Intervensi untuk rawatan kanser oral dan orofarinks: terapi sasaran dan imunoterapi
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
-
"Head and Neck Neoplasms"/
-
"Mouth Neoplasms"/
-
"Gingival Neoplasms"/
-
"Palatal Neoplasms"/
-
"Tongue Neoplasms"/
-
((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).ti,ab.
-
or/1‐6
-
exp Gene therapy/
-
exp Immunotherapy/
-
Molecular targeted therapy/
-
((gene$ or target$ or DNA) adj6 therap$).ti,ab.
-
(immunotherap$ or "immuno therap").ti,ab.
-
Antibodies, Monoclonal/
-
(cetuximab or Erbitux or C‐225 or C225 or "immunoglobulin G1").ti,ab.
-
(panitumumab or ABX‐EGF$ or Amgen or Vectibix).ti,ab.
-
(zalutumumab or 2F8 or HuMax‐EGFr).ti,ab.
-
"anti‐human epidermal growth factor receptor".ti,ab.
-
("EGFR inhibitor$" or "epidermal growth factor inhibitor$").ti,ab.
-
Quinazolines/
-
("tyrosine kinase inhibitor$" or "protein kinase inhibitor$").ti,ab.
-
(erlotinib or CP358774 or CP‐358774 or OSI‐774 or Tarceva or Gefitnib or Iressa or ZD1839 or ZD‐1839 or Gefitero or Gefonib or Geftib or Geftilon or Geftinat).ti,ab.
-
("vascular endothelial growth factor$" adj2 inhibitor$).ti,ab.
-
Vascular Endothelial Growth Factors/ai [Antagonist & Inhibitors]
-
(Bevacizumab or Avastin or Genetech or Altuzan or R435 or R‐435 or rhuMAb‐VEGF or Vandetanib or Zactima or ZD6474 or ZD‐6474 or Caprelsa).ti,ab.
-
Protein kinase inhibitors/
-
("molecular multiple kinase inhibitor$" or Sorafenib or BAY439006 or BAY‐439006 or BAY5459085 or BAY545‐9085 or Nexavar or Sunitinib or SU11248 or SU‐11248 or Sutent or Lapatinib or GW572016 or GW282944X or Tykerb or Tyverb).ti,ab.
-
(trastuzumab or herceptin or lonafarnib or serasar or perifosine or afatinib).ti,ab.
-
Cyclooxygenase 2 Inhibitors/
-
(("COX 2" or COX‐2 or "cyclooxygenase 2") adj2 inhibitor$).ti,ab.
-
(celecoxib or SC58635 or SC‐58635 or celebrex or selekoksib or YM‐177 or Algybrex or celemax or cloxib or coxel or coxtenk or niflem or radicacine or tisorek or orsenal or solexa or celebra or aclarex or celact or celcib or celcox or celecap or celedol or celetop or celib or cobix or colcibra or coxib or eloxib or icel or orthocel or ultracele or artilog or zycel or aubrex or celcoxx or flogoxib or ranselex or reumoxib or cexb).ti,ab.
-
Cyclin dependent kinase inhibitor proteins/
-
((CDK or "cyclin dependent kinase") adj2 inhibitor$).ti,ab.
-
(Seliciclib or Roscovitine or CYC202 or CYC‐202 or Flavopiridol or HMR1275 or HMR‐1275 or L868275 or L868‐275 or Avodenib).ti,ab.
-
"Poly(ADP‐ribose) Polymerases"/ai
-
("poly ADP‐ribose polymerase" adj2 inhibitor$).ti,ab.
-
(PARP adj2 inhibitor$).ti,ab.
-
(Iniparib or 4‐ido‐3‐nitrobenzamide or BSI201 or BSI‐201 or Olaparib or AZD2281 or AZD‐2281).ti,ab.
-
((mTOR or "mammalian target of rapamycin") adj inhibitor$).ti,ab.
-
(Everolimus or RAD001 or RAD‐001 or Certican or SDZRAZ or "immunosuppressive agent$" or afinitor or votubia or sertican or zortress or temsirolimus or CCI779 or CCI‐779 or Torisel).ti,ab.
-
"proteasome inhibitor$".ti,ab.
-
(bortezomib or PS341 or PS‐341 or velcade or LDP341 or LDP‐341 or bortenat or mibor).ti,ab.
-
Histone Deacetylase Inhibitors/
-
("histone acetylation inhibitor$" or "histone deacetylase inhibitor$").ti,ab.
-
(Vorinostat or 18F‐SAHA or M344 or M‐344 or "suberanilohydroxamic acid" or "suberoylanilide hydroxamic acid" or zolinza or romidepsin or astella or FK228 or FK‐228 or FR901228 or FR‐901228 or isodax or depsipeptide).ti,ab.
-
("heat shock protein inhibitor$" or tanespimycin or IP1493 or IP‐1493 or IPI504 or IPI‐504 or retaspimycin or NSC330507 or NSC‐330507).ti,ab.
-
Immunotoxins/
-
(immunotoxin$ or affinotoxin$ or "antibody toxin conjugate$" or "antibody toxin hybrid$" or "chimeric toxin$" or "cytotoxin antibody conjugate$" or "monoclonal antibody toxin conjugate$" or "targeted toxin$" or "toxin carrier" or "toxin conjugate$" or "toxin antibody conjugate$" or "toxin antibody hybrid$").ti,ab.
-
((antisense adj strateg$) or (anti‐sense adj strateg$) or ("anti sense" adj strateg$)).ti,ab.
-
("ONYX 015" or "HF 10").ti,ab.
-
Oncolytic virotherapy/
-
((oncolytic adj5 adenovirus$) or "oncolytic virus$").ti,ab.
-
Cancer vaccines/
-
BCG vaccine/
-
(vaccine$ adj2 (cancer or neoplasm or tumour or tumor)).ti,ab.
-
(vaccine$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab.
-
or/8‐55
-
7 and 56
The above subject search was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised controlled trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011).
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 2. Cochrane Oral Health Group Trials Register search strategy
#1 ((cancer* or tumour* or neoplas* or malignan* or carcinoma* or metatas*)) AND ((oral or intra‐oral* or intraoral* or "intral oral*" or gingiva* or oropharyn* or mouth* or tongue* or cheek* or gum* or palatal* or palate* or "head and neck"))
#2 ((gene* or target* or DNA or immunotherap* or "immuno therap*"))
#3 ((cetuximab or Erbitux or C‐225 or C225 or "immunoglobulin G1" or panitumumab or ABX‐EGF* or Amgen or Vectibix or zalutumumab or 2F8 or HuMax‐EGFr or "anti‐human epidemal growth factor receptor*" or "EGFR inhibitor*" or "epidermal growth factor inhibitor*"))
#4 (("tyrosine kinase inhibitor*" or "protein kinase inhibitor*" or erlotinib or CP358774 or CP‐358774 or OSI‐774 or Tarceva or Gefitnib or Iressa or ZD1839 or ZD‐1839 or Gefitero or Gefonib or Geftib or Geftilon or Geftinat or "vascular endothelial growth factor*"))
#5 ((Bevacizumab or Avastin or Genetech or Altuzan or R435 or R‐435 or rhuMAb‐VEGF or Vandetanib or Zactima or ZD6474 or ZD‐6474 or Caprelsa))
#6 (("molecular multiple kinase inhibitor*" or Sorafenib or BAY4390006 or BAY‐4390006 or BAY5459085 or BAY545‐9085 or Nexavar or Sunitinib or SU11248 or SU‐11248 or Sutent or Lapatinib or GW572016 or GW282944X or Tykerb or Tyverb))
#7 ((trastuzumab or herceptin or Ionafarnib or serasar or perifosine or afatinib))
#8 (("COX 2"or COX‐2 or "cyclooxygenase 2"))
#9 ((celecoxib or SC58635 or SC‐58635 or celebrex or selekoksib or YM‐177 or Algybrex or celemax or cloxib or coxel or coxtenk or niflem or radicacine or tisorek or orsenal or solexa or celebra or aclarex or aclarex or celact or celcib or celcox or celecap or celedol or celetop or celib or cobix or colcibra or coxib or eloxib or icel or orthocel or ultracele or artilog or zycel or aubrex or celcoxx or flogoxib or ranselex or reumoxib or cexb))
#10 (("CDK inhibitor*" or "cyclin dependent kinase inhibitor*" or seliciclib or Roscovitine or CYC202 or CYC‐202 or Flavopiridol or HMR1275 or HMR‐1275 or L868275 or L868‐275 or Avodenib or "poly ADP‐ribose inhibitor*" or "PARP inhibitor*"))
#11 ((iniparib or 4‐ido‐3‐nitrobenzamide or BSI201 or BSI‐201 or Olaparib or AZD2281 or AZD‐2281 or mTOR or "mammalian target of rapamycin" or Everolimus or RAD001 or RAD‐001 or Certican or SDZRAZ or "immunosuppressive agent*" or afinitor or votubia or sertican or zortress or temsirolimus or CCI779 or CCI‐779 or Torisel or "proteasome inhibitor*" or bortezomib or PS341 or PS‐341 or velcade or LDP341 or LDP‐341 or bortenat or mibor))
#12 (("histone acetylation inhibitor*" or "histone deacetylase inhibitor*" or Vorinostat or 18F‐SAHA or M344 or M‐344 or "suberanilohydroxamic acid" or "suberoylanilide hydroxamic acid" or zolinza or romidepsin or astella or FK228 or FK‐228 or FR901228 or FR‐901228 or isodax or depsipeptide or "heat shock protein inhibitor*" or tanespimycin or IP1493 or IP‐1493 or IPI504 or IPI‐504 or retaspimycin or NSC330507 or NSC‐330507))
#13 ((immunotoxin* or affinotoxin* or "antibody toxin conjugate*" or "antibody toxin hybrid*" or "chimeric toxin*" or "cytotoxin antibody conjugate*" or "monoclonal antibody toxin conjugate*" or "targeted toxin*" or "toxin carrier" or "toxin conjugate*" or "toxin antibody conjugate*" or "toxin antibody hybrid*"))
#14 (("antisense strateg*" or "anti‐sense strateg*" or "anti sense stateg*"))
#15 (("ONYX 015" or "HF 10" or "oncolytic adenovirus*" or "oncolytic virus*"))
#16 vaccine* AND (cancer or neoplasm or tumour or tumor)
#17 vaccine* AND ((BCG or "Bacillus Calmette Guerin" or Calmette*))
#18 (#2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17)
#19 (#1 and #18)
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Head and Neck Neoplasms this term only
#2 MeSH descriptor Mouth Neoplasms this term only
#3 MeSH descriptor Gingival Neoplasms this term only
#4 MeSH descriptor Palatal Neoplasms this term only
#5 MeSH descriptor Tongue Neoplasms this term only
#6 ( (cancer* in Title, Abstract or Keywords or tumour* in Title, Abstract or Keywords or tumor* in Title, Abstract or Keywords or neoplas* in Title, Abstract or Keywords or malignan* in Title, Abstract or Keywords or carcinoma* in Title, Abstract or Keywords or metatasta* in Title, Abstract or Keywords) and (oral* in Title, Abstract or Keywords or intra‐oral* in Title, Abstract or Keywords or intraoral* in Title, Abstract or Keywords or "intra oral*" in Title, Abstract or Keywords or gingiva* in Title, Abstract or Keywords or oropharyn* in Title, Abstract or Keywords or mouth* in Title, Abstract or Keywords or tongue* in Title, Abstract or Keywords or cheek* in Title, Abstract or Keywords or gum* in Title, Abstract or Keywords or palatal* in Title, Abstract or Keywords or palate* in Title, Abstract or Keywords or "head and neck" in Title, Abstract or Keywords) )
#7 (#1 or #2 or #3 or #4 or #5 or #6)
#8 MeSH descriptor Gene therapy explode all trees
#9 MeSH descriptor Immunotherapy explode all trees
#10 MeSH descriptor Molecular targeted therapy this term only
#11 ( (gene* in Title, Abstract or Keywords near/6 therap* in Title, Abstract or Keywords) or (target* in Title, Abstract or Keywords near/6 therap* in Title, Abstract or Keywords) or (DNA in Title, Abstract or Keywords near/6 therap* in Title, Abstract or Keywords) )
#12 (immunotherap* in Title, Abstract or Keywords or "immuno therap" in Title, Abstract or Keywords)
#13 MeSH descriptor Antibodies, Monoclonal this term only
#14 (cetuximab in Title, Abstract or Keywords or Erbitux in Title, Abstract or Keywords or C‐225 in Title, Abstract or Keywords or C225 in Title, Abstract or Keywords or "immunoglobulin G1" in Title, Abstract or Keywords)
#15 (panitumumab in Title, Abstract or Keywords or ABX‐EGF* in Title, Abstract or Keywords or Amgen in Title, Abstract or Keywords or Vectibix in Title, Abstract or Keywords)
#16 (zalutumumab in Title, Abstract or Keywords or 2F8 in Title, Abstract or Keywords or HuMax‐EGFr in Title, Abstract or Keywords)
#17 "anti‐human epidermal growth factor receptor*" in Title, Abstract or Keywords
#18 ("EGFR inhibitor*" in Title, Abstract or Keywords or "epidermal growth factor inhibitor*" in Title, Abstract or Keywords)
#19 MeSH descriptor Quinazolines this term only
#20 ("tyrosine kinase inhibitor*" in Title, Abstract or Keywords or "protein kinase inhibitor*" in Title, Abstract or Keywords)
#21 (erlotinib in Title, Abstract or Keywords or CP358774 in Title, Abstract or Keywords or CP‐358774 in Title, Abstract or Keywords or OSI‐774 in Title, Abstract or Keywords or Tarceva in Title, Abstract or Keywords or Gefitnib in Title, Abstract or Keywords or Iressa in Title, Abstract or Keywords or ZD1839 in Title, Abstract or Keywords or ZD‐1839 in Title, Abstract or Keywords or Gefitero in Title, Abstract or Keywords or Gefonib in Title, Abstract or Keywords or Geftib in Title, Abstract or Keywords or Geftilon in Title, Abstract or Keywords or Geftinat in Title, Abstract or Keywords)
#22 ("vascular endothelial growth factor*" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords)
#23 MeSH descriptor Vascular Endothelial Growth Factors this term only
#24 (Bevacizumab in Title, Abstract or Keywords or Avastin in Title, Abstract or Keywords or Genetech in Title, Abstract or Keywords or Altuzan in Title, Abstract or Keywords or R435 in Title, Abstract or Keywords or R‐435 in Title, Abstract or Keywords or rhuMAb‐VEGF in Title, Abstract or Keywords or Vandetanib in Title, Abstract or Keywords or Zactima in Title, Abstract or Keywords or ZD6474 in Title, Abstract or Keywords or ZD‐6474 in Title, Abstract or Keywords or Caprelsa in Title, Abstract or Keywords)
#25 MeSH descriptor Protein kinase inhibitors this term only
#26 ("molecular multiple kinase inhibitor*" in Title, Abstract or Keywords or Sorafenib in Title, Abstract or Keywords or BAY439006 in Title, Abstract or Keywords or BAY‐439006 in Title, Abstract or Keywords or BAY5459085 in Title, Abstract or Keywords or BAY545‐9085 in Title, Abstract or Keywords or Nexavar in Title, Abstract or Keywords or Sunitinib in Title, Abstract or Keywords or SU11248 in Title, Abstract or Keywords or SU‐11248 in Title, Abstract or Keywords or Sutent in Title, Abstract or Keywords or Lapatinib in Title, Abstract or Keywords or GW572016 in Title, Abstract or Keywords or GW282944X in Title, Abstract or Keywords or Tykerb in Title, Abstract or Keywords or Tyverb in Title, Abstract or Keywords)
#27 (trastuzumab in Title, Abstract or Keywords or herceptin in Title, Abstract or Keywords or lonafarnib in Title, Abstract or Keywords or serasar in Title, Abstract or Keywords or perifosine in Title, Abstract or Keywords or afatinib in Title, Abstract or Keywords)
#28 MeSH descriptor Cyclooxygenase 2 Inhibitors this term only
#29 ( ("COX 2" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) or (COX‐2 in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) or ("cyclooxygenase 2" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) )
#30 (celecoxib in Title, Abstract or Keywords or SC58635 in Title, Abstract or Keywords or SC‐58635 in Title, Abstract or Keywords or celebrex in Title, Abstract or Keywords or selekoksib in Title, Abstract or Keywords or YM‐177 in Title, Abstract or Keywords or Algybrex in Title, Abstract or Keywords or celemax in Title, Abstract or Keywords or cloxib in Title, Abstract or Keywords or coxel in Title, Abstract or Keywords or coxtenk in Title, Abstract or Keywords or niflem in Title, Abstract or Keywords or radicacine in Title, Abstract or Keywords or tisorek in Title, Abstract or Keywords or orsenal in Title, Abstract or Keywords or solexa in Title, Abstract or Keywords or celebra in Title, Abstract or Keywords or aclarex in Title, Abstract or Keywords or celact in Title, Abstract or Keywords or celcib in Title, Abstract or Keywords or celcox in Title, Abstract or Keywords or celecap in Title, Abstract or Keywords or celedol in Title, Abstract or Keywords or celetop in Title, Abstract or Keywords or celib in Title, Abstract or Keywords or cobix in Title, Abstract or Keywords or colcibra in Title, Abstract or Keywords or coxib in Title, Abstract or Keywords or eloxib in Title, Abstract or Keywords or icel in Title, Abstract or Keywords or orthocel in Title, Abstract or Keywords or ultracele in Title, Abstract or Keywords or artilog in Title, Abstract or Keywords or zycel in Title, Abstract or Keywords or aubrex in Title, Abstract or Keywords or celcoxx in Title, Abstract or Keywords or flogoxib in Title, Abstract or Keywords or ranselex in Title, Abstract or Keywords or reumoxib in Title, Abstract or Keywords or cexb in Title, Abstract or Keywords)
#31 MeSH descriptor Cyclin‐Dependent Kinase Inhibitor Proteins this term only
#32 ( (CDK in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) or ("cyclin dependent kinase" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) )
#33 (Seliciclib in Title, Abstract or Keywords or Roscovitine in Title, Abstract or Keywords or CYC202 in Title, Abstract or Keywords or CYC‐202 in Title, Abstract or Keywords or Flavopiridol in Title, Abstract or Keywords or HMR1275 in Title, Abstract or Keywords or HMR‐1275 in Title, Abstract or Keywords or L868275 in Title, Abstract or Keywords or L868‐275 in Title, Abstract or Keywords or Avodenib in Title, Abstract or Keywords)
#34 MeSH descriptor Poly(ADP‐ribose) Polymerases this term only
#35 ("poly ADP‐ribose polymerase" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords)
#36 (PARP in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords)
#37 (Iniparib in Title, Abstract or Keywords or 4‐ido‐3‐nitrobenzamide in Title, Abstract or Keywords or BSI201 in Title, Abstract or Keywords or BSI‐201 in Title, Abstract or Keywords or Olaparib in Title, Abstract or Keywords or AZD2281 in Title, Abstract or Keywords or AZD‐2281 in Title, Abstract or Keywords)
#38 ( (mTOR in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) or ("mammalian target of rapamycin" in Title, Abstract or Keywords near/2 inhibitor* in Title, Abstract or Keywords) )
#39 (Everolimus in Title, Abstract or Keywords or RAD001 in Title, Abstract or Keywords or RAD‐001 in Title, Abstract or Keywords or Certican in Title, Abstract or Keywords or SDZRAZ in Title, Abstract or Keywords or "immunosuppressive agent*" in Title, Abstract or Keywords or afinitor in Title, Abstract or Keywords or votubia in Title, Abstract or Keywords or sertican in Title, Abstract or Keywords or zortress in Title, Abstract or Keywords or temsirolimus in Title, Abstract or Keywords or CCI779 in Title, Abstract or Keywords or CCI‐779 in Title, Abstract or Keywords or Torisel in Title, Abstract or Keywords)
#40 "proteasome inhibitor*" in Title, Abstract or Keywords
#41 (bortezomib in Title, Abstract or Keywords or PS341 in Title, Abstract or Keywords or PS‐341 in Title, Abstract or Keywords or velcade in Title, Abstract or Keywords or LDP341 in Title, Abstract or Keywords or LDP‐341 in Title, Abstract or Keywords or bortenat in Title, Abstract or Keywords or mibor in Title, Abstract or Keywords)
#42 MeSH descriptor Histone Deacetylase Inhibitors this term only
#43 ("histone acetylation inhibitor*" in Title, Abstract or Keywords or "histone deacetylase inhibitor*" in Title, Abstract or Keywords)
#44 (Vorinostat in Title, Abstract or Keywords or 18F‐SAHA in Title, Abstract or Keywords or M344 in Title, Abstract or Keywords or M‐344 in Title, Abstract or Keywords or "suberanilohydroxamic acid" in Title, Abstract or Keywords or "suberoylanilide hydroxamic acid" in Title, Abstract or Keywords or zolinza in Title, Abstract or Keywords or romidepsin in Title, Abstract or Keywords or astella in Title, Abstract or Keywords or FK228 in Title, Abstract or Keywords or FK‐228 in Title, Abstract or Keywords or FR901228 in Title, Abstract or Keywords or FR‐901228 in Title, Abstract or Keywords or isodax in Title, Abstract or Keywords or depsipeptide in Title, Abstract or Keywords)
#45 ("heat shock protein inhibitor*" in Title, Abstract or Keywords or tanespimycin in Title, Abstract or Keywords or IP1493 in Title, Abstract or Keywords or IP‐1493 in Title, Abstract or Keywords or IPI504 in Title, Abstract or Keywords or IPI‐504 in Title, Abstract or Keywords or retaspimycin in Title, Abstract or Keywords or NSC330507 in Title, Abstract or Keywords or NSC‐330507 in Title, Abstract or Keywords)
#46 MeSH descriptor Immunotoxins this term only
#47 (immunotoxin* in Title, Abstract or Keywords or affinotoxin* in Title, Abstract or Keywords or "antibody toxin conjugate*" in Title, Abstract or Keywords or "antibody toxin hybrid*" in Title, Abstract or Keywords or "chimeric toxin*" in Title, Abstract or Keywords or "cytotoxin antibody conjugate*" in Title, Abstract or Keywords or "monoclonal antibody toxin conjugate*" in Title, Abstract or Keywords or "targeted toxin*" in Title, Abstract or Keywords or "toxin carrier" in Title, Abstract or Keywords or "toxin conjugate*" in Title, Abstract or Keywords or "toxin antibody conjugate*" in Title, Abstract or Keywords or "toxin antibody hybrid*" in Title, Abstract or Keywords)
#48 ( (antisense in Title, Abstract or Keywords near/2 strateg* in Title, Abstract or Keywords) or (anti‐sense in Title, Abstract or Keywords near/2 strateg* in Title, Abstract or Keywords) or ("anti sense" in Title, Abstract or Keywords near/2 strateg* in Title, Abstract or Keywords) )
#49 ("ONYX 015" in Title, Abstract or Keywords or "HF 10" in Title, Abstract or Keywords)
#50 MeSH descriptor Oncolytic virotherapy this term only
#51 ( (oncolytic in Title, Abstract or Keywords near/5 adenovirus* in Title, Abstract or Keywords) or (oncolytic in Title, Abstract or Keywords near/2 virus* in Title, Abstract or Keywords) )
#52 MeSH descriptor Cancer vaccines this term only
#53 MeSH descriptor BCG vaccine this term only
#54 (vaccine* in Title, Abstract or Keywords and (cancer* in Title, Abstract or Keywords or neoplas* in Title, Abstract or Keywords or tumour* in Title, Abstract or Keywords or tumor* in Title, Abstract or Keywords) )
#55 (vaccine* in Title, Abstract or Keywords and (BCG in Title, Abstract or Keywords or "Bacillus Calmette Guerin" in Title, Abstract or Keywords or Calmette* in Title, Abstract or Keywords) )
#56 (#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55)
#57 (#7 and #56)
Appendix 4. EMBASE (Ovid) search strategy
1. "Head and Neck tumor"/
2. "Mouth tumor"/
3. "Gingiva tumor"/
4. "Jaw tumor"/
5. "Tongue tumor"/
6. ((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).ti,ab.
7. or/1‐6
8. exp Gene therapy/
9. exp Immunotherapy/
10. Molecularly targeted therapy/
11. ((gene$ or target$ or DNA) adj6 therap$).ti,ab.
12. (immunotherap$ or "immuno therap").ti,ab.
13. Monoclonal antibody/
14. (cetuximab or Erbitux or C‐225 or C225 or "immunoglobulin G1").ti,ab.
15. (panitumumab or ABX‐EGF$ or Amgen or Vectibix).ti,ab.
16. (zalutumumab or 2F8 or HuMax‐EGFr).ti,ab.
17. "anti‐human epidermal growth factor receptor".ti,ab.
18. ("EGFR inhibitor$" or "epidermal growth factor inhibitor$").ti,ab.
19. "Quinazoline derivative"/
20. ("tyrosine kinase inhibitor$" or "protein kinase inhibitor$").ti,ab.
21. (erlotinib or CP358774 or CP‐358774 or OSI‐774 or Tarceva or Gefitnib or Iressa or ZD1839 or ZD‐1839 or Gefitero or Gefonib or Geftib or Geftilon or Geftinat).ti,ab.
22. ("vascular endothelial growth factor$" adj2 inhibitor$).ti,ab.
23. (Bevacizumab or Avastin or Genetech or Altuzan or R435 or R‐435 or rhuMAb‐VEGF or Vandetanib or Zactima or ZD6474 or ZD‐6474 or Caprelsa).ti,ab.
24. Protein kinase inhibitor/
25. ("molecular multiple kinase inhibitor$" or Sorafenib or BAY439006 or BAY‐439006 or BAY5459085 or BAY545‐9085 or Nexavar or Sunitinib or SU11248 or SU‐11248 or Sutent or Lapatinib or GW572016 or GW282944X or Tykerb or Tyverb).ti,ab.
26. (trastuzumab or herceptin or lonafarnib or serasar or perifosine or afatinib).ti,ab.
27. Cyclooxygenase 2 Inhibitor/
28. (("COX 2" or COX‐2 or "cyclooxygenase 2") adj2 inhibitor$).ti,ab.
29. (celecoxib or SC58635 or SC‐58635 or celebrex or selekoksib or YM‐177 or Algybrex or celemax or cloxib or coxel or coxtenk or niflem or radicacine or tisorek or orsenal or solexa or celebra or aclarex or celact or celcib or celcox or celecap or celedol or celetop or celib or cobix or colcibra or coxib or eloxib or icel or orthocel or ultracele or artilog or zycel or aubrex or celcoxx or flogoxib or ranselex or reumoxib or cexb).ti,ab.
30. Cyclin dependent kinase inhibitor/
31. ((CDK or "cyclin dependent kinase") adj2 inhibitor$).ti,ab.
32. (Seliciclib or Roscovitine or CYC202 or CYC‐202 or Flavopiridol or HMR1275 or HMR‐1275 or L868275 or L868‐275 or Avodenib).ti,ab.
33. ("poly ADP‐ribose polymerase" adj2 inhibitor$).ti,ab.
34. (PARP adj2 inhibitor$).ti,ab.
35. (Iniparib or 4‐ido‐3‐nitrobenzamide or BSI201 or BSI‐201 or Olaparib or AZD2281 or AZD‐2281).ti,ab.
36. ((mTOR or "mammalian target of rapamycin") adj inhibitor$).ti,ab.
37. (Everolimus or RAD001 or RAD‐001 or Certican or SDZRAZ or "immunosuppressive agent$" or afinitor or votubia or sertican or zortress or temsirolimus or CCI779 or CCI‐779 or Torisel).ti,ab.
38. "proteasome inhibitor$".ti,ab.
39. (bortezomib or PS341 or PS‐341 or velcade or LDP341 or LDP‐341 or bortenat or mibor).ti,ab.
40. Histone Deacetylase Inhibitor/
41. ("histone acetylation inhibitor$" or "histone deacetylase inhibitor$").ti,ab.
42. (Vorinostat or 18F‐SAHA or M344 or M‐344 or "suberanilohydroxamic acid" or "suberoylanilide hydroxamic acid" or zolinza or romidepsin or astella or FK228 or FK‐228 or FR901228 or FR‐901228 or isodax or depsipeptide).ti,ab.
43. ("heat shock protein inhibitor$" or tanespimycin or IP1493 or IP‐1493 or IPI504 or IPI‐504 or retaspimycin or NSC330507 or NSC‐330507).ti,ab.
44. Immunotoxin/
45. (immunotoxin$ or affinotoxin$ or "antibody toxin conjugate$" or "antibody toxin hybrid$" or "chimeric toxin$" or "cytotoxin antibody conjugate$" or "monoclonal antibody toxin conjugate$" or "targeted toxin$" or "toxin carrier" or "toxin conjugate$" or "toxin antibody conjugate$" or "toxin antibody hybrid$").ti,ab.
46. ((antisense adj strateg$) or (anti‐sense adj strateg$) or ("anti sense" adj strateg$)).ti,ab.
47. ("ONYX 015" or "HF 10").ti,ab.
48. Oncolytic virotherapy/
49. ((oncolytic adj5 adenovirus$) or "oncolytic virus$").ti,ab.
50. Cancer vaccine/
51. BCG vaccine/
52. (vaccine$ adj2 (cancer or neoplasm or tumour or tumor)).ti,ab.
53. (vaccine$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab.
54. or/8‐53
55. 7 and 54
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via Ovid:
1. random$.ti,ab.
2. factorial$.ti,ab.
3. (crossover$ or cross over$ or cross‐over$).ti,ab.
4. placebo$.ti,ab.
5. (doubl$ adj blind$).ti,ab.
6. (singl$ adj blind$).ti,ab.
7. assign$.ti,ab.
8. allocat$.ti,ab.
9. volunteer$.ti,ab.
10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. RANDOMIZED CONTROLLED TRIAL.sh.
13. SINGLE BLIND PROCEDURE.sh.
14. or/1‐13
15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
16. 14 NOT 15
Appendix 5. ClinicalTrials.gov search strategy
"oral cancer" and immunotherapy
"head and neck cancer" and immunotherapy
"oral cancer" and "targeted therapy"
"head and neck cancer" and "targeted therapy"
Appendix 6. WHO International Clinical Trials Registry Platform search strategy
"oral cancer" and immunotherapy
"head and neck cancer" and immunotherapy
"oral cancer" and "targeted therapy"
"head and neck cancer" and "targeted therapy"
Appendix 7. American Society of Clinical Oncology conference abstracts search strategy
"oral cancer" immunotherapy
"oral cancer" "targeted therapy"
"head and neck cancer" immunotherapy
"head and neck cancer" "targeted therapy"
Appendix 8. Radiation Therapy Oncology Group clinical trials protocols search strategy
immunotherapy
targeted therapy
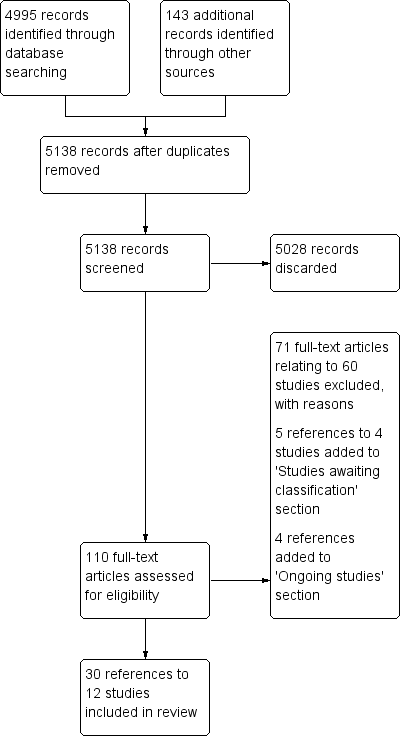
Review flow diagram
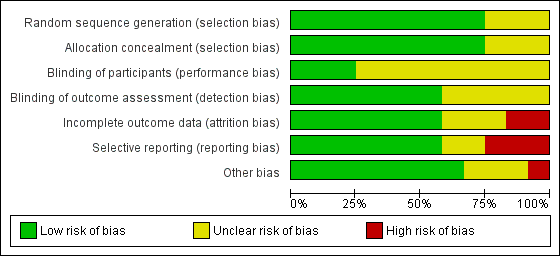
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Monoclonal antibodies (mAb), Outcome 1 Overall survival (5 years).
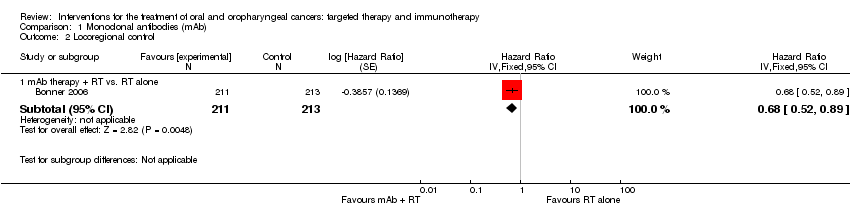
Comparison 1 Monoclonal antibodies (mAb), Outcome 2 Locoregional control.

Comparison 1 Monoclonal antibodies (mAb), Outcome 3 Progression‐free survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 1 Overall survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 2 Locoregional control.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 3 Disease‐free survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 4 Progression‐free survival.

Comparison 3 Immunotherapy, Outcome 1 Overall survival.

Comparison 3 Immunotherapy, Outcome 2 Disease‐free survival.
| Monoclonal antibodies plus standard therapy versus standard therapy alone for the treatment of people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: RT or CRT alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk mAb | |||||
| Overall survival | Low‐risk population* | HR 0.82 | 1421 | ⊕⊕⊕⊝ moderate1 | There was an 18% reduction in death (during the follow‐up period) in the participants treated with EGFR mAb therapies in addition to standard therapies | |
| 160 per 1000 | 133 per 1000 (113 to 156) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 347 per 1000 (301 to 396) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 577 per 1000 (515 to 639) | |||||
| Locoregional control | Low‐risk population* | HR 0.68 | 424 | ⊕⊕⊕⊝ | There was a 32% reduction in recurrence of cancer (during the follow‐up period) in the participants treated with EGFR mAb therapies (cetuximab) in addition to standard therapies | |
| 160 per 1000 | 112 per 1000 (87 to 144) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 297 per 1000 (237 to 370) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 510 per 1000 (421 to 607) | |||||
| Progression‐free survival | We formed 2 subgroups: mAb therapy + RT versus RT alone and mAb therapy + CRT versus CRT alone. There was a significant difference between these subgroups (P value = 0.008; I2 = 86%) and as a result we were unable to pool the data. The subgroup comparing mAb therapy + RT versus RT alone reported a 30% reduction in the number of people whose disease progresses if treated with EGFR mAb in addition to RT (HR 0.70; 95% CI 0.54 to 0.91; P value = 0.006). However, the subgroup comparing mAb therapy + CRT versus CRT alone reported no evidence of a difference in progression‐free survival (HR 1.08; 95% CI 0.89 to 1.32; P value = 0.76) | |||||
| Adverse effects | A subgroup estimate shows evidence of an increase in skin toxicity/acneiform rash (all grades of adverse effects: RR 6.56, 95% CI 5.35 to 8.03; 1311 participants, 2 studies; adverse effects grades ≥ 3: RR 17.72, 95% CI 8.33 to 37.73; 1403 participants, 3 studies) in people treated with cetuximab in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; EGFR: epidermal growth factor receptor; mAb: monoclonal antibody; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once for reporting bias (potential publication bias and poor reporting of EGFR and biopsy data in one study). | ||||||
| Tyrosine kinase inhibitors in addition to standard treatments for people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (either RT or CRT alone) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk Tyrosine kinase inhibitors | |||||
| Overall survival | Low‐risk population* | HR 0.99 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in participant survival in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 159 per 1000 (102 to 239) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 402 per 1000 (275 to 557) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 646 per 1000 (478 to 808) | |||||
| Locoregional control | Low‐risk population* | HR 0.89 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in locoregional control in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 144 per 1000 (88 to 229) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 370 per 1000 (241 to 539) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 607 per 1000 (429 to 791) | |||||
| Disease‐free Survival | Low‐risk population* | HR 1.51 | 60 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants survived without signs or symptoms of oral cavity cancer when treated with tyrosine kinase inhibitors (gefitinib) in addition to standard therapies | |
| 160 per 1000 | 231 per 1000 (101 to 476) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 543 per 1000 (271 to 854) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 795 per 1000 (473 to 980) | |||||
| Progression‐free Survival | Low‐risk population* | HR 0.8 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants stayed alive with stable disease when treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 130 per 1000 (85 to 200) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 340 per 1000 (233 to 486) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 568 per 1000 (415 to 739) | |||||
| Adverse effects | A subgroup estimate showed evidence of an increase in gastrointestinal complaints (all grades of adverse effects: RR 15.53, 95% CI 2.18 to 110.55; 67 participants, 1 study) in people treated with lapatinib in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once due reporting bias (data from a large study (Gregoire 2011) was not available). 2 Downgraded once due to imprecision 3 Downgraded once due to unclear risk of bias across multiple domains. 4 Downgraded once for applicability ‐ only participants receiving RT as a standard therapy were included in this subgroup (no CRT). | ||||||
| Recombinant Interleukin (rIL‐2) in addition to surgery for the treatment of people with oral and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (surgery) alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk rIL‐2 | |||||
| Overall survival | Low‐risk population* | HR 0.52 (0.31 to 0.87) | 201 | ⊕⊝⊝⊝ | There was an 48% reduction in death in the groups treated with rIL‐2 in addition to standard therapy | |
| 160 per 1000 | 87 per 1000 | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 237 per 1000 (149 to 363) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 421 per 1000 (278 to 599) | |||||
| Disease‐free survival | Low‐risk population* | HR 0.66 | 201 | ⊕⊝⊝⊝ | There is no evidence of a difference in the length of time that participants survived without signs or symptoms of cancer when treated with rIL‐2 in addition to standard therapies | |
| 160 per 1000 | 109 per 1000 (72 to 163) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 290 per 1000 (200 to 411) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 500 per 1000 (363 to 657) | |||||
| Adverse effects | There was no evidence of an increase or reduction of nausea/vomiting, stomatitis or leukopenia in participants treated with rIL‐2 in addition to standard therapy (1 study, 30 participants) | |||||
| CI: confidence interval; HR: hazard ratio; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010) 1 Downgraded twice due to high/unclear risk of bias across domains. | ||||||
| Inclusion criteria | Exclusion criteria | |
| Location of cancer | People with primary cancer of the oral cavity (ICD‐O: C01‐06) or oropharynx (ICD‐O: C09‐10 ‐ includes tonsil) | Lip (ICD‐O: C00) cancers |
| Type of cancer | Squamous cell carcinoma | Parotid gland (ICD‐O: C07), unspecified major salivary gland (ICD‐O: C08) |
| ICD‐O: International Classification of Diseases for Oncology. | ||
| ITT analysis | Docetaxel + cisplatin + cetuximab (n = 48) | Docetaxel + cisplatin (n = 44) |
| OS (3 years) | 88% (n = 42) | 74% (n = 33) |
| PFS (3 years) | 70% (n = 34) | 56% (n = 25) |
| ITT: intention to treat; n: number of participants; OS: overall survival; PFS: progression‐free survival. | ||
| Outcome | Grade | No. of studies | No. of patients | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 1.6 Mucositis | All grades | 3 | 1417 | Not estimable due to high heterogeneity (P value = 0.0009; I2 = 86%) between subgroups (cetuximab; nimotuzumab), and within the cetuximab subgroup (P value = 0.0002) I2 = 93%) | |
| Grades ≥ 3 | 4 | 1495 | 1.20 (0.96 to 1.51) (random effects) P value = 0.12 | P value = 0.07; I2 = 58% | |
| 1.7 Dysphagia | All grades | 3 | 1403 | 0.97 (0.92 to 1.03) (fixed effect) P value = 0.37 | P value = 0.60; I2 = 0% |
| Grades ≥ 3 | 3 | 1403 | 0.93 (0.83 to 1.04) (fixed effect) P value = 0.19 | P value = 0.26; I2 = 26% | |
| 1.8 Xerostomia | All grades | 3 | 1417 | 0.97 (0.91 to 1.04) (fixed effect) P value = 0.46 | P value = 0.61; I2 = 0% |
| Grades ≥ 3 | 2 | 1311 | 1.36 (0.80 to 2.31) (fixed effect) P value = 0.25 | P value = 0.60; I2 = 0% | |
| 1.9 Skin toxicity/ acneiform rash | All grades | 3 | 1403 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 99.3%) | |
| 2 (cetuximab) | 1311 | 6.56 (5.35 to 8.03) (fixed effect) P value < 0.00001 | P value = 0.080; I2 = 66% | ||
| 1 (nimotuzumab) | 92 | 1.06 (0.85 to 1.31) P value = 0.61 | Not applicable | ||
| Grades ≥ 3 | 4 | 1495 | Not estimable due to significant difference between subgroups (P value = 0.005; I2 = 87.5%) | ||
| 3 (cetuximab) | 1403 | 17.72 (8.33 to 37.73) (fixed effect) P value < 0.00001 | P value = 0.56; I2 = 0% | ||
| 1 (nimotuzumab) | 92 | 0.20 (0.01 to 4.05) P value = 0.29 | Not applicable | ||
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 2.5 Mucositis | All grades | 2 | 286 | 1.03 (0.94 to 1.11) (fixed effect) P value = 0.55 | P value = 0.44; I2 = 0% |
| Grades ≥ 3 | 2 | 286 | 1.22 (0.96 to 1.56) (fixed effect) P value = 0.10 | P value = 0.53; I2 = 0% | |
| 2.6 Skin toxicity | All grades | 4 | 544 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 95.5%) | |
| 2 (gefitinib) | 286 | 1.03 (0.82 to 1.28) (fixed effect) P value = 0.82 | P value = 0.42; I2 = 0% | ||
| 1 (lapatinib) | 67 | 2.02 (1.23 to 3.32) P value = 0.005 | Not applicable | ||
| 1 (erlotinib) | 191 | 6.57 (3.60 to 12.00) P value < 0.00001 | Not applicable | ||
| Grades ≥ 3 | 4 | 544 | 1.25 (0.54 to 2.88) (random effects) P value = 0.61 | P value = 0.09; I2 = 54% | |
| 2.7 Gastrointestinal | All grades | 2 | 293 | Not estimable due to significant difference between subgroups (P value = 0.007; I2 = 86.2%) | |
| 1 (gefitinib) | 226 | 1.04 (0.98 to 1.11) P value = 0.18 | Not applicable | ||
| 1 (lapatinib) | 67 | 15.53 (2.18 to 110.55) P value = 0.006 | Not applicable | ||
| Grades ≥ 3 | 3 | 484 | 1.11 (0.83 to 1.49) (fixed effect) P value = 0.47 | P value = 0.53; I2 = 0% | |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | BCG‐cell wall preparation + surgery | Surgery alone |
| Overall survival (3 years) | 10/10 | 9/10 |
| Recurrence (3 years) | 3/10 | 4/10 |
| BCG: Bacillus Calmette‐Guérin. | ||
| Outcome | Neoadjuvant chemotherapy (n = 17) | Neoadjuvant chemotherapy + rIL‐2 (n = 16) |
| Locoregional control Complete response | 3/15 | 4/13 |
| Locoregional control Partial response | 9/15 | 6/13 |
| rIL‐2: recombinant interleukin. | ||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 3.3 Nausea/vomiting | All grades | 1 | 30 | 1.24 (0.90 to 1.70) P value = 0.19 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 3.40 (0.15 to 77.34) P value = 0.44 | Not applicable | |
| 3.4 Stomatitis | All grades | 1 | 30 | 1.47 (0.75 to 2.90) P value = 0.27 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 1.71 (0.33 to 8.83) P value = 0.52 | Not applicable | |
| 3.5 Leukopenia | All grades | 1 | 30 | 0.57 (0.22 to 1.50) P value = 0.25 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 0.38 (0.04 to 3.26) P value = 0.38 | Not applicable | |
| 3.6 Increased temperature | All grades | 1 | 24 | 0.67 (0.13 to 3.30) P value = 0.62 | Not applicable |
| 3.7 Moderate‐severe chills | All grades | 1 | 24 | 11.00 (0.67 to 179.29) P value = 0.09 | Not applicable |
| 3.8 Gastrointestinal | All grades | 1 | 24 | 5.00 (0.27 to 94.34) P value = 0.28 | Not applicable |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (5 years) Show forest plot | 3 | 1421 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 1.1 mAb therapy + radiotherapy (RT) vs. RT alone | 2 | 530 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 1.2 mAb therapy + chemoradiotherapy (CRT) vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 2 Locoregional control Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 3 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 3.2 mAb therapy + CRT vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 1.1 TKI + chemoradiotherapy (CRT) vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 2 Locoregional control Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 2.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 3 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 TKI + radiotherapy (RT) vs. RT alone | 1 | 60 | Hazard Ratio (Fixed, 95% CI) | 1.51 [0.61, 3.71] |
| 4 Progression‐free survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| 4.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Surgery ± radiotherapy (RT) + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 2 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Surgery ± RT + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.43, 1.02] |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||

