Saiz insisi yang berbeza untuk phacoemulsification dalam katarak yang berkaitan dengan usia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: parallel‐group RCT Number randomized: 135 eyes of 96 participants in total; 45 eyes of 32 participants in each of the 3 groups Exclusions after randomization: none reported Number analyzed: not reported, assumed to be: 135 eyes of 96 participants in total; 45 eyes of 32 participants in each of the 3 groups Unit of analysis: unclear as trial investigators did not report how 39 participants with both eyes included were analyzed Unit of randomization: unclear as trial investigators did not report whether 39 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: Turkey Mean age (SD): 64.5(NR) overall: 61.5 (8.1) for the B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group 65.8 (13.2) for the larger C‐MICS group 66.2 (12.6) for the standard phacoemulsification with 2.8‐millimeter incision group Gender: 50 men and 46 women overall: 14 men (44%) and 18 women (56%) in the B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group 17 men (55%) and 14 women (45%) in the larger C‐MICS group 19 men (58%) and 14 women (42%) in the standard phacoemulsification with 2.8‐millimeter incision group Inclusion criteria: not reported Exclusion criteria: previous ocular surgery or eye disease that might affect the final visual acuity (amblyopia, corneal scar, glaucoma, retinal or macular disorders, etc.) Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group Intervention 2: larger C‐MICS group Intervention 3: standard phacoemulsification with 2.8‐millimeter incision group The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: VA, pachymetric differences, SIA, phacoemulsification time, CDE (i.e. average ultrasound power), and effective phaco time (time required had 100% power been used throughout), complications Intervals at which outcomes assessed: 1 day, 7 days, 30 days, and 90 days | |
| Notes | Full study name: Coaxial, microcoaxial, and biaxial microincision cataract surgery prospective comparative study Type of study: published Funding sources: not reported Disclosures of interest: No author has a financial or proprietary interest in any material or method mentioned. Study period: November 2006 to September 2008 Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | The study did not report whether participants were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not report how many participants were followed at each time point. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Low risk | Source of funding not reported. Study investigators declared "no author has a financial or proprietary interest in any material or method mentioned" (p740). |
| Methods | Study design: paired‐eye RCT Number randomized: 74 eyes of 37 participants; 37 eyes of 37 participants per group Exclusions after randomization: none reported Number analyzed: 72 eyes of 36 participants at 3 months; 36 eyes of 36 participants per group Unit of analysis: 1 eye of each participant Unit of randomization: both eyes of each participant included; 1 eye is randomized to smaller C‐MICS and the other eye to standard phacoemulsification Losses to follow‐up: 1 participant at 3 months How were missing data handled? excluded from analysis Reported power calculation: no Unusual study design: the study used paired‐eye design, but did not used paired analysis. | |
| Participants | Country: Spain Mean age (SD): 72.97 (NR) years overall; age not reported by group Age range: 52 to 84 years Gender: 12 men (32%) and 25 women (68%) overall; number per group not reported Inclusion criteria: over 50 years of age, bilateral senile cataracts intervened for cataract surgery in both eyes between September 2008 and April 2010 Exclusion criteria: corneal pathology, previous ocular trauma or surgery, infectious and/or inflammatory ocular pathology, and astigmatism exceeding 3 diopters Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: smaller C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision Akreos MI60 IOL (Bausch & Lomb) was inserted for both groups. Length of follow‐up: Planned: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined by the study report: SIA, ECL, CCT, and foveal thickness Intervals at which outcomes assessed: preoperatively and day 1, 1 week, 1 month, and 3 months after surgery | |
| Notes | Full study name: Comparative study of coaxial microincision cataract surgery and standard phacoemulsification Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: September 2008 to April 2010 Trial registration: not reported We emailed the authors and included data provided through email correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The published reported stated that it was a prospective randomized study, but through email correspondence on 24 January 2017, the authors reported assigning treatment by "alternating one‐by‐one." From the published reported: "prospective and randomised study" (p269) |
| Allocation concealment (selection bias) | High risk | The published reported stated that it was a prospective randomized study, but through email correspondence the authors reported assigning treatment by "alternating one‐by‐one." |
| Masking of participants and personnel (performance bias) | High risk | We were informed through email correspondence that participants and personnel were not masked. |
| Masking of outcome assessment (detection bias) | High risk | We were informed through email correspondence that outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | 1 out of 37 participants (less than 3%) lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Trial registration not reported. We were informed through email correspondence that the protocol was not published and the trial was not registered. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Unit of randomization: 1 eye per participant | |
| Participants | Country: Switzerland 75 years for the C‐MICS group 75 years for the standard phacoemulsification group Gender: 22 men (41%) and 28 women (59%) in total: 8 men (32%) and 17 women (68%) in the smaller C‐MICS group 14 men (53%) and 11 women (47%) in the standard phacoemulsification group | |
| Interventions | Intervention 1: smaller C‐MICS with 1.6‐millimeter incision; enlarged to 1.8 mm Length of follow‐up: | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: BCVA, CCT, and ECL | |
| Notes | Full study name: Outcomes of coaxial microincision cataract surgery versus conventional coaxial cataract surgery Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The participants were randomly assigned to have coaxial MICS or conventional coaxial phacoemulsification.”; but sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported. Protocol not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 190 eyes of 151 participants in total: 63 eyes of 52 participants in the smaller C‐MICS group 66 eyes of 52 participants in the larger C‐MICS group 61 eyes of 47 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: 190 eyes of 151 participants in total: 63 eyes of 52 participants in the smaller C‐MICS group 66 eyes of 52 participants in the larger C‐MICS group 61 eyes of 47 participants in the standard phacoemulsification group Unit of analysis: mixed "As the original dataset included both unilateral and bilateral cases, we reported for each comparison the estimates obtained considering all evaluated eyes (unilateral and bilateral cases) and then repeated the analysis after restricting the data set to one eye per patient by choosing one eye randomly (via a computer‐generated algorithm) in bilateral cases (data not shown)." (p262) Unit of randomization: 1 or both eyes per participant were assigned to the same treatment group. Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: not reported, assumed to be in France (all surgeries were performed by the same surgeon (JLF)) 76.6 (7.6) in the smaller C‐MICS group 73.9 (8.4) in the larger C‐MICS group 74.7 (11.3) in the standard phacoemulsification group Age range: not reported 35 women (67%) and 17 men (33%) in the smaller C‐MICS group 37 women (71%) and 15 men (29%) in the larger C‐MICS group 21 women (47%) and 26 men (53%) in the standard phacoemulsification group Inclusion criteria: people undergoing cataract surgery | |
| Interventions | Intervention 1: smaller C‐MICS with MI 60 IOL (Bausch & Lomb, Rochester, NY, USA) inserted Intervention 2: larger C‐MICS with AcrySof Natural SN60WF IOL (Alcon, Fort Worth, TX, USA) inserted Intervention 3: standard phacoemulsification (3.2‐millimeter incision) with Adapt AO IOL (Bausch & Lomb, Rochester, NY, USA) inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: not reported Actual: 1 month | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: keratometric astigmatism (using the same autokeratometer (Tonoref II; Nidek, Aichi, Japan); the Tonoref II measures the corneal curvature based on the projection onto the cornea of 4 near‐infrared rays, scalar comparison of astigmatism, with‐the‐wound change, and against‐the‐wound change | |
| Notes | Full study name: Astigmatic equivalence of 2.2‐mm and 1.8‐mm superior clear corneal cataract incision Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | None of the participants were reported to have been lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry number and protocol were not reported, therefore we could not compare outcomes reported in the published full text and the trial registry/protocol. |
| Other bias | Unclear risk | Trial register number and funding sources were not reported. |
| Methods | Study design: parallel‐group RCT 59 eyes of 43 participants in the smaller C‐MICS group 58 eyes of 42 participants in the standard phacoemulsification group 59 eyes of 43 participants in the smaller C‐MICS group 58 eyes of 42 participants in the standard phacoemulsification group Unit of randomization: unclear whether both eyes of the 32 participants were assigned to the same or different groups | |
| Participants | Country: China 64.8 (8.5) in the smaller C‐MICS group 63.3 (8.8) in the standard phacoemulsification group 21 men (49%) and 22 women (51%) in the smaller C‐MICS group 19 men (45%) and 23 women (55%) in the standard phacoemulsification group (1) people with cataract nucleus graded II‐III, according to the Emery‐Little classification; (2) corneal diopter less than 1.0 diopters; (3) axial lengths 22 to 24.5 mm; (4) endothelial cell density more than 1500 cells/mm2 (1) corneal degeneration, uveitis, glaucoma, high myopia, optic neuropathy, retinopathy, age‐related macular degeneration, history of eye injury, history of LASIK; (2) history of systemic disorders that could not tolerate operation | |
| Interventions | Intervention 1: smaller C‐MICS with Akreos MI60 IOL inserted Intervention 2: standard phacoemulsification with 3.2‐millimeter incision with Adapt‐AO IOL inserted Planned: not reported | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected VA, average ultrasound energy, effective phacoemulsification time, ECL, and corneal topography Adverse events reported: yes, temporary increase in intraocular pressure | |
| Notes | Full study name: 同轴 1. 8mm 微切口白内障超声乳化吸除术的临床研究 [Clinical effects of coaxial 1.8 mm microincision phacoemulsification] Type of study: published full text Funding sources: 陕西省科学技术研究发展计划项目 [Shaanxi Province Science and Technology Research and Development Project (No. 2012K16‐11(5))] Disclosures of interest: not reported Trial registry number: not reported Study period: December 2012 to March 2015 We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “将患者按数字表法随机分为两组" [Participants were randomized into 2 groups using random number tables.] (p1829) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data were reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison. |
| Other bias | Unclear risk | No disclosures of interest, no trial registry number, no protocol |
| Methods | Study design: parallel‐group RCT Number randomized: 60 eyes of 55 participants overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Number randomized: 55 participants of 60 eyes overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: unclear, assumed to be: 60 eyes of 55 participants overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Unit of analysis: unclear, as trial investigators did not report how 5 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 5 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: South Korea Overall mean age: not reported Mean age (SD): 69.7 (NR) overall: 70.6 (9.2) in the larger C‐MICS group 68.8 (7.6) in the standard phacoemulsification group Age range: not reported Gender: 13 men and 15 women in the larger C‐MICS group; 11 men and 16 women in the standard phacoemulsification group Inclusion criteria: not reported Exclusion criteria: "patients with corneal disease, intraocular inflammation, glaucoma, and those who had previously undergone corneal surgery or surgery that could affect high‐order aberrations were excluded" (p1598) | |
| Interventions | Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification (2.8 mm) We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. SA60AT IOL (AcrySof; Alcon, USA) was inserted for both groups. Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary outcome, as defined in study reports: SIA Postoperative change of corneal higher‐order aberrations in each group at 1 month or 3 months after surgery Corneal higher‐order aberrations between the 2 groups preoperatively, at 1 month or 3 months after surgery Intervals at which outcomes assessed: baseline, 1 month and 3 months | |
| Notes | Type of study: published full text Funding sources: Inje University Disclosures of interest: not reported Trial registry number: not reported Study period: July 2006 to December 2006 We attempted to contact the authors but the email was returned undeliverable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: paired‐eye RCT 42 eyes of 42 participants in the larger C‐MICS group 42 eyes of 42 participants in the standard phacoemulsification group 42 eyes of 42 participants in the larger C‐MICS group 42 eyes of 42 participants in the standard phacoemulsification group Unit of randomization: 1 eye per participant | |
| Participants | Country: Korea 64.52 (10.65) in the larger C‐MICS group 65.87 (12.91) in the standard phacoemulsification group | |
| Interventions | Intervention 1: larger C‐MICS group MI60 IOL (Bausch & Lomb, Rochester, NY, USA) was inserted for both groups. | |
| Outcomes | Primary outcome, as defined in study reports: "1. Intraoperative measurements included: 1.1. Ultrasound time (UST) 1.2. Mean cumulative dissipated ultrasound energy (CDE) 1.3. Total balanced salt solution (BSS) use 2. Clinical measurements included preoperative, 1week postoperative, 1month postoperative and 2month postoperative: 2.1. Best corrected visual acuity (BCVA) 2.2. Central corneal thickness (CCT) 2.3. Endothelial cell count (ECC)" Intervals at which outcomes assessed: 1 day, 1 week, 1 month, and 2 months | |
| Notes | Full study name: Comparison of macular thickness and inflammatory cytokine levels after microincision versus small incision coaxial cataract surgery Type of study: published full text Funding sources: "Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2012R1A1A1038648) and the Institute of Clinical Medicine Research of Bucheon St. Mary’s Hospital, Research Fund, BCMC14AA07" (p194) Disclosures of interest: not reported Trial registry number: ISRCTN 69290731 Study period: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Low risk | “One examiner was masked as to whether the images were from the microincision group or the small incision group.” (p191) |
| Incomplete outcome data (attrition bias) | Unclear risk | None of the participants were reported to have been lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the outcomes in the registry have been reported. |
| Other bias | Unclear risk | Disclosures of interest were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 144 eyes of 96 participants overall: 72 eyes of NR participants in the larger C‐MICS group 72 eyes of NR participants in the standard phacoemulsification group Exclusions after randomization: none reported, assumed to be none Number analyzed: 144 eyes of 96 participants overall: 72 eyes of NR participants in the larger C‐MICS group 72 eyes of NR participants in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 48 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 48 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled?: no missing data reported Reported power calculation: no | |
| Participants | Country: South Korea Mean age (SD): 71.49 (NR) overall: 72.30 (10.85) in C‐MICS group 70.67 (8.27) in standard phacoemulsification group Age range: not reported Gender: not reported Inclusion criteria: people with cataract; no specific description Exclusion criteria: "patients with a history of preoperative corneal surgery, patients with diabetic retinopathy or iritis, and those who were previously diagnosed with keratopathy were excluded. The posterior capsular rupture or vitreous loss was excluded from the study." (p709) | |
| Interventions | Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. MI‐60 IOL (Bausch & Lomb, Rochester, NY, USA) was inserted for both groups. Length of follow‐up: Planned: not available | |
| Outcomes | Primary outcome, as defined in study reports: total UST, CDE, average torsional amplitude, fluid amount, case time, CCT increase Adverse events reported: yes, burning of the cornea, no postoperative infection or persistent inflammation Intervals at which outcomes assessed: baseline, 1 day, and 1 month | |
| Notes | Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Authors may have not reported an outcome due to difficulty assigning it. "To assess corneal stress after surgery, it may be helpful to measure the density of endothelial cells and measure the thickness and recovery pattern of the corneal incision site. In the present study, it was difficult to measure endothelial cells in the presence of central corneal edema 1 day after surgery. Therefore, there is a limitation that it is excluded." |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 76 eyes of 71 participants in total: 37 eyes of NR participants in the larger C‐MICS group 39 eyes of NR participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be: 76 eyes of 71 participants total; 37 eyes in the larger C‐MICS group 39 eyes in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 5 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 5 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: Korea Mean age: overall 68.53 years: 66.83 years in the larger C‐MICS group 69.25 years in the standard phacoemulsification group Age range: 57 to 85 overall Gender: 39 men (55%) and 32 women (45%) overall; number per group not reported Inclusion criteria: age‐related cataracts included eyes with grade II‐IV nuclear cataracts (Lens Opacities Classification System (LOCS) III) Exclusion criteria: “presence of pre‐existing corneal disease or degeneration, an incision that required enlarging to insert an IOL, and complicated or excessively prolonged surgery” Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision They type of IOL was not reported; "in both the 2.2 and 2.8 mm groups, the IOL type and the IOL injector system were matched, except for the incision size, to minimize bias." (p201) Length of follow‐up: Planned: 1 month | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: CDE, UST, ECL, and CCT Adverse events reported: not reported Intervals at which outcomes assessed: 1 day before surgery, and postoperatively at 1 day, 1 week, and 1 month | |
| Notes | Full study name: Early changes in corneal edema following torsional phacoemulsification using anterior segment optical coherence tomography and Scheimpflug photography Type of study: published Funding sources: Korea Healthcare Technology R&D Project, Ministry for Health Welfare & Family Affairs, Republic of Korea (A 090573) Disclosures of interest: not reported Study period: May to July 2009 Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Low risk | Source of funding reported, conflicts of interest not reported. "This work was supported by the Korea Healthcare Technology R&D Project, Ministry for Health Welfare & Family Affairs, Republic of Korea (A 090573)." (p203) |
| Methods | Study design: parallel‐group RCT Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant | |
| Participants | Country: China 51.5 (4.2) in C‐MICS 2.2 mm and IOL implantation group 50.2 (3.9) in standard phacoemulsification 3.0 mm and IOL implantation group Age range: 45 to 62 overall: 45 to 62 in coaxial C‐MICS 2.2 mm and IOL implantation group 46 to 61 in standard phacoemulsification 3.0 mm and IOL implantation group 25 men (56%) and 20 women (44%) in C‐MICS 2.2 mm and IOL implantation group 24 men (53%) and 21 women (47%) in standard phacoemulsification 3.0 mm and IOL implantation group | |
| Interventions | Intervention 1: C‐MICS 2.2 mm and IOL implantation Intervention 2: standard phacoemulsification 3.0 mm and IOL implantation Akreos MI60 IOL was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 30 days | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: dry eye symptom score, break‐up time, Schirmer's 1 test, and corneal fluorescein staining score Adverse events reported: yes, temporary increase in intraocular pressure | |
| Notes | Full study name: Changes of the ocular surface and tear film after clear corneal incision phacoemulsification with different incision sizes Type of study: published full text Funding sources: "海南省卫生厅科研项目(No.琼卫2012PT‐28)作者单位:(570102)中国海南省海口市,海南医学院附属医院眼科" (p80) Research of Hainan Provincial Health Department (No.琼卫2012PT‐28) Disclosures of interest: not reported Study period: May 2013 to May 2014 Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Low risk | Source of funding reported, conflicts of interest not reported "海南省卫生厅科研项目(No. 琼卫2012PT‐28) |
| Methods | Study design: parallel‐group RCT 38 eyes of NR participants in the larger C‐MICS group 40 eyes of NR participants in the standard phacoemulsification (3.0‐millimeter incision) group 78 eyes of 56 participants in total: 38 eyes of NR participants in the larger C‐MICS group 40 eyes of NR participants in the standard phacoemulsification (3.0‐millimeter incision) group Unit of analysis: unclear as trial investigators did not report how 22 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 22 participants were assigned to the same or different groups | |
| Participants | Country: China age‐related cataract with age over 50 with preoperation endothelial cell count larger than 1200/mm2 diabetic retinopathy, glaucoma, age‐related macular degeneration, previous operation, posterior capsule rupture during surgery, vitreous loss in the surgery | |
| Interventions | Intervention 1: C‐MICS (2.2‐millimeter incision) Intervention 2: standard phacoemulsification (3.0‐millimeter incision) SN60WF IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 3 months | |
| Outcomes | Primary and secondary outcomes were not differentiated. Outcomes, as defined in the study reports: uncorrected VA, BCVA, corneal astigmatism, SIA | |
| Notes | Full study name: 同轴微切口白内障超声乳化术后角膜散光的临床观察 Observation of corneal astigmatism induced by 2.2‐millimeter microincision coaxial phacoemulsification Type of study: published full text Funding sources: 佛山市卫生局医学科研立项课题 (No. 2011162) Medical scientific research program of Foshan board of health (No. 2011162) Disclosures of interest: not reported Trial registry number: not reported Study period: January to September 2011 We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “将患者按就诊顺序登记并按随机表法分成两组,分别施行 2郾 2mm 同轴微切口白内障超声乳化联合 IOL 植入术及 3.0mm 常规白内障超声乳化联合 IOL 植入术,其中 2.2mm 组 38 眼,3. 0mm 组 40 眼。 “排除增殖期糖尿病视网膜病变、青光眼、老年性黄斑变性等眼部疾病及既 往有内眼手术史的患者。 术中出现后囊膜破裂、玻璃体溢 出等并发症者、不能完成 3mo 随访者予以剔除。 ” (p1465) Randomized table was used to distribute the participants into different groups. Diabetic retinopathy, glaucoma, age‐related macular degeneration, previous operation, posterior capsule rupture during surgery, vitreous loss in the surgery. Postrandomization exclusions exist. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Participants were excluded postrandomization. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry number and protocol were not available for comparison. |
| Other bias | Unclear risk | Funding sources and disclosures of interests were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 120 eyes of 120 participants total; 40 eyes of 40 participants per group Exclusions after randomization: none Number analyzed: 120 eyes of 120 participants total; 40 eyes of 40 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data Reported power calculation: no Unusual study design: no | |
| Participants | Country: China Mean age: overall mean age not reported; 73.95 years for 1.8 mm; 71.37 years for 2.2 mm; 72.48 years for 3.0 mm Gender: 58 men and 62 women overall; 21 men (53%) and 19 women (47%) in the smaller C‐MICS group 18 men (45%) and 22 women (55%) in the C‐MICS (2.2‐millimeter incision) group 19 men (47%) and 21 women (53%) in the standard phacoemulsification (3.0‐millimeter incision) group Inclusion criteria: age between 55 and 85 years, the presence of nuclear or corticonuclear cataract of grades 2.0 to 4.0 (Lens Opacities Classification System III), a transparent central cornea, pupil dilation 7 mm at the time of preoperative examination, and a preoperative central endothelial cell count of 1500 cells/mm2 Exclusion criteria: previous intraocular surgery, glaucoma, pseudoexfoliation, uveitis, high myopia, and diabetes mellitus Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: smaller C‐MICS group with Akreos MI 60 IOL (Bausch & Lomb) inserted Intervention 2: C‐MICS (2.2‐millimeter incision) group with SN60WF IOL (Alcon) inserted Intervention 3: standard phacoemulsification (3.0‐millimeter incision) group with SA60AT IOL (Alcon) inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: UST, surgical time, CDE and the total BSS volume, intraoperative measurements of corneal incision size during surgery, change in maximal clear corneal incision thickness as evaluated by AS‐OCT, BCVA, central cornea ECL, and SIA Intervals at which outcomes assessed: postoperatively at 1 day, 1 week, 1 month, 3 months | |
| Notes | Full study name: Clinical evaluation of three incision size–dependent phacoemulsification systems Type of study: published Funding sources: Natural Science Foundation of China (30973277) and the Key Projects for Hospital Clinical Disciplines Fund of the Chinese Ministry of Health in 2010–2012 (Project No.175 in Document 439 of the Planning and Finance Secretary of Ministry of Health, China) Disclosures of interest: “The authors indicate no financial conflict of interest.” Study period: July 2010 to January 2011 Trial registration: NCT01429532 (ClinicalTrials.gov) We attempted to contact the authors but the email was returned undeliverable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A number between 1 and 120, generated by a random number generator, was assigned to each subject. The number was divided by 3. If the remainder was 1, the patient was assigned to Group I; if the remainder was 2, the patient was assigned to Group II; and if the number was divisible by 3, the patient was assigned to Group III." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Low risk | "Both the technician and the surgeon were masked during postoperative examinations to the participants' group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | "All participants completed all follow‐up visits." |
| Selective reporting (reporting bias) | Low risk | This clinical trial has been registered at www.clinicaltrials.gov, registration number NCT01429532. All outcomes were reported. |
| Other bias | Low risk | Source of funding and conflicts of interest reported. "Publication of this clinical study was supported by the Natural Science Foundation of China (30973277)" (p838) "The authors indicate no financial conflict of interest." (p838) |
| Methods | Study design: parallel‐group RCT Number randomized: 160 eyes of 160 participants total; 80 eyes of 80 participants in each group Exclusions after randomization: none reported Number analyzed: 160 eyes of 160 participants; 80 eyes of 80 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none at 1 month How were missing data handled? not reported Reported power calculation: no Unusual study design: no | |
| Participants | Country: China Mean age (SD): 69 (NR) overall; not reported by group Age range: 48 to 82 years Gender: 84 men (52%) and 76 women (48%) overall; number per group not reported Inclusion criteria: age‐related cataract Exclusion criteria: people with glaucoma, diabetes, or other eye diseases were excluded Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: B‐MICS (1.4‐millimeter incision) group with Acri.Smart46s (Acti.Tec) IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) group with SENSER IOL (AMO) inserted. We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: not reported Actual: 1 month | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected VA and SIA Intervals at which outcomes assessed: preoperatively and postoperatively at 1 day, 1 week, and 1 month | |
| Notes | Full study name: Analysis of the visual quality after bimanual phacoemulsification via micro‐incision Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: August 2005 to February 2006 Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 30 eyes of 30 participants; 15 eyes of 15 participants per group Exclusions after randomization: not reported Number analyzed: not reported Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none How were missing data handled? not reported Reported power calculation: no Unusual study design? none | |
| Participants | Country: Italy Mean age (SD): 69.8 (NR) overall: 70.11 (NR) smaller C‐MICS group 69.44 (NR) C‐MICS (2.2‐millimeter incision) group Gender: not reported Inclusion criteria: age 65 to 75 years, axial length 23.0 to 24.0 mm, corneal astigmatism less than 3.00 diopters, nuclear cataract of grade 4 (nuclear opalescence ‐ NO4, Lens Opacities Classification System III), and corneal endothelial cell count greater than 1200/mm2 Exclusion criteria: anterior segment pathological alterations such as keratoconus, chronic uveitis, zonular dialysis, pseudoexfoliation syndrome, glaucoma, and diabetes mellitus; other ocular pathologies impairing visual function; previous anterior or posterior segment surgery; and intraoperative or postoperative complications Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: smaller C‐MICS Intervention 2: C‐MICS (2.2‐millimeter incision) AcrySof SN60WF IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: not reported | |
| Outcomes | Primary outcome, as defined in study reports: uncorrected VA and BCVA, keratometric astigmatism, endothelial cell count, and corneal thickness at incision site. The amount and axis of astigmatic change induced by the cataract surgery were assessed by calculating the SIA. Power vector analysis of keratometric astigmatic change between preoperative and postoperative values was performed. Intervals at which outcomes assessed: postoperatively at 1 day, 7 days, 30 days, and 90 days | |
| Notes | Full study name: Microcoaxial torsional cataract surgery 1.8 mm versus 2.2 mm: functional and morphological assessment Type of study: published Funding sources: not reported Disclosures of interest: the authors have no financial or proprietary interest in the materials Study period: not reported Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to group 1 or group 2 the day before surgery by block randomization (randomly assigned by computer‐generated numbers)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants and examiners performing preoperative and postoperative controls were masked to the surgical technique used in each case" |
| Masking of outcome assessment (detection bias) | Low risk | "Participants and examiners performing preoperative and postoperative controls were masked to the surgical technique used in each case" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Low risk | Source of funding not reported. "The authors have no financial or proprietary interest in the materials presented herein." (p114) |
| Methods | Study design: parallel‐group RCT Number randomized: 100 eyes of 89 participants overall: 32 eyes of NR participants in the smaller C‐MICS group 38 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 30 eyes of NR participants in standard phacoemulsification with 2.8‐millimeter incision group Exclusions after randomization: none reported Number analyzed: 100 eyes of 89 participants overall: 32 eyes of NR participants in the smaller C‐MICS group 38 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 30 eyes of NR participants in standard phacoemulsification with 2.8‐millimeter incision group Unit of analysis: unclear as trial investigators did not report how 11 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 11 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: South Korea Mean age (SD): 65.2 (NR) overall: 64.5 (10.5) years old in the smaller C‐MICS group 61.8 (15.3) years old in C‐MICS (2.2‐millimeter incision) group 69.4 (10.0) years old in standard phacoemulsification with 2.8‐millimeter incision group Age range: not reported Gender: 30 men and 59 women total; number per group not reported Inclusion criteria: "the subjects with corneal astigmatism less than 2.25 diopters were examined" (p408) Exclusion criteria: "the patients who underwent corneal surgery including refractive surgery, eyes that can not measure the corneal curvature due to corneal opacity, surgery that affects corneal astigmatism during surgery, or other types of intraocular lenses were excluded from the study." (p408) | |
| Interventions | Intervention 1: smaller C‐MICS with MI60 IOL (Bausch & Lomb, Rochester, NY, USA) Intervention 2: C‐MICS (2.2‐millimeter incision) with AcrySof IQ IOL (Alcon, Fort Worth, TX, USA) Intervention 3: standard phacoemulsification (2.8‐millimeter incision) with Akreos AO IOL (Bausch & Lomb) We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: no protocol available Actual: baseline, 1 and 3 months | |
| Outcomes | Primary outcome, as defined in study reports: SIA and high‐order aberrations (coma, trefoil, and spherical aberration) Intervals at which outcomes assessed: baseline, 1 and 3 months | |
| Notes | Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Study period: March 2009 to August 2009 Trial registry number: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources, disclosures of interests were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 94 eyes of 64 participants total; 43 eyes in standard phacoemulsification group, 51 eyes in B‐MICS group Exclusions after randomization: none Number analyzed: 94 eyes of 64 participants; 43 eyes in standard phacoemulsification group, 51 in B‐MICS group Unit of analysis: unclear as trial investigators did not report how 30 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 30 participants were assigned to the same or different groups Losses to follow‐up: standard phacoemulsification group had none reported at 1 month, 3 participants at 3 months and 5 participants at 6 months; B‐MICS had none reported at 1 month, 3 participants at 3 months, and 3 participants at 6 months. How were missing data handled? not reported Reported power calculation: no Unusual study design? no | |
| Participants | Country: Spain Mean age (SD): 70.53 (NR) overall: 69.02 (NR) years for standard phacoemulsification 72.04 (NR) years for B‐MICS Gender: 24 men (37%) and 40 women (63%) overall; number not reported by group Inclusion criteria: people scheduled for elective cataract surgery Exclusion criteria: topographic astigmatism 42.0 diopters, intraoperative use of corneal sutures, or corneal disease Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: B‐MICS (1.5‐ to 2‐millimeter incision) with Acri.Smart 48S IOL (Acri.Tec, Hennigsdorf, Germany) inserted Intervention 2: standard phacoemulsification (2.8‐millimeter incision) with Y601075 (AJL, A ́lava, Spain) Length of follow‐up: Planned: not reported Actual: 6 months | |
| Outcomes | Primary outcome, as defined in study reports: SIA and BCVA Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: preoperatively and postoperatively at 1, 3, and 6 months | |
| Notes | Full study name: Surgically induced astigmatism after biaxial phacoemulsification compared to coaxial phacoemulsification Type of study: published Funding sources: not reported Disclosures of interest: no Study period: not reported Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Neither the patient nor the examiner knew which technique had been applied."; double‐blind clinical trial |
| Masking of outcome assessment (detection bias) | Low risk | "Neither the patient nor the examiner knew which technique had been applied." |
| Incomplete outcome data (attrition bias) | Low risk | 8 out of 94 participants (9.6%) were lost to follow‐up at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: paired‐eye RCT Number randomized: 60 eyes of 30 participants in total; 30 eyes of 30 participants per group Exclusions after randomization: not reported Number analyzed: not reported, assumed to be 60 eyes of 30 participants in total; 30 eyes of 30 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: none | |
| Participants | Country: Bosnia and Herzegovina Mean age (SD): 63.6 (NR) overall: 65.13 (NR) in standard phacoemulsification (3.0‐millimeter incision) group 62.06 (NR) in the larger C‐MICS group Gender: not reported Inclusion criteria: people from everyday operational program cataract surgery at the Eye Clinic of University Clinical Center Tuzla in the period of October 2009 to December 2009. People planned for cataract surgery and who met the inclusion criteria of the study were included. Exclusion criteria: not reported Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: standard phacoemulsification (3.0‐millimeter incision) Intervention 2: larger C‐MICS The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: postoperatively at 1, 7, and 30 days; extended to 90 days for SIA | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected distance VA, corneal keratometry, and corneal astigmatism and SIA were assessed 1, 7, and 30 days after cataract surgery. Intervals at which outcomes assessed: postoperatively at 1, 7, and 30 days; extended to 90 days for SIA | |
| Notes | Full study name: Corneal astigmatism after micro‐incision cataract operation Type of study: published Funding sources: not reported Disclosures of interest: none Study period: October 2009 to December 2009 Trial registration: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | The methods describe 4 outcomes; 2 of them, corneal keratometry and corneal astigmatism, were not found in the results section. |
| Other bias | Low risk | Source of funding not reported. "Conflict of interest: none declared." (p128) |
| Methods | Study design: parallel‐group RCT Number randomized: 362 eyes of 362 participants in total: 211 eyes of 211 participants in C‐MICS 2.2‐millimeter group 151 eyes of 151 participants in standard phacoemulsification 2.8‐millimeter group Exclusions after randomization: none reported Number analyzed: 362 eyes of 362 participants in total: 211 eyes of 211 participants in C‐MICS 2.2‐millimeter group 151 eyes of 151 participants in standard phacoemulsification 2.8‐millimeter group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: none | |
| Participants | Country: China age‐related cataract, undertaking clear corneal incision phacoemulsification + implantation of posterior chamber foldable intraocular lens high myopia, keratopathy, central corneal endothelial cell < 1800/mm2, fundus diseases, glaucoma, diabetic retinopathy, uveitis, trauma, previous operation | |
| Interventions | Intervention 1: C‐MICS 2.2‐millimeter group Intervention 2: standard phacoemulsification 2.8‐millimeter group The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: 1 month | |
| Outcomes | Primary outcome, as defined in study reports: uncorrected VA, BCVA, corneal astigmatism, corneal endothelial cell counting, ultrasonic energy, phacoemulsification time Intervals at which outcomes assessed: 1 week and 1 month | |
| Notes | Full study name: 同轴 2.2 mm 与 2.8 mm 切口白内障超声乳化手术疗效 评价 [Evaluation on curative effect of coaxial 2.2‐ and 2.8‐millimeter incision phacoemulsification for cataract] Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Study period: July 2014 to March 2015 Trial registry number: not reported We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources, disclosures of interests were not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 132 eyes of 98 participants overall: 60 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 72 eyes of NR participants in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: none Number analyzed: not reported Unit of analysis: unclear as trial investigators did not report how 34 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 34 participants were assigned to the same or different groups Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: none | |
| Participants | Country: China Mean age (SD): 72 (NR) overall: 71 (NR) in C‐MICS (2.2‐millimeter incision) 73 (NR) in standard phacoemulsification (3.0‐millimeter incision) Gender: not reported Inclusion criteria: people with cataract with endothelial cell > 2000/mm2 Exclusion criteria: people with corneal degeneration, uveitis, or glaucoma Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: C‐MICS (2.2‐millimeter incision) with AcrySof IQ IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) with AcrySof Natural IOL inserted Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: average ultrasound power, BCVA, SIA, ECL, incidence of capsule rupture and corneal edema Adverse events reported: yes; posterior capsular rupture and corneal edema Intervals at which outcomes assessed: postoperatively at 1 day, 1 month, and 3 months | |
| Notes | Full study name: Clinical evaluation on the coaxial microincision cataract surgery in hard nuclear cataracts Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: October 2011 to October 2012 Trial registration: NCT01385878 (ClinicalTrials.gov) We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. The study included both eyes of some participants in the same group, but did not account for intraperson correlation. |
| Methods | Study design: parallel‐group RCT Number randomized: 100 eyes of 100 participants in total; 50 eyes of 50 participants per group Exclusions after randomization: not reported Number analyzed: 100 eyes of 100 participants in total; 50 eyes of 50 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none How were missing data handled? not reported Reported power calculation: yes: “A sample size of 100 participants was computed ... A difference of 0.175 log units or more was considered ... A sample size of 50 participants in each group would have an 80% power to capture the above‐mentioned difference between the 2 groups” (p566‐7) Unusual study design: no | |
| Participants | Country: India Mean age (NR): 63.54 (NR) years overall: 64.42 (NR) years in the smaller C‐MICS group 62.67 (NR) years in C‐MICS (2.2‐millimeter incision) group Gender: 50 men (50%) and 50 women (50%) overall: 24 men (48%) and 26 women (52%) in the smaller C‐MICS group 26 men (52%) and 24 women (48%) in C‐MICS (2.2‐millimeter incision) group Inclusion criteria: people with uncomplicated ARC. People having nuclear or corticonuclear cataracts of grade 2 to 4 according to the Lens Opacities Classification System III were included. Ages eligible for study: 50 to 80 years Exclusion criteria: glaucoma, shallow anterior chamber (anterior chamber depth < 2.1 mm), pupil dilation less than 6.0 mm, extremely dense cataract, posterior polar cataract, subluxated cataract, white mature cataract, diabetic retinopathy, high myopia (defined as axial length > 25 mm), uveitis, or previous ocular trauma or surgery Equivalence of baseline characteristics (Y/N): yes | |
| Interventions | Intervention 1: corneal incision system with the Stellaris PC system (Bausch & Lomb) using longitudinal ultrasound (C‐MICS 1.8‐millimeter incision) with MI60 IOL (Bausch & Lomb) inserted Intervention 2: corneal incision system with the Infiniti Vision system (Alcon) using torsional ultrasound (C‐MICS 2.2‐millimeter) with AcrySof SN60WF IOL (Alcon) inserted Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports Ingress of trypan blue from the ocular surface into the anterior chamber, SIA at 3 months Intraoperative outcome measures: total surgical time, CDE, volume of BSS, incision enlargement, trypan blue ingress into the anterior chamber Postoperative outcome measures: anterior segment optical coherence tomography ‐ incision morphology, localized Descemet membrane detachment (postoperatively at 1 day, 1 week, or 1 month), endothelial gaping or misalignment (postoperative at 1 day and 1 week), epithelial gaps (postoperatively at 1 day and 1 week), anterior segment inflammation (postoperatively at 1 day, 1 week, or 1 month), corneal endothelial morphology (postoperatively at 3 months), CCT (postoperatively at 1 day, 1 week, or 1 month), corneal clarity (postoperatively at 1 day, 1 week, and 1 month) Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: postoperatively at 1 day, 7 days, 1 month, and 3 months | |
| Notes | Full study name: Incision integrity and postoperative outcomes after microcoaxial phacoemulsification performed using 2 incision‐dependent systems Type of study: published Funding sources: not reported Disclosures of interest: Iladevi Cataract & IOL Research Centre receives occasional travel support from Alcon Laboratories, Inc. No author has a financial or proprietary interest in any material or method mentioned. Study period: September 2011 to February 2012 Trial registration: NCT01385878 (ClinicalTrials.gov) We emailed the authors and included data provided through email correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. We contacted the study investigator, and received the following reply: "Randomization was done using computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | From an email correspondence on 24 January 2017: "we prepared sequenced and sealed envelopes containing one of the two options which were stored in a dedicated box in the operating room. An unscrubbed nurse (1 of 2) opened the envelope and informed the operating surgeon of the incision size and phacoemulsification system to be used just prior to beginning of the surgery." |
| Masking of participants and personnel (performance bias) | Low risk | Both participants and outcome assessors were masked. We contacted the study investigator, and received the following reply: "The patients and outcome assessors were blinded to the technique used, however the surgeon was not. The outcome assessor was another ophthalmologist, and not the operating surgeon." |
| Masking of outcome assessment (detection bias) | Low risk | "This prospective randomized patient‐ and analyzer‐masked clinical trial comprised participants with uncomplicated age‐related cataract"; we contacted the study investigator, and received the following reply: "The patients and outcome assessors were blinded to the technique used, however the surgeon was not. The outcome assessor was another ophthalmologist, and not the operating surgeon." |
| Incomplete outcome data (attrition bias) | Low risk | "No participants were lost to follow‐up." |
| Selective reporting (reporting bias) | Low risk | The study is registered with ClinicalTrials.gov (NCT01385878). The outcomes described in the protocol are consistent with those in the paper. |
| Other bias | Unclear risk | "Iladevi Cataract & IOL Research Centre receives occasional travel support from Alcon Laboratories, Inc. No author has a financial or proprietary interest in any material or method mentioned." (p563) |
| Methods | Study design: parallel‐group RCT Number randomized: 83 participants (129 eyes) in total: NR participants (43 eyes) in C‐MICS (2.2‐millimeter incision) group NR participants (42 eyes) in standard phacoemulsification (2.6‐millimeter incision) group NR participants (44 eyes) in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: none reported Number analyzed: 83 participants (129 eyes) in total: NR participants (43 eyes) in C‐MICS (2.2‐millimeter incision) group NR participants (42 eyes) in standard phacoemulsification (2.6‐millimeter incision) group NR participants (44 eyes) in standard phacoemulsification (3.0‐millimeter incision) group Unit of analysis: unclear as trial investigators did not report how 46 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 46 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled?: no missing data reported Reported power calculation: no | |
| Participants | Country: China Overall mean age: 72 years Mean age (SD): 69 (9) in C‐MICS (2.2‐millimeter incision) group 69 (7) in standard phacoemulsification (2.6‐millimeter incision) group 71 (8) in standard phacoemulsification (3.0‐millimeter incision) group Overall age range: 49 to 83 years Gender: 33 men (40%) and 50 women (60%) Inclusion criteria: people diagnosed with age‐related cataract Exclusion criteria: "patients with corneal disease, glaucoma, uveitis, age‐related maculopathy, high myopia, or history of ocular trauma or surgery were excluded." (p665) Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: C‐MICS (2.2‐millimeter incision) Intervention 2: standard phacoemulsification (2.6‐millimeter incision) (excluded from review) Intervention 3: standard phacoemulsification (3.0‐millimeter incision) AcrySof Natural SN60AT IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: protocol not available | |
| Outcomes | Outcomes, as defined in study reports: SIA, BCVA, CDE, CCT, anterior chamber depth, ultrasound time Intervals at which outcomes assessed: 1 and 3 months | |
| Notes | Full study name: The effect of micro‐incision and small‐incision coaxial phaco‐emulsification on corneal astigmatism Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: September 2006 to February 2007 We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomized into three groups: 43 eyes into the 2.2‐mm group, 42 eyes into the 2.6‐mm group and 44 eyes into the 3.0‐mm group.” (p665) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Patients and examiners were masked to the group assignment.” (p665) |
| Masking of outcome assessment (detection bias) | Low risk | "Patients and examiners were masked to the group assignment.” (p665) |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 280 eyes total: 146 eyes in B‐MICS group 134 eyes in standard phacoemulsification group Exclusions after randomization: none Number analyzed: 280 eyes total: 146 eyes in B‐MICS group 134 eyes in standard phacoemulsification group Unit of analysis: unclear as number of participants in each group was not reported Unit of randomization: unclear as number of participants in each group was not reported Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: no | |
| Participants | Country: China Mean age : 69 years overall: 69 years in B‐MICS group 69 years in standard phacoemulsification group Gender: 126 men (45%) and 154 women (55%) overall: 64 men (44%) and 82 women (56%) in B‐MICS group 62 men (46%) and 72 women (54%) in standard phacoemulsification group Inclusion criteria: not reported Exclusion criteria: people with corneal diseases, glaucoma, and uveitis or surgery history of eye were excluded. Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: B‐MICS (1.5‐millimeter incision) group with Acri.Smart46s (Acti.Tec) IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) group with Staar KS‐1 (Canon) IOL inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary outcome, as defined in study reports: phacoemulsification time, VA, CCT, ECL, SIA Adverse events reported: no Intervals at which outcomes assessed: postoperatively at 1 day and 3 months | |
| Notes | Full study name: Clinical evaluation on the bimanual microincision cataract surgery Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 2006 to May 2007 Trial registration: not reported Data were clarified by an author of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used 'random numbers table' for sequence generation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants were masked to their treatment groups" |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors were masked to their treatment groups" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 89 eyes of 89 participants in total: 45 eyes of 44 participants in the smaller C‐MICS group 44 eyes of 44 participants in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: 1 eye of 1 participant in microincision cataract surgery (1.8‐millimeter incision) group Number analyzed: 80 eyes of 80 participants total; 40 eyes in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: 9 participants How were missing data handled? not reported Reported power calculation: no Unusual study design: no | |
| Participants | Country: China Mean age: 72 years overall; number not reported by group Gender: 29/80 men (36%) and 51/80 women (64%) overall; number not reported by group Inclusion criteria: ARC with astigmatism < 2.00 diopters Exclusion criteria: people with corneal diseases, uveitis, glaucoma, age‐related macular degeneration, high myopia, eye trauma, or surgery history of eyes were excluded. Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: smaller C‐MICS with Akreos MI 60 IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) with Akreos Adapt IOL inserted Length of follow‐up: Planned: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: average ultrasound power, effective phacoemulsification time, uncorrected VA, BCVA, ECL, SIA Intervals at which outcomes assessed: postoperatively at 1 day, 1 week, 1 month, and 3 months | |
| Notes | Full study name: Clinical evaluation on the coaxial 1.8 mm microincision cataract surgery Type of study: published Funding sources: National Eleventh Five‐Year Technology Support Program (2006BAI02B04), Zhejiang Key Creative Group of Technology (2009R50039) Disclosures of interest: not reported Study period: July 2009 to May 2010 Trial registration: not reported Data were clarified by an author of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used random numbers table for sequence generation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants were masked to their treatment groups" |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors were masked to their treatment groups" |
| Incomplete outcome data (attrition bias) | Low risk | 9 out of 89 participants (10%) lost to follow‐up, but reasons not reported. 4 cases dropped out at 1 month, 5 extra cases dropped out at 3 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: parallel‐group RCT Number randomized: 150 eyes of 150 participants in total; 50 eyes of 50 participants in each group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be 150 eyes of 150 participants in total; 50 eyes of 50 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no | |
| Participants | Country: China 70.30 (6.67) in the smaller C‐MICS group 70.68 (6.98) in microincision (2.0‐millimeter) group 70.34 (7.23) in standard phacoemulsification group | |
| Interventions | Intervention 1: smaller C‐MICS group with Akreos MI 60 IOL (Bausch & Lomb) inserted Intervention 3: standard phacoemulsification (3.0‐millimeter incision) group with PY‐60 IOL implantation (Hoya, Japan) inserted | |
| Outcomes | Outcomes, as defined in study reports: intraoperative data and postoperative outcomes including SIA, corneal incision thickness, wavefront aberrations and modulation transfer function of cornea were obtained. | |
| Notes | Full study name: A comparable study of clinical and optical outcomes after 1.8, 2.0 mm microcoaxial and 3.0 mm coaxial cataract surgery Type of study: published full text Funding sources: supported by the Key Program of the National Natural Science Foundation of China (No. 81130018); National Twelfth Five‐Year Plan Foundation of China (No. 2012BAI08B01); Zhejiang Key Innovation Team Project of China (No. 2009R50039); Zhejiang Key Laboratory Fund of China (No. 2011E10006) Disclosures of interest: "none" (p404) Trial registry number: ChiCTR‐TRC‐12002565 Study period: not reported Data were clarified by an author of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | "The postoperative follow‐up was performed by the same independent examiner, who did not perform any of the surgeries." |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was not reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in the trial registry were reported. |
| Other bias | Low risk | Reported no conflict of interest and government‐funded trial |
| Methods | Study design: parallel‐group RCT 84 eyes of 80 participants in the smaller C‐MICS group 84 eyes of 83 participants in the standard phacoemulsification group 84 eyes of 80 participants in the smaller C‐MICS group 84 eyes of 83 participants in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 8 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 8 participants were assigned to the same or different groups | |
| Participants | Country: China 67.5 in the smaller C‐MICS group 69.8 in the standard phacoemulsification group Age range: 50 to 83 years overall: 50 to 83 years in the smaller C‐MICS group 52 to 81 years in the standard phacoemulsification group 45 women and 35 men in the smaller C‐MICS group 38 women and 42 men in the standard phacoemulsification group | |
| Interventions | Intervention 1: smaller C‐MICS group with ZEISS ASPHINA 509M IOL inserted Intervention 2: standard phacoemulsification with 3.2‐millimeter incision group with ZEISS SPHERIS 209M IOL inserted Length of follow‐up: Planned: protocol not available Actual: 1 day, 1 week, and 1 month | |
| Outcomes | Outcomes, as defined in study reports: postoperative astigmatism, uncorrected VA Intervals at which outcomes assessed: 1 day, 1 week, 1 month | |
| Notes | Full study name: Effect of 1.8 coaxial micro‐incision cataract phacoemulsification on corneal astigmatism Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: January 2012 to June 2012 We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
| Methods | Study design: paired‐eye RCT Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant | |
| Participants | Country: China Mean age : 67.7 overall: 66.8 years in the larger C‐MICS group 68.4 years in the standard phacoemulsification 3.0‐millimeter group Age range: 39 to 83 years overall: 39 to 80 years in the larger C‐MICS group 48 to 83 years in the standard phacoemulsification 3.0‐millimeter group Gender: 24 men (53%) and 21 women (47%) in total and by group | |
| Interventions | Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 3.0‐millimeter incision The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported | |
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: VA, corneal endothelial cell counting, CCT, SIA Intervals at which outcomes assessed: 1 day, 1 week, 1 month, 3 months | |
| Notes | Full study name: 不同切口同轴白内障超声乳化术的疗效比较 [Comparison of 2.2‐millimeter microincision and 3.0‐millimeter incision coaxial phacoemulsification] Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: January 2012 to June 2013 We attempted to contact the authors but did not receive any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses to follow‐up or missing data were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources and disclosure of interest not reported. |
ARC: age‐related cataract
AS‐OCT: anterior segment optical coherence tomography
B‐MICS: biaxial microincision cataract surgery
BCVA: best‐corrected visual acuity
BSS: balanced salt solution
C‐MICS: coaxial microincision cataract surgery
CCT: central corneal thickness
CDE: cumulative dissipated energy
ECL: endothelial cell loss
IL‐1alpha: interleukin 1 alpha
IL6: interleukin 6
IOP: intraocular lens
LOCS: Lens Opacities Classification System
NR: not reported
PGE2: prostaglandin E2
RCT: randomized controlled trial
SD: standard deviation
SIA: surgically induced astigmatism
UST: ultrasound time
VA: visual acuity
VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT, but included the wrong intervention as the B‐MICS group's final incision size was 1.7 ± 0.21 mm, which overlapped with 2 of our comparison groups | |
| RCT, but included the wrong intervention as B‐MICS group's final incision size range was from 1.6 to 1.8 mm, which overlapped with 2 of our comparison groups | |
| RCT, but included the wrong intervention as the authors compared phacoemulsification versus manual small‐incision cataract surgery without phacoemulsification, removing cataract mechanically | |
| Not an RCT; this was a cohort study, and wrong intervention as incisions were enlarged before IOL implantation | |
| RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final size overlapped with our comparison groups. | |
| RCT, but wrong intervention as the incisions in standard phacoemulsification group were 2.65 mm, not in the range of 2.75 to 3.2 mm | |
| RCT, but wrong intervention as the incisions in B‐MICS group were enlarged to 2.8 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong intervention as the incisions in B‐MICS group were 1.7 mm | |
| RCT, but wrong intervention as 2.8‐millimeter incision was enlarged to 5.2 mm at the end of surgery | |
| RCT, but wrong participants as incisions were enlarged in B‐MICS group to 1.8 mm before IOL implantation. The final incision size overlapped with the smaller C‐MICS group. | |
| RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong intervention as single stitch was made in all participants, which affected the surgically induced astigmatism | |
| RCT, but wrong comparison as both intervention groups used the same incision size | |
| RCT, but the conference abstract did not report the incision sizes created during microcoaxial and conventional phacoemulsification. Author contact information was not available through the conference website or other publications in PubMed. | |
| RCT, but wrong comparison as the final incision size for the standard phacoemulsification group was 2.65 mm, which was not within our range of 2.75 mm to 3.2 mm | |
| RCT, but wrong comparison as both intervention groups used the same incision size | |
| RCT, but wrong intervention as incision was performed at the steep axis, which affects the evaluation of surgically induced astigmatism | |
| RCT, but wrong intervention as the incision size in the standard phacoemulsification group was 4.0 mm, which was not within our range of 2.75 mm to 3.2 mm | |
| RCT, but wrong intervention as incision was enlarged to 2.0 mm or 3.0 mm for IOL implant | |
| This was a case series, not an RCT, and wrong intervention as the incision for the B‐MICS (1.4‐millimeter) group was enlarged to 3.2 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision size was enlarged, and the size overlapped with our comparison groups. | |
| RCT, but wrong intervention as the incisions in B‐MICS group were enlarged to 2.0 mm for IOL implantation | |
| RCT, but wrong comparison as the study authors compared pulse, burst, and continuous phacoemulsification mode | |
| RCT, but wrong comparison as the intervention involved suturing the corneal incision, which affected the evaluation of surgically induced astigmatism | |
| RCT, but wrong comparison as intervention was manual small‐incision cataract extraction without phacoemulsification | |
| RCT, but wrong intervention as the incision size was enlarged from 1.5 mm to 1.7 mm during IOL insertion | |
| RCT, but wrong intervention as the incision was enlarged from 1.5 mm to 1.7 mm in the B‐MICS group before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision was larger than 1.5 mm. | |
| RCT, but wrong population as the participants "had nuclear or corticonuclear cataract of grade II | |
| Not an RCT; quasi‐RCT as "patients were randomized by date of surgery; on odd dates, patients received a 3‐mm incision and on even dates a 2.2‐mm incision was used for the first eye." (p22) | |
| RCT, but wrong intervention as both incisions enlarged to 2.75 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong comparison as the intervention was manual small‐incision cataract surgery | |
| Not an RCT; study did not report how participants were allocated to treatment groups. However, it was described as a "prospective, observer‐masked study" (p56). | |
| RCT, but wrong intervention as the study compared 2.5‐millimeter (out the range of 2.75‐ to 3.2‐millimeter) mini‐incision coaxial phacoemulsification with 1.8‐millimeter microincision cataract surgery | |
| RCT, but wrong comparison as the intervention was manual small‐incision cataract extraction without phacoemulsification; the cataract was removed using irrigating vectis | |
| Not an RCT; this was a case series of people with nuclear or corticonuclear cataracts | |
| RCT, but the incision size was not reported and the authors could be not be reached | |
| RCT, but wrong intervention as incision size was enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| Animal study (rabbit eyes) | |
| RCT, but wrong comparison as participants were assigned to different IOL groups | |
| RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. | |
| RCT, but wrong comparison as the incisions were enlarged to 3.5 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision size was enlarged, and the final incision size overlapped with our comparison groups. | |
| Authors did not report how participants were assigned to treatment groups, and wrong intervention compared (B‐MICS incision size was 1.7 mm). | |
| RCT, but we attempted to contact the authors as it was unclear if the incision had been enlarged | |
| RCT, but wrong comparison as the B‐MICS incision size was 1.7 mm | |
| RCT, but wrong intervention as the surgery was performed on the steepest corneal meridians, which affects the evaluation of surgically induced astigmatism by usually decreasing the astigmatism |
ARC: age‐related cataract
B‐MICS: biaxial microincision cataract surgery
C‐MICS: coaxial microincision cataract surgery
CCT: controlled clinical trial
IOL: intraocular lens
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 8 | 996 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.30, ‐0.09] |
| Analysis 1.1  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months. | ||||
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 242 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| Analysis 1.2  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months. | ||||
| 3 Mean change of endothelial cell loss at 3 months Show forest plot | 4 | 596 | Mean Difference (IV, Random, 95% CI) | ‐7.23 [‐78.66, 64.20] |
| Analysis 1.3  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months. | ||||
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 5 | 487 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.26, 1.90] |
| Analysis 1.4  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months. | ||||
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 6 | 784 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.33, 0.72] |
| Analysis 1.5 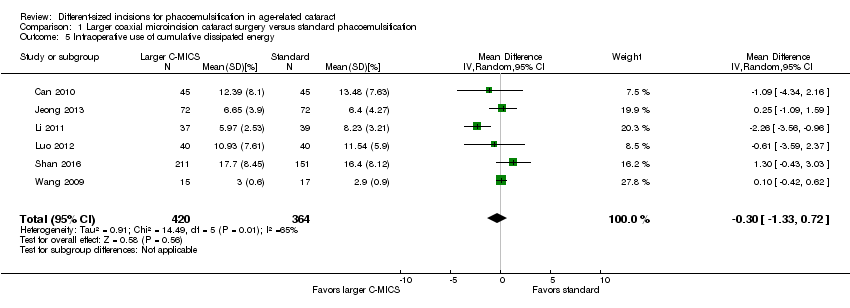 Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy. | ||||
| 6 Intraoperative use of balanced salt solution Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution. | ||||
| 7 Surgical time Show forest plot | 3 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.16] |
| Analysis 1.7 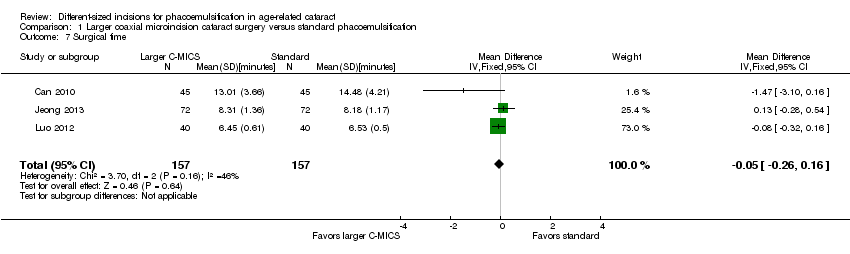 Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time. | ||||
| 8 Phacoemulsification time Show forest plot | 4 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐3.48, 1.56] |
| Analysis 1.8  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time. | ||||
| 9 Corneal edema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 9 Corneal edema. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 5 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.34, ‐0.13] |
| Analysis 2.1  Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months. | ||||
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.03, ‐0.00] |
| Analysis 2.2 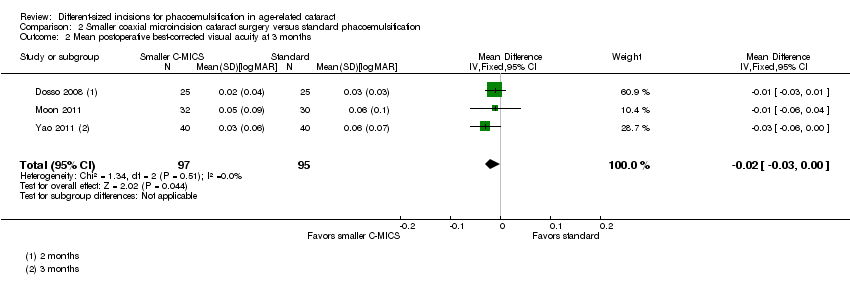 Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months. | ||||
| 3 Mean change of endothelial cell loss at 3 months Show forest plot | 5 | 380 | Mean Difference (IV, Random, 95% CI) | 7.56 [‐67.65, 82.77] |
| Analysis 2.3 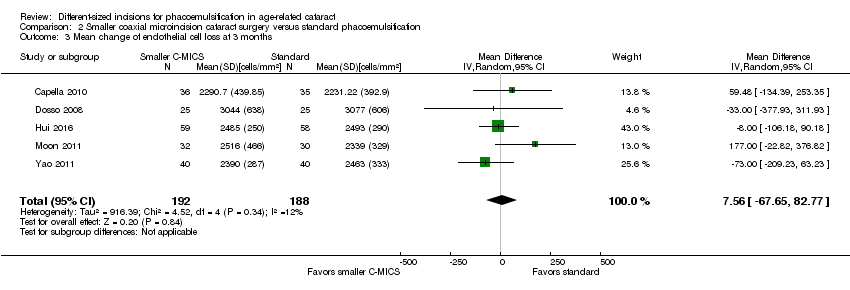 Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months. | ||||
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 3 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐6.29, 3.25] |
| Analysis 2.4  Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months. | ||||
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 2 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐5.43, ‐3.07] |
| Analysis 2.5 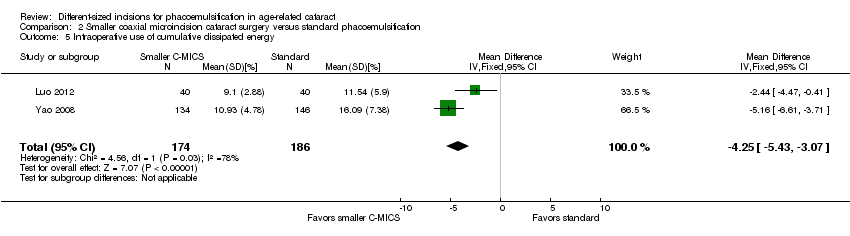 Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy. | ||||
| 6 Intraoperative use of balanced salt solution Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐6.32, 2.46] |
| Analysis 2.6  Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution. | ||||
| 7 Surgical time Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| Analysis 2.7  Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time. | ||||
| 8 Phacoemulsification time Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 8.20 [2.88, 13.52] |
| Analysis 2.8  Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.16] |
| Analysis 3.1  Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months. | ||||
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.04] |
| Analysis 3.2  Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months. | ||||
| 3 Mean endothelial cell loss at 3 months Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 213.00 [11.15, 414.85] |
| Analysis 3.3 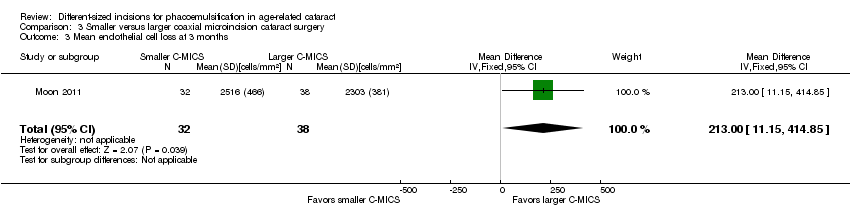 Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 3 Mean endothelial cell loss at 3 months. | ||||
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.70, 3.60] |
| Analysis 3.4 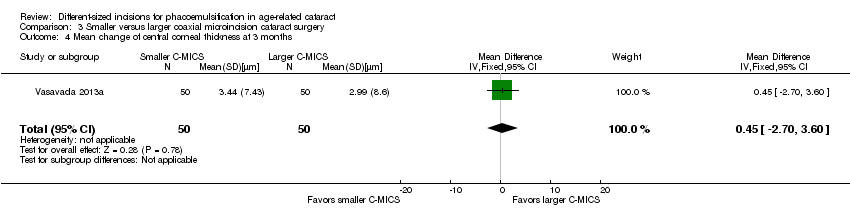 Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 4 Mean change of central corneal thickness at 3 months. | ||||
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 4 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐3.72, 3.07] |
| Analysis 3.5 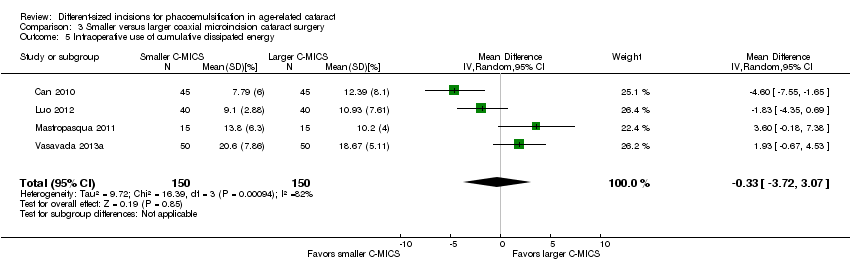 Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 5 Intraoperative use of cumulative dissipated energy. | ||||
| 6 Intraoperative use of balanced salt solution Show forest plot | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐2.45, 4.53] |
| Analysis 3.6 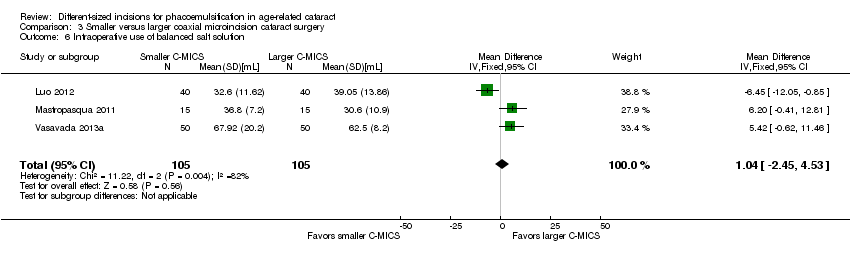 Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 6 Intraoperative use of balanced salt solution. | ||||
| 7 Surgical time Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.12, 0.55] |
| Analysis 3.7  Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 7 Surgical time. | ||||
| 8 Phacoemulsification time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8 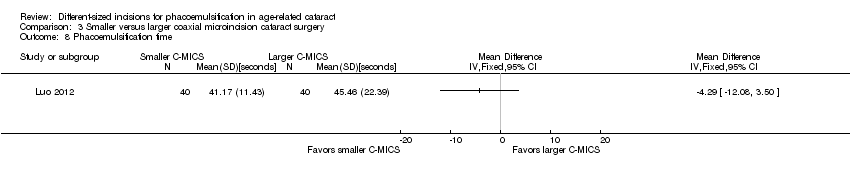 Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 8 Phacoemulsification time. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Analysis 4.1  Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months. | ||||
| 2 Mean postoperative surgically induced astigmatism at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative surgically induced astigmatism at 6 months. | ||||
| 3 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| Analysis 4.3  Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean postoperative best‐corrected visual acuity at 3 months. | ||||
| 4 Mean endothelial cell loss at 3 months Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 55.83 [‐34.93, 146.59] |
| Analysis 4.4 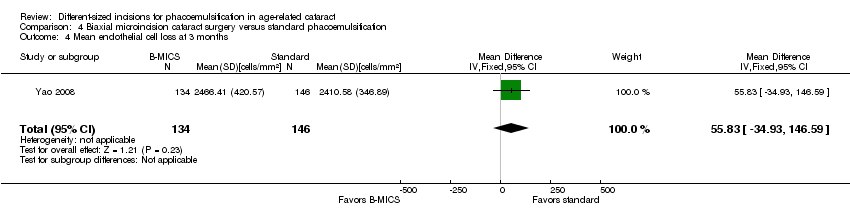 Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean endothelial cell loss at 3 months. | ||||
| 5 Postoperative central corneal thickness at 3 months Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐14.04, 14.24] |
| Analysis 4.5  Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Postoperative central corneal thickness at 3 months. | ||||
| 6 Intraoperative use of cumulative dissipated energy Show forest plot | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐6.58, ‐3.97] |
| Analysis 4.6 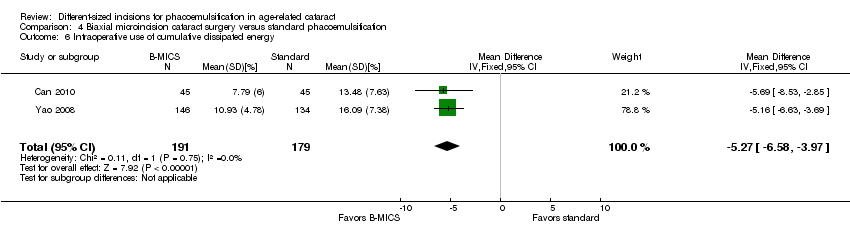 Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of cumulative dissipated energy. | ||||
| 7 Surgical time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.7 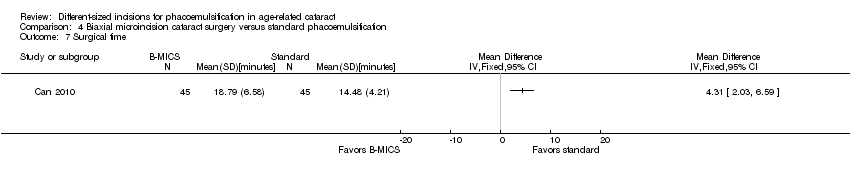 Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time. | ||||
| 8 Phacoemulsification time Show forest plot | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.58 [‐9.52, ‐1.63] |
| Analysis 4.8  Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
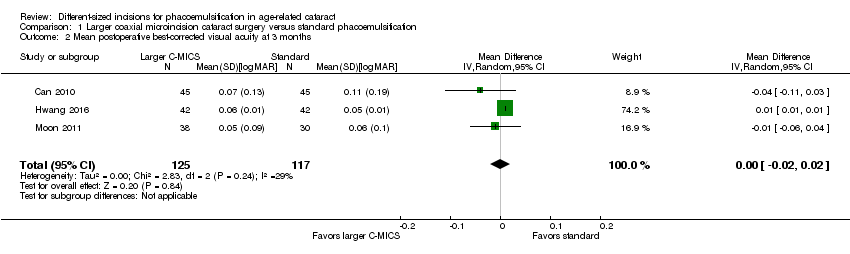
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy.
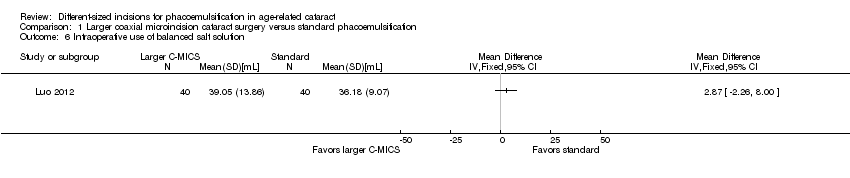
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution.
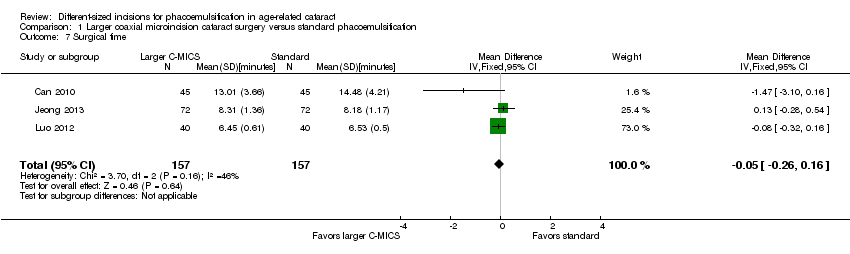
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.

Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 9 Corneal edema.

Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.

Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.
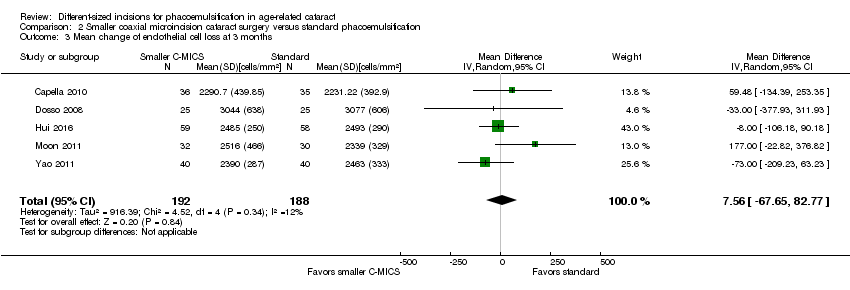
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months.
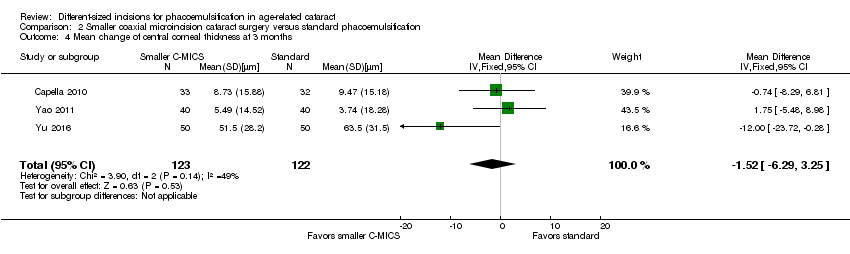
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months.

Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy.
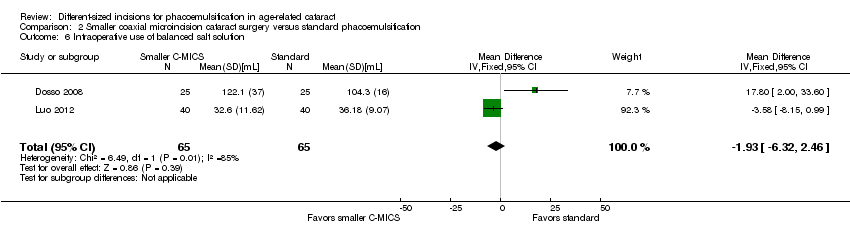
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution.

Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.

Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.

Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
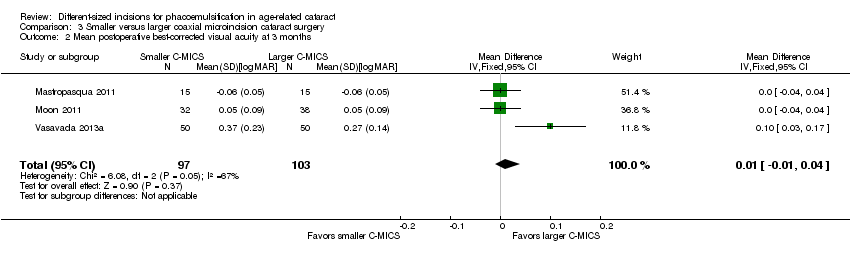
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.

Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 3 Mean endothelial cell loss at 3 months.
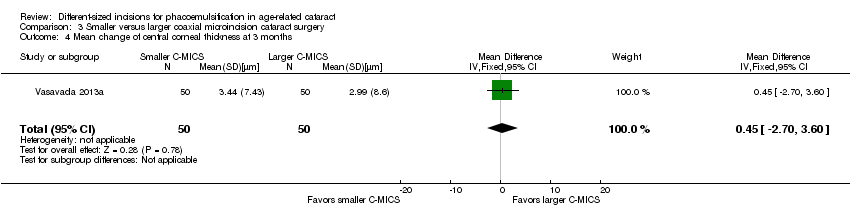
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 4 Mean change of central corneal thickness at 3 months.

Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 5 Intraoperative use of cumulative dissipated energy.

Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 6 Intraoperative use of balanced salt solution.
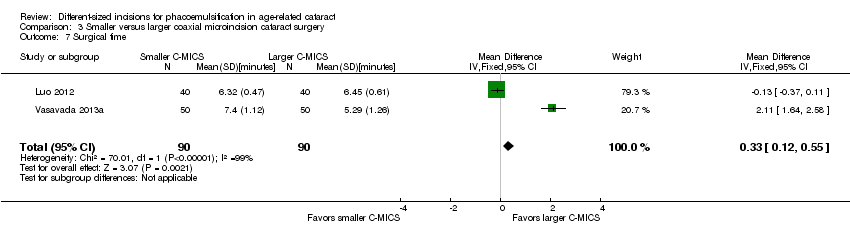
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 7 Surgical time.

Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 8 Phacoemulsification time.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
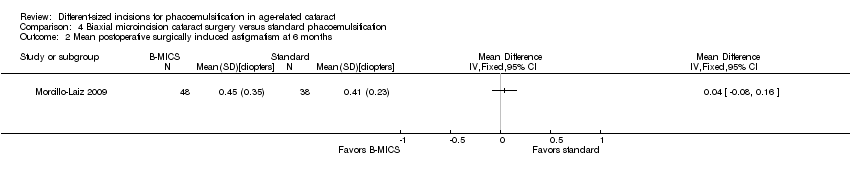
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative surgically induced astigmatism at 6 months.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean postoperative best‐corrected visual acuity at 3 months.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean endothelial cell loss at 3 months.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Postoperative central corneal thickness at 3 months.
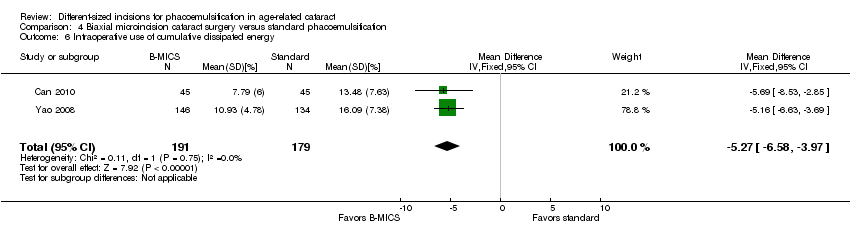
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of cumulative dissipated energy.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.

Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.
| Larger C‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
| Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: larger C‐MICS with 2.2‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | Larger C‐MICS | |||||
| Mean postoperative surgically induced astigmatism Follow‐up: 3 months | The mean surgically induced astigmatism was 0.7 to 1.34 diopters. | The mean surgically induced astigmatism in the intervention groups was 0.19 diopters lower (0.30 to 0.09 diopters lower). | ‐ | 996 | ⊕⊝⊝⊝ | A lower diopter value is a better clinical outcome. |
| Mean postoperative best‐corrected visual acuity Follow‐up: 3 months | The mean best‐corrected visual acuity was 0.05 to 0.11 logMAR. | The mean best‐corrected visual acuity in the intervention groups was 0.00 logMAR lower (0.02 logMAR lower to 0.02 logMAR higher). | ‐ | 242 | ⊕⊕⊝⊝ | |
| Mean endothelial cell loss Follow‐up: 3 months | The mean of endothelial cell loss ranged across control groups was 2054.0 to 2339.0 cells/mm2. | The mean change of endothelial cell loss in the intervention groups was 7.23 cells/mm2 lower (78.66 cells/mm2 lower to 64.20 cells/mm2 higher). | ‐ | 596 (4 RCTs) | ⊕⊕⊝⊝ | Little or no difference between groups is a clinically positive result. |
| Central corneal thickness Follow‐up: 3 months | The mean change of central corneal thickness was 9.24 μm. The mean central corneal thickness ranged across control groups from 546.0 to 580.0 μm. | The mean change of central corneal thickness in the intervention groups was 0.68 μm lower (3.26 μm lower to 1.90 μm higher). | ‐ | 487 (5 RCTs) | ⊕⊕⊝⊝ | Hwang 2016 did not report the standard deviation, but reported that the mean % decrease in central corneal thickness was 1.00 in the 2.2‐millimeter group and 0.31 in the 2.75‐millimeter group. Little or no difference between groups is a clinically positive result. |
| Adverse events (corneal edema) Follow‐up: 3 months | 46 per 1000 | 47 per 1000 (19 to 122) | RR 1.02 (0.40 to 2.63 | 362 (1 RCT) | Wang 2009 reported "no intraoperative complications." | |
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for risk of bias, as one study was at high risk of selection and attrition bias, while another study was at high risk of reporting bias. | ||||||
| Smaller C‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
| Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: smaller C‐MICS with 1.8‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | Smaller C‐MICS | |||||
| Mean postoperative surgically induced astigmatism Follow‐up: 3 months | The mean postoperative surgically induced astigmatism ranged across control groups from 0.03 to 0.94 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was 0.23 lower (0.34 diopters to 0.13 diopters lower). | ‐ | 561 | ⊕⊝⊝⊝ | A lower diopter value is a better clinical outcome. |
| Mean postoperative best‐corrected visual acuity Follow‐up: 2 to 3 months | The mean postoperative best‐corrected visual acuity ranged across control groups from | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.02 logMAR units lower (0.03 logMAR units lower to 0.00 logMAR units). | ‐ | 192 | ⊕⊕⊝⊝ | |
| Mean change of endothelial cell loss Follow‐up: 3 months | The mean change of endothelial cell loss ranged across control groups from 2231.22 to 3077.0 cells/mm2. | The mean change of endothelial cell loss in the intervention groups was | ‐ | 380 | ⊕⊕⊝⊝ | |
| Mean change of central corneal thickness Follow‐up: 3 months | The mean change of central corneal thickness ranged across control groups from 3.74 to 63.5 μm. | The mean change of central corneal thickness in the intervention groups was 1.52 μm lower (6.29 μm lower to 3.25 μm higher). | ‐ | 245 | ⊕⊕⊝⊝ | |
| Adverse events Follow‐up: 3 months | None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for high risk of selection, performance, and detection bias. | ||||||
| Smaller coaxial microincision cataract surgery (C‐MICS) compared with larger C‐MICS for age‐related cataract | ||||||
| Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: smaller C‐MICS with 1.8‐millimeter incision Comparison: larger C‐MICS with 2.2‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Larger C‐MICS | Smaller C‐MICS | |||||
| Mean postoperative surgically induced astigmatism Follow‐up: 3 months | The mean postoperative surgically induced astigmatism ranged across control groups from 0.48 to 1.08 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was | ‐ | 259 | ⊕⊕⊝⊝ | A lower diopter value is a better clinical outcome. |
| Mean postoperative best‐corrected visual acuity Follow‐up: 3 months | The mean postoperative best‐corrected visual acuity ranged across control groups from | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.01 logMAR units higher (0.01 logMAR units lower to 0.04 logMAR units higher). | ‐ | 200 | ⊕⊕⊝⊝ | |
| Mean change of endothelial cell loss Follow‐up: 3 months | The mean change in endothelial cell loss was 2303.0 cells/mm2. | The mean change in endothelial cell loss was 213.00 cells/mm2 higher (11.15 to 414.85 cells/mm2 higher). | ‐ | 70 (1 RCT) | ⊕⊝⊝⊝ | |
| Mean central corneal thickness Follow‐up: 3 months | The mean change in central corneal thickness was 2.99 μm. | The mean change in central corneal thickness was 0.45 μm higher (2.70 μm lower to 3.60 μm higher). | ‐ | 100 | ⊕⊕⊝⊝ | |
| Adverse events Follow‐up: end of trial | None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for unexplained statistical heterogeneity, as the I2 was greater than 70%. | ||||||
| B‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
| Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: B‐MICS with equal to or smaller than 1.5‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | B‐MICS | |||||
| Mean postoperative surgically induced astigmatism Follow‐up: 3 months | The mean postoperative surgically induced astigmatism ranged across control groups from 0.12 diopters to 0.44 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was | ‐ | 368 | ⊕⊕⊕⊝ | A lower diopter value is a better clinical outcome. |
| Mean postoperative best‐corrected visual acuity Follow‐up: 3 months | The mean postoperative best‐corrected visual acuity ranged across control groups from | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.02 logMAR units lower (0.04 logMAR units lower to 0.00 LogMAR units). | ‐ | 464 | ⊕⊕⊝⊝ | |
| Mean endothelial cell loss Follow‐up: 3 months | The mean endothelial cell loss in the control groups was 2410 cells/mm2. | The mean endothelial cell loss in the intervention groups was (34.93 cells/mm2 lower to 146.59 cells/mm2 higher). | ‐ | 280 | ⊕⊕⊝⊝ | |
| Postoperative central corneal thickness Follow‐up: 3 months | The mean change in central corneal thickness in the control group was 546 μm. | The mean change of central corneal thickness in the intervention groups was 0.10 μm higher (14.04 μm lower to 14.24 μm higher). | ‐ | 90 | ⊕⊕⊝⊝ | |
| Adverse events Follow‐up: end of study | None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias, as the trials were at unclear risk of selection and reporting bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 8 | 996 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.30, ‐0.09] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 242 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 3 Mean change of endothelial cell loss at 3 months Show forest plot | 4 | 596 | Mean Difference (IV, Random, 95% CI) | ‐7.23 [‐78.66, 64.20] |
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 5 | 487 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.26, 1.90] |
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 6 | 784 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.33, 0.72] |
| 6 Intraoperative use of balanced salt solution Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Surgical time Show forest plot | 3 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.16] |
| 8 Phacoemulsification time Show forest plot | 4 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐3.48, 1.56] |
| 9 Corneal edema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 5 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.34, ‐0.13] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.03, ‐0.00] |
| 3 Mean change of endothelial cell loss at 3 months Show forest plot | 5 | 380 | Mean Difference (IV, Random, 95% CI) | 7.56 [‐67.65, 82.77] |
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 3 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐6.29, 3.25] |
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 2 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐5.43, ‐3.07] |
| 6 Intraoperative use of balanced salt solution Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐6.32, 2.46] |
| 7 Surgical time Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 8 Phacoemulsification time Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 8.20 [2.88, 13.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.16] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.04] |
| 3 Mean endothelial cell loss at 3 months Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 213.00 [11.15, 414.85] |
| 4 Mean change of central corneal thickness at 3 months Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.70, 3.60] |
| 5 Intraoperative use of cumulative dissipated energy Show forest plot | 4 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐3.72, 3.07] |
| 6 Intraoperative use of balanced salt solution Show forest plot | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐2.45, 4.53] |
| 7 Surgical time Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.12, 0.55] |
| 8 Phacoemulsification time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean postoperative surgically induced astigmatism at 3 months Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 2 Mean postoperative surgically induced astigmatism at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean postoperative best‐corrected visual acuity at 3 months Show forest plot | 3 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 4 Mean endothelial cell loss at 3 months Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 55.83 [‐34.93, 146.59] |
| 5 Postoperative central corneal thickness at 3 months Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐14.04, 14.24] |
| 6 Intraoperative use of cumulative dissipated energy Show forest plot | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐6.58, ‐3.97] |
| 7 Surgical time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Phacoemulsification time Show forest plot | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.58 [‐9.52, ‐1.63] |

