Cierre subcutáneo versus ningún cierre subcutáneo después de los procedimientos quirúrgicos no relacionados con la cesárea
Appendices
Appendix 1. Classification of surgical wounds
| Clean wound
|
| Clean‐contaminated wound
|
| Contaminated wound
|
| Dirty wound
|
Appendix 2. Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL Search Strategies
Ovid Medline
1 exp Wound Closure Techniques/ (38162)
2 exp Sutures/ (12060)
3 (closure or close or closing or sutur*).tw. (300829)
4 or/1‐3 (324065)
5 exp Subcutaneous Tissue/ (1703)
6 (((subcutaneous or sub‐cutaneous) adj5 (fat or adipose or tissue*)) or superficial fascia or hypdermis).tw. (18170)
7 or/5‐6 (19321)
8 4 and 7 (937)
9 randomized controlled trial.pt. (338195)
10 controlled clinical trial.pt. (85043)
11 randomized.ab. (241810)
12 placebo.ab. (134534)
13 clinical trials as topic.sh. (162087)
14 randomly.ab. (173722)
15 trial.ti. (103477)
16 or/9‐15 (782338)
17 (animals not (humans and animals)).sh. (3663525)
18 16 not 17 (720068)
19 8 and 18 (66)
Ovid Embase
1 exp wound closure/ (8769)
2 exp suture/ (28180)
3 (closure or close or closing or sutur*).tw. (400571)
4 or/1‐3 (412703)
5 exp subcutaneous tissue/ (22275)
6 (((subcutaneous or sub‐cutaneous) adj5 (fat or adipose or tissue*)) or superficial fascia or hypdermis).tw. (25061)
7 or/5‐6 (37394)
8 4 and 7 (1710)
9 Randomized controlled trials/ (26220)
10 Single‐Blind Method/ (16988)
11 Double‐Blind Method/ (115513)
12 Crossover Procedure/ (36187)
13 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (1213080)
14 (doubl$ adj blind$).ti,ab. (140490)
15 (singl$ adj blind$).ti,ab. (13290)
16 or/9‐15 (1268250)
17 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ (19613926)
18 human/ or human cell/ (14178567)
19 and/17‐18 (14131914)
20 17 not 19 (5482012)
21 16 not 20 (1091425)
22 8 and 21 (133)
EBSCO CINAHL
S22 S9 AND S21
S21 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 MH "Quantitative Studies"
S19 TI placebo* or AB placebo*
S18 MH "Placebos"
S17 TI random* allocat* or AB random* allocat*
S16 MH "Random Assignment"
S15 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S14 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S13 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S12 TI clinic* N1 trial* or AB clinic* N1 trial*
S11 PT Clinical trial
S10 MH "Clinical Trials+"
S9 S4 AND S8
S8 S5 OR S6 OR S7
S7 TI ( "superficial fascia" or hypdermis ) OR AB ( "superficial fascia" or hypdermis )
S6 TI ( (subcutaneous or sub‐cutaneous) N5 (fat or adipose or tissue*) ) OR AB ((subcutaneous or sub‐cutaneous) N5 (fat or adipose or tissue*) )
S5 (MH "Abdominal Fat")
S4 S1 OR S2 OR S3
S3 TI ( closure or close or closing or sutur* ) OR AB ( closure or close or closing or sutur* )
S2 (MH "Suture Techniques")
S1 (MH "Sutures")
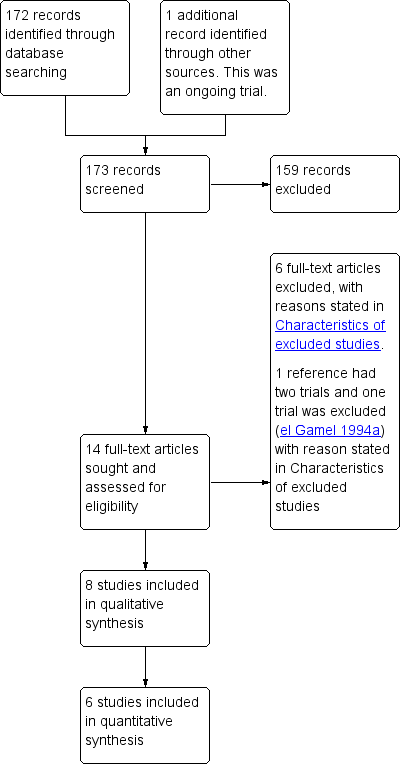
Study flow diagram
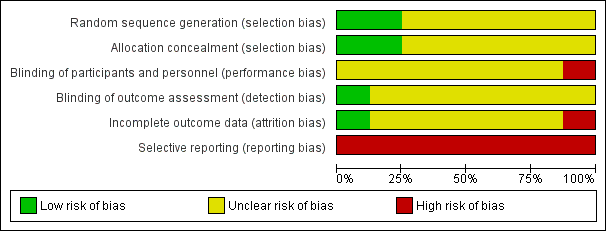
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
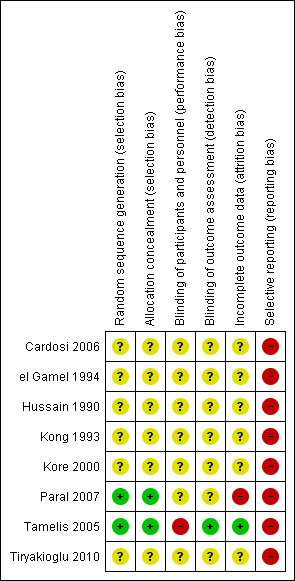
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 1 Superficial surgical site infection.

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 2 Superficial wound dehiscence.
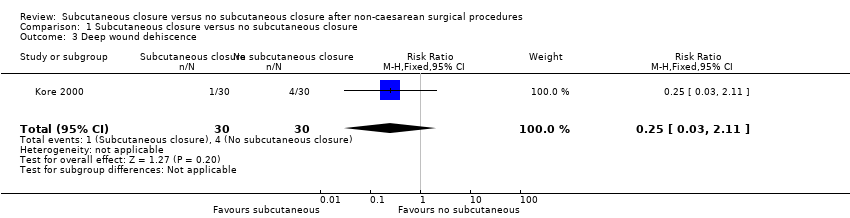
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 3 Deep wound dehiscence.
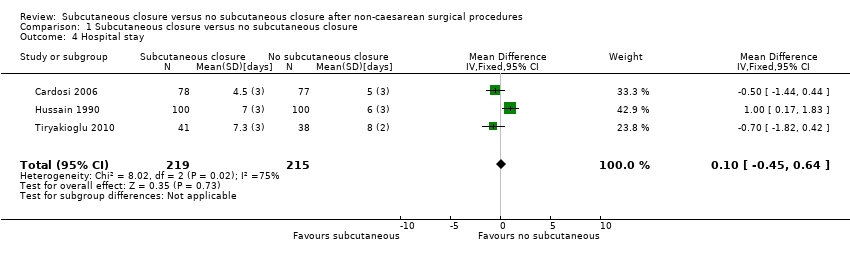
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 4 Hospital stay.
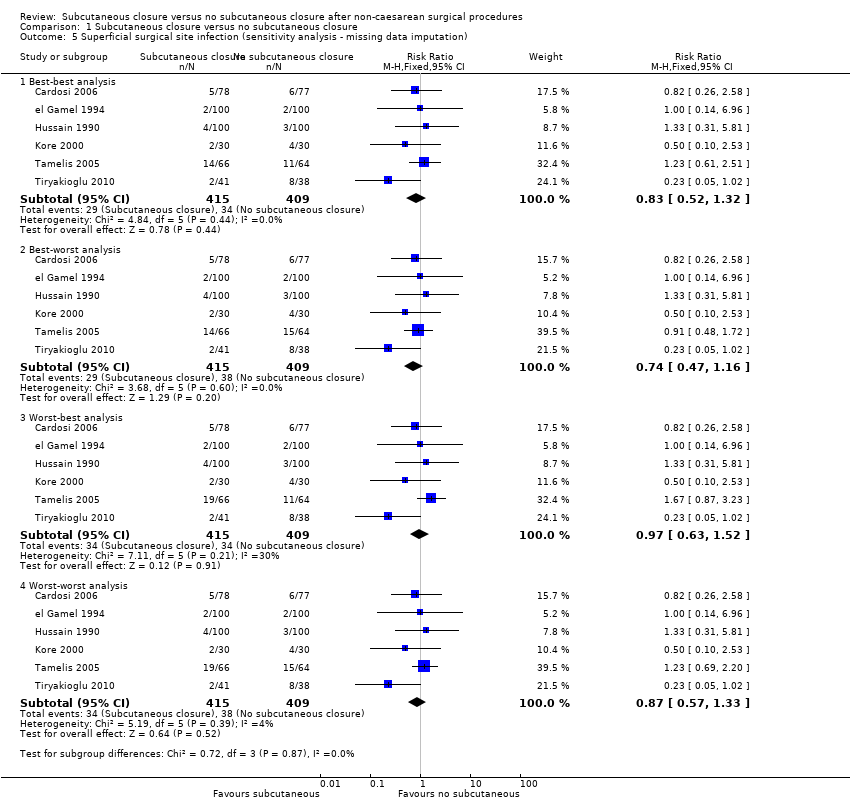
Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 5 Superficial surgical site infection (sensitivity analysis ‐ missing data imputation).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 6 Hospital stay (sensitivity analysis).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 7 Superficial surgical site infection (sensitivity analysis ‐ > 30 days).

Comparison 1 Subcutaneous closure versus no subcutaneous closure, Outcome 8 Superficial wound dehiscence (sensitivity analysis ‐ > 30 days).
| Subcutaneous closure compared to no subcutaneous closure for non‐caesarean surgery | |||||
| Patient or population: participants having non‐caesarean surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No subcutaneous closure | Subcutaneous closure | ||||
| Superficial surgical site infection | 83 per 1000 | 70 per 1000 | RR 0.84 | 815 | ⊕⊝⊝⊝ |
| Superficial wound dehiscence | 103 per 1000 | 58 per 1000 | RR 0.56 | 215 | ⊕⊝⊝⊝ |
| Deep wound dehiscence | 133 per 1000 | 33 per 1000 | RR 0.25 | 60 | ⊕⊝⊝⊝ |
| Hospital stay | The mean hospital stay in the control groups was | The mean hospital stay in the intervention groups was | 434 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The trial(s) was (were) of high risk of bias | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Superficial surgical site infection Show forest plot | 6 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 2 Superficial wound dehiscence Show forest plot | 2 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.41] |
| 3 Deep wound dehiscence Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.11] |
| 4 Hospital stay Show forest plot | 3 | 434 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.45, 0.64] |
| 5 Superficial surgical site infection (sensitivity analysis ‐ missing data imputation) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Best‐best analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| 5.2 Best‐worst analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.16] |
| 5.3 Worst‐best analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.52] |
| 5.4 Worst‐worst analysis | 6 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 6 Hospital stay (sensitivity analysis) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Superficial surgical site infection (sensitivity analysis ‐ > 30 days) Show forest plot | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.19] |
| 8 Superficial wound dehiscence (sensitivity analysis ‐ > 30 days) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

