Resumen de la seguridad del formoterol o salmeterol habitual en adultos con asma: una revisión global de revisiones Cochrane
Referencias
References to included reviews
Ir a:
Additional references
Ir a:

Network of comparisons of serious adverse events from reviews of regular formoterol and salmeterol. Figure 1A shows the numbers of trials and adults on monotherapy versus placebo. Figure 1B shows the numbers of trials and adults on combination therapy versus the same dose of ICS. Adults randomly assigned to other arms in the included trials have not been counted.

Review selection flow diagram.

Formoterol or salmeterol monotherapy versus placebo (with variable background use of ICS).
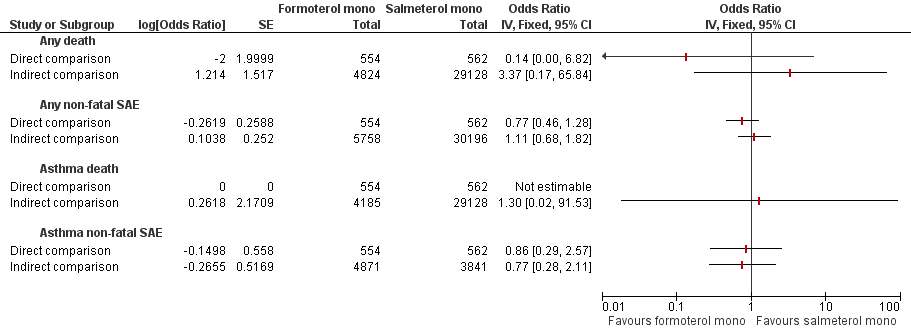
Formoterol monotherapy versus salmeterol monotherapy.

Formoterol or salmeterol combination therapy versus the same dose of ICS.

Formoterol combination therapy versus salmeterol combination therapy.
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 0 per 10000 | not estimable (see comment) | OR 4.49 (0.24 to 84.80) | 4824 (13 studies) | ⊕⊕⊝⊝ | No deaths on placebo, two deaths on formoterol |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 23 per 10000 | 31 per 10000 (20 to 48) | OR 1.33 (0.85 to 2.08) | 29,128 (10 studies) | ⊕⊕⊕⊝ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 20 per 10000 | 27 per 10000 (18 to 43) | OR 1.37 (0.88 to 2.13) | 33,952 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 106 per 10000 | 133 per 10000 (83 to 214) | OR 1.26 (0.78 to 2.04) | 5758 (17 studies) | ⊕⊕⊕⊝ | |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 345 per 10000 | 391 per 10000 (348 to 437) | OR 1.14 (1.01 to 1.28) | 30,196 (13 studies) | ⊕⊕⊕⊕ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 316 per 10000 | 359 per 10000 (322 to 401) | OR 1.14 (1.02 to 1.29) | 35,954 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are very wide, as only two deaths occurred (‐2 points) 2. Confidence intervals are wide enough to include important harm and benefit (‐1 for imprecision) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol monotherapy | Formoterol monotherapy | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 18 per 10000 | 2 per 10000 (0 to 115) | OR 0.14 (0.00 to 6.82) | 1116 | ⊕⊝⊝⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 641 per 10000 | 501 per 10000 (305 to 806) | OR 0.77 (0.46 to 1.28) | 1116 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Open studies (‐1 point) and confidence intervals are very wide indeed, as only one death occurred (‐2 points) 2. Open studies and wide confidence intervals (‐1 point each) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 2 per 10000 | 7 per 10000 (2 to 32) | OR 3.56 (0.79 to 16.03) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 11 per 10000 | 10 per 10000 (3 to 29) | OR 0.90 (0.31 to 2.60) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 7 per 10000 | 10 per 10000 (4 to 24) | OR 1.42 (0.60 to 3.38) | 24,718 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 241 per 10000 | 239 per 10000 (187 to 304) | OR 0.99 (0.77 to 1.27) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 209 per 10000 | 240 per 10000 (191 to 298) | OR 1.15 (0.91 to 1.44) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 228 per 10000 | 244 per 10000 (206 to 288) | OR 1.07 (0.90 to 1.27) | 24,718 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are wide and include important harm and benefit (‐1 for imprecision) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol combination therapy | Formoterol combination therapy | |||||
| Adults who died of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 3 per 10000 | 8 per 10000 (1 to 48) | OR 2.68 | 6769 | ⊕⊕⊝⊝ | Based on data from all formoterol combination trials |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 226 per 10000 | 252 per 10000 (186 to 342) | OR 1.12 | 6769 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are very wide, as only five deaths occurred (‐2 points) 2. Confidence intervals are wide and include important harm and benefit (‐1 point) | ||||||
| Comparison (with additional data from two new trials) | Formoterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | Quality of the evidence | |||
| Outcome (mean duration 14 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 2 | 2924 | 0 | 1900 | Peto OR 4.49 (95% CI 0.24 to 84.80) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 48 | 3401 | 25 | 2357 | Peto OR 1.26 (95% CI 0.78 to 2.04) | 15% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 2495 | 0 | 1690 | Peto OR 4.54 (95% CI 0.07 to 285.25) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 2849 | 10 | 022 | Peto OR 1.09 (95% CI 0.50 to 2.40) | 23% | ⊕⊕⊝⊝ |
| Comparison | Salmeterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 27 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 44 | 14648 | 33 | 14480 | Peto OR 1.33 (95% CI 0.85 to 2.08) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 587 | 15170 | 518 | 15026 | Peto OR 1.14 (95% CI 1.01 to 1.28) | 0% | ⊕⊕⊕⊕ |
| Mortality due to asthma | 13 | 14648 | 3 | 14480 | Peto OR 3.49 (95% CI 1.31 to 9.31) | Data from single trial | ⊕⊕⊕⊕ |
| Non‐fatal SAE due to asthma | 23 | 1994 | 16 | 1847 | Peto OR 1.43 (95% CI 0.75 to 2.71) | 0% | ⊕⊕⊝⊝ |
| Comparison | Formoterol Monotherapy | Salmeterol Monotherapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 26 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 0 | 554 | 1 | 662 | Peto OR 0.14 (95% CI 0.00 to 6.82) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE all‐cause | 28 | 554 | 36 | 662 | Peto OR 0.77 (95% CI 0.46 to 1.28) | 0% | ⊕⊕⊝⊝ |
| Mortality due to asthma | 0 | 554 | 0 | 662 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 6 | 554 | 7 | 662 | Peto OR 0.86 (95% CI 0.29 to 2.57) | 0% | ⊕⊕⊝⊝ |
| Comparison (with additional data from three new trials) | Formoterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 29 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 6 | 6507 | 1 | 4764 | Peto OR 3.56 (95% CI 0.79 to 16.03) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 145 | 6507 | 115 | 4764 | Peto OR 0.99 (95% CI 0.77 to 1.27) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 6507 | 0 | 4764 | Peto OR 7.34 (95% CI 0.15 to 369.72) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 6325 | 30 | 4576 | Peto OR 0.49 (95% CI 0.28 to 0.88) | 0% | ⊕⊕⊕⊝ |
| Comparison | Salmeterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 34 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 7 | 6986 | 7 | 6461 | Peto OR 0.90 (95% CI 0.31 to 2.60) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 167 | 6986 | 135 | 6461 | Peto OR 1.15 (95% CI 0.91 to 1.44) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 6986 | 0 | 6461 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 29 | 6986 | 23 | 6461 | Peto OR 1.12 (95% CI 0.65 to 1.94) | 5% | ⊕⊕⊕⊝ |
| Comparison | Formoterol Combination Therapy | Salmeterol Combination Therapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 24 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 4 | 3453 | 1 | 3316 | Peto OR 2.68 (95% CI 0.44 to 16.14) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 90 | 3453 | 75 | 3316 | Peto OR 1.12 (95% CI 0.82 to 1.53) | 13% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 3453 | 0 | 3316 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 17 | 3081 | 25 | 3082 | Peto OR 0.69 (95% CI 0.37 to 1.26) | 33% | ⊕⊕⊝⊝ |
| 1. Few events were observed leading to wide CIs (including the possibilities of no effect and appreciable harm) 2.There was no independent assessment of the cause of serious adverse events, leading to possible ascertainment bias for disease‐specific outcomes 3. Open studies | |||||||
| Review title | Inclusion criteria | Date of search | No. included studies (all versus placebo or ICS) | No. included studies (adults only versus placebo or ICS) | ||||
| Studies (Randomised trials only) | Participants (Diagnosis of asthma; any age group) | Intervention | Comparison | Primary outcome measures (All‐cause mortality & non‐fatal SAEs) | ||||
| 1. Regular treatment with formoterol for chronic asthma: serious adverse events | Yes | Yes | Inhaled formoterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | January 2012 | 20 (versus placebo) | 15 (versus placebo) |
| 2. Regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2008 | Yes | Yes | Inhaled salmeterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | August 2011 | 24 (versus placebo) | 19 (versus placebo) |
| 3. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013b | Yes | Yes | ICS and formoterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 27 | 20 |
| 4. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013a | Yes | Yes | ICS and salmeterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 40 | 35 |
| 5. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2012b | Yes | Yes | Inhaled formoterol; at least 12 weeks duration; not randomised with ICS | Inhaled salmeterol; at least 12 weeks duration; not randomised with ICS | Yes | January 2012 | 4 | 3 |
| 6. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cates 2010 | Yes | Yes | Inhaled formoterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Inhaled salmeterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Yes | August 2011 | 10 | 10 |
| Study ID from adults in Cates 2012a | % patients on background ICS | (N) | (N) | (N) | Placebo (N) | Mortality data (all‐cause) | Duration (weeks) |
| Formoterol Dose | 48 mcg/day | 24 mcg/day | 12 mcg/day |
|
| ||
| Bensch 2001 | 51 | 135 | 136 | 136 | ✓ | 12 | |
| Busse 2004 | 64 | 80 | 80 | ✓ | 12 | ||
| Corren 2007 | 0 | 123 | 131 | ✓ | 12 | ||
| Corren 2013 | 0 | 111 | 109 | ✓ | 12 | ||
| Ekstrom 1998 | 86 | 135 | 129 |
| 12 | ||
| Ekstrom 1998a | 89 | 114 | 113 |
| 12 | ||
| Fitzgerald 1999 | 100 | 89 | 91 | ✓ | 24 | ||
| LaForce 2005 | 67 | 86 | 91 | ✓ | 12 | ||
| Molimard 2001 | 100 | 130 | 129 | ✓ | 12 | ||
| Nathan 2012 | 0 | 116 | 111 | ✓ | 12 | ||
| Noonan 2006 | 100 | 123 | 125 | ✓ | 12 | ||
| Pleskow 2003 | 44 | 136 | 139 | 141 | ✓ | 12 | |
| SD‐037‐0344 | 100 | 429 | 210 | ✓ | 12 | ||
| Steffensen 1995 | 87 | 103 | 101 |
| 12 | ||
| van der Molen 1997 | 100 | 125 | 114 |
| 24 | ||
| van Schayck 2002 | 95 | 46 | 41 | ✓ | 12 | ||
| Wolfe 2006 | 62 | 525 | 527 | 514 | ✓ | 16 | |
| mean duration 14 weeks | |||||||
| All trials contributed data for non‐fatal serious adverse events of any cause. Corren 2013 and Nathan 2012 have been added to the trials already included in the Cochrane review. | |||||||
| Study ID from adults Cates 2008 | % patients on background ICS | Number on salmeterol | **Dose of salmeterol (mcg/bd) | Number on placebo | Data found on mortality (all‐cause) | Data found on non‐fatal SAE (all‐cause) | Duration (weeks) |
| Adinoff 1998 | 64 | 142 | 50 | 244 |
|
| 36 |
| Boyd 1995 | 100 | 55 | 100 | 64 |
| ✓ | 12 |
| Busse 1998 | 67 | 263 | 50 | 275 |
| ✓ | 12 |
| Chervinsky 1999 | 51 | 176 | 50 | 176 | ✓ |
| 52 |
| D'Alonso 1994a | 21 | 106 | 50 | 108 |
|
| 12 |
| D'Urso 2001 | 93 | 455 | 50 | 456 | ✓ | ✓ | 24 |
| Kavuru 2000 | 0* | 92 | 50 | 82 | ✓ | ✓ | 12 |
| Kemp 1998a | 43 | 149 | 50 | 152 |
|
| 12 |
| Kemp 1998b | 100 | 252 | 50 | 254 |
| ✓ | 12 |
| Lazarus 2001 | 0* | 54 | 50 | 56 | ✓ | ✓ | 16 |
| Nathan 1999 | 0* | 128 | 50 | 129 | ✓ | ✓ | 26 |
| Nathan 2006 | 0* | 91 | 50 | 89 |
| ✓ | 12 |
| Pearlman 1992 | 25 | 78 | 50 | 79 | ✓ |
| 12 |
| Pearlman 2004 | 0* | 92 | 50 | 87 |
| ✓ | 12 |
| Rosenthal 1999 | 0 | 202 | 50 | 206 |
|
| 24 |
| Shapiro 2000 | 0* | 88 | 50 | 93 | ✓ | ✓ | 12 |
| SLMF4002 | 100 | 93 | 100 | 95 | ✓ | ✓ | 26 |
| SMART 2006 | 47 | 13,176 | 50 | 13,179 | ✓ | ✓ | 28 |
| Wolfe 2000 | 33 | 331 | 50 | 167 | ✓ | ✓ | 12 |
| mean duration 27 weeks | |||||||
| * background ICS treatment was withdrawn from all participants ** 50 micrograms is the ex‐actuator dose, but in some studies this is reported as the equivalent delivered dose of 42 micrograms | |||||||
| Study ID from adults in Cates 2012b | N | Age | Formoterol Device | Salmeterol Device | Location | Sponsor | Duration (weeks) |
| Condemi 2001 | 528 | 18+ | Foradil Aerolizer | Serevent Diskus | USA | Novartis | 26 |
| Gabbay 1998 | 127 | 18+ | Foradil Aerolizer | Serevent Diskus | UK | Novartis | 12 |
| Vervolet 1998 | 482 | 18+ | Foradil Aerolizer | Serevent Diskus | Europe | Novartis | 26 |
| Total | 1137 | mean duration 24 weeks | |||||
| All the above trials compared formoterol (Foradil) 12 mcg twice daily with salmeterol 50 mcg twice daily and all participants were taking a background inhaled corticosteroid. | |||||||
| Study ID from adults in Cates 2013b | Age (Years | N on F&ICS | N on ICS Alone | Daily dose of budesonide or other ICS (mcg metered dose) | Daily Dose of Formoterol (mcg metered dose) | Once daily | Twice daily | Combined inhalers | Separate inhalers | DPI | pMDI | Duration weeks |
| Brown 2012 | 12+ | 377 | 364 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Buhl 2003 | 18+ | 352 | 171 | 400 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Chuchalin 2002 | 18+ | 111 | 114 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2007 | 12+ | 123 | 121 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2013 | 12+ | 110 | 113 | 250 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| D5896C00001 | 12+ | 312 | 153 | 400 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Jenkins 2006 | 12+ | 341 | 115 | 1600 | 48 | ✓ | ✓ | ✓ | ✓ | 24 | ||
| Kuna 2006 | 18+ | 409 | 207 | 200 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Meltzer 2012 | 12+ | 182 | 188 | 200 (mometasone) | 20 | ✓ | ✓ | 26 | ||||
| Morice 2007 | 12+ | 462 | 217 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Nathan 2010 | 12+ | 191 | 192 | 400 (mometasone) | 20 | ✓ | ✓ | ✓ | 26 | |||
| Nathan 2012 | 12+ | 115 | 117 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Noonan 2006 | 12+ | 239 | 109 | 400 | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| O'Byrne 2001 | 18+ | 554 | 550 | 400 | 12 | ✓ | ✓ | ✓ | 52 | |||
| O'Byrne 2001a | 18+ | 315 | 312 | 800 | 12 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997 | 18+ | 210 | 213 | 200 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997a | 18+ | 215 | 214 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pearlman 2012 | 12+ | 119 | 119 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Peters 2008 | 12+ | 443 | 133 | 1600 | 48 | ✓ | ✓ | ✓ | 52 | |||
| Price 2002 | 12+ | 250 | 255 | 800 | 24 | ✓ | ✓ | ✓ | 24 | |||
| SD‐039‐0726 | 16+ | 301 | 145 | 200 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Spector 2012 | 12+ | 156 | 155 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Weinstein 2010 | 12+ | 255 | 240 | 800 (mometasone) | 20 | ✓ | ✓ | ✓ | 12 | |||
| Zangrilli 2011 | 12+ | 127 | 123 | 800 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Zetterstrom 2001 | 18+ | 238 | 124 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| mean duration 29 weeks | ||||||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause. Corren 2013, Nathan 2012 and Pearlman 2012 have been added to the trials already included in the Cochrane review. | ||||||||||||
| Study ID from adults in Cates 2013a | Age of Participants (Years) | N on FSC | N on ICS | Daily dose of fluticasone (mcg) | Daily dose of salmeterol (mcg) | Combined Inhaler | Separate Inhalers | Duration (weeks) |
| Aubier 1999 | 12+ | 338 | 165 | 1000 | 100 | ✓ | ✓ | 28 |
| Bailey 2008 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| Bateman 2001 | 12+ | 1709 | 1707 | 200 | 100 | ✓ | 52 | |
| GOAL 2004 | 12+ | 333 | 165 | 200/500/1000 | 100 | ✓ | 12 | |
| Godard 2008 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Ind 2003 | 16+ | 336 | 160 | 500 | 100 | ✓ | 28 | |
| Katial 2011 | 12+ | 306 | 315 | 500 | 100 | ✓ | 52 | |
| Kavuru 2000 | 12+ | 310 | 318 | 200 | 100 | ✓ | 52 | |
| Kerwin 2011 | 12+ | 92 | 90 | 500 | 100 | ✓ | 12 | |
| Koenig 2008 | 12+ | 156 | 156 | 200/500/1000 | 100 | ✓ | 40 | |
| Koopmans 2006 | 18+ | 173 | 177 | 500 | 100 | ✓ | 12 | |
| Lundback 2006 | 18+ | 101 | 102 | 500 | 100 | ✓ | 12 | |
| Murray 2004 | 12+ | 94 | 91 | 200 | 100 | ✓ | 12 | |
| Nathan 2006 | 12+ | 171 | 168 | 220 | 100 | ✓ | 16 | |
| Nelson 2003 | 12+ | 95 | 97 | 200 | 100 | ✓ | 12 | |
| Pearlman 2004 | 12+ | 92 | 89 | 200 | 100 | ✓ | 12 | |
| Renzi 2010 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| Rojas 2007 | 12+ | 180 | 182 | 500 | 100 | ✓ | 12 | |
| SAM30007 | 18+ | 29 | 32 | 200/500/1000 | 100 | ✓ | 30 | |
| SAM40004 | 18+ | 42 | 21 | 200 | 100 | ✓ | 52 | |
| SAM40008 | 18+ | 93 | 93 | 1000 | 100 | ✓ | 26 | |
| SAM40031 | 18+ | 41 | 41 | 200/500/1000 | 100 | ✓ | 52 | |
| SAM40065 | 12+ | 150 | 150 | 200/500/1000 | 100 | ✓ | 40 | |
| SAS30022 | 12+ | 210 | 212 | 500 | 50 | ✓ | 12 | |
| SAS30023 | 12+ | 151 | 155 | 100 | 50 | ✓ | 12 | |
| SAS40036 | 15+ | 172 | 159 | 200 | 100 | ✓ | 16 | |
| SAS40037 | 15+ | 161 | 161 | 200 | 100 | ✓ | 16 | |
| SAS40068 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| SFA103153 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| SFCF4026 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Shapiro 2000 | 12+ | 84 | 84 | 500 | 100 | ✓ | 12 | |
| SLGF75 | 16+ | 14 | 17 | 200 | 100 | ✓ | 12 | |
| Strand 2004 | 18+ | 78 | 72 | 200 | 100 | ✓ | 12 | |
| van Noord 2001 | 12+ | 337 | 172 | 1000 | 100 | ✓ | 12 | |
| Wallin 2003 | 12+ | 18 | 19 | 400 | 100 | ✓ | 12 | |
| mean duration 34 weeks | ||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause | ||||||||
| Study ID from adults in Cates 2010 | N | Duration (weeks) | Formoterol device | Formoterol dose | ICS type and dose | Salmeterol device | Salmeterol dose | ICS type and dose | Duration (weeks) |
| Aalbers 2004 | 439 | 26 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 26 |
| Bodzenta‐Lukaszyk 2011 | 202 | 12 | HFA pMDI with AeroChamber | 10 µg bd | Fluticasone 100 µg or 250 µg bd | HFA pMDI with AeroChamber | 50 µg bd | Fluticasone 100 µg or 250 µg bd | 12 |
| Busse 2008 | 833 | 30 | pMDI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 30 |
| Dahl 2006 | 1397 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Kuna 2007 | 2218 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Maspero 2010 | 404 | 52 | pMDI | 10 µg bd | Mometasone 200 µg or 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg or 500 µg bd | 52 |
| Papi 2007 | 228 | 12 | pMDI | 12 µg bd | Beclomethasone extra fine 200 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| Ringdal 2002 | 428 | 12 | DPI two separate inhalers | 12 µg bd | Budesonide 800 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| SAM 40010 | 373 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 100 µg bd | 12 |
| SAM 40048 | 247 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| mean duration 23 weeks | |||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause | |||||||||
| AMSTAR Criteria | ||||||
| 1. Was an 'a priori' design provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a. Was there duplicate study selection? (0.5 point) | Yes | Yes | Yes | Yes | Yes | No |
| 2b. Was there duplicate data extraction? (0.5 point) | No | No | Yes | Yes | Yes | No |
| 3. Was a comprehensive literature search performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No | No | No | No | No | No |
| 5. Was a list of studies (included and excluded) provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Were the characteristics of the included studies provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Was the likelihood of publication bias assessed? | Yes | Yes | Yes | Yes | Not applicable | Not applicable |
| 11. Was the conflict of interest stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Total criteria met: | 10.5 | 10.5 | 11 | 11 | 10 | 9 |
| Note: item 4 is met with the assessment 'NO', all others 'YES'. We felt that item 2 was 2 separate questions, so we split it into two parts and awarded half a point for each. This differs from the published version of the tool. | ||||||
| Comparison | Adults with an event on control (n) | Total number of adults on control (N) | SAE per 10,000 adults (95% CI) | Mean duration of trials (weeks) | SAE per 10,000 adults per week |
| Formoterol v Placebo | 25 | 2357 | 106 (65 to 147) | 14 | 7.6 |
| Salmeterol v Placebo | 518 | 15026 | 345 (317 to 375) | 27 | 12.8 |
| Formoterol & ICS v ICS | 115 | 4764 | 241 (197 to 285) | 29 | 8.3 |
| Salmeterol & ICS v ICS | 135 | 6461 | 209 (177 to 247) | 34 | 6.1 |
| Study ID | Treatment arm | Cause of death |
| Buhl 2003 | Formoterol and budesonide | Cardiac arrest |
| O'Byrne 2001 | Formoterol and budesonide (separate inhalers) | Status asthmaticus, followed by septic shock |
| Pauwels 1997a | Formoterol and budesonide (separate inhalers) | Suicide |
| Zetterstrom 2001 | Formoterol and budesonide | Suicide |
| Brown 2012 | Formoterol and budesonide | Cerebro‐vascular accident |
| Brown 2012 | Budesonide | Homicide |
| Nathan 2010 | Formoterol and mometasone | Uterine Leiomyosarcoma |
| Jenkins 2006 | Formoterol and budesonide | Pulmonary embolus (but the death occurred after the control budesonide arm was discontinued so was not included in the meta‐analysis) |
| Study ID | Treatment arm | Cause of death |
| Aubier 1999 | salmeterol and fluticasone (separate inhalers) | Bronchial carcinoma (one death) |
| GOAL 2004 | salmeterol/fluticasone | Myocardial infarction (two deaths) and pneumonia (one death) |
| GOAL 2004 | fluticasone | Myocardial infarction (two deaths) |
| Ind 2003 | salmeterol and fluticasone (separate inhalers) | Pneumothorax (one death) |
| Kerwin 2011 | salmeterol/fluticasone | Cardiac disease (one death) |
| Kerwin 2011 | fluticasone | Breast cancer (one death) |
| Koenig 2008 | fluticasone | Cardiac arrest and deep vein thrombosis (one death) |
| Renzi 2010 | fluticasone | Cardiac arrest (one death) |
| SAS40068 | fluticasone | Ventricular hypertrophy and aortic hypoplasia (one death) |
| Strand 2004 | fluticasone | Unknown cause (one death) |
| van Noord 2001 | salmeterol/fluticasone | Leukaemia (one death) |

