Ubat anti‐radang bukan steroid berbanding kortikosteroid untuk mengawal keradangan selepas pembedahan katarak yang tidak rumit
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 50 eyes of 42 participants Per group: NSAID plus corticosteroid: 25 eyes, corticosteroid only: 25 eyes Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: France Age: not reported Gender (per cent): not reported Inclusion criteria: participants scheduled for phacoemulsification Exclusion criteria: complicated surgeries and participants with OCT images with artifacts Equivalence of baseline characteristics: yes, “No significant preoperative difference was detected between the two groups for age, visual acuity and macular thickness.” | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: indomethacin plus dexamethasone postoperatively Corticosteroid: dexamethasone only postoperatively Other medications: all participants also received the antibiotic tobramycin postoperatively Length of follow‐up: Planned: not reported Actual: 1 month | |
| Outcomes | Primary outcome, as defined in study reports: macular thickness measured using OCT Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day before surgery, 1 week and 1 month after surgery | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not described. |
| Masking of outcome assessment (detection bias) | Low risk | “OCT examination and interpretation were done by an expert masked for treatment and exam timing.” |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unable to judge whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not provided; some participants had more than 1 eye included, but authors do not report on how correlation between eyes was handled in the analysis. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 106 eyes of 98 participants Per group: NSAID + corticosteroid: 53, corticosteroid alone: 53 Exclusions after randomization: 1 participant in the ketorolac group canceled surgery Number analyzed (total and per group): Total: at 1 week: 98; at 1 month: 80 Per group: at 1 week: NSAID + corticosteroid: 50, corticosteroid alone: 48; at 1 month: NSAID + corticosteroid: 38, corticosteroid alone: 42 Unit of analysis: eyes Losses to follow‐up: ketorolac group: total of 15 (1 canceled surgery, 3 ketorolac corneal toxicity, 1 Alzheimer’s disease‐related decompensation, 1 urgent orthopedic surgery, 9 missed postop. month appointment); control group: total of 11 (1 postoperative corneal edema, 1 intraoperative complication, 3 voluntarily withdrew from study to avoid extra follow‐up at 1 month, 1 CME detected at 1 week and not included in 1‐month follow‐up, 1 iritis, 1 Alzheimer’s disease‐related decompensation, 3 missed postop month appointment) How were missing data handled?: complete‐case analysis (those with missing data were not included) Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Canada Age: NSAID + corticosteroid mean age = 71.3 ± 10.3 (range 45 to 92); corticosteroid‐alone mean age = 72.4 ± 10.3 (45 to 92) Gender (per cent): NSAID + corticosteroid: 26 males (49.1%) and 27 females (50.9%); corticosteroid alone: 16 males (30.1%) and 37 females (69.8%) Inclusion criteria: first cataract eye surgery, receiving phacoemulsification with intraocular lens implantation Exclusion criteria: hypersensitivity to the NSAID drug class, aspirin/NSAID‐induced asthma, and pregnancy in the 3 trimester Equivalence of baseline characteristics: yes, “Overall, baseline demographics and clinical characteristics were similar in both groups.” | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: ketorolac tromethamine 0.5% 4 times a day beginning 2 days before surgery and for 29 days after surgery plus prednisolone acetate 1% 4 times a day for 1 week followed by twice a day for 1 week Corticosteroid: prednisolone acetate 1% 4 times a day for 1 week followed by twice a day for 1 week Other medications: all participants also received the antibiotic gatifloxacin 0.3% Length of follow‐up: Planned: 1 month Actual: 1 month | |
| Outcomes | Primary outcome, as defined in study reports: difference in total macular volume (TMV) between 1 month and baseline measured using OCT Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: preoperatively, 7 days (range 5 days to 10 days), and 28 days (range 4 to 6 weeks) after surgery | |
| Notes | Type of study: published Funding sources: funded by a Queen’s University grant, Kingston, Ontario, Canada Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned” Study period: June 2006 to May 2007 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | High risk | This study was reported as non‐masked. |
| Masking of outcome assessment (detection bias) | High risk | This study was reported as non‐masked. |
| Incomplete outcome data (attrition bias) | High risk | Participants who dropped out were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 193 eyes of 193 participants Per group: not reported Exclusions after randomization: 31 participants did not complete the study Number analyzed (total and per group): Total: 162 eyes of 162 participants Per group: NSAID + corticosteroid 1: 54, NSAID + corticosteroid 2: 54, placebo + corticosteroid: 54 Unit of analysis: eyes Losses to follow‐up: none (participants were withdrawn because of side effects/rescheduled surgery) How were missing data handled?: intention‐to‐treat analysis Reported power calculation: yes, 80% Unusual study design?: none | |
| Participants | Country: Canada Age: 72.4 ± 8.2 Gender (per cent): 88 (54%) female, 74 (46%) male Inclusion criteria: 18 years or older, cataract, expected to have phacoemulsification with implantation of posterior chamber intraocular lens Exclusion criteria: pre‐existing retinal disease, previous uveitis, previous intraocular surgery, allergy or hypersensitivity to NSAIDs, complicated previous cataract surgery Equivalence of baseline characteristics: yes, "There were no differences in age, sex, or operative eye between the 3 groups." | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid 1: ketorolac 0.5% 4 times a day starting 1 day before surgery and continuing for 4 weeks plus prednisolone 1% 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week NSAID + corticosteroid 2: nepafenac 0.1% 4 times a day starting 1 day before surgery and continuing for 4 weeks plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Corticosteroid: placebo plus prednisolone Other medications: all participants also received the antibiotic gatifloxacin 0.3% Length of follow‐up: Planned: 1 month Actual: 1 month | |
| Outcomes | Primary outcome, as defined in study reports: change in OCT macular cube central subfield thickness, macular cube volume, and average macular cube thickness Secondary outcomes, as defined in study reports: COMTOL HRQOL analysis Adverse events reported: yes Intervals at which outcomes assessed: day 0 or 1, 1 month | |
| Notes | Type of study: published Funding sources: Queen's University educational research grant Disclosures of interest: no financial or proprietary interest Study period: March 2010 to May 2011 Reported subgroup analyses: yes Trial registry #: NCT01395069 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles ... Bottles were labeled with study identification number, patient identification number, expiration date, and emergency contact information only.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Study is reported as double‐masked, but masking of outcome assessors was not described specifically. |
| Incomplete outcome data (attrition bias) | Low risk | “One hundred sixty‐two patients, 54 in each arm, made up the intent‐to‐treat data set” |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: NSAID: 75 eyes of 75 participants, corticosteroid: 75 eyes of 75 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 142 eyes of 142 participants Per group: NSAID: 71 eyes of 71 participants, corticosteroid: 71 eyes of 71 participants Unit of analysis: individual (1 eye per participant) Losses to follow‐up: NSAID: total 4: 1 due to "complications", 3 due to "discontinuation proposal (there were patients who withdrew their consent during the course of this study)"; corticosteroid: total 4: 1 due to "complications", 2 due to "discontinuation proposal", 1 who "did not return to the hospital 2 weeks after surgery" How were missing data handled?: missing data were not included Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Japan Age: NSAID mean age = 66.07 ± 5.51; corticosteroid mean age = 66.23 ± 5.55 Gender (per cent): NSAID: 43.6% male, 56.3% female; corticosteroid: 45% male, 55% female Inclusion criteria: age 55 to 75 years, nuclear hardness of Emery‐Little grade IV or less, and surgery in 1 eye only Exclusion criteria: acute infection or inflammation within 1 month after initiation of the study, allergy to NSAIDs, steroids or fluorescein, history of eye trauma or intraocular disease other than cataract, pseudoexfoliation syndrome, uveitis, glaucoma, diabetes and related complications, kidney disease, asthma or chronic airway disease, uncontrolled hypertension, severe heart failure, myocardial infarction or cerebrovascular disorders, and intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac 0.1% 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery Corticosteroid: topical betamethasone 0.1% 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery Length of follow‐up: Planned: 8 weeks Actual: 8 weeks | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, IOP and flare Secondary outcomes, as defined in study reports: incidence and severity of CME (measured by fluorescein angiography) Adverse events reported: no Intervals at which outcomes assessed: 1 and 3 days, 1, 2, 5, and 8 weeks after surgery | |
| Notes | Type of study: published Funding sources: none reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: April 2004 to September 2005 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | “The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed.” |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is described as double‐masked, but it is unclear who was masked — participants, surgeons, or outcomes assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | The study is described as double‐masked, but it is unclear who was masked — participants, surgeons, or outcomes assessors. |
| Incomplete outcome data (attrition bias) | High risk | 4 participants from each group were randomized but not included in the analysis; of these 3 in one group and 2 in the other group were not included due to withdrawing consent. It is not clear if this was due to the study drug or not. |
| Selective reporting (reporting bias) | Unclear risk | Though IOP was evaluated at every postsurgery visit, the incidence of CME was only evaluated at week 5, for reasons that are not clearly stated. |
| Other bias | Unclear risk | Though the authors report no conflict of interest, no funding source is provided. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 62 eyes Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: not reported Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac 4 times a day Corticosteroid: rimexolone 4 times a day Length of follow‐up: Planned: 2 weeks Actual: 2 weeks | |
| Outcomes | Primary outcome, as defined in study reports: cell and flare measure Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks postoperatively | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there was any loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: 30 eyes of 30 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 60 eyes of 60 participants Per group: 30 eyes of 30 participants Unit of analysis: participants Losses to follow‐up: “All patients completed the follow‐up visits over a 6‐week period” How were missing data handled?: N/A Reported power calculation: Yes, reported in Discussion section and appears to be post hoc ‐ “Statistical analysis showed that 46 patients per arm (80%) power were needed to show difference in FT [foveal thickness] between the two groups” Unusual study design?: no | |
| Participants | Country: Mexico Age: Mean age = 71.9 +/‐ 9.7 years (range 51 to 88) Gender (per cent): 36.6% male, 63.4% female Inclusion criteria: adults 40 years of age or older, regardless of race or gender, who were diagnosed with senile or metabolic cataract or both and were scheduled for surgery by phacoemulsification and IOL implantation inside the capsular bag, with a normal fundoscopy exam (if observance was possible) Exclusion criteria: pregnant or breastfeeding women; history of ocular inflammatory or infectious eye disease; treatment for eye infection within 30 days prior to inclusion in the study; alterations on the eye surface (including dry eye); history of ocular surgery or trauma or both; and knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication. Other exclusion criteria were use of eye medications, including prostaglandin analogues; use of topical or systemic steroids within 30 days prior to inclusion in the study; and use of topical or systemic NSAIDs within 14 days prior to inclusion in the study. People with non‐controlled diabetes mellitus based on clinical history and blood glucose level (≥ 126 mg), proliferative diabetic retinopathy, and/or macular edema were excluded from the study. Preoperative mydriasis less than 6 mm prior to the study; synechiae; ocular alteration preventing adequate mydriasis such as iris atrophy; macular alteration documented by optical coherence tomography, including macular edema of any etiology, macular holes, epiretinal membrane, macular degeneration related to age, and central serous chorioretinopathy; and the use of contact lens in the eye involved during the study were also considered exclusion criteria. Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 0.1% 3 x daily 1 day prior to surgery and continued for 6 weeks plus dexamethasone treatment 4 x daily for 10 days after surgery Corticosteroid: dexamethasone treatment 4 x daily for 10 days after surgery Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 6 weeks Actual: 6 weeks | |
| Outcomes | Primary outcome, as defined in study reports: trans‐operative mydriasis and postoperative macular edema (“FT [foveal thickness] and TMV [total macular volume] at baseline”) measured using macular OCT and the "Fast Macular Thickness Map" scan Secondary outcomes, as defined in study reports: average foveal thickness and total macular volume in the control and nepafenac groups Adverse events reported: “There were no serious treatment‐related adverse events or toxicity related to the use of nepafenac 0.1%” Intervals at which outcomes assessed: 1 day, 2 weeks, 6 weeks after surgery | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: the authors have no conflicts of interest to disclose Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | “The identity of patients receiving preoperative mydriatic or preoperative mydriatic and nepafenac was concealed from the surgeons”. This is reported as a single‐masked study. Since surgeons were masked, we infer that participants were not masked, but it is unclear whether this would create bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | This is reported as a single‐masked study and it is stated that surgeons were masked. It is unclear whether the outcomes assessors were the surgeons or other study personnel. |
| Incomplete outcome data (attrition bias) | Low risk | “All patients completed the follow‐up visits over a 6 week period” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 145 participants Per group: NSAID plus corticosteroid: 73; corticosteroid alone: 72 Exclusions after randomization: people who underwent vitrectomy due to PCR were excluded: 3 people in the NSAID plus corticosteroid group; 4 people in the corticosteroid alone group Number analyzed (total and per group): Total: 138 Per group: NSAID plus corticosteroid: 70; corticosteroid alone: 68 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Greece Age: NSAID plus corticosteroid mean age = 74.3 ± 7.3; corticosteroid alone = 74.0 ± 7.6 Gender (per cent): NSAID plus corticosteroid: 43 (61.4%) men, 27 (38.6%) women; corticosteroid alone: 40 (58.9%) men, 28 (41.1%) women Inclusion criteria: not reported Exclusion criteria: history of intraocular surgery on the eye to be operated; any previous episode of uveitis in the eye to be operated; severe systemic disease (heart failure of the New York Heart Association stage III of IV, end‐stage renal failure, pulmonary failure, receiving chemotherapy); and regular, systemic use of steroid or NSAID during the last 3 months Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: combination of ketorolac tromethamine 0.5%, 1 drop 3 times per day plus dexamethasone 0.1%, 1 drop 4 times per day for 28 days after phacoemulsification Corticosteroid: dexamethasone 0.1%, 1 drop 4 times per day for 28 days after phacoemulsification Other medications: all participants also received the antibiotic tobramycin 0.3% Length of follow‐up: Planned: 42 days Actual: 42 days “On day 35, patients who needed continuation of the treatment were once again evaluated.” “Irrespective of continuation, on day 42 all patients underwent fundoscopy and an Amsler grid test, so as to trace any suspicious signs for the development of clinically significant cystoid macular edema (CME)." | |
| Outcomes | Primary outcome, as defined in study reports: corneal edema, conjunctival hyperemia, anterior chamber or Tyndall reaction Secondary outcomes, as defined in study reports: BCVA Adverse events reported: no Intervals at which outcomes assessed: days 7, 14, 21, and 28 postoperation | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: 1 October 2009 to 10 January 2010 Reported subgroup analyses: no Trial registry #: NCT01103401 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “The study was masked to the patients, i.e. they received unmarked bottles so as to be unaware of which treatment they received”. “Each patient was independently assessed by 2 ophthalmologists at each visit” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) | High risk | “Patients who underwent vitrectomy due to posterior capsule rupture were excluded” |
| Selective reporting (reporting bias) | High risk | “On day 35, patients who needed continuation of the treatment were once again evaluated as above” (results not shown) “All patients reported pain and ocular discomfort lower than 1/10 on the visual analog scale at all time points” (it is unclear whether this includes days 35 and 42) |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 100 individuals, 120 eyes Per group: 50 individuals, 60 eyes Exclusions after randomization: not reported Number analyzed (total and per group): Total: 100 individuals, 120 eyes Per group: NSAID plus corticosteroid: 50 individuals, 60 eyes; corticosteroid alone: 50 individuals, 60 eyes Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: China Age: NSAID plus corticosteroid mean age = 72.08 ± 9.68, corticosteroid only mean age = 75.12 ± 9.42 Gender (per cent): NSAID plus corticosteroid: 30 (50%) women's eyes, 30 (50%) men's eyes; corticosteroid only: 28 (47%) women's eyes, 32 (53%) men's eyes Inclusion criteria: cataract surgery with phacoemulsification and implantation of posterior chamber lens from January 2013 to May 2015; normal heart, lung, kidney, and liver function Exclusion criteria: non‐infectious blepharitis; chronic conjunctivitis; non‐infectious keratitis; sclerotitis; glaucoma; uveitis; ocular bottom diseases; eye trauma history; lens nucleus level II‐III; tear break‐up time ≥ 10 seconds Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: bromfenac sodium 0.1% twice daily for 1 to 3 days preoperatively and 2 weeks postoperatively plus dexamethasone 4 times daily for 1 week postoperation and 3 times daily for another week Corticosteroid alone: dexamethasone 6 times daily for 1 week postoperation and 4 times daily for another week Other medication: all participants also received the antibiotics tobramycin 4 or 6 times daily for 1 week postoperation and 3 or 4 times daily for another week and levofloxacin 4 times daily for 1 to 3 days preoperatively Length of follow‐up: Planned: 2 weeks Actual: 2 weeks | |
| Outcomes | Primary outcome, as defined in study reports: inflammation score, IOP, cystoid macular edema measured with OCT Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1, 7, 14 days | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: none Study period: January 2013 to May 2015 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up and no missing data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; protocol was not available. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 58 eyes of 43 participants Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: not reported Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: not reported Unusual study design?: in some participants, both eyes of a single participant were included, and it is unclear if non‐independence of the eyes was taken into consideration in the analysis | |
| Participants | Country: Turkey Age: not reported Gender (per cent): not reported Inclusion criteria: people who underwent uneventful phacoemulsification surgery Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: topical dexamethasone 0.1% 6 times daily postoperatively, which was tapered and discontinued 6 weeks after surgery plus topical ketorolac 0.5% 4 times daily, beginning 2 days prior to surgery and discontinued 4 weeks after surgery Corticosteroid: topical dexamethasone 0.1% 6 times daily postoperatively, which was tapered and discontinued 6 weeks after surgery Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcome, as defined in study reports: macular thickness; mean foveal thickness; parafoveal and perifoveal thickness (all measured using OCT) Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: week 1, months 1 and 2 postoperatively | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers of participants who were randomized, excluded, lost to follow‐up were not reported. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; protocol was not available. |
| Other bias | Unclear risk | Both eyes of some participants were included, and it is unclear whether non‐independence of the eyes was taken into consideration in the analysis; this study was published in abstract form and full‐text was not available. Declarations of interest were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 116 eyes of 116 participants Per group: NSAID: 57 eyes of 57 participants, corticosteroid: 59 eyes of 59 participants Exclusions after randomization: not reported Number analyzed (total and per group): Total: ITT analysis: 116 eyes of 116 participants; per‐protocol analysis: 101 eyes of 101 participants Per group: ITT analysis: NSAID 57 eyes of 57 participants, corticosteroid: 59 eyes of 59 participants; per‐protocol analysis: NSAID 50 eyes of 50 participants, corticosteroid 51 eyes of 51 participants Unit of analysis (individuals vs eyes): eyes (1 eye per participant) Losses to follow‐up: protocol violations NSAID: 7 (3 failure to follow the appointment schedule, 1 intake of unacceptable concomitant medication, 3 non‐compliance with the study medication); corticosteroid: 8 (4 complicated surgery, 3 failure to follow the appointment schedule, 1 intake of unacceptable concomitant medication) How were missing data handled?: ITT analysis for the efficacy, safety, and tolerance parameters Reported power calculation: no Unusual study design?: none | |
| Participants | Country: not reported Age: NSAID mean = 74, corticosteroid mean = 72 Gender (per cent): NSAID: 26 (45.6%) men and 31 (54.3%) women; corticosteroid: 16 (27.1%) men and 43 (72.9%) women Inclusion criteria: at least 40 years old with visually disabling cataract and scheduled to undergo phacoemulsification with posterior chamber lens implication Exclusion criteria: intraocular pressure greater than 26 mm Hg with or without treatment, any ocular pathology like pseudoexfoliation or diabetic retinopathy, ocular medications other than topical beta‐blockers or artificial tear substitutes, previous intraocular surgery in the operative eye, signs of or history of intraocular inflammation or herpes infection in the operative eye, use of topical or systemic steroids in the 4 weeks preceding surgery, hypersensitivity to NSAIDs or any of the ingredients in the study medications, operative eye being the only useful eye, presence of insulin‐dependent diabetes mellitus or uncontrolled diabetes mellitus of any type, and the presence or likelihood of pregnancy or substance abuse Equivalence of baseline characteristics: “It was found that there was no statistically significant difference between the two groups except with relation to sex (chi‐squared test, p=0.039).” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% (1 drop 4 times daily) for the duration of the study Corticosteroid: prednisolone acetate 1.0% (1 drop 4 times daily) for the duration of the study Length of follow‐up: Planned: 12 to 16 days Actual: 12 to 16 days | |
| Outcomes | Primary outcome, as defined in study reports: sum of flare and cells Secondary outcomes, as defined in study reports: conjunctival hyperemia, visual acuity, slit‐lamp examination, applanation tonometry Adverse events reported: yes, “There were two probably drug‐related adverse events in the prednisolone group and none in the diclofenac group.” Intervals at which outcomes assessed: day 1, 5 to 8, and 12 to 16 postsurgery | |
| Notes | Type of study: published Funding sources: “This study was supported in part by a grant from CIBA Vision Ophthalmics, Bülach, Switzerland.” Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “To ensure masking of the groups, the patients, investigators and study personnel from the investigator site and CIBA Vision Ophthalmics were masked as to the study drug codes until the statistical analysis of the study data was completed.” |
| Masking of outcome assessment (detection bias) | Low risk | “To ensure masking of the groups, the patients, investigators and study personnel from the investigator site and CIBA Vision Ophthalmics were masked as to the study drug codes until the statistical analysis of the study data was completed.” |
| Incomplete outcome data (attrition bias) | Low risk | An ITT analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | It is stated that visual acuity outcomes were collected, but they were not reported, for unknown reasons. |
| Other bias | Unclear risk | The study was funded in part by CIBA Vision, which makes diclofenac sodium (NSAID). |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 100 eyes of 100 participants Per group: 25 each Exclusions after randomization: not reported Number analyzed (total and per group): Total: 100 eyes of 100 participants Per group: 25 each Unit of analysis (individuals vs eyes): individuals Losses to follow‐up: unclear How were missing data handled?: unclear Reported power calculation: no Unusual study design?: none | |
| Participants | Country: not reported Age: 72.8 +/‐ 8.5 Gender (per cent): 55% female, 45% male Inclusion criteria: not reported Exclusion criteria: sensitivity to study medications, monocular status, previous intraocular surgery, diabetes mellitus, history of uveitis, iritis, or intraocular inflammation, use of NSAID during the study or the week before, pupils that dilated no more than 5.0 mm or required mechanical pupil stretching, pregnant, nursing, or planning a pregnancy Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 3 days preoperatively and 3 times every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week NSAID 2 plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 1 day preoperatively and every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week NSAID 3 plus corticosteroid: ketorolac tromethamine 0.4% every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week Corticosteroid: vehicle every 15 minutes in the hour before surgery and topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week Other medications: all participants also received topical gatifloxacin 0.3% 4 times daily for 3 days before cataract surgery and 1 week after surgery Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcomes, as defined in study reports: mydriasis at the onset of surgery and at the time of IOL insertion, inflammation, incidence of clinically significant CME at 2 weeks, participant discomfort requiring additional analgesia, UCVA, BCVA, surgical time, endothelial cell counts, corneal clarity, intraoperative surgical complications, and mean ultrasound and phacoemulsification times Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 2 weeks, 3 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: Drs Donnenfeld, Perry, and Wittpenn are consultants to Allergan Pharmaceuticals Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was based on a random‐number‐generated protocol that was created before initiation of the study. The process ensured the randomization of cataract density in the 4 groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether participants, personnel, or outcome assessors were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether participants, personnel, or outcome assessors were masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were any missing data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective reporting. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: CCT Number randomized (total and per group): Total: 269 eyes of 269 participants Per group: bromfenac 0.9%: 138 eyes of 138 participants; prednisolone acetate 1%: 135 eyes of 135 participants Exclusions after randomization: not reported Number analyzed (total and per group): Total: 222 eyes of 222 participants Per group: bromfenac 0.9%: 113, prednisolone acetate 1%: 109 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: bromfenac 0.9%: 25, prednisolone acetate 1%: 26 How were missing data handled?: data from participants lost to follow‐up were not included in the analysis Reported power calculation: yes Unusual study design?: “Patients not responding to the respective group’s postoperative regimen were given rescue medication, that is, if the degree of anterior chamber inflammation did not improve clinically or if the SOIS remained the same in subsequent clinic visits, the patient in the NSAID group (group I) was given a steroid, and the patient in the steroid group (group II) was given an NSAID”; “Patients were excluded from the study if the FT (foveal thickness) and TMV (total macular volumes) were 2 SDs from the baseline OCT value(s)” | |
| Participants | Country: United States Age: bromfenac mean age = 71.8 ± 8.5, prednisolone mean age = 71.1 ± 10.4 Gender (per cent): bromfenac: 53 (46.9%) male and 60 (53.1%) female; prednisolone: 42 (38.5%) male and 67 (61.5%) female Inclusion criteria: people with visually significant cataracts which were defined as follows: BCVA worse than 20/50; cataracts affecting the activities of daily living; symptomatic, e.g. glares and halos, myopic shift; and/or monocular diplopia. The Lens Opacities Classification System II was used to classify the opacity. Greater than 98% of the operated cataracts were of the age‐related type, nuclear sclerosis with/without cortical changes, to include mature and hard cataracts. The remaining cataracts were of the posterior subcapsular cataract and traumatic types. Specific to this study, type II diabetic patients with nonproliferative diabetic retinopathy without a history of macular edema or photocoagulation therapy were included in this study. Exclusion criteria: proliferative diabetic retinopathy, persistent macular edema secondary to diabetic retinopathy, epiretinal membrane, pre‐existing anterior uveitis, exfoliation syndrome, and exudative macular degeneration Equivalence of baseline characteristics: yes, “No significant differences were observed in age, visual acuity, operation time, and macular edema” | |
| Interventions | Comparison: NSAID vs corticosteroid NSAID: bromfenac 0.09%, 1 drop in operated eye every day starting 3 days before surgery and continuing for a total of 14 days Corticosteroid: prednisolone acetate 1%, 1 drop in the operated eye 4 times a day for 7 days followed by a tapering dose totaling 14 days of treatment Other medications: besifloxacin 0.6%, 1 drop in the operated eye twice a day for 2 days before surgery and 7 days after surgery Length of follow‐up: Planned: 2 months Actual: 82 (73%) of participants in the bromfenac group and 83 (76%) of participants in the prednisolone group returned for their 2‐month postoperative OCT measures | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, degree of anterior chamber inflammation, prevalence of macular edema using foveal thickness and mean thickness of the fovea using OCT (postoperative macular changes falling outside of 2 SDs were considered to have cystoid macular edema) Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months | |
| Notes | Type of study: published Funding sources: “This study was not funded by any government or nongovernment agencies. Ample pharmaceutical agents were made available to all the patients throughout the study to ensure that patients enrolled in the study did not have to pay out of their pocket” Disclosures of interest: “All participants (ophthalmologists and optometrist) in this study do not have any financial and proprietary interests in any of the products included in this study. None of the authors and personnel involved in this study have any competing interests with any of the products mentioned throughout this article.” Study period: 4 April 2011 to 31 August 2011 Reported subgroup analyses: yes, participants with diabetes Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients undergoing cataract surgery on the even week of the month, that is, the second and fourth week of the month, were placed in group I, whereas those scheduled for the odd week of the month, that is, first, third, and fifth (when applicable) week of the month, were placed in group II. The nursing staff at the surgery center was given a study schedule indicating which medications were to be given preoperatively and postoperatively" |
| Allocation concealment (selection bias) | High risk | It was planned in advance to which groups participants would be assigned. |
| Masking of participants and personnel (performance bias) | High risk | “single‐masked” study; “the surgical counselor/scheduler, the operating surgeon, and the evaluating ophthalmologist were 'blinded' about which group the patients will be placed into. This information was known only to the primary author and the nursing staff at the surgery center.”; participants were not masked. |
| Masking of outcome assessment (detection bias) | Low risk | “single‐masked” study; “the surgical counselor/scheduler, the operating surgeon, and the evaluating ophthalmologist were 'blinded' about which group the patients will be placed into. This information was known only to the primary author and the nursing staff at the surgery center.” |
| Incomplete outcome data (attrition bias) | High risk | It is reported that 51 participants were lost to follow‐up, and the data from these participants were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | “Patients not responding to the respective group’s postoperative regimen were given rescue medication, that is, if the degree of anterior chamber inflammation did not improve clinically or if the SOIS remained the same in subsequent clinic visits, the patient in the NSAID group (group I) was given a steroid, and the patient in the steroid group (group II) was given an NSAID”; “Patients were excluded from the study if the FT and TMV were 2 SDs from the baseline OCT value(s)” |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: 20 participants per group Exclusions after randomization: 2; people were excluded from the study if they had any postoperative complication affecting accurate measurements of the flare and cells Number analyzed (total and per group): Total: 58 Per group: NSAID group 1: 19; NSAID group 2: 19; corticosteroid group: 20 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: United States Age: NSAID group 1 mean = 68.1 (range 32 to 85); NSAID group 2 mean = 70.2 (range 48 to 81); corticosteroid group mean = 71.1 (range 49 to 86) Gender (per cent): NSAID group 1: 47.3% men, 52.7% women; NSAID group 2: 57.9% men, 42.1% women; corticosteroid group: 55% men, 45% women Inclusion criteria: people who underwent phacoemulsification with intraocular lens implantation Exclusion criteria: evidence of acute or chronic ocular inflammation, previous surgery in the eye that was operated on, use of any systemic or topical corticosteroids or NSAIDs, known hypersensitivity to any of the study drugs, and lack of consent Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs NSAID vs corticosteroid NSAID 1: diclofenac sodium 0.1% one drop 4 times a day for 1 week, then 2 times a day for 3 weeks NSAID 2: ketorolac tromethamine 0.5% one drop 4 times a day for 1 week, then 2 times a day for 3 weeks Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: 28 days Actual: 28 days | |
| Outcomes | Primary outcome, as defined in study reports: flare, cells, and intraocular pressures Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: postoperative days 1, 7, and 28 | |
| Notes | Type of study: published Funding sources: Hermann Eye Fund, Research to Prevent Blindness and by an unrestricted grant from Allergan Inc. Disclosures of interest: none Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “Postoperatively, drops were supplied by the hospital pharmacy in identical 10‐mL bottles labeled with the drug code and the patient’s identification number. Both patients and study examiners were masked to the content of the bottles” |
| Masking of outcome assessment (detection bias) | Unclear risk | It is unclear whether outcomes assessors were masked. |
| Incomplete outcome data (attrition bias) | Low risk | “Two patients were withdrawn from the study after randomization ... neither patient received study medications. Fifty‐eight patients completed the study” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective reporting. |
| Other bias | Unclear risk | The study was funded by an unrestricted grant from Allergan, the makers of one of the study drugs. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 86 eyes of 70 participants Per group: NSAID group: 43 eyes of 35 participants; corticosteroid group: 43 eyes of 35 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 86 eyes of 70 participants Per group: NSAID group: 43 eyes of 35 participants; corticosteroid group: 43 eyes of 35 participants Unit of analysis: eyes Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: some participants had both eyes included analyses did not take into account the non‐independence of eyes | |
| Participants | Country: Egypt Age: NSAID group: 13 aged 50 to 60, 14 aged 61 to 70, 6 aged > 70; corticosteroid group: 15 aged 50 to 60, 13 aged 61 to 70, 9 aged > 70 Gender (per cent): overall: 44 male and 26 female; NSAID group: 23 male and 12 female; steroid group: 21 male and 14 female Inclusion criteria: people selected for the study had at least 1 risk factor for CME (besides diabetic retinopathy), including history of retinal vein occlusion, presence of epiretinal membrane, or preoperative use of prostaglandin analogues eye drops Exclusion criteria: not reported Equivalence of baseline characteristics: yes, “There was non‐significant (P>0.05) difference between both studied groups regarding the enrollment data (Table 1).” | |
| Interventions | Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID: topical ketorolac tromethamine 0.4% twice daily in addition to topical dexamethasone 0.1% 4 times daily for 12 weeks Corticosteroid: topical dexamethasone 0.1% 4 times daily for 12 weeks postoperatively Length of follow‐up: Planned: 12 weeks Actual: 12 weeks | |
| Outcomes | Primary outcome, as defined in study reports: clinical signs of CME; ocular coherent tomography Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 3, 6, and 12 weeks postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “The authors report no conflicts of interest in this work.” Study period: January 2011 to March 2012 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomized to one of the regimes by asking an independent person to choose one envelope from each container.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not described. Both eyes of some participants were included, and it is unclear whether non‐independence of the eyes was taken into consideration in the analysis. |
| Methods | Study design: parallel RCT Number randomized (total and per group): Total: 75 eyes of 75 participants Per group: NSAID: 40; corticosteroid: 35 Exclusions after randomization: 3 people due to difficulty with the OCT measurement; 10 dropped out because of poor health (n = 8), posterior capsular rupture (n = 1), and epidemic keratoconjunctivitis (n = 1) Number analyzed (total and per group): Total: 62 eyes of 62 participants Per group: NSAID: 31; corticosteroid: 31 Unit of analysis (individuals vs eyes): 1 eye per individual Losses to follow‐up: 10 participants dropped out How were missing data handled?: not included in final results Reported power calculation: no Unusual study design?: the 2 groups had different drug administration schedules; “Considering the side‐effects of long‐term steroid administration, we substituted fluorometholone for betamethasone 1 week postoperatively because of ethical considerations” | |
| Participants | Country: Japan Age: 37 to 84 years Gender (per cent): 54.8% men and 45.2% women Inclusion criteria: people with diabetes who underwent small‐incision phacoemulsification with intraocular lens implantation at the facility between March 2005 and May 2007 Exclusion criteria: foveal thickness of 250 μm or more; severe diabetic retinopathy for which ocular surgery (including photocoagulation) was indicated; use of topical medications for glaucoma, uveitis, and other diseases that cause CME; ocular allergies to bromfenac (NSAID group) or steroids (corticosteroid group); use of systemic steroids or NSAIDs; and serious cardiac, cerebral, or renal disease Equivalence of baseline characteristics: yes, however there was a significant (P = 0.003) difference in glycated hemoglobin despite randomization | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: bromfenac sodium ophthalmic solution twice daily for 6 weeks Corticosteroid: steroidal solution (betamethasone sodium phosphate and neomycin) 4 times daily for 1 week followed by fluorometholone 4 times daily for 5 weeks; “Considering the side‐effects of long term steroid administration, we substituted fluorometholone for betamethasone 1 week postoperatively because of ethical considerations” Length of follow‐up: Planned: 6 weeks postoperatively Actual: 6 weeks postoperatively | |
| Outcomes | Primary outcome, as defined in study reports: BCVA, intraocular pressure, slit‐lamp biomicroscopy, anterior chamber flare, and average perifoveal thickness Secondary outcomes, as defined in study reports: not reported Adverse events reported: “No adverse events occurred in either group” Intervals at which outcomes assessed: preoperatively, and 1 day and 1, 2, 4, and 6 weeks postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “The authors have no financial interest in any aspect of this article.” Study period: enrollment March 2005 to May 2007 Reported subgroup analyses: yes; participants with non‐proliferative diabetic retinopathy in the NSAID group (n = 16) and the corticosteroid group (n = 11) were compared Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors describe using the “envelope method,” but it is unclear how the sequence was generated: “A prospective open‐label trial was conducted using the envelope method”. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | High risk | This was an open‐label trial. |
| Masking of outcome assessment (detection bias) | High risk | This was an open‐label trial. |
| Incomplete outcome data (attrition bias) | High risk | “Three patients were excluded because of difficulty with the OCT measurements. Ten patients (10 eyes) dropped out of the study because of poor health (eight patients), posterior capsular rupture (one patient) and epidemic keratoconjunctivitis (one patient).” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 108 eyes of 108 participants Per group: steroid: 54, NSAID: 54 Exclusions after randomization: none Number analyzed (total and per group): Total: 108 eyes of 108 participants Per group: steroid: 54, NSAID: 54 Unit of analysis: eyes (1 eye per individual) Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: yes, 90% Unusual study design?: none | |
| Participants | Country: Iran Age: total: 68 ± 7 years; diclofenac group: 67 ± 8 years; corticosteroid group: 69 ± 6 years Gender (per cent): diclofenac group: 21 male (38.9%) and 33 female (61.1%); corticosteroid group: 27 male (50%) and 27 female (50%) Inclusion criteria: diabetic patients with non‐proliferative diabetic retinopathy who were candidates for phacoemulsification and IOL implantation Exclusion criteria: eyes with clinically significant macular edema based on the ETDRS or central macular thickness > 260 µm or both; eyes with other accompanying diseases affecting the macula or eyes with history of previous retinal laser photocoagulation or intraocular surgery; eyes with severe cataract that precludes performing acceptable quality optical coherence tomography Equivalence of baseline characteristics: no, “The groups were comparable regarding all the initial characteristics except for the baseline BCVA which was better in the case group (p = 0.036)” | |
| Interventions | Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID: diclofenac sodium 0.1% every 6 hours from 1 week before surgery and up to 6 weeks after surgery plus corticosteroid drops for 1 month after surgery Corticosteroid: corticosteroid drops (not specified) for 1 month after surgery Additional medication: all participants also received antibiotics (unspecified) 4 times a day for 1 week after surgery Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: BCVA, OCT findings, IOP Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes, “None of the eyes developed drug‐related complications throughout the study.” Intervals at which outcomes assessed: preoperatively, day 1, 30, and 90 | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “None of the authors have any financial/conflicting interests to disclose.” Study period: March 2010 to June 2012 Reported subgroup analyses: no Trial registry number: NCT02306031 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study reports: “Before surgical intervention, all included eyes were randomly assigned to the case and control groups according to a permuted randomization,” but it remains unclear how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | The study reports: “Before surgical intervention, all included eyes were randomly assigned to the case and control groups according to a permuted randomization,” but it is unclear whether study personnel knew the permutation sequence. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported to be double‐masked, but details of masking or participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | “OCTs were performed by an experienced optometrist who was masked to the eyes before, and 1 and 3 months after the surgeries” |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | High risk | Funding sources are not provided; “The frequency of the corticosteroid drops was adjusted for each eye”; and visual acuity at baseline was not equivalent between groups. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Unit of analysis: individuals (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: none | |
| Participants | Country: not reported Age: NSAID mean age = 58.8 ± 13.6; corticosteroid mean age = 60.7 ± 11.4 Gender (per cent): NSAID: 17 (56.7%) women and 13 (43.3%) men; corticosteroid: 14 (46.7%) women and 16 (53.3%) men Inclusion criteria: participants undergoing uncomplicated cataract lens implant surgery Exclusion criteria: severe systemic disorders (in particular diabetes), clinically significant ocular disease such as glaucoma, ocular hypertension, or dry eyes, history of intraocular surgery or recent extraocular surgery, history of hypersensitivity to components of the study drug, complicated surgery or significant complications on the first postoperative day, the use of topical or systemic NSAIDs, corticosteroids, or antibiotics within 14 days prior to surgery Equivalence of baseline characteristics: yes, “The baseline parameters were similar in the two study groups.” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac 0.5% one drop 5 times daily Corticosteroid: fluorometholone 0.1% one drop 5 times daily Other medications: all participants also received the antibiotic tobramycin 0.3% (1 drop 5 times daily) Length of follow‐up: Planned: not reported Actual: 14 days | |
| Outcomes | Primary outcome, as defined in study reports: anterior chamber flare and cells Secondary outcomes, as defined in study reports: symptoms of ocular discomfort, pinhole visual acuity, conjunctival hyperemia, local tolerance (burning/stinging and blurring of vision) Adverse events reported: yes, “During the study no adverse events requiring discontinuation from the study occurred.” Intervals at which outcomes assessed: postoperative day 1, 2, 3, 7, and 14 | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Investigators were masked, but it is not reported if participants were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study is reported to be investigator‐masked, but details of masking are not provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were any missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and declarations of interest were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: 30 (5 treatment groups) Exclusions after randomization: “Six patients (4%) were excluded because of the following complications/adverse events: postoperative hyphema of unknown genesis (1 patient; group 2), corneal stromal edema after an ultrasonic time of more than 3 minutes of progressing brunescent cataract (1 patient each in group 1 and 5), traumatic wound rupture with required wound examination (1 patient; group 4) and two patients didn't come to follow up (group 2 and 3)" Number analyzed (total and per group): Total number: 144 eyes of 144 participants Per group: group 1: 29, group 2: 28, group 3: 29, group 4: 29, group:5: 29 Unit of analysis: individuals Losses to follow‐up: 2 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Germany Age: 39 to 88 years; group 1 mean age = 71 ± 1.9 years; group 2 mean age = 69.8 ± 1.9 years; group 3 mean age = 70.7 ± 1.7 years; group 4 mean age = 68.6 ± 1.9 years; group 5 mean age = 72.2 ± 8.9 years Gender (per cent): 82 (54.6%) women, 68 (45.3%) men Inclusion criteria: age‐related cataract, minimally inflammatory cataract surgery Exclusion criteria: glaucoma, deficiencies of the blood‐aqueous barrier, pseudoexfoliation syndrome, uveitis, ocular infections, cornea guttata, diabetes mellitus, diseases of the rheumatic form circle, local or systemic anti‐inflammatory drugs within the last 4 weeks Equivalence of baseline characteristics: unclear; age was not significantly different between groups (P = 0.69), but other characteristics were not mentioned | |
| Interventions | Comparison analyzed: NSAID vs NSAID plus corticosteroid vs corticosteroid alone NSAID 1 (group 1): preservative‐free diclofenac 0.1% eye drops pre‐ and postoperatively NSAID 2 (group 2): diclofenac 0.1% eye drops with preservative pre‐ and postoperatively NSAID 3 (group 3): preservative‐free diclofenac 0.1% only postoperatively NSAID plus corticosteroid (group 4): preservative‐free diclofenac 0.1% pre‐ and postoperatively in combination with dexamethasone‐21‐dihydrogenphosphate 0.1% eye drops (DEXA) Corticosteroid alone (group 5): dexamethasone 0.1% postoperatively Medication timing: Preoperatively: night before surgery, and on the day of surgery 3, 2, 1.5, 1, and ½ hours preoperatively Postoperatively: immediately after the surgery, during changing of bandages, and in the following night; they were also applied from day 1 to 7 postoperatively 5 times a day every 3 hours Length of follow‐up: Planned: 7 days postop Actual: 7 days postop | |
| Outcomes | Primary outcome, as defined in study reports: aqueous flare measured by laser flare‐cell meter FC‐1000 (photon counts/ms), postoperative IOP measured by Goldmann applanation tonometer (mm Hg) and BCVA Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes (postoperative hyphema of unknown genesis, corneal stromal edema after an ultrasonic time of over 3 minutes of progressing brunescent cataract, traumatic wound rupture with required wound examination) Intervals at which outcomes assessed: preoperative (day before surgery), about 6 hours postoperatively, 1, 3, 7 days postoperatively | |
| Notes | Type of study: published Funding sources: study medications were provided by the company “Dr. Mann Pharma, Berlin” Disclosures of interest: authors report no financial interest Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Low risk | Double‐blind study design. All eye drops were put into identical‐looking 1‐dose ampoules. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 participants were lost to follow‐up, but may have been included in data analysis. There is a discrepancy in the text, which states that participants have been excluded from the study, but the text underneath Table 1 states that the “mean values are from 30 patients per group” when there would have been fewer than 30. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | A pharmaceutical company provided study medications. |
| Methods | Study design: parallel RCT Number randomized (total and per group): Total: 60 Per group: NSAID: 30; corticosteroid: 30 Exclusions after randomization: Number analyzed (total and per group): Total: 59 Per group: NSAID: 30; corticosteroid: 29 Unit of analysis: individuals Losses to follow‐up: 1 participant in the corticosteroid group was excluded from the study because of a pre‐existing preoperative epithelial defect. 3 participants in the NSAID group and 1 in the corticosteroid group missed the 3‐day follow‐up, and 1 participant in each group missed the 7‐day follow‐up. How were missing data handled?: not directly addressed in text; possibly not included in results for those specific time points Reported power calculation: no Unusual study design?: none | |
| Participants | Country: United States Age: NSAID mean age = 67.3; corticosteroid mean age = 68.5 Gender (per cent): 35% men and 63% women Inclusion criteria: over 18 years of age, with a potential visual acuity of 20/40 or better in the fellow eye, had a visually significant cataract in the study eye, and were willing to comply with all postoperative instructions Exclusion criteria: pregnant, nursing, or of childbearing age and not using a medically acceptable form of birth control or had previous surgery in the eye, uveitis, insulin‐requiring diabetes, advanced glaucoma, a history of recent oral NSAID use, a known sensitivity to the study medications, or an ocular or systemic disease that could interfere with the study Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac tromethamine 0.5% ophthalmic solution 4 drops daily for 1 week and then 2 drops daily for the remainder of the study Corticosteroid: loteprednol etabonate 0.5% ophthalmic suspension 4 drops daily for 1 week and then 2 drops daily for the remainder of the study Length of follow‐up: Planned: 30 +/‐ 7 days postoperatively Actual: 30 +/‐ 7 days postoperatively | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, external examination, slit‐lamp examination including IOP measurement, and Kowa LFCM cell and flare measurement Secondary outcomes, as defined in study reports: not reported Adverse events reported: there were no adverse events in either group Intervals at which outcomes assessed: day 1, 4, 7, and 30 | |
| Notes | Type of study: published Funding sources: unrestricted grant from Allergan Inc. Disclosures of interest: “None of the authors has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “The medications were given in identical bottles, and none of the patients, investigators, or technical staff knew which medication was given” |
| Masking of outcome assessment (detection bias) | Low risk | “The medications were given in identical bottles, and none of the patients, investigators, or technical staff knew which medication was given” |
| Incomplete outcome data (attrition bias) | Unclear risk | “One patient in the loteprednol group was excluded from the study because of a pre‐existing preoperative epithelial defect. Three patients in the ketorolac group and 1 in the loteprednol group missed the 3‐day follow‐up and one patient in each group missed the 7‐day follow‐up”. The authors do not address how the missing data were handled; implies that these measurements were not considered, but the percentage of missing data is small. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | This study is funded by an unrestricted grant from Allergan, which manufactures one of the study drugs. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 91 eyes of 91 participants Per group: NSAID 1 plus corticosteroid: 28; NSAID 2 plus corticosteroid: 32; corticosteroid alone: 31 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 91 eyes of 91 participants Per group: NSAID 1 plus corticosteroid: 28; NSAID 2 plus corticosteroid: 32; corticosteroid alone: 31 Unit of analysis: eye (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: no | |
| Participants | Country: South Korea Age: total mean age = 66.9 ± 8.5 years (range 33 to 78 years); NSAID 1 plus corticosteroid mean age = 66.9 ± 11.1; NSAID 2 plus corticosteroid mean age = 67.5 ± 7.0; corticosteroid alone mean age = 66.8 ± 8.1 Gender (per cent): overall: 41 men (45.1%) and 50 women (54.9%); NSAID 1 plus corticosteroid: 13 men (46.4%) and 15 women (53.6%); NSAID 2 plus corticosteroid: 15 men (46.9%) and 17 women (53.1%); corticosteroid: 13 men (41.9%) and 18 women (58.1%) Inclusion criteria: males or non‐pregnant females aged between 20 and 80 years Exclusion criteria: poor general condition, including high blood pressure, poor blood glucose control, or renal failure; history of ocular trauma or disease; history of intraocular surgery; systemic or topical NSAIDs or corticosteroids use within 4 weeks of enrollment; known hypersensitivity to salicylates or other NSAIDs; and use of alpha‐1 adrenergic antagonist or other analogous systemic medications that could increase the tendency for miosis during the operation (intraoperative floppy iris syndrome) Equivalence of baseline characteristics: yes, “Overall, baseline demographics and clinical characteristics, including preoperative ocular surface status and macular thickness and volume, were similar among the groups.” | |
| Interventions | Comparison: NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid alone NSAID 1: 1 drop of bromfenac sodium hydrate ophthalmic solution 0.1% twice daily beginning 3 days before surgery and 2 drops at 20‐minute intervals 2 hours before surgery plus prednisolone acetate 1% applied 4 times daily for 4 weeks NSAID 2: 1 drop of ketorolac 0.45% ophthalmic solution twice‐daily beginning 1 day prior to cataract surgery and 2 drops at 20‐minute intervals 2 hours before surgery, with continued application twice‐daily throughout the first 2 postoperative weeks plus prednisolone acetate 1% applied 4 times daily for 4 weeks Corticosteroid: prednisolone acetate 1% eye drops applied 4 times daily for 4 weeks Other medications: all participants also received topical gatifloxacin 0.3% Length of follow‐up: Planned: 4 weeks Actual: 4 weeks | |
| Outcomes | Primary outcome, as defined in study reports: intraoperative change in pupil size, postoperative anterior chamber inflammation control, change in macular thickness and volume measured using OCT, and ocular surface status after operation Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: postoperative day 1, 7, and 28 | |
| Notes | Type of study: published Funding sources: "This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A1A2058907)." Disclosures of interest: “The authors have no financial conflicts of interest” Study period: November 2013 to June 2014 Reported subgroup analyses: yes Trial registry #: NRF 2013R1A1A2058907 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | “... and the study medications were masked to both the surgeon and examiners.” It is unclear whether participants were masked. |
| Masking of outcome assessment (detection bias) | Low risk | “... and the study medications were masked to both the surgeon and examiners.” |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT (single center) Number randomized (total and per group): Total: 212 eyes of 108 participants Per group: group 1: 53; group 2: 50; group 3: 58; group 4: 51 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eye Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: both eyes of some participants were independently assigned to interventions and analyzed without taking into account the non‐independence of eyes | |
| Participants | Country: Japan Age: mean 72.6 years, range 48 to 88 years Gender (per cent): not reported Inclusion criteria: not reported Exclusion criteria: any ocular complications such as uveitis, glaucoma, pigmentary retinopathy, or diabetic retinopathy, and intraoperative complications including iris damage, posterior capsular rupture, vitreous prolapse Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID 1 (group 1): polymethylmethacrylate lens and diclofenac sodium 3 times daily Corticosteroid 1 (group 2): polymethylmethacrylate lens and corticosteroid drops 3 times daily NSAID 2 (group 3): silicone lens and diclofenac sodium 3 times daily Corticosteroid 2 (group 4): silicone lens and corticosteroid drops 3 times daily Notes: study medication was initiated 1 day after the operation; all participants received dibekacin (Panimycin) 3 times daily Length of follow‐up: Planned: 90 days Actual: 90 days | |
| Outcomes | Primary outcome, as defined in study reports: anterior chamber flare Secondary outcomes, as defined in study reports: none Adverse events reported: yes Intervals at which outcomes assessed: day 1 and 3, week 1 and 2, and month 1 and 3 | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 1996 to January 1997 Reported subgroup analyses: not reported Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers of participants who were excluded, lost to follow‐up, and analyzed were not reported. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | For some participants, both eyes were independently assigned to interventions and analyzed without taking into account the non‐independence of eyes; funding source and declarations of interest were not reported. |
| Methods | Study design: parallel RCT Number randomized (total and per group): Total: 180 eyes of 180 participants Per group: 60 eyes of 60 participants Exclusions after randomization: 9 Number analyzed (total and per group): Total: 180 eyes of 180 participants Per group: 60 eyes of 60 participants Unit of analysis (individuals vs eyes): individuals (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not included in results for specific observation times with missing data Reported power calculation: yes, 80% Unusual study design?: none | |
| Participants | Country: Sweden Age: 64 to 85 years of age Gender (per cent): NSAID: 33% women and 67% men; corticosteroid: 37% women and 63% men; saline: 32% women and 68% men Inclusion criteria: people with no other known eye disease, enrolled for cataract surgery Exclusion criteria: pseudoexfoliation syndrome, small pupils (< 5 mm after pharmacological dilation), dark brown iris, glaucoma, uveitis, diabetes, treatment with topical medications, or anti‐inflammatory drugs, eyes with a dark brown iris (due to being more prone postoperative inflammation) Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% 4 times daily for 1 week and then 2 times daily for 3 weeks Corticosteroid: postoperative treatment with dexamethasone phosphate 0.1% 4 times daily for 1 week and then 2 times daily for 3 weeks Control: saline 0.9% Length of follow‐up: Planned: 4 years Actual: 4 years | |
| Outcomes | Primary outcome, as defined in study reports: inflammatory response, visual acuity, intraocular pressure, capsulotomy rate, corneal reactions Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: “The inflammatory reaction in the anterior chamber was measured with laser flare photometry preoperatively and 1, 3, and 8 days, 2 and 4 weeks, 2 and 6 months, and 1, 2, and 4 years postoperatively” | |
| Notes | Type of study: published Funding sources: Alcon and Leiras provided drug, the pharmacy at St Göran’s Hospital, Stockholm provided masked bottles Disclosures of interest: “The authors have no proprietary interest in any of the products or equipment mentioned in this article” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by a computer in blocks of 6 participants (randomly permuted blocks, SAS/PLAN procedure). |
| Allocation concealment (selection bias) | Low risk | See above |
| Masking of participants and personnel (performance bias) | Low risk | “On the first day after surgery, the patients received three coded bottles containing one of the treatment solutions”. All bottles were white and non‐transparent. They were delivered from the pharmacy with identical labels except for the randomization number. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether the outcome assessors were masked. |
| Incomplete outcome data (attrition bias) | High risk | The authors did not report data from withdrawn participants. |
| Selective reporting (reporting bias) | High risk | Not all follow‐up points were reported for rate of inflammatory symptoms in participants, striate keratopathy, or Nd:YAG. |
| Other bias | Unclear risk | Study drugs were provided by the respective pharmaceutical companies |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 217 eyes of 217 participants Per group: NSAID: 104, corticosteroid: 113 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 217 eyes of 217 participants Per group: NSAID: 104, corticosteroid: 113 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: China Age: NSAID mean age = 72.2 ± 10.8, corticosteroid mean age = 71.6 ± 8.5 Gender (per cent): NSAID: 35 (33.7%) men and 69 (66.3%) women; corticosteroid: 46 (40.7%) men and 67 (59.3%) women Inclusion criteria: diagnosed with type II diabetes mellitus but without diabetic retinopathy and given phacoemulsification and intraocular lens implantation Exclusion criteria: diabetic retinopathy, age‐related macular degeneration, macular membrane, or retinal vascular disease, people with intraoperative complications Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac 1% for 4 weeks postoperatively and dexamethasone 4 times daily for 1 day preoperatively and 4 weeks postoperatively Corticosteroid: dexamethasone 4 times daily for 4 weeks postoperatively Other medications: all participants also received the topical antibiotic tobramycin Length of follow‐up: Planned: 4 weeks Actual: 4 weeks | |
| Outcomes | Primary outcome, as defined in study reports: cystoid macular edema incidence measured using OCT Secondary outcomes, as defined in study reports: central field retinal thickness and BCVA Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 and 4 weeks | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 2009 to December 2010 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Low risk | Published reports appear to include all expected outcomes. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 84 eyes of 84 participants Per group: NSAID plus corticosteroid: 42, corticosteroid alone: 42 Exclusions after randomization: 5; “Three subjects in the treatment group (NSAID plus corticosteroid) and two subjects in the control group (corticosteroid alone) had unreliable preoperative OCT scans because of dense posterior subcapsular cataracts" Number analyzed (total and per group): Total: 79 eyes of 79 participants Per group: NSAID plus corticosteroid: 39, corticosteroid alone: 40 Unit of analysis: individual (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: yes, 90% power Unusual study design?: none | |
| Participants | Country: United States Age: NSAID plus corticosteroid mean age = 74.0 ± 9.00 (range 51 to 90); corticosteroid alone mean age = 70.3 ± 8.0 (range 44 to 88) Gender (per cent): NSAID plus corticosteroid: 19 (47.5%) men and 21 (52.5%) women; corticosteroid alone: 18 (46.2%) men and 21 (53.9%) women Inclusion criteria: people planning to have cataract surgery by KLC at the Ambulatory Care Center, the University of North Carolina Hospitals Exclusion criteria: factors that could increase the risk of postcataract CME, including medically treated diabetes mellitus, history of uveitis, use of topical prostaglandin analogues for glaucoma, history of earlier intraocular surgery in the same eye, retinal vascular disease, and macular degeneration, abnormal preoperative OCT measurements Equivalence of baseline characteristics: no, “The mean age of patients in the treatment group was slightly greater than that in the control group, 73.95 and 70.33 years, respectively (P=0.0460)" | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 0.1% 3 times a day for 1 month plus prednisolone acetate 1% 4 times a day for 1 month Corticosteroid alone: prednisolone acetate 1% 4 times a day for 1 month Other medications: all participants also received the antibiotic moxifloxacin 0.5% 4 times a day for 10 days Length of follow‐up: Planned: not reported Actual: corticosteroid alone mean = 68.98 ± 13.98 days, NSAID plus corticosteroid mean = 73.31 ± 21.58 | |
| Outcomes | Primary outcome, as defined in study reports: changes of macular thickness in OCT Secondary outcomes, as defined in study reports: total macular volume and BCVA Adverse events reported: yes, “There were no adverse events reported by patients using nepafenac.” Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months | |
| Notes | Type of study: published Funding sources: “This work was supported in part by Research to Prevent Blindness, Inc., New York, NY.” Disclosures of interest: the authors report no financial interest Study period: not reported Reported subgroup analyses: no Trial registry #: NCT00494494 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomised according to even/odd subject identification number, using computer‐generated random numbers, to the control group (standard of care only) or to the treatment group (standard of care plus nepafenac).” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | It is unclear whether participants and personnel were masked; participants in the treatment group had an extra bottle of eye drops to use. |
| Masking of outcome assessment (detection bias) | Low risk | “Postoperative follow‐up was at 1 day, 1 week, 1 month, and 2 months. At the 2 months visits, technicians, who were masked to treatment, measured ETDRS BCVA, and OCT scans were performed.” “Experienced ophthalmic photographers, who were masked to treatment, obtained Stratus OCT (Carl Zeiss Meditec, Inc., San Francisco, CA, USA) scans using the fast macular thickness protocol." |
| Incomplete outcome data (attrition bias) | Unclear risk | 5 participants who were randomized were not analyzed due to unreliable preoperative OCT scans. It is unclear how incomplete outcome data were addressed. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: the study is ongoing: “Additional patients are being enrolled in the study in order to confirm these findings.” | |
| Participants | Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: people scheduled for cataract extraction Exclusion criteria: pre‐existing retinal disease, diabetes, previous intraocular surgery, dense cataract preventing preoperative retinal evaluation, or were unable to come to the site for follow‐up visits Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac sodium 0.1% 2 days prior to surgery and for 4 weeks following surgery plus postoperative topical steroid Corticosteroid: postoperative topical steroid only Length of follow‐up: Planned: 6 weeks Actual: not reported | |
| Outcomes | Primary outcome, as defined in study reports: presence of CME measured using OCT Secondary outcomes, as defined in study reports: visual acuity testing, Amsler grid testing, dilated fundus examination Adverse events reported: no Intervals at which outcomes assessed: postoperatively and 6 weeks | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | Participants in the NSAID group received eye drops to take at home, while participants in the steroid‐only group did not receive any medication to take at home. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and disclosures of interest were not provided; this is an ongoing study and initial results were reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 62 eyes of 62 participants Per group: NSAID: 31, corticosteroid: 31 Exclusions after randomization: 12 total, 6 eyes from each group; “The reasons for exclusion in the diclofenac group were as follows: one eye showed sensitivity to fluorescein sodium, three eyes presented insufficient pupil dilation, and two eyes had glaucoma. The causes in the fluorometholone group were as follows: Three eyes presented insufficient pupil dilation, one eye had uveitis, one subject had diabetes, and one subject had hypertension.” Number analyzed (total and per group): Total: 50 eyes of 50 participants Per group: NSAID: 25, corticosteroid: 25 Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Japan Age: NSAID mean age = 65.4 ± 7.0, corticosteroid mean age = 65.8 ± 7.1 Gender (per cent): NSAID: 13 (52%) men and 12 (48%) women, corticosteroid: 10 (40%) men and 15 (60%) women Inclusion criteria: between 50 and 70 years of age, subjected to unilateral surgery or to have 6 months’ span between surgeries in people with bilateral cataract Exclusion criteria: eyes encountering acute ocular infection or inflammation during the first month of the study; eyes showing sensitivity to diclofenac or fluorometholone; eyes showing sensitivity to fluorescein sodium; eyes with insufficient dilation; eyes with a history of other ocular surgeries; eyes with pseudoexfoliation syndrome; eyes with a history of trauma; eyes with uveitis, glaucoma, or other disorders; eyes with complication of diabetes and kidney disorders; people with uncontrollable hypertension; and eyes encountering rupture of the posterior capsule, vitreous loss, and other complications during a cataract/IOL implantation procedure Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: topical diclofenac 0.1% 4 times before surgery and 3 times daily for 5 weeks after surgery Corticosteroid: fluorometholone 0.1% 4 times before surgery and 3 times daily for 5 weeks after surgery Length of follow‐up: Planned: 5 weeks Actual: 5 weeks | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, intraocular pressure, blood pressure, laser Doppler flowmetry, flare, fluorescein fundus angiography Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: baseline and 2 days, 1, 2, 5 weeks postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: no disclosures of interest Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that “Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method ...,” but it is unclear what the envelope method is. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported to be double‐masked, but details of masking or participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | “CME, captured by fluorescein angiography at 2 and 5 weeks after surgery, was analyzed by one of the authors (NK) in a masked fashion and graded with a method explained previously.” “Laser flarimetry was conducted to measure the amount of aqueous flare by one of the authors (SA) in a masked fashion.” |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Exclusions after randomization: “One patient was excluded because the patient wanted a bilateral procedure immediately after signing up for the study” Number analyzed (total and per group): Total: 59 eyes of 59 participants Per group: NSAID: 30, corticosteroid: 29 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 participant in the NSAID group had macular degeneration that precluded fluorescein fundus angiography and dropped out of the study, and another participant was dropped due to an unwillingness to attend the examination. 2 participants in the corticosteroid group dropped out, 1 because a humeral fracture prevented fluorescein fundus angiography and another due to posterior lens capsule rupture during surgery How were missing data handled?: all participants were included in the safety evaluation; it was not reported how losses to follow‐up were handled Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Japan Age: NSAID group: 16 (53.3%) were 18 to 64 years and 14 (46.7%) were ≥ 65 years, corticosteroid group: 12 (41.4%) were 18 to 64 years and 17 (58.6%) were ≥ 65 years Gender (per cent): NSAID group: 16 (53.5%) men and 14 (46.7%) women, corticosteroid group: 16 (55.2%) men and 13 (44.8%) women Inclusion criteria: people older than 20 years who had phacoemulsification cataract extraction and IOL implantation between October 2007 and April 2008 at Shohzankai Medical Foundation, Miyake Eye Hospital Exclusion criteria: people were excluded if they (1) had taken systemic, topical, or ointment steroidal agents within 14 days of surgery; (2) had an intraocular or periocular injection of steroidal agents within 90 days of surgery; (3) had taken systemic or topical NSAIDs within 7 days of surgery; (4) had a history of ophthalmic surgery (including laser surgery) or of ocular trauma that could affect the study results; (5) had pseudoexfoliation syndrome; (6) had a history of chronic or recurring ocular inflammation (e.g. uveitis or scleritis); (7) had diabetic retinopathy; (8) had an ocular anomaly (e.g. aniridia, congenital cataract); (9) had iris atrophy; (10) had disorders that would preclude improvement in visual function; (11) had macular edema; (12) had severe corneal epithelial disorder (e.g. corneal ulcer); (13) had no visual function in the contralateral eye; (14) were scheduled to have other ocular surgery from baseline to 5 weeks after cataract surgery; (15) had secondary IOL implantation; (16) were allergic to or might have been sensitive to NSAIDs, amfenac, or fluorometholone; (17) had a positive skin reaction to fluorescein; (18) had a tendency to bleed or were currently on anticoagulants; (19) had prostaglandin‐type treatment for glaucoma within 4 days of surgery; (20) had been included in a previous study of prostaglandin‐type antiglaucoma drugs; (21) had joined another clinical study within 30 days of the study; (22) had ocular infection; (23) had uncontrollable diabetes mellitus; (24) had severe liver, kidney, or heart disorder; (25) might have been pregnant or were currently breastfeeding; (26) had other factors determined to be unsuitable for the study. Equivalence of baseline characteristics?: yes, “There was no statistically significant difference in any [patient characteristics and surgical] parameter between the 2 groups.” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: nepafenac 0.1% 1 drop 3 times a day from 1 day before surgery until 5 weeks after surgery Corticosteriod: fluorometholone 0.1% 1 drop 3 times a day from 1 day before surgery until 5 weeks after surgery Length of follow‐up: Planned: 5 weeks Actual: 5 weeks | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, intraocular pressure, fundus examination, slit‐lamp examination, ocular coherence tomography, fluorescein angiography, laser flare‐cell meter Secondary outcomes, as defined in study reports: the study did not differentiate between primary and secondary outcomes Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1, 2, and 5 weeks postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: Drs Miyake and Numaga are consultants to Alcon Japan Ltd. Study period: surgery between October 2007 and April 2008 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This was reported as a double‐masked study, and it is reported that outcome assessors were masked. However, it is unclear whether participants and/or other study personnel were masked. |
| Masking of outcome assessment (detection bias) | Low risk | The presence and grade of CME was determined in a masked manner. |
| Incomplete outcome data (attrition bias) | High risk | Some participants were excluded from analyses when there were problems with the measurement; one participant was excluded due to surgical complications. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | Drs Miyake and Numaga are consultants to Alcon Japan Ltd., which manufacturers the study drugs |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 72 eyes of 72 patients Per group: NSAID: 25, corticosteroid: 23, combined treatment: 24 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: not reported Losses to follow‐up: 1 participant in the corticosteroid group developed CME at 1 month after cataract surgery in the left eye and was withdrawn from the study How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Japan Age: NSAID group mean age = 73.6 (range 48 to 86), corticosteroid group mean age = 69.7 (range 41 to 83), combined group mean = 71.4 (range 46 to 86) Gender (per cent): NSAID group: 8 (32%) men and 17 (68%) women; corticosteroid group: 6 (26%) men and 17 (74%) women; combined group: 7 (29%) men and 17 (71%) women Inclusion criteria: people scheduled to undergo routine phacoemulsification combined with IOL implantation Exclusion criteria: people with corneal disease, glaucoma, uveitis, pseudoexfoliation syndrome, diabetes, or other pathologies that might affect treatment responses or evaluations; people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery Equivalence of baseline characteristics: yes, “There was no significant differences between groups in gender or age.” “There were no statistically significant differences between the groups in surgical data, such as the nucleus degrees of the lens, surgical time, phacoemulsification time, and amount of irrigation fluid.” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid vs NSAID plus corticosteroid NSAID: 0.1% bromfenac 2 times daily for 2 months Corticosteroid: 0.1% betamethasone 4 times daily for 1 month and then fluorometholone 4 times daily for 1 month NSAID plus corticosteroid: combined 0.1% bromfenac 2 times daily for 2 months plus 0.1% betamethasone 4 times daily for 1 month and then fluorometholone 4 times daily for 1 month Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcome, as defined in study reports: inflammatory reaction using laser flare cell photometry Secondary outcomes, as defined in study reports: BCVA, intraocular pressure, aqueous flare, corneal thickness measured using pachymetry and corneal endothelial cell count Adverse events reported: no Intervals at which outcomes assessed: 1, 2, and 3 days, 1 and 2 weeks, and 1 and 2 months postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: February to August 2006 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and conflicts of interest were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 79 eyes of 79 participants Per group: NSAID plus corticosteroid: 38, corticosteroid alone: 41 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: yes, “A post hoc power calculation was performed at the completion of the study (day 28), as this time point may reflect the clinical performance of diclofenac.” Unusual study design?: none | |
| Participants | Country: Greece Age: NSAID plus corticosteroid mean age = 76.68 ± 10.72; corticosteroid alone mean age = 76.71 ± 8.82 Gender (per cent): NSAID plus corticosteroid: 12 (31.6%) men and 26 (68.4%) women; corticosteroid alone: 15 (36.6%) men and 26 (63.4%) women Inclusion criteria: not reported Exclusion criteria: presence of corneal abnormalities; history of intraocular surgery; preoperative ECC < 1500 cells/mm2; history of uveitis, diabetes, and age‐related macular degeneration; regular, systemic use of steroid or NSAIDs during the previous 3 months; and intraoperative complication, such as posterior capsule rupture, vitreous loss, lost nucleus, zonule dehiscence, and wound leak Equivalence of baseline characteristics: yes, ”The groups had no statistically significant differences for age, gender, BCVA, ECC, CCT, and macular thickness.” | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac 0.1% 1 drop 3 times a day plus dexamethasone 0.1% 1 drop 4 times a day Corticosteroid: dexamethasone 0.1% 1 drop 4 times a day Other medications: all participants also received the antibiotic chloramphenicol 0.5% Length of follow‐up: Planned: 28 days Actual: 28 days | |
| Outcomes | Primary outcome, as defined in study reports: BCVA, macular thickness, endothelial cell density, central corneal thickness Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: postoperative days 1, 14, 28 | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “No competing financial interests exist” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random was done using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing outcome data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 105 eyes of 105 participants Per group: 35 in each group Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individuals (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: not reported Age: mean age 68.61 ± 0.98 Gender (per cent): 45 males (42.9%), 60 females (57.1%) Inclusion criteria: people undergoing phacoemulsification and posterior chamber IOL Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs NSAID vs corticosteroid NSAID 1: diclofenac sodium 0.1% 4 times daily for 1 month thereafter 3 times daily for remainder NSAID 2: ketorolac 0.5% 4 times daily for 1 month thereafter 3 times daily for remainder Corticosteroid: dexamethasone 0.1% 4 times daily for 1 month thereafter 3 times daily for remainder Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: biotolerance (epithelial staining), comfort (0‐to‐3 subjective scale), and inflammatory response (flare, hyperemia, edema, and IOP) Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: 5 postoperative visits; timing not reported | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This was a “double masked” study, but details of masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | This was a “double masked” study, but details of masking of outcome assessors were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear if there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 157 eyes of 157 participants Per group: NSAID: 57, corticosteroid group 1: 59, corticosteroid group 2: 41 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 155 eyes of 155 participants Per group: NSAID group: 56, corticosteroid group 1: 58, corticosteroid group 2: 41 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 6 participants discontinued treatment due to adverse events, 1 participant was withdrawn from the study because of surgical complications and 1 for noncompliance How were missing data handled?: data from the 8 participants who were withdrawn were included if the participant had a least 1 postoperative visit. The data from 1 participant in the prednisolone group and 1 participant in the dexamethasone group were excluded from the analysis Reported power calculation: no Unusual study design?: “To provide partial masking of the two agents — ketorolac in the form of a solution and prednisolone in the form of a suspension — dexamethasone, a glucocorticoid solution, was added as a third study drug.” | |
| Participants | Country: not reported Age: mean age ± SD: NSAID mean age = 67.3 ± 12.1 (range 26 to 85), corticosteroid group 1 mean age = 71.8 ± 9.7 (range 44 to 87), corticosteroid group 2 mean age = 69.5 ± 12.1 (range 30 to 98) Gender (per cent): NSAID group: 28 (50%) men and 28 (50%) women, corticosteroid group 1: 28 (48%) men and 30 (52%) women, corticosteroid group 2: 19 (46%) men and 22 (54%) women Inclusion criteria: adult candidates for routine cataract extraction and posterior chamber intraocular lens implantation, undergoing surgery in 1 eye only Exclusion criteria: people who had received any glucocorticoid or cyclooxygenase inhibitor within 1 week of surgery, people with known hypersensitivity to any study drug or fluorescein, serious uncontrolled systemic disease, women who were pregnant or lactating Equivalence of baseline characteristics: yes, “With the exception of iris color, no significant differences were found among the three treatment groups in demographic measures, baseline intraocular pressure, operative eye selection, or surgical procedure.” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid vs corticosteroid NSAID: 0.5% ketorolac sterile ophthalmic solution 3 times daily from 1 day before surgery to 4 weeks after surgery Corticosteroid 1: 1% prednisolone acetate sterile ophthalmic suspension 3 times daily from 1 day before surgery to 4 weeks after surgery Corticosteroid 2: 0.1% dexamethasone sodium phosphate sterile ophthalmic solution from 1 day before surgery to 4 weeks after surgery Length of follow‐up: Planned: 6 weeks Actual: 6 weeks | |
| Outcomes | Primary outcome, as defined in study reports: cells and flare in the anterior chamber, fluorescein leakage Secondary outcomes, as defined in study reports: other clinical signs of inflammation: edema of the lid; hyperemia, ciliary flush, and chemosis of the conjunctiva; superior edema, central edema, and Descemet's folds of the cornea; and the presence of hyphema and fibrin in the anterior chamber Adverse events reported: yes Intervals at which outcomes assessed: prior to surgery, day 1 or 2 and 5, week 2, 4, and 6 | |
| Notes | Type of study: published Funding sources: “This study was sponsored by a grant from Syntex Laboratories Inc., Palo Alto, California" Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study was described as partially masked due to the formulation of the study drugs, so participants and personnel could determine assignment to prednisolone, they could not distinguish between ketorolac and dexamethasone. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Participants were excluded only when they did not complete any study visits (n = 2). |
| Selective reporting (reporting bias) | Unclear risk | A protocol was mentioned but not cited, so it is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | Funded by Syntex Laboratories Inc., but they did not manufacture any of the study drugs. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 52 eyes of 52 participants Per group: NSAID: 25, corticosteroid: 27 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 52 eyes of 52 participants Per group: NSAID: 25, corticosteroid: 27 Unit of analysis: not reported Losses to follow‐up: none, “All patients enrolled completed the study.” How were missing data handled?: N/A Reported power calculation: yes, the study had power of at least 80% Unusual study design?: none | |
| Participants | Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: participants undergoing phacoemulsification with posterior chamber intraocular lens implantation Exclusion criteria: history of systemic or ocular inflammation (iritis, uveitis), taking oral or topical ophthalmic corticosteroids or NSAIDs, if they had other ocular disease such as glaucoma, corneal disease, or diabetic retinopathy Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% 4 times per day Corticosteroid: prednisolone acetate 1% 4 times per day Length of follow‐up: Planned: 1 month Actual: 1 month | |
| Outcomes | Primary outcome, as defined in study reports: IOP, cell and flare measurements for postoperative inflammation Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 1 month postoperatively | |
| Notes | Type of study: published Funding sources: the investigation was supported in part by grants from Michael and Gina Ricciardi, Wantagh, NY, Leonard and Silva Marx, Mammaroneck, NY, and Research to Prevent Blindness, New York, NY Disclosures of interest: “The authors have no commercial or proprietary interest in any of the drugs discussed in the article. Dr. Roberts serves on the CIBA Vision Ophthalmics Physician Advisory Panel.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported as double‐masked, but it is unclear who among participants, personnel, and outcome assessors was masked. |
| Masking of outcome assessment (detection bias) | Low risk | “Neither examiner knew in which of the study groups the patient was enrolled, nor did the examiners know the results of the other’s examination.” |
| Incomplete outcome data (attrition bias) | Low risk | “All patients enrolled completed the study. No postoperative visits were missed.” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | The study was funded in part by Ciba Vision Ophthalmics, the manufacturer of one of the study drugs. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 40 eyes of 40 participants Per group: NSAID: 20, corticosteroid: 20 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individuals (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Cuba Age: NSAID mean age = 73 ± 9, corticosteroid mean age = 72 ± 8 Gender (per cent): NSAID: 45% men and 55% women, corticosteroid: 60% men and 40% women Inclusion criteria: people who underwent phacoemulsification for cataract Exclusion criteria: people who had ophthalmological or systemic disease, showed alterations in pupillary dilation Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID: diclofenac sodium 0.1% 1 drop every 4 hours 2 days prior to surgery, 1 drop every 30 minutes 2 hours prior to surgery, and 1 drop every 4 hours during the 1st week postoperatively plus prednisolone eye drops Corticosteroid: prednisolone eye drops Other medications: all participants also received routine treatment eye drops (phenylephrine and tropicamide, chloramphenicol) Length of follow‐up: Planned: 1 month Actual: 6 days | |
| Outcomes | Primary outcome, as defined in study reports: the presence of hyperemia conjunctival and cells in the anterior chamber Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 6 days, 1 month | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: during 2010 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were incomplete outcome data or any missing data. |
| Selective reporting (reporting bias) | High risk | The study was planned for 1‐month follow‐up, but data from 1 month are not presented. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: not reported Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: 120 eyes of 120 participants Per group: NSAID 1 plus corticosteroid: 33, NSAID 2 plus corticosteroid: 30, NSAID 3 plus corticosteroid: 31, corticosteroid alone: 26 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: India Age: overall mean age = 61.14 ± 9.76, NSAID 1 plus corticosteroid mean age = 63.48 ± 9.60, NSAID 2 plus corticosteroid mean age = 59.63 ± 8.96, NSAID 3 plus corticosteroid mean age = 60.42 ± 10.72, corticosteroid alone mean age = 60.77 ± 9.65 Gender (per cent): overall: 67 men (55.8%) and 53 women (44.2%); NSAID 1 plus corticosteroid: 19 men (57.6%) and 14 women (26.4%); NSAID 2 plus corticosteroid: 15 men (50%) and 15 women (50%); NSAID 3 plus corticosteroid: 18 men (58.1%) and 13 women (41.9%); corticosteroid alone: 15 men (57.7%) and 11 women (42.3%) Inclusion criteria: people with visually significant age‐related cataract who were 40 years or older of either sex having phacoemulsification with posterior chamber IOL implantation Exclusion criteria: prior ocular trauma or intraocular surgery; history of uveitis; eyes with corneal disease, pseudoexfoliation syndrome, or ocular infection; history of coexistent ocular disease such as glaucoma, optic atrophy, or ocular tumors; history of use of topical steroids, NSAIDs, or prostaglandins within 2 weeks of enrollment; eyes with complications from diabetic mellitus; uncontrolled hypertension; any connective tissue disorders, serious renal, hepatic, endocrine, pulmonary, cardiac, neurologic, rheumatic, psychiatric, or cerebral dysfunction; and ocular allergy to ketorolac, bromfenac, or nepafenac Equivalence of baseline characteristics: yes | |
| Interventions | Comparison: NSAID plus corticosteroid vs NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac 0.4% 3 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued NSAID 2 plus corticosteroid: bromfenac 0.09% 2 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued NSAID 3 plus corticosteroid: nepafenac 0.1% 3 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued Corticosteroid: prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued Other medication: all participants also received moxifloxacin 0.5% 6 times a day Length of follow‐up: Planned: 8 weeks Actual: 8 weeks | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, IOP, laser flare photometry, and fundus examination Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: day 1, weeks 1, 2, 4, and 8 | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: all surgeries were performed between July 2013 and June 2014 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly divided into 1 of 4 groups using a computer‐generated random‐number table” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described. |
| Masking of participants and personnel (performance bias) | High risk | Not a masked study |
| Masking of outcome assessment (detection bias) | High risk | Not a masked study |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there was any loss to follow‐up and how missing data were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; a protocol was not available. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 125 participants Per group: 25 Exclusions after randomization: none Number analyzed (total and per group): Total: 150 participants Per group: 25 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: 125 people undergoing a phacoemulsification were randomized to 5 different therapy groups. 25 people undergoing the ECCE procedure were also included in the study because they showed higher postoperative flare values, but they were not randomized to 1 of the 5 groups | |
| Participants | Country: Germany Age: mean age 69 years Gender (per cent): not reported Inclusion criteria: age‐related cataract, stationary cataract extraction with posterior chamber lenses implantation Exclusion criteria: other diseases of the eye, diabetes mellitus, diseases of the rheumatic form circle, anti‐inflammatory general therapy Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid vs combinations of NSAIDs plus corticosteroids NSAID: flurbiprofen 0.03% (Ocuflur) Corticosteroid: prednisolone acetate 1% (Inflanefran forte) NSAID plus corticosteroid 1: prednisolone acetate 0.12% (Inflanefran), flurbiprofen 0.03% (Ocuflur) NSAID plus corticosteroid 2: prednisolone acetate 1% (Inflanefran forte), flurbiprofen 0.03% (Ocuflur) NSAId plus corticosteroid 3: prednisolone hemisuccinate 2.5%, flurbiprofen 0.03% (Ocuflur) Additional ECCE procedure (non‐randomised): fortified topical corticosteroid therapy with prednisolone acetate 1% (Inflanefran forte) each hour Length of follow‐up: Planned: 3 days Actual: 3 days | |
| Outcomes | Primary outcome, as defined in study reports: aqueous flare and aqueous cell counts measured by laser flare‐cell photometer FC‐1000 (photon counts/ms) Secondary outcomes, as defined in study reports: definition of a “fibrin border”: high postoperative flare values that will lead to a fibrin exudation within 24 hours, and if a fortified topical corticosteroid therapy prevents a fibrin exudation Adverse events reported: no Intervals at which outcomes assessed: preoperative, 1 and 3 days postoperative | |
| Notes | Type of study: published Funding sources: the laser flare‐cell photometer was provided by the company Pharm‐Allergan GmbH, Ettlingen; other funding sources were not mentioned Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there was any loss to follow‐up or missing data. |
| Selective reporting (reporting bias) | Unclear risk | The authors refer to a study protocol because they added to it 25 participants who underwent another surgery form (ECCE). It is not clear if they also changed the selection of outcomes. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: intra‐individual RCT Number randomized (total and per group): Total: 36 eyes of 18 participants Per group: 18 eyes of 18 participants Exclusions after randomization: 2 eyes (1 individual) Number analyzed (total and per group): Total: 34 eyes of 17 participants Per group: 17 eyes of 17 participants Unit of analysis: eyes Losses to follow‐up: 1 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: analysis does not take account of paired‐eye design | |
| Participants | Country: Japan Age: 71 (57 to 85) Gender (per cent): 23.5% men, 76.5% women Inclusion criteria: bilateral age‐related cataract; underwent phacoemulsification and intraocular lens implantation in both eyes Exclusion criteria: diabetes mellitus, autoimmune diseases, history of chronic corneal or conjunctival diseases or both, and those who wore contact lenses Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac sodium 0.1% plus dexamethasone Corticosteroid: dexamethasone Additional ECCE procedure (non‐randomized): fortified topical corticosteroid therapy with prednisolone acetate 1% (Inflanefran forte) each hour. Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcome, as defined in study reports: vital stainings, tear film break‐up time, and corneal sensitivity; tear function tests; inflammation in the anterior chamber; specular microscopy and corneal thickness; epithelial barrier function Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1 month | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | This was an unmasked study. |
| Masking of outcome assessment (detection bias) | High risk | This was an unmasked study. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant was excluded from analysis due to posterior capsule rupture in 1 eye. There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 263 eyes of 263 participants Per group: nepafenac plus prednisolone acetate: 133, vehicle plus prednisolone acetate: 130 Exclusions after randomization: not reported Number analyzed (total and per group): Total: intention to treat: 251, safety analysis: 253 Per group: nepafenac plus prednisolone acetate: 125, vehicle plus prednisolone acetate: 126 Unit of analysis: eyes (1 eye per individual) Losses to follow‐up: 3 serious events in the vehicle group led to participant discontinuation; 4 other participants discontinued study due to non‐serious adverse events How were missing data handled?: ITT analysis, last observation carried forward method was used to impute missing macular thickness and BCVA data; no imputations for missing data were made in the safety analysis Reported power calculation: no Unusual study design?: none | |
| Participants | Country: United States Age: nepafenac plus prednisolone acetate mean age = 66.6 ± 9.3, vehicle plus prednisolone acetate mean age = 66.4 ± 9.7 Gender (per cent): nepafenac plus prednisolone acetate: 42 male (33.6%), 83 female (66.4%); vehicle plus prednisolone acetate: 51 male (40.5%), 75 female (59.5%) Inclusion criteria: male and female diabetic (type 1 or type 2) patients of any race/ethnicity aged 18 years and older having an existing diagnosis of nonproliferative diabetic retinopathy that required cataract extraction with planned implantation of a posterior chamber intraocular lens (at least 50% of all enrolled participants were required to have moderate to severe nonproliferative diabetic retinopathy, as defined by the International Clinical Diabetic Retinopathy Disease Severity Scale) Exclusion criteria: no significant corneal staining scores at baseline and no history of dry eye syndrome; conditions that caused macular edema including pre‐existing histories of retinal vein occlusions, ocular surgeries, inflammatory eye diseases, ocular infections, congenital ocular anomalies, and ocular traumas; people with baseline cysts or the presence of macular traction and epiretinal membrane; people using concomitant medication such as topical or systemic NSAIDs and steroids which could have interfered with the assessment of the study outcome measures Equivalence of baseline characteristics: “The demographic and baseline characteristics of the patients in the nepafenac and vehicle groups were generally similar” | |
| Interventions | Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 3 times daily on the day prior to cataract surgery and for 90 days plus prednisolone acetate ophthalmic suspension four times daily for 2 weeks postsurgery Corticosteroid: vehicle 3 times daily on the day prior to cataract surgery and for 90 days plus prednisolone acetate ophthalmic suspension four times daily for 2 weeks postsurgery Length of follow‐up: Planned: 90 days Actual: 90 days | |
| Outcomes | Primary outcome, as defined in study reports: efficacy included the percentage of participants who developed macular edema (≥ 30% increase in central subfield macular thickness from baseline) and the percentage of participants with decreases of more than 5 letters in BCVA from day 7 to 90 Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: days 1, 7, 14, 30, 60, and 90 | |
| Notes | Type of study: published Funding sources: Alcon Research Disclosures of interest: RS, LA, GJJ, RPL, JL, HJR, KS, and TW are paid consultants for Alcon Research Ltd. (Fort Worth, TX). DS is an employee of Alcon Research Ltd. Medical writing support, which was funded by Alcon Research Ltd., was provided by Cullen T Vogelson and Usha Sivaprasad of Illuminated Research LLC (Fort Worth, TX) Study period: enrollment between November 2008 and July 2010 Reported subgroup analyses: yes, by nonproliferative diabetic retinopathy classification Trial Registry #: NCT00782717 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Study is reported as double‐masked, but it is unclear whether this refers to participants, surgeons, or other study personnel. |
| Masking of outcome assessment (detection bias) | Low risk | “Morphological features, including intraretinal cysts, were analyzed by the reading center in a masked fashion” and "... study personnel at each investigational center who were responsible for administering the study medication on day 0 did not assess adverse events or the corneal staining results." |
| Incomplete outcome data (attrition bias) | Unclear risk | This study used an ITT analysis with a last observation carried forward method for macular thickness and BCVA data, however 12 participants who were enrolled were not included in the analysis, and the reason for this was not explained. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | High risk | Eight study authors are paid consultants to Alcon Japan Ltd., which manufacturers one of the study drugs. Alcon Japan Ltd. also provided funding for medical writing support. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 38 eyes of 38 participants Per group: NSAID: 18, corticosteroid: 20 Exclusions after randomization: 7 total: NSAID: 4, corticosteroid: 3 Number analyzed (total and per group): Total: 31 Per group: NSAID: 14, corticosteroid: 17 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 in the NSAID group How were missing data handled?: not included Reported power calculation: no Unusual study design?: none | |
| Participants | Country: United States Age: NSAID mean age = 70.6, corticosteroid mean age = 67.6 Gender (per cent): NSAID: 7 (50%) men and 7 (50%) women; corticosteroid: 6 (35.3%) men and 11 (64.7%) women Inclusion criteria: older than 18 years, had potential visual acuity of 20/40 or better in the fellow eye, had a visually significant cataract in the study eye, and were willing to comply with all postoperative instructions Exclusion criteria: pregnant, nursing, or of childbearing age and not using a medically acceptable form of birth control or if they had prior surgery in the eye or had uveitis, diabetes requiring insulin, advanced glaucoma, a history of recent oral NSAID use, a known sensitivity to the study medications, or an ocular or systemic disease that could interfere with the study Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac tromethamine 0.5% 4 times a day for 1 week, then 2 times a day Corticosteroid: rimexolone 1% 4 times a day for 1 week, then 2 times a day Length of follow‐up: Planned: 30 days Actual: 30 days | |
| Outcomes | Primary outcome, as defined in study reports: ocular and systemic symptoms, Kowa cell and flare measurement, visual acuity, an external examination, a slit‐lamp examination, and IOP measurement Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: preoperative assessment then 1, 4, 7, and 30 days postoperative | |
| Notes | Type of study: published Funding sources: “Funded by an unrestricted grant from Allergan, Inc.” Disclosures of interest: “None of the authors has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Low risk | “Treatment was masked to both patient and investigator.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Participants who did not complete the study were not included in the analysis (n=4 in the NSAID group (22%) and 3 in the corticosteroid group (15%). |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | This study was funded by an unrestricted grant from Allergan, which manufactures 1 of the study drugs. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 91 participants Per group: ketorolac plus prednisolone: 42, prednisolone: 49 Exclusions after randomization: “Five patients did not complete the trial in the placebo group: 1 patient withdrew because of adverse events (burning); 1 was excluded because of intraoperative complications; 1 refused to undergo fluorescein angiography; and 2 patients were discontinued because of protocol violations. Five patients did not complete the study in the ketorolac group: 4 patients were discontinued because of protocol violations and 1 was discontinued because of intraoperative complications.” Number analyzed (total and per group): Total: 81 Per group: ketorolac plus prednisolone: 37, prednisolone: 44 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: none How were missing data handled?: excluded Reported power calculation: yes, 80% power Unusual study design?: “Patients who were initially enrolled and randomized into the study were excluded if complications occurred during cataract surgery (e.g., posterior capsule rupture, vitreous loss, retained cortical material, or an IOL not placed in the capsular bag). Patients were also excluded if they did not follow instructions or if they did not show up for appointments” | |
| Participants | Country: Brazil Age: ketorolac plus prednisolone mean age = 67.1 ± 10.8, prednisolone alone mean age = 66.1 ± 8.7 Gender (per cent): ketorolac plus prednisolone: 21 male (56.8%) and 16 female (43.2%), prednisolone alone: 22 male (50%) and 22 female (50%) Inclusion criteria: people with nuclear cataract density of 2 and 3 determined by LOCS II (> 50 years old), with indication for cataract surgery with intraocular lens implantation under local anesthesia Exclusion criteria: diabetes, NSAID use, use of topical eye drops (including antiglaucoma drugs), history of uveitis, macular disease, pseudoexfoliation syndrome, congenital ocular abnormalities, cataract density of 1 and 4 determined by LOCS II, and previous intraocular surgery or injections into the eye Equivalence of baseline characteristics: yes, “Baselines demographics were similar in all groups. There were no differences regarding age (P = 0.617), preoperative BCVA (P = 0.153), or gender distribution (P = 0.656)” | |
| Interventions | Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 3 days before surgery and 5 weeks postoperatively and prednisolone acetate 1% 4 times daily Corticosteroid: hypromellose/dextran 70 as a placebo 4 times daily for 3 days before surgery plus prednisolone acetate 1% 4 times daily Other medications: all participants were also treated with gatifloxacin ophthalmic solution 0.3% 4 times daily for 3 days preoperatively and 4 times daily for 7 days postoperatively Length of follow‐up: Planned: 5 weeks Actual: 5 weeks | |
| Outcomes | Primary outcome, as defined in study reports: incidence of angiographic CME 5 weeks after surgery Secondary outcomes, as defined in study reports: mean change in BCVA, clinical CME incidence, IOP, and retinal thickness measured using OCT Adverse events reported: yes Intervals at which outcomes assessed: 1 day and 5 weeks postoperatively | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “No competing financial interests exist.” Study period: February 2011 to March 2012 Reported subgroup analyses: no Trial registry #: NTC01542190 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was performed in a simple manner. Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. When a patient was included in the study, a pharmacist provided the patient with a small individual envelope, and after viewing the random number, the patient took the respective eye drop bottle” |
| Allocation concealment (selection bias) | Low risk | “The randomization was performed in a simple manner. Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. When a patient was included in the study, a pharmacist provided the patient with a small individual envelope, and after viewing the random number, the patient took the respective eye drop bottle” |
| Masking of participants and personnel (performance bias) | Low risk | “The surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients.” “All study participants were blinded to their treatment assignment” |
| Masking of outcome assessment (detection bias) | Low risk | “The surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients.” |
| Incomplete outcome data (attrition bias) | High risk | 5 participants in the ketorolac plus prednisolone group (1 due to adverse event; 1 due to intraoperative complication; 1 refused to undergo fluorescein angiography; 2 protocol violations) and 5 participants in the prednisolone alone group (1 intraoperative complication; 4 protocol violations) did not complete the study and were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Unclear risk | Funding sources were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 120 eyes of 102 participants Per group: NSAID: 40 eyes, corticosteroid 1: 39 eyes, corticosteroid 2: 41 eyes Exclusions after randomization: corticosteroid 1: 2 eyes, corticosteroid 2: 2 eyes Number analyzed (total and per group): Total: 116 Per group: NSAID: 40, corticosteroid 1: 37, corticosteroid 2: 39 Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: by day 28, corticosteroid group 1: 4, NSAID group: 3, corticosteroid group 2: 4 How were missing data handled?: excluded from the study Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Thailand Age: NSAID mean age = 61.8 ± 12.8; corticosteroid group 1 mean age = 63.3 ± 12.2; corticosteroid group 2 mean age = 63.2 ± 11.4 Gender (per cent): NSAID: 14 (35%) men and 26 (65%) women; corticosteroid group 1: 14 (37.8%) men and 23 (62.2%) women; corticosteroid group 2: 15 (42.9%) men and 20 (57.1%) women Inclusion criteria: eyes of non‐diabetic patients that had undergone phacoemulsification and intraocular lens implantation Exclusion criteria: glaucoma, hypersensitivity to the study drugs, using any corticosteroids or NSAIDs in the previous 2 months, previous intraocular surgery, and accompanying ocular disease or corneal lesions that interfered with intraocular examination Equivalence of baseline characteristics: yes, “From baseline data collect on post‐operative day 1 prior to the application of the drugs, there was no significant difference among groups in visual acuity, intraocular pressure, inflammation of conjunctiva and cornea, and number of cells and flare in the anterior chamber.” “There was no significant difference in demographic data among the 3 groups as shown in Table 1.” | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid vs corticosteroid NSAID: ketorolac tromethamine 0.5% 4 times a day for 28 days Corticosteroid 1: topical prednisolone acetate 1% 4 times a day for 28 days Corticosteroid 2: fluorometholone acetate 0.1% 4 times a day for 28 days Length of follow‐up: Planned: 28 days Actual: 28 days | |
| Outcomes | Primary outcome, as defined in study reports: BCVA, intraocular pressure, slit‐lamp biomicroscopy, grading of cells and flare in the anterior chamber, and ocular symptoms Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: weekly | |
| Notes | Type of study: published Funding sources: the study drugs were provided by Allergan (Thailand) and Alcon Laboratories (Thailand) Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study is reported as investigator‐masked but it is unclear whether participants were also masked to which study medication they received. |
| Masking of outcome assessment (detection bias) | Low risk | “Baseline and four successive weekly ocular examinations were performed by a single ophthalmologist who was masked to allocation.” |
| Incomplete outcome data (attrition bias) | High risk | Participants who were lost to follow‐up were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | A protocol is mentioned but not cited, so it is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Conflicts of interest were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: 50 Exclusions after randomization: 24 Number analyzed (total and per group): Total: 126 eyes of 126 participants Per group: ketorolac + corticosteroid group: 40, nepafenac + corticosteroid group: 41, corticosteroid only: 40 Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: 8 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Brazil Age: mean age 65.36 ± 7.3 years Gender (per cent): 44% men, 56% women Inclusion criteria: older than 40 years; age‐related cataract; a normal ophthalmological examination besides senile cataract Exclusion criteria: previous ocular surgery; central endothelial cell count < 2000 cells/mm2; glaucoma or IOP > 21 mm Hg; amblyopia; retinal abnormalities; steroid or immunosuppressive treatment, connective tissue diseases, or an allergy or hypersensitivity to NSAIDs; complicated cataract surgery (e.g. posterior capsule rupture, vitreous loss, or an IOL not placed in the capsular bag) Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac tromethamine 0.4% 4 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 1% 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week NSAID 2 plus corticosteroid: nepafenac 0.1% 3 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Corticosteroid: artificial tear substitute 4 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Other medication: all participants received moxifloxacin 0.5% 4 times a day starting 2 days before surgery and for 7 days after surgery Length of follow‐up: Planned: 12 weeks Actual: 12 weeks | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity and refraction; central corneal thickness and endothelial cell density; preoperative and postoperative thickness, values of the retinal layers; the percentage of participants in whom the postoperative checkup revealed a change in central subfields retinal thickness; the percentage of participants to be diagnosed with macular edema Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1, 4, 12 weeks | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: the authors report no competing interest Study period: recruitment between 4 June 2013 and 7 October 2013 Reported subgroup analyses: no Trial registry #: NCT02084576 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned in a 1:1:1 ratio to one of three treatment groups using a computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | "All investigators were masked with regard to treatment group." |
| Masking of outcome assessment (detection bias) | Low risk | "All investigators were masked with regard to treatment group." |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear if there was attrition bias. The authors state that 8 participants were lost to follow‐up and 8 were discontinued due to protocol violations and that their data were not included in the results. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: RCT Number randomized (total and per group): Total: 100 eyes Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: 98 eyes of 98 participants Per group: not reported Unit of analysis: eyes Losses to follow‐up: 2 eyes How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: not reported Age: mean = 76.0 ± 6.4 years (range 50 to 81) Gender (per cent): 40 men (40.8%), 48 women (49.0%) Inclusion criteria: people scheduled for clear corneal phacoemulsification cataract surgery Exclusion criteria: not reported Equivalence of baseline characteristics: not reported | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% Corticosteroid: prednisolone acetate 1.0% Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcome, as defined in study reports: postoperative discomfort, redness, BCVA, signs of inflammation, and intraocular pressure Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: not reported | |
| Notes | Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 participants did not complete the study, but the reasons were not described. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and declarations of interest were not reported. |
| Methods | Study design: RCT Number randomized (total and per group): Total: 240 participants Per group: 60 Exclusions after randomization: none Number analyzed (total and per group): Total: 167 participants Per group: group 1: 40, group 2: 43, group 3: 43, group 4: 41 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: yes, only 167 (70%) completed the study How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: China Age: mean age = 73.4 ± 9.2 (range 46 to 92) Gender (per cent): 46.3% men and 53.8% women Inclusion criteria: people undergoing phacoemulsification with posterior chamber IOL Exclusion criteria: (1) people who were suffering from any ocular diseases that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy, or amblyopia; (2) people who were suffering from any systemic diseases that might affect treatment responses or evaluations, such as diabetes mellitus; (3) potentially pregnant women; (4) people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery and people with contraindication to oral steroids, such as those with peptic ulcer, cancer, or tuberculosis; (5) people who had surgical complications, such as posterior capsule rupture or hyphema, and (6) people with particular diseases that might affect surgery in the eyes, such as limitation of pupil dilation Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID 1 (group 1): ophthalmic bromfenac sodium 0.1% twice per day for 1 month postoperatively NSAID 2 (group 2): ophthalmic bromfenac sodium 0.1% twice per day for 2 months postoperatively Corticosteroid 1 (group 3): ophthalmic fluorometholone 0.1% 3 times a day for 1 month postoperatively Corticosteroid 2 (group 4): ophthalmic dexamethasone 0.1% 3 times a day for 1 month postoperatively Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Primary outcome, as defined in study reports: BCVA Secondary outcomes, as defined in study reports: IOP, endothelial cell density, photon count value, and retinal foveal thickness Adverse events reported: yes (reported as no drug‐related adverse events) Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months | |
| Notes | Type of study: published Funding sources: this study was supported by grants from Zhejiang Key Innovation Team Project of China (grant no. 2009R50039) and Zhejiang Key Laboratory Fund of China (no. 2011E10006) Disclosures of interest: not reported Study period: October 2010 to December 2011 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | “The drugs were applied topically to the assigned patients open‐labeled. The same physician served as the medical monitor and assigned one of the drugs to each patient." |
| Masking of outcome assessment (detection bias) | Unclear risk | “The drugs were applied topically to the assigned patients open‐labeled. The same physician served as the medical monitor and assigned one of the drugs to each patient. “ |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: RCT Number randomized (total and per group): Total: 578 participants Per group: not reported Exclusions after randomization: 32 participants did not undergo surgery: 18 decided not to participate in the study, 5 were lost to follow‐up, 5 were discontinued because of protocol violations, and 4 could not participate because of insurance issues Number analyzed (total and per group): Total: 546 participants Per group: NSAID + corticosteroid: 268, corticosteroid: 278 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: 5 were lost to follow‐up prior to surgery, 11 were discontinued due to adverse events How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: United States Age: mean age = 70 Gender (per cent): 291 (53.3%) women and 255 (46.7%) men Inclusion criteria: as determined by the individual investigator, each eligible patient had 20/20 BCVA potential without any evidence of macular abnormality, including age‐related macular changes, epiretinal membranes, or other retinal‐vascular anomalies. People with systemic diseases were eligible only if there were no ocular manifestations of the disease (e.g. diabetic patients with normal retinal exams) Exclusion criteria: people initially enrolled and randomized into the study were subsequently disqualified if vitreous loss or capsular disruption/rupture occurred during surgery. Participants could also be exited from the study if, on postoperative day 1, the surgeon felt the amount of inflammation was greater than expected and, in his best clinical judgement, more aggressive anti‐inflammatory treatment was indicated Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: ketorolac 0.4% plus prednisolone 1% Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: 4 weeks Actual: 4 weeks; “Patients could also return for an additional visit between days 43 and 50 (week 6) at the discretion of the investigator” | |
| Outcomes | Primary outcome, as defined in study reports: incidence of CME Secondary outcomes, as defined in study reports: these included an assessment of retinal (foveal) thickness (based on OCT measurements), Snellen BCVA, and contrast sensitivity Adverse events reported: yes Intervals at which outcomes assessed: day 1, between days 8 and 12 (week 1), and between days 26 and 34 (week 4) | |
| Notes | Type of study: published Funding sources: this study was supported by an unrestricted educational grant from Allergan Inc., Irvine, CA Disclosures of interest: the authors indicate no financial conflict of interest. Drs Wittpenn, Silverstein, and Heier are consultants for Allergan Inc. Dr Heier is also a consultant for Ista Pharmaceuticals. Study period: 5 June 2005 to 10 August 2006 Reported subgroup analyses: no Trial Registry #: NCT00348244 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A central coordination center (IMEDS Inc, Riverside, California, USA; [M.E.]) generated the allocation sequence, enrolled participants, and assigned participants to their treatment groups.“ |
| Allocation concealment (selection bias) | Low risk | See above |
| Masking of participants and personnel (performance bias) | Low risk | “All investigators were masked with regard to treatment group. The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles.“ |
| Masking of outcome assessment (detection bias) | Unclear risk | “All investigators were masked with regard to treatment group. The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles.“ |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Unrestricted educational grant from Allergan Inc. The authors declare no financial conflict of interest. The authors are also consultants for Allergan. |
| Methods | Study design: RCT Number randomized (total and per group): Total: 179 eyes of 179 participants Per group: not reported Exclusions after randomization: 10 Number analyzed (total and per group): Total: 179 participants Per group: group 1: 61, group 2: 60, group 3: 58 Unit of analysis: participants Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Turkey Age: group 1 mean age = 65.28 ± 9.90, group 2 mean age = 62.25 ± 11.57, group 3 mean age = 64.78 ± 9.18 Gender (per cent): group 1: 54.1% men and 45.9% women; group 2: 60.0% men and 40.0% women; group 3: 63.8% men and 36.2% women Inclusion criteria: not reported Exclusion criteria: people with a history of intraocular surgery; any complication during cataract surgery; glaucoma; uveitis; vitreoretinal pathology; history of diabetes mellitus, hypertension, or cardiac disease; or topical or systemic drug use were excluded from the study Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid 1 (group 1): indomethacin 0.1% 4 times daily for 3 days preoperatively and 1 month postoperatively plus topical prednisolone acetate 1% 4 times daily for 1 month NSAID plus corticosteroid 2 (group 2): indomethacin 0.1% 4 times daily for 1 month postoperatively plus topical prednisolone acetate 1% 4 times daily for 1 month Corticosteroid (group 3): prednisolone acetate 1% 4 times daily for 1 month Other medications: all participants also received the antibiotic ofloxacin 0.3% 4 times daily for 1 week Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: BCVA, angiographic CME Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 3 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Low risk | Fluorescein leakage to diagnose angiographic CME was evaluated by a masked observer. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear whether there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 160 eyes of 160 participants Per group: 80 in each group Exclusions after randomization: 5 in the nepafenac group (1 cortical remnants in the anterior chamber on the postoperative day; 1 loose zonular fibers intraoperatively requiring a placement of a capsule tension ring; 1 improper dosing of study medication; 1 use of non‐allowed concomitant medicine; and 1 appearance of general health problems judged to be unrelated to the study treatments); 2 in the control group (participant decision). “Eyes with intraoperative difficulties such as loose zonular fibers, extended operating time, or residual cortical material or with complications such as posterior capsule rupture and vitreous loss were excluded from the study after randomization.” Number analyzed (total and per group): Total: 152 eyes of 152 participants Per group: NSAID plus corticosteroid: 75, corticosteroid alone: 77 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 in the control group How were missing data handled?: 8 were not included in the ITT analysis Reported power calculation: yes, 80% Unusual study design?: some exclusions after randomization based on operation difficulties | |
| Participants | Country: Sweden Age: NSAID plus corticosteroid mean age = 70.4 ± 7.4 (range 51.0 to 84.0), corticosteroid alone mean age = 68.3 ± 7.5 (range 51.0 to 83.0) Gender (per cent): NSAID plus corticosteroid: 27 males (36.0%) and 48 females (64.0%), corticosteroid alone: 27 males (35.1%) and 50 females (64.9%) Inclusion criteria: people between the ages of 45 and 85 years scheduled for cataract surgery under local anesthesia Exclusion criteria: people with small pupils (< 5.0 mm after pharmacologic dilation), dark brown irides, exfoliation syndrome, history of uveitis, glaucoma, macular degeneration, or any vision‐impairing eye disorder except cataract, diabetic patients, pregnant women, and people using any topical or systemic anti‐inflammatory treatment or with hypersensitivity to any of the given study treatments Equivalence of baseline characteristics: yes, there were no statistically significant differences in these parameters between the 2 groups | |
| Interventions | Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac ophthalmic suspension 0.1% starting 2 days before surgery and given 30 minutes before surgery 3 times 5 minutes apart and dexamethasone 0.1% 3 times a day for 3 weeks after surgery Corticosteroid: dexamethasone 0.1% 3 times a day for 3 weeks after surgery and a placebo (dextran 70‐hypromellose (Tears Naturale II Polyquad)) starting 2 days before surgery and given 30 minutes before surgery 3 times 5 minutes apart Length of follow‐up: Planned: 6 weeks Actual: 6 weeks | |
| Outcomes | Primary outcome, as defined in study reports: change in total macular volume 6 weeks after cataract surgery compared with baseline, proportion of participants with macular swelling of at least 10 mm 6 weeks postoperatively compared with baseline Secondary outcomes, as defined in study reports: change in total macular volume 3 weeks postoperatively compared with baseline; proportion of participants with macular swelling of at least 10 mm 3 weeks postoperatively compared with baseline; visual acuity at 3 and 6 weeks; anterior segment inflammation measured with a laser flare meter at 1 day, 3 and 6 weeks; ocular pain sensation during surgery and 24 hours and 3 weeks postoperatively; ocular discomfort and photophobia 1 day postoperatively and during the 3‐week treatment course; and intraoperative pupil dilation measured with a caliper Adverse events reported: yes Intervals at which outcomes assessed: preoperative examination within 1 week of surgery and 1 day, 3 and 6 weeks postoperatively | |
| Notes | Type of study: published Funding sources: “Supported by Alcon Research Ltd, Fort Worth, Texas, USA, and S.A. Alcon‐Couvreur N.V., Puurs, Belgium, which produced and provided the masked eye drop bottles. Partially supported by Alcon, Inc., Sweden. Financial support was also provided through the regional agreement on Medical training and Clinical research (ALF) between Stockholm County Council and Karolinska Institutet (20120623). The statistical analyses were supported by G. Andersson Foundation.” Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | "double‐masked” study; “Nepafenac and placebo suspensions were supplied in identical bottles labeled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not explicitly reported. |
| Incomplete outcome data (attrition bias) | High risk | An ITT analysis was used, but 8 participants were not included. |
| Selective reporting (reporting bias) | Unclear risk | This trial was registered at the European Clinical Trials Database, but the record could not be retrieved. |
| Other bias | High risk | The study was supported by Alcon Research Ltd., which manufactures the NSAID used. Alcon Research Ltd provided the masked eyedrop bottles |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 140 participants Per group: 35 per group Exclusions after randomization: none Number analyzed (total and per group): Total: 140 participants Per group: 35 per group Unit of analysis: eye Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: yes, 80% Unusual study design?: none | |
| Participants | Country: Brazil Age: ketorolac 0.4% mean age = 66 ± 8; nepafenac 0.1% mean age = 66 ± 7; prednisolone 1% mean age = 67 ± 8; placebo mean age = 67 ± 6 Gender (per cent): ketorolac 0.4%: 15 male (42.9%) and 20 female (57.1%); nepafenac 0.1%: 14 male (40%) and 21 female (60%); prednisolone 1%: 13 male (37.1%) and 22 female (62.9%); placebo: 12 male (34.3%) and 23 female (65.7%) Inclusion criteria: people with nuclear cataract density of 2 and 3 by LOCS II (> 50 years old), with indication for cataract surgery with intraocular lens implant, under local anesthesia Exclusion criteria: diabetes, hypertension, people using NSAID, alpha‐blocker, topical eye drops (including antiglaucoma drugs), history of uveitis, macular disease, pseudoexfoliation syndrome, congenital ocular abnormalities, cataract density of 1 and 4 by LOCS II, and previous intraocular surgery Equivalence of baseline characteristics: yes, “Baseline demographic and clinical characteristics were similar in all groups. There were no differences regarding ages (P = 0.930), neither in age‐related cataract density (P = 0.852), nor in gender distribution (P = 0.896), ultrasound time (P = 0.986) and surgical time (P = 0.666)” | |
| Interventions | Comparison: NSAID vs NSAID vs corticosteroid vs placebo NSAID 1: ketorolac tromethamine 0.4% 3 times daily for 2 days prior to surgery NSAID 2: nepafenac 0.1% 3 times daily for 2 days prior to surgery Corticosteroid: prednisolone acetate 1% 3 times daily for 2 days prior to surgery Placebo: carboxymethylcellulose sodium 0.5% 3 times daily for 2 days prior to surgery Other medications: gatifloxacin 4 times daily for 2 days prior to surgery Length of follow‐up: Planned: N/A Actual: N/A | |
| Outcomes | Primary outcome, as defined in study reports: number of participants with pupil ≥ 6 mm (vertical and horizontal diameters) at the end of the surgery Secondary outcomes, as defined in study reports: number of participants with pupil ≥ 6 mm (vertical and horizontal diameters) at the beginning of the surgery (prior to the corneal section) Adverse events reported: yes Intervals at which outcomes assessed: before and after surgery | |
| Notes | Type of study: published Funding sources: none Disclosures of interest: “None declared” Study period: eligible participants were recruited from March 2009 to March 2010 Reported subgroup analyses: no Trial Registry #: NCT00865540, Conep ‐ National Research Ethics Committee – Brazil – 0816.0.146.000‐10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was done in a blocking manner. Each of the four intervention groups received 35 different numbers from a random number table.” |
| Allocation concealment (selection bias) | Low risk | “These numbers were transferred to small individual envelopes and also fixed at one of the opaque eye drop bottles. It helped not only to randomize the patients but also to mask the treatment groups until data analysis. Four small envelopes, one of each intervention group, were sealed and placed into a larger envelope, totalizing 35 large envelopes containing four small individual envelopes in each one. This comprises the block length of four to assure that every four patients have received all four interventions. When a patient was included in the study, a pharmacist took for him a small individual envelope and after discovering the random number, she took the respective eye drop bottle. The surgeon and the ophthalmologist who collected the data did not know the randomized groups.” |
| Masking of participants and personnel (performance bias) | Low risk | “These eye drops were administered 48 hours before surgery by mask fashion, three times daily for two days prior to surgery”; “The surgeon and the ophthalmologist who collected the data did not know the randomized groups.” “The eye drop group was revealed to the researchers once recruitment, data collection, and statistical analyses were complete. All study participants were masked to treatment assignment.” |
| Masking of outcome assessment (detection bias) | Low risk | “The surgeon and the ophthalmologist who collected the data did not know the randomized groups." “The eye drop group was revealed to the researchers once recruitment, data collection, and statistical analyses were complete.” |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up and there were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 220 eyes of 220 participants Per group: 110 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 220 eyes of 220 participants Per group: 110 Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: China Age: mean age = 71 (range 55 to 87) Gender (per cent): 98 (44.5%) men and 122 (55.5%) women Inclusion criteria: cataract patient having uneventful phacoemulsification and IOL implantation Exclusion criteria: younger than 18 years of age; used steroid hormone or NSAID within 1 week; severe cornea dysfunction; severe cardiovascular, lung, liver, kidney failure; diabetes patients; glaucoma patients; pregnant or breastfeeding women Equivalence of baseline characteristics: yes | |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: pranoprofen plus dexamethasone Corticosteroid: dexamethasone Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: the proportion of participants with intraocular inflammation Secondary outcomes, as defined in study reports: the proportion of participants with CME Adverse events reported: yes Intervals at which outcomes assessed: 1, 2 weeks, 1, 3 months after surgery | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: 2007 to 2008 Reported subgroup analyses: no Trial registry #: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were reported for all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
BCVA: best‐corrected visual acuity
CCT: central corneal thickness
CME: cystoid macular edema
COMTOL: Comparison of Ophthalmic Medications for Tolerability Questionnaire
ECC: endothelial cell count
ECCE: extracapsular cataract extraction
ETDRS: Early Treatment Diabetic Retinopathy Study
FT: foveal thickness
HRQOL: health‐related quality of life
IOL: intraocular lens
IOP: intraocular pressure
ITT: intention‐to‐treat
LFCM: laser flare‐cell meter
LOCS II: Lens Opacities Classification System, version II
N/A: not applicable
Nd:YAG: neodymium‐doped yttrium aluminium garnet
NSAID: non‐steroidal anti‐inflammatory drug
OCT: optical coherence tomography
PCR: posterior capsule rupture
RCT: randomized controlled trial
SD: standard deviation
TMV: total macular volume
UCVA: uncorrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Used extracapsular cataract extraction rather than phacoemulsification; oral NSAID and corticosteroid were used rather than topical drops | |
| Study not intended to assess vision outcomes | |
| Some participants received extracapsular cataract extraction and results were not separated | |
| Letter to the editor | |
| No NSAID treatment arm, used intracapsular cataract extraction | |
| Only one treatment group; no comparison | |
| Corticosteroids were not used | |
| No NSAID treatment arm; used extracapsular cataract extraction for some participants without separating out results | |
| Compared NSAID plus corticosteroid with placebo | |
| Unclear which type of cataract extraction surgery was used | |
| Multiple types of cataract surgery, results are not divided | |
| Type of cataract surgery is unclear | |
| Used extracapsular cataract extraction rather than phacoemulsification | |
| Unclear if groups are randomized | |
| Corticosteroids were not used | |
| Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest | |
| Used extracapsular cataract extraction | |
| Does not specify type of cataract surgery | |
| Unclear whether the study is randomized | |
| Interventions do not fit criteria for review | |
| Participants had cataract, glaucoma, and strabismus surgery | |
| Unclear which type of surgery | |
| Corticosteroids were not used | |
| Multiple surgical techniques employed, outcomes are aggregated | |
| Unclear whether the study is randomized | |
| Unclear whether the study is randomized | |
| Not a clinical trial | |
| Both study arms received NSAID plus corticosteroid | |
| Comparisons unclear; trial record does not provide detail | |
| Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest | |
| Dosage study, both groups received a corticosteroid and a different dose of an NSAID | |
| Study compares 2 corticosteroids, both study arms received NSAID plus corticosteroid | |
| Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest | |
| Participants underwent extracapsular cataract extraction | |
| Could not confirm whether trial was randomized | |
| Corticosteroids were not used | |
| Multiple types of surgeries, results are not divided | |
| Study compares 2 NSAIDs, both study arms received NSAID plus corticosteroid | |
| Letter to the editor | |
| Not topical corticosteroids, surgery method unclear | |
| Participants underwent extracapsular cataract extraction | |
| Parrticipants underwent extracapsular cataract extraction | |
| Participants underwent extracapsular cataract extraction | |
| Participants underwent extracapsular cataract extraction | |
| Type of cataract surgery is not specified | |
| Participants underwent extracapsular cataract extraction | |
| Quasi‐randomized trial | |
| Participants were not randomized | |
| Multiple types of surgeries, phacoemulsification plus vitrectomy | |
| Type of cataract surgery is not specified |
NSAID: non‐steroidal anti‐inflammatory drug
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: parallel‐group RCT Number enrolled: 240 |
| Participants | Country: China Condition: ocular inflammation and CME after phacoemulsification Gender: both Age: minimum 46, maximum 92 Inclusion criteria: people with age‐related cataract undergoing phacoemulsification with posterior chamber intraocular lens implantation Exclusion criteria: people with any ocular disease that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy, or amblyopia; people with any systemic disease that might affect treatment responses or evaluations, such as diabetes mellitus; potentially pregnant women; people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID: bromfenac sodium Corticosteroid 1: fluorometholone Corticosteroid 2: dexamethasone 0.1% Length of follow‐up: Planned: not reported |
| Outcomes | Outcomes: photon count value, retinal foveal thickness, intraocular pressure, BCVA, endothelial cell density Intervals at which outcomes assessed: not reported |
| Notes | Funding source: Zhejiang Key Innovation Team Project of China (grant no. 2009R50039), Zhejiang Key Laboratory Fund of China (no. 2011E10006) Study period: not reported Trial registry #: ChiCTR‐TRC‐12002600 |
| Methods | Study design: parallel‐group RCT Number enrolled: 62 |
| Participants | Country: Italy Condition: cataract, pseudoexfoliation syndrome Gender: both Age: 60 years and older Inclusion criteria: cataract, pseudoexfoliation syndrome Exclusion criteria: history of ocular inflammation or trauma, previous intraocular surgery, corneal haze, retinal vascular disease, diabetic retinopathy, variation of the foveal profile at OCT (macular edema, epiretinal membrane), moderate to severe age‐related macular degeneration |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: bromfenac 0.09% twice a day for 2 weeks starting the day after surgery plus dexamethasone 0.1% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Corticosteroid: dexamethasone 0.1% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Other medications: all participants also received the antibiotic tobramycin 0.3% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Length of follow‐up: Planned: 4 weeks |
| Outcomes | Outcomes: change from baseline in anterior chamber inflammation measured by laser flare photometry, proportion of participants with central macular thickness greater than 300 μm, proportion of participants with BCVA equal to 20/20, proportion of participants with no ocular pain Intervals at which outcomes assessed: 1, 3 days, 1, 4 weeks |
| Notes | Study period: November 2013 to October 2014 Trial registry #: NCT02137161 and EUCTR2013‐002066‐39‐IT |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Czech. Awaiting translation. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Chinese. Awaiting translation. |
| Methods | Study design: parallel‐group RCT Number enrolled: 200 |
| Participants | Country: India Condition: senile cataract Gender: both Age: 40 years and older Inclusion criteria: "indication for cataract extraction with implantation of the intraocular lens and uncomplicated cataract surgery" Exclusion criteria: "topical/inhaled/systemic steroid 14 days before surgery, topical/inhaled/systemic NSAIDs within 7 days of surgery, eyes with cells and flare and ocular pain in preoperative baseline examination, corneal opacity, suspected hypersensitivity to NSAIDs, chronic or recurrent inflammatory eye disease, diabetic patient, severe dry eye patient, cases in which there is intraoperative complications, glaucoma patient, patient having macular pathology" |
| Interventions | Comparison analyzed: NSAIDs vs corticosteroid NSAIDs (3 groups): nepafenac 0.1%, bromfenac 0.09%, and ketorolac 0.5% Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: one month |
| Outcomes | Outcomes: "postoperative inflammation was evaluated subjectively by intraocular pressure, slit‐lamp assessment of signs of inflammation, including conjunctival hyperemia, ocular pain, and aqueous cells and flare." Intervals at which outcomes assessed: baseline, 1 week, 2 weeks, and one month |
| Notes | Funding source: reported "nil" Study period: not reported Trial registry #: none reported |
| Methods | Study design: parallel‐group RCT Number enrolled: 40 |
| Participants | Country: United States Condition: not reported Gender: both Age: 18 years and older Inclusion criteria: person must have a visually significant age‐related cataract, in the planned operated eye, 18 years of age or older, the vision in the fellow, unoperated eye should have a potential visual acuity of 20/40 or better as determined by the principal investigator, person must desire cataract extraction, be willing and able to comply with scheduled visits and other study procedures Exclusion criteria: advanced glaucomatous damage, any abnormality preventing reliable applanation tonometry in operated eye, contact lens use during the active treatment portion of the trial in the operated eye, any concurrent infectious/non‐infectious conjunctivitis, keratitis or uveitis in either eye, any history of allergic hypersensitivity or poor tolerance to any component of the preparations used in this trial, pregnant or nursing mothers and females of childbearing potential not practicing a reliable and medically acceptable method of birth control, any clinically significant, serious, or severe medical or psychiatric condition, participation (or current participation) in any investigational drug or device trial within the previous 30 days prior to the start date of this trial, intraocular conventional surgery within the past 3 months or intraocular laser surgery within 1 month in the operated eye, required use of other topical medications during the active portion of the trial except prophylactic antibiotic, topical lid care, tear replacement solutions, or glaucoma medications, other ocular surgery at the time of the cataract extraction, use of topical or oral antiprostaglandins or corticosteroids as well as aspirin products (> 81 mg) during the active treatment portion of the trial. If patient wants to participate in the trial and can stop the medication, he/she can be enrolled after 7‐day washout period. |
| Interventions | Comparison analyzed: NSAID vs corticosteroid NSAID + corticosteroid: ketorolac tromethamine 0.4% (Acular) Corticosteroid: loteprednol etabonate ophthalmic suspension 0.5% (Lotemax) Length of follow‐up: Planned: not reported |
| Outcomes | Outcomes: not reported Intervals at which outcomes assessed: not reported |
| Notes | Funding source: not reported Study period: February 2006 to September 2007 Trial registry #: NCT00366691 |
| Methods | Study design: parallel‐group RCT Number enrolled: 97 |
| Participants | Country: Greece Condition: cataracts Gender: both Age: 55 to 95 years Inclusion criteria: phacoemulsification (due to cataract), uneventful phacoemulsification surgery Exclusion criteria: disruption of the anterior lens capsule, age‐related macular degeneration, proliferative diabetic retinopathy |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: ketorolac tromethamine 0.5% plus dexamethasone 0.1% Corticosteroid: dexamethasone Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 28 days |
| Outcomes | Outcomes: corneal edema, conjunctival redness, anterior chamber reaction Intervals at which outcomes assessed: 28, 42 days |
| Notes | Study period: January 2009 to April 2009 Trial registry #: NCT00992355 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Italian. Awaiting translation. |
BCVA: best‐corrected visual acuity
CME: cystoid macular edema
NSAID: non‐steroidal anti‐inflammatory drug
OCT: optical coherence tomography
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | PRevention of Macular EDema after cataract surgery (PREMED) |
| Methods | Study design: parallel‐group RCT Anticipated enrollment: 926 non‐diabetic and 209 diabetic patients |
| Participants | Country: Austria, Belgium, Germany, Hungary, Netherlands, Portugal, Spain Condition: cystoid macular edema, cataract, diabetes mellitus Inclusion criteria: all people undergoing routine phacoemulsification (1 eye per person), willing and/or able to comply with the scheduled visits and other study procedures, able to communicate properly and understand instructions, accepting possible off‐label use of intravitreal bevacizumab and/or subconjunctival preservative‐free triamcinolone acetonide Exclusion criteria: general exclusion criteria for participation in this study are: age below 21 years old, participation in another clinical study, post‐traumatic cataract, combined surgery, functional monoculus, previous ocular surgery, progressive glaucoma with severe visual field defects, use of antiglaucomatous medication or steroid‐induced IOP elevation requiring IOP‐lowering treatment, IOP ≥ 25 mm Hg, history of any intraocular inflammation or uveitis, history of pseudoexfoliation syndrome, which is expected to cause perioperative complications, history of Fuchs' endothelial dystrophy or cornea guttata 3+, history of retinal vein occlusion, any macular pathology that might influence visual acuity other than diabetic macular edema, use of intravitreal bevacizumab or ranibizumab in the previous 6 weeks or intravitreal aflibercept in the previous 10 weeks, use of intra‐ or periocular corticosteroid injection in the previous 4 months, current use of topical NSAIDs or corticosteroids, use of systemic corticosteroids (≥ 20 mg prednisolone or equivalence), history of relevant adverse events including serious adverse events occurring after administration of NSAIDs, acetylsalicylic acid, sodium sulphite, corticosteroids, or bevacizumab, contraindications for use of topical NSAIDs, topical or subconjunctival corticosteroids, or intravitreal bevacizumab or related drugs. Non‐diabetic patients with a history of CME will be excluded from participation in the study. Additionally, diabetic patients will be excluded from participation in case of: macular edema with a central subfield macular thickness ≥ 450 µm, very severe NPDR or proliferative DR requiring panretinal photocoagulation or vitrectomy, vitreous hemorrhage present during preoperative visit(s), cerebrovascular accident, myocardial infarction, or other thromboembolic events in the previous 3 months, a history of recurrent thromboembolic events, a history of severe systemic bleeding in the previous 3 months, major surgery in the previous 3 months, history of glaucoma |
| Interventions | Comparison analyzed: NSAID vs corticosteroid vs NSAID plus corticosteroid NSAID in non‐diabetics: bromfenac 0.9% Corticosteroid in non‐diabetics: dexamethasone 0.1% NSAID plus corticosteroid in non‐diabetics: bromfenac 0.9% plus dexamethasone 0.1% NSAID plus corticosteroid in diabetics: bromfenac 0.9% plus dexamethasone 0.1% NSAID plus 2 corticosteroids in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus triamcinolone acetonide NSAID plus corticosteroid plus anti‐VEGF in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus bevacizumab NSAID plus 2 corticosteroids plus anti‐VEGF in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus triamcinolone acetonide plus bevacizumab Length of follow‐up: Planned: 12 weeks |
| Outcomes | Primary outcome, as defined in study reports: change in central subfield mean macular thickness as a measurement of efficacy; "The primary endpoint is the change in central subfield mean macular thickness in the 1 mm area (central subfield macular thickness, CSMT) as compared to baseline within the first 6 weeks postoperatively" Secondary outcomes, as defined in study reports: number of participants who develop clinically significant macular edema as a measurement of efficacy; "The secondary endpoint is the occurrence of postoperative clinically significant macular edema (CSME) within 12 weeks postoperatively". Other secondary outcome measures: change in corrected distance visual acuity as a measurement of efficacy, change in retinal thickness in the central inner circle (3 mm) as a measurement of efficacy, intraocular pressure as a measurement of safety, health‐related quality of life as a measurement of efficacy and tolerability, number of participants experiencing adverse events as a measurement of safety and tolerability, change in retinal thickness in the central outer circle (6 mm) as a measurement of efficacy, change in macular volume as a measurement of efficacy, vision‐related quality of life as a measurement of efficacy and tolerability, cost‐effectiveness Adverse events reported: yes Intervals at which outcomes assessed: 1, 2 weeks, 1, 3 months after surgery |
| Starting date | July 2013 |
| Contact information | Prof. Rudy MM Nuijts, MD, PhD, +31 43 387 1594, [email protected] Laura HP Wielders, MD, +31 43 387 1594, [email protected] |
| Notes | Study sponsor: Maastricht University Medical Center |
| Trial name or title | Nepafenac Once Daily for Macular Edema (Study 1 and 2) |
| Methods | Study design: parallel‐group RCT Number enrolled: 615 and 605 randomized in Study 1 and Study 2, respectively |
| Participants | Country: United States Condition: non‐proliferative diabetic retinopathy Gender: both Age: 18 years and older Inclusion criteria: planned cataract extraction by phacoemulsification with implantation of a posterior chamber intraocular lens, history of type 1 or 2 diabetes and non‐proliferative diabetic retinopathy (mild, moderate, or severe) in the study eye, BCVA of 73 letters or worse in the study eye with expectation of improvement after surgery, understand and sign an informed consent document, other protocol‐defined inclusion criteria may apply Exclusion criteria: pre‐existing macular edema in the study eye, history in the study eye of retinal detachment, wet age‐related macular degeneration, chronic or recurrent inflammatory eye disease, or prior procedures, planned cataract surgery in the fellow eye after randomization and prior to the Day 90 postoperative study visit or through study exit, planned multiple procedures for the study eye during the cataract/intraocular lens implantation surgery, use of exclusionary medications including NSAIDs and steroids as specified in protocol, participation in any other clinical study within 30 days of the screening visit, females of childbearing potential who are breastfeeding, have a positive urine pregnancy test at screening, are not willing to undergo a urine pregnancy test upon entering or exiting the study, intend to become pregnant during the study, or do not agree to use adequate birth control methods for the duration of the study, other protocol‐defined exclusion criteria may apply |
| Interventions | Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: nepafenac 0.3% 1 drop 1 day prior to surgery through 90 days following surgery plus prednisolone acetate Corticosteroid: prednisolone acetate Length of follow‐up: Planned: 90 days |
| Outcomes | Outcomes: proportion of participants with BCVA improvement of ≥ 15 letters from preoperative baseline, proportion of participants who develop macular edema Intervals at which outcomes assessed: not reported |
| Starting date | Study period: June 2013 to May 2015 Trial registry #: NCT01853072 and NCT01872611 |
| Contact information | Clinical Project Lead, Alcon Research |
| Notes | Study sponsor: Alcon Research |
anti‐VEGF: anti‐vascular endothelial growth factor
CME: cystoid macular edema
DR: diabetic retinopathy
IOP: intraocular pressure
NPDR: non‐proliferative diabetic retinopathy
NSAID: non‐steroidal anti‐inflammatory drug
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cell values at one week Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 NSAIDs versus corticosteroids, Outcome 1 Mean cell values at one week. | ||||
| 1.1 Measured with cell meter (cells per 0.075 mm3) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.19, 0.99] |
| 2 Mean flare values at one week Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 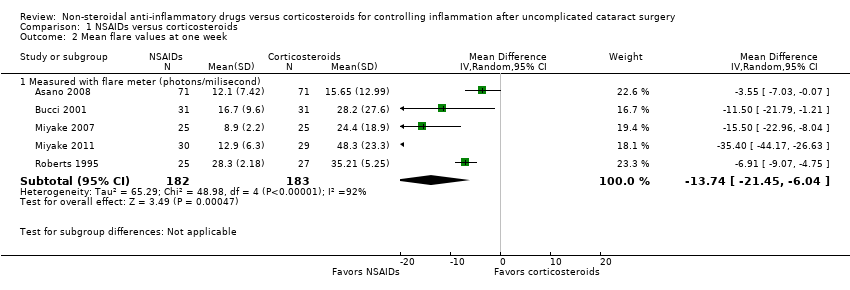 Comparison 1 NSAIDs versus corticosteroids, Outcome 2 Mean flare values at one week. | ||||
| 2.1 Measured with flare meter (photons/milisecond) | 5 | 365 | Mean Difference (IV, Random, 95% CI) | ‐13.74 [‐21.45, ‐6.04] |
| 3 Proportion of participants with cystoid macular edema one month postoperative Show forest plot | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.17, 0.41] |
| Analysis 1.3 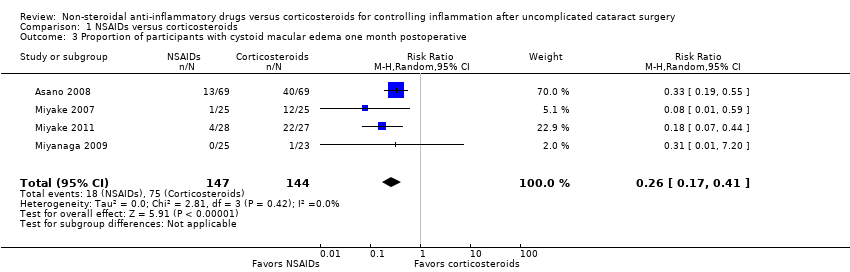 Comparison 1 NSAIDs versus corticosteroids, Outcome 3 Proportion of participants with cystoid macular edema one month postoperative. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with cystoid macular edema at one week Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.97] |
| Analysis 2.1  Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 1 Proportion of participants with cystoid macular edema at one week. | ||||
| 2 Proportion of participants with cystoid macular edema at one month Show forest plot | 7 | 1213 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.23, 1.06] |
| Analysis 2.2  Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 2 Proportion of participants with cystoid macular edema at one month. | ||||
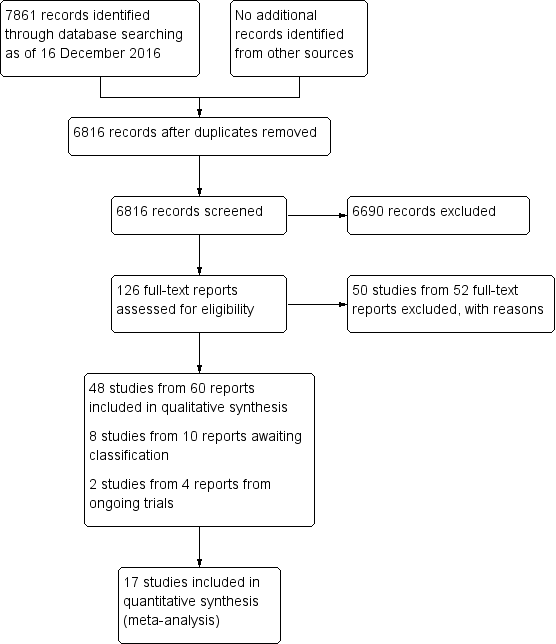
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 NSAIDs versus corticosteroids, outcome: 1.1 Mean cell values at one week.

Forest plot of comparison: 1 NSAIDs versus corticosteroids, outcome: 1.2 Mean flare values at one week.

Forest plot of comparison: 2 NSAIDs plus corticosteroids versus corticosteroids alone, outcome: 2.1 Proportion of participants with cystoid macular edema at one week.
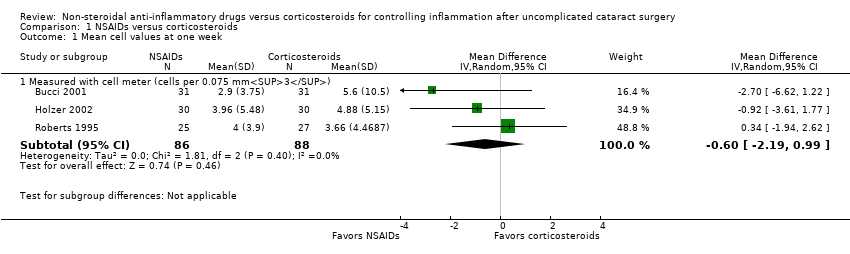
Comparison 1 NSAIDs versus corticosteroids, Outcome 1 Mean cell values at one week.

Comparison 1 NSAIDs versus corticosteroids, Outcome 2 Mean flare values at one week.
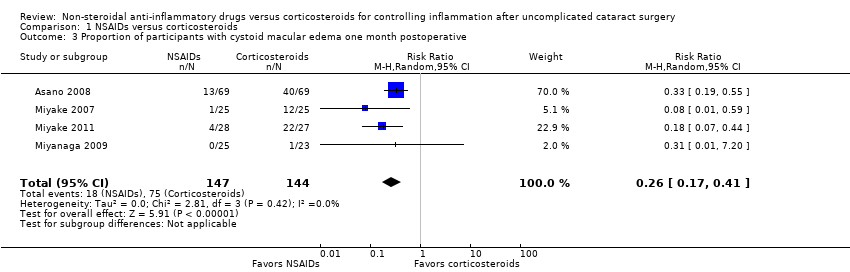
Comparison 1 NSAIDs versus corticosteroids, Outcome 3 Proportion of participants with cystoid macular edema one month postoperative.

Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 1 Proportion of participants with cystoid macular edema at one week.

Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 2 Proportion of participants with cystoid macular edema at one month.
| NSAIDs compared with corticosteroids for controlling inflammation after uncomplicated cataract surgery | ||||||
| Patient or population: people who received phacoemulsification Intervention: NSAID Comparison: corticosteroid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroid | NSAID | |||||
| Intraocular inflammation measured by anterior chamber cell and flare 1 week | Cell values | — | 174 (3 RCTs) | ⊕⊕⊕⊝1 | The cell values presented were measured using a Kowa cell meter. 2 additional studies measured cells using a slit lamp, but we were unable to combine these data as the studies used different scales to report the number of cells. | |
| The mean cell value ranged from 3.7 to 5.8. | The mean cell value was0.24 cells lower (1.65 lower to 1.16 higher). | |||||
| Flare values | — | 365 (5 RCTs) | ⊕⊕⊝⊝1,2 | The flare values presented were measured using a Kowa cell meter. There was high statistical heterogeneity among the included studies (I2 = 92%). | ||
| The mean flare ranged from 15.65 to 48.3 photons/ms. | The mean flare was 13.74 photons/ms lower (21.47 lower to 6 lower). | |||||
| Intraocular inflammation measured by proportion of participants with corneal edema 1 week | 133 per 1000 | 103 per 1000 (35 to 305) | RR 0.77 (0.26 to 2.29) | 114 (1 RCT) | ⊕⊕⊝⊝3,4 | |
| Proportion of participants with best‐corrected visual acuity of 20/40 1 week | None of the included studies reported on this outcome. | |||||
| Proportion of participants with cystoid macular edema 1 week | 521 per 1000 | 135 per 1000 (89 to 214) | RR 0.26 (0.17 to 0.41) | 291 | ⊕⊕⊝⊝1,5 | None of the included studies reported the proportion of participants with cystoid macular edema at 1 week, our intended outcome of interest. The data shown here are for 4 studies that reported on the presence of macular edema at 1 month. |
| Time to cessation of treatment | None of the included studies reported on this outcome. | |||||
| Adverse events | None of the included studies reported on this outcome. | |||||
| Economic outcomes | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence due to risk of bias: the studies included in this meta‐analysis were poorly reported. | ||||||
| NSAIDs plus corticosteroids compared with corticosteroids alone for controlling inflammation after uncomplicated cataract surgery | ||||||
| Patient or population: people who received phacoemulsification Intervention: NSAID plus corticosteroid Comparison: corticosteroid alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroid alone | NSAID plus corticosteroid | |||||
| Intraocular inflammation measured by anterior chamber cell and flare 1 week | None of the included studies reported on this outcome. | |||||
| Intraocular inflammation measured by proportion of participants with corneal edema 1 week | 912 per 1000 | 976 per 1000 (894 to 1000) | RR 1.07 (0.98 to 1.16) | 138 | ⊕⊕⊕⊝1 | |
| Proportion of participants with best‐corrected visual acuity of 20/40 1 week | None of the included studies reported on this outcome. | |||||
| Proportion of participants with cystoid macular edema 1 week | 47 per 1000 | 8 per 1000 | RR 0.17 (0.03 to 0.97) | 220 | ⊕⊕⊝⊝2,3 | 7 additional studies (including 1213 participants) reported on the presence of cystoid macular edema at 1 month postoperatively. The meta‐analysis showed that the group that received a combination of NSAID plus corticosteroid had a lower risk of macular edema at 1 month compared with the group that received a corticosteroid only, however there was uncertainty in the measurement (RR 0.50, 95% CI 0.23 to 1.06) |
| Time to cessation of treatment | None of the included studies reported on this outcome. | |||||
| Adverse events | See comment | — | — | — | Only 2 studies reported on adverse events. 1 reported that there were no adverse events related to NSAID use, but that 1 participant randomized to NSAIDs plus corticosteroid had heterogeneous retinal detachment as a complication of cataract surgery. Another study used the COMTOL questionnaire to ask participants about the frequency and severity of side effects; 3 of the top 5 most commonly reported side effects were markers of ocular discomfort: burning, redness, and blurred vision. The adverse events reported in this study were not separated by intervention group. | |
| Economic outcomes | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the certainty of the evidence due to high risk of reporting bias: the study reported collecting data for certain time points, but results were not reported. | ||||||
| Cells/Grade | Cells per field |
| 0 | Less than 1 |
| 0.5+ | 1 to 5 cells |
| 1+ | 6 to 15 cells |
| 2+ | 16 to 25 cells |
| 3+ | 26 to 50 cells |
| 4+ | More than 50 cells |
| Flare/Grade | Description |
| 0 | None to trace |
| 1+ | Faint |
| 2+ | Moderate (iris and lens details clear) |
| 3+ | Marked (iris and lens details hazy) |
| 4+ | Intense (fibrin or plastic aqueous) |
| Grade | Degree of ocular discomfort | Description |
| 0 | None | Absent |
| 1 | Mild | You experience ocular discomfort, but it does not interfere at all with your completion of daily tasks. |
| 2 | Moderate | You experience ocular discomfort and it slows you down, but you are able to carry out work of a light or sedentary nature (e.g. light house work, office work). |
| 3 | Severe | Your experience of ocular discomfort makes you completely unable to carry out any work activities. |
| Primary Outcomes | Secondary outcomes | |||||||||
| Study | Expected outcome: Proportion of participants with corneal edema >grade 0 | Expected outcome: Proportion of participants with anterior chamber cells and flare >grade 1 | Reported outcome: Cells and flare as a continuous measure | Expected outcome: Proportion of participants with BCVA of 20/40 or better | Reported outcome: Visual acuity as a continuous measure | Expected outcome: Proportion of participants with CME | Reported outcome: OCT outcomes or FFA outcomes measured continuously (macular thickness, total macular volume, macular cube volume, fluorescein leakage,etc) | Expected outcome: Time to cessation of treatment for inflammation | Expected outcome: Adverse events | Expected outcome: Economic outcomes (cost) |
| Studies comparing an NSAID to a corticosteroid | ||||||||||
| Yes | Yes | Yes | ||||||||
| Yes | ||||||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | |||||||||
| Data in figure only | Yes | Yes | ||||||||
| Yes | Yes | |||||||||
| Yes | Yes | |||||||||
| Partial | ||||||||||
| Yes | Yes | |||||||||
| Yes | Yes | Yes | Yes | |||||||
| Yes | Yes | Yes | Yes | |||||||
| Yes | ||||||||||
| Yes | Yes | |||||||||
| Yes | ||||||||||
| Yes | Yes | |||||||||
| Studies comparing an NSAID plus a corticosteroid to a corticosteroid alone | ||||||||||
| Yes | ||||||||||
| Data in figure only | Yes | |||||||||
| Yes | ||||||||||
| Yes | ||||||||||
| Yes | Yes | |||||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | |||||||||
| Yes | Yes | |||||||||
| Yes | Yes | Yes | ||||||||
| Partial | Yes | |||||||||
| Data in figure only | Yes | |||||||||
| Partial | Yes | |||||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | Yes | Yes | |||||||
| Data in figure only | Yes | Yes | Yes | Yes | ||||||
| Yes | Yes | |||||||||
| Studies with other combinations of NSAIDs and corticosteroids | ||||||||||
| Yes | ||||||||||
| Yes | Yes | Yes | Yes | |||||||
| Yes | ||||||||||
| Data in figure only | Yes | Yes | ||||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | Yes | Yes | |||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | Yes | ||||||||
| Data in figure only | ||||||||||
| Yes | Yes | Yes | Yes | |||||||
| Yes | Yes | Yes | ||||||||
| Yes | Yes | |||||||||
| Yes | ||||||||||
| Partial refers to studies missing data or not reporting all data needed to perform a meta‐analysis (such as reporting means without standard deviations) and studies reporting data at time points that were not of interest for this review. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cell values at one week Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Measured with cell meter (cells per 0.075 mm3) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.19, 0.99] |
| 2 Mean flare values at one week Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Measured with flare meter (photons/milisecond) | 5 | 365 | Mean Difference (IV, Random, 95% CI) | ‐13.74 [‐21.45, ‐6.04] |
| 3 Proportion of participants with cystoid macular edema one month postoperative Show forest plot | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.17, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with cystoid macular edema at one week Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.97] |
| 2 Proportion of participants with cystoid macular edema at one month Show forest plot | 7 | 1213 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.23, 1.06] |

