نقش درمان فارماکولوژیک عوامل خطرساز عروقی برای کاهش مرگومیر و حوادث قلبیعروقی در بیماران مبتلا به آنوریسم آئورت شکمی
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study type: double‐blind randomised controlled trial Study aim: to test the hypothesis that, at 30 days and 6 months after vascular surgery, the perioperative administration of metoprolol reduces the incidence of cardiac complications defined as cardiac death, nonfatal myocardial infarction (MI), congestive heart failure (CHF), unstable angina, and dysrhythmias requiring treatment. Country: Canada Setting: 3 tertiary care centres: General Campus, Hamilton Health Sciences; Victoria Campus, London Health Sciences; and Kingston General Hospital between 1999 and 2002. Recruitment: all patients undergoing vascular surgery were screened for eligibility. Elective vascular surgical patients are evaluated by internists, cardiologists, or anaesthesiologists in preoperative clinics. Screening was also undertaken on the wards when applicable | |
| Participants | Inclusion criteria: patients with American Society of Anesthesiology class 3 or less and undergoing abdominal aortic surgery and infrainguinal or axillofemoral revascularisation Exclusion criteria: current or recent β‐blocker use, current amiodarone use, airflow obstruction requiring treatment, history of CHF, history of atrioventricular block, previous adverse drug reactions to β‐blockers, and previous participation in the MaVS study Gender: placebo group 184 M/66 F; metoprolol group 193 M/53 F Age: placebo participants mean 65.9 ± 10.0 years; metoprolol participants mean 66.4 ± 10.0 years Co‐morbidities: Prior MI: 30 placebo, 37 metoprolol Angina: 25 placebo, 18 metoprolol Diabetes mellitus on treatment: 37 placebo, 54 metoprolol Permanent pacemaker: 1 placebo, 0 metoprolol AAA subgroup: 116 placebo, 111 metoprolol | |
| Interventions | Treatment: metoprolol administered orally or intravenously. Participants weighing ≥ 75 kg received metoprolol 100 mg; participants weighing between 40 and 75 kg received metoprolol 50 mg; and participants weighing ≤ 40 kg received metoprolol 25 mg OR intravenously at 1 mg/mL for 15 minutes. Intravenous (IV) treatment was converted to oral as soon as oral intake was tolerated Control: placebo administered orally as tablet or given intravenously as saline 0.2 mL/kg (to a maximum of 15 mL), diluted with 20 mL of saline for 15 minutes Duration: metoprolol or placebo given orally 2 hours preoperatively. Within 2 hours of surgery, metoprolol or placebo were give intravenously or orally. IV drug administered over 15 minutes every 6 hours. Oral administration was twice daily. Treatment lasted for 5 days or until hospital discharge, whichever occurred sooner Co‐interventions: short‐acting vasoactive medications including phenylephrine, ephedrine, nitroglycerine, and low‐dose dopamine were allowed. Open‐label β‐blocker use was strongly discouraged except when deemed absolutely necessary by the attending physician. Circumstances for open‐label use were generally for rapid heart rate control. Intraoperatively, esmolol, if deemed absolutely necessary, was allowed | |
| Outcomes | Primary outcome: composite of cardiac complications at 30 days postoperation including; cardiac death1, nonfatal MI2, CHF3, unstable angina4, and dysrhythmia requiring treatment defined as atrial fibrillation or ventricular dysrhythmias5 1Cardiac death was defined as either the ultimate cause of death traceable to an initiating cardiac complication or death in which the cause was not clearly identifiable or was insufficient to account for the demise in a patient who was not expected to succumb at the time of death. 2Nonfatal MI within 3 postoperative deaths diagnosed if ≥ 1 of the following present: chemical evidence of MI or new Q waves > 0.04 s on 2 contiguous leads. Beyond 3 days, nonfatal MI was determined by attending physicians with supporting documentation of hospital chart, troponins, and pre‐ and postoperative electrocardiograms. 3Unstable angina diagnosed by attending physician when anginal symptoms necessitated a change in medications, coronary revascularisation, or intensive care admission. 4CHF was diagnosed clinically with the requisite radiographic evidence. 5Dysrhythmia requiring treatment was defined as one of the following: ventricular fibrillation requiring counter shock, ventricular tachycardia requiring counter shock or medication, or atrial fibrillation > 15 minutes in duration requiring counter shock or medication. Secondary outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was constructed in blocks of 4 by the study statistician" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Methods of concealment of allocation are not stated. Insufficient information to permit judgement of low or high risk of selection bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, investigators, and all caretakers were blinded to the study randomisation. Blinding of randomisation was maintained throughout clinical decisions on reducing or discontinuing the study medication" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All data were collected by the participating centres and evaluated by the adjudication committee in a blinded fashion" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Completion of the study protocol was similar in the placebo (77.6%) and treatment groups (75.2%). Discontinuation of the study protocol was also similar in the placebo and treatment groups; primary outcome event (30 and 25,respectively); patient/family/physician preference (27 and 14, respectively); open‐label β‐blockers (24 and 14, respectively); patient death (3 and 0, respectively), atrioventricular block (2 and 3, respectively), bronchospasm (1 and 4, respectively); and other reason (11 and 13, respectively)." Comment: All missing data accounted for and similarly balanced across the two treatment groups. Low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Quote: "Our results show that the RRR achieved with perioperative metoprolol in the vascular population is smaller than previously reported and is not significant" Comment: Authors commented on study results in relation to expected outcomes from other published reports. Furthermore, all of the primary and secondary pre‐specified outcomes were reported. |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Abbreviations: CHF: congestive heart failure; IV: intravenous; MI: myocardial infarction; RRR: relative risk reduction
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The study did not report if there was a subgroup of participants with abdominal aortic aneurysm (AAA). We attempted to contact the study author to see if these data were available but we could not make contact | |
| The study authors reported that 6.6% of participants had undergone vascular surgery but it did not report the number, if any, with AAA. We attempted to contact the study author to see if these data were available but we could not make contact | |
| This study examined beta‐blocker‐related complications in patients undergoing vascular surgery. We contacted the study authors for outcome data for AAA participants but they did not respond to communication | |
| The principal investigator of the DECREASE Study was dismissed for misconduct including failing to obtain patient written informed consent and negligent data collection. A full copy of the report issued by the Erasmum Medical Centre can be found here: Erasmus MC Follow‐Up Committee 2012 | |
| A subgroup of 56 participants underwent a AAA repair but specific outcome data for these participants were not presented. Through personal communication, the study author confirmed that these data were not available | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if these data were available but we could not make contact | |
| Of the 262 participants studied, 66% were taking antiplatelets, 19% anticoagulants, 23% calcium antagonists, 33% angiotensin‐converting enzyme (ACE) inhibitors and 15% were taking angiotensin II receptors prior to randomisation. Outcomes in this study could not be attributed to one specific drug and therefore we excluded this study | |
| Prospective study that measured the incidence of perioperative myocardial ischaemic injury in high‐risk vascular surgery patients. It was not a randomised controlled trial and it did not administer drugs | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if these were available but we could not make contact | |
| Intervention is curcumin, which is a natural health product | |
| Patients in this study were taking co‐medications (angiotensin drugs, calcium channel blockers, beta blockers, acetylsalicylic acid, clopidogrel) that we planned to assess in this review. Outcomes in this study could not be attributed to one specific drug and therefore we excluded this study | |
| Of the 103 participants included in this study, 38% underwent aortic repair. However the study did not present outcome data for this subgroup. We attempted to retrieve these data but the study authors did not respond to our communication | |
| Following personal communication, the study author confirmed that data for the AAA participants were not available | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if the data were available but we could not make contact | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if the data were available but we could not make contact | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if the data were available but we could not make contact | |
| The study did not report if there was a subgroup of participants with AAA. We attempted to contact the study author to see if the data were available but we could not make contact |
Abbreviations: AAA: abdominal aortic aneurysm; ACE: angiotensin‐converting enzyme.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 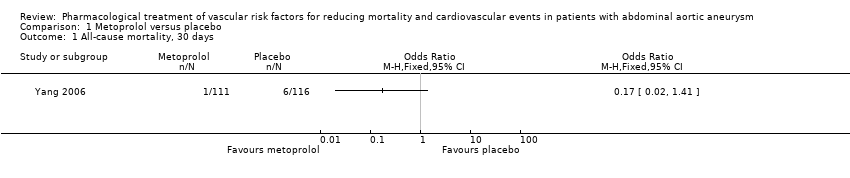 Comparison 1 Metoprolol versus placebo, Outcome 1 All‐cause mortality, 30 days. | ||||
| 2 Cardiovascular death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Metoprolol versus placebo, Outcome 2 Cardiovascular death, 30 days. | ||||
| 3 AAA‐related death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Metoprolol versus placebo, Outcome 3 AAA‐related death, 30 days. | ||||
| 4 Nonfatal cardiovascular event, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Metoprolol versus placebo, Outcome 4 Nonfatal cardiovascular event, 30 days. | ||||
| 5 All‐cause mortality, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 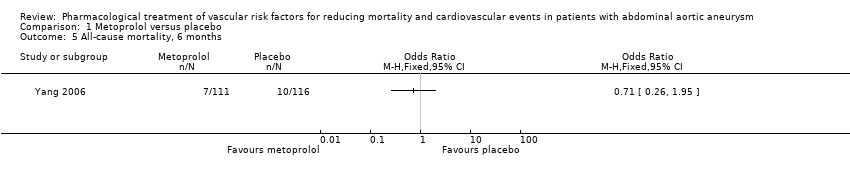 Comparison 1 Metoprolol versus placebo, Outcome 5 All‐cause mortality, 6 months. | ||||
| 6 Cardiovascular death, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 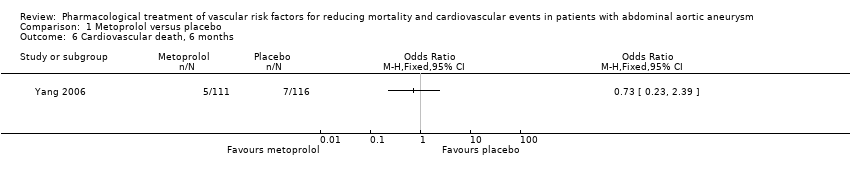 Comparison 1 Metoprolol versus placebo, Outcome 6 Cardiovascular death, 6 months. | ||||
| 7 Nonfatal cardiovascular event, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7 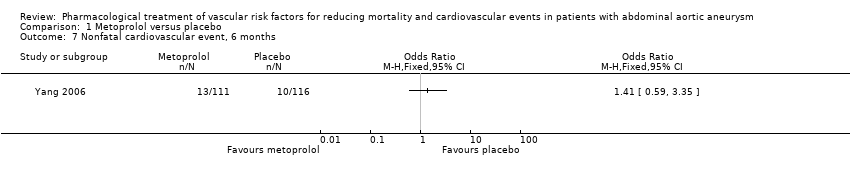 Comparison 1 Metoprolol versus placebo, Outcome 7 Nonfatal cardiovascular event, 6 months. | ||||
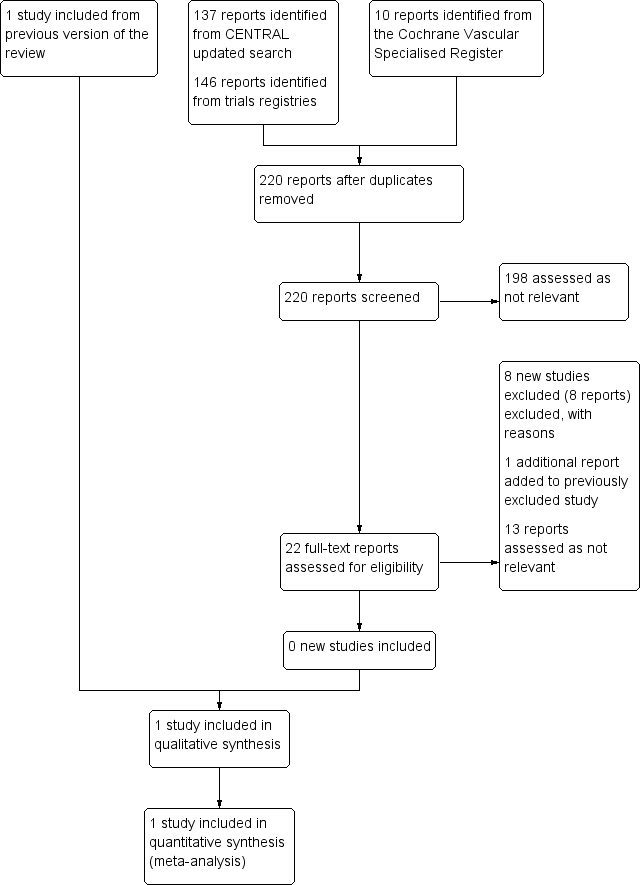
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Metoprolol versus placebo, Outcome 1 All‐cause mortality, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 2 Cardiovascular death, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 3 AAA‐related death, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 4 Nonfatal cardiovascular event, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 5 All‐cause mortality, 6 months.
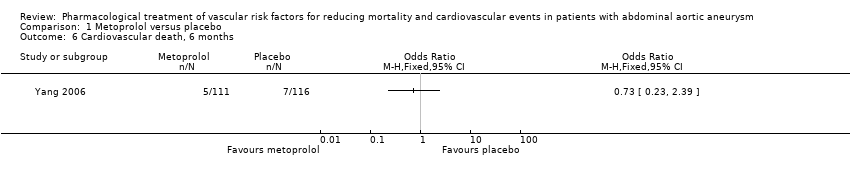
Comparison 1 Metoprolol versus placebo, Outcome 6 Cardiovascular death, 6 months.

Comparison 1 Metoprolol versus placebo, Outcome 7 Nonfatal cardiovascular event, 6 months.
| Metoprolol compared to placebo for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm (AAA) | ||||||
| Patient or population: patients of any age with AAA less than 30 mm in diameter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with metoprolol | |||||
| All‐cause mortality, 30 days1 | Study population | OR 0.17 | 227 | ⊕⊕⊝⊝ | — | |
| 52 per 1000 | 9 per 1000 | |||||
| Cardiovascular death, 30 days3 | Study population | OR 0.20 | 227 | ⊕⊕⊝⊝ | — | |
| 43 per 1000 | 9 per 1000 | |||||
| AAA‐related death, 30 days4 | Study population | OR 1.05 | 227 | ⊕⊕⊝⊝ | — | |
| 9 per 1000 | 9 per 1000 | |||||
| Nonfatal cardiovascular event, 30 days5 | Study population | OR 1.44 | 227 | ⊕⊕⊝⊝ | — | |
| 78 per 1000 | 108 per 1000 | |||||
| All‐cause mortality, 6 months1 | Study population | OR 0.71 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 63 per 1000 | |||||
| Cardiovascular death, 6 months3 | Study population | OR 0.73 | 227 | ⊕⊕⊝⊝ | — | |
| 60 per 1000 | 45 per 1000 | |||||
| AAA‐related death, 6 months4 | See comments | See comments | See comments | See comments | The incidence of AAA‐related death was not measured at six months. | |
| Nonfatal cardiovascular event, 6 months5 | Study population | OR 1.41 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 117 per 1000 | |||||
| *The risk with placebo was the average risk in the placebo group (i.e. the number of participants with events divided by total number of participants of the placebo group included in the meta‐analysis). The risk in the metoprolol group (and its 95% CI) is based on the assumed risk in the placebo group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Death from all causes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Cardiovascular death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 AAA‐related death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Nonfatal cardiovascular event, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 All‐cause mortality, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Cardiovascular death, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Nonfatal cardiovascular event, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

