Comparaison de la revascularisation endovasculaire et d'une approche conservatrice pour le traitement de la claudication intermittente
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel 2‐arm RCT Number of sites: 1 Sample size estimation: not reported Follow‐up: 3, 6, 9, 12, 15 months and 6 years | |
| Participants | Country and setting: United Kingdom, Oxford Regional Vascular Service Inclusion criteria ‐ Stable unilateral claudication, with failure of conservative treatment for ≥ 3 months ‐ Treadmill claudicating distance < 375 meters ‐ Angiographically significant lesion(s) suitable for treatment by angioplasty, as agreed upon by both surgeon and radiologist Exclusion criteria: none Number of participants assessed and randomised: 36 participants fulfilled the entry criteria and were randomised Demographics ‐ Age (years): Group 1: mean 63.6 (SD 8.9); Group 2: mean 62.2 (SD 8.6) ‐ Gender (male): Group 1: 15 (75%); Group 2: 12 (75%) | |
| Interventions | Group 1: endovascular revascularisation without stenting, n = 20 (n = 30 at 6 years' follow‐up) Group 2: supervised exercise therapy for 6 months (2 sessions/week, 30 minutes/session), n = 16 (n = 26 at 6 years' follow‐up) Compliance with interventions ‐ Group 1: Two angioplasties were not successful ‐ Group 2: Mean attendance over 6 months of exercise therapy was 0.89 sessions/week Mortality ‐ Group 1: 4 participants after 6 years' follow‐up ‐ Group 2: 6 participants after 6 years' follow‐up Loss to follow‐up ‐ Group 1: 4 participants after 6 years' follow‐up ‐ Group 2: 5 participants after 6 years' follow‐up | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, procedure‐related complications | |
| Notes | Source of funding: Oxford District Research Committee Notes: New participants were added to the study after initial publication in 1990. Meta‐analysis for long‐term walking distances used numbers of participants at 6 years' follow‐up. Authors' conclusion: "In patients with mild or moderate claudication, who do not require an immediate therapeutic response, supervised exercise therapy may ultimately produce greater symptomatic improvement than PTA." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Simple randomisation" was performed according to trial authors; exact randomisation technique was not reported |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment process |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | High risk | Not analysed according to intention‐to‐treat principle; participants who had technically unsuccessful angioplasties were excluded from analysis; at 1‐year follow‐up, walking distance in only 5 participants (25%) in the revascularisation group and in 7 participants (44%) in the exercise group assessed; characteristics of withdrawals not adequately discussed; new participants added to study after 1 year of follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Unclear risk | Sample size estimation not adequately discussed; low number of participants in whom primary endpoint was assessed (study underpowered) |
| Methods | Study design: parallel 2‐arm RCT Number of sites: 10 Sample size estimation: 210 participants to detect 30% difference in maximum walking distance between the 2 treatment groups based on 90% power, type I error rate of 0.01, and anticipating 10% censoring Follow‐up: 1, 6, and 12 months | |
| Participants | Country and setting: Netherlands, university and non‐university hospitals Inclusion criteria ‐ Patients with stable intermittent claudication ‐ One or more vascular stenoses > 50% diameter reduction at the aortoiliac and/or femoropopliteal level established by non‐invasive vascular imaging ‐ Maximum walking distance between 100 and 500 meters as assessed on a graded treadmill Exclusion criteria ‐ Targeted lesion deemed unsuitable for revascularisation ‐ Prior treatment for the targeted lesion (including exercise therapy) ‐ Limited life expectancy ‐ Limited ambulation due to any other condition than intermittent claudication not allowing participant to follow treadmill training Number of participants assessed and randomised: 666 participants assessed, 212 participants randomised to 1 of the treatment groups Demographics ‐ Age (years): Group 1: mean 64 (SD 9); Group 2: mean 66 (SD 10) ‐ Gender (male): Group 1: 60 (57%); Group 2: 72 (68%) | |
| Interventions | Group 1: endovascular revascularisation with selective stenting plus supervised exercise therapy, n = 106 Group 2: supervised exercise therapy for 12 months (2 to 3 sessions/week 0 to 3 months, 1 session/week 3 to 6 months, 1 session/mo 6 to 12 months, 60 minutes/session), n = 106 Compliance with interventions ‐ Group 1: endovascular revascularisation technically successful in 102 (96%) participants, on average per participant 30 sessions exercise followed ‐ Group 2: on average per participant 43 sessions exercise followed Mortality ‐ Group 1: 1 participant ‐ Group 2: 3 participants Loss to follow‐up ‐ Group 1: 5 participants ‐ Group 2: 8 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, procedure‐related complications, SF‐36 Physical Functioning, SF‐36 Physical Role, SF‐36 Bodily Pain, SF‐36 General Health, VascuQol | |
| Notes | Source of funding: grant from Netherlands organization for health research and development Authors conclusion: "Among patients with intermittent claudication after 1 year of follow‐up, a combination therapy of endovascular revascularization followed by supervised exercise resulted in significantly greater improvement in walking distances and health‐related quality‐of‐life scores compared with supervised exercise only." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using Web‐based randomisation software based on minimisation method |
| Allocation concealment (selection bias) | Low risk | Central Web‐based allocation |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Low risk | Independent outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Analysis based on an intention‐to‐treat principle, censoring at 12 months low (6%) and comparable between groups |
| Selective reporting (reporting bias) | Low risk | All requested relevant outcome measures provided by study authors |
| Other bias | Low risk | No other forms of bias identified |
| Methods | Study design: parallel 2‐arm RCTs: (1) femoropopliteal disease trial; (2) aortoiliac disease trial Number of sites: 9 Sample size estimation: 170 participants in each trial based on 90% power and significance level of 0.05 to detect a difference of 60 metre improvement in absolute walking distance between groups Follow‐up: 6, 12, and 24 months | |
| Participants | Country and setting: United Kingdom, university and non‐university hospitals Inclusion criteria ‐ Positive outcome on the Edinburgh Claudication Questionnaire ‐ ABPI < 0.9 or > 0.9 with a positive stress test (fall of > 30 mmHg in Doppler blood pressure following a treadmill test at 4 km/h, 10 slope for 1 minute) ‐ Aortoiliac or femoropopliteal target lesion amenable to endovascular revascularisation as demonstrated by duplex mapping or diagnostic arteriography Exclusion criteria ‐ Symptoms too mild to consider angioplasty or so severe that intervention was mandatory ‐ Critical limb ischaemia (absolute Doppler blood pressure < 50 mmHg or presence of ulcers or gangrene with Doppler pressure > 50 mmHg) ‐ Concomitant disease such as musculoskeletal or cardiac that was prohibitive to exercise Number of participants assessed and randomised: 144 participants assessed, 127 participants randomised (93 participants in the femoropopliteal trial and 34 participants in the aortoiliac trial) Demographics Femoropopliteal disease trial ‐ Age (years): Group 1: 63.9 (SD: 9.0); Group 2: 68.5 (SD: 9.4) ‐ Gender (male): Group 1: 33 (69%); Group 2: 26 (58%) Aortoiliac disease trial ‐ Age (years): Group 1: mean 63.9 (SD 8.6); Group 2: mean 62.5 (SD 9.8) ‐ Gender (male): Group 1: 12 (62%); Group 2: 10 (67%) | |
| Interventions | Group 1: endovascular revascularisation with selective stenting plus supervised exercise therapy, n = 48 (femoropopliteal disease trial), and n = 19 (aortoiliac disease trial) Group 2: supervised exercise therapy for 6 months (≥ 1 session/week, 30 minutes/session), n = 45 (femoropopliteal disease trial), and n = 15 (aortoiliac disease trial) Compliance with interventions Femoropopliteal trial ‐ Group 1: in 11/44 participants, endovascular revascularisation recorded as failed, 62% attended available weekly exercise classes ‐ Group 2: 61% attended available weekly exercise classes Aortoiliac trial ‐ Group 1: in 2/19 participants, endovascular revascularisation recorded as failed, 53% attended available weekly exercise classes ‐ Group 2: 48% attended available weekly exercise classes Mortality Femoropopliteal trial ‐ Group 1: 2 participants ‐ Group 2: 2 participants Aortoiliac trial ‐ Group 1: 1 participants ‐ Group 2: 2 participants Loss to follow‐up Femoropopliteal trial ‐ Group 1: 3 participants ‐ Group 2: 6 participants Aortoiliac trial ‐ Group 1: 4 participants ‐ Group 2: 1 participants | |
| Outcomes | Absolute walking distance, initial claudication distance, number of secondary interventions, SF‐36 physical health score , SF‐36 physical mental score, procedure‐related complications | |
| Notes | Source of funding: Camelia Botnar Arterial Research Foundation with independent educational grants from Bard Ltd., Boston Scientific Ltd., and Cook Authors' conclusion: "PTA confers adjuvant benefit over supervised exercise and best medical therapy in terms of walking distances and ABPI 24 months after PTA in patients with stable mild to moderate intermittent claudication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate randomisation technique using "randomly permuted blocks of unequal size generated by Stata" |
| Allocation concealment (selection bias) | Low risk | Central allocation, "performed by the trial manager via a laptop computer whilst on site at each centre" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Low risk | Analysis based on an intention‐to‐treat principle, censoring at 24 months moderate (17%) and comparable between groups |
| Selective reporting (reporting bias) | Unclear risk | Prespecified maximum walking distance reported; for pain‐free walking distance, no absolute distances reported during follow‐up |
| Other bias | Unclear risk | Intended recruitment based on power calculations 170 participants in each trial, eventually including 93 participants in the femoropopliteal trial and 34 participants in the aortoiliac trial |
| Methods | Study design: parallel 3‐arm RCT Number of sites: 4 Sample size estimation: 21 participants (7 per group) required to detect a 75% reduction in the thrombin antithrombin complex in treatment groups with 80% power and a P value of 0.05 Follow‐up: 3 and 6 months | |
| Participants | Country and setting: United Kingdom, Department of Vascular Surgery at University of Birmingham Inclusion criteria ‐ Confirmed mild to moderate intermittent claudication (defined as absolute claudication distance (of 50 to 500 m on a treadmill) due to infrainguinal disease ‐ Suitable for unilateral infrainguinal endovascular revascularisation and participation in a supervised exercise programme ‐ 3 to 6 months stabilised on best medical therapy before consideration for study entry Exclusion criteria ‐ Significant aortoiliac disease ‐ Equally severe bilateral symptoms (making them unsuitable for unilateral angioplasty) ‐ Previous ipsilateral infrainguinal intervention ‐ Unable to exercise to absolute claudication distance on treadmill Number of participants assessed and randomised: 372 participants screened for entry; from them 23 participants randomised to 1 of 3 treatment arms Demographics ‐ Age (years): Group 1: median 67 (IQR 57 to 77); Group 2: median 67 (IQR 58 to 71); Group 3: median 67 (IQR 57 to 77) ‐ Gender (male): Group 1: 6 (67%); Group 2: 6 (86%); Group 3: 4 (57%) | |
| Interventions | Group 1: endovascular revascularisation without stenting plus best medical therapy, n = 9 Group 2: supervised exercise therapy plus best medical therapy for 12 weeks (2 sessions/week, 60 minutes/session), n = 7 Group 3: best medical therapy based on cardiovascular risk factor management, n = 7 (for analysis, this group was labelled as 'no therapy') Compliance with interventions: not reported Mortality ‐ Group 1: 0 participants ‐ Group 2: 0 participants ‐ Group 3: 0 participants Loss to follow‐up ‐ Group 1: 0 participants ‐ Group 2: 1 participant ‐ Group 3: 0 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance | |
| Notes | Source of funding: Health Technology Assessment Grant Authors' conclusion: "The addition of lower limb revascularization by PTA to best medical therapy in patients with intermittent claudication due to infra‐inguinal disease results in a medium‐term improvement in the resting procoagulant and hypo fibrinolytic state." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants underwent "central randomisation", yet exact randomisation technique not specified |
| Allocation concealment (selection bias) | Unclear risk | Study did not address allocation concealment process |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Low risk | During 6‐month follow‐up, only 1 participant withdrew (4%) from the study |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Unclear risk | Study was not powered to consider walking distances as primary endpoint; study was closed early including only 10% of required participants |
| Methods | Study design: parallel 3‐arm RCT Number of sites: 1 Sample size estimation: 60 participants in each treatment arm based on 80% power, α = 0.05, and anticipating a 20% dropout rate to detect a 20% difference between treatment arms in physical function domain of SF‐36 Follow‐up: 1,3, 6, and 12 months | |
| Participants | Country and setting: United Kingdom, Vascular Surgical Unit of a university hospital Inclusion criteria ‐ Symptomatic unilateral intermittent claudication ‐ Femoropopliteal lesion amenable to angioplasty (as discussed in a multi‐disciplinary meeting) ‐ Symptoms stable after 3 months on best medical therapy Exclusion criteria ‐ Critical limb ischaemia ‐ Incapacitating systemic disease ‐ Inability to tolerate treadmill testing (unrelated to limb ischaemia) ‐ Significant Ischaemic changes on ECG during treadmill testing ‐ Ipsilateral vascular surgery or peripheral angioplasty within previous 6 months Number of participants assessed and randomised: 1157 participants were assessed for inclusion; from them 178 participants were randomised to 1 of 3 treatment arms Demographics ‐ Age (years): Group 1: median 69.5 (95% CI 64 to 79); Group 2: median 70 (95% CI 63 to 75); Group 3: median 69 (95% CI 63 to 76) ‐ Gender (male): Group 1: 33 (57%); Group 2: 37 (62%); Group 3: 37 (62%) | |
| Interventions | Group 1: endovascular revascularisation without stenting plus supervised exercise therapy, n = 58 Group 2: endovascular revascularisation without stenting, n = 60 Group 3: supervised exercise therapy for 12 weeks (3 sessions/week), n = 60 Compliance with interventions: not reported Mortality ‐ Group 1: 1 participant ‐ Group 2: 0 participants ‐ Group 3: 0 participants Loss to follow‐up ‐ Group 1: 10 participants ‐ Group 2: 8 participants ‐ Group 3: 14 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, SF‐36 Physical Function, SF‐36 Role Physical, SF‐36 Bodily Pain, SF‐36 General Health, SF‐36 Vitality, SF‐36 Social, SF‐36 Emotional, SF‐36 Mental, VascuQol, self‐reported maximum walking distance | |
| Notes | Source of funding: BJS research bursary, European Society of Vascular Surgery research grant, and support from the Academic Vascular Surgical Unit, University of Hull Authors' conclusion: "For patients with intermittent claudication due to femoropopliteal disease, PTA, supervised exercise and PTA plus supervised exercise were all equally effective in improving walking distance and quality of life after 12 months." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomised into one of the three treatment arms"; exact sequence generation method not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Low risk | Sufficient number of participants (82%) analysed after 1 year of follow‐up; censoring comparable between groups |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Low risk | No other forms of bias identified |
| Methods | Study design: parallel 4‐arm RCT Number of sites: 22 Sample size estimation: Allowing 30% premature withdrawal, 252 participants would be needed to have 80% power to detect relevant difference between supervised exercise and stenting groups. Sample size was adjusted to 217 after removal of stenting plus supervised exercise arm owing to slow enrolment. Follow‐up: 6 and 18 months | |
| Participants | Country and setting: United States, university and non‐university hospitals Inclusion criteria ‐ Symptoms of moderate to severe intermittent claudication (ability to walk 2 to 11 minutes on a graded treadmill test) ‐ Objective evidence of a haemodynamically significant aortoiliac arterial stenosis established by non‐invasive vascular testing Exclusion criteria ‐ Critical limb ischaemia ‐ Comorbid conditions limiting participants' walking ability ‐ More than 25% deviation between 2 treadmill tests at baseline ‐ Total aortoiliac occlusion from the level of the renal arteries to the inguinal ligaments Number of participants assessed and randomised: 999 participants screened, 119 participants randomised to 1 of 4 treatment arms Demographics ‐ Age (years): Group 1: mean 65 (SD 10); Group 2: mean 64 (SD 10); Group 3: mean 62 (SD 8) ‐ Gender (male): Group 1: 32 (70%); Group 2: 21 (49%); Group 3: 16 (73%) | |
| Interventions | Group 1: endovascular revascularisation with primary stenting plus claudication pharmacotherapy (cilostazol), n = 46 Group 2: supervised exercise therapy for 26 weeks (3 sessions/week, 1 hour/session) supplemented by 12‐month telephone‐based (1 to 2 calls/mo) programme to adhere and maintain adherence plus claudication pharmacotherapy (cilostazol), n = 43 Group 3: claudication pharmacotherapy, including cilostazol 100 mg twice daily and advice on home exercise and diet, n = 22 Group 4: endovascular revascularisation plus supervised exercise therapy and claudication pharmacotherapy (cilostazol), n = 8 (inclusion in this study arm stopped prematurely and study arm excluded from further analysis) Compliance with interventions ‐ Group 1: 43 participants received assigned intervention, all procedures technically successful, > 90% adherence to cilostazol treatment ‐ Group 2: 29 (71%) participants attended at least 70% of 78 scheduled exercise sessions, > 90% adherence to cilostazol treatment ‐ Group 3: > 90% adherence to cilostazol treatment Mortality ‐ Group 1: 0 participants ‐ Group 2: 1 participant ‐ Group 3: 0 participants Loss to follow‐up ‐ Group 1: 5 participants ‐ Group 2: 8 participants ‐ Group 3: 4 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, procedure‐related complications, SF‐12 Physical, SF‐12 Mental, Walking Impairment Questionnaire Score, Peripheral Artery Questionnaire Score, hourly free‐living steps on pedometer | |
| Notes | Source of funding: grants from National Heart, Lung, and Blood Institute. Financial support from Cordis/Johnson & Johnson (Warren, NJ), eV3 (Plymouth,MN), and Boston Scientific (Natick, MA). Cilostazol was donated to all study participants by Otsuka America, Inc (San Francisco, CA). Pedometers were donated by Omron Healthcare, Inc (Lake Forest, IL). Krames Staywell (San Bruno, CA) donated print materials on exercise and diet. Notes: The endovascular revascularisation plus supervised exercise therapy treatment arm was stopped after including 8 participants owing to slow enrolment. Authors' conclusion: "Both supervised exercise and endovascular revascularization had better 18‐month outcomes than medical treatment. Both treatments provided comparable durable improvement in functional status and in quality of life up to 18 months. The durability of claudication exercise interventions merits its consideration as a primary claudication treatment." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A real‐time Web‐based randomisation programme was used to randomise participants |
| Allocation concealment (selection bias) | Low risk | Central Web‐based allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Low risk | Study reported to be an "observer‐blinded" randomised trial |
| Incomplete outcome data (attrition bias) | Low risk | All analyses according to intention‐to‐treat principle; censoring was moderate (10%) after 6 months' follow‐up and was well balanced between treatment groups |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Unclear risk | Intended recruitment based on power calculations was 252 participants; eventually 119 participants were included in the study |
| Methods | Study design: parallel 2‐arm RCT Number of sites: 1 Sample size estimation: From power calculations, a total sample size of 158 participants was needed with the assumption of a maximum dropout rate of 25% and 80% power to detect relevant differences between the 2 groups. Follow‐up: 6 and 12 months | |
| Participants | Country and setting: Sweden, Department of Vascular Surgery at university hospital Inclusion criteria ‐ Stable (≥ 6 months) intermittent claudication, without any other important activity‐limiting medical condition ‐ Aged ≤ 80 years Exclusion criteria ‐ Very mild claudication symptoms ‐ Weight > 120 kg ‐ Inability to understand the Swedish language Number of participants assessed and randomised: 464 participants screened for inclusion; of these, 205 participants were eligible, and eventually 158 participants were randomised in the trial Demographics ‐ Age (years): Group 1: mean 68 (SD 7); Group 2: mean 68 (SD 6) ‐ Gender (male): Group 1: 41 (52%); Group 2: 38 (48%) | |
| Interventions | Group 1: invasive vascular procedure including open surgery or endovascular revascularisation with primary stenting in the aortoiliac segment and selective stenting in the femoropopliteal segment plus claudication pharmacotherapy (cilostazol 100 mg twice daily), home‐based exercise training advice, and cardiovascular risk factor management, n = 79 (of these, 52 participants received an endovascular revascularisation procedure) Group 2: non‐invasive management including claudication pharmacotherapy (cilostazol 100 mg twice daily), home‐based exercise training advice, and cardiovascular risk factor management, n = 79 Compliance with interventions ‐ Group 1: 70 participants received invasive treatment; from them, 52 participants received an endovascular intervention, 60% adherence to cilostazol treatment at 12 months, no data on exercise adherence ‐ Group 2: 60% adherence to cilostazol treatment at 12 months; no data on exercise adherence Mortality ‐ Group 1: 1 participant of the 52 participants receiving endovascular revascularisation ‐ Group 2: 0 participants Lost to follow up: ‐ Group 1: 2 participants of 52 participants receiving endovascular revascularisation ‐ Group 2: 3 participants | |
| Outcomes | Maximum walking distance, intermittent claudication distance, number of secondary interventions, procedure‐related complications, SF‐36 Physical Function, SF‐36 Role Physical, SF‐36 Bodily Pain, SF‐36 General Health, SF‐36 Vitality, SF‐36 Social, SF‐36 Emotional, SF‐36 Mental Health, and VascuQol | |
| Notes | Source of funding: Study was funded by the Fred G. and Emma E. Kanolds Foundation/Gothenburg Medical Society; Helena Ahlin Foundation; Odd Fellow, Karlstad, Sweden; Swedish Heart and Lung Foundation; and Hjalmar Svensson Foundation. Notes: Outcome data from the subgroup of 52 participants who received an endovascular revascularisation at baseline in the invasive treatment group were provided by study authors and were included in the analyses in this systematic review. Authors' conclusion: "An invasive treatment strategy improves health‐related quality of life and intermittent claudication distance after 1 year in patients with stable lifestyle‐limiting claudication receiving current medical management. Long‐term follow‐up data and health‐economic assessments are warranted to further establish the role for revascularization in intermittent claudication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via computerised randomisation software based on minimisation method |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed using computerised randomisation software |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Low risk | All analysis performed by intention‐to‐treat principle; censoring during 12‐month follow‐up low and well balanced between treatment groups |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported in the results section |
| Other bias | Low risk | No other forms of bias identified |
| Methods | Study design: parallel 2‐arm RCT Number of sites: 1 Sample size estimation: approximately 100 participants in each group; thus a total of 200 participants to detect a difference of 20% in QoL between groups assuming type I error of 5% and power of 80% Follow‐up: 3, 12, and 24 months | |
| Participants | Country and setting: Norway, Centre of Vascular Surgery at university hospital Inclusion criteria ‐ < 80 years of age ‐ Symptomatic IC > 3 months ‐ Ankle brachial index < 0.9 without pain at rest and/or ischaemic skin changes ‐ Lesion feasible for angioplasty evaluated by angiography ‐ Subjective pain‐free walking distance < 400 metres ‐ Ability to exercise on a treadmill Exclusion criteria ‐ Previous vascular or endovascular surgery ‐ Diabetic skin ulceration ‐ Renal insufficiency (defined as serum creatinine > 150 mmol/L) ‐ Oral anticoagulant medication ‐ Suffering from a physical or mental disorder expected to impede compliance Number of participants assessed and randomised: 826 participants were assessed for inclusion; finally 56 participants could be included and randomised to 1 of 2 treatment groups. Demographics ‐ Age (years): Group 1: median 68 (25 to 75 percentiles: 56 to 72); Group 2: median 69 (25 to 75 percentiles: 61 to 75) ‐ Gender (male): Group 1: 16 (57%); Group 2: 15 (54%) | |
| Interventions | Group 1: endovascular revascularisation with primary stenting for iliac occlusions and selective stenting for iliac stenoses plus optimal medical treatment, n = 28 Group 2: optimal medical treatment including active smoking cessation, advice on home‐based exercise therapy, individual nutritional advice, and acetylsalicylic acid 160 mg daily to all participants and cardiovascular risk factor management, n = 28 (for analysis, this group was labelled as 'no therapy') Compliance with interventions ‐ Group 1: All procedures were technically successful. ‐ Group 2: No data were provided on home‐based exercise therapy compliance. Mortality ‐ Group 1: 1 participant ‐ Group 2: 0 participants Loss to follow‐up ‐ Group 1: 1 participant ‐ Group 2: 4 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, SF‐36 Physical Function, SF‐36 Role Physical, SF‐36 Bodily Pain, SF‐36 General Health, SF‐36 Vitality, SF‐36 Social, SF‐36 Emotional, SF‐36 Mental, SF‐36 Health Transition, Claudication Score (5 domains), visual analogue scale | |
| Notes | Source of funding: unrestricted grants from Pfizer AS, Norway Authors conclusion: "Early intervention with PTA in addition to optimal medical treatment seems to have a generally more positive effect compared to optimal medical treatment only, on haemodynamic, functional as well as quality of life aspects during the first 2 years in patients with intermittent claudication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computerized randomisation list" was used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Selective reporting (reporting bias) | Low risk | Censoring during 12‐month follow‐up minimal (9%) and well balanced between groups |
| Other bias | Unclear risk | Intended recruitment based on power calculations was 200 participants; eventually 56 participants were included in the study, |
| Methods | Study design: parallel 2‐arm RCT Number of sites: 1 Sample size estimation: 68 participants in each arm based on 80% power and significance level of 0.05 to detect 25% difference in improvement in physical functioning dimension of SF‐36 between the 2 groups Follow up: 1, 6, 12 months and 7 years | |
| Participants | Country and setting: Netherlands, outpatient clinic at a non‐university hospital Inclusion criteria ‐ Rutherford category 1, 2, or 3 claudication with duration ≥ 3 months ‐ Maximum pain‐free walking distance < 350 metres ‐ Ankle‐brachial index < 0.9 at rest or decreasing by more than 0.15 after the treadmill test ‐ ≥ 1 vascular stenoses of > 50% diameter reduction at the iliac or femoropopliteal level on magnetic resonance angiography Exclusion criteria ‐ Abdominal aortic aneurysm, life‐incapacitating cardiac disease (New York Heart Association Class III and higher) ‐ Multi‐level disease (i.e. same‐side stenoses at both iliac and femoral levels, requiring multiple revascularisation procedures) ‐ Isolated tibial artery disease ‐ Lesions deemed unsuitable for revascularisation ‐ Prior treatment for the lesion (including exercise therapy) Number of participants assessed and randomised: 293 participants assessed, 151 participants randomised to 1 of 2 treatment groups Demographics ‐ Age (years): Group 1: mean 65 (SD 11); Group 2: mean 66 (SD 9) ‐ Gender (male): Group 1: 44 (59%); Group 2: 39 (52%) | |
| Interventions | Group 1: endovascular revascularisation with selective stenting, n = 76 Group 2: supervised exercise therapy for 24 weeks (2 sessions/week, 30 minutes/session), n = 75 Compliance with interventions ‐ Group 1: In 4 participants, revascularisation failed technically ‐ Group 2: Per participant, on average 33 (SD 10) sessions of exercise followed Mortality ‐ Group 1: 5 participants (after 7 years: 15) ‐ Group 2: 3 participants (after 7 years: 17) Loss to follow‐up ‐ Group 1: 2 participants (after 7 years: 14) ‐ Group 2: 0 participants (after 7 years: 22) | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, procedure‐related complications, SF‐36 Physical Functioning, SF‐36 Physical Role, SF‐36 Bodily Pain, SF‐36 General Health, VascuQol | |
| Notes | Source of funding: not applicable Authors conclusion: "After 6 and 12 months, patients with intermittent claudication benefited equally from either endovascular revascularization or supervised exercise." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computer generated block‐randomized list" was used, prepared in advance by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Allocation was "sealed for every particular participant." |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Low risk | Walking distance evaluated by an independent assessor blinded to assigned treatment |
| Incomplete outcome data (attrition bias) | Low risk | All analysis according to intention‐to‐treat principle; after 12 months' follow‐up, censoring minimal (7%) and well balanced between the 2 groups |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Low risk | No other forms of bias identified |
| Methods | Study design: parallel 2‐arm RCT Number of sites: 1 Sample size estimation: 54 participants based on 90% power and significance level of 0.05 to detect 40% difference in number of participants with symptomatic improvement between intervention and control groups Follow‐up: 3, 6, and 24 months | |
| Participants | Country and setting: United Kingdom, outpatient department of a university hospital Inclusion criteria ‐ Predominantly unilateral intermittent claudication ‐ Lesion suitable for endovascular revascularisation Exclusion criteria ‐ Previous angioplasty or arterial surgery to the symptomatic leg ‐ Iliac occlusion or > 10 cm length femoropopliteal occlusion, multiple stenoses or diffuse disease with long stenoses ‐ Participants taking oral anticoagulants ‐ Duration of symptoms < 1 month ‐ Inability to manage the treadmill examination ‐ Any psychiatric illness or other reason making follow‐up difficult. Number of participants assessed and randomised: 425 participants assessed, 62 participants randomised Demographics ‐ Age (years): Group 1: mean 60.6 (range 44 to 73); Group 2: mean 62.6 (range 45 to 78) ‐ Gender (male): Group 1: 23 (77%); Group 2: 28 (88%) | |
| Interventions | Group 1: endovascular revascularisation without stenting, n = 30 Group 2: conventional medical treatment including low‐dose aspirin plus advice on smoking and exercise, n = 32 (for analysis, this group was labelled as 'no therapy') Compliance with interventions: not reported Mortality ‐ Group 1: 0 participants ‐ Group 2: 2 participants Loss to follow‐up ‐ Group 1: 1 participant ‐ Group 2: 2 participants | |
| Outcomes | Maximum walking distance, pain‐free walking distance, number of secondary interventions, Nottingham health profile scores, self‐reported maximum walking distance | |
| Notes | Source of funding: grant from the Scottish Home and Health Department, cost of balloon catheters from Meadox, UK Authors' conclusion: "Two years after PTA, patients had less extensive disease than medically treated patients, but this did not translate into a significant advantage in terms of improved walking or quality of life." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out via a computerised random allocation system |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out via a computerised random allocation system |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not possible given the nature of the intervention (endovascular revascularisation) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors not adequately discussed in the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Censoring minimal (5%) and comparable between groups, yet participants in the control group who underwent angioplasty or surgery excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures as specified in the methods section were reported |
| Other bias | Unclear risk | Source of funding: cost of balloon catheters from Meadox, UK |
ABI: ankle brachial pressure index.
IC: intermittent claudication.
IQR: interquartile range.
PTA: percutaneous transluminal angioplasty.
QoL: quality of life.
RCT: randomised controlled trial.
SD: standard deviation.
SF‐36: Short Form‐36.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| All participants received endovascular revascularisation | |
| Two endovascular revascularisation techniques were compared; no non‐interventional treatment group was included | |
| Two endovascular revascularisation techniques were compared; no non‐interventional treatment group was included | |
| Intervention group included participants with open and endovascular revascularisation; no data could be provided for the subgroup of participants receiving endovascular revascularisation | |
| Participant assignment to a specific group of treatment was not randomised | |
| Participants were followed up to 4 weeks. No relevant outcome measures for this systematic review were reported | |
| Walking distances were recorded only up to 1 month follow‐up; no long‐term data were provided. In addition, no other relevant outcome measures for this systematic review were reported | |
| All participants received endovascular revascularisation | |
| Abstract data only with incomplete results; no additional data could be provided |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | SUPERvised Exercise Therapy or Immediate PTA for Intermittent Claudication in Participants With an Iliac Artery Obstruction |
| Methods | Study design: multi‐centre randomised controlled trial Sites: 15 Dutch hospitals Sample size estimation: 400 participants to detect a clinically relevant difference in quality‐adjusted life‐years between the 2 groups based on 90% power and 2‐sided significance level of 0.05 Follow up: 1 week, 1, 6, and 12 months |
| Participants | Consecutive outpatients with intermittent claudication due to aortoiliac disease with a walking distance between 100 and 300 metres on a treadmill at 3.2 km/h and 10% incline. All participants must have an iliac artery obstruction with a diameter reduction ≥ 50% |
| Interventions | Group 1: endovascular revascularisation with selective stenting Group 2: supervised exercise therapy for 6 months |
| Outcomes | Maximum walking distance, pain‐free walking distance, complications, treatment failures, additional interventions, costs, AMC linear disability score, VascuQol, Short‐Form 36, EuroQol |
| Starting date | Inclusion started in September 2011 |
| Contact information | |
| Notes | Owing to slow enrolment, inclusion stopped prematurely (241 participants included per May 2015; www.superstudie.nl). |
| Trial name or title | Primary Stenting vs Conservative Treatment in Claudicants ‐ A Study on Quality of Life (NCT01230229) |
| Methods | Study design: randomised controlled trial Estimated enrolment: 100 participants Follow‐up: 12 and 24 months |
| Participants | Patients with stable intermittent claudication (Fontaine IIa and IIb) due to superficial femoral artery disease |
| Interventions | Group 1: endovascular revascularisation with primary stenting (self‐expanding stent) Group 2: best medical treatment including an exercise programme |
| Outcomes | Primary: improvement in quality of life scores (Short Form‐36 and EuroQol‐5D surveys) Secondary: ABI, walking distances, cost parameters |
| Starting date | January 2010 |
| Contact information | Hans Lindgren, MD; e‐mail: [email protected] |
| Notes | Planned recruitment and randomisation of 100 participants; estimated study completion date June 2017 (https://clinicaltrials.gov/ct2/show/study/NCT01230229?term=endovascular+and+claudication#desc) |
ABI: ankle brachial pressure index.
AMC: academic medical centre.
PTA: percutaneous transluminal angioplasty.
QoL: quality of life.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.31, 1.08] |
| Analysis 1.1 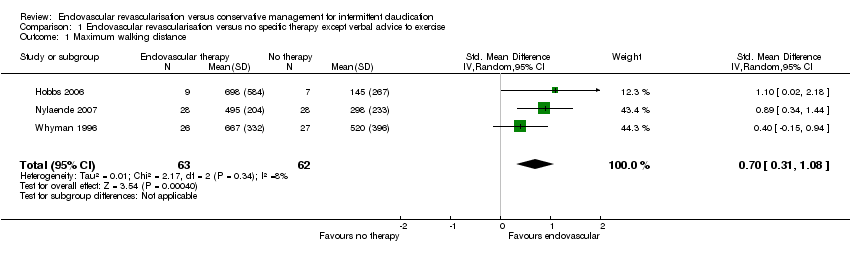 Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 1 Maximum walking distance. | ||||
| 2 Maximum walking distance (long‐term) Show forest plot | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.30, 1.63] |
| Analysis 1.2  Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 2 Maximum walking distance (long‐term). | ||||
| 3 Pain‐free walking distance Show forest plot | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.90, 1.68] |
| Analysis 1.3 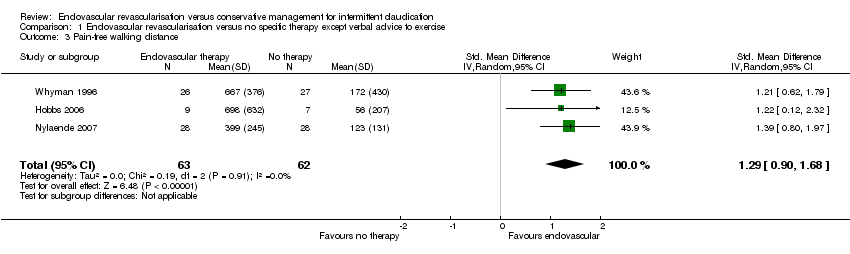 Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 3 Pain‐free walking distance. | ||||
| 4 Pain‐free walking distance (long‐term) Show forest plot | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.45, 1.82] |
| Analysis 1.4 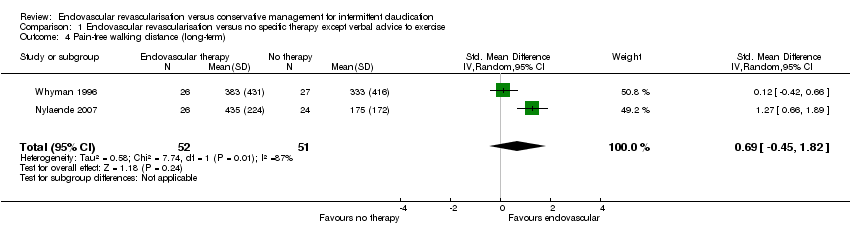 Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 4 Pain‐free walking distance (long‐term). | ||||
| 5 Secondary invasive interventions Show forest plot | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.12, 5.28] |
| Analysis 1.5 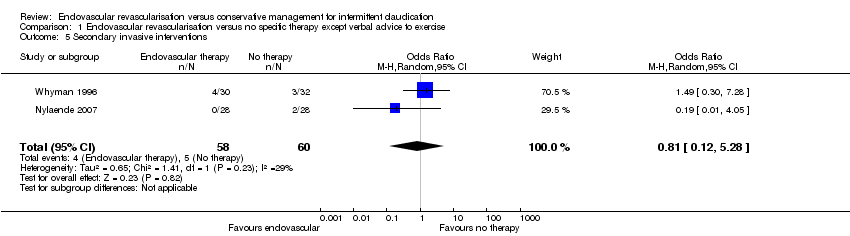 Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 5 Secondary invasive interventions. | ||||
| 6 Mortality Show forest plot | 3 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.13, 4.44] |
| Analysis 1.6 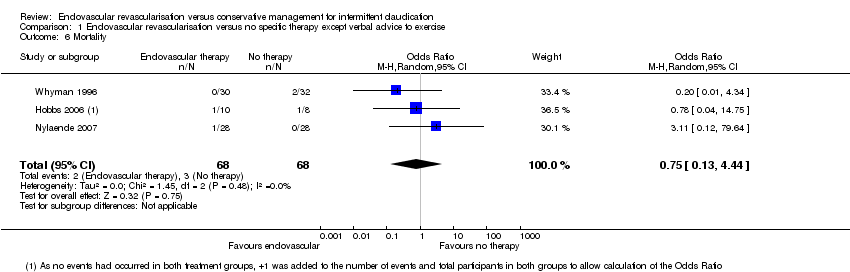 Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 6 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 5 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.87, 0.04] |
| Analysis 2.1  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 1 Maximum walking distance. | ||||
| 2 Maximum walking distance (long‐term) Show forest plot | 3 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.36, 0.32] |
| Analysis 2.2  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 2 Maximum walking distance (long‐term). | ||||
| 3 Pain‐free walking distance Show forest plot | 5 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.38, 0.29] |
| Analysis 2.3  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 3 Pain‐free walking distance. | ||||
| 4 Pain‐free walking distance (long‐term) Show forest plot | 2 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.26, 0.48] |
| Analysis 2.4 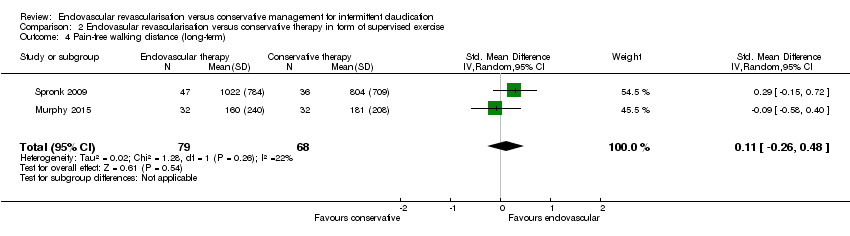 Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 4 Pain‐free walking distance (long‐term). | ||||
| 5 Secondary invasive interventions Show forest plot | 4 | 395 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.70, 2.80] |
| Analysis 2.5  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 5 Secondary invasive interventions. | ||||
| 6 Quality of life (disease‐specific) Show forest plot | 3 | 301 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.04, 0.41] |
| Analysis 2.6  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 6 Quality of life (disease‐specific). | ||||
| 7 Mortality Show forest plot | 5 | 435 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.00] |
| Analysis 2.7  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 7 Mortality. | ||||
| 8 Sensitivity analysis: maximum walking distance Show forest plot | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.98, ‐0.07] |
| Analysis 2.8  Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 8 Sensitivity analysis: maximum walking distance. | ||||
| 9 Sensitivity analysis: pain‐free walking distance Show forest plot | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.46, 0.23] |
| Analysis 2.9 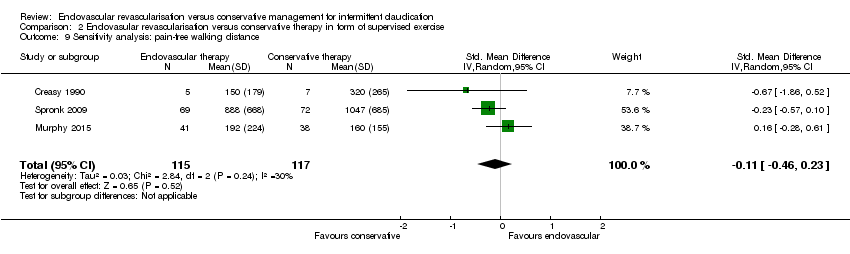 Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 9 Sensitivity analysis: pain‐free walking distance. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 1 Maximum walking distance. | ||||
| 1.1 Supervised exercise therapy | 3 | 432 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.13, 0.64] |
| 1.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 2 Maximum walking distance (long‐term) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 2 Maximum walking distance (long‐term). | ||||
| 2.1 Supervised exercise therapy | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 1.18 [0.65, 1.70] |
| 2.2 Pharmacotherapy | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.09, 1.36] |
| 3 Pain‐free walking distance Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3 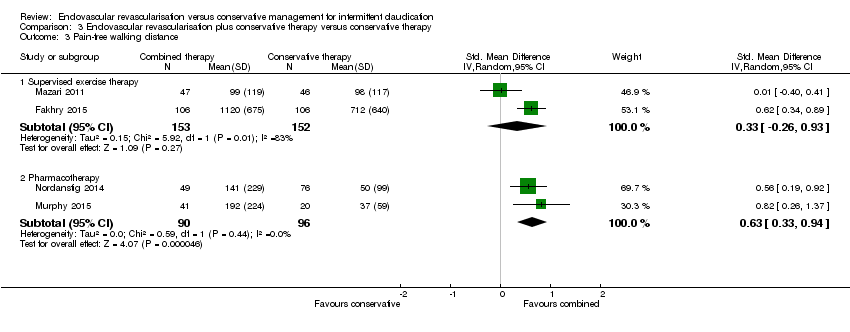 Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 3 Pain‐free walking distance. | ||||
| 3.1 Supervised exercise therapy | 2 | 305 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.26, 0.93] |
| 3.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.33, 0.94] |
| 4 Pain‐free walking distance (long‐term) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4 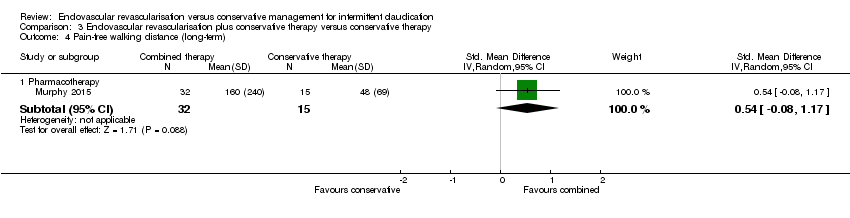 Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 4 Pain‐free walking distance (long‐term). | ||||
| 4.1 Pharmacotherapy | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.08, 1.17] |
| 5 Secondary invasive interventions Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 5 Secondary invasive interventions. | ||||
| 5.1 Supervised exercise therapy | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.55] |
| 5.2 Pharmacotherapy | 2 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [0.49, 6.83] |
| 6 Quality of life (disease‐specific) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.6 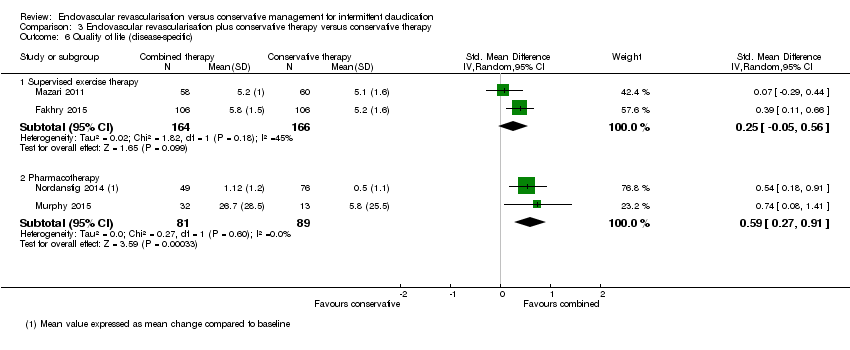 Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 6 Quality of life (disease‐specific). | ||||
| 6.1 Supervised exercise therapy | 2 | 330 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.56] |
| 6.2 Pharmacotherapy | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.27, 0.91] |
| 7 Mortality Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.7 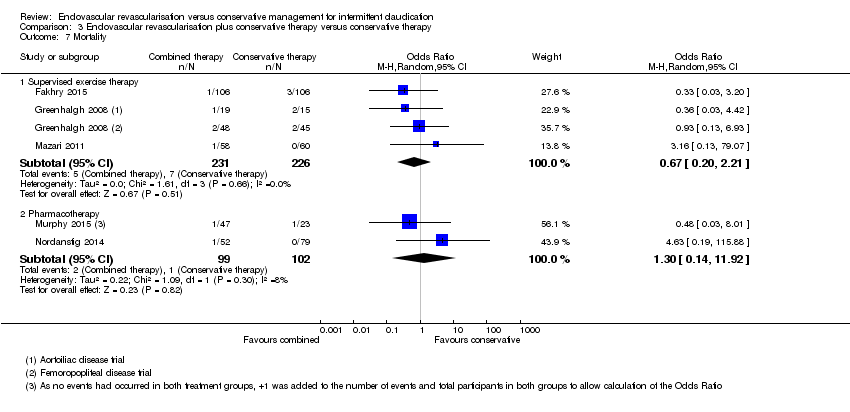 Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 7 Mortality. | ||||
| 7.1 Supervised exercise therapy | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.20, 2.21] |
| 7.2 Pharmacotherapy | 2 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.14, 11.92] |
| 8 Sensitivity analysis: maximum walking distance Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.8 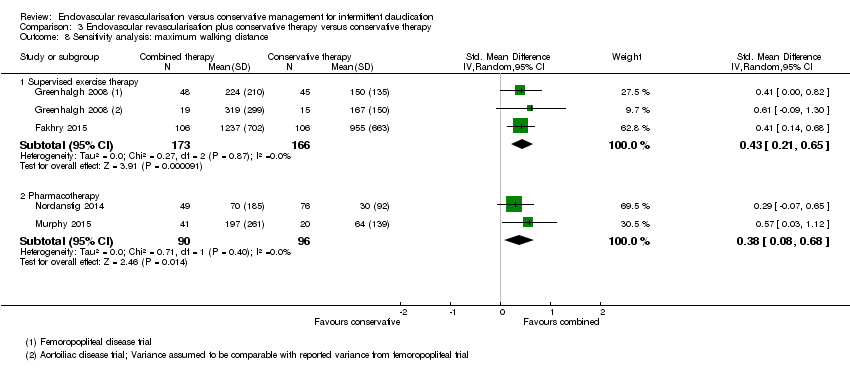 Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 8 Sensitivity analysis: maximum walking distance. | ||||
| 8.1 Supervised exercise therapy | 2 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.21, 0.65] |
| 8.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 9 Sensitivity analysis: pain‐free walking distance Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.9  Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 9 Sensitivity analysis: pain‐free walking distance. | ||||
| 9.1 Supervised exercise therapy | 1 | 212 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.34, 0.89] |
| 9.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.33, 0.94] |

Study flow diagram.
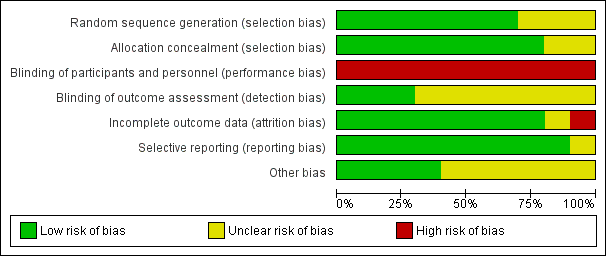
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
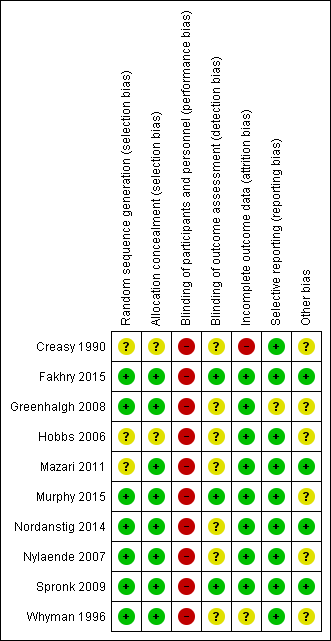
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
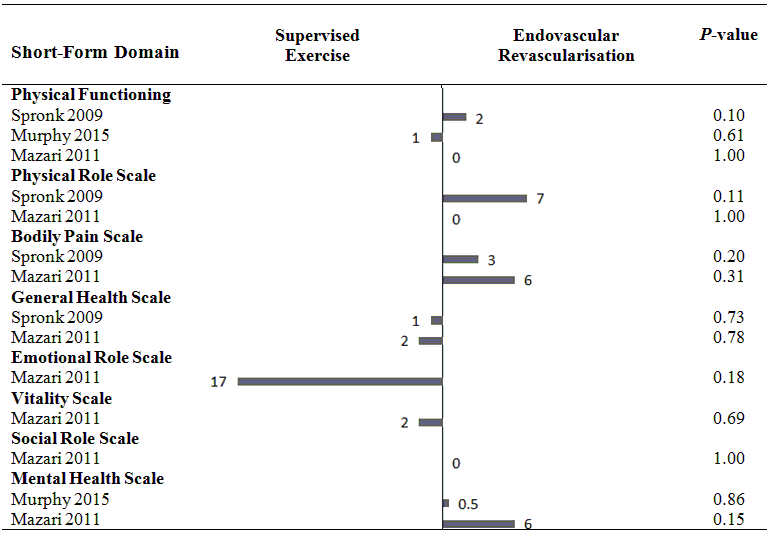
Health‐related quality of life (mean differences between groups).
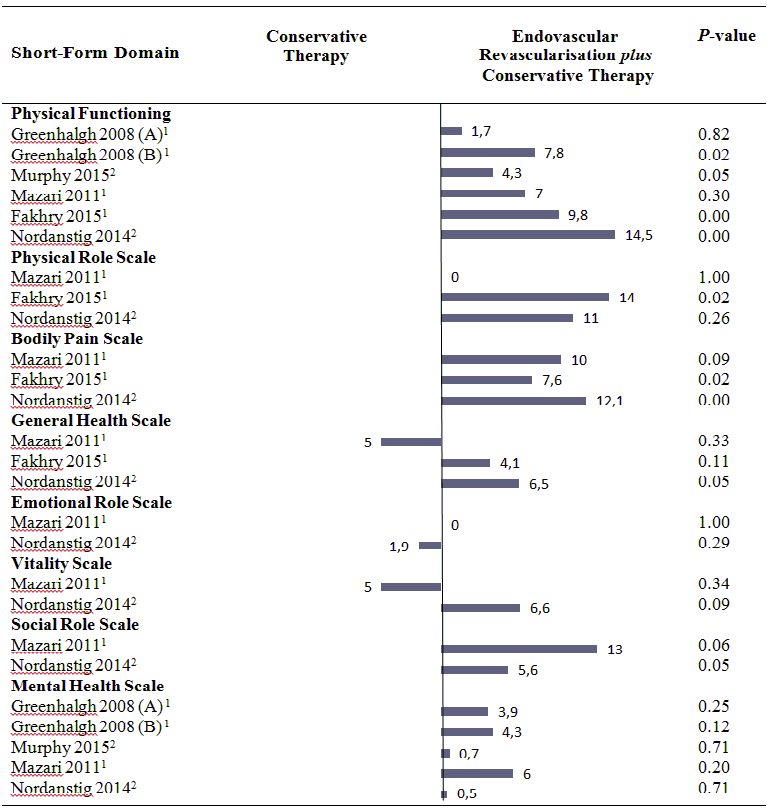
Health‐related quality of life (mean differences between groups).
A: Femoropoliteal trial.
B: Aortoiliac trial.
1 In this study, the comparison was endovascular revascularisation plus supervised exercise versus supervised exercise.
2 In this study, the comparison was endovascular revascularisation plus pharmacotherapy with cilostazol versus cilostazol.
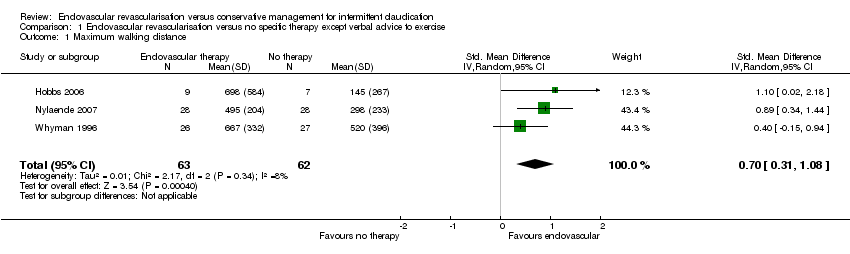
Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 1 Maximum walking distance.

Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 2 Maximum walking distance (long‐term).

Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 3 Pain‐free walking distance.

Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 4 Pain‐free walking distance (long‐term).

Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 5 Secondary invasive interventions.

Comparison 1 Endovascular revascularisation versus no specific therapy except verbal advice to exercise, Outcome 6 Mortality.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 1 Maximum walking distance.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 2 Maximum walking distance (long‐term).
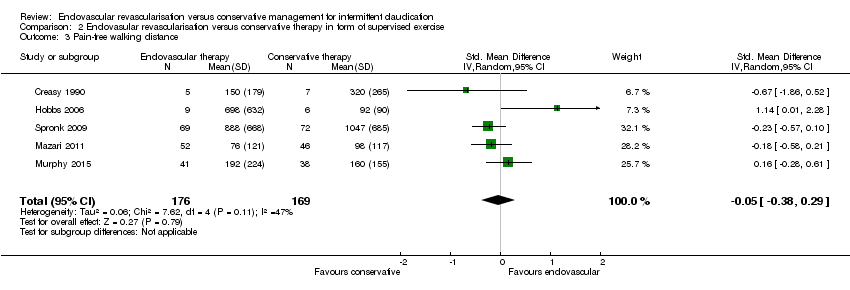
Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 3 Pain‐free walking distance.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 4 Pain‐free walking distance (long‐term).
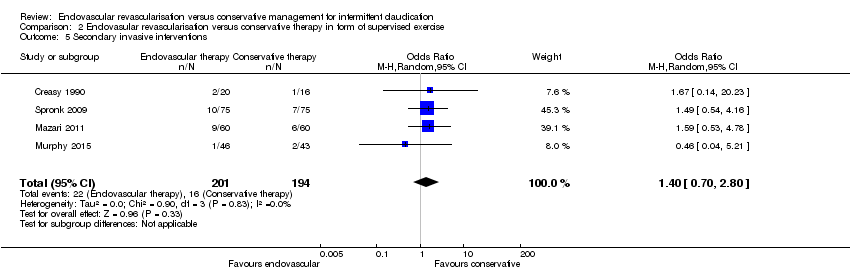
Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 5 Secondary invasive interventions.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 6 Quality of life (disease‐specific).

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 7 Mortality.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 8 Sensitivity analysis: maximum walking distance.

Comparison 2 Endovasular revascularisation versus conservative therapy in form of supervised exercise, Outcome 9 Sensitivity analysis: pain‐free walking distance.
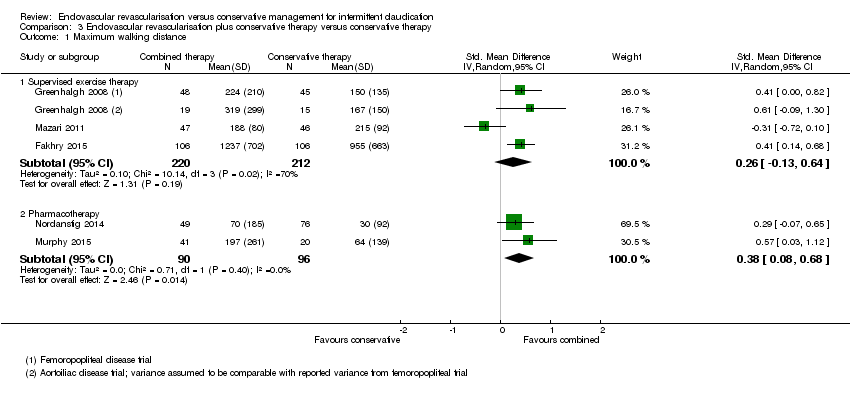
Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 1 Maximum walking distance.

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 2 Maximum walking distance (long‐term).

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 3 Pain‐free walking distance.
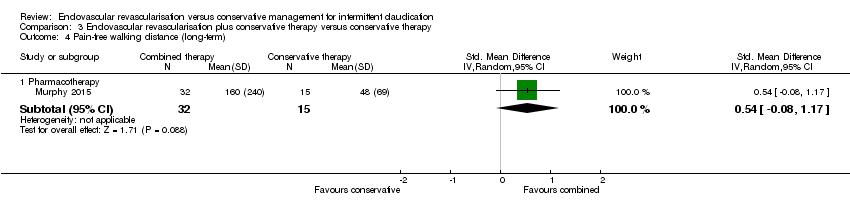
Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 4 Pain‐free walking distance (long‐term).

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 5 Secondary invasive interventions.

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 6 Quality of life (disease‐specific).

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 7 Mortality.

Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 8 Sensitivity analysis: maximum walking distance.
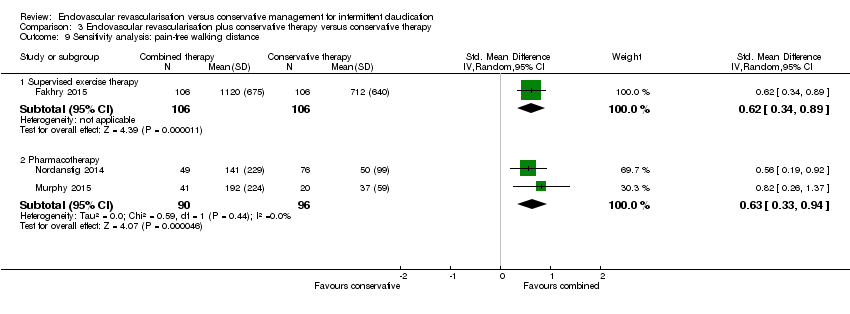
Comparison 3 Endovascular revascularisation plus conservative therapy versus conservative therapy, Outcome 9 Sensitivity analysis: pain‐free walking distance.
| Endovascular revascularisation compared with no specific therapy for intermittent claudication except verbal advice to exercise | ||||||
| Patient or population: intermittent claudication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with no specific therapy | Risk with endovascular revascularisation | |||||
| Maximum walking distance | ‐ | Mean maximum walking distance in the intervention group was 0.70 standard deviations higher (0.31 higher to 1.08 higher). | ‐ | 125 | ⊕⊕⊕⊝ | |
| Maximum walking distance (long‐term) | ‐ | Mean maximum walking distance at long term in the intervention group was 0.67 standard deviations higher (0.30 lower to 1.63 higher). | ‐ | 103 | ⊕⊕⊝⊝ | |
| Pain‐free walking distance | ‐ | Mean pain‐free walking distance in the intervention group was 1.29 standard deviations higher (0.90 higher to 1.68 higher). | ‐ | 125 | ⊕⊕⊕⊝ | |
| Pain‐free walking distance (long‐term) | ‐ | Mean pain‐free walking distance at long term in the intervention group was 0.69 standard deviations higher (0.45 lower to 1.82 higher). | ‐ | 103 | ⊕⊕⊝⊝ | |
| Secondary invasive interventions | Study population | OR 0.81 | 118 | ⊕⊕⊕⊝ | ||
| 83 per 1000 | 69 per 1000 | |||||
| Quality of life (disease‐specific) | See comments. | See comments. | ‐ | ‐ | ‐ | One study reported no significant differences in disease‐specific QoL between study groups after 2 years without providing data |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Pooled standardised mean differences were interpreted using rules of thumb (< 0.40 = small, 0.40 to 0.70 = moderate, > 0.70 = large effect) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Treatment consisted of cardiovascular risk factor management and only verbal exercise advice without any form of supervision. | ||||||
| Endovascular revascularisation compared with conservative therapy for intermittent claudication | ||||||
| Patient or population: intermittent claudication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conservative therapy | Risk with endovascular revascularisation | |||||
| Maximum walking distance | ‐ | Mean maximum walking distance in the intervention group was 0.42 standard deviations lower (0.87 lower to 0.04 higher). | ‐ | 345 | ⊕⊕⊕⊝ | |
| Maximum walking distance (long‐term) | ‐ | Mean maximum walking distance at long term in the intervention group was 0.02 standard deviations lower (0.36 lower to 0.32 higher). | ‐ | 184 | ⊕⊕⊕⊝ | |
| Pain‐free walking distance | ‐ | Mean pain‐free walking distance in the intervention group was 0.05 standard deviations lower (0.38 lower to 0.29 higher). | ‐ | 345 | ⊕⊕⊕⊝ | |
| Pain‐free walking distance (long‐term) | ‐ | Mean pain‐free walking distance at long term in the intervention group was 0.11 standard deviations higher (0.26 lower to 0.48 higher). | ‐ | 147 | ⊕⊕⊕⊝ | |
| Secondary invasive interventions | Study population | OR 1.40 | 395 | ⊕⊕⊕⊕ | ||
| 82 per 1000 | 112 per 1000 | |||||
| Quality of life (disease‐specific) | ‐ | Mean quality of life (disease‐specific) in the intervention group was 0.18 standard deviations higher (0.04 lower to 0.41 higher). | ‐ | 301 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Pooled standardised mean differences were interpreted using rules of thumb (< 0.40 = small, 0.40 to 0.70 = moderate, > 0.70 = large effect), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Supervised exercise consisted of only supervised exercise in four studies and a combination of supervised exercise and pharmacotherapy with cilostazol in one study. | ||||||
| Endovascular revascularisation plus conservative therapy compared with conservative therapy alone for intermittent claudication | ||||||
| Patient or population: intermittent claudication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conservative therapy | Risk with endovascular revascularisation plus conservative therapy | |||||
| Conservative therapy consists of supervised exercise | ||||||
| Maximum walking distance | ‐ | Mean maximum walking distance in the intervention group was 0.26 standard deviations higher (0.13 lower to 0.64 higher). | ‐ | 432 | ⊕⊕⊕⊝ | |
| Maximum walking distance (long‐term) | ‐ | Mean maximum walking distance at long term in the intervention group was 1.18 standard deviations higher (0.65 higher to 1.70 higher). | ‐ | 106 | ⊕⊕⊝⊝ | |
| Pain‐free walking distance | ‐ | Mean pain‐free walking distance in the intervention group was 0.33 standard deviations higher (0.26 lower to 0.93 higher). | ‐ | 305 | ⊕⊕⊕⊝ | |
| Pain‐free walking distance (long‐term) | See comments. | See comments. | ‐ | ‐ | ‐ | None of the studies reported any quantitative data on pain‐free walking distance at long term |
| Secondary invasive interventions | Study population | OR 0.27 | 457 | ⊕⊕⊕⊕ | ||
| 164 per 1000 | 50 per 1000 | |||||
| Quality of life (disease‐specific) | ‐ | Mean quality of life (disease‐specific) in the intervention group was 0.25 standard deviations higher (0.05 lower to 0.56 higher). | ‐ | 330 | ⊕⊕⊕⊝ | |
| Conservative therapy consists of pharmacotherapy (cilostazol) | ||||||
| Maximum walking distance | ‐ | Mean maximum walking distance in the intervention group was 0.38 standard deviations higher (0.08 higher to 0.68 higher). | ‐ | 186 | ⊕⊕⊕⊕ | |
| Maximum walking distance (long‐term) | ‐ | Mean maximum walking distance at long term in the intervention group was 0.72 standard deviations higher (0.09 higher to 1.36 higher). | ‐ | 47 | See comments. | Only 1 small RCT included in this analysis, no meaningful grading of quality of evidence possible |
| Pain‐free walking distance | ‐ | Mean pain‐free walking distance in the intervention group was 0.63 standard deviations higher (0.33 higher to 0.94 higher). | ‐ | 186 | ⊕⊕⊕⊕ | |
| Pain‐free walking distance (long‐term) | ‐ | Mean pain‐free walking distance at long‐term in the intervention group was 0.54 standard deviations higher (0.08 lower to 1.17 higher). | ‐ | 47 | See comments. | Only one small RCT included in this analysis, no meaningful grading of quality of evidence possible |
| Secondary invasive interventions | Study population | OR 1.83 | 199 | ⊕⊕⊕⊕ | ||
| 69 per 1000 | 120 per 1000 | |||||
| Quality of life (disease‐specific) | ‐ | Mean quality of life (disease‐specific) in the intervention group was 0.59 standard deviations higher (0.27 higher to 0.91 higher). | ‐ | 170 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Pooled standardised mean differences were interpreted using rules of thumb (< 0.40 = small, 0.40 to 0.70 = moderate, > 0.70 = large effect), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 Conservative therapy consisted of supervised exercise in three studies and of pharmacotherapy with cilostazol and only advice to exercise in two studies. | ||||||
| Study | Groin haematoma | Artery dissection |
| 3/20 | 1/20 | |
| 5/106 | 2/106 | |
| 8/67 | 1/67 | |
| nr | 2/46 | |
| 1/52 | nr | |
| 6/75 | 1/75 | |
| nr: not reported. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.31, 1.08] |
| 2 Maximum walking distance (long‐term) Show forest plot | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.30, 1.63] |
| 3 Pain‐free walking distance Show forest plot | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.90, 1.68] |
| 4 Pain‐free walking distance (long‐term) Show forest plot | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.45, 1.82] |
| 5 Secondary invasive interventions Show forest plot | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.12, 5.28] |
| 6 Mortality Show forest plot | 3 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.13, 4.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 5 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.87, 0.04] |
| 2 Maximum walking distance (long‐term) Show forest plot | 3 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.36, 0.32] |
| 3 Pain‐free walking distance Show forest plot | 5 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.38, 0.29] |
| 4 Pain‐free walking distance (long‐term) Show forest plot | 2 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.26, 0.48] |
| 5 Secondary invasive interventions Show forest plot | 4 | 395 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.70, 2.80] |
| 6 Quality of life (disease‐specific) Show forest plot | 3 | 301 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.04, 0.41] |
| 7 Mortality Show forest plot | 5 | 435 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.00] |
| 8 Sensitivity analysis: maximum walking distance Show forest plot | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.98, ‐0.07] |
| 9 Sensitivity analysis: pain‐free walking distance Show forest plot | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.46, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Supervised exercise therapy | 3 | 432 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.13, 0.64] |
| 1.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 2 Maximum walking distance (long‐term) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Supervised exercise therapy | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 1.18 [0.65, 1.70] |
| 2.2 Pharmacotherapy | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.09, 1.36] |
| 3 Pain‐free walking distance Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Supervised exercise therapy | 2 | 305 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.26, 0.93] |
| 3.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.33, 0.94] |
| 4 Pain‐free walking distance (long‐term) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Pharmacotherapy | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.08, 1.17] |
| 5 Secondary invasive interventions Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Supervised exercise therapy | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.55] |
| 5.2 Pharmacotherapy | 2 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [0.49, 6.83] |
| 6 Quality of life (disease‐specific) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Supervised exercise therapy | 2 | 330 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.56] |
| 6.2 Pharmacotherapy | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.27, 0.91] |
| 7 Mortality Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Supervised exercise therapy | 3 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.20, 2.21] |
| 7.2 Pharmacotherapy | 2 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.14, 11.92] |
| 8 Sensitivity analysis: maximum walking distance Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Supervised exercise therapy | 2 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.21, 0.65] |
| 8.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 9 Sensitivity analysis: pain‐free walking distance Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Supervised exercise therapy | 1 | 212 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.34, 0.89] |
| 9.2 Pharmacotherapy | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.33, 0.94] |

