Música para el insomnio en adultos
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
CENTRAL, 2014 (5), searched 22 May 2015 [67 records]
#1 MeSH descriptor: [Music] explode all trees
#2 MeSH descriptor: [Music Therapy] explode all trees
#3 music*
#4 (#1 or #2 or #3)
#5 MeSH descriptor: [Sleep] explode all trees
#6 MeSH descriptor: [Sleep Disorders] explode all trees
#7 sleep*
#8 insomnia*
#9 wakeful*
#10 sleepless*
#11 dyssomn*
#12 (#5 or #6 or #7 or #8 or #9 or #10 or #11)
#13 (#4 and #12)
PubMed (ncbi.nlm.nih.gov/pubmed)
PubMed, 1950 to current, searched 22 May 2015 [91 records]
#1 Music [Mesh]
#2 Music therapy [Mesh]
#3 music*
#4 (#1 OR #2 OR #3)
#5 Sleep [Mesh]
#6 Sleep disorders [Mesh]
#7 sleep*
#8 insomnia*
#9 wakeful*
#10 sleepless*
#11 dyssomn*
#12 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 randomized controlled trial [Publication Type]
#14 controlled clinical trial [Publication Type]
#15 clinical trial [Publication Type]
#16 Clinical Trials as Topic [Mesh]
#17 randomized [Title/Abstract]
#18 placebo [Title/Abstract]
#19 randomly [Title/Abstract]
#20 trial [Title/Abstract]
#21 (#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20)
#22 (#4 AND #12 AND #21)
Embase
Embase (Elsevier), 1980 to current, searched 22 May 2015 [194 records]
#1. 'music'/exp
#2. 'music therapy'/exp
#3. music*
#4. 'music'/exp OR 'music therapy'/exp OR music*
#5. 'sleep'/exp
#6. 'sleep disorder'/exp
#7. sleep*
#8. insomnia*
#9. wakeful*
#10. sleepless*
#11. dyssomn*
#12. 'sleep'/exp OR 'sleep disorder'/exp OR sleep* OR insomnia* OR wakeful* OR sleepless* OR dyssomn*
#13. 'randomized controlled trial'/de
#14. 'controlled clinical trial'/de
#15. 'clinical trial'/de
#16. 'clinical trial (topic)'/exp
#17. randomized:ab
#18. placebo:ab
#19. randomly:ab
#20. trial:ab
#21. 'randomized controlled trial'/de OR 'controlled clinical trial'/de OR 'clinical trial'/de OR 'clinical trial (topic)'/exp OR randomized:ab OR placebo:ab OR randomly:ab OR trial:ab
#22. 'music'/exp OR 'music therapy'/exp OR music* AND ('sleep'/exp OR 'sleep disorder'/exp OR sleep* OR insomnia* OR wakeful* OR sleepless* OR dyssomn*) AND ('randomized controlled trial'/de OR 'controlled clinical trial'/de OR 'clinical trial'/de OR 'clinical trial (topic)'/exp OR randomized:ab OR placebo:ab OR randomly:ab OR trial:ab)
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
CINAHL (EBSCOhost), 1982 to current, searched 22 May 2015 [177 records]
S1 MH music
S2 MH music therapy
S3 music*
S4 (S1 OR S2 OR S3)
S5 MH sleep
S6 MH sleep disorders
S7 sleep*
S8 insomnia*
S9 wakeful*
S10 sleepless*
S11 dyssomn*
S12 (S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11)
S13 (S4 AND S12)
PsycINFO
PsycINFO (Proquest), 1967 to current, searched 22 May 2015 [55 records]
S1 SU.EXACT.EXPLODE("Music")
S2 SU.EXACT.EXPLODE("Music Therapy")
S3 music*
S4 SU.EXACT.EXPLODE("Music") OR SU.EXACT.EXPLODE("Music Therapy") OR music*
S5 SU.EXACT.EXPLODE("Sleep")
S6 SU.EXACT.EXPLODE("Sleep Disorders")
S7 sleep*
S8 insomnia*
S9 wakeful*
S10 sleepless*
S11 dyssomn*
S12 SU.EXACT.EXPLODE("Sleep") OR SU.EXACT.EXPLODE("Sleep Disorders") OR sleep* OR insomnia* OR wakeful* OR sleepless* OR dyssomn*
S13 SU.EXACT.EXPLODE("Clinical Trials")
S14 ab(randomized) OR ti(randomized)
S15 ab(placebo) OR ti(placebo)
S16 ab(randomly) OR ti(randomly)
S17 ab(trial) OR ti(trial)
S18 SU.EXACT.EXPLODE("Clinical Trials") OR (ab(randomized) OR ti(randomized)) OR (ab(placebo) OR ti(placebo)) OR (ab(randomly) OR ti(randomly)) OR (ab(trial) OR ti(trial))
S19 (SU.EXACT.EXPLODE("Music") OR SU.EXACT.EXPLODE("Music Therapy") OR music*) AND (SU.EXACT.EXPLODE("Sleep") OR SU.EXACT.EXPLODE("Sleep Disorders") OR sleep* OR insomnia* OR wakeful* OR sleepless* OR dyssomn*) AND (SU.EXACT.EXPLODE("Clinical Trials") OR (ab(randomized) OR ti(randomized)) OR (ab(placebo) OR ti(placebo)) OR (ab(randomly) OR ti(randomly)) OR (ab(trial) OR ti(trial)))
Web of Science databases
Science Citation Index Expanded, Social Sciences Citation Index and Arts and Humanities Citation Index , 1970 to current
Conference Proceedings Citation Index ‐ Science and Conference Proceedings Citation Index ‐ Social Science and Humanities, 1990 to current
Searched 22 May 2015 [69 records]
#1 TOPIC: (music*)
#2 TOPIC: (sleep*)
#3 TOPIC: (insomnia*)
#4 TOPIC: (wakeful*)
#5 TOPIC: (sleepless*)
#6 TOPIC: (dyssomn*)
#7 (#2 OR #3 OR #4 OR #5 OR #6)
#8 TOPIC: (randomized controlled trial)
#9 TOPIC: (controlled clinical trial)
#10 TOPIC: (clinical trial)
#11 TOPIC: (trial)
#12 TITLE: (randomized)
#13 TITLE: (placebo)
#14 TITLE: (randomly)
#15 TITLE: (trial)
#16 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 (#1 AND #7 AND #16)
SCOPUS
SCOPUS (Elsevier), 1960 to current, searched 22 May 2015 [178 records]
1 TITLE‐ABS‐KEY(music*)
2 TITLE‐ABS‐KEY(sleep*)
3 TITLE‐ABS‐KEY(insomnia*)
4 TITLE‐ABS‐KEY(wakeful*)
5 TITLE‐ABS‐KEY(sleepless*)
6 TITLE‐ABS‐KEY(dyssomn*)
7 (TITLE‐ABS‐KEY(sleep*)) OR (TITLE‐ABS‐KEY(insomnia*)) OR (TITLE‐ABS‐KEY(wakeful*)) OR (TITLEABS‐KEY(sleepless*)) OR (TITLE‐ABS‐KEY(dyssomn*))
8 TITLE‐ABS‐KEY(trial*)
9 TITLE‐ABS‐KEY(randomized)
10 TITLE‐ABS‐KEY(randomly)
11 TITLE‐ABS‐KEY(placebo)
12 (TITLE‐ABS‐KEY(trial*)) OR (TITLE‐ABS‐KEY(randomized)) OR (TITLE‐ABS‐KEY(randomly)) OR (TITLEABS‐KEY(placebo))
13 (TITLE‐ABS‐KEY(music*)) AND ((TITLE‐ABS‐KEY(sleep*)) OR (TITLE‐ABS‐KEY(insomnia*)) OR (TITLEABS‐KEY(wakeful*)) OR (TITLE‐ABS‐KEY(sleepless*)) OR (TITLE‐ABS‐KEY(dyssomn*))) AND ((TITLEABS‐KEY(trial*)) OR (TITLE‐ABS‐KEY(randomized)) OR (TITLE‐ABS‐KEY(randomly)) OR (TITLEABS‐KEY(placebo)))
Répertoire International de Littérature Musicale (RILM)
RILM (EBSCOhost), 1969 to current, searched 22 May 2015 [n = 56 records]
S1 music*
S2 sleep*
S3 insomnia*
S4 (S2 OR S3)
S5 SU therapy
S6 trial*
S7 TI randomized OR AB randomized
S8 (S5 OR S6 OR S7)
S9 (S1 AND S4 AND S8)
ClinicalTrials.gov (clinicaltrials.gov/)
ClinicalTrials.gov, searched 22 May 2015 [n = 34 records]
music* AND (sleep* OR insomnia*)
Current Controlled Trials (controlled‐trials.com)
Current Controlled Trials, searched 22 May 2015 [n = 3 records]
music* AND (sleep* OR insomnia*)
Appendix 2. Handsearched journals
-
Australian Journal of Music Therapy (1990 to 2014, vol 25).
-
British Journal of Music Therapy (1987 to December 2014, vol 28 (2)).
-
Canadian Journal of Music Therapy (1993 to 2014, vol 20 (1)).
-
New Zealand Journal of Music Therapy (2010 to 2013, vol 11).
-
Nordic Journal of Music Therapy (1992 to May 2015, vol 24 (3)).
-
Music and Medicine (2009 to April 2015, vol 7(2)).
-
Music Therapy Perspectives (2004 to 2014, vol 32 (2)).
-
Music Therapy Today (online) (2001 to 2014, vol 10(1)).
-
Voices (online) (2001 to 2015, vol 15(2)).
-
The International Journal of Arts Medicine (1991 to 1999).
-
Journal of Music Therapy (1980 to Fall 2014, vol 51(3)).
-
The Arts in Psychotherapy (1980 to April 2015, vol 43).
-
Musik‐,Tanz‐ und Kunsttherapie (German) (1995 to January 2014, vol 25(1)).
-
Musiktherapeutische Umschau (German) (1980 to 2014, vol 35(4)).
-
Musik und Gesundsein (German) (2011 to 2015, vol. 27).
Appendix 3. Additional methods archived for future updates of this review
| Analysis | Methods |
| Measures of treatment effect | Dichotomous data For dichotomous data, we will present the results as summary odd ratios (OR) with 95% confidence intervals (CI). Continuous data The standardized mean difference (SMD) will be used to combine trials that measure the same outcome, but use different scales. All outcomes will be presented with 95% CIs. If a trial provided multiple interchangeable measures of the same construct at the same time point, we will calculate the average SMD across these outcomes and the average of their estimated variances. Where trials report the same outcomes using continuous and dichotomous measures, we will re‐express ORs as SMDs allowing dichotomous and continuous data to be pooled together as described in section nine of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Ordinal data Ordinal data measured on shorter scales will be analysed as dichotomous data by combining categories, and the intervention effect will be expressed using OR. |
| Unit of analysis issues | Cluster‐randomised trials We anticipate that trials using clustered randomisation will have controlled for clustering effects. In case of doubt, we will contact the first authors to ask for individual participant data to calculate an estimate of the intracluster correlation coefficient (ICC). If this is not possible, we will obtain external estimates of the ICC from a similar trial or from a study of a similar population as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When the ICC is established, we will use it to reanalyse the trial data. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC Cross‐over trials Cross‐over trials will be analysed using combined data from all study periods, or using first period data if combined data is not available Trials with more than two treatment arms If more than one of the interventions is a music intervention, and there is sufficient information in the trial to assess the similarity of the interventions, we will combine similar music interventions to allow for a single pair‐wise comparison |
| Dealing with missing data | We will explore the impact of including studies with high levels of missing data by performing sensitivity analyses based on consideration of best and worst case scenarios. The potential impact of missing data on the findings of the review will be addressed in the 'Discussion' section of the review |
| Assessment of heterogeneity | Where significant heterogeneity is present, we intend to investigate it by conducting a subgroup analysis based on the participant clinical characteristics and interventions of the included studies (see subsection on 'Subgroup analyses' below) |
| Assessment of reporting bias | If sufficient study data are available for individual outcomes, we will draw and inspect funnel plots for evidence of reporting or publication bias. We will assess funnel plot asymmetry visually and statistically by means of the Bee and Mazumdar (Begg 1994) and the Egger et al tests (Egger 1997), if sufficient studies are available; ten or more studies are recommended. If asymmetry is suggested by visual assessment or detected in any of these tests, we will perform exploratory analyses to investigate if it reflects a publication bias or a true relationship between trial size and effect size |
| Subgroup analyses | We will conduct the following subgroup analyses.
|
| Sensitivity analysis | We will conduct a sensitivity analysis excluding trials using inadequate methods of blinding personnel |
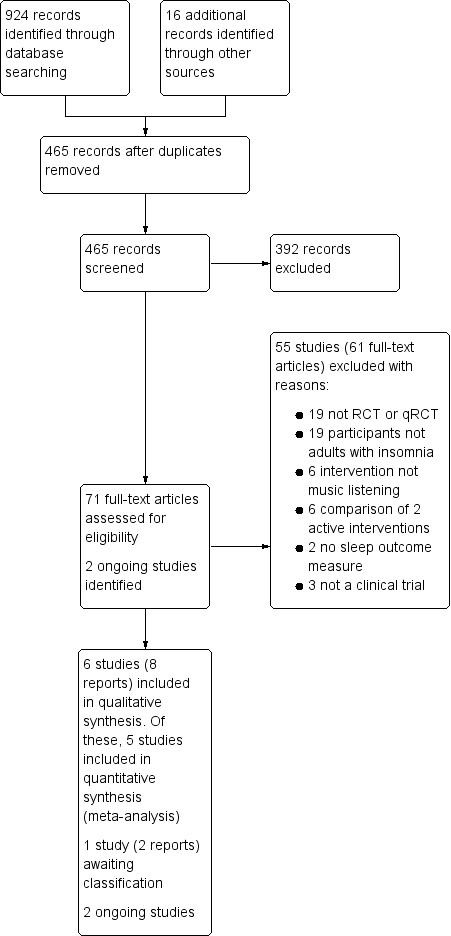
Study flow diagram
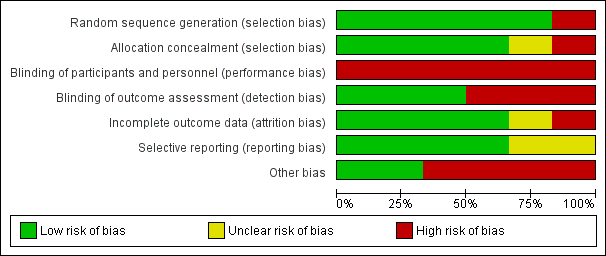
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
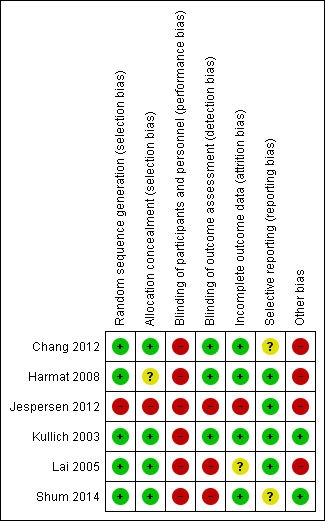
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial

Forest plot of comparison: 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, outcome: 1.1 Sleep quality: Pittsburgh Sleep Quality Index (PSQI) ‐ immediately post‐treatment.

Forest plot of comparison: 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, outcome: 1.2 Subgroup (PSQI) by music selection ‐ immediately post‐treatment.

Forest plot of comparison: 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, outcome: 1.3 Subgroup (PSQI) by relaxation instructions ‐ immediately post‐treatment.
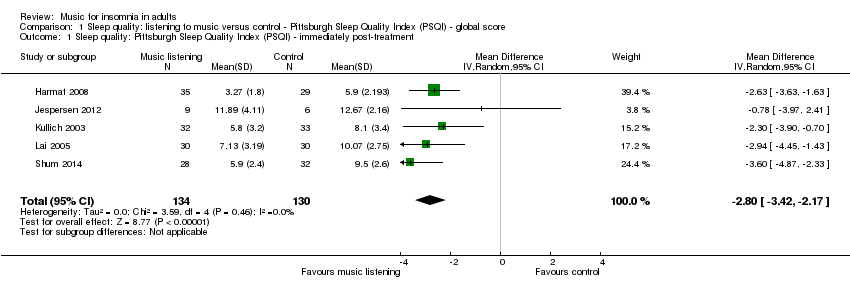
Comparison 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, Outcome 1 Sleep quality: Pittsburgh Sleep Quality Index (PSQI) ‐ immediately post‐treatment.

Comparison 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, Outcome 2 Subgroup (PSQI) by music selection ‐ immediatly post‐treatment.

Comparison 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, Outcome 3 Subgroup (PSQI) by relaxation instructions ‐ immediately post‐treatment.
| Listening to music compared to no treatment or treatment‐as‐usual (TAU) for adults with insomnia | ||||||
| Patient or population: adults with insomnia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or TAU | Listening to music | |||||
| Sleep quality ‐ immediately post‐treatment Follow‐up: 21 to 35 days | The mean score in the intervention groups was | 264 | ⊕⊕⊕⊝ | A lower score indicates better sleep quality (i.e. fewer sleep problems). The change is about the size of one standard deviation which is considered a clinically relevant change. The studies included participants with a complaint of poor sleep (PSQI > 5)¹. | ||
| Sleep onset latency ‐ immediately post‐treatment Follow‐up: 3 days | See comment | See comment | 50 | ⊕⊕⊝⊝ | The one trial reporting this outcome found no evidence of an effect of the intervention. The data were not available for analysis. The study included participants that had reported poor sleep for at least one month (PSQI > 5)¹. | |
| Total sleep time ‐ immediately post‐treatment | See comment | See comment | 50 | ⊕⊕⊝⊝ | The one study reporting this outcome found no evidence of an effect of the intervention. The data were not available for analysis. The study included participants that had reported poor sleep for at least one month (PSQI > 5)¹. | |
| Sleep interruption ‐ immediately post‐treatment | See comment | See comment | 50 | ⊕⊕⊝⊝ | The one study reporting this outcome found no evidence of an effect of the intervention. The data are not available for analysis. The study included participants that had reported poor sleep for at least one month (PSQI > 5)¹. | |
| Sleep efficiency ‐ immediately post‐treatment | See comment | See comment | 50 | ⊕⊕⊝⊝ | The one study reporting this outcome found no evidence of an effect of the intervention. The data were not available for analysis. The study included participants that had reported poor sleep for at least one month (PSQI > 5)¹. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Pittsburgh Sleep Quality Index. 0 indicates good sleep quality and 21 indicates severe sleep problems. Clinical cut off > 5 (Buysse 1989). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sleep quality: Pittsburgh Sleep Quality Index (PSQI) ‐ immediately post‐treatment Show forest plot | 5 | 264 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐3.42, ‐2.17] |
| 2 Subgroup (PSQI) by music selection ‐ immediatly post‐treatment Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Researcher‐selected music | 3 | 144 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐3.24, ‐1.60] |
| 2.2 Participant‐selected music (choice among researcher pre‐selected music) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐4.28, ‐2.42] |
| 3 Subgroup (PSQI) by relaxation instructions ‐ immediately post‐treatment Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Music listening alone | 3 | 149 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐3.92, ‐1.78] |
| 3.2 Music listening and relaxation instructions | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐3.74, ‐1.54] |

