Cierre subcutáneo versus ningún cierre subcutáneo después de los procedimientos quirúrgicos no relacionados con la cesárea
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010425.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 enero 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KS Gurusamy conceived the review question; developed and co‐ordinated the review; secured funding; completed the first draft of the review; advised on, wrote, edited and made an intellectual contribution to the review; approved the final version prior to submission, and is its guarantor.
Clare Toon extracted data, made an intellectual contribution to the review and approved the final version prior to submission.
Brian Davidson conceived the review question; secured funding; made an intellectual contribution; advised on part of the review and approved the final version prior to submission.
Contributions of editorial base
Nicky Cullum: edited the review; advised on methodology, interpretation and review content.
Susan O'Meara: Editor; approved the final review prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; and edited the review.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Rachel Richardson: edited the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
National Institute for Health Research, the health research wing of the UK Government Department of Health funds K Gurusamy to complete this review.
-
The National Institute for Health Research (NIHR) is the sole funder of the Cochrane Wounds Review Group, UK.
Declarations of interest
This project was funded by the National Institute for Health Research (NIHR).
Disclaimer
Department of Health disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS (National Health Service), or the Department of Health.
KS Gurusamy: no known conflict of interest
Clare Toon: no known conflict of interest
Brian Davidson: no known conflict of interest
Acknowledgements
The authors would like to acknowledge the contribution of the Wounds Group editor (Julie Bruce), peer referees (Susanne Hempel; Robert Wyllie; Christine Fyfe), Statistical editor (Elmer Villanueva), and copy editor Elizabeth Royle, for their role in improving the review. To Ms Tina Sedaghati, who helped with the translation of Nouraei 2010.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Jan 21 | Subcutaneous closure versus no subcutaneous closure after non‐caesarean surgical procedures | Review | Kurinchi Selvan Gurusamy, Clare D Toon, Brian R Davidson | |
| 2013 Mar 28 | Subcutaneous closure versus no subcutaneous closure after non‐obstetric surgical procedures | Protocol | Kurinchi Selvan Gurusamy, Brian R Davidson | |
Differences between protocol and review
A new outcome "Impact to the patient (in terms of return to activity and return to work) and to the healthcare funder (in terms of costs related to dressings or treatment related to wound complications)" was added, as this outcome will permit assessment of the impact of any difference in incidence of wound complications for both participants and healthcare funders.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
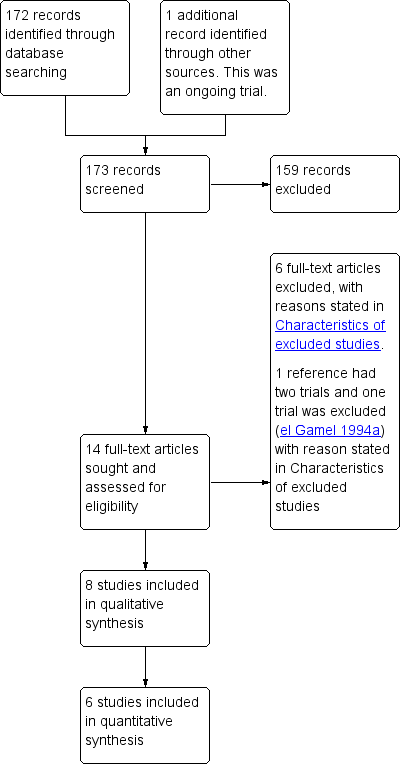
Study flow diagram
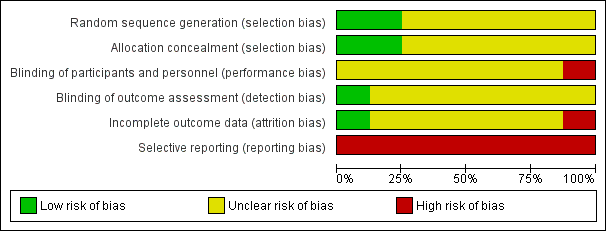
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
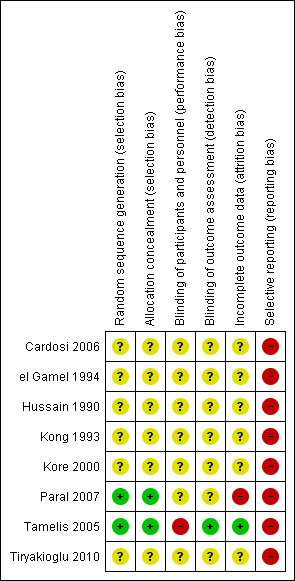
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 1 Superficial surgical site infection.

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 2 Superficial wound dehiscence.
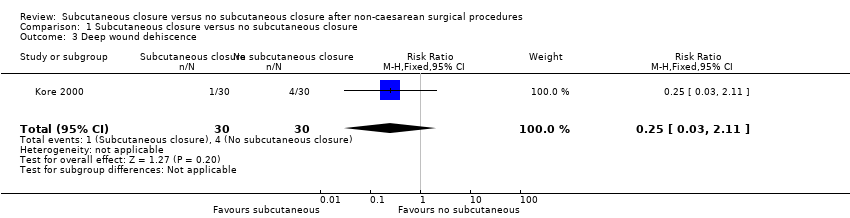
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 3 Deep wound dehiscence.
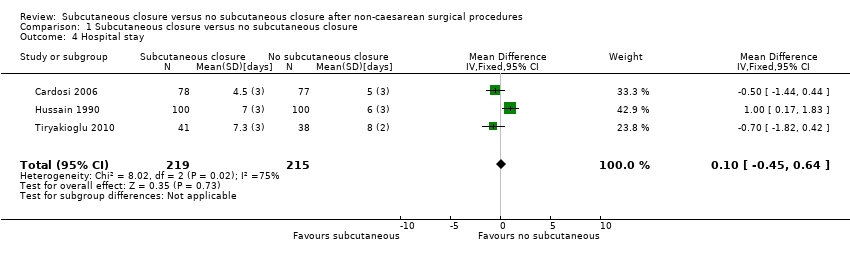
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 4 Hospital stay.
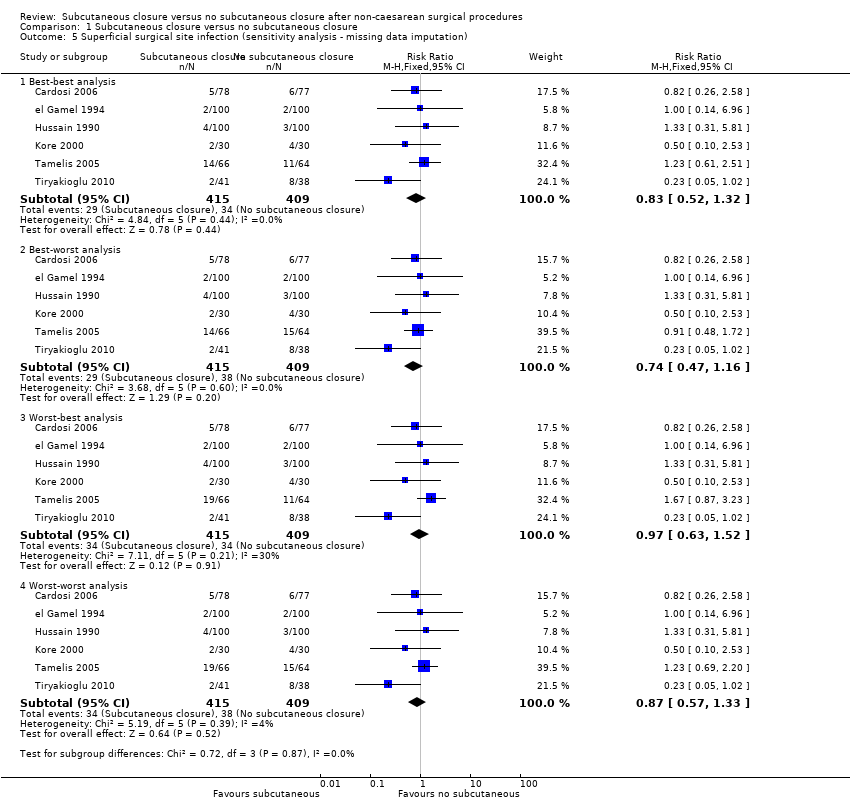
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 5 Superficial surgical site infection (sensitivity analysis ‐ missing data imputation).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 6 Hospital stay (sensitivity analysis).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 7 Superficial surgical site infection (sensitivity analysis ‐ > 30 days).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 8 Superficial wound dehiscence (sensitivity analysis ‐ > 30 days).
| Subcutaneous closure compared to no subcutaneous closure for non‐caesarean surgery | |||||
| Patient or population: participants having non‐caesarean surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No subcutaneous closure | Subcutaneous closure | ||||
| Superficial surgical site infection | 83 per 1000 | 70 per 1000 | RR 0.84 | 815 | ⊕⊝⊝⊝ |
| Superficial wound dehiscence | 103 per 1000 | 58 per 1000 | RR 0.56 | 215 | ⊕⊝⊝⊝ |
| Deep wound dehiscence | 133 per 1000 | 33 per 1000 | RR 0.25 | 60 | ⊕⊝⊝⊝ |
| Hospital stay | The mean hospital stay in the control groups was | The mean hospital stay in the intervention groups was | 434 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The trial(s) was (were) of high risk of bias | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Superficial surgical site infection Show forest plot | 6 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 2 Superficial wound dehiscence Show forest plot | 2 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.41] |
| 3 Deep wound dehiscence Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 4 Hospital stay Show forest plot | 3 | 434 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.45, 0.64] |
| 5 Superficial surgical site infection (sensitivity analysis ‐ missing data imputation) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Best‐best analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| 5.2 Best‐worst analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.16] |
| 5.3 Worst‐best analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.52] |
| 5.4 Worst‐worst analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 6 Hospital stay (sensitivity analysis) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Superficial surgical site infection (sensitivity analysis ‐ > 30 days) Show forest plot | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.19] |
| 8 Superficial wound dehiscence (sensitivity analysis ‐ > 30 days) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

