یوگا برای آسم
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 12): 20 x 1‐hour in‐class yoga sessions in a group setting (2 times per week for 10 weeks) and 10 x 30‐minute sessions at home (1 time per week for 10 weeks)
Control group (n = 8): usual care
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of their assignment status could subconsciously affect their performance, especially in more subjective measures such as quality of life. Lung function measures may more determined by the biological, objective effects of the intervention and therefore less vulnerable to performance bias irrespective of blinding. Overall, we assessed this study to be at high risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No active control and no procedure intended to blind outcome assessors were mentioned. In the case of self reported outcomes such as quality of life, the participant is the outcome assessor and therefore knowledge of assignment status could affect the outcome. Objective measures such as lung function may be less vulnerable to detection bias irrespective of blinding. Overall, we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on withdrawal or loss to follow‐up of participants was provided |
| Selective reporting (reporting bias) | High risk | The pre‐post changes in FEV1, PEFR, and FVC of the 2 groups were measured but not reported in detail due to lack of statistical significance. Quote: "There were no differences in FEV1, FVC, or PEFR in either group prior to the intervention, and no changes were demonstrated after the intervention." |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Participants were asked to keep their dose of inhaled steroid constant throughout the first 6 months, unless they had an asthma exacerbation. Yoga group (n = 30): 15‐minute home use of Pink City Lung Exerciser twice a day for 6 months
Control group (n = 29): 15‐minute home use of placebo Pink City Lung Exerciser twice a day for 6 months | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible subjects were then allocated to one of the three treatment groups using the next available number from computer generated numbers, randomised in blocks of six, and using sealed envelopes prepared independently." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible subjects were then allocated to one of the three treatment groups using the next available number from computer generated numbers, randomised in blocks of six, and using sealed envelopes prepared independently." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to some degree (quote: "Subjects were only given details of their treatment"). In addition, this is a placebo‐controlled trial in which participants were unlikely to determine the differences between groups. We thus considered the outcomes assessed in this study to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The assessor was not told which breathing technique subjects were using and subjects were asked not to mention it." For participant‐reported outcomes, the participant, who was unaware of group assignment, was the outcome assessor. We thus considered this study to be at low risk of performance bias |
| Incomplete outcome data (attrition bias) | Low risk | Although 13 of the initially randomised 59 participants discontinued the study and were thus not included in the final analysis, "the number of participants failing to complete and the reasons given were similar" between groups. Specifically, of the 29 participants allocated PCLE placebo device, 7 discontinued (6 lack of time/no perceived benefit, 1 no reason given); of the 30 participants allocated PCLE, 6 discontinued (5 lack of time/no perceived benefit, 1 health reasons (eye problems)) |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 12): 3‐hour sessions 5 times per week for 3 weeks of yoga (postures, breathing, cleansing, relaxation)
Control group (n = 12): usual care | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. This study may be less vulnerable to performance bias as the only outcomes measured were lung function and adverse events, which may be more determined by the biological, objective effects of the intervention and therefore less likely to be affected by the participants' and/or personnel's awareness of the intervention status. However, we assessed this study to be at high risk of performance bias overall |
| Blinding of outcome assessment (detection bias) | High risk | The paper did not mention any procedures intended to blind outcome assessors. Even if no blinding was applied, assessments of lung function by spirometry are less likely to be biased by outcome assessors' awareness of the intervention status. Adverse event recording may be more at risk of bias from knowledge of participant's group assignment. Overall, we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | High risk | 3 participants in the control group discontinued, and "their lung function measurements were not included in further evaluation". Although "no significant changes were discernible between the baseline values of these three and those of others", there could still be a high risk of bias because the sample size was so small (12 in the yoga group versus 12 in the control group). In addition, the 3 participants who withdrew did so for asthma‐related reasons. Quote: "Three subjects from the control group had to undergo treatment oral steroids use due to acute exacerbations of their asthma". The final results of the trial were thus prone to bias |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 138): yogic intervention for 30 min per day in the morning, 5 days a week for a period of 6 months, in addition to standard medical treatment
Control group (n = 138): standard medical treatment | |
| Outcomes |
| |
| Notes | 1. This study is available as abstract only. No details of the results were provided. 2. 17 participants in the yoga group and 18 participants in the control group dropped out during the study. The results presented in this report are based on the data collected from the 241 participants who completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "A total of 276 subjects were included in the study after randomization which was done by computer generated random number table." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of the assignment status could subconsciously affect their quality of life, asthma symptom score, and asthma medication usage and to a lesser extent, their performance on lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | The paper did not mention any procedures intended to blind outcome assessors. Even if no blinding was applied, assessments of lung function by spirometry were less likely to be biased by outcome assessors' knowledge of the assignment status. However, for participant‐reported outcomes such as quality of life, symptom score, and asthma medication usage, the participant, who was aware of assignment status, is the outcome assessor. Overall, we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 17 participants in the yoga group and 18 participants in the control group dropped out during the study. The results presented in this report are based on the data collected from the 241 participants who completed the study only. No information was provided regarding the characteristics and outcomes of the participants who dropped out |
| Selective reporting (reporting bias) | Low risk | All study outcomes were mentioned in the report, although no details were provided |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 24): 20‐minute session of ujjayi pranayama (postures and breathing) and shavasana (relaxation) twice a day for 6 weeks
Control group (n = 24): no intervention
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. However, this study may be less vulnerable to performance bias as the only outcome measured was lung function, which may be more determined by the biological, objective effects of the intervention and therefore less likely to be affected by the participants' and/or personnel's awareness the intervention status. Despite this, we assessed this study to be at high risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | The paper did not mention any procedures intended to blind outcome assessors. Even if no blinding was applied, assessments of lung function by spirometry are less likely to be biased by outcome assessors' awareness of the intervention status. For this reason, we judged this study to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Table 1 indicates no withdrawal or loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 12): 50‐minute daily sessions of yoga (postures, breathing, relaxation, discussion) for 4 weeks; remained on normal medication.
Control group (n = 12): not reported; remained on normal medication | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Participants were given information about the study objective, voluntary participation and told to their treatment. They were also told about the activities that are going to be practiced and were also informed as they can withdraw from participation at any stage." No active control. In this case, participants' knowledge of the assignment status could subconsciously affect their asthma medication usage and to a lesser extent their reporting of asthma attacks and their performance in lung function tests such as PEFR |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A physician who was blinded to the groups helped to complete the questionnaire and conducted the peak expiratory flow meter test." However, as asthma medication use and asthma attacks per week are likely to be self reported subjective outcomes, and participants were aware of group allocation, we considered this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | The tables indicate no withdrawal or loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | High risk | A number of 'errors' were found in the paper, e.g. inconsistent data in table 2 and table 4. This raises concern about the quality, i.e. at least the reporting quality, of the study |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 53): 2.5‐hour sessions of yoga training programme (postures, breathing, meditation, lectures) daily for 2 weeks; 65‐minutes yoga daily for 54 months
Control group (n = 53): usual care (continued taking their usual drugs) | |
| Outcomes |
| |
| Notes | 25 participants dropped out of the study: 7 after 6 months' of follow‐up, 7 after 12 months, 2 after 18 months, 4 after 24 months, and 5 after 30 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is more like a matched cohort study than a RCT. The two groups of participants were balanced on the matched factors, but not necessarily on others. The randomisation was conducted separately within every pair of two participants, i.e. it was conducted a total of 53 times, once for each pair. However, to randomise or to just subjectively assign two participants for each pair into different groups is the same in terms of potential to introduce bias, because other factors than the matched ones could not be balanced in this way. Quote: "Fifty three pairs of patients matched for age and sex and type, severity, and duration of asthma were selected from a bigger group who came to our outpatient clinic for yoga therapy. One from each pair was randomly selected for training in yoga, and the other served as a control." |
| Allocation concealment (selection bias) | High risk | After the assignment status of 1 participant in a pair was determined, the intervention to be received by the other member of the pair, who had not been recruited, was determined. Whether or not to recruit a coming patient could thus be affected by the staff's knowledge of the assignment scheme, which could lead to selection bias |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of the assignment status could subconsciously affect their asthma medication usage and asthma severity score and to a lesser extent their reporting of asthma attacks and performance on lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | The paper did not mention any procedures intended to blind the outcome assessment, and in the case of participant‐reported outcomes such as asthma severity score and medication usage, the participant, who was aware of group assignment, is the outcome assessor. We therefore assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Although 25 participants (24%) dropped out at the end of the study, their outcomes were still recorded. In this review we used records that covered almost all participants |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 40): pranayama yoga breathing: 60 minutes each day for 3 to 5 days, and then during the 3 months' follow‐up practice the exercises at home for 15 minutes twice daily; take medications in accordance with the physician's instructions Control group (n = 40): usual care (routine pharmacological management) | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Low risk | Quote: "The method of allocation was concealed in sequentially numbered, sealed, opaque envelopes. An independent observer who performed the randomisation procedure was not involved in conducting intervention and collecting the outcome measures." |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of the assignment status could subconsciously affect their quality of life and asthma control, and to a lesser extent, their reporting of adverse events and their performance in lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | The paper did not mention any procedures intended to blind outcome assessment. Even if no blinding was applied, assessments of lung function by spirometry and adverse events may be less likely to be biased by participant's and/or outcome assessors' knowledge of the assignment status, while participant‐reported outcomes are at higher risk of bias. Overall, we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | 4 participants from the yoga group were excluded from analysis due to non‐compliance with exercise. The baseline characteristics of the 4 participants were not presented or compared with those of other participants in the yoga group. However, compared to the sample size of 80, the drop‐out rate was low. We thus considered the risk of bias arising from this issue to be low |
| Selective reporting (reporting bias) | High risk | Adverse events were recorded but not reported. Quote: "Exacerbations and adverse events were recorded for all the groups." |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 29): 90‐minute sessions twice weekly for 4 weeks of Iyengar yoga, including 15 asanas (postures), pranayama (breathing), and dhyana (meditation); rescue inhaler use was allowed. Control group (n = 33): 90‐minute sessions twice weekly for 4 weeks of sham intervention of basic muscle stretching exercises; rescue inhaler use was allowed | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the interest of maintaining small class sizes for the intervention, participants were divided into 5 consecutive cohorts. In each cohort, participants were randomly assigned on the basis of software generated (SAS version 8.2; SAS Institute Inc, Cary, NC) blocked random assignment to a yoga intervention group or a stretching control group." |
| Allocation concealment (selection bias) | Low risk | Quote: "At enrolment, each participant was assigned an identification number, which was later coded to his or her allocation. All allocations were maintained in sealed envelopes that were unavailable to outcomes assessors to maintain masking." |
| Blinding of participants and personnel (performance bias) | Low risk | This is a double‐masked controlled clinical trial. Quote: "all participants were told that they were receiving 'complementary care body conditioning' for asthma management, and Sanskrit words, including yoga, asana, pranayama, and dhyana, were not used with participants." We therefore considered participants to be unaware of group assignment status and the study to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Outcomes were evaluated at baseline, at the end of the training sessions, and then monthly for 3 months by an investigator masked to treatment assignment." Participant‐reported outcomes were also considered to be at low risk of bias as the participants were unaware of group assignment |
| Incomplete outcome data (attrition bias) | High risk | 17 participants (27%) withdrew, 6 on yoga and 11 on control. Intention‐to‐treat analysis was performed, but there remains a risk of attrition bias inflating the results as more participants withdrew on control |
| Selective reporting (reporting bias) | High risk | Details on results of secondary outcomes were not reported |
| Other bias | High risk | The baseline FEV1/FVC (P = 0.02) and FEV 25‐75% (P = 0.03) were not comparable between intervention and control groups. Quote: "Although not all baseline values were significantly different, the intervention group consistently exhibited more disability for all spirometry measurements and asthma severity assessments than controls." |
| Methods |
| |
| Participants |
| |
| Interventions | Participants were initially stabilised on drugs until no further symptomatic improvement occurred. Then:
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. Although lung function (FEV1, FVC, and FEV1/FVC ratio) may be more determined by the biological, objective effects of the intervention and therefore less likely to be affected by the participants' and/or personnel's knowledge of the assignment status, we still considered this study to be at high risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | The paper did not mention any procedures intended to blind outcome assessors. Even if no blinding was applied, assessment of lung function by spirometry is less likely to be biased by outcome assessors' awareness of the intervention status. For this reason we judged this study to be low risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Table 1 indicates no withdrawal or loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 22): 15 minutes twice daily for 2 weeks of Pink City Lung Exerciser use
Control group (n = 22): 15 minutes twice daily for 2 weeks of placebo Pink City Lung Exerciser use
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Low risk | This is a randomised, double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) | Low risk | The paper did not mention any procedures intended to blind the outcome assessors. However, even if no blinding was applied, assessments of lung function by spirometry and adverse events are less likely to be biased by outcome assessors' knowledge of the assignment status, and the participants, who were unaware of assignment status, were the outcome assessors for the other measures, such as symptom score |
| Incomplete outcome data (attrition bias) | Low risk | The 4 participants who withdrew from the study were not included in the analysis. However, compared to the sample size of 44, the drop‐out rate was low; we thus considered the risk of bias arising from this issue as low. Quote: "4 subjects withdrew from the study; 1 found the lung exercises to be inconvenient and had nausea during the first period (with the placebo exerciser), and 3 had respiratory tract infection during the second period (2 with the PCL exerciser). Complete data are presented for 18 subjects." |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | The medication for asthma was kept same throughout the study period. Yoga group (n = 30): 50 minutes daily for 2 months of yoga (breathing, postures, meditation, and lifestyle modification)
Control group (n = 30): usual care | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of the assignment status could subconsciously affect their quality of life and to a lesser extent their performance in lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | The paper did not mention any procedures intended to blind the outcome assessment. Assessment of lung function by spirometry was less likely to be biased by the outcome assessors' knowledge of the assignment status, while the participant, who was aware of group assignment, is the outcome assessor for quality of life. Overall, we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | The 4 participants who withdrew from the study were not included in the analysis. However, compared to the sample size of 60, the 4 excluded participants represented a small number and were thus unlikely to exert substantial influence on the results |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | High risk | There were a number of 'errors' in the paper. For example, the abstract and methods reported that there were 60 participants in total. However, the results section reported that "four subjects withdrew from the study; one found the lung exercises to be inconvenient, and three had respiratory tract infection. Hence complete data are presented for 60 subjects", indicating there should be 64 participants in total. On the other hand, table 1 indicated that there were only 30 participants in total |
| Methods |
| |
| Participants |
| |
| Interventions | All participants remained on their prescribed treatment during the study.
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. The participants' knowledge of the assignment status could subconsciously affect their quality of life and asthma medication usage, and to a lesser extent the frequency and severity of asthma attacks and performance on lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | No procedures intended to blind outcome assessment were mentioned. The assessments of lung function by spirometry were less likely to be affected by outcome assessors' knowledge of the assignment status, but for participant‐reported outcomes, such as quality of life and attacks per week, the participant, who was aware of group assignment, was the outcome assessor. Overall, we judged this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | The paper mentioned nothing about withdrawal or loss to follow‐up of participants |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 9): 55‐minute classes 3 times weekly for 16 weeks of yoga
Control group (n = 8): not reported | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | No active control. In this case, the participants' knowledge of the assignment status could subconsciously affect their asthma medication usage and severity and frequency scores and to a lesser extent their performance in lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "During the study period, the records of both groups were coded. Decoded data were unavailable to the principal investigators. The investigating physicians did not know which patients were undergoing the yoga intervention." For objective outcomes such as lung function, we considered this study to be at lower risk of bias, but for participant‐reported outcomes we considered the study to be at high risk of bias. Overall, we judged this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | All 17 participants completed the study, and there were no dropouts |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
| Methods |
| |
| Participants |
| |
| Interventions | Yoga group (n = 30): a comprehensive yoga‐based lifestyle modification and stress management program for 4 hours a day for 2 weeks, in addition to conventional care (including normal rescue medication use).
Control group (n = 30): a session on health education relevant to their illness, in addition to conventional care (including normal rescue medication use) | |
| Outcomes |
| |
| Notes | 1 participant in the yoga group and 2 participants in the control group discontinued midway in the study. The results presented in this report are based only on the data collected from the 57 participants who completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | High risk | This is an open‐label RCT. In this case, the participants' knowledge of the assignment status could subconsciously affect their quality of life and asthma medication usage and to a lesser extent their performance on lung function tests |
| Blinding of outcome assessment (detection bias) | High risk | This is an open‐label RCT. The reporting and/or evaluation of quality of life and asthma medication use could thus be subconsciously affected by participant's and/or outcome assessors' knowledge of the assignment status. Lung function measures may be less vulnerable to detection bias, but overall we assessed this study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "However, one subject in the yoga group, and two subjects in the control group discontinued midway in the study. The results presented in this report are based on the data collected from only the 57 subjects who completed the study (yoga group, n = 29; control group, n = 28)." However, compared to the sample size of 60, the drop‐out rate was low. We thus considered the risk of bias arising from this issue to be low |
| Selective reporting (reporting bias) | Low risk | All study outcomes were reported with details |
| Other bias | Low risk | No evidence of other bias was found |
AQLQ: Asthma Quality of Life Questionnaire
AQOL: Assessment of Quality of Life
FEF 25‐75%: forced expiratory flow between 25% and 75% of vital capacity
FEV1: forced expiratory volume in one secondFRC: functional residual capacity
FVC: forced vital capacity
MVV: maximal voluntary ventilation
PCLE: Pink City Lung Exerciser
PD20: provocative dose of inhaled histamine or methacholine required to produce a 20% fall in FEV1
PEFR: peak expiratory flow rate
RCT: randomised controlled trial
Rtot: total airway resistance
RV: residual volume
SF‐36: 36‐item Short Form Health Survey
SGRQ: St. George's Respiratory Questionnaire
SVC: slow vital capacity
TLC: total lung capacity
VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The outcome measure (biochemical profile) in this report is not relevant to this review. The results on outcomes relevant to the present review were reported by Kant 2013, which has already been included | |
| It is not a randomised study | |
| The intervention (Buteyko technique) is not yogic | |
| The intervention (Papworth method) is not yogic | |
| It is not a randomised study | |
| It is not a randomised study | |
| It is not a randomised study | |
| The intervention included nutritional manipulation, yoga techniques, and journaling, and the net comparison of intervention vs control was not yoga alone | |
| The control group received relaxation methods, group discussion, and cognitive behaviour therapy, and the net comparison of intervention vs control was not yoga alone | |
| It is not a randomised study | |
| The control group practiced meditation, and the net comparison of intervention vs control was not yoga alone | |
| It is not a randomised study |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in AQLQ score Show forest plot | 5 | 375 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.37, 0.77] |
| Analysis 1.1  Comparison 1 Yoga vs usual care/sham intervention, Outcome 1 Change in AQLQ score. | ||||
| 1.1 Yoga breathing alone vs. control | 2 | 196 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Combination of yoga breathing, postures and meditation vs. control | 3 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.47, 1.22] |
| 2 Asthma symptom Show forest plot | 3 | 218 | Std. Mean Difference (Fixed, 95% CI) | 0.37 [0.09, 0.65] |
| Analysis 1.2 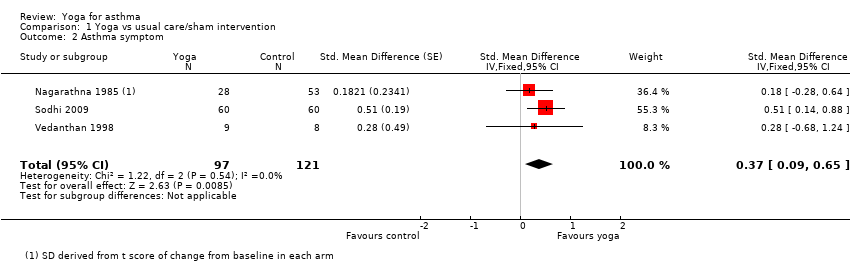 Comparison 1 Yoga vs usual care/sham intervention, Outcome 2 Asthma symptom. | ||||
| 3 FEV1 Show forest plot | 10 | 583 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.08, 0.70] |
| Analysis 1.3  Comparison 1 Yoga vs usual care/sham intervention, Outcome 3 FEV1. | ||||
| 4 FEV1 change from baseline Show forest plot | 7 | 340 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| Analysis 1.4  Comparison 1 Yoga vs usual care/sham intervention, Outcome 4 FEV1 change from baseline. | ||||
| 5 FVC Show forest plot | 6 | 376 | Std. Mean Difference (Random, 95% CI) | 0.67 [0.20, 1.14] |
| Analysis 1.5  Comparison 1 Yoga vs usual care/sham intervention, Outcome 5 FVC. | ||||
| 6 FEV1/FVC Show forest plot | 6 | 435 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐1.63, 2.87] |
| Analysis 1.6  Comparison 1 Yoga vs usual care/sham intervention, Outcome 6 FEV1/FVC. | ||||
| 7 PEFR Show forest plot | 7 | 455 | Std. Mean Difference (Random, 95% CI) | 0.73 [0.36, 1.09] |
| Analysis 1.7  Comparison 1 Yoga vs usual care/sham intervention, Outcome 7 PEFR. | ||||
| 8 FEF25‐75% Show forest plot | 3 | 197 | Std. Mean Difference (Random, 95% CI) | 0.45 [‐0.28, 1.19] |
| Analysis 1.8  Comparison 1 Yoga vs usual care/sham intervention, Outcome 8 FEF25‐75%. | ||||
| 9 Medication usage (frequency) Show forest plot | 3 | 228 | Std. Mean Difference (Fixed, 95% CI) | 0.69 [0.41, 0.96] |
| Analysis 1.9 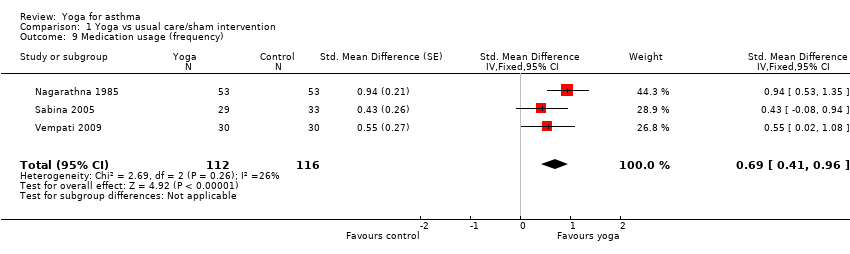 Comparison 1 Yoga vs usual care/sham intervention, Outcome 9 Medication usage (frequency). | ||||
| 10 Medication usage (percentage of participants with decreasing dosage) Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.35 [1.29, 22.11] |
| Analysis 1.10 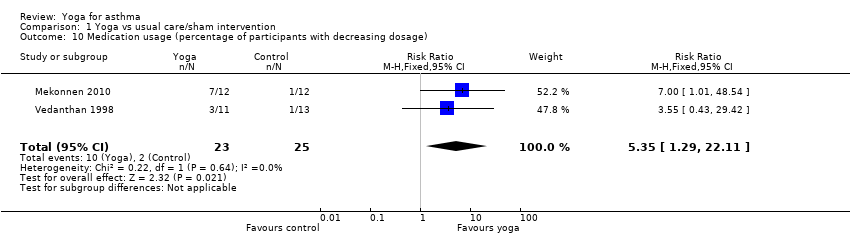 Comparison 1 Yoga vs usual care/sham intervention, Outcome 10 Medication usage (percentage of participants with decreasing dosage). | ||||
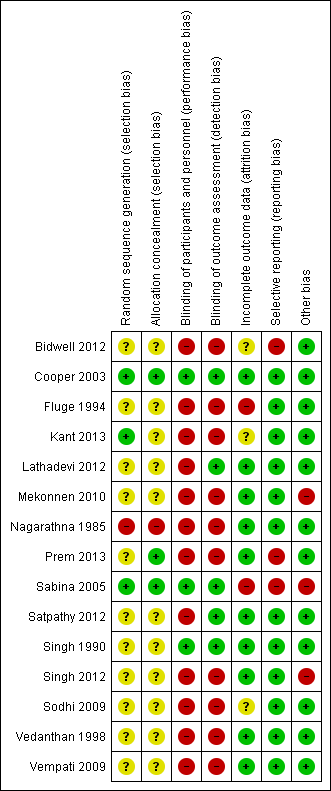
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.
![Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.1 Change in AQLQ score [7 pt scale].](/es/cdsr/doi/10.1002/14651858.CD010346.pub2/media/CDSR/CD010346/image_n/nCD010346-AFig-FIG03.png)
Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.1 Change in AQLQ score [7 pt scale].

Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.2 Asthma symptom.
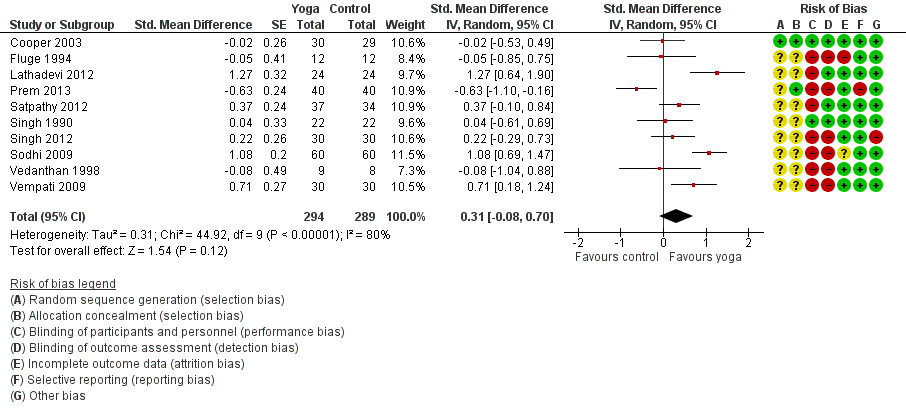
Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.3 FEV1.
![Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.4 FEV1 change from baseline [litres].](/es/cdsr/doi/10.1002/14651858.CD010346.pub2/media/CDSR/CD010346/image_n/nCD010346-AFig-FIG06.png)
Forest plot of comparison: 1 Yoga vs usual care/sham intervention, outcome: 1.4 FEV1 change from baseline [litres].

Comparison 1 Yoga vs usual care/sham intervention, Outcome 1 Change in AQLQ score.

Comparison 1 Yoga vs usual care/sham intervention, Outcome 2 Asthma symptom.

Comparison 1 Yoga vs usual care/sham intervention, Outcome 3 FEV1.
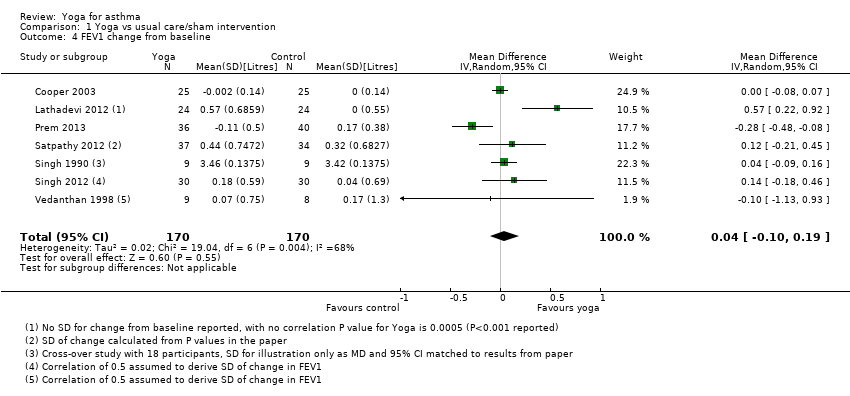
Comparison 1 Yoga vs usual care/sham intervention, Outcome 4 FEV1 change from baseline.

Comparison 1 Yoga vs usual care/sham intervention, Outcome 5 FVC.

Comparison 1 Yoga vs usual care/sham intervention, Outcome 6 FEV1/FVC.
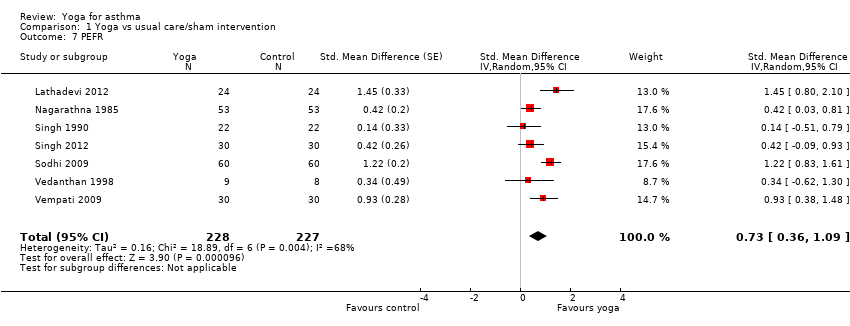
Comparison 1 Yoga vs usual care/sham intervention, Outcome 7 PEFR.
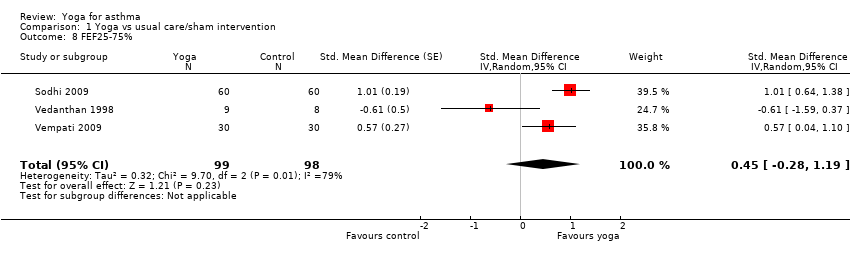
Comparison 1 Yoga vs usual care/sham intervention, Outcome 8 FEF25‐75%.
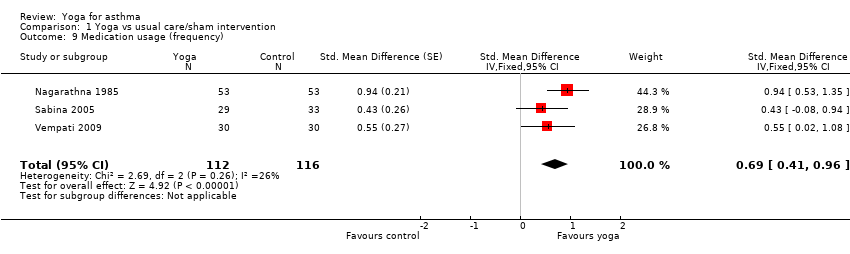
Comparison 1 Yoga vs usual care/sham intervention, Outcome 9 Medication usage (frequency).

Comparison 1 Yoga vs usual care/sham intervention, Outcome 10 Medication usage (percentage of participants with decreasing dosage).
| Yoga compared with usual care or sham intervention for asthma | ||||||
| Patient or population: People with asthma (mostly mild or moderate) Settings: Outpatient clinic and at home (studies conducted in Ethiopia, Germany, India, UK, and USA) Intervention: Yoga (duration no more than 6 months on average; range 2 weeks to 54 months) Comparison: Usual care or sham intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care or sham intervention | Yoga | |||||
| Quality of life (Asthma Quality of Life Questionnaire, with 32 items, 0 to 7 points per item) | The mean points per item of Asthma Quality of Life Questionnaire ranged from 4.06 to 4.50 points across control groups | The mean change from baseline in the intervention groups was on average 0.57 units higher (95% CI 0.37 to 0.77) | ‐ | 375 (5) | ⊕⊕⊕⊖ moderate1 | Minimal clinically important difference: 0.5 |
| Asthma symptoms (different severity scores; change from baseline) | The mean severity score ranged from 0.83 to 1.05 points across control groups on different scales | The mean severity score in the intervention groups was on average 0.37 SD units lower (95% CI 0.09 to 0.65) | ‐ | 243 (3) | ⊕⊕⊕⊖ moderate2 | Lower score indicates improvement Nagarathna 1985 and Sodhi 2009a used a 3‐point scoring system for severity of asthma symptoms from 1 (mild) to 3 (severe) Vedanthan 1998 used a 5‐point scoring system from A (no symptoms) to E (very severe symptoms). No established minimal clinically important difference in these scores is available |
| Asthma control (weekly number of attacks) | The mean weekly number of attacks ranged cross control groups from 0.58 to 2.10 | See comment | ‐ | 226 (2) | ⊕⊕⊖⊖ low3 | Two studies showed benefit, but the results were not combined due to very high heterogeneity between them |
| Forced expiratory volume in one second (change from baseline FEV1 (L)) | The mean FEV1 ranged across control groups from 2.24 to 4.19 L | The mean FEV1 in the intervention groups was on average 0.04 L higher (95% CI ‐0.10 to 0.19) | ‐ | 340 (7) | ⊕⊖⊖⊖ very low4 | ‐ |
| Reduced asthma medication usage | 8 per 100 | 43 per 100 (11 to 100) | RR 5.35 (1.29 to 22.11) | 48 (2) | ⊕⊕⊖⊖ low5 | ‐ |
| Adverse events | ‐ | ‐ | ‐ | 108 (3) | ⊕⊖⊖⊖ very low6 | Fluge 1994 reported 3 participants from the control group required oral steroids treatment due to acute exacerbations of their asthma, as compared with none in the yoga group. Sabina 2005 reported no adverse events associated with yoga or the control. In Singh 1990, 1 participant in the yoga group reported mild dyspnoea during yoga using the Pink City Lung Exerciser |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for study limitations; four out of five studies contributing to this outcome are at high risk of performance and detection bias, and one study is at high risk of attrition bias (see Figure 1). 2Downgraded once for study limitations; all three studies contributing to this outcome are at high risk of performance and detection bias, and one study is at high risk of selection bias (see Figure 1). 3Downgraded for (1) study limitations: both studies contributing to this outcome are at high risk of performance and detection bias, and one study is also at high risk of selection bias (see Figure 1), and (2) inconsistency: the studies could not be combined in a meta‐analysis due to very high levels of heterogeneity. 4Downgraded for (1) study limitations: six out of the seven studies contributing to this outcome are at unclear risk of selection bias (see Figure 1), (2) inconsistency: we detected substantial heterogeneity (I² = 68%) in the meta‐analysis, and (3) imprecision: the confidence intervals include both the possibility of harm or benefit of the intervention. 5Downgraded for (1) study limitations: both studies contributing to this outcome are at high risk of performance and detection bias and at unclear risk of selection bias, and one study is at high risk of other biases (see Figure 1), and (2) imprecision: despite the confidence intervals excluding no difference, the breadth of the confidence intervals and the small numbers of participants in the analysis reduces our confidence in the estimate. 6Downgraded for (1) study limitations: one study reporting adverse events is at high risk of performance, detection, and attrition bias, another is at high risk of attrition and reporting bias, and a third is at unclear risk of selection bias (see Figure 1), (2) imprecision: the very small number of studies reporting very rare events reduced our confidence in this outcome, and (3) potential publication bias due to no mention of adverse events (which were specified explicitly as one of the outcomes of interest in their research protocol) in Prem 2013. We decided not to pool these results. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in AQLQ score Show forest plot | 5 | 375 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.37, 0.77] |
| 1.1 Yoga breathing alone vs. control | 2 | 196 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Combination of yoga breathing, postures and meditation vs. control | 3 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.47, 1.22] |
| 2 Asthma symptom Show forest plot | 3 | 218 | Std. Mean Difference (Fixed, 95% CI) | 0.37 [0.09, 0.65] |
| 3 FEV1 Show forest plot | 10 | 583 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.08, 0.70] |
| 4 FEV1 change from baseline Show forest plot | 7 | 340 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 5 FVC Show forest plot | 6 | 376 | Std. Mean Difference (Random, 95% CI) | 0.67 [0.20, 1.14] |
| 6 FEV1/FVC Show forest plot | 6 | 435 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐1.63, 2.87] |
| 7 PEFR Show forest plot | 7 | 455 | Std. Mean Difference (Random, 95% CI) | 0.73 [0.36, 1.09] |
| 8 FEF25‐75% Show forest plot | 3 | 197 | Std. Mean Difference (Random, 95% CI) | 0.45 [‐0.28, 1.19] |
| 9 Medication usage (frequency) Show forest plot | 3 | 228 | Std. Mean Difference (Fixed, 95% CI) | 0.69 [0.41, 0.96] |
| 10 Medication usage (percentage of participants with decreasing dosage) Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.35 [1.29, 22.11] |

