergoing amputations are advanced age, sedentary lifestyle, longstanding arterial disease and an identifiable hypercoagulable condition (Yeager 1995). While surgery itself is an important and established risk for VTE, it is thought that the slower blood flow proximal to the ligated veins combined with the endothelial trauma to the veins during amputation predisposes amputees to a higher risk of deep vein thrombosis (DVT) than other surgical patients (Lastória 2006).
Description of the intervention
There are two types of thromboprophylaxis, pharmacological and mechanical. The type of prophylaxis administered depends upon individual patient risk, amputation level and expected level of activity following amputation. Both pharmacological and mechanical prophylaxis have been shown to reduce the incidence of VTE in post‐surgical patients. However, pharmacological prophylaxis is associated with adverse events such as heparin‐induced thrombocytopenia (HIT) (a relative decrease of platelets in the blood) and bleeding. Clinicians have to consider the risk of VTE against the risk of adverse events. Unlike pharmacological prophylaxis, methods of mechanical prophylaxis are not associated with increased risk of bleeding. However, mechanical prophylaxis is not appropriate for people with peripheral arterial disease, arteriosclerosis, gangrene or double lower limb amputation. Furthermore, applying mechanical prophylaxis to just one leg may not prevent DVT in the proximal veins on the side undergoing amputation.
How the intervention might work
Pharmacological prophylaxis
There are several pharmacological agents for the prevention of VTE.
1. Heparins
Unfractionated heparin (UFH) acts as an anticoagulant, thus preventing the formation of clots or extensions of existing clots within the blood. It binds to the enzyme inhibitor antithrombin, which inactivates the effect of thrombin and other coagulation enzymes involved in blood clotting (X, IX, XI and XII) (Chuang 2001). UFH can be administered intravenously or by subcutaneous injection (UK National Clinical Guidelines 2010). UFH has a short half‐life of approximately one hour and therefore requires frequent or continuous infusions (Eikelboom 2002). However, its lower weight derivative (low molecular weight heparin (LMWH)) has a half‐life of four to five hours, allowing once daily dosing (Weitz 2004). Heparin can be used as a prophylactic measure or to treat VTE. Heparin can cause serious side effects, including heparin‐induced thrombocytopenia (HIT), defined as a decrease in platelet count during or following exposure to heparin (Warkentin 2004). Evidence has shown that approximately 1% to 5% of people on heparin will develop HIT (Baglin 1997; Kelton 2002). HIT is a serious and potentially life‐threatening condition (Franchini 2005).
2. Factor Xa inhibitor
Fondaparinux also binds to antithrombin but, unlike heparin, it does not inhibit thrombin. Instead, the binding of fondaparinux to antithrombin selectively inactivates factor Xa thus inhibiting coagulation (Bauer 2002). It has a longer half‐life than heparin, allowing it to be administered once daily as a subcutaneous injection (UK National Clinical Guidelines 2010). Fondaparinux has been shown to be as effective and as safe as adjusted dose, intravenous administration of UFH in the initial treatment of pulmonary embolism (PE) (Matisse 2003). Furthermore, as fondaparinux does not interact with platelets, there is no risk of HIT (Bauer 2001). Fondaparinux is more expensive than heparin, which may limit its use. Another factor Xa inhibitor, rivaroxaban, has been approved in Canada and Europe for the prevention of VTE in people who have undergone elective total hip or knee replacement. The National Institute for Health and Clinical Excellence published guidelines reviewing evidence from four randomised controlled trials involving over 12,000 people undergoing total hip or knee replacement (NICE 2009). Results showed that although rivaroxaban is at least as effective as LMWH in the prevention of VTE, it is associated with a higher rate of major bleeding.
3. Vitamin K antagonists
Warfarin is a synthetic derivative of coumarin, a chemical found naturally in many plants. It acts as a vitamin K antagonist, which, in turn, reduces the amount of active clotting factors thereby producing a state of anticoagulation (Holford 1986). The effect of warfarin is not immediate. It is administered at a variable dose that is adjusted until it reaches a therapeutic level defined by an international normalised ratio (INR) of 2.5 (Ansell 2008). The response to warfarin varies according to several factors including, age, genetic status, medications, diet, underlying medical conditions and smoking status (UK National Clinical Guidelines 2010). Due to the time taken to achieve a therapeutic level sufficient to protect against thromboembolic events, people undergoing surgery stop warfarin upon admission to hospital and heparin, which provides immediate anticoagulation, is given (UK National Clinical Guidelines 2010). Bleeding is the primary adverse event associated with warfarin. Although the effects of warfarin can be reversed through discontinuation of the drug, or administration of vitamin K or prothrombin concentrate, the reversal is permanent and often difficult to achieve (UK National Clinical Guidelines 2010).
4. Direct thrombin inhibitors
Direct thrombin inhibitors (DTIs) act as anticoagulants by directly inhibiting the enzyme thrombin and thus delaying blood clotting. The DTI dabigatran has been approved in Canada and Europe for thromboprophylaxis for hip and knee replacement, while other DTIs are undergoing clinical development. Unlike warfarin, DTIs are immediately effective and do not require dose adjustment and close monitoring (UK National Clinical Guidelines 2010). However, the anticoagulation effect of DTIs is non‐reversible.
5. Antiplatelets
Aspirin has an antiplatelet effect by inhibiting the production of thromboxane. Guidelines have recommended aspirin as primary prophylaxis for the prevention of VTE following orthopaedic surgery (Falck‐Ytter 2012). Prolonged use of aspirin has been shown to increase the risk of gastrointestinal bleeding and stomach ulcers, particularly when taken with warfarin (Sørensen 2000).
Mechanical prophylaxis
Mechanical devices work to reduce venous stasis in the leg, a known risk factor for VTE (Morris 2004). They are non‐invasive and, unlike pharmacological prophylaxis, there is no risk of bleeding. Depending on the estimated risk of VTE, mechanical devices can be used alone or in combination with pharmacological prophylaxis (UK National Clinical Guidelines 2010). Methods include antiembolism stockings (AES), intermittent pneumatic compression devices (IPCD) and foot impulse devices (FID), also known as foot pumps.
1. Antiembolism stockings (AESs)
AESs exert graded circumferential pressure from the distal to the proximal regions of the leg. They have two potential actions in preventing DVT in immobile people. First, exerting graduated compression increases blood flow velocity, which promotes venous return; and second, preventing passive venous distension is thought to prevent subendothelial tears and the activation of clotting factors (UK National Clinical Guidelines 2010). AESs are contraindicated in people with peripheral arterial disease and arteriosclerosis.
2. Intermittent pneumatic compression devices (IPCDs)
IPCDs comprise inflatable cuffs placed around the foot, calf or thigh that are intermittently inflated, powered by a pneumatic pump. The inflation and deflation process stimulates the calf muscle pump, thus forcing blood in the deep veins up towards the heart. This increases the velocity of blood flow in the deep veins of the leg thereby reducing venous stasis and hypertension (Goucke 1989). Calf compression with an IPCD has also been shown to enhance fibrinolysis, a process that augments thrombus breakdown and prevents clot formation (Storti 1996).
3. Foot impulse devices (FIDs)
FIDs (or foot pumps) act by creating the effect of walking in immobilised people. During ambulation, the muscle pump in the foot forces blood in the deep veins upwards towards the heart. This action increases venous outflow in the deep veins, thereby reducing stasis and venous hypertension and preventing the formation of blood clots in the legs (Gardner 1983). FIDs recreate this physiological process through artificially stimulating the foot pump, thereby mimicking the effect of walking (UK National Clinical Guidelines 2010).
Why it is important to do this review
Evidence from observational studies in people undergoing major lower extremity amputation supports the use of pharmacological thromboprophylaxis (Burke 2000; Harper 1973; Hill 2010; Huang 2005; Lastória 2006; Yeager 1995). Indeed, one study reported that DVT occurred in 50% of people following major lower extremity amputation without prophylaxis (Harper 1973). In one randomised trial comparing LMWH and UFH for amputations, the incidence of DVT was similar in each group at about 10% (Lastória 2006). Nevertheless, some evidence suggests that despite the use of pharmacological thromboprophylaxis (with LMWH), the rate of VTE in people undergoing amputation remains high (Struijk‐Mulder 2010a). Mechanical prophylaxis, including IPCDs and AESs, has been proposed as a useful addition to pharmacological prophylaxis (Struijk‐Mulder 2010b) but this is not always appropriate in amputees with contraindications.
The Scottish Intercollegiate Guidelines Network recommend thromboprophylaxis in all people undergoing all major lower extremity amputations (SIGN 2010). With conflicting reports regarding the efficacy of thromboprophylaxis, it is imperative to determine the most effective. To date, no systematic review has been conducted examining the effectiveness of thromboprophylaxis in preventing VTE in people undergoing amputation.
Objectives
To determine the effectiveness of thromboprophylaxis in preventing VTE in people undergoing major amputation of the lower extremity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised controlled trials in which people undergoing an amputation of the lower extremity were allocated to receive different types of thromboprophylaxis. We included published studies and studies in progress if preliminary results were available. Non‐English studies were also eligible for inclusion in the review.
Types of participants
People undergoing major unilateral or bilateral amputation (hip disarticulation, transfemoral (above knee), knee disarticulation, ankle disarticulation and transtibial (below knee) of the lower extremity). Patients undergoing minor amputations of the toes or a portion of the foot were excluded.
Types of interventions
-
Pharmacological prophylaxis (LMWH, UFH, factor Xa inhibitors, vitamin K antagonists, DTIs, antiplatelets)
-
Mechanical prophylaxis (AES, IPCD, FID) (for unilateral amputees only)
Comparisons included:
-
one pharmacological prophylaxis versus another (e.g. LMWH versus UFH);
-
one mechanical prophylaxis versus another (e.g. AES versus FID);
-
pharmacological prophylaxis versus mechanical prophylaxis;
-
pharmacological plus mechanical prophylaxis versus mechanical prophylaxis alone;
-
prophylaxis (pharmacological or mechanical, or both) versus placebo.
Types of outcome measures
Primary outcomes
-
Total VTE (non‐fatal and fatal)
-
Symptomatic or asymptomatic non‐fatal VTE (DVT or PE) confirmed by diagnostics tests, including helical computed tomography (CT), CT angiography and ventilation‐perfusion lung scintigraphy for diagnosis of PE; and complete compression ultrasonography and impedance plethysmography (test in which air is used to determine circulatory capacity) for diagnosis of DVT
Secondary outcomes
-
All‐cause mortality.
-
Adverse events (HIT).
-
Bleeding, as defined by the Bleeding Academic Research Consortium Definition for Bleeding (Mehran 2011):
-
type 0 ‐ no bleeding;
-
type 1 ‐ no actionable bleeding;
-
type 2 ‐ overt bleeding requiring either non‐surgical intervention or hospitalisation, or prompting evaluation;
-
type 3 ‐ overt bleeding with a drop in haemoglobin (Hb). Subdivided into 3a (Hb drop 3 to 5 g/dL, 3b (Hb drop > 5 g/dL or 3c (intracranial haemorrhage);
-
type 5 ‐ fatal bleeding.
Search methods for identification of studies
Electronic searches
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched May 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 4), part of The Cochrane Library, (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
The following trial databases were searched in May 2013 by the TSC for details of ongoing and unpublished studies using the terms (amputation and (leg or lower)):
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/);
-
ClinicalTrials.gov (clinicaltrials.gov/);
-
Current Controlled Trials (www.controlled‐trials.com/).
Searching other resources
Non‐English trials were translated where required and reference lists of identified studies were searched.
Data collection and analysis
Selection of studies
One review author (LR) used the selection criteria to identify trials for inclusion and the second review author (AR) independently confirmed this selection. Any disagreements were resolved by discussion.
Data extraction and management
Two review authors (LR, AR) independently extracted the data. Information about the trial design, type of amputation, baseline characteristics of participants and type of prophylaxis was recorded. Non‐fatal and fatal VTE data were recorded as the primary outcome measures. Information on bleeding and deaths was collected in accordance with the secondary outcome measures. Authors of the included studies were contacted if further information or clarification was required. Any disagreements in data extraction and management were resolved by discussion.
Assessment of risk of bias in included studies
Two review authors (LR, AR) independently used the Cochrane Collaboration's 'Risk of bias' tool for assessing risk of bias for each of the included studies (Higgins 2011). The tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting and any other relevant biases. Any disagreements were resolved by discussion.
Measures of treatment effect
Analysis was based on intention‐to‐treat data from the individual clinical trials. As the primary and secondary outcomes were all binary measures, odds ratios (ORs) were computed using a random‐effects model and 95% confidence intervals (CI) of the effect sizes were calculated.
Unit of analysis issues
The unit of analysis within each trial was the individual patient.
Dealing with missing data
Information about dropouts, withdrawals and other missing data was sought and, if not reported, study authors were contacted for this information.
Assessment of heterogeneity
The inclusion of studies on a wide range of medical treatments is likely to result in a high degree of heterogeneity. We planned to assess the heterogeneity between pooled studies by using the Chi2 test regarding the characteristics and quality of the included studies. We planned to use the I2 statistic to measure the degree of inconsistency between studies, with results of 50% representing moderate to substantial heterogeneity (Deeks 2011). However, only two studies were included in this review. The studies tested different drugs and it was not possible to pool the data. Therefore, heterogeneity between studies was not assessed.
Assessment of reporting biases
We planned to assess reporting biases such as publication bias by funnel plots. There are many reasons for funnel plot asymmetry and we consulted the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011) to aid the interpretation of the results. However, as only two studies were included in the review it was not possible to test for funnel plot asymmetry.
Data synthesis
The review authors independently extracted the data. One review author (LR) inputted the data into Review Manager (RevMan 2011) while the second review author (AR) cross‐checked the data entry. Any discrepancies were resolved by consulting the source publication.
A random‐effects model was used to analyse the data.
Subgroup analysis and investigation of heterogeneity
Where possible, we attempted to analyse clinically relevant subgroups based on type of prophylaxis and participant groupings. Subgroupings included:
-
DVT or PE;
-
indication for amputation;
-
level of amputation (transfemoral, transtibial, exarticulation knee);
-
history of VTE;
-
diabetes;
-
PVD (arterial, venous and lymphatic disease);
-
smoking;
-
body mass index (BMI);
-
duration of prophylaxis.
Sensitivity analysis
We planned to conduct sensitivity analysis to examine the stability of the results in relation to the quality of the included studies. However, as only two studies were included in the review, it was not feasible to perform a sensitivity analysis.
Results
Description of studies
Results of the search
See: Figure 1
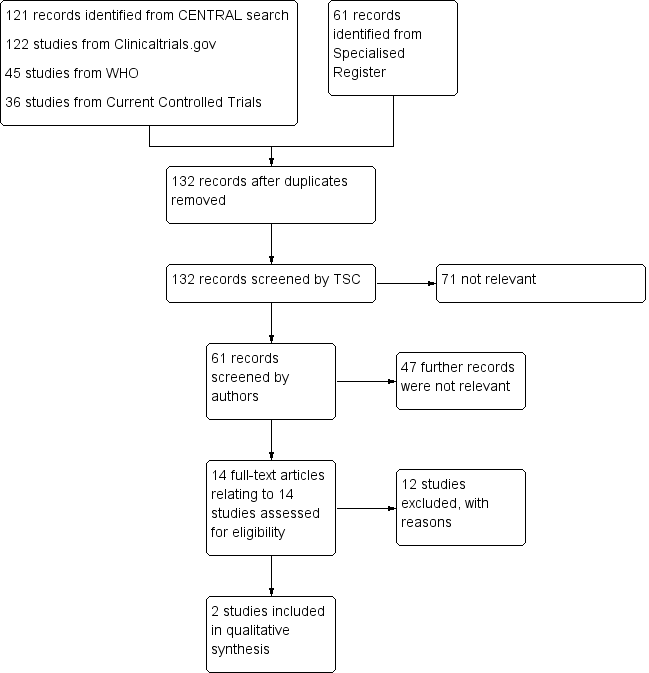
Study flow diagram.
Included studies
See: Characteristics of included studies
One study was an open‐label randomised controlled trial in which 75 patients undergoing elective or emergency lower limb amputation for critical limb ischaemia (CLI) were randomised to receive subcutaneous UFH (5000 IU twice daily) or 40 mg enoxaparin daily (Lastória 2006). Patients with a previous VTE or a contraindication to anticoagulant prophylaxis were excluded from the study. Prophylaxis began 12 hours before elective surgery or, in emergency cases, on the first day postoperatively. DVT was assessed by daily clinical examination and duplex ultrasound before surgery and again between five and eight days after surgery. DVT was diagnosed as an 'abnormal venous flow and evidence of intraluminal thrombi confirmed with compressive ultrasonography'. Of the 75 patients, 30 underwent an above‐knee amputation while the remaining 45 had a below‐knee amputation. Thirty‐four patients were randomised to UFH and 41 were randomised to LMWH.
The second study (Williams 1978) was a randomised, double‐blind trial in which 134 patients undergoing lower extremity amputation for the complications of ischaemic vascular disease were randomised to receive low dose heparin or placebo. Sixty‐nine patients with an amputation (53 above knee and 16 below knee) received 10,000 USP units/ml of sodium heparin subcutaneously at a rate of 0.5 ml every 12 hours for two weeks or until discharge. Sixty‐five patients (41 above knee and 24 below knee) received saline. Treatment was started approximately two hours before surgery. Lung perfusion scans were performed before surgery and then at weekly intervals for the duration of treatment. In patients with a new pulmonary perfusion defect, a pulmonary arteriography was performed and if that was unclear, an angiogram was done. PE was diagnosed if a new lobar, segmental or subsegmental perfusion defect appeared which was not attributable to an infiltrate, pleural effusion, vascular congestion or atelectasis appearing on the chest roentgenogram.
Excluded studies
See: Characteristics of excluded studies
Twelve studies were excluded (Atkins 1978; Covey 1975; Di Serio 1985; Haas 2005; Huttenen 1977; Kakkar 1978; Kakkar 2005; Kill 1979; Mantz 2011; Nicolaides 1972; Pachter 1977; Strand 1975), 11 of which were excluded as they did not include participants undergoing amputations (Atkins 1978; Covey 1975; Di Serio 1985; Haas 2005; Huttenen 1977; Kakkar 1978; Kakkar 2005; Kill 1979; Mantz 2011; Nicolaides 1972; Strand 1975). One study (Pachter 1977) was excluded as the authors did not state what the control treatment was. Additionally, the methods of diagnosing DVT and PE were not reported.
Risk of bias in included studies
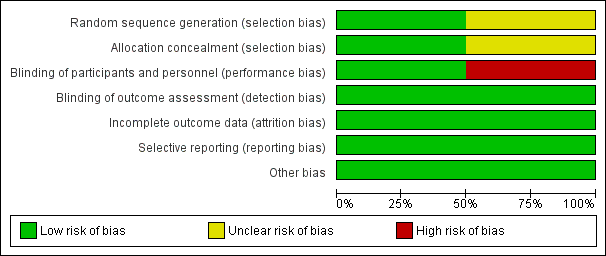
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
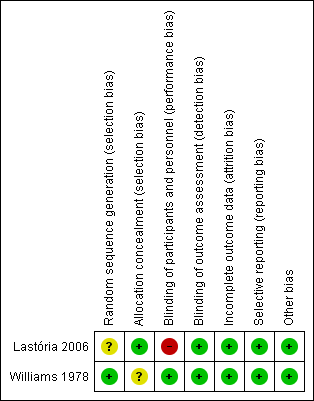
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although both studies were randomised, the study by Lastória 2006 did not provide any information on the method of randomisation used and therefore the risk of selection bias could not be assessed. The study by Williams 1978 used a random number table and therefore was at low risk of selection bias. In terms of concealing the allocation of treatment, the study by Lastória 2006 used serial number envelopes while the study by Williams 1978 did not report the methods used.
Blinding
Of the two studies, one was open label (Lastória 2006) and therefore at a high risk of performance bias. The remaining study (Williams 1978) blinded both study participants and personnel and was therefore at low risk of performance bias. Both studies blinded outcome assessors to the treatment and were deemed to be at low risk of detection bias.
Incomplete outcome data
Both studies accounted for all incomplete outcome data and were judged to be at low risk of attrition bias.
Selective reporting
Both studies reported data on all pre‐specified primary and secondary outcomes and were therefore at low risk of reporting bias.
Other potential sources of bias
Both studies appeared to be free from other sources of bias.
Effects of interventions
Two studies (Lastória 2006; Williams 1978) were included in the review. One (Lastória 2006) compared UFH against LMWH and the second (Williams 1978) compared UFH with a placebo. No studies on mechanical prophylaxis, such as compression devices, were included in the review.
Unfractionated heparin (UFH) versus low molecular weight heparin (LMWH)
In the one study (Lastória 2006) comparing UFH with LMWH, four out of 34 participants randomised to UFH had a DVT compared to four out of 41 randomised to LMWH (OR 1.23, 95% CI 0.28 to 5.35). The study authors reported the number of participants with above and below knee amputations but did not present the results according to amputation type. The authors were contacted for this data but they did not respond to our communication. No bleeding events, adverse events or deaths occurred in either treatment group.
Heparin versus placebo
In the one study comparing UFH with a placebo (Williams 1978), the incidence of PE was similar in the heparin (14/69) and placebo (13/65) groups (OR 1.02, 95% CI 0.44 to 2.37). When analysed by type of amputation, heparin offered no significant improvement over placebo: above knee amputation OR of 0.79 (95% CI 0.31 to 1.97), below knee amputation OR of 1.53 (95% CI 0.09 to 26.43). The incidence of postoperative bleeding was less than 10% in both treatment groups, although the exact figures were not reported and the study authors did not respond to requests for these data. One participant randomised to placebo was removed prematurely from the study due to excessive bleeding. The study authors also reported that 10 participants died during the study. Of those 10, five underwent a post‐mortem and three were found to have had a recent PE, all of whom were on placebo. There were no reports of other adverse events.
Discussion
Summary of main results
Only two studies were found which fulfilled the eligibility criteria for inclusion in this review. One study (Lastória 2006) compared the effectiveness of LWMH with UFH in the prevention of DVT up to eight days post‐amputation. Neither drug was found to be significantly superior and no bleeding occurred in the study population. The second study (Williams 1978) was concerned with PE and found that heparin offered no significant improvement over a placebo regardless of the level of amputation. Neither study used mechanical prophylaxis in addition to pharmacological prophylaxis.
Overall completeness and applicability of evidence
At present, there is a severe lack of evidence concerning the effectiveness of pharmacological and mechanical prophylaxis in the prevention of VTE in patients with lower limb amputation. One study was open label and therefore at a high risk of performance bias. The two studies tested different drugs and outcomes and therefore the results could not be pooled in a meta‐analysis. Only one study presented data according to the level of amputation. Neither study used mechanical prophylaxis in addition to the drugs administered. Furthermore, no study on mechanical prophylaxis was included in the review.
Quality of the evidence
The quality of reporting in the studies was generally good. The risk of selection bias was unclear as one study (Lastória 2006) failed to report the process of randomisation while the second study (Williams 1978) did not state the methods used to conceal allocation of treatment. The study by Lastória was open label and therefore at high risk of performance bias (Lastória 2006). Both study authors accounted for all missing data and reported data on all primary and secondary outcomes and therefore minimised the chances of attrition and performance bias. The two studies included in this review were based on relatively small sample sizes. The effect estimates from our results were imprecise and the confidence intervals were compatible with both benefit and harm.
Potential biases in the review process
None of the authors of this review were involved in any of the included or excluded studies. Furthermore, none have any commercial or other conflict of interest. The search was as comprehensive as possible, and all studies were independently assessed for inclusion by two review authors. We are confident that we have included all relevant studies and we have attempted to reduce bias in the review process. However, the possibility remains that we may have missed studies which have not been published.
Agreements and disagreements with other studies or reviews
Many studies have measured the effectiveness of drug prophylaxis on the prevention of VTE after surgery. However, the majority of these have been conducted in patients undergoing hip or knee replacement. Other studies on more general types of surgery have been done but specific data for patients undergoing amputation is rarely presented. Research on mechanical forms of prophylaxis in patients with amputation is scarce. To date, no systematic review has attempted to assess the effectiveness of prophylaxis in the prevention of VTE specifically in patients undergoing amputation.
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Unfractionated heparin versus low molecular weight heparin, Outcome 1 Deep vein thrombosis (DVT).

Comparison 2 Heparin versus placebo, Outcome 1 Pulmonary embolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis (DVT) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary embolism Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Above knee amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Below knee amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

