Penggunaan hyaluronidase sebagai tambahan kepada blok mata anestetik setempat untuk mengurangkan sakit intraoperatif dalam kalangan dewasa
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel group, prospective, masked randomized controlled single centre study in the UK. Study dates not stated. | |
| Participants | 92 adults (extracapsular cataract extraction phacoemulsification and trabeculectomy) received peribulbar block. Number of participants: hyaluronidase group; 44 control group ; 48. Mean age : hyaluronidase group: 72 years; control group : 75 years. | |
| Interventions | Hyaluronidase group ; lignocaine 2% with adrenaline 1:200,000, + bupivacaine 0.5% + hyaluronidase 150 IU/mL. Control group: lignocaine 2% with adrenaline 1:200,000 + bupivacaine 0.5%. 10 mL peribulbar injection using a standardized technique. | |
| Outcomes | Akinesia, objective analgesia assessed by surgeon, subjective analgesia assessed by participant after surgery. VAS 0 to 10 for subjective and objective pain scores were stratified into a dichotomous pain/no pain. | |
| Notes | Conflict of interest and funding sources not documented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomization described. |
| Allocation concealment (selection bias) | Unclear risk | Masked allocation but no details described |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All groups were reported on. |
| Blinding of participants and personnel (performance bias) | Low risk | Masking of participants described. |
| Blinding of outcome assessment (detection bias) | Low risk | Double masking described. |
| Methods | Parallel group, randomized double blind design in the UK. Study dates were not stated. | |
| Participants | 60 consecutive adults for elective intra‐ocular surgery. Number of participants: 20 per group. Results for low dose and higher dose were combined for analysis. Mean age: hyaluronidase (low dose) group: 74 years; hyaluronidase (higher dose) group: 73 years; control group: 72 years. | |
| Interventions | Hyaluronidase (low dose) group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75% + hyaluronidase 50 IU/mL. Hyaluronidase (higher dose) group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75% + hyaluronidase 150 IU/mL. Control group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75%. No sedation and premedication given. | |
| Outcomes | Speed of onset, akinesia, analgesia, top‐up frequency, incidence of harm. Analgesia measured by assessing participant's reaction to insertion of superior rectus suture and by direct questioning during procedure. 3 point scoring system used. Akinesia was the primary outcome measure. | |
| Notes | Conflict of interest and funding sources not documented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment but no details described. |
| Allocation concealment (selection bias) | Unclear risk | Masked allocation but no details described. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported on. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Composition of the local anaesthetic solution was not known to the anaesthetist", but no further details of masking described. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor (surgeon) masked. |
| Methods | Parallel group, prospective randomized controlled double masked, single centre trial in Leeds, the UK. Study dates; not stated. | |
| Participants | 20 adults undergoing routine cataract surgery. Data for 1 participant in hyaluronidase group were incomplete and excluded from analysis. Participants were ASA 1‐3. Number of participants in analysis: hyaluronidase group: 9; control group: 10. Mean age: hyaluronidase group: (73.8 years; control group: 74 years). Exclusion criteria; refusal, language problems, history of allergy to amide local anaesthetics or hyaluronidase or pre‐existing extra ocular muscle palsy. | |
| Interventions | Hyaluronidase group: lignocaine 2% + hyaluronidase 15 IU/mL. Control group: lignocaine 2%. Sub‐Tenon's block. Total volume of local anaesthesia 5 mL with no premedication sedation. | |
| Outcomes | Akinesia, depth of anaesthetic fluid spread on ultrasound, surgical conditions, pain during operation measured by visual analogue scale (VAS). SD pain scores unavailable. Attempts to contact authors for more clarification unsuccessful. | |
| Notes | Authors declared no conflict of interest and received no funding from private or public bodies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization. |
| Allocation concealment (selection bias) | Low risk | Coded syringes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 participant in treatment group excluded after randomization, due to incomplete data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were masked. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor masked. |
| Methods | Parallel group, prospective randomized double masked placebo controlled trial with a multicentre design in Germany. Study dates: 29 July 2003 to 2 November 2004. | |
| Participants | 80 adults undergoing elective cataract surgery with retrobulbar block. No participant dropped out. Number of participants: hyaluronidase group: 40; control group: 40. Mean (SD) age: hyaluronidase group: 76 ± 11 years; control group; 74±10 years. Inclusion criteria; adults aged >18 years, elective surgery, no active ocular disease and informed consent obtained in written form. Exclusion criteria; known intolerance to hyaluronidase, pregnancy, lack of co‐operation, history of alcohol or drug abuse, or local anaesthetic complications. | |
| Interventions | Hyaluronidase group: 5 mL 1% mepivacaine + 75 IU/mL hyaluronidase. Control group: 5 mL 1% mepivacaine + placebo (special batch of Hyalase without active ingredient). | |
| Outcomes | Primary end point; complete akinesia after 5 minutes. Secondary end points; akinesia at other times, top‐up injections, ptosis, time to anaesthesia, pain (VAS) immediately after surgery and 3 hours postsurgery and efficacy and tolerability for participant and surgeon. Adverse events recorded. First pain assessment point, planned before surgery then reported for immediately after surgery. | |
| Notes | No conflict or financial interest reported by author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization not described in detail. |
| Allocation concealment (selection bias) | Low risk | Double masked as per German legal framework. |
| Incomplete outcome data (attrition bias) | Low risk | No participant dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported on. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo control, double masked. |
| Blinding of outcome assessment (detection bias) | Low risk | Masked according to federal law. |
| Methods | Parallel group, randomized double masked controlled trial in 1 centre in UK. Study dates: not stated. | |
| Participants | 150 adults for elective cataract surgery with sub‐Tenon's block. Number of participants: hyaluronidase group: 76; control group: 74. Mean age; in hyaluronidase group; 77.14 years; control group; 76.51 years. Groups similar in terms of age, sex and proportion of blocks administered by each investigator. Exclusion criteria: people with learning difficulties, dementia, profound deafness and known adverse reaction to lignocaine or hyaluronidase. | |
| Interventions | Hyaluronidase group: 3 mL 2% lignocaine/adrenaline + hyaluronidase 30 IU/mL. Control group: 3 mL 2% lignocaine/adrenaline. | |
| Outcomes | Akinesia, post‐injection and immediate postoperative pain. Pain measured using VAS (0‐10 cm). SD pain scores not available with attempts to obtain more clarification from authors unsuccessful. | |
| Notes | Declaration of funding sources and conflict of interest not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Masked syringes. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported accordingly. |
| Blinding of participants and personnel (performance bias) | Low risk | Masking of participants not described. Masking of personnel (operative surgeon, independent assistant and nursing staff) was described. |
| Blinding of outcome assessment (detection bias) | Low risk | Double masked design. |
| Methods | Parallel group, randomized double masked trial in 1 centre in Iran. Study dates: February 2011 to July 2011. | |
| Participants | 44 adults initially recruited from a referral eye centre (Nikookari Eye Hospital) to undergo elective cataract surgery (phacoemulsification) under sub‐Tenon block. 2 participants did not meet the criteria and were excluded. Number of participants: hyaluronidase group: 21; control group: 21. Mean (SD) ages: hyaluronidase group: 65.62 ± 3.01 years; control group; 67 ± 4.4 years. Groups comparable for gender and age. Exclusion criteria: people with deafness and allergy to lidocaine or hyaluronidase. | |
| Interventions | Hyaluronidase group: 2 mL 2% lidocaine + hyaluronidase 150 IU/mL Control group: 2 mL 2% lidocaine. Ampoules were identical in appearance with a printed code (A or B). Codes disclosed for statistical analysis only at end of study. | |
| Outcomes | Akinesia, participant and surgical satisfaction with yes/no questions, postoperative pain via VAS scoring using a standard VAS chart. Participants were given appropriate explanation on usage of chart. | |
| Notes | No declaration of funding sources or conflict of interest reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive numbers assigned to participants on admission by a staff member not involved in study. |
| Allocation concealment (selection bias) | Low risk | Coded syringes. |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions after randomization reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Masking of participants, personnel by coded syringes. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor masked by coded syringes. |
| Methods | Randomized double masked study conducted at State University of Campinas ‐ Unicamp, Brazil. Study dates: not stated. | |
| Participants | 57 adults undergoing elective extracapsular cataract extraction on an outpatient basis. Participant's physical statuses described as ASA 1‐ 3. Number of participants: hyaluronidase group: 29; control group: 28. Sex: 31 men (54.4%); 26 women (45.6%). Age range (overall): 45‐ 89 years; mean (SD) overall: 67.73 (± 10.65). Groups homogeneous in relation to sex, age and physical condition. Peribulbar injection given as a block by double needle injection with 25x7 mm needle, administered 4 mL at lower temporal with super‐medial inclination of about 15°and 3 mL (nasal‐superior). Anaesthetic solution prepared without knowledge of ophthalmologist who performed the block. | |
| Interventions | Hyaluronidase group: ropivacaine 1% + hyaluronidase 100 IU/mL. Control group: ropivacaine 1%. | |
| Outcomes | Onset time to akinesia, need for supplementary injections and pain assessed by VAS (0‐10). | |
| Notes | No declaration of funding sources or conflict of interest reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals documented, all participants included initially were assessed and reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Masking described in participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment masked. |
ASA: American Society of Anesthesiology Classification; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Pain not assessed formally using a rating scale. Instead, augmentation eye drops given if participant complained of pain during procedure. Therefore, pain not measured but reported. | |
| 2 participants in the hyaluronidase group received local anaesthetic drops for pain intraoperatively. However, pain not formally measured, therefore, inclusion criteria not met. | |
| Pain not formally measured using a rating scale. | |
| Pain not measured as per rating scale and top‐ups primarily given to achieve akinesia initially. | |
| Surgeon assessed quality of analgesia and pain, not measured by asking participant. No formal method of assessing pain described. | |
| On further inspection, not a randomized trial. | |
| Adequate pain relief defined as lack of complaint or response from participant. Did not constitute pain measurement and no formal assessment of pain described in the study. | |
| Presence or absence of hyaluronidase not masked. | |
| Poster presentation, Further details could not be obtained about randomization, masking and results. | |
| Part one of third study was not randomized. Part two of this study compared two different concentrations of hyaluronidase only. | |
| Intraoperative pain not measured (e.g. by asking participant). It was assumed that the participants would spontaneously voice their pain during operation. Therefore, study did not measure any of our stipulated outcomes. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
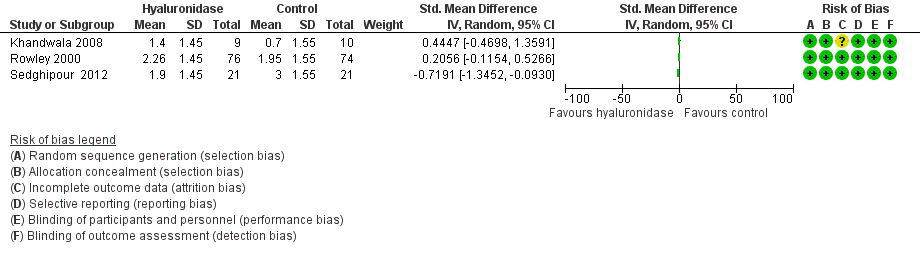
| 1 Intraoperative pain (reported continuous) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Hyaluronidase versus control, Outcome 1 Intraoperative pain (reported continuous). | ||||
| 2 Intraoperative pain (reported dichotomous) Show forest plot | 4 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |
| Analysis 1.2 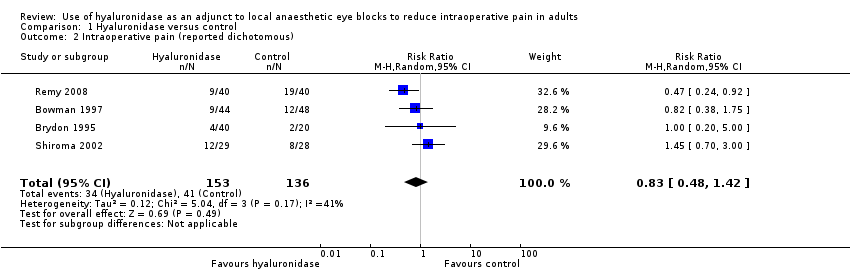 Comparison 1 Hyaluronidase versus control, Outcome 2 Intraoperative pain (reported dichotomous). | ||||
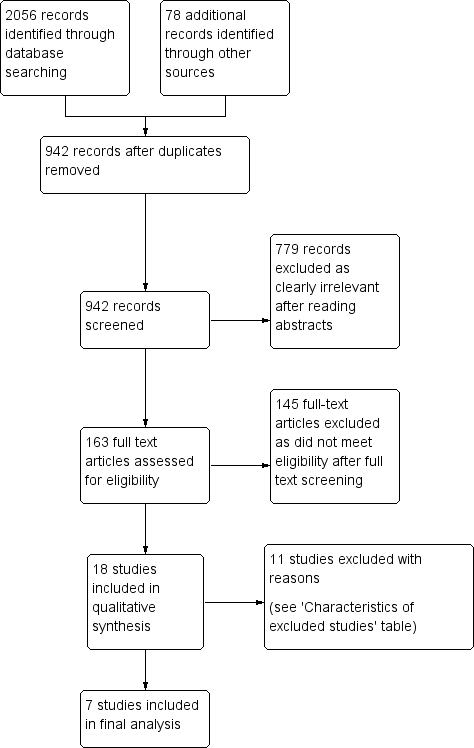
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.1 Intraoperative pain (measured by analogue rating scales; reported continuous).

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.2 Intraoperative pain (measured by analogue rating scales; reported dichotomous).

Comparison 1 Hyaluronidase versus control, Outcome 1 Intraoperative pain (reported continuous).

Comparison 1 Hyaluronidase versus control, Outcome 2 Intraoperative pain (reported dichotomous).
| Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | ||||||
| Patients or population: adults (aged ≥ 18 years) undergoing ophthalmic surgery under local anaesthetic eye blocks. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no hyaluronidase | Risk with hyaluronidase | |||||
| Intraoperative pain (reported dichotomous) | RR 0.83 | 289 | ⊕⊕⊝⊝ | ‐ | ||
| 301 per 1000 | 250 per 1000 | |||||
| Intraoperative pain (reported continuous) | 3 trials looked at effect of hyaluronidase on reduction of intraoperative pain measured by rating scales. Results were reported as continuous data. 2 studies did not provide the SMD, which measures the effect in a clinical setting, the results could not be meta‐analysed and hence were reported narratively (Khandwala 2008; Rowley 2000). Among the 3 trials covering 211 participants (Khandwala 2008: Mean difference 0.70; Rowley 2000: Mean difference 0.31; Sedghipour 2012: Mean difference ‐1.10), only the Sedghipour study with 42 participants, which is a high quality study, showed a statistically significant (at the 5 % level) reduction in pain in the hyaluronidase group (P = 0.04). The remaining 2 studies with 169 participants showed no statistically significant (at the 5 % level) reduction of pain intraoperatively with hyaluronidase (Khandwala 2008: P = 0.5; Rowley 2000: n.s). These studies were also of high quality and low risk of bias. Khandwala and colleagues had an unclear attrition bias as 1/10 participants in the treatment group was dropped after randomization with no clear explanation. | ‐ | 211 | ⊕⊕⊝⊝ | ‐ | |
| Incidence of harm | None of the studies reported harms in relation to hyaluronidase. | ‐ | (0 studies) | ‐ | ‐ | |
| Participant satisfaction | Significantly better satisfaction in these well designed studies with low risk of bias (Remy 2008; Sedghipour 2012). The studies included 122 participants and showed higher satisfaction scores in the treatment group (P < 0.05). | ‐ | 122 | ⊕⊕⊕⊝ | ‐ | |
| Surgical satisfaction | Surgical satisfaction was reportedly superior with hyaluronidase in the larger 2 studies (Remy 2008: P < 0.001; Sedghipour 2012: P = 0.02) and not significantly different in 1 small study (Khandwala 2008: P = 0.96). | ‐ | 141 | ⊕⊕⊕⊝ | ‐ | |
| Economic outcomes or cost calculations | None of the included studies reported economic outcomes or cost calculations | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n.s: not statistically significant; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to marked heterogeneity with a calculated I2 > 50%. 2Downgraded one level for imprecision due to wide 95% confidence intervals, reflecting uncertainty in the direction of effect estimate. 3Downgraded one level for imprecision and inconsistency in measurement, lack of data and small sample size. 4Downgraded one level because of imprecision secondary to small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraoperative pain (reported continuous) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Intraoperative pain (reported dichotomous) Show forest plot | 4 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |

