Administración de suplementos de coenzima Q10 para la prevención primaria de las enfermedades cardiovasculares
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT cross‐over design (analysed as parallel group, using data only from the first 3 months of intervention before patients crossed over to the other therapy) | |
| Participants | 34 outpatients with primary hypercholesterolaemia (LDL‐cholesterol > 190 mg/dl, triglycerides < 200 mg/dl, 5 ° percentile < HDL‐cholesterol < 95 ° percentile of a reference population recruited in Italy | |
| Interventions | After a drug‐free controlled diet period participants were randomly allocated to 2 treatment groups: Intervention (statin + CoQ10): simvastatin (20 mg/day) plus CoQ10 (100 mg/day) for 3 months Control (statin): simvastatin (20 mg/day) for 3 months After this first 3‐month (90‐day) phase the 2 groups were crossed over | |
| Outcomes | Blood pressure, total cholesterol, HDL‐cholesterol, LDL‐cholesterol, triglycerides | |
| Notes | Age, sex and ethnicity of participants were not specified. Number of participants randomised to each arm was not specified. Outcome data were not fully reported. We could not find any contact details for the authors of this paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | High risk | Outcomes for LDL‐cholesterol, HDL‐cholesterol and triglycerides are not reported |
| Other bias | Unclear risk | Insufficient information to judge |
| Methods | RCT of parallel‐group design | |
| Participants | 40 participants (11 men and 29 postmenopausal women, 60.7 ± 5.7 years) with mild hypercholesterolaemia (5.90 ± 0.96 mmol/L) taking a regular HMG‐CoA reductase inhibitor treatment were recruited from newspaper advertisements from Eastern Finland in Spring 1997. BMI of participants was 26.9 ± 3.6 kg/m2 Exclusion criteria: regular intake of antioxidants, any drug with antioxidative properties, acetyl‐salicylic acid or other investigational products within the last month, malabsorption, treatment with oral oestrogen, use of anticoagulants, manifest insulin diabetes, cancer or other severe diseases that could cause difficulties in the participation Participants were recruited to 4 arms: α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol and placebo | |
| Interventions | Intervention (CoQ10): 10 participants were randomised. Oil‐based CoQ10 (2 x 100 mg daily) for 3 months. All capsules contained soybean oil. Participants were advised to take capsules in the morning and evening with meals (2 capsules in the morning and 2 in the evening) and to maintain their statin treatment, smoking and normal exercise and dietary habits during the study Control (placebo): 10 participants were randomised. Placebo capsules contained soybean oil. Participants were advised to take placebo capsules in the morning and evening with meals (2 capsules in the morning and 2 in the evening) and to maintain their statin treatment, smoking and normal exercise and dietary habits during the study | |
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | 4‐arm trial (α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol and placebo). We used the CoQ10 and placebo arms only. Age and sex were not specified in each arm of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Insufficient information to judge |
| Methods | RCT of parallel‐group design | |
| Participants | 50 obese adults of either sex, greater than 20 years of age, and whose BMI was greater than 25 kg/m2 were recruited by advertisement in Seoul, Republic of Korea Exclusion criteria: taking vitamins or antioxidants, taking lipid‐lowering drugs such as statins or fenofibrates, uncontrolled hypertension (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg), uncontrolled diabetes mellitus with a fasting blood sugar ≥ 150 mg/dL, hyperlipidaemia (triglycerides ≥ 400mg/dL or total cholesterol ≥ 250 mg/dL), or a history of cardiovascular disease | |
| Interventions | Intervention (CoQ10): 26 participants (11 men and 15 women, mean age 42.7 ± 11.3 years) were given a 200 mg coenzyme Q10 pill once a day for 12 weeks Control (placebo): 25 participants (10 men and 15 women, mean age 42.5 ± 11.2 years) were given a placebo pill once a day for 12 weeks | |
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 30% lost to follow‐up overall |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Report states "the strict exclusion criteria were meant to reduce confounding factors but may have resulted in a relatively healthy study population that attenuated the favourable effects of CoQ10." Also states, "the small sample size may have resulted in a Type II error" |
| Methods | RCT of parallel‐group design | |
| Participants | 49 Japanese, hypercholesteraemic (above 220 mg/dL) patients of either sex Exclusion criteria: pregnant or lactating women; women of childbearing potential; patients with familial hypercholesterolaemia; patients taking other lipid‐lowering drugs such as fibrates or bile acid‐binding resins and other drugs known to affect statin metabolism, such as fibrates, cyclosporine, tamoxifen, corticosteroids, macrolide antibiotics and others; patients taking antioxidants such as ascorbic acid and tocopherol | |
| Interventions | After a 4‐week dietary lead‐in period (less than 300 mg/day of low cholesterol diet), patients were randomised as follows: Intervention (statin + CoQ10): 24 participants (6 men and 18 women, mean age 61 ± 8 years) took atorvastatin (10 mg/day) for 16 weeks plus CoQ10 (100 mg/day) for 12 weeks Control (statin + placebo): 25 participants (8 men and 17 women, mean age 60 ± 8 years) took atorvastatin (10 mg/day) for 16 weeks plus placebo for 12 weeks Patients were instructed not to change their dietary and smoking habits throughout the study | |
| Outcomes | Serum total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | Nobody could discriminate the soft capsule of placebo containing only safflower oil from the CoQ10 capsule by appearance, odour and taste | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Plasma triglyceride levels in the placebo arm were slightly higher than those in the CoQ10 arm at baseline |
| Methods | RCT of parallel‐group design | |
| Participants | 52 patients of either sex, with essential hypertension whose blood pressure was higher than 150/90 mmHg were selected at random from the outpatient clinic of The Center for Adult Diseases, Osaka, Japan. 20 patients (8 men, 12 women, mean age 60 years) with low CoQ level and low SDH‐Q reductase activity were randomised Patients receiving conventional therapy for hypertension such as thiazide, beta‐blocker or vasodilators were allowed to enter the study. In the CoQ10 arm, 2 participants were taking thiazide, 3 were taking beta‐blockers, 2 were taking a combination of both of these medications and 3 had no therapy. In the control (placebo) group, 2 participants were taking thiazide, 4 were taking beta‐blockers, 1 was taking a combination of both of these medications, 1 was taking alpha‐blockers and 2 had no therapy. Therapy was continued during the trial period without any change Exclusion criteria: serious complications such as angina pectoris, myocardial infarction or cerebral vascular diseases were not included | |
| Interventions | Each patient entered a lead‐in period of at least 4 weeks, during which their symptoms and blood pressure were stable. The intervention group (4 men, 6 women; mean age 59.5 ± 2.6 years) received 3 capsules daily. Each capsule contained 33.3 mg CoQ10. The control group (4 men, 6 women; mean age 61.3 ± 3.3 years) received 3 capsules daily of inactive placebo | |
| Outcomes | Systolic blood pressure, diastolic blood pressure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
| Methods | RCT of parallel‐group design | |
| Participants | 44 patients (22 male, 22 female) with previous statin‐related myalgia, recruited from New Zealand Exclusion criteria: acute myocardial infarction or cerebral vascular accident within 3 months, alanine aminotransferase or aspartate aminotransferase > 3 times the upper level of normal, calculated glomerular filtration rate 45 ml/min, decompensated heart failure, warfarin treatment and antioxidant vitamin supplementation | |
| Interventions | Before randomisation, patients underwent a 2‐week washout of coenzyme Q10 supplements and lipid‐modifying therapies, except for ezetimibe (n = 4) The intervention group (12 men, 10 women; mean age 59 ± 2 years) received CoQ10 capsules (200 mg/day) for 12 weeks in combination with upward dose titration of simvastatin from 10 mg/day, doubling every 4 weeks if tolerated to a maximum of 40 mg/day. The control group (10 men, 12 women; mean age 59 ± 2 years) received placebo for 12 weeks in combination with upward dose titration of simvastatin from 10 mg/day, doubling every 4 weeks if tolerated to a maximum of 40 mg/day | |
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Insufficient information to judge |
BMI: body mass index
DBP: diastolic blood pressure
HMG‐CoA: hydroxy‐methylglutaryl‐coenzyme A
RCT: randomised controlled trial
SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No outcomes of interest measured | |
| Participants not healthy (29% had CHD and 45% had diabetes) | |
| Not a suitable intervention or control (both include vitamin E) | |
| Not a suitable control group (vitamin E supplementation) | |
| Short‐term trial (follow‐up period was 2 weeks) | |
| Not a suitable intervention (CoQ10 was combined with a Mediterranean diet) | |
| Short‐term trial (follow‐up period was 10 weeks) | |
| No outcomes of interest measured | |
| No outcomes of interest measured | |
| Short‐term trial (follow‐up period was 2 months) | |
| Not a suitable control group (vitamin E supplementation). Short‐term trial (follow‐up period was 30 days) | |
| Participants not healthy (56% with CAD) | |
| No outcomes of interest measured | |
| Short‐term trial (follow‐up period was 4 weeks) | |
| Short‐term trial (follow‐up period was 4 weeks) | |
| No outcomes of interest measured | |
| Short‐term trial (follow‐up period was 4 weeks) | |
| Short‐term trial (follow‐up period was 8 hours) | |
| Participants not healthy (haemodialysis patients) | |
| Short‐term trial (follow‐up period was 20 days) | |
| Short‐term trial (follow‐up period was 4 weeks) | |
| No outcomes of interest measured | |
| Participants not healthy (53% with diabetes and 30% with CVD) | |
| No outcomes of interest measured | |
| No outcomes of interest measured |
CHD: coronary heart disease
CoQ10: co‐enzyme Q10
CVD: cardiovascular disease
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT of parallel‐group design |
| Participants | 60 patients with statin‐associated myopathy |
| Interventions | Randomisation 1: 200 mg CoQ10 daily or the corresponding placebo 4 subgroups were studied: (1) Group Q10Se, 200 mg of CoQ10 (active) + 200 µg of selenium (active) (daily) (2) Group Q10SePla, 200 mg CoQ10 (active) + selenium placebo (daily) (4) Group Q10PlaSePla, CoQ10 placebo and selenium placebo |
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure |
| Notes | Contacted author and received response but still waiting for specific data |
CAD: coronary artery disease
CoQ10: co‐enzyme Q10
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Q10 for Gulf War Veterans |
| Methods | Randomised, double‐blind (participant, investigator, outcomes assessor), placebo‐controlled, cross‐over study |
| Participants | 46 Gulf War veterans with chronic health problems (Gulf War Illness). Minimum age: 18 years. maximum age: n/a. Gender: both Inclusion criteria: |
| Interventions | Coenzyme Q10 at 100 mg 3 times a day or 300 mg 3 times a day or matching placebo for 3.5‐month periods |
| Outcomes | Quality of life (subjective health; syndrome defining symptoms (fatigue, muscle pain, muscle strength; and cognition)) |
| Starting date | Date of first enrolment: July 2008 |
| Contact information | Beatrice A Golomb, MD, PhD, Principal Investigator University of California San Diego La Jolla |
| Notes | This study is ongoing, but not recruiting participants Emailed to find out further information (http://gulfstudy.ucsd.edu/Contact_Us.htm) but no response received |
| Trial name or title | A double‐blind, randomised, placebo‐controlled, 12‐week, cross‐over study to assess the effect of coenzyme Q10 treatment on 24‐hour mean ambulatory systolic and diastolic blood pressure in inadequately treated hypertensive patients with the metabolic syndrome |
| Methods | Randomised, controlled, cross‐over trial |
| Participants | Age minimum: 25 years Inclusion criteria: hypertension (average sitting systolic BP of > 139 mmHg or > 129/80 if patient has type 2 diabetes) and stabilised on antihypertensives for at least 1 month. The metabolic syndrome Exclusion criteria: uncontrolled hypertension. Cerebrovascular accident within 12 months prior. Taking warfarin treatment or antioxidant vitamin supplements |
| Interventions | Coenzyme Q10 (100 mg twice daily) or placebo (twice daily) for 12 weeks via oral capsule, followed by a 4‐week washout period, then 12 weeks of the alternate 'treatment' |
| Outcomes | Systolic and diastolic blood pressure |
| Starting date | Date of first enrolment: December 2008 |
| Contact information | Name: Sarah Molyneux Address: Canterbury Health Laboratories Biochemistry Unit, P.O. Box 151, Christchurch, 8140, New Zealand Telephone: +64 3 3641594 Email: [email protected] |
| Notes | Recruitment status: Closed. Follow‐up complete Contacted Sarah Molyneux for information but no response received |
| Trial name or title | The study of coenzyme Q10 supplementation on blood pressure, inflammation factors and adiponectin in hypertensive patients |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used. Assignment: parallel |
| Participants | Age minimum: 35 Inclusion criteria: willingness to co‐operate in the project and complete a written informed consent, diagnosis of hypertensive patients based on diagnostic criteria and clinical examination by a physician, no pregnant or breastfeeding women, the absence of any autoimmune disorders, cardiovascular or renal diseases, lack of nutritional supplements during the past 6 months Exclusion criteria: diabetes mellitus type 1 or type 2, the history of disease such as myocardial infarction, cardiac dysfunction, cardiac arrhythmia, angina and kidney disease, autoimmune diseases like MS, rheumatism, etc., BMI > 40, factors causing secondary hypertension, supplementation of vitamin, mineral or other nutritional supplements, alcohol or drug use, incidence of severe side effects, non‐compliance with study protocol |
| Interventions | Coenzyme Q10, 100 mg orally, once daily for 3 months Placebo, 100 mg, once daily for 3 months |
| Outcomes | Hypertension. Time point: at baseline, and 6 and 12 weeks after baseline assessment. Method of measurement: Measured by standard mercury blood pressure measuring device sitting |
| Starting date | Date of first enrolment: March 2011 |
| Contact information | Name: Dr Hassan Mozaffari Address: 3rd floor, Central Building of Shahid Sadughi University of Medical Sciences and Health Services, Shahid Bahonar Sq. Yazd, Islamic Republic of Iran Telephone: +00 98 3517249333 Email: [email protected] Affiliation: Shahid Sadughi University of Medical Sciences and Health Services Name: Seied Mohammad Mohammadi Zarchi Address: Shahid Sadughi University of Medical Sciences and Health Services,Yazd Diabetes Research Center Yazd, Islamic Republic of Iran Telephone: 00989123468307 Email: [email protected] Affiliation: Shahid Sadughi University of Medical Sciences and Health Services |
| Notes | Target sample size: 72. Recruitment status: complete Contacted Dr Hassan Mozaffari and Seied Mohammad Mohammadi Zarchi. Response was received from Dr Hassan Mozaffari to say the trial was done but they are unable to write the article. Dr Hassan Mozaffari was contacted again to find out further information on who is responsible for writing the article/reasons why they are unable to write the article |
| Trial name or title | Effect of Q10 in the treatment of metabolic syndrome |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used. Assignment: parallel |
| Participants | Inclusion criteria: 1. Having the metabolic syndrome according to the NCEP ATP III definition: Exclusion criteria: Exclusion criteria: Age minimum: 35 |
| Interventions | Intervention group: Q10, 100 mg soft gel capsule twice a day for 3 months Placebo group: placebo tablets containing 100 mg of soy oil twice a day for 3 months |
| Outcomes | Primary: HDL‐cholesterol, blood pressure, TG (at the beginning of the study and 3 months after intervention) Secondary: LDL‐cholesterol, total cholesterol (at the beginning of the study and 3 months after intervention) |
| Starting date | Date of first enrolment: October 2012 |
| Contact information | Name: Dr Gholamreza Usefzadeh Address: Shariati Ave, Kerman, Islamic Republic of Iran Telephone: +00 98 3412459003 Email: [email protected] Affiliation: Kerman Physiology Research Center Name: Dr Azadeh Najarzadeh Address: Bahonar Sq Yazd, Islamic Republic of Iran Telephone: +00 98 3516238505 Email: [email protected] Affiliation: Shahid Sadoghi University of Medical Sciences |
| Notes | Recruitment status complete Contacted Dr Gholamreza Usefzadeh and Dr Azadeh Najarzadeh for further information but no response was received |
| Trial name or title | Randomised, double‐blind, placebo‐controlled, cross‐over study to investigate the effect of dietary supplementation with coenzyme Q10 on endothelial function in males with the metabolic syndrome who are concurrently on statin treatment |
| Methods | Randomised, controlled, cross‐over trial |
| Participants | Age minimum: 35 years Inclusion criteria: Waist circumference >= 94 cm Exclusion criteria: Pre‐treatment low‐density lipoprotein (LDL)‐cholesterol < 1.5 mmol/L or > 6.5 mmol/L |
| Interventions | Coenzyme Q10 dietary supplement is a tablet (100 mg) to be taken orally, twice daily for 3 months. There is a 1‐month washout period between intervention and control or vice versa depending on treatment allocation |
| Outcomes | Markers of cardiovascular risk measured from blood tests Quality of life Systolic and diastolic blood pressure |
| Starting date | Date of first enrolment: October 2008 |
| Contact information | Name: Jo Young Address: Lipid and Diabetes Research Group First Floor, Hagley Hostel Christchurch Hospital, Private Bag 4710, Christchurch 8140, New Zealand Telephone: +64 3 364 1186 Email: [email protected] |
| Notes | Contacted Jo Young for further information. Received response to say the trial was completed and they are currently writing the manuscript for publication |
NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III
BMI: body mass index
BP: blood pressure
CDC: Centers for Disease Control and Prevention
MS: multiple sclerosis
TG: Triglycerides
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic blood pressure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 CoQ10, Outcome 1 Systolic blood pressure. | ||||
| 2 Diastolic blood pressure Show forest plot | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐5.20, 1.96] |
| Analysis 1.2  Comparison 1 CoQ10, Outcome 2 Diastolic blood pressure. | ||||
| 3 Total cholesterol Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.10, 0.70] |
| Analysis 1.3  Comparison 1 CoQ10, Outcome 3 Total cholesterol. | ||||
| 4 HDL‐cholesterol Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| Analysis 1.4  Comparison 1 CoQ10, Outcome 4 HDL‐cholesterol. | ||||
| 5 Triglycerides Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.42, 0.52] |
| Analysis 1.5 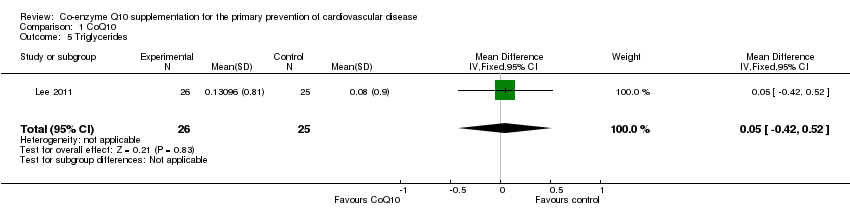 Comparison 1 CoQ10, Outcome 5 Triglycerides. | ||||
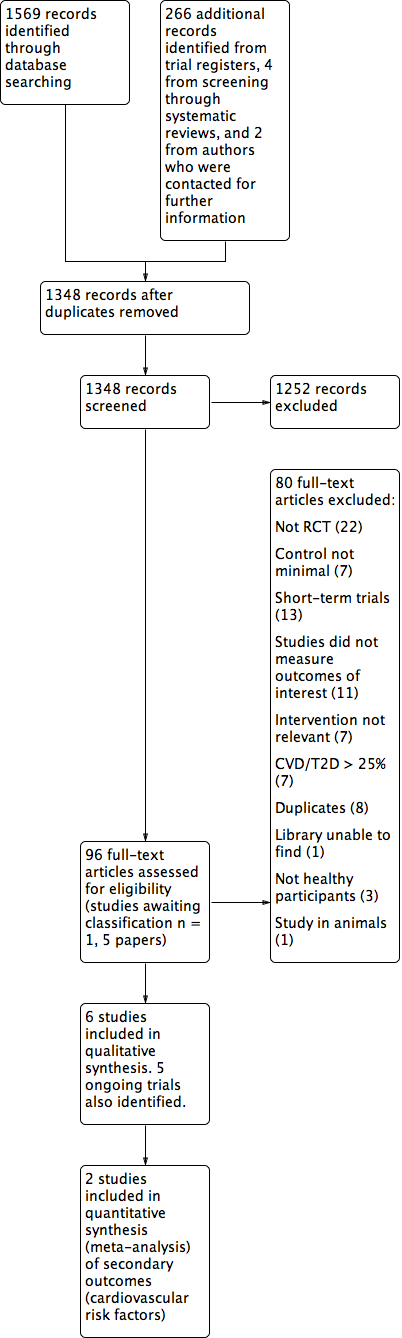
Study flow diagram.
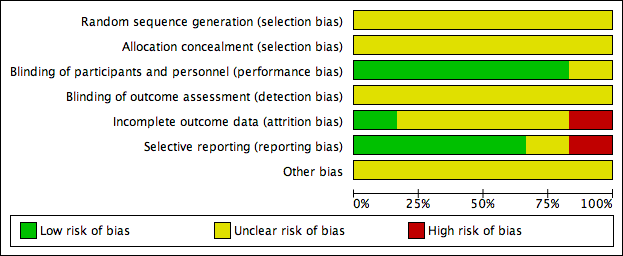
'Risk of bias' graph: authors' judgements about each risk of bias item presented as percentages across all included studies.
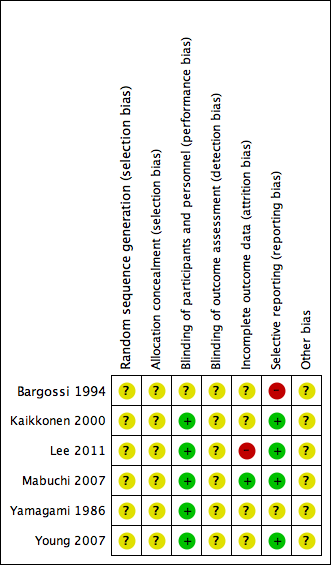
'Risk of bias' summary: authors' judgements about each risk of bias item for each included study.
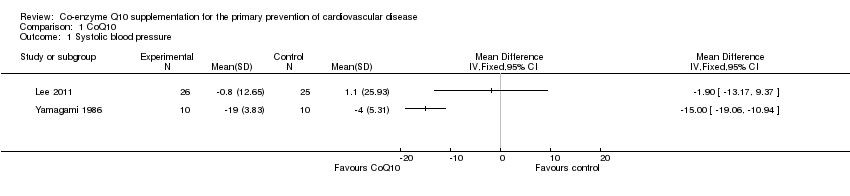
Comparison 1 CoQ10, Outcome 1 Systolic blood pressure.
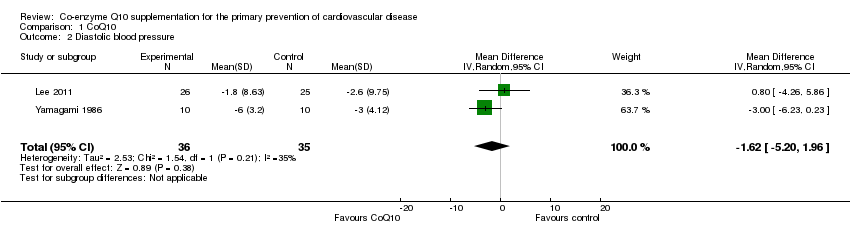
Comparison 1 CoQ10, Outcome 2 Diastolic blood pressure.

Comparison 1 CoQ10, Outcome 3 Total cholesterol.
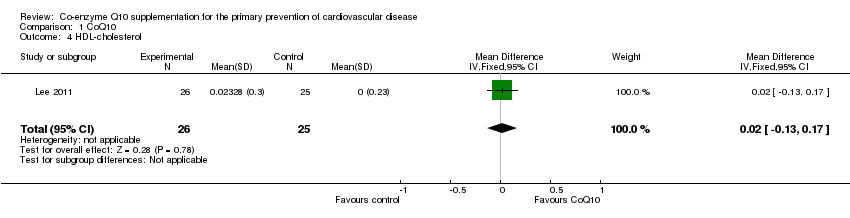
Comparison 1 CoQ10, Outcome 4 HDL‐cholesterol.
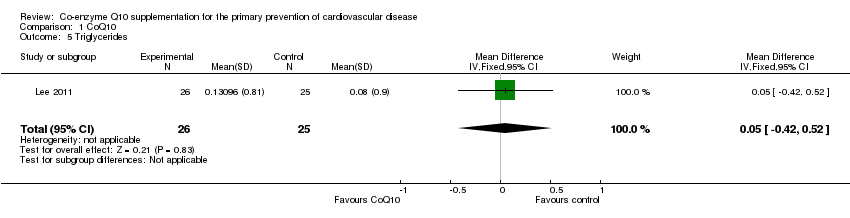
Comparison 1 CoQ10, Outcome 5 Triglycerides.
| Study | Country | Baseline CoQ10 level | Dose of CoQ10 level studied (mg/day) | Statin therapy | Duration of CoQ10 intervention |
| Italy | Plasma 1.20 mg/dL equivalent to 12,000 µg/L (intervention group) 1.08 mg/dL equivalent to 10,800 µg/L (control group) | 100 | Yes | 90 days | |
| Finland | Plasma 0.83 ± 0.04 µmol/L equivalent to 716.57 ± 34.53 µg/L (intervention group) 1.07 ± 0.10 µmol/L equivalent to 923.77 ± 86.33 µg/L (control group) | 200 | Yes | 3 months | |
| Seoul, Republic of Korea | Serum 0.63 ± 0.25 µg/ml equivalent to 630 ± 250 µg/L (intervention group) 0.65 ± 0.27 µg/ml equivalent to 650 ± 270 µg/L (control group) | 200 | No | 12 weeks | |
| Japan | Plasma 1.113 ± 0.444 µmol/L equivalent to 960.90 ± 383.32 µg/L (intervention group) 1.180 ± 0.282 µmol/L equivalent to 1018.74 ± 243.46 µg/L (control group) | 100 | Yes | 12 weeks | |
| Japan | Serum 0.704 ± 0.04 µg/ml equivalent to 704 ± 40µg/L (intervention group) 0.626 ± 0.05 µg/ml equivalent to 626 ± 50 µg/L (control group) | 100 | No | 12 weeks | |
| New Zealand | Plasma 1.3 (1.0 to 1.4) µmol/L equivalent to 1122.34 ± (863.34 to 1208.68) µg/L (intervention group) 1.4 (1.1 to 1.8) µmol/L equivalent to 1208.68 ± (949.67 to 1554.01) µg/L (control group) | 200 | Yes | 12 weeks | |
| Conversions: Molecular mass CoQ10 is 863.34 g | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic blood pressure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Diastolic blood pressure Show forest plot | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐5.20, 1.96] |
| 3 Total cholesterol Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.10, 0.70] |
| 4 HDL‐cholesterol Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| 5 Triglycerides Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.42, 0.52] |

