Tratamiento de heridas con presión negativa para el tratamiento de las heridas del pie en los pacientes con diabetes mellitus
Resumen
Antecedentes
Las heridas del pie en los pacientes con diabetes mellitus (DM) son un problema de salud prevalente y grave a nivel mundial. Los pacientes con DM son propensos a desarrollar úlceras del pie y, si estas no cicatrizan, también pueden ser sometidos a cirugía de amputación del pie que da lugar a heridas posquirúrgicas. El tratamiento de heridas con presión negativa (THPN) es una tecnología utilizada actualmente en todo el mundo para el cuidado de las heridas. El THPN incluye la aplicación de un apósito de heridas conectado a una máquina de aspiración al vacío. La máquina aplica una presión negativa (o vacío) controlada cuidadosamente, que aspira cualquier exudado de la herida y del tejido lejos del área tratada hacia un recipiente. Se necesita un resumen claro y actual de la evidencia existente para facilitar la toma de decisiones con respecto al uso de los apósitos.
Objetivos
Evaluar los efectos del tratamiento de heridas con presión negativa comparado con la atención estándar u otros tratamientos coadyuvantes en la cicatrización de las heridas del pie en pacientes con DM en cualquier contexto de atención.
Métodos de búsqueda
En enero de 2018, para esta primera actualización de la revisión, se hicieron búsquedas en el registro especializado del Grupo Cochrane de Heridas (Cochrane Wounds Specialised Register); Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials [CENTRAL]); Ovid MEDLINE (incluyendo In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. También se realizaron búsquedas de estudios en curso y no publicados en los registros de ensayos clínicos, y se escanearon las listas de referencias de los estudios incluidos relevantes, revisiones, metanálisis e informes de tecnología sanitaria para identificar estudios adicionales. No hubo ninguna restricción en cuanto al idioma, la fecha de publicación o el ámbito de los estudios. Se identificaron seis estudios adicionales para su inclusión en la revisión.
Criterios de selección
Ensayos controlados aleatorios (ECA) publicados o no publicados que evaluaron los efectos de cualquier tipo de THPN en el tratamiento de las heridas del pie en pacientes con DM, independientemente de la fecha o el idioma de publicación. Se hizo un esfuerzo especial para identificar los estudios no publicados.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios, evaluaron el riesgo de sesgo y extrajeron los datos. Los desacuerdos iniciales se resolvieron mediante discusión, o mediante la inclusión de un tercer autor de revisión, cuando fue necesario. Se presentaron y analizaron los datos por separado para las úlceras del pie y las heridas posoperatorias.
Resultados principales
11 ECA (972 participantes) cumplieron los criterios de inclusión. Los tamaños de muestra de los estudios variaron de 15 a 341 participantes. Un estudio tuvo tres brazos y todos se incluyeron en la revisión. Los 10 estudios restantes tenían dos brazos. Dos estudios se centraron en las heridas posteriores a la amputación y todos los otros estudios incluyeron las úlceras del pie en los pacientes con DM. Diez estudios compararon el THPN con apósitos; y un estudio comparó el THPN administrado a 75 mmHg con THPN administrado a 125 mmHg. Las medidas de resultado primarias de esta revisión fueron el número de heridas cicatrizadas y el tiempo hasta la cicatrización de las heridas.
THPN en comparación con apósitos para las heridas posoperatorias
Dos estudios (292 participantes) compararon THPN con apósitos húmedos para las heridas en las heridas posoperatorias (heridas posteriores a la amputación). Sólo un estudio especificó un tiempo de seguimiento, que fue de 16 semanas. Este estudio (162 participantes) informó un aumento en el número de heridas cicatrizadas en el grupo de THPN en comparación con el grupo de apósitos (cociente de riesgos [CR] 1,44; intervalo de confianza [IC] del 95%: 1,03 a 2,01; evidencia de baja certeza disminuida por el riesgo de sesgo y la imprecisión). Este estudio también informó que el tiempo mediano hasta la cicatrización fue 21 días más corto con el THPN en comparación con los apósitos húmedos (cociente de riesgos instantáneos [CRI] calculado por los autores de la revisión 1,19; IC del 95%: 1,21 a 2,99; evidencia de baja certeza disminuida por el riesgo de sesgo y la imprecisión). Los datos de dos estudios indicaron que no está claro si hay una diferencia entre los grupos en el riesgo de amputación (CR 0,38; IC del 95%: 0,14 a 1,02; 292 participantes; evidencia de baja certeza, disminuida una vez por el riesgo de sesgo y dos veces debido a la imprecisión).
THPN en comparación con apósitos para las úlceras del pie
Hubo ocho estudios (640 participantes) en este análisis y los tiempos de seguimiento variaron entre los estudios. Seis estudios (513 participantes) informaron la proporción de heridas cicatrizadas y fue posible agrupar los datos de cinco estudios. Los datos agrupados (486 participantes) indicaron que el THPN puede aumentar el número de heridas cicatrizadas en comparación con los apósitos (CR 1,40; IC del 95%: 1,14 a 1,72; I² = 0%; evidencia de baja certeza, disminuida una vez por el riesgo de sesgo y una vez debido a la imprecisión). Tres estudios evaluaron el tiempo de curación, pero solo un estudio informó datos utilizables. Este estudio informó que el THPN reduce el tiempo hasta la cicatrización en comparación con los apósitos (cociente de riesgos instantáneos [CRI] calculado por los autores de la revisión 1,82; IC del 95%: 1,27 a 2,60; 341 participantes; evidencia de baja certeza, disminuida una vez por el riesgo de sesgo y una vez debido a la imprecisión).
Los datos de tres estudios (441 participantes) indican que es posible que en los pacientes asignados a THPN se redujera el riesgo de amputación en comparación con los pacientes asignados a los apósitos (CR 0,33; IC del 95%: 0,15 a 0,70; I² = 0%; evidencia de certeza baja, disminuida una vez por el riesgo de sesgo y una vez debido a la imprecisión).
THPN a baja presión en comparación con THPN a alta presión para las úlceras del pie
Un estudio (40 participantes) comparó THPN a 75 mmHg y THPN a 125 mmHg. El tiempo de seguimiento fue de cuatro semanas. No hubo datos sobre los resultados primarios. No hubo una diferencia clara en el número de heridas cerradas o cubiertas con cirugía entre los grupos (CR 0,83; IC del 95%: 0,47 a 1,47; evidencia de baja certeza, disminuida una vez por el riesgo de sesgo y dos veces debido a imprecisión grave) ni en los eventos adversos (CR 1,50; IC del 95%: 0,28 a 8,04; evidencia de baja certeza, disminuida una vez por el riesgo de sesgo y dos veces debido a imprecisión grave).
Conclusiones de los autores
Hubo evidencia de certeza baja para indicar que el THPN, comparado con apósitos para las heridas, puede aumentar la proporción de heridas cicatrizadas y reducir el tiempo hasta la cicatrización de las heridas posoperatorias y las úlceras del pie en los pacientes con DM. En las comparaciones de diferentes presiones de THPN para el tratamiento de las úlceras del pie en los pacientes con DM, no está claro si hay una diferencia en el número de heridas cerradas o cubiertas con cirugía, ni en los eventos adversos. Ninguno de los estudios incluidos proporcionó evidencia sobre el tiempo hasta la cirugía de cierre o recubrimiento, la calidad de vida relacionada con la salud ni la relación entre coste y efectividad. Las limitaciones en la evidencia de los ECA actuales indican que se necesitan ensayos adicionales para reducir la incertidumbre alrededor de la toma de decisiones con respecto al uso del THPN para tratar las heridas del pie en pacientes con DM.
PICO
Resumen en términos sencillos
Tratamiento de heridas con presión negativa para el tratamiento de las heridas del pie en los pacientes con diabetes mellitus
¿Cuál era el objetivo de esta revisión?
Se revisó la evidencia acerca de si el tratamiento de heridas con presión negativa (THPN) es efectivo en el tratamiento de las heridas del pie en los pacientes con diabetes mellitus. Los investigadores de Cochrane recopilaron y analizaron todos los estudios relevantes (ensayos controlados aleatorios; estudios clínicos en los que las personas se asignaron al azar a uno de dos o más grupos de tratamiento) para responder a esta pregunta y encontraron 11 estudios relevantes.
Mensajes clave
No hay seguridad acerca de si el THPN es efectivo para el tratamiento de las heridas del pie en los pacientes con diabetes mellitus. Hay alguna evidencia de baja certeza de que el THPN aumenta el número de heridas cicatrizadas en comparación con los apósitos, y que puede reducir el tiempo de cicatrización. No hay seguridad acerca de la efectividad de presiones diferentes del THPN sobre la cicatrización de las heridas. En términos generales, la confiabilidad de la evidencia aportada por los ensayos fue demasiado baja para tener seguridad en cuanto a los efectos beneficiosos y perjudiciales del THPN para el tratamiento de las heridas del pie en los pacientes con diabetes.
¿Qué estudió esta revisión?
La diabetes mellitus es una afección frecuente que provoca concentraciones sanguíneas altas de glucosa y que afecta a alrededor de 2 800 000 personas en el Reino Unido (cerca del 4,3% de la población). Algunas personas con diabetes pueden desarrollar úlceras en los pies. Estas heridas pueden tardar mucho tiempo en cicatrizar, pueden ser dolorosas e infectarse. La ulceración del pie en los pacientes con diabetes también puede dar lugar a un riesgo mayor de amputación de partes del pie o la pierna. En general, los pacientes con diabetes tienen un riesgo mayor de amputación del miembro inferior que los pacientes sin diabetes.
El THPN es un tratamiento que se utiliza actualmente para las heridas, incluidas las úlceras de la pierna. El THPN incluye la aplicación de un apósito para heridas conectado a una máquina de aspiración al vacío que aspira cualquier exudado de la herida y del tejido lejos del área tratada hacia un recipiente. El uso del THPN está en aumento en todo el mundo. Sin embargo, es caro en comparación con tratamientos para las heridas como los apósitos.
Se deseaba determinar si el THPN podría ayudar a que las heridas del pie cicatrizaran más rápido y de forma efectiva en los pacientes con diabetes. Se deseaba saber si los pacientes tratados con THPN presentaban algún efecto secundario. También hubo interés en la repercusión del THPN sobre la calidad de vida del paciente.
¿Cuáles fueron los principales resultados de la revisión?
En enero de 2018 se buscaron los ensayos controlados aleatorios que compararon el THPN con otros tratamientos para las úlceras del pie u otras heridas abiertas en el pie en los pacientes con diabetes. Se encontraron 11 ensayos con 972 adultos. Los números de participantes en cada ensayo variaron de 15 a 341 y los tiempos de seguimiento (observación) de los ensayos variaron de cuatro a 16 semanas cuando se especificó. No todos los estudios señalaron cómo fueron financiados. Dos estudios fueron financiados por un fabricante del THPN.
Existe evidencia de baja certeza que indica que el THPN puede ser efectivo en la cicatrización posoperatoria de las heridas del pie y de las úlceras del pie en los pacientes con diabetes en comparación con los apósitos para heridas, con respecto a la proporción de heridas cicatrizadas y tiempo de cicatrización. En la comparación de diferentes presiones del THPN para las úlceras del pie en los pacientes con diabetes, no está claro si hay una diferencia en el número de heridas cerradas o cubiertas mediante cirugía, ni en los efectos adversos. No hay evidencia disponible sobre el tiempo hasta la cirugía de cierre o recubrimiento, la calidad de vida relacionada con la salud ni la relación entre coste y efectividad.
¿Qué grado de actualización tenía esta revisión?
Se hicieron búsquedas de estudios que se habían publicado hasta enero de 2018.
Authors' conclusions
Summary of findings
| NPWT compared with dressings for postoperative wounds | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: 16 weeks | Study population | RR 1.44 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 559 per 1000 | |||||
| Time to healing Follow‐up: 16 weeks | Study population | HR 1.91 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 609 per 1000 | |||||
| Amputations Follow‐up: 16 weeks or unspecified | Study population | RR 0.38 | 292 (2 studies) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 60 per 1000 | 23 per 1000 | |||||
| Number of wounds closed or covered with surgery | 954 per 1000 | 1000 per 1000 | RR 1.02 | 130 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — |
| Adverse events Follow‐up: 16 weeks | Study population | RR 0.96 | 162 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 541 per 1000 | 520 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias: some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias. | ||||||
| NPWT compared with dressings for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: unclear for 4 studies and 8–16 weeks for the other 3 studies | Study population | RR 1.40 | 486 | ⊕⊕⊝⊝ Lowa,b | — | |
| 406 per 1000 | 540 per 1000 | |||||
| Time to healing Follow‐up: unclear for 2 studies and 16 weeks for the other study | Study population | — | 468 (3 studies) | ⊕⊕⊝⊝ Lowa,b | 3 studies reported HR, median and mean (1 each) and we were unable to pool any data for this comparison. | |
| See comment | See comment | |||||
| Amputations Follow‐up: unclear for 4 studies and 16 weeks for the other study | Study population | RR 0.33 | 441 | ⊕⊕⊝⊝ Lowa,b | — | |
| 114 per 1000 | 38 per 1000 | |||||
| Number of wounds closed or covered with surgery Follow‐up: unclear | Study population | RR 1.02 | 129 | ⊕⊕⊝⊝ Lowa,b | — | |
| 714 per 1000 | 729 per 1000 | |||||
| Adverse events | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence Follow‐up: 6–10 months | Study population | RR 0.50 (0.10 to 2.53) | 60 | ⊕⊝⊝⊝ Very lowa,c | — | |
| 133 per 1000 | 66 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias (no blind outcome assessment). | ||||||
| Low‐pressure compared with high‐pressure NPWT for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: low‐pressure NPWT (75 mmHg) Comparison: high‐pressure NPWT (125 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with low compared with high pressure of NPWT | |||||
| Proportion of wounds healed | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Time to ulcer healing | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Amputation | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Number of wounds closed or covered with surgery Follow‐up: 4 weeks | Study population | RR 0.83 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 600 per 1000 | 498 per 1000 | |||||
| Adverse events Follow‐up: 4 weeks | Study population | RR 1.50 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 100 per 1000 | 150 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded three levels: once for risk of bias (some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias); twice for very serious imprecision with a small sample size and limited reported information to quantify imprecision. | ||||||
Background
Description of the condition
Diabetes mellitus (DM) is a chronic condition caused by impaired regulation of blood glucose levels. Normally the hormone insulin regulates blood glucose, but in people with type 1 DM production of insulin no longer occurs. Type 2 DM is characterised by cellular insensitivity to insulin and reduced insulin secretion. In the UK approximately 90% of people with DM have type 2 (Diabetes UK 2010).
Worldwide in 2017, there were over 425 million adults with DM (five million of whom die of the disease annually), and the prevalence of diabetes is expected to reach over 640 million (1 in 10) by 2040 (IDF 2017). In the UK adult population, the prevalence of diagnosed DM is approximately 3.7 million people (Diabetes UK 2017a). In the USA, the 2015 prevalence of diagnosed DM (all ages) was approximately 9% (CDC 2015), and in Canada in 2008/2009, for those over one year of age, it was 6.8% (Public Health Agency of Canada 2011). However, many cases of DM are undiagnosed and when these are included, the adjusted 2010 prevalence estimates increase to 10.3% for the USA, 9.2% for Canada, 7.8% for India and 10.8% for Mexico. The global prevalence of DM is projected to rise further up to the late 2030s, largely driven by ageing populations, obesity and increasingly sedentary lifestyles (Shaw 2010). Almost half of all deaths attributable to high blood glucose occur before the age of 70 years and the World Health Organization (WHO) projects that diabetes will be the seventh leading cause of death in 2030 (WHO 2016).
DM is a serious health problem because of its associated complications including microvascular complications such as retinopathy, nephropathy and neuropathy (damage to the retina, kidney and nerves); and macrovascular complications including cardiovascular, cerebrovascular and peripheral arterial disease (PAD). The particular combination of peripheral neuropathy (nerve damage) and peripheral vascular disease (damaged veins) contributes to the development of foot ulceration, which may lead to surgical debridement or amputation of the foot or lower limb.
Foot wounds in people with diabetes mellitus
There are two main types of foot wounds that can affect people with DM, foot ulcers and surgical wounds to the foot; these are summarised below.
Foot ulcers
Both PAD and neuropathy are risk factors for the development of chronic foot ulceration in people with DM (Pecoraro 1990; Reiber 1999). PAD and neuropathy can occur separately (the ischaemic foot (PAD) or the neuropathic foot (neuropathy)), or in combination (the neuroischaemic foot). Foot ulceration is reported to affect 15% or more of people with DM at some time in their lives (Reiber 1996; Singh 2005). Estimates of the prevalence of foot ulceration vary, but around 1% to 4% of people with DM have foot ulcers at any given time (Abbott 2002; Kumar 1994). Figures for 2008 showed that, for those people with DM in receipt of US Medicare, the prevalence of the presence of least one foot ulcer was 8% (Margolis 2011).
An ulcer forms as a result of damage to the epidermis (outermost layer of skin) and subsequent loss of underlying tissue. A foot ulcer is specifically defined by the International Consensus on the Diabetic Foot as a wound that extends through the full thickness of the skin below the level of the ankle (Apelqvist 2000a). This definition is not concerned with duration of the ulcer (although some definitions of chronic ulceration require a duration of six weeks or more), and includes ulcers that extend to muscle, tendon and bone.
The severity of foot ulcers in people with DM can be graded using a number of systems. The Wagner wound classification system was one of the first described and has, historically, been widely used, although it is now rarely used in clinical practice. This system assesses ulcer depth and the presence of osteomyelitis (bone infection) or gangrene and grades ulcers as: grade 0 (pre‐ or post‐ulcerative lesion), grade 1 (partial/full‐thickness ulcer), grade 2 (probing to tendon or capsule), grade 3 (deep with osteitis (inflammation of the bone)), grade 4 (partial foot gangrene) and grade 5 (whole foot gangrene) (Wagner 1981). Newer grading systems, such as the PEDIS system (Schaper 2004), the University of Texas Wound Classification System (Oyibo 2001), and SINBAD (Ince 2008), have been developed since, with the SINBAD system being the best validated (Karthikesalingam 2010).
Foot ulcers in people with DM have a serious impact on health‐related quality of life, particularly with respect to physical functioning and role‐limitations due to physical and emotional issues (Nabuurs‐Franssen 2005; Ribu 2006). They also represent a major use of health resources, incurring costs not only for dressings, but also staff costs (for podiatrists, nurses, doctors), costs for tests and investigations, antibiotics and specialist footwear. In 2010 to 2011 the estimated National Health Service (NHS) spend on foot ulceration and amputation in people with DM in England was GBP 639 million to GBP 662 million (Diabetes UK 2017b). The economic impact is also high in terms of the personal costs to patients and carers, for example, costs associated with lost work time and productivity while the patient is unable to bear weight or is hospitalised. As many as 85% of foot‐related amputations are preceded by ulceration (Apelqvist 2000b; Pecoraro 1990).
In terms of ulcer healing, one meta‐analysis of trials in which people with neuropathic ulcers received good wound care, reported that 24% of ulcers completely healed by 12 weeks and 31% by 20 weeks (Margolis 1999). Reasons for delayed healing can include: infection (especially osteomyelitis (bone infection)), comorbidities such as peripheral vascular disease and end‐stage renal disease, and the size and depth of an ulcer at presentation. Even when ulcers do heal, the risk of recurrence is high. Pound 2005 reported that 62% of people with ulcers (from a sample of 231 people) became ulcer‐free at some stage over a 31‐month observation period, however, 40% of the ulcer‐free group went on to develop a new, or recurrent, ulcer after a median of 126 days. Indeed, the ulcer recurrence rate over five years can be as high as 70% (Dorresteijn 2010; Van Gils 1999). Failure of ulcers to heal may result in amputation, and people with DM have a 10‐ to 20‐fold higher risk of losing a lower limb, or part of a lower limb, to non‐traumatic amputation than people without DM (Morris 1998; Wrobel 2001).
Surgical wounds to the foot
The risk of lower limb amputation is much greater for people with DM than for those without. The major underlying pathophysiological conditions associated with amputation are neuropathy and ischaemia. Lower limb amputation can have devastating consequences for people's health status and health‐related quality of life (Tennvall 2000), as well as having a large financial impact on healthcare providers and users. In the UK, from 1 April 2007 to 31 March 2010, a total of 16,693 lower limb amputations were recorded in people with DM (Holman 2012). Of these 10,216 were classed as minor amputations (usually defined as below the ankle joint), and 6477 as major amputations (usually defined as above the ankle joint). The cost of diabetic foot care in 2010 to 2011 was estimated at GBP 580 million, almost 0.6% of NHS expenditure in England. Of hospital admissions with recorded diabetes, 8.8% included ulcer care (GBP 219 million) or amputation (GBP 55 million) (Kerr 2014). In the US, the 2008 prevalence of lower extremity amputation in Medicare recipients was 1.8%, with a total mean annual Medicare reimbursement cost for each person with DM and a lower extremity amputation estimated at USD 54,000. Ulcers are often considered to be chronic wounds, while postsurgical amputation sites are considered to be acute wounds, unless they do not heal (Ubbink 2008).
As well as amputation, debridement is regarded as an important component of the treatment of 'chronic' foot wounds, such as ulcers or non‐healing surgical wounds, in people with DM, and can sometimes be undertaken as a surgical procedure. Debridement involves removal of dead tissue and callus, along with pressure‐relief/off‐loading, treatment of infection and revascularisation, where necessary. As in other areas of wound care, sharp (surgical) debridement of diabetic foot wounds is recommended in guidelines in order to promote wound healing by 'converting' a chronic wound to an acute wound via removal of dead tissue and slough (Steed 2006). While this practice is common, there is little evidence that surgical debridement promotes healing of diabetic foot wounds (Eneroth 2008; Lebrun 2010), but debridement of necrotic tissue with eschar from wounds, including diabetic foot wounds, can sometimes be a requirement prior to the use of wound treatments such as negative pressure wound therapy (NPWT) (KCI 2018).
Description of the intervention
Any intervention that promotes healing, or reduces amputation rates, or both, in foot wounds in people with DM would make an important difference, and a number of health technologies are marketed as impacting on these outcomes. However, the evidence for the clinical‐ and cost‐effectiveness of these technologies is frequently lacking. A suite of Cochrane Reviews (Dumville 2011a; Dumville 2011b; Dumville 2012a; Dumville 2012b), and an associated mixed treatment comparison (Dumville 2012c), found no robust evidence to suggest that any one dressing was more effective than another in terms of healing foot ulcers in people with DM. A similar conclusion was drawn following a systematic review by the International Working Group of the Diabetic Foot (Game 2012).
NPWT is a technology that is currently used widely in wound care. NPWT is promoted for use on complex wounds, including foot wounds in people with DM, as an adjunct (additional) therapy to standard care (Guy 2012). NPWT involves the application of a wound dressing through which a negative pressure (or vacuum) is applied, with wound and tissue fluid being collected into a canister. The intervention was developed in the 1990s, and the uptake of NPWT in the healthcare systems of high‐income countries has been dramatic. One US Department of Health report estimated that between 2001 and 2007 Medicare payments for NPWT pumps and associated equipment increased from USD 24 million to USD 164 million (an increase of almost 600%) (Department of Health and Human Services 2009). Initially only one NPWT manufacturer supplied NPWT machines (the V.A.C (vacuum‐assisted closure) system: Kinetic Concepts Inc (KCI), San Antonio, TX); however, as the NPWT market has grown, several different commercial NPWT systems have been developed, with machines becoming smaller and more portable. Indeed, the most recent introduction to the market is a single use, or 'disposable,' negative pressure product. Ad hoc, homemade, negative pressure devices are also used, especially in resource‐poor settings. These devices tend to use simple wound dressings, such as gauze, or transparent occlusive (non‐permeable) dressings, with negative pressure generated in hospital by vacuum suction pumps.
Several different healthcare professionals prescribe and apply NPWT, and it is now used both in secondary and primary (community) care, particularly following the introduction of ambulatory systems. While the NPWT systems outlined above differ in a number of respects, such as type of pressure (constant or cyclical) applied to the wound, the material in contact with the surface of the wound and also the type of dressing used, the principle of applying a negative pressure to the wound in a closed environment is the same for all products. The place of NPWT in the treatment pathway and the rationale for its use vary based on different types of wound and local treatment protocols. For open wounds that have been debrided but are still waiting for soft tissue cover, National Institute for Health and Care Excellence (NICE) guidelines recommend that NPWT is considered as an intermediate wound dressing prior to further surgical intervention. Thus, NPWT would be used for a short period of time on an open, postsurgical wound, with a key aim of reducing infection risk.
How the intervention might work
NPWT ostensibly assists in wound management by collecting high volumes of wound exudate, reducing the frequency of dressing changes by keeping anatomically challenging wounds (such foot wounds) clean, and reducing odour. However, manufacturers also suggest that the application of mechanical force to the wound provides biologically plausible processes by which wound healing is promoted (i.e. the drawing together of wound edges, increased perfusion, and the removal of infectious material and exudate) (KCI 2018; Huang 2014). NPWT might have a beneficial effect by encouraging off‐loading (i.e. reducing the weight taken on the foot, as some NPWT systems make ambulation difficult) and preventing unnecessary dressing changes and repeated exposures to the environment. The molecular effects of negative pressure on the wound bed are still being investigated (Glass 2014).
There are some potentially negative aspects associated with NPWT; these include wound maceration (softening due to exposure to liquid), retention of dressings, and wound infection as well as other injuries (FDA 2011). NPWT devices are usually worn continually by patients during treatment, they can interfere with mobility, and, anecdotally, are often noisy, which prevents some people from sleeping.
Why it is important to do this review
NPWT is an expensive, yet widely used, health technology for the management of complex wounds, and there is potential for its use to increase. In the UK, NPWT can now be prescribed by primary care physicians (who may not have specific training in wound care). A Cochrane Review that examined the clinical effectiveness of NPWT for treating chronic wounds had been previously published, but was withdrawn from publication in acknowledgment of the fact that the topic area was too broad, and that separate reviews addressing a single wound type (pressure ulcers, venous leg ulcers and foot ulcers in people with DM) would provide a more focused summary of evidence. There is a great deal of focus on the use of NPWT, and it is an area of high research activity and so is a priority area for review. This updated review includes all foot wounds in people with DM (both surgical and non‐surgical): this scope means that, for people with DM, we present evidence from foot wounds caused by surgical debridement and recent amputation, in addition to evidence for the effects of NPWT on non‐surgically treated foot ulcers or other non‐healing foot wounds. This approach provides an up‐to‐date and comprehensive overview of evidence for NPWT for all types of foot wound in people with DM, with a focus on considering the type of diabetic foot wound to which current evidence relates.
A Cochrane review that comprehensively identifies, interrogates, presents and synthesises evidence of the effects of NPWT on the outcomes of foot wounds in people with DM is a valuable piece of research. The review is relevant to clinical policy and consumer decision‐makers in providing a robust overview of current evidence, and to researchers and funders in highlighting areas of uncertainty that may be addressed by future research. This is relevant, since the draft NICE clinical guideline, Diabetic foot problems: prevention and management (NICE 2016), recommends that NPWT is considered as a treatment after surgical debridement for diabetic foot ulcers on the advice of the multidisciplinary foot care service.
This is the first update of this review: the update is required since there are new trials to be added to the review which previously reported inconclusive findings.
Objectives
To assess the effects of negative pressure wound therapy compared with standard care or other therapies in the treatment of foot wounds in people with DM in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomised controlled trials (RCTs) that evaluated the effects of any brand of NPWT in the treatment of foot wounds in people with DM, irrespective of publication status or language of publication.
Types of participants
Trials recruiting people with type 1 or type 2 DM (as defined by the study authors), with foot wounds below the ankle, regardless of underlying aetiology (i.e. ischaemic, neuropathic or neuroischaemic). This included diabetic foot ulcers, or wounds resulting from amputation or other surgical treatment, or both. We included trials involving people of any age and from any setting.
Where trials with broad inclusion criteria recruited people with DM with foot wounds as part of a larger chronic wound study population (e.g. alongside participants with pressure ulcers or leg ulcers), we excluded these trials unless the results for the subgroup of people with DM with foot wounds were reported separately or were available from authors on request.
Types of interventions
Any brand of NPWT (including studies that investigated homemade or ad hoc negative pressure devices) compared with standard care (such as advanced wound dressings and gauze) or other treatments, so that the primary intervention of interest was NPWT (both commercial and non‐commercial treatments). We included RCTs in which the use of a specific NPWT intervention during the treatment period was the only systematic difference between treatment groups. We anticipated that likely comparisons would include the use of NPWT during the care pathway compared with no use of NPWT or comparison of different types/brands of NPWT used during the care pathway.
Types of outcome measures
We listed primary and secondary outcomes below. If a study was otherwise eligible (i.e. correct study design, population and intervention/comparator) but did not report a listed outcome, then we contacted the study authors where possible to establish whether an outcome of interest here was measured but not reported. If we remained unsure whether an outcome was measured or not, the study was included. We reported outcome measures at the latest time point available (assumed to be length of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this was different from latest time point available). For all outcomes, we planned to class assessment of outcome measures from:
-
one week or less to eight weeks as short term;
-
eight weeks to 16 weeks as medium term;
-
more than 16 weeks as long term.
Primary outcomes
-
Complete wound healing
-
Time to wound healing within a specific time period, correctly analysed using survival, time‐to‐event, approaches, ideally with adjustment for relevant covariates such as size of wound at baseline (start of trial). We assumed that the period of time in which healing could occur was the duration of the trial, unless otherwise stated.
-
Number of wounds completely healed during follow‐up (frequency of complete healing).
-
Where studies reported both of these outcomes, our plan was to present all data in a summary outcome table for reference, but give 'time to healing' primacy. As planned, when time was analysed as a continuous measure but it was not clear whether all ulcers had healed, we documented the use of this outcome in the study but did not summarise, or otherwise use, the data in any meta‐analysis. We accepted study authors' definitions of what constituted a healed wound.
-
Amputation
-
Major amputation (defined as any amputation above the ankle joint).
-
Minor amputation (defined as any amputation below the level of the ankle joint).
-
Secondary outcomes
-
Proportion of wounds closed or covered with surgery: complete wound closure as the result of delayed surgical closure but without subsequent wound healing (i.e. the wounds were surgically closed but not yet healed). The inclusion of this outcome represents a change from the protocol; see Differences between protocol and review for more details.
-
Time to closure or coverage surgery: NPWT is often not used until complete wound healing but until a point where the wound is ready for further treatment such as closure surgery. The inclusion of this outcome represents a change from the protocol; see Differences between protocol and review for more details.
-
Participant health‐related quality of life/health status (measured using a standardised generic questionnaire such as EQ‐5D, 36‐item Short Form (SF‐36), 12‐item Short Form (SF‐12) or six‐item Short Form (SF‐6) or wound‐specific questionnaires such as the Cardiff Wound Impact Schedule at noted time points. These reported data were adjusted for the baseline score. We did not include ad hoc measures of quality of life that were not likely to be validated and would not have been common to multiple trials.
-
Other adverse events (measured using survey/questionnaire/data capture process or visual analogue scale), where a clear methodology for the collection of adverse event data was provided. This would include making it clear whether (i) events were reported at the participant level or if multiple events per person were reported; and (ii) that an appropriate adjustment was made for data clustering. Where available, we extracted data on all serious and all non‐serious adverse events. We did not extract individual types of adverse events such as pain or infection, which require specific assessment under this outcome, rather we used the assessment of any event classed as adverse by the participant or health professional, or both, during the trial.
-
Within‐trial cost‐effectiveness analysis comparing mean differences in effects with mean cost differences between the two arms: data extracted were incremental mean cost per incremental gain in benefit (incremental cost‐effectiveness ratio (ICER)). The inclusion of this outcome represents a change from the protocol; see Differences between protocol and review for more details.
-
Wound recurrence: we accepted study author definitions of wound recurrence unless it was clear that the term had not been used to describe the return of a wound that was previously healed.
Search methods for identification of studies
Electronic searches
In January 2018, we searched the following electronic databases to identify reports of relevant clinical trials:
-
Cochrane Wounds Group Specialised Register (searched 10 January 2018);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 10 January 2018);
-
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 10 January 2018);
-
Ovid Embase (1974 to 10 January 2018);
-
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 January 2018).
Appendix 1 shows the search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries for unpublished and ongoing studies in the area. We searched for trials evaluating NPWT and explored these records for those pertaining to foot wounds in people with DM as defined above:
-
ClinicalTrials.gov (www.clinicaltrials.gov) (28 February 2018);
-
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) (28 February 2018);
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu/ctr‐search/search) (28 February 2018).
Appendix 1 shows the search strategies for clinical trial registries.
Searching other resources
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
When necessary, we contacted authors of key papers and abstracts to request further information about their trials.
We also examined the content of the European Wound Management Association conference proceedings (2012 to 2017) and systematic reviews in the field that might have referred to data we had not found, and contacted key manufacturers (KCI, and Smith & Nephew) to ask about unpublished (as well as ongoing) work.
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Dumville 2013a), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors independently assessed the titles and abstracts of retrieved studies for relevance. After this initial assessment, we obtained full copies of all studies considered to be potentially relevant. Two review authors independently checked the full papers for eligibility; we resolved disagreements by discussion and, where required, the input of a third review author. We recorded all reasons for exclusion of studies for which we had obtained full copies in the Characteristics of excluded studies table. We completed a PRISMA flowchart to summarise this process (Liberati 2009).
Data extraction and management
We extracted and summarised details of the eligible studies using a data extraction sheet. Two review authors extracted data independently and resolved disagreements by discussion, drawing on a third review author where required. Where data were missing from reports, we attempted to contact the study authors to obtain this information. We included studies published in duplicate once, but extracted the maximal amount of data. We extracted the following data, where possible:
-
country of origin;
-
participants' type of DM;
-
wound aetiology (e.g. PAD);
-
type of wound, including site on foot;
-
unit of investigation (per participant) (i.e. single wound, or foot, or patient, or multiple wounds on the same participant);
-
care setting;
-
number of participants randomised to each trial arm;
-
eligibility criteria and key baseline participant data;
-
details of the dressing/treatment regimen received by each group;
-
details of any co interventions;
-
number of postamputation/debridement wounds closed surgically;
-
primary and secondary outcome(s) (with definitions);
-
outcome data for primary and secondary outcomes (by group);
-
duration of follow‐up;
-
number of withdrawals (by group);
-
adverse events;
-
publication status of study; and
-
source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane tool for assessing risk of bias (Higgins 2011a) (Appendix 2). This tool addresses six specific domains, namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance, issues with unit of investigation). We assessed blinding of participants and health professionals, and blinded outcome assessment separately. Blinding to reduce the risk of performance bias is often not possible in device trials but it can be minimised, for example, in some cases using blinded panels to make care decisions. To avoid detection bias, blinded outcome assessment is key in open trials. Hróbjartsson 2012 argued that the estimated effects of experimental interventions in RCTs tended to be considerably more optimistic when they were based on non‐blinded assessment of subjective outcomes compared with blinded assessment.
For our assessment, we were aware that blinding of participants and health professionals to treatment received would not be possible, but it was important to understand if, and how, studies had compensated for this where required. We completed a 'Risk of bias' table for each eligible study and resolved disagreements about risk of bias assessment by discussion. Where possible, when a lack of reported information resulted in an unclear decision, we contacted authors for clarification.
We classed studies with an assessment of high risk of bias for the randomisation sequence domain or the allocation concealment domain or the blinded outcome assessment domain (for specified outcome) (or a combination of these) as being at overall high risk of bias. We also considered the potential for performance and measurement bias for each primary and secondary outcome extracted.
Measures of treatment effect
Where possible, we grouped studies according to wound type. Where possible, we presented the outcome results for each trial with 95% confidence intervals (CI). We reported estimates for dichotomous outcomes (e.g. ulcers healed during a particular time period) as risk ratios (RR). We used the RR rather than odds ratio (OR), since, when event rates are high, as is the case for many trials reporting wound healing, ORs (when interpreted as RR) can give an inflated impression of the effect size (Deeks 2002). We planned to report outcomes relating to continuous data (e.g. percentage change in ulcer area) as mean differences (MD) and overall effect size (with 95% CI). Where a study reported data on time‐to‐healing (the probability of healing over a consecutive time period) we planned to report and plot these data (where possible) using hazard ratio (HR) estimates. However, where the HR was not reported, but data regarding the number of events and the P value for a log rank test (reported to at least two significant figures) were reported, we employed methods proposed by Parmar 1998 to calculate the HR indirectly. Where log rank test P values were published to only one significant figure, the robustness of the calculated HR for the highest possible P value was investigated to test robustness of estimates. HRs and associated 95% CIs were then calculated using the inverse variance option in Review Manager 5 (Review Manager 2014).
Unit of analysis issues
We recorded whether trials presented outcomes in relation to a wound, a foot, a participant or as multiple wounds on the same participant. We also recorded occasions where multiple wounds on a participant were (incorrectly) treated as independent within a study, rather than having within‐participant analysis methods applied. This was recorded as part of the risk of bias assessment. For wound healing and amputation, unless otherwise stated, where the number of wounds appeared to equal the number of participants, we treated the participant as the unit of analysis. For other adverse event outcomes, in order to facilitate further analyses, we aimed to establish whether data were presented at the level of the participant, because in this area there is potential for data to refer to multiple events occurring to a single person (or wound per person), which means that data cannot be analysed further without violating the assumption of independence.
Where studies randomised at the participant level and measured outcomes at the wound level (e.g. wound healing), we treated the participant as the unit of analysis when the number of wounds assessed appeared equal to the number of participants (e.g. one wound per person).
Where there were instances of clustered data, that is where a proportion of individually randomised trial participants had outcome data collected and reported on multiple wounds, this was not treated as a cluster trial since not all participants would have multiple wounds. Rather this was a trial that incorrectly included a mixture of individual and clustered data. We noted these trials and recorded the issue in the risk of bias assessment. Data were extracted and presented but not the subject of any further analyses.
We planned only to incorporate clearly conducted fully clustered trials into meta‐analyses if the trial was analysed correctly. Where a cluster trial had been conducted but incorrectly analysed, we recorded this as part of the 'Risk of bias' assessment. If possible we planned to approximate the correct analyses based on Cochrane Handbook for Systematic Reviews of Interventions guidance (Higgins 2011b) using information on:
-
the number of clusters (or groups) randomised to each intervention group; or the average (mean) size of each cluster;
-
the outcome data ignoring the cluster design for the total number of participants (e.g. number or proportion of participants with events, or means and standard deviations (SD)); and
-
an estimate of the intracluster (or intraclass) correlation coefficient (ICC).
Where multiple trial arms were reported in a single trial, we planned to include only the relevant arms. If two interventions or more interventions were compared with control and eligible for the same meta‐analysis, we planned to pool the intervention arms and compare them with control. If the study data could not be analysed correctly, we extracted outcome data and presented them but did not analysed them further.
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants post randomisation from the analysis, or ignoring those participants who are lost to follow‐up compromises the randomisation, and potentially introduces bias into the trial. In individual studies, where data on the proportion of ulcers healed were presented, we assumed that if randomised participants were not included in an analysis, their wound did not heal (i.e. they would be considered in the denominator but not the numerator). Where a trial did not specify participant group numbers prior to dropout, we presented only complete‐case data. In a time‐to‐healing analysis using survival analysis methods, dropouts should be accounted for as censored data. Hence all participants contributed to the analysis. Such analysis assumes that dropouts are missing at random (i.e. not associated with time to healing). We presented data for area change, and for all secondary outcomes, as a complete‐case analysis.
Assessment of heterogeneity
We considered both clinical and statistical heterogeneity. Wherever appropriate, that is, where studies appeared similar in terms of wound type, intervention type, duration and outcome type, we pooled data using meta‐analysis (conducted using Review Manager 5 (Review Manager 2014)). We planned to assess statistical heterogeneity using the Chi² test (a significance level of P less than 0.1 was considered to indicate heterogeneity) and the I² estimate (Higgins 2003). The I² estimate examines the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I² higher than 50% indicate a high level of heterogeneity. In the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I² over 50%), we envisioned using a random‐effects model; however, we did not anticipate pooling studies where heterogeneity was very high (I² over 75%) (Deeks 2011). Where there was no clinical or statistical heterogeneity, we used a fixed‐effect model.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of small‐study effects, that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small‐study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial's size or precision (Sterne 2011). We planned to present funnel plots for meta‐analyses comprising 10 RCTs or more using Review Manager 5 (Review Manager 2014).
Data synthesis
We were unable to pre specify the amount of clinical, methodological and statistical heterogeneity in the included studies. Thus, we used a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow CIs. We would only have used a fixed‐effect approach when clinical and methodological heterogeneity was assessed to be minimal, and the assumption that a single underlying treatment effect was being estimated held. Chi² and I² statistics were used to quantify heterogeneity but were not used to guide choice of model for meta‐analysis (Kontopantelis 2012). We would have exercised caution when meta‐analysed data were at risk of small‐study effects because use of a random‐effects model may be unsuitable here. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we planned to assess the impact of the approach using sensitivity analyses to compare results from alternate models, but this was not implemented (Thompson 1999).
We presented data using forest plots where possible. For dichotomous outcomes, we presented the summary estimate as an RR with 95% CI. Where continuous outcomes were measured, we presented an MD with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to use the inverse variance method on the estimated HR and standard error, when reported or calculated from available data. Unfortunately, it was not possible for us to plot (and, if appropriate, to pool) estimates of HRs and 95% CIs for time‐to‐event data, as there were insufficient data presented in the study reports. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds had healed, we documented use of the outcome in the study, but did not summarise or use these data in any meta‐analysis.
We obtained pooled estimates of the treatment effect using Review Manager 5 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We considered whether there was potential heterogeneity between wound dressings used in control groups (i.e. advanced dressings (non‐antimicrobial), antimicrobial dressings or basic contact dressings) as there is no single dressing to suit all scenarios (Wounds International 2013). Where there was evidence of between‐trial heterogeneity in trial‐level co interventions, especially off‐loading, we envisaged a subgroup analysis being conducted based on variations in co interventions (e.g. all trial participants reported to receive adequate off‐loading protocol/advice being compared with trial participants who received unclear advice about off‐loading); however, this was not required. Finally, depending on the number and heterogeneity of included studies, we considered using meta‐regression to investigate wound aetiology as a possible explanatory variable but this analysis also was not possible.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the effect of the removal of studies classed at high risk of bias for any domain, but this was not possible due to lack of available data.
'Summary of findings' tables
We used the principles of the GRADE system to assess the certainty of the body of evidence associated with specific outcomes (Guyatt 2008), and constructed 'Summary of findings' tables using GRADEpro GDT software (GRADEpro GDT 2015).
These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We included the following main outcomes in the 'Summary of findings' tables:
-
proportion of wounds healed;
-
time to ulcer healing;
-
amputation;
-
number of wounds closed or covered with surgery;
-
adverse events;
-
cost‐effectiveness;
-
wound recurrence.
For relevant outcomes reported for comparisons not listed above, we present a GRADE assessment without a 'Summary of findings' table.
When evaluating the 'Risk of bias' domain, we downgraded the GRADE assessment only when we classified a study as being at high risk of bias for one or more domains, or when the 'Risk of bias' assessment for selection bias was unclear (this was classified as unclear for the generation of the randomisation sequence domain and the allocation concealment domain). We downgraded the GRADE assessment when the 'Risk of bias' assessment for blinding was unclear (this was classified as unclear for the performance bias domain and the detection bias domain) as well as at high risk of bias. We did not downgrade for unclear 'Risk of bias' assessments in other domains.
We selected an informal optimal information size of 300 for binary outcomes, following the GRADE default value (Guyatt 2011). We also followed GRADE guidance and downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
Results
Description of studies
Results of the search
The initial version of this review included five studies (Dumville 2013a). This is the first update and six studies have been added (Dalla‐Paola 2010; Lavery 2014; Nain 2011; Vaidhya 2015; Zhang 2017; Zhu 2014). We present the results of the search in the PRISMA diagram (Figure 1).
The literature search for this 2018 update yielded 208 abstracts: we sought 23 full‐text articles for further scrutiny. From the 23 articles, we included six studies. There are no studies awaiting classification. See Characteristics of included studies and Characteristics of excluded studies tables for full details of the studies identified. We contacted all trial authors for additional information and missing data; any responses are noted in relevant tables. Four studies are ongoing: ACTRN12612000885897; ChiCTR‐TRC‐12002700; DRKS00000059; and ISRCTN64926597. To date, only ISRCTN64926597 has begun to recruit participants (see Ongoing studies).
Included studies
This review includes 11 studies randomising 972 participants. Ten studies had two arms (Armstrong 2005; Blume 2008; Dalla‐Paola 2010; Karatepe 2011; Lavery 2014; Mody 2008; Nain 2011; Vaidhya 2015; Zhang 2017; Zhu 2014), and one had three arms (Novinščak 2010). All studies were parallel studies.
Three studies were undertaken in the USA (Armstrong 2005; Blume 2008; Lavery 2014); two in China (Zhang 2017; Zhu 2014); one in Italy (Dalla‐Paola 2010); one in Croatia (Novinščak 2010); three in India (Mody 2008; Nain 2011; Vaidhya 2015); and one in Turkey (Karatepe 2011).
Populations evaluated in the studies were people with DM and foot wounds resulting from amputation in two studies (Armstrong 2005; Dalla‐Paola 2010), and people with DM and foot ulcers in all the other studies (Blume 2008; Karatepe 2011; Lavery 2014; Mody 2008; Nain 2011; Novinščak 2010; Vaidhya 2015; Zhang 2017; Zhu 2014). Two studies reported their funding source: Armstrong 2005 and Blume 2008 received funding from KCI – manufacturers of the V.A.C. intervention.
Comparison arms received a variety of treatments including:
-
dressings:
-
advanced moist wound therapy (non‐antimicrobial dressing): Armstrong 2005 (moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings); Dalla‐Paola 2010 (alginate, hydrofibre, silver‐dressing or polyurethanes); Blume 2008 (advanced moist wound therapy dressings, predominantly hydrogels and alginates);
-
antimicrobial dressing: Zhang 2017 (0.5% dilute iodoform gauze and Vaseline gauze); Zhu 2014 (povidone and lipid dressing);
-
basic contact dressing: Karatepe 2011 (sterilised gauze); Mody 2008 (moist gauze); Nain 2011 (saline moistened gauze); Novinščak 2010 (moist dressings and dry gauze); Vaidhya 2015 (saline moistened gauze);
-
-
different pressures of NPWT: Lavery 2014 (75 mmHg and 125 mmHg).
Trials had a range of follow‐up periods:
-
four weeks (Lavery 2014);
-
eight weeks (Nain 2011; Novinščak 2010);
-
16 weeks (Armstrong 2005; Blume 2008); or
-
unclear (Dalla‐Paola 2010; Karatepe 2011; Mody 2008; Vaidhya 2015; Zhang 2017; Zhu 2014).
In terms of primary outcomes, four studies reported time to healing data (Armstrong 2005; Blume 2008; Karatepe 2011; Zhu 2014), seven reported proportion of wounds healed (Armstrong 2005; Blume 2008; Mody 2008; Nain 2011; Novinščak 2010; Zhang 2017; Zhu 2014); five reported data on amputations recorded during study follow‐up (Armstrong 2005; Blume 2008; Vaidhya 2015; Zhang 2017; Zhu 2014); and one reported amputations after the follow‐up period (Dalla‐Paola 2010). For further details, see Table 1.
| Study | Wound characteristics | Comparison | Length of follow‐up | NPWT pathways | Time to healing | Number of wounds completely healed | Amputation | Number of wounds closed or covered with surgery | Time to closure or coverage surgery | Adverse events | Health‐related quality of life | Cost‐effectiveness | Wound recurrence |
| Diabetic foot amputation to trans‐metatarsal level | Group A: NPWT (V.A.C. system), dressing changes every 48 h. Treatment conducted until wound closure or completion of 112‐day assessment (n = 77) Group B: moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings (n = 85) | 16 weeks | After amputation (close/open wounds; if open wounds, secondary intention), NPWT delivered through the V.A.C. system; or standard care with moist wound therapy. | Kaplan‐Meier median time to healing Group A: 56 days (IQR 26 to 92) Group B: 77 days (IQR 40 to 122) Log‐rank taken as P = 0.005 There was no difference noted in time to healing for acute or chronic wounds. | Group A: 43/77 (55.8%) Group B: 33/85 (38.8%) Of healed wounds –healed by secondary intention (without primary/surgical wound closure) Group A: 31/43 (72.1%) Group B: 25/33 (75.8%) Remaining wounds were closed following surgery. | Number of participants undergoing further amputation Group A: 2/77 (2.3%) Major = 0 Minor = 2 Group B: 9/85 (10.6%) Major = 5 Minor = 4 | Not reported | Not reported | Participants who had ≥ 1 adverse events Group A: 40/77 (51.9%) Group B: 46/85 (54.1%) Participants who had ≥ 1 treatment‐related adverse events Group A: 9/77 (11.7%) 1 classified serious Group B: 11/85 (12.9%) 5 classified as serious | Not reported | Not reported | Not reported | |
| Ulceration of the foot in people with diabetes | Group A: NPWT (V.A.C. system), applied according to manufacturer’s instructions (n = 172) Group B: advanced moist wound therapy dressings used according to guidelines/local protocols (n = 169) | 16 weeks | NPWT was continued until ulcer closure. | Kaplan‐Meier median time to healing Group A: 96 days (95% CI 75.0 to 114.0) Group B: could not be estimated Log‐rank taken as P = 0.001 | Group A: 3/172 (42.4%) Group B: 8/169 (28.4%) (6 participants excluded in paper as did not receive treatment, added back into denominator here; ITT 172/169) | Number of participants undergoing amputation* Group A: 7/172 (4.1%) Major = 5 Minor = 2 Group B: 17/169 (10.1%) Major = 4 Minor = 13 | Not reported | Not reported | Limited data: not extracted | Not reported | Not reported | Not reported | |
| Infected open amputations or surgical dehiscence of minor amputations in people with diabetes | Group A: V.A.C. therapy following surgical debridement (n = 65) Group B: advanced dressings following surgical debridement (n = 65) | Not specified. End of therapy was defined as complete coverage of the wound with epithelial tissue. | Duration of therapy depended on the functional parameters of the wound area. | Not reported | Not reported | Number of participants undergoing further amputation (major) Group A: 3/65 (4.6%) Group B: 5/65 (7.7%) | Group A: 63/65 (96.9%) Group B: 62/65 (95.4%) | Group A (n = 65): 65 days (SD 16) Group B (n = 65): 98 days (SD 45) P = 0.005 These data reported as time to "complete closure of the wound" was reached. Unclear if it is mean or median; unclear if "complete closure" means "time to healing of grafted wound" or "time to surgical closure;" unclear if it is a valid measure as not sure all ulcers have healed. Author contacted – waiting for response. | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: NPWT (V.A.C. system) (n = 30) Group B: conventional wound care treatment: based on text in report taken to be dry gauze (n = 37) | Not specified. Last assessment 1 month after healing | Not specified | Median time to healing Group A: 4.4 weeks Group B: 3.9 weeks Mean value presented but not extracted. No specific P value presented (< 0.05) | Not reported | Not reported | Not reported | Not reported | Not reported | SF‐36: data not presented | Not reported | Not reported | |
| Diabetic foot wounds, after incision and drainage | Group A: NPWT with Group B: 125 mmHg of pressure | 4 weeks | NPWT was continued for 4 weeks | Not reported | Not reported | Not reported | Group A: 10/20 (50%) Group B: 12/20 (60%) | Not reported | Group A: study related 2/20 (10%); non‐study related 1/20 (5%) Group B: study related 1/20 (5%); non‐study related 1/20 (5%) | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: locally constructed NPWT (n = 6) Group B: wet‐to‐dry gauze (n = 9) | Not specified: until healing or loss to follow‐up | People receiving TNP only in hospital | Not reported | By secondary intention: Group A: 1/6 (16.6%) Group B: 1/9 (11.0%) | Not reported | By delayed primary closure: Group A: 0/6 (0%) Group B: 3/9 (33%) | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: negative pressure dressing (n = 15) | 8 weeks | Ulcers were treated until the wound was closed surgically or spontaneously, or until completion of the 56 days (8 weeks) assessment whichever was earlier. | Not reported | Group A: 12/15 (80%) Group B: 9/15 (60%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Complicated diabetic foot ulcers | Group A: NPWT (n = 7) Group B: dressings (moist) (n = 12) Group C: classic gauze (n = 8) | 8 weeks | Treatment was monitored for the first 2 months. | Not reported | Group A: * could not be calculated (90%) Group B: 9/12* (75%) Group C: 4/8* (50%) *Figure calculated by review author We obtained data (only proportions) from the study author but were unable to use these to calculate number of healed wounds. It seemed this outcome was measured but was not able to use the data in meta‐analysis. | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wound | Group A: NPWT (n = 30) | Not specified | Interventions discontinued for participants in whom failure or complications | Not reported | Not reported | Data for alternative therapy or amputation: Group A: 3/30 (10%) Group B: 7/30 (23.3%) | Wounds were ready for either skin grafting or secondary suturing (end point) Group A: 27/30 (90%) Group B: 23/30 (67.7%) | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Limited data: not extracted | Not reported | |
| Chronic diabetic ulcers | Group A: vacuum sealing drainage (n = 20) Group B: gauze dressing (n = 20) | Not reported | Interventions were administered in hospital | Not reported | Group A: 17/20 (85%) Group B: 13/20 (65%) | Group A: 1/20 (5%) Group B: 2/20 (10%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wounds | Group A: vacuum sealing drainage (n = 30) | Not reported Follow‐up to 6–10 months for wounds recurrence | Vacuum sealing drainage administered when necessary at several time points | Not reported properly – not all ulcers healed | Group A: 7/30 (23%) Group B: 5/30 (17%) | Group A: 0 Group B: 6/30 (20%) | Of healed wounds by secondary surgery (skin/flap grafting): Group A: 23/30 Group B: 19/24 | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Not reported | Group A: 2 Group B: 4 Follow‐up time: 6–10 months |
h: hour; IQR: interquartile range; ITT: intention to treat; n: number of participants; NPWT: negative pressure wound therapy; SF‐36: 36‐item Short Form; TNP: topical negative pressure.
In terms of secondary outcomes, five studies reported number of wounds closed or covered with surgery (Dalla‐Paola 2010; Lavery 2014; Mody 2008; Vaidhya 2015; Zhu 2014), two reported adverse events (Armstrong 2005; Lavery 2014), and one reported wound recurrence (Zhu 2014).
Excluded studies
Twenty‐eight studies were excluded after investigation of the full text. Eight studies had study populations with multiple wound types and we were unable to obtain separate data on people with DM and foot wounds; nine studies were not considered to be RCTs; nine studies focused on biochemical and related outcomes and, due to the very short follow‐up, we considered that relevant outcomes were not measured (they were not reported); and two studies evaluated NPWT as part of a range of treatments, so this intervention was not the only difference between trial groups. See Characteristics of excluded studies for further details.
See Figure 1 for PRISMA diagram.
Risk of bias in included studies
See Figure 2; Figure 3 risk of bias assessment by study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequacy of randomisation process
All included studies were described as 'randomised' with six studies providing information to confirm that adequate sequence generation had taken place (Armstrong 2005; Blume 2008; Dalla‐Paola 2010; Karatepe 2011; Lavery 2014; Mody 2008); these were at low risk of bias for this domain (all studies used computer‐generated sequences). The remaining five studies did not describe how randomisation took place, and were at unclear risk of bias for this domain.
Allocation concealment
Two of the 11 studies were low risk of bias for allocation concealment (Armstrong 2005; Blume 2008). Both studies employed 'sealed envelopes containing opaque, black paper labelled with assigned treatment and participant ID number that were sequentially numbered and provided to each site,' which we deemed to be robust. The remaining studies did not contain enough detail for us to make a judgement for this domain, and so were at unclear risk of bias.
Blinding
All studies were at unclear risk of blinding bias. We note that while Armstrong 2005, Blume 2008, and Lavery 2014 appeared to undertake some blinded outcome assessment, we questioned the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias. There was no indication that the decision to undertake closure or amputation was guided by the protocol to ensure that there were no differences in performance between groups for reasons other than the treatment received (e.g. surgery was an option only when wounds reached a particular size or condition), or was undertaken by a blinded committee to ensure consistency between groups. Given the non‐blinded status of health professionals to treatment received, there may have been the potential for performance bias in promoting surgery (closure or amputation) in one group compared with the other.
Incomplete outcome data
Seven studies were at low risk of bias for attrition bias (Armstrong 2005; Dalla‐Paola 2010; Lavery 2014; Mody 2008; Vaidhya 2015; Zhang 2017; Zhu 2014). Three studies were at unclear risk of bias: Blume 2008 reported a small number of post‐randomised exclusions, as well as being unclear about whether there was a large number of early censoring in the analysis; Karatepe 2011 and Novinščak 2010 reported very little information regarding participant flow through the study. Nain 2011 was at high risk of bias as it was unclear how many people underwent amputation.
Other potential sources of bias
We assessed Nain 2011 and Novinščak 2010 as being at unclear risk of other bias because the data presented in the studies did not consistently match or lacked clarification, which may have resulted in bias. We judged Armstrong 2005 and Blume 2008 to be at unclear risk of bias for this domain as they were funded by an NPWT manufacturer. All other studies were judged as being at low risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison NPWT compared with dressings for postoperative foot wounds in people with diabetes mellitus; Summary of findings 2 NPWT compared with dressings for foot ulcers in people with diabetes mellitus; Summary of findings 3 Low‐pressure compared with high‐pressure NPWT for foot ulcers in people with diabetes mellitus
Outcome data are summarised in Table 1.
Postoperative wounds
Comparison 1. Negative pressure wound therapy compared with dressings
Two studies with 292 participants (medium‐term follow‐up or unspecified follow‐up) compared NPWT with dressing for amputation wounds (Armstrong 2005; Dalla‐Paola 2010).
Primary outcome: proportion of wounds healed
One study reported proportion of wounds healed (Armstrong 2005). The study randomised 162 participants with DM who had previously undergone foot amputation (to the trans‐metatarsal level) to receive NPWT (dressing changed every 48 hours) or treatment with alginate, hydrocolloid, foam or hydrogel dressings. Participants were followed for 16 weeks. This study reported an increased number of healed wounds in the NPWT group compared with the dressings group (RR 1.44, 95% CI 1.03 to 2.01; low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision) (Analysis 1.1). This means that people in the NPWT group had 1.44 times the 'risk' (likelihood) of healing compared with people in the moist dressing group.
In total, 12/77 (22%) participants in the NPWT group had wounds classed as healed following closure via surgery compared with 8/85 (9%) participants in the dressing group. It was not clear from the report when a surgically closed wound was classed as healed. We contacted the trial authors and they replied that "surgically closed wounds were classed as healed based on the same criteria as the open wounds. Epithelialized with no drainage. Typically that was between 2–4 weeks after closure for both groups depending on the surgeon's assessment."
While participants with 'wounds healed' did undergo blinded outcome assessment, health professionals were aware of treatment received during the study and could decide to close wounds via surgery, which risks introducing performance bias into the findings. There was no indication in the study report that this decision to stop NPWT treatment and recommend surgery was guided by specific decision rules (e.g. size of wound), or was made in a blinded fashion. Thus, potentially, different numbers and types of participants within groups may have had wounds 'closed'.
Primary outcome: time to ulcer healing
One study reported time to healing (complete wound closure) (Armstrong 2005). The study reported a significantly shorter time in the NPWT group (median time to healing of 56 days) compared with the moist dressing group (median time to healing 77 days). We noted that these reported figures did not agree with the Kaplan‐Meier curve reported in the paper, where median values seemed to be higher.
The authors reported that the results of the time to wound closure analysis were statistically significant (P = 0.005: results from a log rank test). Using the observed numbers of events and total numbers in each group together with the reported P value to calculate the log HR and its standard error (Parmar 1998), we calculated the log HR to be 0.645 (0.69 where maximum P value of log rank test assumed, as only reported to one significant figure) with a standard error of 0.23, which gives an HR of 1.91 (95% CI 1.21 to 2.99). Thus, our calculations suggest that, at any point during follow‐up, the hazard (or chance) of healing in participants allocated to NWPT was 1.9 times that of participants allocated to the moist dressing group; NPWT may decrease the time to healing compared with dressings (low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision) (Analysis 1.2). There was the potential for the time to healing outcome to be biased by the undertaking of closure surgery in a non‐blinded and non‐protocol‐driven manner.
Primary outcome: amputations
Two studies reported amputation (Armstrong 2005; Dalla‐Paola 2010). Only Dalla‐Paola 2010 specified major and minor amputations. We decided to carry out meta‐analysis without distinguishing between these two subgroups. It is uncertain whether there is a clear difference between NPWT and wounds treated with dressings in number of amputations ((5/142 (3%) with NPWT versus 14/145 (11%) with dressings; RR 0.38, 95% CI 0.14 to 1.02; very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision) (Analysis 1.3). Ten of the amputations in the dressing group and three in the NPWT group were classed as major. Also, it was not clear whether decisions about amputation were covered by decision rules in the protocol to avoid any potential performance bias.
Secondary outcome: number of wounds closed or covered with surgery
One study (130 participants) reported data on number of wounds closed or covered with surgery (Dalla‐Paola 2010). Based on the findings of this single study it is uncertain whether there is a difference between NPWT or dressings in number of wounds closed or covered with surgery (RR 1.02, 95% CI 0.95 to 1.09; very low‐ certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision) (Analysis 1.4).
Secondary outcome: adverse events
One study reported adverse events (Armstrong 2005). From the study report it is uncertain whether there is a difference in the number of participants experiencing one or more adverse events in the NPWT group compared with the moist dressing group (40/77 (52%) with NPWT versus 46/85 (54%) with dressings; RR 0.96, 95% CI 0.72 to 1.28; very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision) (Analysis 1.5).
Summary of NPWT compared with wound dressings for postoperative wounds
Low‐certainty evidence reporting the hazard or 'chance' of healing over time suggests that there may be a benefit for postoperative foot wounds in participants with DM being treated with NPWT compared with dressings. Low‐certainty evidence also shows that NPWT may decrease the time to healing compared with dressings. There is very low‐certainty evidence on number of wounds closed or covered with surgery, adverse events and amputations, suggesting that it is uncertain whether there is a clear difference between the treatments (summary of findings Table for the main comparison).
Foot ulcers in people with diabetes mellitus
Comparison 2. Negative pressure wound therapy compared with dressings
Eight studies with 640 participants (medium‐term, long‐term or unspecified follow‐up) compared NPWT with dressings for foot ulcers.
Primary outcome: proportion of wounds healed
Six studies (513 participants; medium‐term, long‐term or unspecified follow‐up) reported proportion of wounds healed (Blume 2008; Mody 2008; Nain 2011; Novinščak 2010; Zhang 2017; Zhu 2014). Five studies with 486 participants contributed data to this comparison (Novinščak 2010 was not included as actual numbers of participants healed were not provided) (Analysis 2.1).
Evidence from five pooled studies (n = 486) suggests that NPWT may increase the number of completely healed wounds compared with dressings (RR 1.40, 95% CI 1.14 to 1.72; I² = 0%; low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision).
Subgroup analyses
Of the prespecified subgroup analyses, we were only able to conduct the comparison based on different wound dressings in control groups. The results of this analysis are shown in Analysis 2.1. There is no evidence of a difference between these subgroups (test for subgroup differences: P = 0.85).
Primary outcome: time to ulcer healing
Three studies (468 participants; long‐term or unspecified follow‐up) reported time to ulcer healing data.
Blume 2008 presented a Kaplan‐Meier curve and reported that time to complete wound closure was significantly shorter in the NPWT group, with median time to healing of 96 days (95% CI 75 to 114), compared with the moist dressing group, in which the median number of participants healed was not reached over the 16‐week follow‐up.
A log rank test returned a P value of 0.001. Using the method recommended in Parmar 1998 we calculated the log HR as 0.598 (0.581 where maximum P value of log rank test assumed as only reported to one significant figure) with a standard error of 0.182, which gave an HR of 1.82 (95% CI 1.27 to 2.60). These calculations suggest that, at any point during follow‐up, the hazard (or chance) of healing for participants allocated to NWPT was 1.8 times that of participants allocated to the moist dressing group.
Using the additional analyses outlined we concluded that NPWT may decrease the time to healing compared with dressings (low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision). There was potential for the time to healing outcome to have been affected by the undertaking of closure surgery in a non‐blinded and non‐protocol‐driven way.
Karatepe 2011 reported that median time to healing was 3.9 weeks in the NPWT group compared with 4.4 weeks in the gauze group (P < 0.05, reported by the trial authors) (very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision). However, limited data were presented and an HR could not be calculated.
Zhu 2014 reported the mean time to healing of the healed wounds (mean: 30.32 (SD 3.80) days in the NPWT group compared with 60.51 (SD 8.22) days in the traditional dressing group; P < 0.05, reported by the trial authors); however, as not all wounds healed in this study, it was not appropriate to further analyse mean time to healing data (very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision).
We did not pool data for this comparison as we were unable to convert all results into a single suitable measure with associated variance measures.
Primary outcome: amputations
Three studies (441 participants; long‐term or unspecified follow‐up) reported amputation (without distinguishing between major and minor amputations) (Blume 2008; Zhang 2017; Zhu 2014). The pooled study evidence suggests that NPWT may decrease amputations compared with dressings (RR 0.33, 95% CI 0.15 to 0.70; I² = 0%; low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision) (Analysis 2.2).
A fourth study reported data for alternative therapy or amputation (which we reported narratively rather than pooling them into an analysis; 3/30 participants with NPWT versus 7/30 participants with control) (Vaidhya 2015).
The other four studies did not report relevant data about amputation (Karatepe 2011; Mody 2008; Nain 2011; Novinščak 2010).
Secondary outcome: number of wounds closed or covered with surgery
Three studies (129 participants; unspecified follow‐up) reported number of wounds closed or covered with surgery (Mody 2008; Vaidhya 2015; Zhu 2014). The pooled study evidence suggests there is no clear difference between NPWT and dressing‐treated wounds in number of wounds closed or covered with surgery (RR 1.02, 95% CI 0.85 to 1.24; I² = 28%; low‐certainty evidence, downgraded once for serious risk of bias and once for serious imprecision) (Analysis 2.3).
Secondary outcome: wound recurrence
One study (60 participants; long‐term follow‐up) reported data on wound recurrence (Zhu 2014). The reported duration of follow‐up was six to 10 months. Based on the findings of this single study we are uncertain whether NPWT reduces the risk of wound recurrence compared with dressings (RR 0.50, 95% CI 0.10 to 2.53; very low‐certainty evidence, downgraded once for serious risk of bias and twice due to very serious imprecision) (Analysis 2.4).
Summary of NPWT compared with wound dressings for foot ulcers
Available trial evidence from five studies with 486 participants shows that NPWT may increase the number of completely healed wounds compared with dressings (low‐certainty evidence). Data from one study (342 participants) suggests that NPWT may decrease the time to healing compared with dressings (low‐certainty evidence). Data from three studies (441 participants) suggests treatment with NPWT may reduce the risk of amputation compared with dressings (low‐certainty evidence). Data from three studies (129 participants) shows no clear difference in number of wounds closed or covered with surgery (low‐certainty evidence). It is uncertain whether the incidence of wound recurrence differed between groups (very low‐certainty evidence from one study with 60 participants) (summary of findings Table 2).
Comparison 3. NPWT 75 mmHg versus 125 mmHg
One study (40 participants, short‐term follow‐up) compared NPWT 75 mmHg versus 125 mmHg (Lavery 2014). The study randomised 40 people with DM with foot ulcers to receive NPWT 75 mmHg or NPWT 125 mmHg. Participants were followed‐up for four weeks.
Primary outcome: proportion of wounds healed
The study did not report proportion of wounds healed.
Primary outcome: time to ulcer healing
The study did not report time to ulcer healing.
Primary outcome: amputations
The study did not report amputations.
Secondary outcome: number of wounds closed or covered with surgery
Based on the findings of this single study we are uncertain whether there is a difference between NPWT 75 mmHg and NPWT 125 mmHg in terms of the number of wounds closed or covered with surgery (RR 0.83, 95% CI 0.47 to 1.47; very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision) (Analysis 3.1).
Secondary outcome: adverse events
Based on the findings of this single study we are uncertain whether there is a difference between NPWT 75 mmHg and NPWT 125 mmHg in terms of number of adverse events (RR 1.50, 95% CI 0.28 to 8.04; very low‐certainty evidence, downgraded once for serious risk of bias and twice for very serious imprecision) (Analysis 3.2).
Summary of low compared with high pressure of NPWT for diabetic foot ulcers
It is uncertain whether there is a difference in the number of wounds closed or covered with surgery and adverse events between NPWT 75 mmHg or NPWT 125 mmHg groups (very low‐certainty evidence; summary of findings Table 3). There were no data on primary outcomes.
Discussion
See: summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3.
Summary of main results
We included 11 studies with 972 participants in the review. Ten studies compared NPWT with dressings (two for amputation wounds and eight for foot ulcers in people with DM); one study compared NPWT at 75 mmHg and 125mmHg for the treatment of foot ulcers.
NPWT compared with dressings in postoperative wounds
There is low‐certainty evidence to suggest that NPWT may be effective in healing postoperative foot wounds compared with wound dressings in terms of the proportion of wounds healed and time to healing. It is uncertain whether there is a difference in number of wounds closed or covered with surgery, adverse events and amputations between the treatment groups (very low‐certainty evidence).
NPWT compared with dressings in diabetic foot ulcers
There is low‐certainty evidence to suggest that NPWT may be effective in healing ulcers compared with wound dressings in terms of the proportion of wounds healed and time to healing. There is low‐certainty evidence suggesting NPWT may reduce the risk of amputation, but that there is no clear difference in the number of wounds closed or covered with surgery between the treatment groups. It is uncertain whether the incidence of wound recurrence differs between groups (very low‐certainty evidence).
Low compared with high pressure NPWT in diabetic foot ulcers
It is uncertain whether there is a difference in number of wounds closed or covered with surgery and adverse events between treatment with NPWT 75 mmHg or NPWT 125 mmHg (very low‐certainty evidence). There were no data on primary outcomes.
Overall completeness and applicability of evidence
The included studies recruited adults with DM with foot wounds involving postoperative amputation wounds and foot ulcers. The included studies compared NPWT with dressings and compared NPWT applied at different pressures for treating multiple wounds.
Although we identified 11 studies, many of these did not report, or did not fully report, the primary outcomes of this review: wound healing and amputation. Therefore, usable data on key outcomes were limited and often unavailable. Only a minority of studies reported enough data to enable us to calculate the most appropriate measure of time‐to‐event data – an HR. Where this was not available, we were in some cases able to report a mean time to healing or a relative risk of healing for a particular time point. Neither of these measures was ideal and both may have given an impression of either an effect or a lack of effect which was not truly present, particularly where the event rate was high. For the secondary outcomes, apart from the number of wounds closed or covered with surgery and adverse events, the other outcomes were reported in single studies. All evidence is of low or very low certainty because of risk of bias and imprecision.
The included studies took place in a range of settings and countries, including low‐ to middle‐income countries. The geographical distribution of the studies reflected the concentration of disease burden outside of Western high‐income countries. The use of NPWT for the treatment of foot wounds in people with DM was similar in that the treatment was used on the most serious wounds that could not be easily covered or closed during initial surgery. The treatment aim in most studies was to close the wounds in the near future, which seemed to reflect common practice in this area. Beyond this, treatment protocol varied across studies in terms of frequency of dressing change and dressing type; however, these variations are common in clinical practice. We grouped all dressing treatments as one control group, which we acknowledge is a broad grouping. The generalisability from such a grouping is unclear and the evidence will need to be considered alongside the results of further studies when these become available.
Quality of the evidence
The certainty of the available evidence is low or very low. This is due to the risk of bias, small sample size and wide CIs that included both an effect and no effect or even a harm of the intervention. We downgraded the evidence certainty due to the high risk of bias for the randomisation sequence domain or the allocation concealment domain or the blinded outcome assessment domain or a combination of these. We also downgraded the evidence certainty if the randomisation sequence domain and the allocation concealment domain were both at unclear risk of bias; similarly, we downgraded the evidence certainty if the performance bias and detection bias were both assessed as unclear risk of bias.
We noted that while Armstrong 2005, Blume 2008, and Lavery 2014 appeared to undertake some blinded outcome assessment, we questioned the potential impact of non‐blinded decisions regarding the use of further surgery and the increased risk of performance bias. Given the non‐blinded status of health professionals to treatment received, there may have been the potential for performance bias in promoting surgery (closure or amputation) in one group compared with the other. The two largest studies included in this review, Armstrong 2005 and Blume 2008, were similar in design (both were funded by the manufacturer of V.A.C., i.e. KCI) although they evaluated different types of foot wounds. While these studies were deemed to be at low risk of bias for random sequence generation and allocation concealment, the risk of performance and detection bias for both was unclear, since study reports suggested that key decisions regarding the treatment of wounds, such as closure surgery and further amputation, were made by unblinded health professionals and were not guided by a trial protocol in a way that would minimise potential performance bias. This issue has been noted in other reviews (e.g. Medical Advisory Secretariat 2006), and the validity of combining wounds closed by secondary intention and those closed by surgery questioned. For Blume 2008, it was also unclear whether the study's analysis was as close to an intention‐to‐treat analysis as would be possible with the data collected. The sample sizes of the remaining studies were quite small, leading to imprecision and wide CIs; which in turn led to an overall assessment of very low‐certainty evidence.
We also noted that the included studies had limited information about the receipt of important adjunctive therapies such as off‐loading. While these therapies were often noted as being delivered where required, it would be useful to know whether their delivery was balanced between study groups, as they are such an important part of routine care.
Potential biases in the review process
Following the upgrade of Review Manager 5 in recent years, new methods which were not previously considered were subsequently included in the review. These changes have been highlighted in the Differences between protocol and review section. These additions only serve to ensure a more robust process and methodology; therefore, we do not consider them to be of concern.
We made a concerted effort to prevent biases during the review process by ensuring an extensive literature search and strict adherence to the published protocol. In this, as in other areas, all RCT data should be available in the public domain to enable decision‐making to be informed by the most comprehensive evidence base possible. However, previous work highlighted the large number of RCTs of NPWT that have either been terminated, or have been completed but remain unpublished (Peinemann 2008). Extensive searching here did not locate further unpublished studies beyond those previously identified (Peinemann 2008). However, there may well be other studies of which we are not aware. We also noted that some studies were excluded because they evaluated interventions on multiple wound types, and specific data for foot wounds in people with DM were not available.
The protocol was not specific with regard to wound closure by surgery; we made a decision in this update to include the number of wounds closed or covered with surgery and time to closure or coverage surgery as secondary outcomes. Changing the outcomes of a review is often a potential source of bias. However, wound closure by surgery is a clinically important outcome and the fact that it was not included in the protocol represented an oversight on our part. The inclusion of the outcomes in the review was not driven by the data available in the included studies. We also made a decision to include cost effectiveness rather than resource use as a secondary outcome, in view of the importance of this in determining the implementation of relatively high‐cost interventions such as NPWT.
Agreements and disagreements with other studies or reviews
We found one systematic review that evaluated the clinical efficacy of NPWT in treating foot ulcers in people with DM (Liu 2017 – no relation to this Cochrane Review author). There was some overlap between this review and our Cochrane Review. The review included 11 studies that were classed as RCTs, however we excluded two of these studies (McCallon 2000, 10 participants; Sun 2007, 38 participants), as they used alternation and four further studies did not measure relevant outcome data (Eginton 2003; Sajid 2015; and Sun 2007 measured change in size data and Sepulveda 2009 measured granulation data). Liu 2017 highlighted the positive findings in complete ulcer healing from Armstrong 2005; Blume 2008; Karatepe 2011; Nain 2011; Vaidhya 2015; and McCallon 2000. Liu 2017 also highlighted the positive findings in reducing amputation from Armstrong 2005; and Blume 2008. However, Liu 2017 did not conduct a GRADE assessment, so while our review drew similar conclusions, we included an additional six RCTs and used GRADE assessment to highlight the low certainty in many findings due to risk of bias and imprecision. Additionally, there were also differences between reviews in the analytical approaches taken. This Cochrane Review contains HRs derived from reported data to allow evaluation of the 'chance' of healing over time for some of the comparisons; this is a more robust measure of the outcome than mean time to healing or the occurrence of healing events at a single time point.
We found another systematic review with a title suggesting a focus on foot ulcers in people with DM (Noble‐Bell 2008). The review included four studies that were classed as RCTs; however, we excluded two of these from our review (Etoz 2007, 24 participants; McCallon 2000, 10 participants), as they used alternation and we considered this a quasi‐randomised method of allocation. We excluded the third study from our review as it did not measure relevant outcome data (Eginton 2003). We included the fourth study in our review (Armstrong 2005). The Noble‐Bell 2008 review highlighted the positive findings from Armstrong 2005, while recommending further larger RCTs in a wider number of diabetic foot‐wound groups. We summarised the same RCT findings, but recommend more cautious interpretation of Armstrong 2005.
Finally, NICE guidelines reviewed the data regarding use of NPWT for treatment of foot wounds in people with DM (NICE 2016). They included three studies: two of which we included here (Armstrong 2005; Blume 2008), and one which we excluded (as above; Etoz 2007). The review conducted within the guideline also found that "two RCTs with a total number of 497 participants showed that participants who received NPWT with standard wound care were significantly less likely to have an amputation, and significantly more likely to have complete wound closure, when compared with participants who received standard wound care alone." However, the GRADE assessment of the evidence in the NICE guideline regarded this as low‐quality evidence. The NICE Guideline Development Group recommended that, "a health economic evaluation should be carried out to further assess its [NPWT] cost effectiveness as an adjunctive treatment for diabetic foot problems." The Guideline Development Group also "recommended the use of the intervention in the context of a clinical trial or as a rescue therapy to prevent amputation" (NICE 2016). The findings from our review agreed that further robust RCT research would help to reduce uncertainty regarding the effectiveness of NPWT in the treatment of foot wounds in people with DM. Robust studies should focus on ensuring confidence that differences in outcomes, such as healing and amputations, can be attributed to the intervention, rather than occurring as a result of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
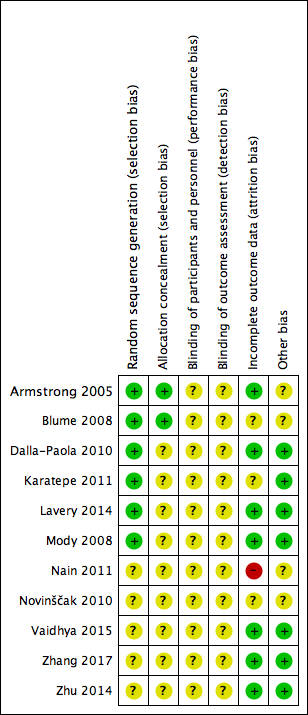
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
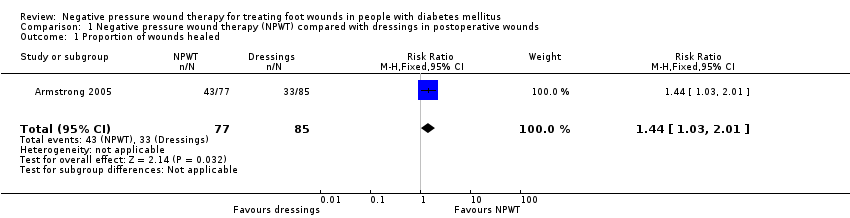
Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 1 Proportion of wounds healed.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 2 Time to healing.
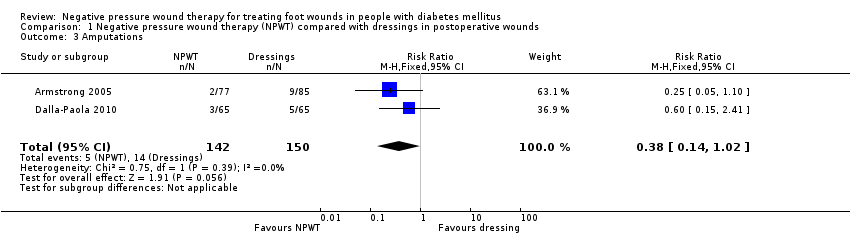
Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 3 Amputations.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 4 Number of wounds closed or covered with surgery.

Comparison 1 Negative pressure wound therapy (NPWT) compared with dressings in postoperative wounds, Outcome 5 Adverse events.
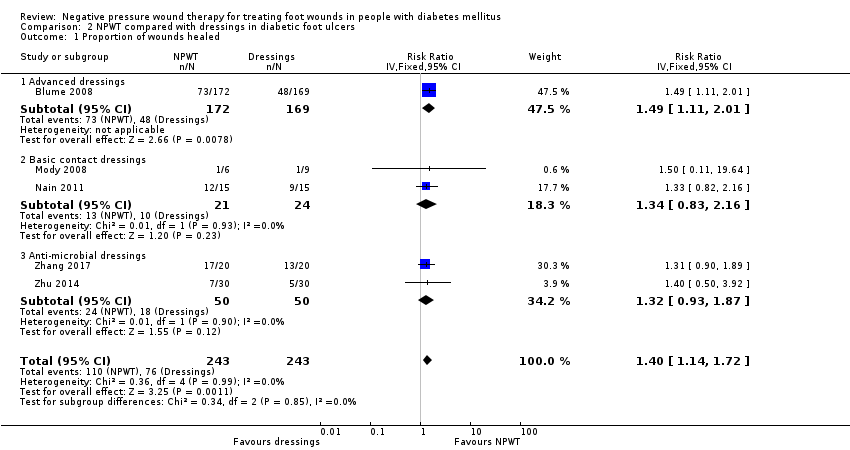
Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 1 Proportion of wounds healed.

Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 2 Amputations.

Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 3 Number of wounds closed or covered with surgery.
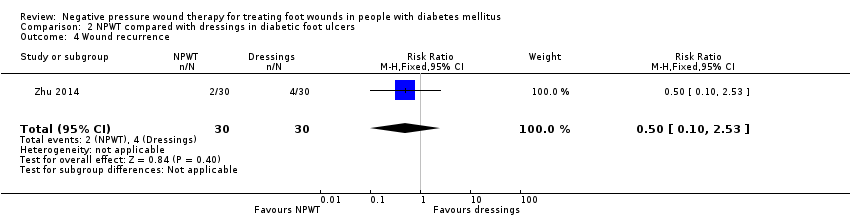
Comparison 2 NPWT compared with dressings in diabetic foot ulcers, Outcome 4 Wound recurrence.

Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 1 Number of wounds closed or covered with surgery.

Comparison 3 Low compared with high pressure of NPWT in diabetic foot ulcers, Outcome 2 Adverse events.
| NPWT compared with dressings for postoperative wounds | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: 16 weeks | Study population | RR 1.44 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 559 per 1000 | |||||
| Time to healing Follow‐up: 16 weeks | Study population | HR 1.91 | 162 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 388 per 1000 | 609 per 1000 | |||||
| Amputations Follow‐up: 16 weeks or unspecified | Study population | RR 0.38 | 292 (2 studies) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 60 per 1000 | 23 per 1000 | |||||
| Number of wounds closed or covered with surgery | 954 per 1000 | 1000 per 1000 | RR 1.02 | 130 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — |
| Adverse events Follow‐up: 16 weeks | Study population | RR 0.96 | 162 (1 study) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 541 per 1000 | 520 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias: some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias. | ||||||
| NPWT compared with dressings for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: NPWT Comparison: dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with NPWT compared with dressings | |||||
| Proportion of wounds healed Follow‐up: unclear for 4 studies and 8–16 weeks for the other 3 studies | Study population | RR 1.40 | 486 | ⊕⊕⊝⊝ Lowa,b | — | |
| 406 per 1000 | 540 per 1000 | |||||
| Time to healing Follow‐up: unclear for 2 studies and 16 weeks for the other study | Study population | — | 468 (3 studies) | ⊕⊕⊝⊝ Lowa,b | 3 studies reported HR, median and mean (1 each) and we were unable to pool any data for this comparison. | |
| See comment | See comment | |||||
| Amputations Follow‐up: unclear for 4 studies and 16 weeks for the other study | Study population | RR 0.33 | 441 | ⊕⊕⊝⊝ Lowa,b | — | |
| 114 per 1000 | 38 per 1000 | |||||
| Number of wounds closed or covered with surgery Follow‐up: unclear | Study population | RR 1.02 | 129 | ⊕⊕⊝⊝ Lowa,b | — | |
| 714 per 1000 | 729 per 1000 | |||||
| Adverse events | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | — |
| Wound recurrence Follow‐up: 6–10 months | Study population | RR 0.50 (0.10 to 2.53) | 60 | ⊕⊝⊝⊝ Very lowa,c | — | |
| 133 per 1000 | 66 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias (no blind outcome assessment). | ||||||
| Low‐pressure compared with high‐pressure NPWT for diabetic foot ulcers | ||||||
| Patient or population: treating foot wounds in people with diabetes mellitus Setting: hospital Intervention: low‐pressure NPWT (75 mmHg) Comparison: high‐pressure NPWT (125 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with low compared with high pressure of NPWT | |||||
| Proportion of wounds healed | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Time to ulcer healing | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Amputation | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Number of wounds closed or covered with surgery Follow‐up: 4 weeks | Study population | RR 0.83 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 600 per 1000 | 498 per 1000 | |||||
| Adverse events Follow‐up: 4 weeks | Study population | RR 1.50 | 40 | ⊕⊝⊝⊝ Very lowa | ‐ | |
| 100 per 1000 | 150 per 1000 | |||||
| Cost‐effectiveness | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| Wound recurrence | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NPWT: negative pressure wound therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded three levels: once for risk of bias (some blinded outcome assessment, but not sure the potential impact of non‐blinded decisions regarding the use of further surgery and the risk of performance bias); twice for very serious imprecision with a small sample size and limited reported information to quantify imprecision. | ||||||
| Study | Wound characteristics | Comparison | Length of follow‐up | NPWT pathways | Time to healing | Number of wounds completely healed | Amputation | Number of wounds closed or covered with surgery | Time to closure or coverage surgery | Adverse events | Health‐related quality of life | Cost‐effectiveness | Wound recurrence |
| Diabetic foot amputation to trans‐metatarsal level | Group A: NPWT (V.A.C. system), dressing changes every 48 h. Treatment conducted until wound closure or completion of 112‐day assessment (n = 77) Group B: moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings (n = 85) | 16 weeks | After amputation (close/open wounds; if open wounds, secondary intention), NPWT delivered through the V.A.C. system; or standard care with moist wound therapy. | Kaplan‐Meier median time to healing Group A: 56 days (IQR 26 to 92) Group B: 77 days (IQR 40 to 122) Log‐rank taken as P = 0.005 There was no difference noted in time to healing for acute or chronic wounds. | Group A: 43/77 (55.8%) Group B: 33/85 (38.8%) Of healed wounds –healed by secondary intention (without primary/surgical wound closure) Group A: 31/43 (72.1%) Group B: 25/33 (75.8%) Remaining wounds were closed following surgery. | Number of participants undergoing further amputation Group A: 2/77 (2.3%) Major = 0 Minor = 2 Group B: 9/85 (10.6%) Major = 5 Minor = 4 | Not reported | Not reported | Participants who had ≥ 1 adverse events Group A: 40/77 (51.9%) Group B: 46/85 (54.1%) Participants who had ≥ 1 treatment‐related adverse events Group A: 9/77 (11.7%) 1 classified serious Group B: 11/85 (12.9%) 5 classified as serious | Not reported | Not reported | Not reported | |
| Ulceration of the foot in people with diabetes | Group A: NPWT (V.A.C. system), applied according to manufacturer’s instructions (n = 172) Group B: advanced moist wound therapy dressings used according to guidelines/local protocols (n = 169) | 16 weeks | NPWT was continued until ulcer closure. | Kaplan‐Meier median time to healing Group A: 96 days (95% CI 75.0 to 114.0) Group B: could not be estimated Log‐rank taken as P = 0.001 | Group A: 3/172 (42.4%) Group B: 8/169 (28.4%) (6 participants excluded in paper as did not receive treatment, added back into denominator here; ITT 172/169) | Number of participants undergoing amputation* Group A: 7/172 (4.1%) Major = 5 Minor = 2 Group B: 17/169 (10.1%) Major = 4 Minor = 13 | Not reported | Not reported | Limited data: not extracted | Not reported | Not reported | Not reported | |
| Infected open amputations or surgical dehiscence of minor amputations in people with diabetes | Group A: V.A.C. therapy following surgical debridement (n = 65) Group B: advanced dressings following surgical debridement (n = 65) | Not specified. End of therapy was defined as complete coverage of the wound with epithelial tissue. | Duration of therapy depended on the functional parameters of the wound area. | Not reported | Not reported | Number of participants undergoing further amputation (major) Group A: 3/65 (4.6%) Group B: 5/65 (7.7%) | Group A: 63/65 (96.9%) Group B: 62/65 (95.4%) | Group A (n = 65): 65 days (SD 16) Group B (n = 65): 98 days (SD 45) P = 0.005 These data reported as time to "complete closure of the wound" was reached. Unclear if it is mean or median; unclear if "complete closure" means "time to healing of grafted wound" or "time to surgical closure;" unclear if it is a valid measure as not sure all ulcers have healed. Author contacted – waiting for response. | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: NPWT (V.A.C. system) (n = 30) Group B: conventional wound care treatment: based on text in report taken to be dry gauze (n = 37) | Not specified. Last assessment 1 month after healing | Not specified | Median time to healing Group A: 4.4 weeks Group B: 3.9 weeks Mean value presented but not extracted. No specific P value presented (< 0.05) | Not reported | Not reported | Not reported | Not reported | Not reported | SF‐36: data not presented | Not reported | Not reported | |
| Diabetic foot wounds, after incision and drainage | Group A: NPWT with Group B: 125 mmHg of pressure | 4 weeks | NPWT was continued for 4 weeks | Not reported | Not reported | Not reported | Group A: 10/20 (50%) Group B: 12/20 (60%) | Not reported | Group A: study related 2/20 (10%); non‐study related 1/20 (5%) Group B: study related 1/20 (5%); non‐study related 1/20 (5%) | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: locally constructed NPWT (n = 6) Group B: wet‐to‐dry gauze (n = 9) | Not specified: until healing or loss to follow‐up | People receiving TNP only in hospital | Not reported | By secondary intention: Group A: 1/6 (16.6%) Group B: 1/9 (11.0%) | Not reported | By delayed primary closure: Group A: 0/6 (0%) Group B: 3/9 (33%) | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot ulcers | Group A: negative pressure dressing (n = 15) | 8 weeks | Ulcers were treated until the wound was closed surgically or spontaneously, or until completion of the 56 days (8 weeks) assessment whichever was earlier. | Not reported | Group A: 12/15 (80%) Group B: 9/15 (60%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Complicated diabetic foot ulcers | Group A: NPWT (n = 7) Group B: dressings (moist) (n = 12) Group C: classic gauze (n = 8) | 8 weeks | Treatment was monitored for the first 2 months. | Not reported | Group A: * could not be calculated (90%) Group B: 9/12* (75%) Group C: 4/8* (50%) *Figure calculated by review author We obtained data (only proportions) from the study author but were unable to use these to calculate number of healed wounds. It seemed this outcome was measured but was not able to use the data in meta‐analysis. | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wound | Group A: NPWT (n = 30) | Not specified | Interventions discontinued for participants in whom failure or complications | Not reported | Not reported | Data for alternative therapy or amputation: Group A: 3/30 (10%) Group B: 7/30 (23.3%) | Wounds were ready for either skin grafting or secondary suturing (end point) Group A: 27/30 (90%) Group B: 23/30 (67.7%) | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Limited data: not extracted | Not reported | |
| Chronic diabetic ulcers | Group A: vacuum sealing drainage (n = 20) Group B: gauze dressing (n = 20) | Not reported | Interventions were administered in hospital | Not reported | Group A: 17/20 (85%) Group B: 13/20 (65%) | Group A: 1/20 (5%) Group B: 2/20 (10%) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Diabetic foot wounds | Group A: vacuum sealing drainage (n = 30) | Not reported Follow‐up to 6–10 months for wounds recurrence | Vacuum sealing drainage administered when necessary at several time points | Not reported properly – not all ulcers healed | Group A: 7/30 (23%) Group B: 5/30 (17%) | Group A: 0 Group B: 6/30 (20%) | Of healed wounds by secondary surgery (skin/flap grafting): Group A: 23/30 Group B: 19/24 | Not reported properly – not all ulcers reached this point | Not reported | Not reported | Not reported | Group A: 2 Group B: 4 Follow‐up time: 6–10 months | |
| h: hour; IQR: interquartile range; ITT: intention to treat; n: number of participants; NPWT: negative pressure wound therapy; SF‐36: 36‐item Short Form; TNP: topical negative pressure. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.03, 2.01] |
| 2 Time to healing Show forest plot | 1 | 162 | Hazard Ratio (Fixed, 95% CI) | 1.91 [1.21, 2.99] |
| 3 Amputations Show forest plot | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 4 Number of wounds closed or covered with surgery Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| 5 Adverse events Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 486 | Risk Ratio (IV, Fixed, 95% CI) | 1.40 [1.14, 1.72] |
| 1.1 Advanced dressings | 1 | 341 | Risk Ratio (IV, Fixed, 95% CI) | 1.49 [1.11, 2.01] |
| 1.2 Basic contact dressings | 2 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.34 [0.83, 2.16] |
| 1.3 Anti‐microbial dressings | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [0.93, 1.87] |
| 2 Amputations Show forest plot | 3 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.70] |
| 3 Number of wounds closed or covered with surgery Show forest plot | 3 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.24] |
| 4 Wound recurrence Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wounds closed or covered with surgery Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.47, 1.47] |
| 2 Adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.04] |