مداخلات درمانی برای سرطانهای ناحیه دهان و اوروفارنژیال: درمان هدفمند و ایمونوتراپی
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Location of trial: US and Canada Number of centres: > 150 Trial ID: RTOG 0522; NCT00265941 Recruitment period: November 2005 to March 2009 Funding source: NCI U10 CA21661, U10 CA37422 and Bristol‐Myers Squibb | |
| Participants | Inclusion criteria: people with stage III‐IV carcinoma of the oropharynx, larynx and hypopharynx; having Zubrod performance of 0‐1 and meeting pre‐defined blood chemistry criteria Exclusion criteria: prior malignancy; primary tumour in OC, nasopharynx, sinus or salivary glands; prior excision of primary tumour; prior treatment (chemotherapy or RT); severe active co‐morbidity (defined) or pregnancy Sex (M/F): 88%/12% Age at baseline (years): Gp A: range 34‐76, median 58; Gp B: range 31‐79, median 57 OP: Gp A: 312/444 (70%), Gp B: 313/447 (70%) Number randomised: 940 Number evaluated: 891 | |
| Interventions | Comparison: cisplatin + RT + cetuximab vs. cisplatin + RT Gp A (n = 444): loading dose cetuximab 400 mg/m2 and 6‐7 weekly doses of cetuximab 250 mg/m2 given in conjunction with RT 70‐72 Gy (6 wk) + 2 cycles of cisplatin (q3 weeks) Gp B (n = 447): RT 70‐72 Gy (6 wk) + 2 cycles of cisplatin (q3 weeks) | |
| Outcomes | Primary: PFS Secondary: OS, locoregional failure, distant metastasis, acute and late adverse events, quality of life (reported separately) Duration of follow‐up: median follow‐up 3.8 years | |
| Notes | Sample size calculation: "RTOG 0522 was initially designed with a sample size of 720 patients to detect a 25% reduction in the hazard associated with disease‐free survival with 80% power and a one‐sided test at the P=.025 level. Selected reported adverse events: most frequently reported (top 5 + any event category) included in Table 8 8: for full listing of reported adverse effects, see original study reference Toxicity evaluation assessment tool: National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, computer‐generated central randomisation: "Patients were...randomly assigned to...arm A or...arm B in a 1:1 ratio using permuted block random assignment" |
| Allocation concealment (selection bias) | Low risk | Central allocation revealed to investigator after participant registration |
| Blinding of participants (performance bias) | Unclear risk | Open label, no placebo. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms; however, primary outcomes considered to be objective (e.g. OS) |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes evaluated according to pre‐specified objective criteria and reviewed by blinded observers |
| Incomplete outcome data (attrition bias) | Low risk | 90% of participants received 2 cisplatin cycles and 74% of participants randomised to cetuximab received loading dose plus ≥ 6 doses. Of 940 participants accrued, 2 lost to follow‐up and 43 were ineligible on review. Similar numbers lost from each group |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Intervention contamination between groups. Conduct of study affected by interim results (recruited additional participants after interim results indicated control group demonstrated better PFS/DFS outcomes than anticipated; sample size was changed several times mid‐study) |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy.
| Methods | Location of trial: Germany Number of centres: 1 Trial ID: prior to trial registrations Recruitment period: period of 3 years ‐ dates not reported Funding source: Grant no. Bi 230/2, awarded by the Deutsche Forschungsgemeinschaft | |
| Participants | Inclusion criteria: participants had histologically confirmed HNSCC with tumour stage T1/2, N0‐2M0 Exclusion criteria: not reported Sex (M/F): 14/10 Age at baseline (years): mean 68 OC: Gp A: n = 10; Gp B: n = 8 OP: Gp A: n = 0; Gp B: n = 2 Lip cancers: Gp A: n = 2; Gp B: n = 2 Number randomised: 24 Number evaluated: 24 | |
| Interventions | Comparison: BCG‐CWP+ surgery versus surgery alone Gp A (n = 10): 3 mL BCG‐CWP was injected under local anaesthesia in a fan like manner over the entire tumour very slowly. Surgery was then performed when the inflammatory infiltrate had subsided (mean 24 d, range 16‐43 d) Gp B (n = 10): surgery only | |
| Outcomes | Primary: recurrence, survival Secondary: changes in clinical, laboratory‐chemical and immunological findings pre and post BCG‐CWP therapy Duration of follow‐up: 1 year | |
| Notes | Adverse events: included in Table 9 Toxicity evaluation assessment tool: not reported Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised". Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants (performance bias) | Unclear risk | Not reported, no placebo. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms; however, primary outcomes considered to be objective |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, no placebo |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in outcomes |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Location of trial: international; US (73 centres), Australia (5), South Africa (5), France (5), Israel (3), UK (3), Belgium (2), Spain (2), Poland (2), Switzerland (2), Sweden (1), Germany (1), New Zealand (1) Number of centres: 105 Trial ID: Recruitment period: April 1999 to March 2002 Funding: ImClone Systems, New York, Merck (Darmstadt Germany) and National Institutes of Health grant (CA06294) | |
| Participants | Inclusion criteria: patients with stage III or IV, non‐metastatic measurable SCC of oropharynx, hypopharynx or larynx. Medically suitable, KPS > 60 and normal haematopoietic, renal and hepatic function Exclusion criteria: previous cancer or chemotherapy in past 3 years, or previous surgery or RT for head and neck cancer Sex (M/F): Gp A: 171/40, Gp B: 169/44 Age at baseline: Gp A: median 56 years, Gp B median 58 years OC: none OP: Gp A: 118/211(56%), Gp B: 135/213 (63%) Number randomised: 424 Number evaluated: 424 | |
| Interventions | Comparison: cetuximab + RT vs. RT alone Gp A (n = 211): high‐dose RT + cetuximab Gp B (n = 213): high‐dose RT alone On study registration all investigators selected 1 of 3 RT regimens:
then twice daily; mornings: 1.8 Gy/fraction 5 fractions/wk for 2.4 wk afternoons: 1.5 Gy/fraction, 5 fractions/wk for 2.4 wk Duration of follow‐up: 5 years | |
| Outcomes | LRC, OS, PFS, ORR, safety | |
| Notes | Sample size: to detect an increase in the rate of LRC at 1 year, from 44% to 57% it was calculated the 208 participants per treatment group were required to give 90% power at the 5% significance level with use of a 2‐sided log‐rank test Adverse events note: different percentages presented in later publication (2010; Table 2) Selected reported adverse events: most frequently reported (top 6) included in Table 8 8: for full listing of reported adverse effects, see original study reference's Table 4) Toxicity evaluation assessment tool: toxicity criteria of the Radiation Therapy Oncology Group (1995) Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was stratified for KPS (60‐80 vs 90‐100), nodal involvement (N0 vs N+) tumour stage (T1‐3 vs T4) and radiation fractionation regimen (1x/day vs 2x/day vs concom boost). A minimisation method was used in the randomisation of patients to receive RT + cetuximab or RT alone)" |
| Allocation concealment (selection bias) | Low risk | RT regimens were chosen prior to randomisation based on preset clinical criteria. Allocation was obtained using an Interactive Voice Response System, which informed investigators of study number and treatment group |
| Blinding of participants (performance bias) | Unclear risk | Open‐label, no placebo. Knowledge of allocated treatment may have influenced participants' care. |
| Blinding of outcome assessment (detection bias) | Low risk | Investigator‐generated data were submitted for blind review by an independent committee of experts |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis for efficacy included all randomised participants |
| Selective reporting (reporting bias) | Low risk | Planned and expected primary outcomes reported in full |
| Other bias | Low risk | No other biases identified |
| Methods | Location of trial: Torino, Italy Number of centres: 5 centres Trial ID: not given Recruitment period: May 1990 to December 1996 Funding: Italian Association for Cancer Research and from the Italian Ministry for the Universities and Scientific and Technological Research | |
| Participants | Inclusion criteria: patients with histologically confirmed resectable stage T2‐4, M0, N0‐N3, SCC of OC or oropharynx. KPS at least 70%, aged 18‐75 years, medically fit for surgery, WBC > 4000/mm3, creatinine < 1.4 mg/dL, platelets > 150,000/mm3, AAT and ALP levels < 1.5 normal Exclusion criteria: patients with infiltration of the pterygopalatine fossa and carotid artery were classed as inoperable. Patients with tumours of the anterior floor of the mouth and the base of the tongue were excluded due to their high risk of cervical metastasis and need for bilateral neck dissection. Patients with previous treatment for this or any other malignancy and the need for steroid management were excluded Age at baseline (years): Gp A: median 62, Gp B: median 61 OC: Gp A: n = 64; Gp: B n = 61 (125 evaluated) OP: Gp A: n = 36; Gp B: n = 40 (76 evaluated) OC + OP: 201 evaluated Number randomised: 220 (110 and 110) Number evaluated: 201 | |
| Interventions | Comparison: rIL‐2 + surgery (± RT) versus surgery (± RT) Gp A (n = 100): daily course of rIL‐2 (5000 units in 1 mL phosphate buffered saline/albumin) in the ipsilateral cervical lymph node chain for 10 d prior to surgery. 2 administration sites in each person. Surgery ± RT. Further 5‐d courses were given monthly in the residual contralateral chain for 1 year after surgery or after RT when this was necessary Gp B (n = 101): surgery ± RT Duration of follow‐up: median 64 months (minimum of 2 years) | |
| Outcomes | Primary: OS Secondary: DFS, recurrence, new primary tumours | |
| Notes | Sample size: planned number of participants was 250, because a 5‐year DFS rate of 55% was presumed for the control group, and a 10% increase in survival was presumed in the rIL‐2 group Adverse events: included in Table 9 9 Toxicity evaluation assessment tool: not reported Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned randomly to the 2 treatment groups by a central office (Chiron Medical Department, Amsterdam, The Netherlands). Randomisation stratified by participant gender and date of birth, tumour site (OC or oropharynx), TNM classification, and KPS score. Method of sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation conducted by central office. Concealment not specifically reported |
| Blinding of participants (performance bias) | Unclear risk | Not reported, no placebo. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms; however, primary outcomes considered to be objective |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 10 participants lost from rIL‐2 group (due to interruption of planned treatment after randomisation) and 9 lost from control group (4 died due to myocardial infarction, 5 refused to take part). Reasons different in each group and probably related to allocated treatment |
| Selective reporting (reporting bias) | Low risk | Planned and expected outcomes reported |
| Other bias | Low risk | Unplanned interim analysis resulted in early closure of study. Guidance from Higgins 2011 is that simulation evidence suggests that inclusion of stopped early trials in meta‐analyses will not lead to substantial bias |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy.
| Methods | Location of trial: International; Belgium, Czech Republic, Germany, India, Poland, Serbia, Taiwan, US Number of centres: 26 Trial ID: NCT00229723 Recruitment period: November 2004 to June 2008 Funding source: AstraZeneca | |
| Participants | Inclusion criteria: patients with previously untreated histologically confirmed stage III/IV non‐metastatic HNSCC with measurable disease (by Response Evaluation Criteria in Solid Tumours ‐ (RECIST)) , aged ≥ 18 years, WHO performance status 0‐1, life expectancy ≥ 12 wk Exclusion criteria: buccal, mucosal carcinoma and post nasal space, thyroid or salivary gland tumours, stage III laryngeal carcinoma or metastatic disease, disease invading mandible, simultaneous primary tumours, abnormal renal or liver function, neutrophils < 1 x 109/L, platelets < 100 x 109/L, pregnancy or breastfeeding Sex (M/F): 87.6%/12.4% Age at baseline (mean (SD)) (years): Gp A: 53.1 (8.36), Gp B: 52.5 (12.37), Gp C: 53.4 (10.59), Gp D: 54.3 (8.56), Gp E: 50.8 (9.29), Gp F: 53.4 (6.22), Gp G: 54.9 (9.95) OC: Gp A: 4; Gp B: 0; Gp C: 2; Gp D: 1; Gp E: 3; Gp F: 2; Gp G: 3; total 15 OP: Gp A: 26; Gp B: 12; Gp C: 15; Gp D: 19; Gp E: 13; Gp F: 15; Gp G: 10; total 110 OC + OP: 125/226 (55%) Number randomised: 226 Number evaluated: 83 who completed study prior to interim analysis | |
| Interventions | Comparisons: gefitinib 250 mg/d vs. gefitinib 500 mg/d vs. placebo (in concomitant and maintenance phases) Gp A (n = 60): placebo (both concomitant and maintenance treatment phases) Gp B (n = 24): gefitinib 250 mg/d orally concomitant and placebo maintenance phase Gp C (n = 31): gefitinib 500 mg/d orally and placebo maintenance phase Gp D (n = 31): gefitinib 250 mg/d orally concomitant and gefitinib 250 mg/d maintenance Gp E (n = 24): gefitinib 500 mg/d orally concomitant and gefitinib 500 mg/d maintenance Gp F (n = 34): placebo in concomitant phase and gefitinib 250 mg/d maintenance Gp G (n = 22): placebo in concomitant phase and gefitinib 500 mg/d maintenance In the concomitant phase, all participants received CRT comprising cisplatin (100 mg/m2 IV every 21 d (3 cycles) plus 3D conformal RT 70 Gy in 35 fractions over 7 wk) plus intervention tablets administered daily from the day of randomisation to the end of RT (8‐9 wk in total) Maintenance phase lasted up to 2 years Duration of follow‐up: 2 years | |
| Outcomes | Primary: LRC at 2 years Secondary: LRC at 1 year, CRR, ORR, PFS, OS, safety and tolerability Duration of follow‐up: 2 years | |
| Notes | 7 arm trial ‐ only 1 comparison (gefitinib concomitant vs. placebo (Gp B+C vs. A), included in this review's primary outcomes Note: data are held by the pharmaceutical company that produces gefitinib, unable to interrogate reported HRs Adverse events: included in Table 10 10 (CRT + gefitinib (250/500 mg) vs. CRT + placebo concomitant phase only: Gp A: n = 110 (Gps B+C+D+E); Gp B: n = 116 (Gps A+F+G)) Toxicity evaluation assessment tool: National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation scheme was prepared by Astra Zeneca statistical personnel …. and codes were allocated strictly sequentially as patients entered the study" |
| Allocation concealment (selection bias) | Low risk | Central randomisation schedule; however, concealment not specifically reported |
| Blinding of participants (performance bias) | Low risk | "Each patient received an identical kit of two matching bottles of tablets and took one tablet from each bottle every day |
| Blinding of outcome assessment (detection bias) | Low risk | "Placebo controlled double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcomes were not reported for each randomised group and it was difficult to determine the numbers of participants excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Planned outcomes of LRC, CRR and ORR reported but, but only for combinations of groups. Data on PFS and OS not found |
| Other bias | Low risk | No other sources of bias identified |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) |
CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor.
| Methods | Location of trial: international (9 countries) Number of centres: unclear Trial ID: NCT00387127, EudraCT#: 2005‐003767‐23 Recruitment period: November 2006 to January 2009 Funding source: GlaxoSmithKline | |
| Participants | Inclusion criteria: eligible patients had histologically confirmed, unresected stage III/IVA/IVB HNSCC of at least 1 of the following sites: OC, oropharynx, hypopharynx or larynx. Other inclusion criteria included: European Cooperative Oncology Group Performance Status 0, 1 or 2; adequate renal, hepatic and haematological function and normal left ventricular ejection fraction Exclusion criteria: patients with T1N1, T2N1 or metastatic disease and patients who had received any prior or current treatment for invasive HNSCC were excluded. Initially, only patients with EGFR over‐expression (3+ by immunohistochemistry) were included. This inclusion criterion was later amended to allow inclusion of all participants regardless of EGFR status Sex (M/F): 90%/10% Age at baseline (years): Gp A: range 44‐66, median 56; Gp B: range 34‐69, median 57 OC: 5 (7%) OP: 43 (64%) OC + OP: 48 Number randomised: 67 Number evaluated: 67 | |
| Interventions | Comparison: lapatinib + CRT vs. CRT alone Gp A (n = 34): concomitant lapatinib 1500 mg/d + CRT + lapatinib only in maintenance phase Gp B (n = 33): CRT + placebo + placebo during maintenance phase Both groups received CRT comprising cisplatin 100 mg/m2 on d 1, 22 and 43 of the RT course. RT was delivered once daily (dose < 2.5 Gy/fraction to a total dose of 65 Gy (IMRT) or 70 Gy (2D or 3D RT) over 6.5‐7 wk to the gross site with 50 Gy to elective nodal areas Duration of follow‐up: 40 months | |
| Outcomes | Primary: CRR at 6 months Secondary: PFS, OS, LRC, distant relapse, biomarkers and safety | |
| Notes | Small, short study. Primary outcome CRR at 6 months (to indicate whether further trials are justified) and likely insufficient power to detect a clinically important benefit of lapatinib if this is present Adverse events: included in Table 10 10 Adverse events note: selectively reported numerical data only provided for adverse events not comparable in incidence during concomitant phase. All adverse events otherwise presented graphically, and data not reliably extractable Toxicity evaluation assessment tool: not reported Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Phase II randomised controlled trial sponsored by GlaxoSmithKline. Method of sequence generation not clearly reported but randomisation stratified by nodal status (N0‐1 vs. N2‐3) and tumour location |
| Allocation concealment (selection bias) | Low risk | Central randomisation schedule; however, concealment not specifically reported. There were nine centres so it is likely that the randomisation was done centrally by GSK. |
| Blinding of participants (performance bias) | Low risk | Double blind ‐ placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | "All scans and clinical data for the primary end‐point were reviewed centrally by an independent review board (BioClinica, Newtown, PA, USA), and all readers were blinded to treatment" |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis for efficacy |
| Selective reporting (reporting bias) | Unclear risk | Planned clinical outcomes reported in full; adverse events selectively reported numerically (all otherwise available graphically) |
| Other bias | Unclear risk | GSK who funded the study were involved in the preparation of the trial report. |
| Methods | Location of trial: Korea Number of centres: multicentre, Seoul National University Hospital; Clinical Research Center for Solid Tumor [sic] Trial ID: NCT0623558; CRSCT‐L0002 Recruitment period: not reported. April 2008‐? Funding source: Merck KGaA Darmstadt, Germany | |
| Participants | Inclusion criteria: > 18 years old; either sex; unresectable LN‐HNSCC due to either tumour fixation, involvement of basal skull or lymph node fixation or LA‐HNSCC with low surgical curability on the basis of advanced disease (T3‐T4) or regional lymph node extension (N2‐3, except for T1N2), or LA‐HNSCC, which is a candidate for organ preservation (primary tumour to be in OC, oropharynx, hypopharynx or larynx); ECOG performance status 0 or 1; informed consent; ALT/AST < 2.5 x ULN; serum albumin level > 3.0 g/dL; serum ALP < 2.5 x ULN; bilirubin < 1.5 mg/dL; serum creatinine < 1.5 x ULN; WBC > 3000/mm3; absolute neutrophil count > 1500/mm3; haemoglobin > 9 g/dL Exclusion criteria: previous chemotherapy or RT for LA‐HNSCC; targeted‐lesions RT with 6 months; previous EGFR pathway‐targeting therapy; prior cancer surgery (excluding diagnostic biopsy in 4 wk prior to recruitment; distant metastatic disease; heart failure/coronary artery disease/myocardial infarction in prior 6 months; known allergy to study treatment; pregnancy/lactation; investigational agent receipt in prior 28 d; previous malignancy in prior 5 years (excluding adequately treated in‐situ cervical cancer/non‐melanoma skin cancer; legal incapacity; diagnosis of another cancer within 5 years, peripheral neuropathy or hearing disorder of > grade 2 by NCI‐CTCAE, co‐morbidity or biological disorder contraindicating administration of systemic chemotherapy or RT Sex (M/F): overall: 82/10; Gp A: 46/2; Gp B: 36/8 Age at baseline (years): overall: median 59, range 29‐73; Gp A: median 58, range 40‐73; Gp B: median 61, range 29‐73 OC: overall: n = 14 (15%); Gp A: n = 4 (8%); Gp B: n = 10 (23%) Number randomised: 92 (Gp A: n = 48; Gp B: n = 44) | |
| Interventions | Comparison: RT + docetaxel + cisplatin + cetuximab vs. RT + docetaxel + cisplatin Gp A (n = 48): RT (weekly for 9 wk) + docetaxel (3 x d 1 of 3 wk cycles; 75 mg/m2) + cisplatin (3 x d 1 of 3 wk cycles; 75 mg/m2; followed by weekly 30 mg/m2 for 9 wk) + cetuximab (weekly for 9 wk; first weekly dose 400 mg/m2; followed by weekly 250 mg/m2 for 9 wk) | |
| Outcomes | Primary: ORR Secondary: completion rate of induction and CCRT, PFS, OS and toxicities | |
| Notes | Adverse events: published as conference poster although not legible from PDF copy Socioeconomic status: not reported, full study report currently unpublished other than abstract and poster Sample size calculation: not reported. Method of analysis: ITT Lost‐to‐follow‐up: incompletion rationale provided only for Gp A (n = 8), not indicated for Gp B (n = 4) Adverse events note: toxicity data split by CRT induction or concurrent CRT Selected reported adverse events: most frequently reported (top 6) included in Table 8 8: for full listing of reported adverse effects, see conference poster Toxicity evaluation assessment tool: National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation. |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Blinding of participants (performance bias) | Unclear risk | Not reported, no placebo used. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms; however, primary outcomes considered to be objective |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up rationale not reported for Gp B |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | Compliance (completion of therapy) reported: Gp A: n = 40; Gp B: n = 40 |
| Methods | Location of trial: Italy Number of centres: multicentre (number of centres unclear) Trial ID: not reported Recruitment period: August 1995 to August 1996 Funding source: CNR Rome, AP "clinical applications of oncological research" contract 96.00588.PF39 | |
| Participants | Inclusion criteria: adults aged 18‐75 years, with histologically confirmed technically resectable or unresectable stage III‐IV HNSCC, with measurable disease, ECOG performance status 0‐1 and a life expectancy of at least 6 months, WBC count > 4000/ml3, platelet count > 100,000/ml3, serum bilirubin < 2 mg/dL, creatinine clearance > 60 mL/minute. Participants must have adequate nutritional and liquid supply, at least a 4‐wk interval from whatever surgery and 2 wk from the taking of biopsy specimen Exclusion criteria: previous treatment for the actual disease, any infection requiring antibiotic treatment, distant metastases, a history or the presence of a second malignancy except for basal cell carcinoma of the skin and cervical carcinoma in situ. Serious hearing impairment, clinical evidence of congestive heart failure grade III‐IV (New York Heart Classification), drug‐uncontrolled angina pectoris, drug‐uncontrolled clinically significant cardiac arrhythmia, drug or chronic alcohol addiction, women of childbearing age without a negative pregnancy test, previous or present hypercalcaemia Sex (M/F): 29/4 Age at baseline (years): mean 54.5, range 38‐72 OC: Gp A: n = 2; Gp B: n = 2 OP: Gp A: n = 6; Gp B: n = 9 OC + OP: total n = 19 Number randomised: 33 Number evaluated: 28 (3 participants refused treatment, 2 died during treatment) | |
| Interventions | Comparison: rIL‐2 + chemotherapy vs. chemotherapy alone Gp A (n = 16): 3 cycles of chemotherapy (Al Sarraf regimen) + 4.5 MIU/d rIL‐2, subcutaneously on d 8‐12 and 15‐19 of chemotherapy, repeated 3 times for each cycle Gp B (n = 17): 3 cycles of chemotherapy (Al Sarraf regimen) Both groups received the same chemotherapy regimen; 100 mg/m2 cisplatin IV as a 60‐minute infusion on d 1, with a standard pre‐ and post‐hydration protocol with forced diuresis by 250 mL 18% mannitol, plus 1000 mg/m2/d 5‐fluorouracil on d 1‐5 (120 h) as a continuous infusion by peripheral vein. 3 cycles planned unless disease progression or unacceptable toxicity necessitated a break | |
| Outcomes | Primary: objective tumour regression (complete or partial), OS and time to disease progression Secondary: toxicity Duration of follow‐up: > 18 months | |
| Notes | The sample size necessary to demonstrate a 20% difference between the 2 regimens at the significance level of α = 0.05 with a power of β = 0.80 was calculated to be 95 participants per arm An interim analysis, carried out on the first 30 participants, is presented in this study Selected reported adverse events: most frequently reported (top 6) included in Table 9 9: for full listing of reported adverse effects, see original study reference (Table 4) Toxicity evaluation assessment tool: WHO Recommendations for Grading of Acute and Subacute Toxicity (1981) Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned". Centralised. Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "The randomisation was centralized in the Division of Medical Oncology, S. Giovanni Hospital, Turin." Concealment not reported specifically |
| Blinding of participants (performance bias) | Unclear risk | Open label, no placebo. Knowledge of allocated treatment may have influenced participants' care receipt. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment not reported, but used a centralised system. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was very high ‐ although rationale was provided and a balance of participants lost‐to‐follow‐up reported across arms |
| Selective reporting (reporting bias) | Low risk | Planned outcomes of response to treatment and toxicity reported in full. OS and time to disease progression also mentioned |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Location of trial: US Number of centres: 10 centres Trial ID: NCT00410826 Recruitment period: December 2006 to October 2011 Funding source: supported by University of Washington and grants from Genentech and Astellas Pharma Global Development | |
| Participants | Inclusion criteria: biopsy‐confirmed stage III, IVA or IVB HNSCC with identified primary tumour in the nasopharynx (WHO type I), OC, oropharynx, hypopharynx or larynx; ECOG performance status of 0‐2, calculated creatinine clearance > 55 mL/minute; adequate liver function; platelet count > 100,000/dL; and neutrophil count > 1250/dL Exclusion criteria: prior chemotherapy or EGFR‐directed therapy, prior attempt at complete surgical resection, prior RT to the head and neck, prior history of HNSCC, pregnant or breastfeeding women, people with cardiac history precluding hydration required for cisplatin. Prior second malignancy was allowed if person was without evidence of disease Sex (M/F): 175/28 Age at baseline: not stated OC: 15 (7%) OP: 137 (67%) OC + OP: 152 (75%) Number randomised: 204 Number evaluated: 204 | |
| Interventions | Comparison: erlotinib + CRT vs. CRT alone Gp A (n = 99): erlotinib 150 mg/d (orally or through endogastric tube), started 1 wk prior to CRT, and continued throughout Gp B (n = 105): CRT only, no placebo used Both groups received cisplatin 100 mg/m2 on d 1, 22 and 43 given with hydration. Cisplatin was only administered during RT, which was either 66‐70 Gy in 30‐35 fractions (either iIMRT or 3D) of 2‐2.2 Gy/fraction, 5 x weekly. Sites of potential/occult disease received 50 Gy | |
| Outcomes | Primary: CR (definitions provided) Secondary: PFS, OS, adverse effects Duration of follow‐up: 60 months | |
| Notes | Sample size calculation: the sample size of 204 randomly assigned participants was estimated to have 80% power to detect a difference between a 60% CRR in arm B and a 40% CRR in arm A, by a 2‐sided test of independence with 5% type I error, assuming a 5% drop‐off rate Adverse events notes: partial reporting of toxicities by grade Serious adverse events*: bespoke category of "Serious adverse events (SAEs)" reported without explanation of criteria/grades included ‐ not originating from National Cancer Institute assessment tool Selected reported adverse events: most frequently reported (top 6) included in Table 10 10: for full listing of reported adverse effects, see original study reference (Table 2) Toxicity evaluation assessment tool: National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) Adverse events analysis method: per‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was stratified by centre, performance status (ECOG 0/1 vs 2), and degree of nodal involvement (N0/1 vs N2/3)." Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Central allocation. Concealment not specifically reported though |
| Blinding of participants (performance bias) | Unclear risk | Open label, no placebo used. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms; however, primary outcomes considered to be objective |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported for assessors. Refers to "Central review of all clinical and pathological outcomes was conducted without knowledge of treatment group" |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for withdrawals clearly described and efficacy analysis by ITT |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes of this Phase II trial reported; partial reporting of adverse events |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Location of trial: India Number of centres: 3 centres Trial ID: h‐R3/SCCHN/001/IND Recruitment period: September 2004‐July 2005 Funding source: Biocon Ltd. (Indian pharmaceutical company, Bangalore, India) | |
| Participants | Inclusion criteria: aged 18‐70 years; histologically confirmed stage III or IVA (T1‐T4a, N0‐N2) HNSCC; suitable for concurrent CRT/RT; KPS of P60%; life expectancy of > 6 months; adequate haematological function (WBC count > 4000/mm3; absolute neutrophil count 1500/mm3; platelets > 100,000/mm3; total bilirubin 61.2 mg/dL; AST; ALT 62.5 x normal limit (37 and 40 U/L, respectively); serum creatinine < 1.4 mg/dL); people contraindicated for surgery owing to prohibitive morbidity or compromised quality of life Exclusion criteria: nasopharyngeal carcinoma; history of malignancy other than non‐melanoma skin cancer or carcinoma‐in situ of the cervix; evidence of distant metastases/concurrent secondary malignancy or T4b lesion; chemotherapy within 3 months before enrolment; prior RT to the head and neck; prior immunotherapy; increased risk of lethal infections and pharmacokinetic interactions with nimotuzumab; history of allergy with similar biological compounds; pregnant/lactating women; people on other investigational drugs/devices Age at baseline (years): Gp A: mean 49.9 (SD 10.6); Gp B: mean 53.7 (SD 9.0); Gp C: mean 58.7 (SD 8.2); Gp D: mean 58.7 (SD 8.1) (P value not reported) Sex (M/F): overall: 82/10 (89%/11%); Gp A: 20/3; Gp B: 21/2; Gp C: 21/2; Gp D: 20/3 (P value not reported) OC: overall: 19.6% (n = 18); Gp A: 22% (n = 5); Gp B: 17% (n = 4); Gp C: 13% (n = 3); Gp D: 26% (n = 6) (P value not reported) Number randomised: 92 Number evaluated: 92 (n = 68 (74%) attrition). Gp A: lost to follow‐up n = 4, died n = 10; Gp B: lost to follow‐up n = 2, died n = 17; Gp C: withdrew (serious adverse events) = 1, died n = 14; Gp D: lost to follow‐up n = 3, died n = 17) Analysis: ITT | |
| Interventions | Comparison: RT + cisplatin + nimotuzumab vs. RT + cisplatin and RT + nimotuzumab vs. RT Gp A: (n = 23) RT (60‐66 Gy; 2 Gy/fraction/d; 5 d/wk for 6‐6.5 wk) + cisplatin (50 mg dilution in 1 L saline; by IV over 2 h; 1 d/wk for 6 wk) + nimotuzumab (4 x 50 mg dilution in 0.9% saline; by IV over 1 h; 1 d/wk for 6 wk) Gp B: (n = 23) RT (60‐66 Gy; 2 Gy/fraction/d; 5 d/wk for 6‐6.5 wk) + cisplatin (50 mg dilution in 1 L saline; by IV over 2 h; 1 d/wk for 6 wk) Gp C: (n = 23) RT (60‐66 Gy; 2 Gy/fraction/d; 5 d/wk for 6‐6.5 wk) + nimotuzumab (4 x 50 mg dilution in 0.9% saline; by IV over 1 h; 1 d/wk for 6 wk) Gp D: (n = 23) RT (60‐66 Gy; 2 Gy/fraction/d; 5 d/wk for 6‐6.5 wk) Duration of follow‐up: 60 months | |
| Outcomes | OS, PFS, adverse events Duration of follow‐up: 60 months | |
| Notes | Sample size calculation: Quote "the sample size was 17 patients per arm, calculated by assuming P < 0.05 (5%), β < 0.2 (20%), power = 0.80 (80%), and a confidence interval (CI) of 95%" Adverse events notes: to compare intervention (adjunctive nimotuzumab) with control (standard therapy: CRT or RT), adverse events presented as follows. Intervention: Gp A+C (CRT/RT + nimotuzumab) n = 46 Control: Gp B+D (CRT/RT) n = 46 Control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore all grade mucositis data not used in analysis "Nimotuzumab was administered 3 d after cisplatin in order to identify AEs [adverse events] related to either intervention separately" Selected reported adverse events: most frequently reported (top 6) included in Table 8 8: for full listing of reported adverse effects, see original study reference's Table 3 Toxicity evaluation assessment tool: Common Toxicity Criteria of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each group was further randomised in a 1:1 fashion ‐ Group I into CRT + nimotuzumab or CRT and Group II into RT + nimotuzumab or RT." Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants (performance bias) | Unclear risk | Open label, no placebo control. Knowledge of allocated treatment may have influenced patients' care receipt and perception of symptoms. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition explained, and data analysed as ITT |
| Selective reporting (reporting bias) | High risk | All outcomes reported, including adverse events; however, derived HR values from graphs do not correspond with textually reported HRs. Confidence Intervals and P values also inconsistent with significance statements reported |
| Other bias | Low risk | No other apparent biases |
| Methods | Location of trial: Cuba Number of centres: unclear Trial ID: not reported Recruitment period: July 2002 to February 2007 Funding source: not reported | |
| Participants | Inclusion criteria: epithelial tumours of head and neck confirmed by cytological or histological techniques, in stages III or IV (advanced locoregional disease), who were suitable candidates for RT, were recruited. Measurable lesions, aged ≥ 18 years, ECOG performance status ≤ 2, life expectancy > 6 months and normal functioning of the organs and of the bone marrow as defined by absolute neutrophil count ≥ 1.5 x 109/L, platelet count ≥ 100 x 109/L, serum creatinine level ≤ ULN and ALT and AST level < 2.5 x ULN Exclusion criteria: prior RT or chemotherapy, concurrent active cancer, any uncontrolled intercurrent illness and pregnancy or lactation Sex (M/F): 81/24 Age at baseline (years): Gp A: mean 59.48, Gp B: mean 65.88 OC: 28 (26%) OP: 72 (69%) OC + OP: 100 Number randomised: 106 Number evaluated: 106 | |
| Interventions | Comparison: nimotuzumab + RT vs. placebo + RT Gp A (n = 54): 6 doses of nimotuzumab IV in 250 mL saline + planned RT of 2 Gy/fraction, 5 fractions/wk to total dose of 60‐66 Gy Gp B (n = 52): 6 doses of placebo IV in 250 mL saline + planned RT of 2 Gy/fraction, 5 fractions/wk to total dose of 60‐66 Gy No modified fractionation, IMRT or concomitant boost RT was carried out | |
| Outcomes | Primary: CRR Secondary: OS Duration of follow‐up: 24 months | |
| Notes | Poorly reported. Information presented for some of the participants format was inconsistent and data were incomplete Adverse events note: derived numbers do not result in reported percentages (equating instead to incomplete patient integers). Corresponding whole participant numbers used Selected reported adverse events: narrative and partial reporting of adverse in publication. Included in Table 8 8 Toxicity evaluation assessment tool: Common Toxicity Criteria of the US National Cancer Institute Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed centrally through a validated simple randomisation system (ASAL) version 1.2 |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed centrally through a validated simple randomisation system (ASAL) version 1.2 |
| Blinding of participants (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported for assessors. Refers to "the final determination of response was done by a centralized independent committee composed by radiologists, head and neck surgeons and pathologists who performed a blinded revision of the investigators generated data" ‐ does not indicate if original assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | The quality of the report was poor and we were unable to eliminate the presence of possible other biases. May be due to translation into English. There was incomplete reporting of some data e.g. EGFR expression, and why some participants had biopsies and some did not. |
| Methods | Location of trial: India Number of centres: 1 Trial ID: not given Recruitment period: December 2008 to August 2010 Funding source: not given | |
| Participants | Inclusion criteria: adult patients aged 40‐65 years, male or female, previously untreated, with measurable and evaluable disease, irrespective of EGFR status, KPS score > 70, biopsy‐confirmed SCC, locally advance OC cancer, normal haematology parameters, renal function and liver function tests normal before recruitment Exclusion criteria: Patients who were previously treated with either chemotherapy or RT Sex (M/F): overall: 45/15; Gp A: 25/5; Gp B: 23/7 Age at baseline (years): Gp A: median 55; Gp B: median 53 Gp A: alveolus 3, buccal mucosa 13, tongue 7, retromolar trigone 5, hard palate 2 Gp B: alveolus 3, buccal mucosa 15, tongue 11, retromolar trigone 1, hard palate 0 Number randomised: 60 (Gp A 30, Gp B 30) Number evaluated: 60 | |
| Interventions | Comparison: gefitinib + RT vs. RT alone Gp A: RT (70 Gy over 7 wk) + gefitinib (250 mg orally daily for 7 d before starting RT and continued up to 90 d) Gp B: RT (70 Gy over 7 wk) alone (no placebo) | |
| Outcomes | Primary: CR, PR, no response and disease progression Secondary: grade of mucositis, skin reaction, haematological toxicity, incidence and grade of diarrhoea and vomiting 20‐month follow‐up shown in survival data but no follow‐up time stated in text | |
| Notes | Very poorly written paper. Incomplete outcome data, selective reporting. No calculation of HR Adverse events note: quote: "The acute toxicities in both the arms are not significantly different. [...] The common side effects expected due to Gefitinib like diarrhoea, nausea, and vomiting were not significantly increased in the concurrent Gefitinib arm compared with the RT only arm. There is no significant difference in hematological, hepatic, and renal toxicity" Selected reported adverse events: narrative and partial reporting of adverse events in publication. Mucositis and skin reactions presented in Tables 3 and 4 in original study reference. Included in Table 10 10 Toxicity evaluation assessment tool: Acute and late radiation reactions: toxicity criteria of the Radiation Therapy Oncology Group (1995) Adverse events analysis method: ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation is not given, the authors only stated, "A total of 60 patients were randomised by simple randomisation to two treatment arms." The term 'imbalanced randomisation' was used but no explanation of this term was given |
| Allocation concealment (selection bias) | Unclear risk | Concealment not specifically reported |
| Blinding of participants (performance bias) | Unclear risk | No placebo was used so participants could not be blinded. Knowledge of allocated treatment may have influenced participants' care receipt and perception of symptoms. |
| Blinding of outcome assessment (detection bias) | Low risk | "The participants and the outcome adjudicators were blinded about allocation to treatment arms" |
| Incomplete outcome data (attrition bias) | High risk | Incomplete information about number of participants included in outcome assessment. Numbers and reasons for withdrawals not given |
| Selective reporting (reporting bias) | High risk | Some outcomes only partially reported e.g. adverse events |
| Other bias | Unclear risk | The quality of the report was poor and we were unable to eliminate the presence of possible other biases |
3D: three dimensional; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; CCRT: concurrent chemoradiotherapy; CR: complete response; CRR: complete response rate; CRT: chemoradiotherapy; d: day; DFS: disease‐free survival; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; F: female; Gp: group; h: hour; HNSCC: head and neck squamous cell carcinoma; HR: hazard ratio; ID: identification number; IMRT: intensity‐modulated radiotherapy; ITT: intention to treat; IV: intravenous; KPS: Karnofsky Performance Status; LA‐HNSCC: locally advanced head and neck squamous cell carcinoma; LN‐HNSCC: lymph node head and neck squamous cell carcinoma; LRC: locoregional control; M: male; n: number of participants; NCI‐CTCAE: National Cancer Institute ‐ Common Terminology Criteria for Adverse Events; OC: oral cavity; OP: oropharyngeal; ORR: objective response rate; OS: overall survival; PFS: progression‐free survival; PR: partial response; q3 weeks: every 3 weeks; rIL‐2: recombinant interleukin; RT: radiotherapy; SCC: squamous cell carcinoma; SD: standard deviation; ULN: upper limit of normal; WBC: white blood cell; WHO: World Health Organization; wk: week.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No targeted therapy used | |
| Participants had laryngeal or hypopharyngeal cancers | |
| The proportion of participants with oral cavity cancer was < 50% | |
| Adjuvant levamisole | |
| Targeted therapy used as adjuvant | |
| Not randomised controlled trial | |
| Only a very small minority of participants had oropharyngeal cancer | |
| Only 12 weeks' follow‐up | |
| < 50% or participants have oral cavity or oropharyngeal cancers | |
| Review of trial without original data | |
| Not randomised controlled trial | |
| Participants had recurrent or metastatic disease | |
| Cannot compare trial arms (cisplatin and panitumumab were direct comparators) | |
| < 6 months' follow‐up | |
| < 6 months' follow‐up | |
| Adjuvant therapy | |
| Participants were post surgery (targeted therapy not primary treatment) | |
| Looking at different radiotherapy regimens. Arms not compar able for immunotherapy | |
| Participants had recurrent or metastatic cancer (NCT00634595) | |
| Adjuvant trial | |
| Study withdrawn prior to enrolment of any participants (EORTC 22071‐24071; NCT01142414) | |
| Participants were post surgery (targeted therapy not primary treatment) | |
| Adjuvant trial | |
| Maintenance therapy after primary treatment | |
| Arms of trial not comparable (other differences between trial arms than just targeted therapy) | |
| < 6 months' follow‐up | |
| Trial arms not comparable (other differences between trial arms than just targeted therapy) | |
| Trial arms not comparable (other differences between trial arms than just targeted therapy) | |
| Trial arms not comparable (other differences between trial arms than just targeted therapy) | |
| Trial arms not comparable (other differences between trial arms than just targeted therapy) | |
| Adjuvant trial | |
| Adjuvant trial | |
| Trial arms not comparable | |
| < 50% of participants have oral cavity and oropharyngeal cancers | |
| Planned protocol for proposed randomised controlled trial (from T Naito who translated from Japanese) | |
| Arms of trial not comparable (other differences between trial arms than just targeted therapy) | |
| Adjuvant therapy | |
| Adjuvant therapy | |
| Participants have recurrent or metastatic disease | |
| Adjuvant therapy | |
| Several participants had undergone surgery prior to targeted therapy treatment | |
| Abstract only, no subsequent publication identified. Insufficient information to include | |
| All groups received cetuximab | |
| < 6 months' follow‐up | |
| < 50% of participants oral cavity or oropharyngeal cancers and all had already received surgery ± radiotherapy | |
| < 50% of participants had either oral cavity or oropharyngeal cancers | |
| Not randomised controlled trial | |
| < 6 months' follow‐up (from translation by Chunjie Li) | |
| Adjuvant therapy | |
| Adjuvant therapy | |
| Abstract only, unclear what type of cancer or whether there was prior treatment. No subsequent publication identified. Insufficient information to include | |
| Immunotherapy not randomly allocated | |
| Adjuvant levamisole therapy | |
| Insufficient information in study report to include. Numbers of participants randomised or evaluated not stated, no baseline characteristics, no details of whether allocation was truly randomised | |
| Comparison of different does of erlotinib, < 6 months' follow‐up | |
| Not randomised controlled trial | |
| Participants had recurrent disease | |
| < 50% oral cavity and oropharyngeal cancers (from translation by Chunjie Li) | |
| < 6 months' follow‐up (from translation by Chunjie Li) | |
| < 50% oral cavity and oropharyngeal cancers (from translation by Chunjie Li) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Phase II randomised trial of RT, cetuximab, and pemetrexed with or without bevacizumab in locally advanced HNSCC Location of trial: University of Pittsburgh Medical Center, US Number of centres: > 1 Recruitment period: unclear Funding: University of Pittsburgh, Eli Lilly and Company, Genentech, Inc |
| Participants | Inclusion criteria: patients with previously untreated stage III/IV HNSCC of the oropharynx, larynx or hypopharynx; PS 0‐1; no history of bleeding; and adequate laboratory parameters, no evidence of distant metastasis Exclusion criteria: patients who are receiving any other investigational agents; people with uncontrolled intercurrent illness including, but not limited to, ongoing or active infection or psychiatric illness/social situations that would limit compliance with study requirements; people should not have prior history of a serious reaction to a monoclonal antibody; people with known hypersensitivity to Chinese hamster ovary cell products or other recombinant human antibodies are not eligible. Pregnant or breastfeeding women Sex (M/F): not stated Age at baseline (years): ≥ 18 OP: 65 (82%) Number randomised: 79 (Gp A 35, Gp B 41) Number evaluated: 59? unclear |
| Interventions | Comparison: cetuximab + pemetrexed + RT vs. cetuximab + pemetrexed + RT + bevacizumab Gp A: RT 2 Gy/d to 70 Gy, cetuximab 250 mg/m2 weekly, after a loading dose of 400 mg/m2 1 wk prior starting RT, and pemetrexed 500 mg/m2 every 21 d x 3 cycles Gp B: RT 2 Gy/d to 70 Gy, cetuximab 250 mg/m2 weekly, after a loading dose of 400 mg/m2 1 wk prior starting RT, and pemetrexed 500 mg/m2 every 21 d x 3 cycles plus bevacizumab 15 mg/kg every 21 d x 3 cycles during RT followed by bevacizumab maintenance x 8 cycles, with antibiotic prophylaxis |
| Outcomes | Primary: progression‐free survival Secondary: time to local progression, time to regional progression, overall survival and toxicity profiles Duration of follow‐up: 2 years |
| Notes | Review author (HW) contacted Ethan Argiris (May 2015) who is currently undertaking analysis of the results. Awaiting publication of trial report |
| Methods | A comparative study of a monoclonal antibody against EGFR (nimotuzumab) used in combination with chemoradiation versus chemoradiation alone in the treatment of locally advanced inoperable HNSCC Location of trial: unclear Number of centres: unclear Recruitment period: unclear Funding: unclear |
| Participants | Inclusion criteria: locally advanced inoperable HNSCC Exclusion criteria: no information given in abstract Sex (M/F): not stated Age at baseline: not stated Number randomised: 56 Number evaluated: 50 |
| Interventions | Comparison: nimotuzumab + CRT vs. CRT alone Gp A: cisplatin 30 mg/m2 repeated weekly for 6‐7 cycles along with external beam RT 64‐70 Gy (200 cGy/d for 5 d/wk for 6‐7 wk) + nimotuzumab 200 mg weekly for 6‐7 cycles Gp B: cisplatin 30 mg/m2 repeated weekly for 6‐7 cycles along with external beam RT 64‐70 Gy (200 cGy/d for 5 d/wk for 6‐7 wk) |
| Outcomes | Primary: tumour response evaluation according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria version 1.1; safety analysis using Radiation Therapy Oncology Group Acute Radiation Morbidity Scoring Criteria Duration of follow‐up: 6 months |
| Notes | Review author (HW) emailed author 12 May 2015 to request full report to make sure that no outcomes of interest for this review were reported in the trial as secondary outcomes and to request the proportion of participants with OC and OP tumours ‐ request for data not responded to. Awaiting publication of trial report |
| Methods | Efficacy and toxicity of tyrosine kinase inhibitor along with platinum based concurrent chemo‐irradiation in locally advanced squamous cell carcinoma of head and neck Location of trial: unclear Number of centres: unclear Recruitment period: October 2007 to January 2008 Funding: unclear |
| Participants | Inclusion criteria: locoregionally advanced histopathologically confirmed HNSCC excluding nasopharynx, nasal and paranasal cavities, people must have minimum age of 18 years, KPS score > 60% with life expectancy of > 6 months and adequate haematological, renal and hepatic function Exclusion criteria: no information given in abstract Sex: not stated Age at baseline: not stated OC/OP: not stated Number randomised: 100 Number evaluated: unclear |
| Interventions | Comparison: gefitinib + CRT vs. CRT alone Gp A (n = 50): weekly cisplatin (30 mg/m2) with concurrent RT (70 Gy/35# with conventional portals) plus daily gefitinib (250 mg) Gp B (n = 50): weekly cisplatin (30 mg/m2) along with concurrent RT (70 Gy/35# with conventional portals) |
| Outcomes | Primary: progression‐free survival Secondary: response rate, overall survival, toxicity Duration of follow‐up: 2 years |
| Notes | Review author (HW) emailed author 12 May 2015 to request full report. No information on percentage of OC and OP ‐ request for data not responded to |
| Methods | The IRESSA novel head and neck chemotherapy evaluation study: a randomised phase II study to investigate the feasibility and benefits of combining ZD1839 and cisplatin/5‐fluorouracil, as induction therapy, in people with locally and advanced HNSCC Location of trial: international (US ‐ 2 centres, Belgium ‐ 3 centres, Czech Republic ‐ 3 centres, Germany ‐ 4 centres, India ‐ 4 centres, Poland ‐ 5 centres, Serbia ‐ 2 centres, Taiwan ‐ 2 centres) Number of centres: 25 Recruitment period: unclear Funding: AstraZeneca Alternative trial ID: 2004‐000358‐21 |
| Participants | Inclusion criteria: Patients with histologically confirmed primary HNSCC, measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST), presence of stage III or IVA HNSCC confirmed histologically by biopsy or by fine needle aspiration at the time of diagnosis, no previous surgery to the tumour except for biopsy and not scheduled for surgery, no previous chemotherapy or RT for any neoplasm/tumour, no previous anti‐EGFR therapy, life expectancy 12 wk, World Health Organization PS of 0 or 1 Exclusion criteria: buccal mucosal carcinomas and post‐nasal space (nasopharyngeal carcinoma); thyroid, sinus or salivary gland tumours and grade III laryngeal carcinoma; early‐stage III cancers (T1N1 or T2N1); extensive disease (T4b) or metastatic disease (M1); disease invading the mandible; presence of simultaneous primary tumours; known severe hypersensitivity to ZD1839 or any of the excipients of this product; known severe hypersensitivity to cisplatin or any of the excipients of this product; any evidence of clinically active interstitial lung disease (people with chronic, stable, radiographic changes who are asymptomatic need not be excluded), other co‐existing malignancies or malignancies diagnosed within the last 5 years with the exception of basal cell carcinoma or cervical cancer in situ, serum bilirubin 3 times the upper limit of the reference range, alanine aminotransferase or aspartate aminotransferase > 5 times the upper limit of the reference range; calculated creatinine clearance (Cockcroft and Gault) 60 mL/minute; serum calcium above the upper limit of the reference range, as judged by the investigator; any evidence of severe or uncontrolled systemic diseases (e.g. unstable or uncompensated respiratory, cardiac, hepatic or renal disease); evidence of any other significant clinical disorder or laboratory finding that makes it undesirable for the person to participate in the study; pregnancy or breastfeeding; concomitant use of phenytoin, carbamazepine, barbiturates, rifampicin, amifostine, erythropoietin or St. John's Wort; concomitant use of CYP3A4 inhibitors (e.g. itraconazole); treatment with a non‐approved or investigational drug within 30 d before day 1 of study treatment; evidence of ototoxicity or other neurotoxicity; concurrent treatment with other experimental drugs, anticancer agents or both Sex (M/F): not stated Age at baseline: ≥ 18 years OC/OP numbers: not given Number randomised: 224 Number evaluated: not given |
| Interventions | Comparison: gefitinib + CRT vs. CRT alone Gp A: radiation + cisplatin; followed by placebo as maintenance therapy (RT and cisplatin dose not given) Gp B: gefitinib 250 mg + cisplatin + RT; followed by placebo as maintenance therapy Gp C: gefitinib 500 mg + cisplatin + RT; followed by placebo as maintenance therapy Gp D: gefitinib 250 mg + cisplatin + RT; followed by gefitinib 250 mg as maintenance therapy Gp E: gefitinib 500 mg + cisplatin + RT; followed by gefitinib 500 mg as maintenance therapy Gp F: placebo + cisplatin + RT; followed by gefitinib 250 mg as maintenance therapy Gp G: placebo + cisplatin + RT; followed by gefitinib 500 mg as maintenance therapy |
| Outcomes | Primary: progression‐free survival (from concomitant use and maintenance use) Secondary: complete response, tumour response, overall survival, safety and tolerability Duration of follow‐up: 2 years |
| Notes | Large trial run by AstraZeneca. Review author (HW) contacted the trial manager on 21 April 2015. Submitted a data request to AstraZeneca and MedImmune (April 2015) to include in review update |
CRT: chemoradiotherapy; d: day; EGFR: epidermal growth factor receptor; F: female; Gp: group; HNSCC: head and neck squamous cell carcinoma; M: male; OC: oral cavity; OP: oropharyngeal; KPS: Karnofsky Performance Status; PS: performance status; RT: radiotherapy; wk: week.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | DAHANCA 19: the Importance of the EGFr‐inhibitor Zalutumumab for the Outcome After Curative Radiotherapy for HNSCC |
| Methods | Location of trial: Denmark Number of centres: 1 Trial ID: NCT00496652 (DAHANCA 19) Recruitment period: still recruiting Funding source: not specifically reported, although trial register indicates Danish Head and Neck Cancer Group as being the sole sponsor Estimated enrolment: 619 |
| Participants | Inclusion criteria: aged > 18 years; histological confirmation of SCC of pharynx, larynx (with exception of Stage I larynx/stage I+II glottis larynx); curative intent; no prior treatment; WHO performance 0‐2; no prior EGFR inhibitor treatment; informed consent; able to fully participate (treatment and follow‐up); women using contraceptive device Exclusion criteria: carcinoma of rhinopharynx or unknown origin, distal metastases, prior/current malignant disease (with exception of planocellular skin cancer) Sex: both included Age at baseline: > 18 years |
| Interventions | Comparison: RT + cisplatin + zalutumumab vs. RT + cisplatin + placebo Gp A: RT 66‐68 Gy, 2 Gy/fraction, 6 fractions/week + weekly cisplatin 40 mg/m2 during RT for stage III and IV participants + zalutumumab 8 mg/kg every week during RT + the week before start of RT (as loading dose) Gp B: RT 66‐68 Gy, 2 Gy/fraction, 6 fraction/week + weekly cisplatin 40 mg/m2 during RT for stage III and IV participants + placebo |
| Outcomes | Primary: locoregional control Secondary: disease‐specific survival, overall survival, acute and late toxicity Duration of follow‐up: 5 years |
| Starting date | November 2007 |
| Contact information | Jesper Grau Eriksen: [email protected] |
| Notes | 5‐year follow‐up period has now ended and data are under analysis |
| Trial name or title | A Phase II, Randomized, Open‐Label, Single Centre Study in Patients with Advanced Head and Neck Cancer to Investigate Efficacy and Safety of Standard Chemoradiation and Add‐on Concurrent Cetuximab ± Consolidation Cetuximab |
| Methods | Location of trial: Switzerland Number of centres: 1 Trial ID: NCT01435252 Recruitment period: September 2011 to December 2017 Funding source: University of Zurich Estimated enrolment: 60 |
| Participants | Inclusion criteria: T3‐4 NX M0; TX N2b‐3 M0 (N2b only if ≥ 3 ipsilateral nodes involved) or total gross tumour volume > 70 cc (any T, any N, M0), or both; biopsy proven squamous cell cancer; primary tumour location in OC, oropharynx, hypopharynx or larynx; people with cancer of unknown primary syndrome in case they have advanced lymph node metastases (TX N2b‐3 M0 (N2b only if ≥ 3 ipsilateral nodes involved); indication for chemoradiation (RT + cisplatin); curative treatment intent; start of chemoradiation within the recruitment time frame; WHO/ECOG performance status: 0‐1; no previous chemotherapy or RT for cancer of the head and neck; women of childbearing potential and male participants must agree to use a medically effective means of birth control throughout their participation in the treatment phase of the study (until at least 60 days following the last study treatment; informed consent prior to study entry Exclusion criteria: cancer of the nasopharynx; any neoadjuvant chemotherapy prior to screening; treatment with other investigational drugs within 4 weeks; history of malignancy other than basal cell skin cancer unless disease free for a minimum of 3 years; uncontrolled claudication, bleeding, or thromboembolic disorders at screening; patients receiving heparin, warfarin or phenprocoumon therapy are ineligible; current uncontrolled cardiac disease: unstable angina, New York Heart Association (NYHA) Grade II or greater congestive heart failure, history of myocardial infarction within 12 months, significant arrhythmias; left ventricular function < 45% (determination of left ventricular function required when history of cardiac disease); history of stroke within 6 months; major surgical procedure or significant traumatic injury within 28 days prior to screening; anticipation of need for major surgical procedure Sex: both included Age at baseline: 18‐75 years |
| Interventions | Comparison: chemoradiation + cetuximab + consolidation cetuximab vs. chemoradiation + cetuximab Gp A: chemoradiation (up to 70 Gy in combination with weekly cisplatin 40 mg/m2) in combination with concurrent cetuximab (loading dose 400 mg/m2, concurrent dose 250 mg/m2 weekly). 2 weeks after end of chemoradiation the consolidation phase will start and participants will receive 500 mg/m2 cetuximab biweekly x 6 over 12 weeks Gp B: chemoradiation (up to 70 Gy in combination with weekly cisplatin 40 mg/m2) in combination with concurrent cetuximab (loading dose 400 mg/m2, concurrent dose 250 mg/m2 weekly) |
| Outcomes | Primary: locoregional tumour control Secondary: progression‐free survival; overall survival; metastasis rate; metastasis‐free survival; biological surrogate markers; safety and tolerability Duration of follow‐up: 2 years |
| Starting date | September 2011 |
| Contact information | Oliver Riesterer, Leitender Arzt +41442553748, [email protected] |
| Notes |
| Trial name or title | Randomized, Placebo‐Controlled, Phase 2 Study of Induction Chemotherapy with Cisplatin/Carboplatin, and Docetaxel with or without Erlotinib in Patients with Head and Neck Squamous Cell Carcinomas Amenable for Surgical Resection |
| Methods | Location of trial: US Number of centres: 1 Trial ID: NCT01927744 Recruitment period: December 2013 to December 2020 Funding source: MD Anderson Cancer Center, Astellas Pharma Inc., OSI Pharmaceuticals, Kadoorie Foundation Estimated enrolment: 100 |
| Participants | Inclusion criteria: suspected or histologically/cytologically confirmed head and neck squamous cell cancer of the OC, stage III, IVA or IVB (according to the American Joint Committee on Cancer 7th edition). Patients with a suspected lesion may be enrolled and a baseline biopsy will be obtained as part of the study. If squamous cell histology is not confirmed, patients will be discontinued from the study; patients must have surgically resectable disease, in the opinion of the treating physician; aged ≥ 18 years; ECOG Performance Status ≤ 2; adequate bone marrow, hepatic and renal function defined by: ANC ≥ 1.5 x 109/L, platelet count ≥ 100 x 109/L, ALT ≤ 1.5 x ULN, total bilirubin ≤ ULN (people with Gilbert's syndrome are eligible, even if total bilirubin is > ULN), alkaline phosphatase ≤ 2.5 x ULN, serum creatinine ≤ 1.5 x ULN; people with reproductive potential (e.g. women menopausal for < 1 year and not surgically sterilised) must practice effective contraceptive measures for the duration of study drug therapy and for at least 30 days after completion of study drug therapy; women of childbearing potential must provide a negative pregnancy test (serum or urine) ≤ 14 days prior to treatment initiation; written informed consent to participate in the study according to the investigational review board Exclusion criteria: histology other than SCC; primary sites other than OC; prior chemotherapy or biological therapy for the same HNSCC; prior chemotherapy or biological therapy for a different previous HNSCC is allowed; history of poorly controlled gastrointestinal disorders that could affect the absorption of the study drug (e.g. Crohn's disease, ulcerative colitis) (people requiring feeding tubes are permitted); other active solid malignancies within 2 years prior to randomisation, except for basal cell or squamous cell skin cancer or in situ cervical or breast cancer or superficial melanoma; serious underlying medical condition that would impair the ability of the person to receive protocol treatment, in the opinion of the treating physician; history of allergic reactions to compounds of similar chemical composition to the study drugs (docetaxel, cisplatin, carboplatin, erlotinib or Sex: both Age at baseline: ≥ 18 years |
| Interventions | Comparison: chemotherapy + erlotinib vs. chemotherapy + placebo Gp A: docetaxel 75 mg/m2 by vein followed by cisplatin 75 mg/m2 or carboplatin AUC 6 mg/minute/mL by vein on day 1 of each 21‐day cycle for a maximum of 3 cycles, plus erlotinib 150 mg by mouth daily continuously until the day before surgery Gp B: docetaxel 75 mg/m2 by vein followed by cisplatin 75 mg/m2 or carboplatin AUC 6 mg/minute/mL by vein on day 1 of each 21‐day cycle for a maximum of 3 cycles, plus placebo 150 mg by mouth daily continuously until the day before surgery |
| Outcomes | Primary: pathological complete response defined as:
Bayesian probit model used to assess the main effect of treatment, nodal status and biomarker, and treatment by biomarker interaction No secondary outcomes given |
| Starting date | December 2013 |
| Contact information | William N. William Jr., MD 713‐792‐6363 University of Texas MD Anderson Cancer Center, Houston, TX, US |
| Notes |
| Trial name or title | A Phase I/II Randomized Study to Determine the Maximum Tolerated Dose, Safety, Pharmacokinetics and Antitumor Activity of Debio 1143 Combined with Concurrent Chemo‐radiation Therapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck |
| Methods | Location of trial: France (3 centres)/Switzerland (1 centre) Number of centres: 4 Trial ID: NCT02022098/Debio 1143‐201 Recruitment period: not given Funding source: Debiopharm International SA Estimated enrolment: 118 |
| Participants | Inclusion criteria: meets protocol‐specified criteria for qualification and contraception; willing and able to comply with study procedures and restrictions related to food, drink and medications; voluntarily consents to participate and provides written informed consent prior to any protocol‐specific procedures Exclusion criteria: has history or current use of non‐prescription medications, dietary supplements or drugs (including nicotine and alcohol) outside protocol‐specified parameters; has signs, symptoms or history of any condition that, per protocol or in the opinion of the investigator, might compromise: the safety or well‐being of the participant or study staff, the safety or well‐being of the participant's offspring (such as through pregnancy or breast‐feeding), the analysis of results Sex: both Age at baseline: 18‐75 years |
| Interventions | Comparison: Debio 1143 + chemotherapy + RT vs. chemotherapy + placebo Gp A: Debio 1143 in solution form administered orally or by feeding tube (while fasting) daily for 14 days every 3 weeks (on days 1‐14, 22‐35 and 43‐56). 3 cycles of cisplatin administered in a 1‐hour intravenous infusion on days 2, 23 and 44. Cisplatin administered 0.5 hours after Debio 1143. Standard fraction RT to the primary tumour delivered daily for 5 days/week over 7 weeks Gp B: placebo solution administered orally or by feeding tube (while fasting) daily for 14 days every 3 weeks (on days 1‐14, 22‐35 and 43‐56). 3 cycles of cisplatin administered in a 1‐hour intravenous infusion on days 2, 23 and 44. Cisplatin administered 0.5 hours after Debio 1143. Standard fraction RT to the primary tumour delivered daily for 5 days/week over 7 weeks |
| Outcomes | Primary: locoregional control at 18 months Secondary: complete response rate, overall response rate, locoregional control at 6 months, progression‐free survival at 12 and 24 months, distant relapse, overall survival at 24 months, adverse events, late toxicity, laboratory abnormalities |
| Starting date | October 2013 |
| Contact information | Claudio Zanna, Jerôme Douchain, +41 21 321 01 11 |
| Notes |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; AUC: area under the curve; CDC: Centers for Control and Prevention; ECOG: Eastern Cooperative Oncology Group; Gp: group; HNSCC: head and neck squamous cell carcinoma; OC: oral cavity; PTT: prothrombin time; RT: radiotherapy; SCC: squamous cell carcinoma; ULN: upper limit of normal; WHO: World Health Organization.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (5 years) Show forest plot | 3 | 1421 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| Analysis 1.1  Comparison 1 Monoclonal antibodies (mAb), Outcome 1 Overall survival (5 years). | ||||
| 1.1 mAb therapy + radiotherapy (RT) vs. RT alone | 2 | 530 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 1.2 mAb therapy + chemoradiotherapy (CRT) vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 2 Locoregional control Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Monoclonal antibodies (mAb), Outcome 2 Locoregional control. | ||||
| 2.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 3 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Monoclonal antibodies (mAb), Outcome 3 Progression‐free survival. | ||||
| 3.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 3.2 mAb therapy + CRT vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| Analysis 2.1  Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 1 Overall survival. | ||||
| 1.1 TKI + chemoradiotherapy (CRT) vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 2 Locoregional control Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| Analysis 2.2  Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 2 Locoregional control. | ||||
| 2.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 3 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 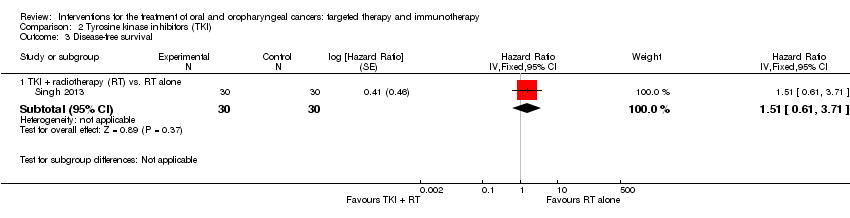 Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 3 Disease‐free survival. | ||||
| 3.1 TKI + radiotherapy (RT) vs. RT alone | 1 | 60 | Hazard Ratio (Fixed, 95% CI) | 1.51 [0.61, 3.71] |
| 4 Progression‐free survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| Analysis 2.4 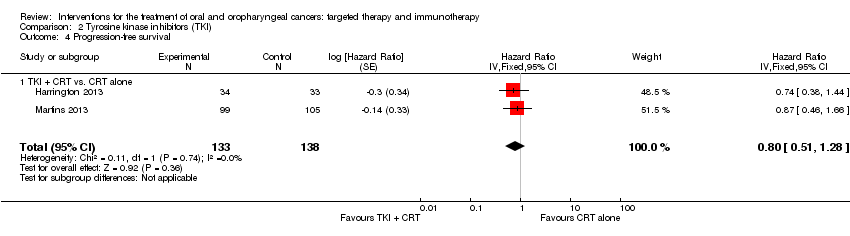 Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 4 Progression‐free survival. | ||||
| 4.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Immunotherapy, Outcome 1 Overall survival. | ||||
| 1.1 Surgery ± radiotherapy (RT) + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 2 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Immunotherapy, Outcome 2 Disease‐free survival. | ||||
| 2.1 Surgery ± RT + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.43, 1.02] |
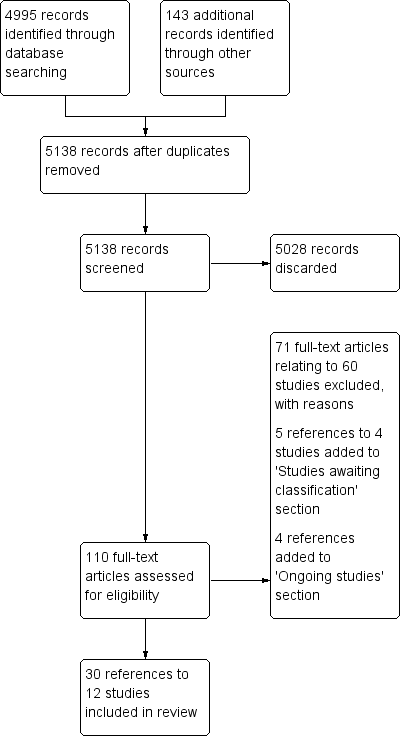
Review flow diagram
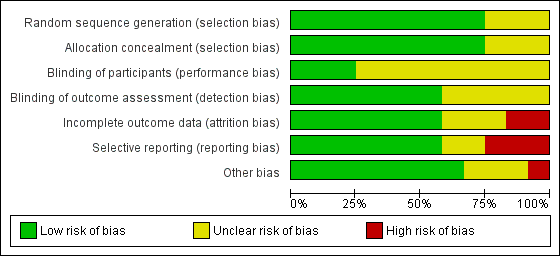
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Monoclonal antibodies (mAb), Outcome 1 Overall survival (5 years).
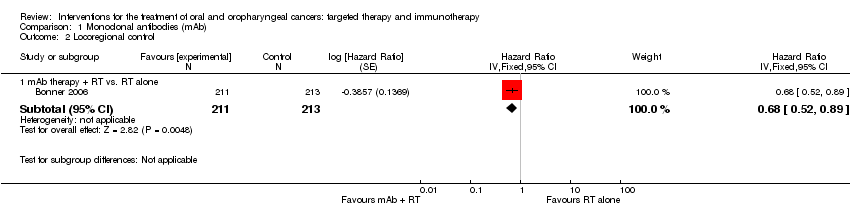
Comparison 1 Monoclonal antibodies (mAb), Outcome 2 Locoregional control.

Comparison 1 Monoclonal antibodies (mAb), Outcome 3 Progression‐free survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 1 Overall survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 2 Locoregional control.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 3 Disease‐free survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 4 Progression‐free survival.

Comparison 3 Immunotherapy, Outcome 1 Overall survival.

Comparison 3 Immunotherapy, Outcome 2 Disease‐free survival.
| Monoclonal antibodies plus standard therapy versus standard therapy alone for the treatment of people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: RT or CRT alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk mAb | |||||
| Overall survival | Low‐risk population* | HR 0.82 | 1421 | ⊕⊕⊕⊝ moderate1 | There was an 18% reduction in death (during the follow‐up period) in the participants treated with EGFR mAb therapies in addition to standard therapies | |
| 160 per 1000 | 133 per 1000 (113 to 156) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 347 per 1000 (301 to 396) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 577 per 1000 (515 to 639) | |||||
| Locoregional control | Low‐risk population* | HR 0.68 | 424 | ⊕⊕⊕⊝ | There was a 32% reduction in recurrence of cancer (during the follow‐up period) in the participants treated with EGFR mAb therapies (cetuximab) in addition to standard therapies | |
| 160 per 1000 | 112 per 1000 (87 to 144) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 297 per 1000 (237 to 370) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 510 per 1000 (421 to 607) | |||||
| Progression‐free survival | We formed 2 subgroups: mAb therapy + RT versus RT alone and mAb therapy + CRT versus CRT alone. There was a significant difference between these subgroups (P value = 0.008; I2 = 86%) and as a result we were unable to pool the data. The subgroup comparing mAb therapy + RT versus RT alone reported a 30% reduction in the number of people whose disease progresses if treated with EGFR mAb in addition to RT (HR 0.70; 95% CI 0.54 to 0.91; P value = 0.006). However, the subgroup comparing mAb therapy + CRT versus CRT alone reported no evidence of a difference in progression‐free survival (HR 1.08; 95% CI 0.89 to 1.32; P value = 0.76) | |||||
| Adverse effects | A subgroup estimate shows evidence of an increase in skin toxicity/acneiform rash (all grades of adverse effects: RR 6.56, 95% CI 5.35 to 8.03; 1311 participants, 2 studies; adverse effects grades ≥ 3: RR 17.72, 95% CI 8.33 to 37.73; 1403 participants, 3 studies) in people treated with cetuximab in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; EGFR: epidermal growth factor receptor; mAb: monoclonal antibody; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once for reporting bias (potential publication bias and poor reporting of EGFR and biopsy data in one study). | ||||||
| Tyrosine kinase inhibitors in addition to standard treatments for people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (either RT or CRT alone) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk Tyrosine kinase inhibitors | |||||
| Overall survival | Low‐risk population* | HR 0.99 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in participant survival in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 159 per 1000 (102 to 239) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 402 per 1000 (275 to 557) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 646 per 1000 (478 to 808) | |||||
| Locoregional control | Low‐risk population* | HR 0.89 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in locoregional control in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 144 per 1000 (88 to 229) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 370 per 1000 (241 to 539) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 607 per 1000 (429 to 791) | |||||
| Disease‐free Survival | Low‐risk population* | HR 1.51 | 60 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants survived without signs or symptoms of oral cavity cancer when treated with tyrosine kinase inhibitors (gefitinib) in addition to standard therapies | |
| 160 per 1000 | 231 per 1000 (101 to 476) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 543 per 1000 (271 to 854) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 795 per 1000 (473 to 980) | |||||
| Progression‐free Survival | Low‐risk population* | HR 0.8 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants stayed alive with stable disease when treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 130 per 1000 (85 to 200) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 340 per 1000 (233 to 486) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 568 per 1000 (415 to 739) | |||||
| Adverse effects | A subgroup estimate showed evidence of an increase in gastrointestinal complaints (all grades of adverse effects: RR 15.53, 95% CI 2.18 to 110.55; 67 participants, 1 study) in people treated with lapatinib in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once due reporting bias (data from a large study (Gregoire 2011) was not available). 2 Downgraded once due to imprecision 3 Downgraded once due to unclear risk of bias across multiple domains. 4 Downgraded once for applicability ‐ only participants receiving RT as a standard therapy were included in this subgroup (no CRT). | ||||||
| Recombinant Interleukin (rIL‐2) in addition to surgery for the treatment of people with oral and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (surgery) alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk rIL‐2 | |||||
| Overall survival | Low‐risk population* | HR 0.52 (0.31 to 0.87) | 201 | ⊕⊝⊝⊝ | There was an 48% reduction in death in the groups treated with rIL‐2 in addition to standard therapy | |
| 160 per 1000 | 87 per 1000 | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 237 per 1000 (149 to 363) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 421 per 1000 (278 to 599) | |||||
| Disease‐free survival | Low‐risk population* | HR 0.66 | 201 | ⊕⊝⊝⊝ | There is no evidence of a difference in the length of time that participants survived without signs or symptoms of cancer when treated with rIL‐2 in addition to standard therapies | |
| 160 per 1000 | 109 per 1000 (72 to 163) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 290 per 1000 (200 to 411) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 500 per 1000 (363 to 657) | |||||
| Adverse effects | There was no evidence of an increase or reduction of nausea/vomiting, stomatitis or leukopenia in participants treated with rIL‐2 in addition to standard therapy (1 study, 30 participants) | |||||
| CI: confidence interval; HR: hazard ratio; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010) 1 Downgraded twice due to high/unclear risk of bias across domains. | ||||||
| Inclusion criteria | Exclusion criteria | |
| Location of cancer | People with primary cancer of the oral cavity (ICD‐O: C01‐06) or oropharynx (ICD‐O: C09‐10 ‐ includes tonsil) | Lip (ICD‐O: C00) cancers |
| Type of cancer | Squamous cell carcinoma | Parotid gland (ICD‐O: C07), unspecified major salivary gland (ICD‐O: C08) |
| ICD‐O: International Classification of Diseases for Oncology. | ||
| ITT analysis | Docetaxel + cisplatin + cetuximab (n = 48) | Docetaxel + cisplatin (n = 44) |
| OS (3 years) | 88% (n = 42) | 74% (n = 33) |
| PFS (3 years) | 70% (n = 34) | 56% (n = 25) |
| ITT: intention to treat; n: number of participants; OS: overall survival; PFS: progression‐free survival. | ||
| Outcome | Grade | No. of studies | No. of patients | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 1.6 Mucositis | All grades | 3 | 1417 | Not estimable due to high heterogeneity (P value = 0.0009; I2 = 86%) between subgroups (cetuximab; nimotuzumab), and within the cetuximab subgroup (P value = 0.0002) I2 = 93%) | |
| Grades ≥ 3 | 4 | 1495 | 1.20 (0.96 to 1.51) (random effects) P value = 0.12 | P value = 0.07; I2 = 58% | |
| 1.7 Dysphagia | All grades | 3 | 1403 | 0.97 (0.92 to 1.03) (fixed effect) P value = 0.37 | P value = 0.60; I2 = 0% |
| Grades ≥ 3 | 3 | 1403 | 0.93 (0.83 to 1.04) (fixed effect) P value = 0.19 | P value = 0.26; I2 = 26% | |
| 1.8 Xerostomia | All grades | 3 | 1417 | 0.97 (0.91 to 1.04) (fixed effect) P value = 0.46 | P value = 0.61; I2 = 0% |
| Grades ≥ 3 | 2 | 1311 | 1.36 (0.80 to 2.31) (fixed effect) P value = 0.25 | P value = 0.60; I2 = 0% | |
| 1.9 Skin toxicity/ acneiform rash | All grades | 3 | 1403 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 99.3%) | |
| 2 (cetuximab) | 1311 | 6.56 (5.35 to 8.03) (fixed effect) P value < 0.00001 | P value = 0.080; I2 = 66% | ||
| 1 (nimotuzumab) | 92 | 1.06 (0.85 to 1.31) P value = 0.61 | Not applicable | ||
| Grades ≥ 3 | 4 | 1495 | Not estimable due to significant difference between subgroups (P value = 0.005; I2 = 87.5%) | ||
| 3 (cetuximab) | 1403 | 17.72 (8.33 to 37.73) (fixed effect) P value < 0.00001 | P value = 0.56; I2 = 0% | ||
| 1 (nimotuzumab) | 92 | 0.20 (0.01 to 4.05) P value = 0.29 | Not applicable | ||
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 2.5 Mucositis | All grades | 2 | 286 | 1.03 (0.94 to 1.11) (fixed effect) P value = 0.55 | P value = 0.44; I2 = 0% |
| Grades ≥ 3 | 2 | 286 | 1.22 (0.96 to 1.56) (fixed effect) P value = 0.10 | P value = 0.53; I2 = 0% | |
| 2.6 Skin toxicity | All grades | 4 | 544 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 95.5%) | |
| 2 (gefitinib) | 286 | 1.03 (0.82 to 1.28) (fixed effect) P value = 0.82 | P value = 0.42; I2 = 0% | ||
| 1 (lapatinib) | 67 | 2.02 (1.23 to 3.32) P value = 0.005 | Not applicable | ||
| 1 (erlotinib) | 191 | 6.57 (3.60 to 12.00) P value < 0.00001 | Not applicable | ||
| Grades ≥ 3 | 4 | 544 | 1.25 (0.54 to 2.88) (random effects) P value = 0.61 | P value = 0.09; I2 = 54% | |
| 2.7 Gastrointestinal | All grades | 2 | 293 | Not estimable due to significant difference between subgroups (P value = 0.007; I2 = 86.2%) | |
| 1 (gefitinib) | 226 | 1.04 (0.98 to 1.11) P value = 0.18 | Not applicable | ||
| 1 (lapatinib) | 67 | 15.53 (2.18 to 110.55) P value = 0.006 | Not applicable | ||
| Grades ≥ 3 | 3 | 484 | 1.11 (0.83 to 1.49) (fixed effect) P value = 0.47 | P value = 0.53; I2 = 0% | |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | BCG‐cell wall preparation + surgery | Surgery alone |
| Overall survival (3 years) | 10/10 | 9/10 |
| Recurrence (3 years) | 3/10 | 4/10 |
| BCG: Bacillus Calmette‐Guérin. | ||
| Outcome | Neoadjuvant chemotherapy (n = 17) | Neoadjuvant chemotherapy + rIL‐2 (n = 16) |
| Locoregional control Complete response | 3/15 | 4/13 |
| Locoregional control Partial response | 9/15 | 6/13 |
| rIL‐2: recombinant interleukin. | ||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 3.3 Nausea/vomiting | All grades | 1 | 30 | 1.24 (0.90 to 1.70) P value = 0.19 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 3.40 (0.15 to 77.34) P value = 0.44 | Not applicable | |
| 3.4 Stomatitis | All grades | 1 | 30 | 1.47 (0.75 to 2.90) P value = 0.27 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 1.71 (0.33 to 8.83) P value = 0.52 | Not applicable | |
| 3.5 Leukopenia | All grades | 1 | 30 | 0.57 (0.22 to 1.50) P value = 0.25 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 0.38 (0.04 to 3.26) P value = 0.38 | Not applicable | |
| 3.6 Increased temperature | All grades | 1 | 24 | 0.67 (0.13 to 3.30) P value = 0.62 | Not applicable |
| 3.7 Moderate‐severe chills | All grades | 1 | 24 | 11.00 (0.67 to 179.29) P value = 0.09 | Not applicable |
| 3.8 Gastrointestinal | All grades | 1 | 24 | 5.00 (0.27 to 94.34) P value = 0.28 | Not applicable |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (5 years) Show forest plot | 3 | 1421 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 1.1 mAb therapy + radiotherapy (RT) vs. RT alone | 2 | 530 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 1.2 mAb therapy + chemoradiotherapy (CRT) vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 2 Locoregional control Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 3 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 3.2 mAb therapy + CRT vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 1.1 TKI + chemoradiotherapy (CRT) vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 2 Locoregional control Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 2.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 3 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 TKI + radiotherapy (RT) vs. RT alone | 1 | 60 | Hazard Ratio (Fixed, 95% CI) | 1.51 [0.61, 3.71] |
| 4 Progression‐free survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| 4.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Surgery ± radiotherapy (RT) + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 2 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Surgery ± RT + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.43, 1.02] |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||

