Tratamiento de heridas con presión negativa para el tratamiento de las heridas del pie en los pacientes con diabetes mellitus
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 2‐arm RCT undertaken in the USA (in wound and academic centres) | |
| Participants | 162 adults Inclusion criteria: presence of: wound from a diabetic foot amputation to the transmetatarsal level of the foot; adequate perfusion; University of Texas grade 2 or 3 Exclusion criteria: people presenting with: active Charcot arthropathy of the foot, wounds resulting from burns, venous insufficiency, untreated cellulitis or osteomyelitis (after amputation), collagen vascular disease, malignant disease in the wound; or people treated with: corticosteroids, immunosuppressive drugs or chemotherapy, NPWT (in the last 30 days), growth factors, normothermic therapy; hyperbaric medicine, bioengineered tissue products (in the last 30 days) Key baselines covariates: Wound area (cm²): Group A: 22.3 (SD 23.4) Group B: 19.2 (SD 17.6) Wound duration (months): Group A: 1.2 (SD 3.9) Group B: 1.8 (SD 5.9) 75.3% of the study population had wounds that were < 30 days' duration (classed as acute wounds by the author) and 24.7% had wounds that were > 30 days' duration (classed as chronic wounds by authors). | |
| Interventions | Group A (n = 77): NPWT (V.A.C. system). No information provided regarding the pressure applied or the cycle (e.g. constant/cyclical etc); dressing changes every 48 h. Treatment conducted until wound closure or completion of 112 day assessment. Group B (n = 85): moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings – adhering to standardised guidelines at the discretion of attending clinician. Dressings changed every other day unless recommended by treating clinician. All participants received: off‐loading therapy, preventatively and therapeutically as indicated – a pressure relief sandal or walker was provided for all participants; sharp debridement within 2 days of randomisation and as deemed necessary by treating clinician; and measurement of prealbumin, albumin and glycosylated haemoglobin levels in 7 days before entering the study. Low pre study albumin levels resulted in consultation with nutritionist, and dietary supplement initiated if needed. | |
| Outcomes | Primary review outcomes: number of wounds completely healed (defined as 100% re‐epithelialisation without drainage and INCLUDED closure via surgery where the decision for surgical closure was made by treating clinician); time to wound healing; amputation Secondary review outcomes: other adverse events (serious and non‐serious); resource use | |
| Notes | Follow‐up: 112 days (16 weeks) Outcome assessment: based on data from wound assessments and digital photographs taken by treatment clinicians at days 0, 7, 14, 28, 42, 56, 84 and 112 A secondary analysis of trial data reported that 75% of wounds were ≤ 1 month in duration (classed by authors as acute) and 25% were > 1 month in duration (classed by authors as chronic). We noted that mean baseline values for ulcer duration were obviously very skewed. Funding: study funded by KCI – manufacturers of the V.A.C. intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was accomplished by using www.randomizer.org to generate 15 blocks of 10 random numbers each." Comment: adequate methodology |
| Allocation concealment (selection bias) | Low risk | Quote: "numbers were systematically assigned to each treatment group, and sealed envelopes containing opaque, black paper labelled with assigned treatment and patient ID number were sequentially numbered and provided to each site. The black paper was added to ensure that the contents of the envelopes were not visible prior to opening." Comment: adequate methodology |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the decision for surgical closure of amputation wounds was decided individually by the physician investigator." Comment: it is understandably not possible to blind participants or investigators to whether or not they received NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about closure surgery that could then have resulted in more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "neither patients nor investigators were masked to the randomised treatment assignment… However, notes that the masking component of the study dealt specifically with planimetry measurements from digital photographs … concordance between the investigator and the digital planimetry provided independent confirmation of the primary efficacy endpoint of complete wound healing." Comment: assessment of healing seems to have had a blinded component |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Unclear risk | Potential funding bias; no evidence of other bias |
| Methods | 2‐arm RCT undertaken in the USA | |
| Participants | 342 adults; 341 randomised; ITT 335 Inclusion criteria: stage 2 or 3 (Wagner’s scale) calcaneal, dorsal or planter foot ulcer; ulcer ≥ 2 cm² in area after debridement; adequate blood perfusion (various tests and cut‐offs reported) Exclusion criteria: recognised active Charcot disease; ulcers resulting from electrical, chemical or radiation burns; collagen vascular disease; ulcer malignancy; untreated osteomyelitis or cellulitis; uncontrolled hyperglycaemia; inadequate lower extremity perfusion; pregnant or nursing mothers; or ulcer treatment within 30 days of trial start with normothermic or hyperbaric oxygen therapy, corticosteroids, immunosuppressive drugs, chemotherapy, recombinant or autologous growth factor products, skin and dermal substitutes; or use of any enzymic debridement treatment. Key baselines covariates: Wound area (cm²): Group A: 13.5 (SD 18.2) Group B: 11.0 (SD 12.7) Wound duration (months) Group A: 6.6 (SD 10.8) Group B: 6.9 (SD 12.2) | |
| Interventions | Group A (n = 172): NPWT (V.A.C. system) applied according to manufacturer’s instructions, but no information provided about the pressure applied or the cycle (e.g. constant/cyclical, etc.). Treatment continued until wound closure, or until there was sufficient granulation tissue formation for healing by primary and secondary intention. Group B (n = 169): advanced moist wound therapy dressings used according to guidelines/local protocols – noted as being predominantly hydrogels and alginates. All participants received: assessment and debridement of ulcers within 2 days of randomisation; off‐loading therapy as deemed necessary | |
| Outcomes | Primary review outcomes: number of wounds completely healed (defined as 100% re‐epithelialisation without drainage or dressing requirement and INCLUDED closure via surgery where the decision for surgical closure was made by treating clinician); time to wound healing; amputation Secondary review outcomes: other adverse events (serious and non‐serious) | |
| Notes | Follow‐up: 112 days (16 weeks) Outcome assessment: participants examined weekly for the first 4 weeks and then every other day until day 112, or ulcer closure by any means. Participants achieving closure were followed up at 3 and 9 months Funding: study funded by KCI – manufacturers of the V.A.C. intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was accomplished by generating blocks of numbers through http://www.randomizer.org." Comment: adequate methodology |
| Allocation concealment (selection bias) | Low risk | Quote: "numbers were assigned to a treatment group and sealed in opaque envelopes containing black paper labelled with treatment and patient ID. Envelopes were sequentially numbered before clinical trial site distribution. At patient randomisation, treatment was assigned on the basis of the next sequentially labelled envelope." Comment: adequate methodology |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We note that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "blinded photographic evaluation was conducted." Comment: while the main report has no discussion of blinded outcome assessment, it is mentioned in the conference abstract describing the study. However as with Armstrong 2005, we noted that unblinded health professionals in 1 group were able to make decisions about undertaking closure surgery that could then have resulted more wounds being closed (and classed as healed) or amputated. As a result of this, we classed the risk of bias for this domain as unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 3 participants were excluded from analysis in each arm as they did not receive the trial treatment allocated. There were relatively low numbers of exclusions, although ideally data on these participants would have been included in the RCT report. Additionally, 31% of participants in the NPWT group and 25% in the dressing group were classed as being 'discontinued' for reasons that included adverse events, ineffective treatment and death. It is not clear whether participants who were discontinued for reasons other than death were also censored from the analysis, rather than being followed up. If discontinuation did result in censoring in this open trial it may have introduced bias. |
| Other bias | Unclear risk | Potential funding bias; no evidence of other bias |
| Methods | 2‐arm RCT undertaken in Italy | |
| Participants | 130 adults. Inclusion criteria: people presenting with infected open amputations or surgical dehiscence of minor amputations of level II‐III A‐B according to the University of Texas Diabetic Wound Classification Exclusion criteria: people with bleeding wounds or untreated osteomyelitis. In those cases of recent debridement of the wound a minimum 24‐h period was awaited before applying a V.A.C. dressing. Key baselines covariates: Wound area (cm²): not reported Wound level University of Texas: Group A: II: n = 20; III: n = 45 Group B: II: n = 22; III: n = 43 | |
| Interventions | Group A (n = 65): V.A.C. therapy (V2) following surgical debridement Group B (n = 65): advanced dressings (control group, C2) following surgical debridement (dressings were changed 3 times per week and during every dressing change the wound bed was inspected. Control group received advanced dressings such as alginate, hydrofibre, silver‐dressing or polyurethanes. The choice of dressing mostly depended on the amount of exudate and presence of infection.) | |
| Outcomes | Primary review outcomes: number of wounds completely healed (further); amputation (after follow‐up period) Secondary review outcomes: number of wounds closed or covered with surgery; time to closure or coverage surgery | |
| Notes | Follow‐up period: end of therapy defined as complete coverage of the wound with epithelial tissue Funding: not reported Only Study II included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using a computerized randomization procedure." Comment: adequate methodology |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted in more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "clinicians (non‐blinded, participating in the study) evaluated the wound bed and made a subjective estimation of the depth of the wound and of the quality of the wound bed." "A photographic documentation was carried out upon enrolment in the study, during the intermediate phase and at the end of the therapy. A planimetry of superficial wounds was done to evaluate the dimensions of ulcerated wounds." "Presence and quantity of granulation tissue was also documented and microbiological examinations (after wound debridement, based on wound biopsies) were repeated. All patients with clinical signs of infection, after microbiological examination, were treated with targeted antibiotic therapy." Comment: as a result of this, we classed the risk of bias for this domain as unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in Turkey | |
| Participants | 67 adults Inclusion criteria: diabetic foot ulcers Exclusion criteria: not reported Key baselines covariates: Wound area (cm²): Group A: 35.7 (SD 6.4) Group B: 29.7 (SD 5.2) Wound duration (weeks): Group A: 11.3 (SD 9.2) Group B: 8.8 (SD 7.2) | |
| Interventions | Group A (n = 30): NPWT (V.A.C. system) Group B (n = 37): conventional wound care treatment (described as daily wound care, debridement and treatment of gangrenous tissue where required and use of sterilised gauze dressing). Clinical measures included standard diabetic treatment, daily wound care including antiseptic bath, debridement, toe removal for gangrene when necessary and wound care with conventional methods or V.A.C. | |
| Outcomes | Primary review outcomes: time to healing Secondary review outcomes: health‐related quality of life measured with SF‐36 (not clearly reported) | |
| Notes | Follow‐up: final SF‐36 form completed 1 month after wound healing (mean in 4th month of study). Outcome assessment: healing time calculated as the time from hospital admission to re‐epithelisation. Table 2 titled as "Duration of granulation" but the table content presented "time to healing." Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation of the patients was arranged by the free use web based system (http://www.tufts.edu\˜gdall/PLAN.HTM)." Comment: classed as an adequate method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants and investigators to whether or not they receive NPWT |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in the USA | |
| Participants | 40 participants Inclusion criteria: people with DM aged 21–90 years, surgical lower extremity wounds (diabetic foot wounds after incision and drainage or amputation for infection), and ankle‐brachial indices > 0.70 Exclusion criteria: not reported Key baselines covariates: Wound area (cm²): Group A: 20.1 (SD 14.3) Group B: 34.6 (SD 32.9) Wound volume (cm³): Group A: 35.1 (SD 33.0) Group B: 65.3 (SD 69.9) History of amputation: Group A: 65% Group B: 65% Wound duration: not reported | |
| Interventions | Group A (n = 20): 75 mmHg continuous pressure with a silicone‐coated dressing (Engenex with Bio‐Dome Technology; ConvaTec, Skillman, NJ) Group B (n = 20): 125 mmHg continuous pressure with a polyurethane foam dressing (V.A.C. with GranuFoam dressing; Kinetic Concepts, Inc., San Antonio, TX) | |
| Outcomes | Primary review outcomes: no review relevant outcome reported Secondary review outcomes: number of wounds closed or covered with surgery; adverse events (we used data from Table 1 in the paper – 3 vs 2; however, discrepancy between table and text which suggests 3 vs 1) | |
| Notes | Follow‐up: 4 weeks Both NPWT devices were changed 3 times per week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised from a computer‐generated list" Comment: classed as an adequate method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted in more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in India | |
| Participants | 48 participants (recruited from inpatient wards), 15 of whom were reported to have DM and a foot ulcer. Data for these 15 participants only were presented Inclusion criteria: people admitted to general surgery, physical medicine and rehabilitation wards and referred by the surgical consultants for care of an acute or chronic extremity, sacral or abdominal wound that could not be treated with primary closure Exclusion criteria: ischaemic wounds; or wounds: in anatomical locations where an adequate seal around the wound site could not be obtained; with exposed bowel or blood vessels; with necrotic tissue that could not be debrided; with communicating fistulae; with malignancy; with recent grafts; or presence of osteomyelitis; or receiving therapeutic anticoagulation Key baselines covariates (foot ulcers in people with DM only): Wound area (cm²): Group A: 25.7 (SD 9.7) Group B: 48.1 (SD 53.5) Wound duration (days): Group A: 8.5 (SD 8.3) Group B: 5.2 (SD 2.3) | |
| Interventions | Group A (n = 6): locally constructed (homemade) device: a sterilised, porous packing material obtained from a local source was cut to fit the wound. A 14‐French suction catheter was tunnelled into the packing material, which then was placed into the wound cavity. A sterile adhesive plastic drape (Dermincise, Vygon, UK) was cut to overlap the surrounding skin and applied over the packing material, forming an airtight seal. Tubing was used to attach the free end of the suction catheter to a wall suction canister. The TNP timer was placed in circuit between the wall suction apparatus and the wall suction canister The TNP timer, constructed from local electronics, was designed to cycle wall suction intermittently using a simple timed switch and a system of valves. For the study protocol, the timer was set to cycle for 2 minutes on, followed by 5 minutes off. Wall suction pressure was set at 125 mmHg. In sensitive wounds, suction was reduced to a tolerable level (usually 50–100 mmHg) until it could be comfortably increased. For oedematous wounds, the suction was kept on a continuous setting until oedema had been reduced and an intermittent regimen could be followed. The dressing was changed every 2 days unless otherwise scheduled by the treating physician. Wounds were debrided as required to keep the wound bed free of necrotic tissue. Participants receiving NPWT who no longer required hospitalisations for their primary diagnosis, or could not afford to remain in the hospital, remained in the study with conventional wound dressings in the outpatient setting, but outcomes were analysed in the original treatment groups. Group B (n = 9): saline‐soaked gauze and dry pads used to cover the wound. Dressing changes typically performed twice daily; frequency adjusted according to the judgement of the treating physician. Wounds in both treatment groups were debrided before dressing application. | |
| Outcomes | Primary review outcomes: number of wounds completely healed (satisfactory healing defined as complete wound closure by secondary intention or wound readiness for delayed primary closure as determined by the study investigator and treating surgeon) Secondary review outcomes: number of wounds closed or covered with surgery | |
| Notes | Participants were followed until wound closure or being lost to follow‐up for a mean of 26.3 days (SD 18.5) in the control and 33.1 days (SD 37.3) in the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "wounds that met inclusion and exclusion criteria were assessed for size (in a manner that allowed blinding) and then block‐randomized using a concealed computer‐generated table in a 1‐to‐2 ratio of TNP closure versus conventional wound dressing." Comment: adequate method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "following enrolment, wound size was assessed using computer‐aided measurements of digital photographs and block‐randomized to the study arms using a concealed allocation table." Comment: unclear how allocation concealment was conducted |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Seems that participants were analysed in groups as randomised |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in India | |
| Participants | 30 participants Inclusion criteria: age group 20–75 years, ulcer area 50–200 cm², diagnosis of DM made by American Diabetes Association Criteria Exclusion criteria: aged < 20 years or > 75 years; obvious septicaemia; osteomyelitis; wounds resulting from venous insufficiency; malignant disease in a wound; people being treated with corticosteroids, immunosuppressive drugs or chemotherapy; any other serious pre‐existing cardiovascular, pulmonary and immunological disease. Key baselines covariates: not reported | |
| Interventions | Group A: negative‐pressure dressing therapy. Foam‐based dressing covered with adhesive drape. An evacuation tube embedded in the foam was connected to a fluid collection canister contained within a portable vacuum/suction machine. Subatmospheric (negative) pressure was applied within a range of –50 mmHg to –125 mmHg intermittently 3 times a day. NPWT dressings were changed when required. Subsequently, the control group received twice daily saline‐moistened gauze dressings. Group B: twice daily dressing changes with saline‐moistened gauze Cointerventions: wounds underwent initial sharp debridement to remove necrotic tissue and slough as far as possible. Standard antibiotic regimens were administered to all participants which consisted of broad‐spectrum antibiotics initially and later according to the culture sensitivity report. | |
| Outcomes | Primary review outcomes: number of wounds completely healed (complete healing defined as 100% wound closure with re‐epithelialisation or scab with no wound drainage present and no dressing required; complete responders: complete healing of lower limb ulcers) Secondary review outcomes: no review relevant outcome reported | |
| Notes | Follow‐up: 8 weeks Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly divided into two groups – study group and control group." Comments: not reported how sequence for randomisation was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants and investigators to whether or not they receive NPWT. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Quote: "the patients who underwent below knee amputation were excluded from this analysis." Comment: surely this is attrition bias. We do not know how many people underwent amputation (it was unclear what the 80% vs 60% refer to. In the text it said that 9 wounds in the A group as 60% at 4 weeks). |
| Other bias | Unclear risk | Not reported |
| Methods | 3‐arm RCT undertaken in Croatia | |
| Participants | 27 adult inpatients Inclusion criteria: complicated diabetic ulcer (Wagner 2–5) managed to international guidelines for treatment protocol (confirmed with the author that these were all foot wounds) Exclusion criteria: revascularisation, reconstruction and amputation procedures were not considered in this study. Key baselines covariates: not reported Wound duration (months): not reported | |
| Interventions | Group A (n = 7): NPWT Group B (n = 12): moist dressings Group C (n = 8): classic gauze Surgical debridement, off‐loading, comorbidity treatment and appropriate wound care were performed. | |
| Outcomes | Primary review outcome: healing rate (author defined as wound closure – personal contact) Secondary review outcomes: no review relevant outcome reported | |
| Notes | Follow‐up: 2 months, extracted from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
| Methods | 2‐arm RCT undertaken in India | |
| Participants | 60 participants Inclusion criteria: people with ulcers on dorsum of foot of size > 10 cm². Adequate blood circulation was assessed by doing lower limb arterial Doppler. Exclusion criteria: people with osteomyelitis, peripheral vascular disease or malignancy Key baselines covariates: not reported | |
| Interventions | Group A: NPWT dressing (a usual suction machine generating pressure of −80 to −150 mmHg, Ryle's tube, piece of foam cut according to size and shape of ulcer, and adhesive transparent dressing (OpSite by Smith & Nephews, UK). The suction was applied 30 minutes on and 30 minutes off.) Group B: conventional dressing (cleaning with povidine iodine solution with or without hydrogen peroxide and applying moist gauze to wound and dressing closed by cotton bandage) All participants were given medical therapy for DM and antibiotics given according to culture and sensitivity patterns. All foot ulcers were surgically debrided prior to initiation of NPWT or conventional treatment. In the NPWT group, dressings were changed every 48–72 h. In the control group, conventional dressings were applied at the time of surgical debridement and changed twice a day thereafter. Participants with failure of dressings were treated with other methods of dressing. | |
| Outcomes | Primary review outcomes: amputation (data for alternative therapy or amputation) Secondary review outcomes: number of wounds closed or covered with surgery | |
| Notes | Follow‐up: end point of study was when wound was ready for either skin grafting or secondary suturing. Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "sixty patients were randomised into either the experimental NPWT group or conventional dressing group (control)." Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in China | |
| Participants | 40 participants Inclusion criteria: clinical diagnosis of type 2 DM, wound was consistent with the diagnosis of a chronic wound, 2 ≤ Wagner grade ≤ 4, continuous existence of the diabetic foot lesion for a minimum of 1 month. Exclusion criteria: refusal to give written informed consent; aged < 18 years; pregnancy; presence of expected non‐compliance with the requirements of the study estimated by investigator at time point of inclusion; necrotic tissue that could not be debrided; malignancy of the wound; severe heart disease, heart failure, unstable angina pectoris, myocardial infarction or severe systemic infection; severe renal insufficiency, with a serum creatinine level > 106 μmol/L; liver dysfunction, with alanine aminotransferase levels > 125 U/L or glutamic‐oxalacetic transaminase level > 87.5 U/L; application of immunosuppressive agents and growth factors; poor compliance, death or unable to complete the course of treatment (during treatment); contraindications for surgery or people did not agree to having surgery. Key baselines covariates: Wound area and wound duration not reported | |
| Interventions | Group A: vacuum sealing drainage group: wounds cleaned and disinfected by repeatedly washing with sterilised physiological saline, hydrogen peroxide and iodine solution and then covered with negative‐pressure material according to the shape and size after debridement; dressing changed every 7 days. Negative pressure was maintained at –120 to –400 mmHg Group B: routine dressing: 0.5% dilute iodoform gauze and Vaseline gauze dressing, changed every other day. | |
| Outcomes | Primary review outcomes: number of wounds completely healed (described as "cured"); amputation Secondary review outcomes: no review relevant outcome reported | |
| Notes | Infiltration of the wound surface, granulation tissue growth and epithelium of the wound surface were observed every 7 days for 1 month. Funding: Science and Technology Grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted in more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Low risk | No evidence of other risk of bias |
| Methods | 2‐arm RCT undertaken in China | |
| Participants | 60 participants Inclusion criteria: duration of DM 10–20 years; mean fasting blood glucose at admission ≥ 10 mmol/L; diabetic foot by Wagner grading method of ≥ 2; diabetic foot ulcers distributed in the distal end of the toe, toe plantar joints, heel, ankle and 1/3 lower leg Exclusion criteria: DM not diagnosed; cancerous ulcer or ulcer malignant, osteomyelitis; taking certain uncommon drugs, chemotherapy, dialysis; difficult to control high blood sugar (glycosylated haemoglobin > 12%) Key baselines covariates: Wound area (cm²): Group A: 39.9 (SD 19.8) Group B: 40.4 (SD 20.4) Wound duration (days) Group A: 51.4 (SD 36.3) Group B: 52.6 (SD 27.6) | |
| Interventions | Group A: vacuum sealing drainage group, conventional treatment combined with the vacuum sealing drainage technology Group B: traditional treatment group, regulating blood sugar level, dressing and traditional debridement Cointerventions: all participants received blood sugar control and debridement | |
| Outcomes | Primary review outcomes: number of wounds completely healed (defined as cured wound: no amputation is needed); amputation Secondary review outcomes: number of wounds closed or covered with surgery; wound recurrence | |
| Notes | Follow‐up: not specified for wound healing; ulcer recurrence was observed in 6–10 months Outcome assessment: healing time calculated only for cured wounds (no amputation needed); preparation time described as time for skin/flap grafting Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: it is understandably not possible to blind participants and investigators to whether or not they receive NPWT. However, given this, it is important that any decision‐making that might be affected by performance bias is recognised and blinding is introduced where possible. We noted that unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted more wounds being closed (and classed as healed) or amputated in 1 group compared with the other. As a result of this, we classed the risk of bias for this domain as unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no evidence of incomplete outcome data |
| Other bias | Low risk | No evidence of other risk of bias |
DM: diabetes mellitus; h: hour; ITT: intention‐to‐treat population; n: number of participants; NPWT: negative pressure wound therapy; RCT: randomised controlled trial; SD: standard deviation; SF‐36: 36‐item Short Form; TNP: topical negative pressure (synonym for NPWT).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included multiple wounds types. Unable to obtain diabetic foot wound data separately | |
| Included multiple wounds types. Unable to obtain diabetic foot wound data separately | |
| Randomised crossover trial; no relevant outcome reported | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Not an RCT, as participants allocated using alternation | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Not an RCT | |
| Included multiple wound types. Unable to obtain diabetic foot wound data separately | |
| Not an RCT, as participants allocated using odd and even numbers (quasi‐randomised study) | |
| Treatment with NPWT was not the only systematic difference between groups (intervention group receiving NPWT also received autologous fibroblasts and skin grafting) | |
| Not an RCT, as participants allocated using alternation. Coin flipped for first participant and then participants allocated by alternation | |
| Not an RCT, as "stratified sequential allocation method" used | |
| Not a diabetic foot wound study population | |
| Included multiple wound types. Unable to obtain diabetic foot wound data separately | |
| Included multiple wound types. Unable to obtain diabetic foot wound data separately | |
| The investigators described a non‐random component in the sequence generation process. | |
| Included wounds in people with diabetes in regions other than the foot (legs and back). Unable to obtain diabetic foot wound data separately | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Crossover design and no relevant outcome reported | |
| NPWT was not the only difference between trial arms. | |
| Included multiple wounds types. Unable to obtain diabetic foot wound data separately | |
| The investigators described a non‐random component in the sequence generation process. | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). | |
| Due to focus on biochemical and related outcomes and the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported). |
NPWT: negative pressure wound therapy; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A pilot randomised controlled trial of negative pressure wound therapy (NPWT) in hospital in the home (HITH) to treat post‐operative foot wounds |
| Methods | RCT |
| Participants | Men and women aged > 18 years; postoperative foot amputation to the transmetatarsal level of foot ≥ 5 cm² to ≤ 20 cm² measured by digital planimetry |
| Interventions | NPWT vs standard care |
| Outcomes | Proportion of wounds healed; time to healing; frequency of treatment; wound recurrence; resources used/costs; recruitment rates; pain and health‐related quality of life |
| Starting date | 17 August 2012 |
| Contact information | |
| Notes | Not yet recruiting |
| Trial name or title | A prospective multicenter assessment of Foryou NPWT security and effectiveness in promoting the healing of diabetic foot ulcer |
| Methods | RCT |
| Participants | People with type 1 or type 2 DM and with DFUs, including amputation wounds, were considered suitable for NPWT by the author of this study |
| Interventions | NPWT vs advanced wound dressing treatment |
| Outcomes | Change in wound area; complete healing rate |
| Starting date | 1 August 2012 |
| Contact information | |
| Notes | Recruitment status not updated |
| Trial name or title | Treatment of diabetic foot wounds by vacuum‐assisted closure |
| Methods | RCT |
| Participants | Men and women aged > 18 years with diabetic foot wounds |
| Interventions | NPWT vs standard conventional moist wound therapy |
| Outcomes | Time until complete (100%) wound closure |
| Starting date | 1 August 2009 |
| Contact information | Private Universität Witten/Herdecke GmbH Institut für Forschung in der Operativen Medizin, Ostmerheimer Str. 200, 51109 Cologne, Germany |
| Notes | Recruiting suspended before start date |
| Trial name or title | Comparing treatments for diabetic foot ulcers |
| Methods | RCT |
| Participants | Adults aged ≥ 18 years with DM and a foot ulcer |
| Interventions | Group 1: TAU Group 2: TAU + HD Group 3: TAU + HD + NPWT Group 4: TAU + HD + DCD Group 5: TAU + HD + DCD + NPWT |
| Outcomes | Reduction in index ulcer area size; time to healing |
| Starting date | April 2017 |
| Contact information | |
| Notes | Recruitment status: recruiting Overall trial end date: 31 March 2022 |
DCD: decellularised dermal allograft; DM: diabetes mellitus; DFU: diabetic foot ulcer; HD: hydrosurgical debridement; NPWT: negative pressure wound therapy; RCT: randomised controlled trial; TAU: treatment as usual.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.03, 2.01] |
| Analysis 1.1  Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 1 Proportion of wounds healed. | ||||
| 2 Time to healing Show forest plot | 1 | 162 | Hazard Ratio (Fixed, 95% CI) | 1.91 [1.21, 2.99] |
| Analysis 1.2  Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 2 Time to healing. | ||||
| 3 Amputations Show forest plot | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| Analysis 1.3  Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 3 Amputations. | ||||
| 4 Number of wounds closed or covered with surgery Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| Analysis 1.4  Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 4 Number of wounds closed or covered with surgery. | ||||
| 5 Adverse events Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.28] |
| Analysis 1.5  Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 5 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 486 | Risk Ratio (IV, Fixed, 95% CI) | 1.40 [1.14, 1.72] |
| Analysis 2.1  Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 1 Proportion of wounds healed. | ||||
| 1.1 Advanced dressings | 1 | 341 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [1.11, 2.01] |
| 1.2 Basic contact dressings | 2 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.34 [0.83, 2.16] |
| 1.3 Anti‐microbial dressings | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [0.93, 1.87] |
| 2 Amputations Show forest plot | 3 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.70] |
| Analysis 2.2  Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 2 Amputations. | ||||
| 3 Number of wounds closed or covered with surgery Show forest plot | 3 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.24] |
| Analysis 2.3  Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 3 Number of wounds closed or covered with surgery. | ||||
| 4 Wound recurrence Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.53] |
| Analysis 2.4  Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 4 Wound recurrence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wounds closed or covered with surgery Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.47, 1.47] |
| Analysis 3.1  Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 1 Number of wounds closed or covered with surgery. | ||||
| 2 Adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.04] |
| Analysis 3.2  Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 2 Adverse events. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
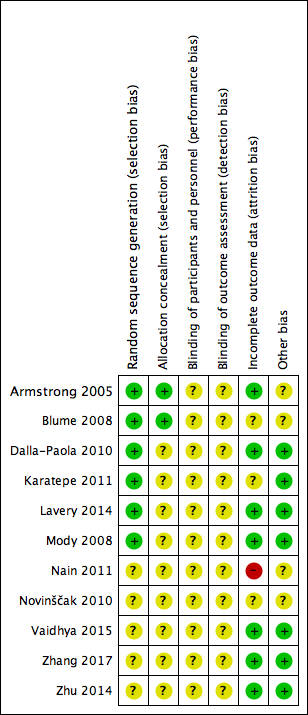
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
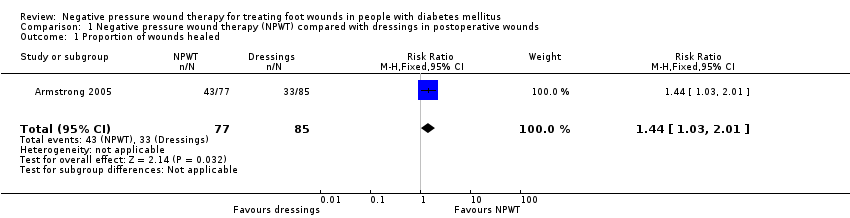
Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 1 Proportion of wounds healed.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 2 Time to healing.
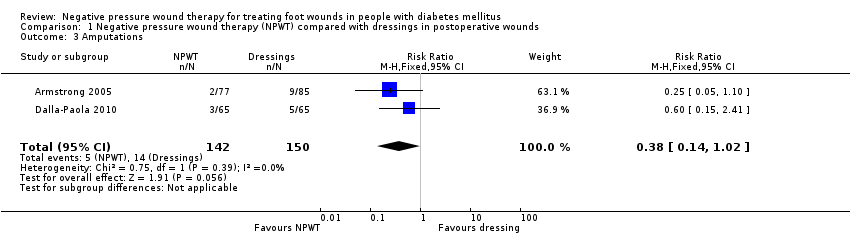
Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 3 Amputations.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 4 Number of wounds closed or covered with surgery.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 5 Adverse events.
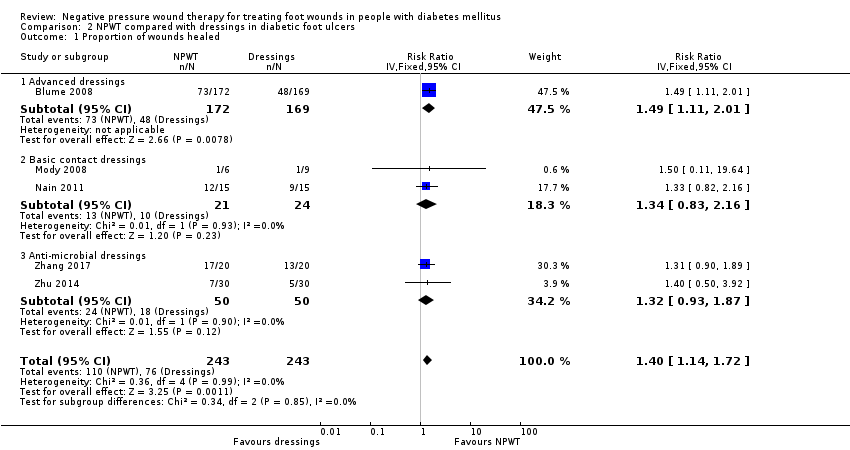
Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 1 Proportion of wounds healed.

Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 2 Amputations.

Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 3 Number of wounds closed or covered with surgery.
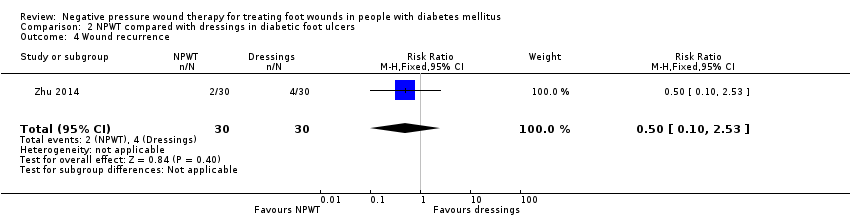
Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 4 Wound recurrence.

Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 1 Number of wounds closed or covered with surgery.

Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 2 Adverse events.
| NPWT compared with dressings for postoperative wounds | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: 16 weeks | Study population | RR 1.44 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 559 per 1000 | |||||
| Time to healing Follow‐up: 16 weeks | Study population | HR 1.91 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 609 per 1000 | |||||
| Amputations Follow‐up: 16 weeks or unspecified | Study population | RR 0.38 | 292 (2 studies) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 60 per 1000 | 23 per 1000 | |||||
| Number of wounds closed or covered with surgery | 954 per 1000 | 1000 per 1000 | RR 1.02 | 130 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — |
| Adverse events Follow‐up: 16 weeks | Study population | RR 0.96 | 162 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 541 per 1000 | 520 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias: some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias. | ||||||
| NPWT compared with dressings for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: unclear for 4 studies and 8–16 weeks for the other 3 studies | Study population | RR 1.40 | 486 | ⊕⊕⊝⊝ Lowa,b | — | |
| 406 per 1000 | 540 per 1000 | |||||
| Time to healing Follow‐up: unclear for 2 studies and 16 weeks for the other study | Study population | — | 468 (3 studies) | ⊕⊕⊝⊝ Lowa,b | 3 studies reported HR, median and mean (1 each) and we were unable to pool any data for this comparison. | |
| See comment | See comment | |||||
| Amputations Follow‐up: unclear for 4 studies and 16 weeks for the other study | Study population | RR 0.33 | 441 | ⊕⊕⊝⊝ Lowa,b | — | |
| 114 per 1000 | 38 per 1000 | |||||
| Number of wounds closed or covered with surgery Follow‐up: unclear | Study population | RR 1.02 | 129 | ⊕⊕⊝⊝ Lowa,b | — | |
| 714 per 1000 | 729 per 1000 | |||||
| Adverse events | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence Follow‐up: 6–10 months | Study population | RR 0.50 (0.10 to 2.53) | 60 | ⊕⊝⊝⊝ Very lowa,c | — | |
| 133 per 1000 | 66 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias (no blind outcome assessment). | ||||||
| Low‐pressure compared with high‐pressure NPWT for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: low‐pressure NPWT (75 mmHg) Comparison: high‐pressure NPWT (125 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with low compared with high pressure of NPWT | |||||
| Proportion of wounds healed | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Time to ulcer healing | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Amputation | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Number of wounds closed or covered with surgery Follow‐up: 4 weeks | Study population | RR 0.83 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 600 per 1000 | 498 per 1000 | |||||
| Adverse events Follow‐up: 4 weeks | Study population | RR 1.50 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 100 per 1000 | 150 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded three levels: once for risk of bias (some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias); twice for very serious imprecision with a small sample size and limited reported information to quantify imprecision. | ||||||
| Study | Wound characteristics | Comparison | Length of follow‐up | NPWT pathways | Time to healing | Number of wounds completely healed | Amputation | Number of wounds closed or covered with surgery | Time to closure or coverage surgery | Adverse events | Health‐related quality of life | Cost‐effectiveness | Wound recurrence |
| Diabetic foot amputation to trans‐metatarsal level | Group A: NPWT (V.A.C. system), dressing changes every 48 h. Treatment conducted until wound closure or completion of 112‐day assessment (n = 77) Group B: moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings (n = 85) | 16 weeks | After amputation (close/open wounds; if open wounds, secondary intention), NPWT delivered through the V.A.C. system; or standard care with moist wound therapy. | Kaplan‐Meier median time to healing Group A: 56 days (IQR 26 to 92) Group B: 77 days (IQR 40 to 122) Log‐rank taken as P = 0.005 There was no difference noted in time to healing for acute or chronic wounds. | Group A: 43/77 (55.8%) Group B: 33/85 (38.8%) Of healed wounds –healed by secondary intention (without primary/surgical wound closure) Group A: 31/43 (72.1%) Group B: 25/33 (75.8%) Remaining wounds were closed following surgery. | Number of participants undergoing further amputation Group A: 2/77 (2.3%) Major = 0 Minor = 2 Group B: 9/85 (10.6%) Major = 5 Minor = 4 | Not reported | Not reported | Participants who had ≥ 1 adverse events Group A: 40/77 (51.9%) Group B: 46/85 (54.1%) Participants who had ≥ 1 treatment‐related adverse events Group A: 9/77 (11.7%) 1 classified serious Group B: 11/85 (12.9%) 5 classified as serious | Not reported | Not reported | Not reported | |
| Ulceration of the foot in people with diabetes | Group A: NPWT (V.A.C. system), applied according to manufacturer’s instructions (n = 172) Group B: advanced moist wound therapy dressings used according to guidelines/local protocols (n = 169) | 16 weeks | NPWT was continued until ulcer closure. | Kaplan‐Meier median time to healing Group A: 96 days (95% CI 75.0 to 114.0) Group B: could not be estimated Log‐rank taken as P = 0.001 | Group A: 3/172 (42.4%) Group B: 8/169 (28.4%) (6 participants excluded in paper as did not receive treatment, added back into denominator here; ITT 172/169) | Number of participants undergoing amputation* Group A: 7/172 (4.1%) Major = 5 Minor = 2 Group B: 17/169 (10.1%) Major = 4 Minor = 13 | Not reported | Not reported | Limited data: not extracted | Not reported | Not reported | Not reported | |
| Infected open amputations or surgical dehiscence of minor amputations in people with diabetes | Group A: V.A.C. therapy following surgical debridement (n = 65) Group B: advanced dressings following surgical debridement (n = 65) | Not specified. End of therapy was defined as complete coverage of the wound with epithelial tissue. | Duration of therapy depended on the functional parameters of the wound area. | Not reported | Not reported | Number of participants undergoing further amputation (major) Group A: 3/65 (4.6%) Group B: 5/65 (7.7%) | Group A: 63/65 (96.9%) Group B: 62/65 (95.4%) | Group A (n = 65): 65 days (SD 16) Group B (n = 65): 98 days (SD 45) P = 0.005 These data reported as time to "complete closure of the wound" was reached. Unclear if it is mean or median; unclear if "complete closure" means "time to healing of grafted wound" or "time to surgical closure;" unclear if it is a valid measure as not sure all ulcers have healed. Author contacted – waiting for response. | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: NPWT (V.A.C. system) (n = 30) Group B: conventional wound care treatment: based on text in report taken to be dry gauze (n = 37) | Not specified. Last assessment 1 month after healing | Not specified | Median time to healing Group A: 4.4 weeks Group B: 3.9 weeks Mean value presented but not extracted. No specific P value presented (< 0.05) | Not reported | Not reported | Not reported | Not reported | Not reported | SF‐36: data not presented | Not reported | Not reported | |
| Diabetic foot wounds, after incision and drainage | Group A: NPWT with Group B: 125 mmHg of pressure | 4 weeks | NPWT was continued for 4 weeks | Not reported | Not reported | Not reported | Group A: 10/20 (50%) Group B: 12/20 (60%) | Not reported | Group A: study related 2/20 (10%); non‐study related 1/20 (5%) Group B: study related 1/20 (5%); non‐study related 1/20 (5%) | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: locally constructed NPWT (n = 6) Group B: wet‐to‐dry gauze (n = 9) | Not specified: until healing or loss to follow‐up | People receiving TNP only in hospital | Not reported | By secondary intention: Group A: 1/6 (16.6%) Group B: 1/9 (11.0%) | Not reported | By delayed primary closure: Group A: 0/6 (0%) Group B: 3/9 (33%) | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: negative pressure dressing (n = 15) | 8 weeks | Ulcers were treated until the wound was closed surgically or spontaneously, or until completion of the 56 days (8 weeks) assessment whichever was earlier. | Not reported | Group A: 12/15 (80%) Group B: 9/15 (60%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Complicated diabetic foot ulcers | Group A: NPWT (n = 7) Group B: dressings (moist) (n = 12) Group C: classic gauze (n = 8) | 8 weeks | Treatment was monitored for the first 2 months. | Not reported | Group A: * could not be calculated (90%) Group B: 9/12* (75%) Group C: 4/8* (50%) *Figure calculated by review author We obtained data (only proportions) from the study author but were unable to use these to calculate number of healed wounds. It seemed this outcome was measured but was not able to use the data in meta‐analysis. | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wound | Group A: NPWT (n = 30) | Not specified | Interventions discontinued for participants in whom failure or complications | Not reported | Not reported | Data for alternative therapy or amputation: Group A: 3/30 (10%) Group B: 7/30 (23.3%) | Wounds were ready for either skin grafting or secondary suturing (end point) Group A: 27/30 (90%) Group B: 23/30 (67.7%) | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Limited data: not extracted | Not reported | |
| Chronic diabetic ulcers | Group A: vacuum sealing drainage (n = 20) Group B: gauze dressing (n = 20) | Not reported | Interventions were administered in hospital | Not reported | Group A: 17/20 (85%) Group B: 13/20 (65%) | Group A: 1/20 (5%) Group B: 2/20 (10%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wounds | Group A: vacuum sealing drainage (n = 30) | Not reported Follow‐up to 6–10 months for wounds recurrence | Vacuum sealing drainage administered when necessary at several time points | Not reported properly – not all ulcers healed | Group A: 7/30 (23%) Group B: 5/30 (17%) | Group A: 0 Group B: 6/30 (20%) | Of healed wounds by secondary surgery (skin/flap grafting): Group A: 23/30 Group B: 19/24 | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Not reported | Group A: 2 Group B: 4 Follow‐up time: 6–10 months | |
| h: hour; IQR: interquartile range; ITT: intention to treat; n: number of participants; NPWT: negative pressure wound therapy; SF‐36: 36‐item Short Form; TNP: topical negative pressure. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.03, 2.01] |
| 2 Time to healing Show forest plot | 1 | 162 | Hazard Ratio (Fixed, 95% CI) | 1.91 [1.21, 2.99] |
| 3 Amputations Show forest plot | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 4 Number of wounds closed or covered with surgery Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| 5 Adverse events Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 486 | Risk Ratio (IV, Fixed, 95% CI) | 1.40 [1.14, 1.72] |
| 1.1 Advanced dressings | 1 | 341 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [1.11, 2.01] |
| 1.2 Basic contact dressings | 2 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.34 [0.83, 2.16] |
| 1.3 Anti‐microbial dressings | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [0.93, 1.87] |
| 2 Amputations Show forest plot | 3 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.70] |
| 3 Number of wounds closed or covered with surgery Show forest plot | 3 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.24] |
| 4 Wound recurrence Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wounds closed or covered with surgery Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.47, 1.47] |
| 2 Adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.04] |


