Le traitement par anti‐IgE dans l'aspergillose broncho‐pulmonaire allergique chez les patients atteints de mucoviscidose
Résumé scientifique
Contexte
La mucoviscidose est une maladie multisystémique autosomique récessive dont la prévalence est d'environ 1 pour 3500 naissances vivantes. L'aspergillose broncho‐pulmonaire allergique est une maladie pulmonaire causée par une hypersensibilité induite par l'aspergillus, avec une prévalence de 2 à 15 % chez les personnes atteintes de mucoviscidose. Le traitement de base comprend les corticostéroïdes et l'itraconazole. Le traitement par corticostéroïdes pendant des périodes prolongées, ou de façon répétée pour des exacerbations d'aspergillose broncho‐pulmonaire allergique, pourrait entraîner de nombreux effets indésirables. L'anticorps monoclonal anti‐IgE, l'omalizumab, a amélioré le contrôle de l'asthme chez les asthmatiques gravement allergiques. Le médicament est administré sous forme d'injection sous‐cutanée toutes les deux à quatre semaines. L'aspergillose broncho‐pulmonaire allergique étant également une affection résultant d'une hypersensibilité à des allergènes spécifiques, comme dans le cas de l'asthme, elle pourrait être un candidat au traitement par anticorps anti‐IgE. Par conséquent, la thérapie anti‐IgE, utilisant des agents comme l'omalizumab, pourrait être un traitement potentiel de l'aspergillose broncho‐pulmonaire allergique chez les personnes atteintes de mucoviscidose. Ceci est une version mise à jour de la revue.
Objectifs
Évaluer l'efficacité et les effets indésirables du traitement par anti‐IgE pour l'aspergillose broncho‐pulmonaire allergique chez les patients atteints de mucoviscidose.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre des essais cliniques du groupe Cochrane sur la mucoviscidose, compilé à partir de recherches dans des bases de données électroniques et de recherches manuelles dans des journaux et livrets de résumés de conférences. Nous avons également consulté les références bibliographiques des revues et articles pertinents. Date des dernières recherches: 09 septembre 2021.
Nous avons effectué des recherches dans deux registres d'essais en cours (Clinicaltrials.gov et le système des essais de l'OMS). Date des dernières recherches: 16 août 2021.
Critères de sélection
Les essais contrôlés randomisés et quasi randomisés comparant la thérapie anti‐IgE à un placebo ou à d'autres thérapies pour l'aspergillose broncho‐pulmonaire allergique chez les patients atteints de mucoviscidose.
Recueil et analyse des données
Deux auteurs de la revue ont indépendamment extrait les données et évalué le risque de biais dans les études incluses. Ils avaient prévu d'effectuer l'analyse des données à l'aide de Review Manager.
Résultats principaux
Une seule étude, portant sur 14 participants, était éligible pour être incluse dans la revue. L'étude en double aveugle a comparé une dose quotidienne de 600 mg d'omalizumab à un placebo, en association avec de l'itraconazole deux fois par jour et des corticostéroïdes oraux à une dose quotidienne maximale de 400 mg. Le traitement a duré six mois mais l'étude a été abandonnée et les données complètes n’étaient pas disponibles. Nous avons contacté l'investigateur de l'étude et avons été informés que l'étude avait été abandonnée en raison de l'incapacité à recruter des participants dans l'étude malgré toutes les tentatives raisonnables. Un ou plusieurs effets secondaires graves ont été rencontrés chez six participants sur neuf (66,67 %) et chez un sur cinq (20 %) dans le groupe omalizumab et le groupe placebo respectivement.
Conclusions des auteurs
Il n’existe pas suffisamment de données probantes concernant l'efficacité et la tolérance du traitement par anti‐IgE (omalizumab) chez les personnes atteintes de mucoviscidose et d'aspergillose broncho‐pulmonaire allergique. Il est nécessaire de mener des études prospectives randomisées et contrôlées de grande taille sur le traitement par anti‐IgE chez les personnes atteintes de mucoviscidose et d'aspergillose bronchopulmonaire allergique, avec des mesures de critères de jugement cliniques et biologiques telles que les besoins en stéroïdes, les exacerbations de l'aspergillose bronchopulmonaire allergique et la fonction pulmonaire.
PICOs
Résumé simplifié
L’utilisation d’un traitement par anti‐IgE dans l'aspergillose broncho‐pulmonaire allergique chez les personnes atteintes de mucoviscidose
Problématique de la revue
Nous avons examiné les données probantes concernant l'effet du traitement par anti‐IgE pour traiter l'aspergillose broncho‐pulmonaire allergique chez les personnes atteintes de mucoviscidose.
Contexte
La mucoviscidose est une maladie héréditaire qui n'est pas rare dans les pays occidentaux. L'aspergillose broncho‐pulmonaire allergique est une maladie pulmonaire causée par une sensibilité extrême à l'aspergillus (un champignon) et pourrait survenir chez 2 à 15 % des personnes atteintes de mucoviscidose. Les corticostéroïdes et les traitements antifongiques constituent le pilier du traitement de l'aspergillose broncho‐pulmonaire allergique, mais l'utilisation prolongée ou répétée de corticostéroïdes pourrait entraîner des effets secondaires graves. L'aspergillose broncho‐pulmonaire allergique se produit suite à l'action des anticorps IgE (un type de protéine). Un médicament qui agit contre ces anticorps IgE (thérapie anti‐IgE), comme l'omalizumab, pourrait être un traitement potentiel de l'aspergillose broncho‐pulmonaire allergique chez les personnes atteintes de mucoviscidose. Le médicament est administré sous forme d'injection sous la peau toutes les deux à quatre semaines. Cette revue a pour but de montrer si le traitement par anti‐IgE de l'aspergillose broncho‐pulmonaire allergique chez les personnes atteintes de mucoviscidose est efficace et de mettre en évidence les éventuels effets secondaires.
Date de la recherche
Les données probantes sont à jour jusqu’au: 09 septembre 2021.
Caractéristiques des études
Nous n'avons pu inclure qu'une seule petite étude dans la revue (14 participants) et celle‐ci a été abandonnée car trop peu de personnes se sont portées volontaires pour participer à l'étude. L'étude a duré six mois et a comparé des injections d'omalizumab (Xolair®) sous la peau du bras ou de la cuisse à des injections de placebo (traitement factice ne contenant aucun médicament actif). Les volontaires ont reçu 600 mg d'omalizumab ou un placebo de façon quotidienne en même temps que de l'itraconazole (un antifongique) deux fois par jour et des corticostéroïdes oraux avec une dose quotidienne maximale de 400 mg.
Principaux résultats
Les résultats complets de l'étude n'ont pas été publiés. Seuls des résultats limités sur les effets secondaires ont été publiés en ligne. Six volontaires sur neuf (66,67 %) dans le groupe omalizumab et un volontaire sur cinq (20 %) dans le groupe placebo ont signalé un ou plusieurs effets secondaires graves.
En raison du manque de données probantes, nous ne sommes pas en mesure de faire des recommandations en faveur ou contre l'utilisation d'un traitement par anti‐IgE (omalizumab) chez les personnes atteintes de mucoviscidose et d'aspergillose broncho‐pulmonaire allergique. Des recherches supplémentaires sur ce traitement sont nécessaires.
Authors' conclusions
Background
Please refer to the glossary for the explanation of clinical terms (Appendix 1).
Description of the condition
Cystic fibrosis (CF), an autosomal recessive multisystem disorder, is characterized by the obstruction and infection of respiratory airways, malabsorption, and various other manifestations and complications (Rowe 2005). With an approximate prevalence of 1 in 3500 live births, CF is more frequent in northern Europe, North America, Australia and New Zealand (Rowe 2005). The mutation in the CF transmembrane regulator (CFTR) gene, with resulting dysfunction of epithelialized surfaces, is the predominant pathogenetic feature in CF. Pulmonary lesions and complications are a major cause of morbidity and mortality in people with CF. Allergic bronchopulmonary aspergillosis (ABPA) is a lung disease caused by aspergillus‐induced hypersensitivity, which usually occurs in susceptible people with bronchial asthma and CF (Agarwal 2009; Greenberger 2002; Tillie‐Leblond 2005). The prevalence of ABPA in people with CF has been described as ranging from 2% to 15% (Becker 1996; Carneiro 2008; Geller 1999; Mastella 2000; Skov 2005; Stevens 2003; Taccetti 2000). These prevalence studies included either just adults or both adults and children. Although, sensitization to aspergillus is not uncommon in people with CF (31% to 59% of people with CF), only a fraction of these individuals develop ABPA (Hemmann 1998; Valletta 1993). The pathophysiology of ABPA is not yet clear. The aspergillus spores attach and penetrate the pre‐activated epithelium in genetically susceptible individuals with CF and form hyphae (Knutsen 2011). The aspergillus antigens from hyphae activate the body’s immune response with a release of inflammatory cytokines (especially IL‐4) which cause bronchial or bronchiolar inflammation and destruction (Knutsen 2003; Knutsen 2011).
The Cystic Fibrosis Foundation Consensus Conference laid down diagnostic criteria for ABPA in CF as well as criteria for screening for ABPA in people with CF (Stevens 2003). Clinical features of ABPA in CF include an acute exacerbation of symptoms, weight loss and a marked increase in productive coughing (Stevens 2003). The presence of ABPA in people with CF has been associated with a decline in lung function (Nepomuceno 1999). If untreated, ABPA may progress to bronchiectasis or pulmonary fibrosis, or both, with significant morbidity and mortality. The mainstay of treatment for ABPA includes corticosteroids and itraconazole. The treatment with corticosteroids may be required for prolonged periods or repeatedly for exacerbations of ABPA, which may lead to many adverse effects. A Cochrane review found no evidence for the use of itraconazole or other antifungal agents for ABPA in people with CF (Elphick 2014).
Description of the intervention
The monoclonal anti‐IgE antibody, omalizumab, has produced a new approach to intervene in the allergic pathway; this new approach is directed to an epitope expressed on the Cɛ3 domain of IgE that binds to high‐ and low‐affinity receptors (Shields 1995). Omalizumab has been shown to decrease circulating free IgE levels by the formation of trimeric and hexameric complexes. These complexes are then cleared by the reticuloendothelial system and there is no activation of a complement system (Corne 1997). In people with asthma, omalizumab inhibits early and late phases of bronchoconstriction induced by allergens, hyper‐responsiveness, and skin‐prick test results (Fahy 1997). In a randomized controlled study, omalizumab treatment improved asthma control in severely allergic asthmatics, reducing inhaled corticosteroid requirements without a worsening of symptom control or an increase in rescue medication use (Holgate 2004). It has been shown to decrease eosinophil counts and also levels of interleukin (IL)‐2+ and IL‐13+ T‐lymphocytes in people with allergic asthma (Noga 2006). Omalizumab is usually given subcutaneously every two to four weeks and the dose is calculated by body weight and baseline total serum IgE levels (Holgate 2004; van der Ent 2007). In a Cochrane review, omalizumab had been found to be effective and safe in allergic asthma (Normansell 2014).
How the intervention might work
Allergic bronchopulmonary aspergillosis is a pulmonary hypersensitivity disease induced by Aspergillus fumigatus (A. fumigatus) (Tillie‐Leblond 2005). In people with CF and ABPA, a number of immunological responses to antigens of A. fumigatus can be observed, such as peripheral blood eosinophilia, immediate cutaneous reactivity, increased levels of total serum IgE, the presence of precipitating antibodies and increased specific serum IgE and IgG antibodies to A. fumigatus (Stevens 2003). Thus, immunomodulatory drugs may be effective for treating ABPA. Omalizumab, an anti‐IgE antibody has been found to be effective in treating asthma with a strong allergy component. Since ABPA is also a condition resulting from hypersensitivity to specific allergens, it may be a candidate for therapy using anti‐IgE antibodies.
Why it is important to do this review
Currently, corticosteroids and antifungal therapy are the mainstay of treatment for ABPA. Corticosteroids may lead to serious side‐effects when used for prolonged periods or repeatedly for exacerbations of ABPA. Therefore, it is prudent to have a therapy for ABPA with a steroid‐sparing effect. Anti‐IgE therapy, using agents such as omalizumab, may be such a potential therapy for ABPA in people with CF. A few observational studies (Emiralioglu 2016; Koutsokera 2020; Nové‐Josserand 2017; Perisson 2017) had shown beneficial effects of omalizumb in CF with ABPA, but a reviw of randomized controlled trials will provide robust evidence for use of omalizumab in CF with ABPA.
This is an updated version of a previously published review (Jat 2018).
Objectives
To evaluate the efficacy and adverse effects of anti‐IgE therapy for ABPA in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs were eligible for inclusion. Cross‐over and cluster‐RCTs were not eligible for inclusion.
Types of participants
People with CF and ABPA. Diagnosis of CF should be in accordance with the criteria laid down by the Cystic Fibrosis Foundation Consensus Report (Farrell 2008); ABPA should be diagnosed using the Rosenberg‐Patterson criteria (Rosenberg 1977), Nelson’s criteria (Nelson 1979), Greenberger’s criteria (Greenberger 2002) or the Cystic Fibrosis Foundation Consensus Criteria (Stevens 2003). There was no limit to age or disease severity for participants included in the review.
Types of interventions
Anti‐IgE therapy compared to placebo or other therapies for ABPA in people with CF. We considered all doses of anti‐IgE therapy in the review.
Types of outcome measures
Primary and secondary outcomes are as follows.
Primary outcomes
-
Number of participants responding to anti‐IgE therapy (as defined by a decrease in oral corticosteroid dose by 50% or more in comparison to baseline)
-
Number of participants requiring rescue therapy with corticosteroids
-
Adverse effects
-
mild (do not lead to discontinuation of treatment)
-
moderate (lead to a change in treatment)
-
severe (lead to hospitalisation or are life‐threatening)
-
Secondary outcomes
-
Lung function
-
forced expiratory volume in one second (FEV₁)
-
peak expiratory flow rate (PEFR)
-
forced vital capacity (FVC)
-
ratio of FEV1/FVC
-
-
Time until steroid use ceases
-
Number of ABPA exacerbations
-
Pulmonary exacerbations requiring treatment (oral or nebulised or intravenous (IV) or combination)
-
Hospitalisation
-
number of admissions
-
number of days in hospital
-
Search methods for identification of studies
We searched for all relevant published and unpublished studies without restrictions on language (we did not exclude studies reported in a language other than English), year or publication status.
Electronic searches
We identified relevant studies from the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's CF Trials Register using the term: allergic bronchopulmonary aspergillosis.
The CF Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group's website.
Date of last search of CF Trials Register: 09 September 2021.
We searched two ongoing trial registries (apps.who.int/trialsearch/; clinicaltrials.gov); details of these searches are provided in the appendices (Appendix 2).
Searching other resources
We also checked the reference lists of any identified studies for additional potentially relevant studies. We contacted authors of the included study to find any ongoing or unpublished relevant studies.
Data collection and analysis
Selection of studies
All three authors independently assessed the results of the searches for any potentially relevant studies. The authors then retrieved and independently assessed the full text of the single study for inclusion, as per the criteria listed above. The authors did not identify any further studies and therefore did not list any studies in any other sections (Excluded studies; Studies awaiting classification; Ongoing studies). There were no disagreements to resolve by discussion.
Data extraction and management
Two review authors (KRJ and AK) independently extracted as much data as possible using a standardized data collection form in accordance with Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), which included the following data: source; eligibility; study methods; participants and settings; interventions and comparisons; outcomes; results; adverse outcomes; and miscellaneous details (e.g. funding source of the study, or potential conflicts of interest). We resolved any disagreement which arose by discussion.
The authors planned to assess all primary and secondary outcomes at 'up to three months', 'over three and up to six months’ and 'over six and up to 12 months' of treatment and to consider any other time‐points reported. There are no efficacy study data available, but there are some adverse event data available on the website clinicaltrials.gov that were reported after six months of treatment.
Assessment of risk of bias in included studies
Two review authors (KRJ and AK) independently assessed the risk of bias in the included study using the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The authors assessed the risk of bias for the domains listed below.
-
Allocation sequence generation
-
Concealment of allocation
-
Blinding of participants and investigators
-
Incomplete outcome data
-
Selective outcome reporting
-
Any other potential risk of bias
The authors judged each of these criteria to have a low, high or unclear risk of bias and resolved any disagreement by discussion. The authors produced figures for a 'Risk of bias graph' and 'Risk of bias summary' using Review Manager 5.1 (RevMan 2014).
Measures of treatment effect
The authors planned to analyse dichotomous variables by calculating the risk ratio (RR). They planned to express continuous variables as a mean difference (MD). They will express continuous variables as standardized mean difference (SMD) if different scales are used for an outcome in different studies in future updates of review. The authors planned to analyse time‐to‐event data using the hazard ratio (HR). They intended to express the overall results with 95% confidence intervals (CI). Finally, the authors planned to report the numbers of ABPA exacerbations and hospital admissions as a rate ratio (ratio of events per person years) in experimental and control group (Deeks 2011).
Unit of analysis issues
The authors planned to include only randomized and quasi‐randomized controlled parallel trials in the review; the included trial was randomized. They planned to exclude both cross‐over and cluster‐randomized trials.
Dealing with missing data
One author (KRJ) contacted study investigators via email to request some missing outcome data; in response, the authors received a small amount of additional information other than that available on website and have added this to the review. The authors also considered taking the following steps to deal with any missing data in future.
-
Perform sensitivity analyses to assess the sensitivity of any assumptions, if made in future updates.
-
Address the potential impact of missing data on the findings of the review in the 'Discussion' section.
-
For missing standard deviations (SDs) of continuous outcome data, to calculate the SD from study statistics (e.g. CIs, standard errors, T values, P values or F values); if it was still not possible to calculate the SD, then they planned to impute it from other studies in the meta‐analysis as per details given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If data are still not available, the authors will generate analyses making assumptions to create the best and worst case scenarios. In this case, the authors planned to undertake a sensitivity analysis (see below).
-
If the review authors include more studies in future, they plan to explore the impact of including studies with missing outcome data in the overall assessment of results by a sensitivity analysis.
Where possible, the authors planned to extract data to allow an intention‐to‐treat (ITT) analysis, which aims to include all participants randomized into a trial irrespective of what happened subsequently.
Assessment of heterogeneity
The current review included only one study. in future updates of review if additional studies become available, the authors will assess clinical and methodological heterogeneity before pooling data in a meta‐analysis. We will carry out an assessment for statistical heterogeneity initially visually and then using a Chi² test and also using the I² statistic. Using the Chi² test, a low P value (P < 0.1) or a large Chi² statistic relative to its degree of freedom will provide evidence of heterogeneity of intervention effects (i.e. the variation in effect estimates beyond chance). The authors will interpret the value of the I² statistic as follows:
-
0% to 40% ‐ might not be important;
-
40% to 60% ‐ may represent moderate heterogeneity;
-
60% to 75% ‐ may represent substantial heterogeneity; and
-
75% to 100% ‐ considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
One author (KRJ) contacted study investigators through email to provide missing outcome data, but no additional missing data were available as the trial was stopped prematurely.
If sufficient numbers of included studies are available in future updates of review, the authors will assess publication bias, by using funnel plots in Review Manager (RevMan 2014).
Data synthesis
If a sufficient number of included studies are available in future updates of review, the authors will perform meta‐analyses using Review Manager (RevMan 2014). We plan to use a fixed‐effect model for pooled data analysis; however, if there is important statistical heterogeneity identified (I² statistic is more than 50%) we will also use a random‐effects model in a sensitivity analysis among studies.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of included studies (at least 10) are available in future updates of review and if the authors identify substantial heterogeneity (over 50%), we will explore it by performing the following subgroup analyses:
-
children (up to and including 18 years of age) versus adults (over 18 years of age);
-
different stages of ABPA (five stages according to Patterson (Patterson 1982): (1) acute; (2) remission; (3) exacerbation; (4) corticosteroid‐dependent asthma; and (5) fibrosis (end stage));
-
colonization of participants with Staphylococcus aureus or Pseudomonas aeruginosa or both or neither.
Sensitivity analysis
Further, the authors will perform a sensitivity analysis to explore whether the heterogeneity is a result of different risk of bias. If sufficient numbers of trials are available (at least 10) they will undertake sensitivity analyses as follows in future updates of review:
-
repeating meta‐analysis after exclusion of studies with a high risk of bias due to concealment of allocation;
-
repeating meta‐analysis after exclusion of studies in which the outcome evaluation was not blinded;
-
repeating meta‐analysis after imputing missing data as best possible and worst possible outcome; and
-
comparing the difference of pooling analysis results by using a fixed‐effect model and a random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We will use the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) to assess the quality of the body of evidence for each outcome in the summary of findings tables (Schünemann 2019b). We will assess the certainty of evidence based on five domains: the risk of bias within the studies contributing to the outcome; relevance to our population of interest (indirectness); unexplained heterogeneity or inconsistency; imprecision of the results; and high risk of publication bias. As a starting point, we will assess all the evidence as high certainty , but will downgrade once if risk of bias is serious and twice if we deem the risk to be very serious across the five domains. We will assess each outcome individually and give it a high, moderate, low or very low rating for the certainty of evidence. Where we downgrade the certainty of the evidence, we will describe our reasons and supporting information for our decisions in the footnotes for each summary of findings table.
We will present a separate summary of findings table for each comparison that we include in the review. For each outcome we will report the illustrative risk with and without the intervention and the magnitude and direction of effect. For dichotomous outcomes, we will report absolute and corresponding risk as a RR (95% CI). For continuous data, we will report the absolute and corresponding risk as MD (95% CI). We will also present the number of studies contributing to the outcome and the number of participants (Schünemann 2019a). We plan to present the following outcomes.
-
Number of participants responding to anti‐IgE therapy
-
Number of participants requiring rescue therapy with corticosteroids
-
Adverse effects
-
FEV₁
-
Number of ABPA exacerbations
-
Number of hospitalizations
Results
Description of studies
The following section is about description of studies.
Results of the search
Only one study for inclusion in the review was found through the search strategy (Figure 1).
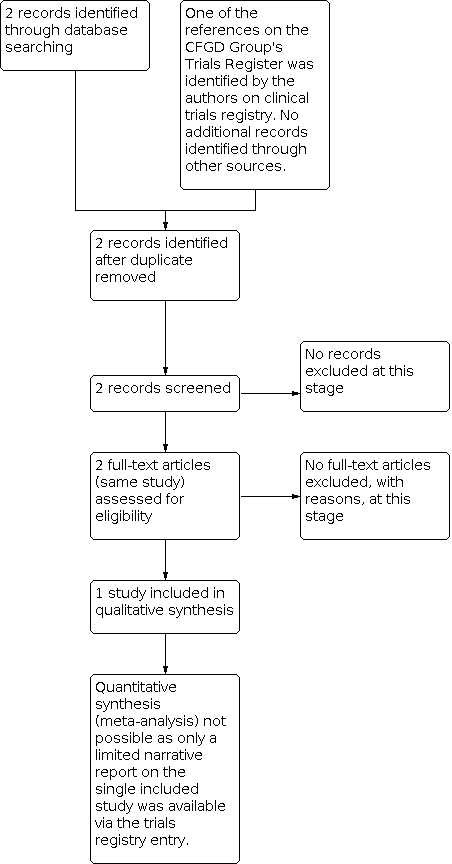
Study flow diagram.
Included studies
Ony one study which evaluated the safety and efficacy of omalizumab for the treatment of ABPA in people with CF was included in the review (NCT00787917). The study details are only available at online trials registries and the characteristics of the study are shown in Characteristics of included studies table.
Trial design
The study was double‐blinded, randomized and placebo‐controlled. Assignment was of parallel design. The study was multicentre, conducted in five countries (Belgium, Germany, Italy, Netherlands, and UK) (NCT00787917). The double‐blind phase of the study was followed by an open‐label period of six months for those participants who completed the double‐blinded phase and continued the same regimen of omalizumab they had received in the double‐blinded phase. No placebo was used in the open‐label period, hence the data from this period are not eligible for inclusion in the review.
Participants
Study participants were individuals with CF complicated by ABPA and aged 12 years and older (except for Italy where participants were up to and including 18 years of age). Both males and females with oral corticosteroid use for ABPA flare and total serum IgE levels greater than or equal to 500 IU/mL were eligible for enrolment. The study enrolled nine (five females) participants in the intervention group and five (four females) participants in the placebo group. The mean (SD) age of participants in the intervention group was 21 (4.1) years and in the placebo group 28 (9.5) years (NCT00787917).
Interventions
In the double‐blind phase of the study, the participants in the experimental arm received omalizumab (Xolair®) in the form of subcutaneous injections (into the upper arm in the area of the deltoid or to the thigh) of 600 mg daily for six months along with itraconazole twice daily, while receiving oral corticosteroids, with a maximum daily dose of 400 mg. The participants in the control arm received placebo in place of omalizumab in the same regimen as the experimental arm.
Outcomes measured
Primary outcome measures were:
-
the change from baseline in the percentage of participants requiring rescue with corticosteroids; and
-
the time to deviation from the protocol‐prescribed steroid‐tapering regimen.
Secondary outcome measures were:
-
change in ABPA exacerbation rates;
-
change in FEV1 from baseline;
-
time to steroid‐free state;
-
change from baseline in average oral corticosteroid use;
-
percentage of participants responding to omalizumab, as defined by a reduction in oral corticosteroid dose use of 50% or more as compared to baseline.
Most of the outcome measures were assessed at six and 12 months of study, except for FEV1 which was assessed at three and six months (NCT00787917).
Excluded studies
The search strategy revealed only one relevant study that was included in the review. No studies were excluded.
Risk of bias in included studies
The risk of bias for the only included study (NCT00787917) is shown in Characteristics of included studies, risk of bias graph (Figure 2) and risk of bias summary (Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There was unclear risk of bias for sequence generation and allocation concealment for the study as information was not available (NCT00787917).
Blinding
There was low risk bias as the study was described as double‐blinded; where participant, caregiver, investigator, and outcome assessor were masked to treatment assignment (NCT00787917).
Incomplete outcome data
There was a high risk of bias for incomplete outcome data because a large proportion of enrolled participants dropped out. Of the nine participants enrolled in the intervention group only four completed the double‐blind phase of the study, and of the five participants in the placebo group only three completed this phase. The five participants who dropped out of the intervention group did so for the following reasons: one due to adverse events; one due to lack of efficacy; and three due to administrative problems. Both participants who dropped out of the placebo group did so due to administrative problems.
Selective reporting
There is a high risk of selective reporting since data related to all the outcome measures were not reported on the website, even for those participants who completed the trial before it was terminated.
Other potential sources of bias
There was high risk of bias as the study was terminated early and results were not published in any journal.
Effects of interventions
Details of the included study are available at www.clinicaltrials.gov only (NCT00787917). It was terminated early and results for any outcome measures except for adverse effects are not available online (www.clinicaltrials.gov).
The study reported one or more non‐serious side effects in all participants in the double‐blinded phase (Table 1; NCT00787917). One or more serious side effects were encountered in six out of nine (66.67%) and one out of five (20.00%) participants in omalizumab group and placebo group respectively (Table 1).
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
|---|---|---|
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
AE: adverse event
CF: cystic fibrosis
SAE: serious adverse event
Discussion
Summary of main results
Only one study was eligible for inclusion in the review, but that was terminated prematurely (NCT00787917).The study details are available at www.clinicaltrials.gov only and full results were not published. Only results relating to adverse effects were available; and serious side effects were encountered more frequently in the omalizumab group compared to the placebo group.
Overall completeness and applicability of evidence
There is lack of evidence for the efficacy and safety of anti‐IgE therapy for ABPA in people with CF.
Quality of the evidence
It is difficult to comment on the quality of the only included study as there is not sufficient information available. A summary of findings table could not be created because of the lack of included studies.
Potential biases in the review process
The search strategy was broad enough to include all relevant studies. However, only one study with incomplete information was eligible for inclusion in the review (NCT00787917).
Agreements and disagreements with other studies or reviews
A few case reports have described the effectiveness of omalizumab for ABPA in people with CF. In 2007, for the first time, van der Ent reported the use of a single dose of omalizumab in a 12‐year‐old girl with CF and ABPA who showed a rapid and good improvement of respiratory symptoms and lung functions (van der Ent 2007). Zirbes reported the use of omalizumab in three children with CF and ABPA (three males aged 12.9 years, 12.8 years and 17 years) who were steroid‐dependent with significant side effects from chronic steroid therapy (Zirbes 2008). After the start of omalizumab (300 mg to 375 mg subcutaneously every two weeks), these children showed significant and sustained clinical improvements and all were able to discontinue steroids. In a third study, Kanu reported on a girl aged 13 years with CF and ABPA who was poorly controlled on steroids; she received 300 mg omalizumab subcutaneously every two weeks and her clinical symptoms and lung function improved markedly to a level that she had not been able to achieve with steroids or antibiotics (Kanu 2008). In another study, two teenagers with CF and ABPA exacerbations who were reluctant to undertake a further course of oral steroids, started subcutaneous injections of 375 mg omalizumab twice monthly (Lebecque 2009). Both respiratory symptoms and lung functions improved rapidly and the therapy was gradually withdrawn without any recurrence after 20 weeks of follow up.
In contrast to the above case reports, in a study by Brinkman, the use of 300 mg omalizumab for four weeks in a 15‐year‐old with CF and steroid‐dependent ABPA was not successful and the individual deteriorated again after an initial improvement of lung function, remaining steroid dependent over 12 months of omalizumab treatment (Brinkmann 2010). In a recent observational study ( Koutsokera 2020), authors reported use of omalizumab in 27 adult CF patients with difficult to treat asthma (n=16) and ABPA (n=11) with improvement in lung function and decrease in steroid use. A retrospective study of 18 patients (mean age 17.1 years) of CF with ABPA reported stabilization of lung function and decresase in use of oral steroids after use of omalizaumb without significant adverse effects (Perisson 2017). A French study of omalizumab usse for 3 months in 32 CF patients (21 adulst and 11 children) with ABPA reported steroid sparing effects, though no change in lung functions (Nové‐Josserand 2017). Emiralioglu et al reported steroid sparing effect of omalizumb in six patients of CF with ABPA (Emiralioglu 2016). These observational studies suggest beneficial effect of omalizumab in CF patients with ABPA, but RCTs are needed to prove it.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
|---|---|---|
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
| AE: adverse event | ||

