Intervenciones para el melanoma in situ, incluido el léntigo maligno
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010308.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 19 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
TT was the contact person with the editorial base.
TT and AK co‐ordinated contributions from the co‐authors and wrote the final draft of the review.
TT and AK screened papers against eligibility criteria.
TT and AK obtained data on ongoing and unpublished studies.
TT and AK appraised the quality of papers.
TT and AK extracted data for the review and sought additional information about papers.
TT and AK entered data into RevMan.
TT and AK (with the help of all authors) analysed and interpreted data.
TT and AK worked on the methods sections.
ZA and TT drafted the clinical sections of the background and responded to the clinical comments of the referees.
TT and AK (with the help of all authors) responded to the methodology and statistics comments of the referees.
PP was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
TT is the guarantor of the update.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
-
Dessau Medical Center, Dessau, Germany.
Provided internet facilities
-
Freie Universität Berlin, Germany.
Provided online literature
-
Faculty of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Provided internet facilities and online literature
-
Hospital of Skin and Venereal Diseases, Thessaloniki, Greece.
Provided internet facilities
-
Women's College Research Institute, University of Toronto, Toronto, Canada.
Provided internet facilities and online literature
-
University Hospital of North Norway, Harstad, Troms, Norway.
Provided internet facilities and online literature
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Thrasivoulos Tzellos: Nothing to declare.
Athanassios Kyrgidis: Nothing to declare.
Simone Mocellin: Nothing to declare.
An‐Wen Chan: Nothing to declare.
Pierluigi Pilati: Nothing to declare.
Zoe Apalla: Nothing to declare.
Acknowledgements
We wish to thank Dr Mark Hyde, corresponding author of the included study (Hyde 2012), for replying to our requests and providing access to primary data of the study. We would also like to thank Shaheen Haque Hussain, co‐author of the protocol (Apalla 2013), who withdrew her name from the author list of the review.
The Cochrane Skin Group editorial base wishes to thank Sam Gibbs, who was the Cochrane Dermatology Editor for this review; Matthew Grainge and Thomas Chu, who were the Statistical Editors; Ching‐Chi Chi, who was Methods Editor; the clinical referees, Susan O'Connell and Eleni Linos; and the consumer referee, Colette O'Sullivan.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 19 | Interventions for melanoma in situ, including lentigo maligna | Review | Thrasivoulos Tzellos, Athanassios Kyrgidis, Simone Mocellin, An‐Wen Chan, Pierluigi Pilati, Zoe Apalla | |
| 2013 Jan 31 | Interventions for melanoma in situ, including lentigo maligna | Protocol | Zoe Apalla, Thrasivoulos Tzellos, Athanassios Kyrgidis, Simone Mocellin, An‐Wen Chan, Shaheen Haque Hussain, Pierluigi Pilati | |
Differences between protocol and review
The order of authors in the byline has changed after agreement with all authors; we changed the contact author, and the new contact author is Thrasivoulos Tzellos.
In our protocol, we reported that a 'Summary of findings' table would include only primary outcomes. We decided to include both primary and secondary outcomes for reasons of better readability and presentation, since there was only one study included.
With regard to 'Types of outcome measures', we decided to swap our primary and secondary outcomes so 'Histological or clinical complete clearance rate' is now our first primary outcome, and 'Clinical or histological local recurrence rate' is our first secondary outcome. The reasons for this change, which was a unanimous decision, were 1) In order for recurrence rate to be measurable, a complete clearance rate is a prerequisite, 2) we identified no outcomes pertaining to 'Clinical or histological local recurrence rate' in this instance of the review.
After discussion with all authors, we rephrased 'Histological or clinical complete clearance rate' as 'Histological or clinical complete response rate', because this is a more common and universal way of expressing this outcome in oncology. The nature of the outcome did not change with this modification; however, we feel that readability has increased.
Taking into account the mechanism of action of the treatments used in the included studies, we added 'Inflammatory response' as a secondary outcome to Types of outcome measures.
Notes
A search of Medline and Embase in January 2016 did not identify any new relevant studies, confirming the authors' thoughts. Thus, an update has not been considered necessary at this time. Our Information Specialist will run a new search in January 2017 to re‐assess whether an update is needed.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Aminoquinolines [administration & dosage];
- Antineoplastic Agents [*administration & dosage];
- Antineoplastic Combined Chemotherapy Protocols [administration & dosage];
- Combined Modality Therapy [methods];
- Hutchinson's Melanotic Freckle [*therapy];
- Imiquimod;
- Melanoma [*therapy];
- Melanoma, Cutaneous Malignant;
- Nicotinic Acids [administration & dosage];
- Randomized Controlled Trials as Topic;
- Skin Neoplasms [*therapy];
Medical Subject Headings Check Words
Female; Humans; Male;
PICO
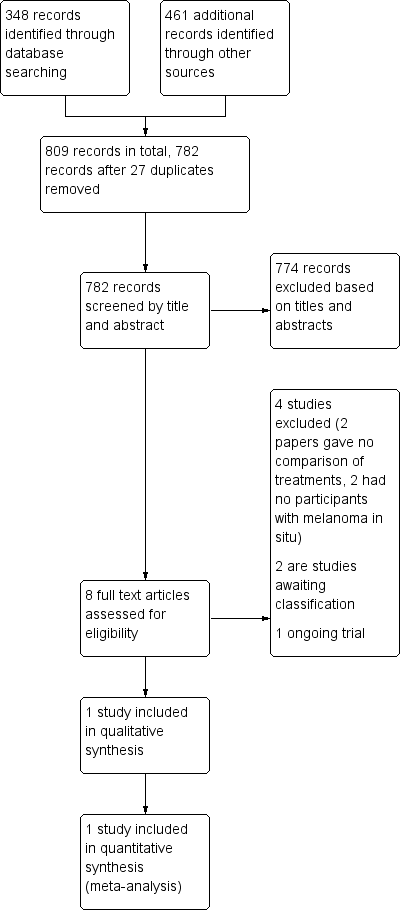
Study flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 1 Primary outcome: Histological complete response (ITT).
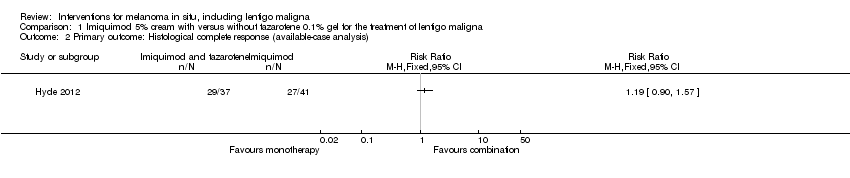
Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 2 Primary outcome: Histological complete response (available‐case analysis).

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 3 Secondary outcome: Discontinuation of treatment because of harms.
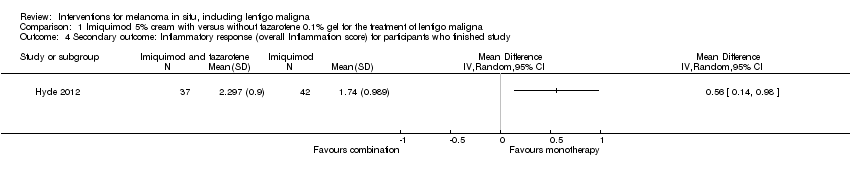
Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 4 Secondary outcome: Inflammatory response (overall Inflammation score) for participants who finished study.

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 5 Secondary outcome: Inflammatory response (overall Inflammation score) for all participants.

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 6 Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3).
| Imiquimod 5% cream with tazarotene 0.1% gel compared with Imiquimod 5% cream alone for melanoma in situ, including lentigo maligna | ||||||
| Patient or population: participants with melanoma in situ, including lentigo maligna | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Imiquimod 5% cream alone | Imiquimod 5% cream with tazarotene 0.1% gel | |||||
| Primary outcome: Histological complete response (intention‐to‐treat) | 587 per 1000 | 657 per 1000 | RR 1.12 | 90 | ⊕⊕⊝⊝ | ‐ |
| Primary outcome: Histological complete response ( available‐case analysis) | 659 per 1000 | 784 per 1000 | RR 1.19 | 78 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Discontinuation of treatment because of harms | 22 per 1000 | 136 per 1000 | RR 6.27 | 90 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3) | 591 per 1000 | 815 per 1000 | RR 1.38 | 88 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (overall Inflammation score) for all participants | ‐ | The mean overall inflammation score (inflammatory response) for all participants in the intervention groups was 0.60 higher | ‐ | 88 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| GRADE = Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| Medical term | Explanation |
| Intraepidermal | something confined within the epidermis |
| Preinvasive | a malignant tumour that remains constricted in the epithelium, while the dermo‐epidermal junction preserves its integrity |
| Eschar | a dry scab or slough formed on the skin as a result of a burn or by the action of a corrosive or caustic substance |
| Invasive | a malignant tumour that breaks the dermo‐epidermal junction and invades the dermis |
| Lentigo maligna | a subtype of preinvasive intraepidermal melanoma, associated specifically with chronic solar exposure |
| Vehicle | an excipient or a menstruum, a substance, usually without therapeutic action, used as a medium to give bulk for the administration of medicines |
| Apoptosis | a genetically directed process of cell self‐destruction that is marked by the fragmentation of nuclear DNA. It is activated either by the presence of a stimulus or removal of a suppressing agent or stimulus, and is a normal physiological process eliminating DNA‐damaged, superfluous, or unwanted cells. It is a synonym for programmed cell death |
| Actinic keratosis | rough, scaly macule or patch of skin that it is considered precancerous and is associated with chronic sun exposure |
| Lentigo senilis | a flat brownish, pigmented spot on the skin, due to increased deposition of melanin and an increased number of melanocytes |
| Seborrheic keratosis | a benign skin tumour that occurs singly or in clusters on the surface of the skin, is usually light to dark brown or black in colour, and typically has a warty texture often with a waxy appearance |
| Patch | a greater than 1 cm circumscribed area of discolouration on the skin |
| Cytokine | a small protein released by cells that has a specific effect on the interactions between cells, on communications between cells, or on the behavior of cells |
| Innate immunity | immunity that occurs naturally as a result of a person's genetic constitution or physiology and does not arise from a previous infection or vaccination |
| Blinding | the concealment of group assignment ‐ to either the treatment or control group ‐ from the knowledge of participants, investigators in a clinical trial, or both |
| Tumour islands | aggregates of tumour cells |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: Histological complete response (ITT) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Primary outcome: Histological complete response (available‐case analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Secondary outcome: Discontinuation of treatment because of harms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Secondary outcome: Inflammatory response (overall Inflammation score) for participants who finished study Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Secondary outcome: Inflammatory response (overall Inflammation score) for all participants Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

