Tratamiento anti‐IgE para la aspergilosis broncopulmonar alérgica en pacientes con fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010288.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
| Roles and responsibilities | |
| Task | Who will undertake the task? |
| Protocol stage: draft the protocol | All three authors |
| Review stage: select which trials to include (2 + 1 arbiter) | All three authors |
| Review stage: extract data from trials (2 people) | KRJ, AK |
| Review stage: enter data into RevMan | KRJ, AK |
| Review stage: carry out the analysis | KRJ, DKW |
| Review stage: interpret the analysis | All three authors |
| Review stage: draft the final review | All three authors |
| Update stage: update the review | All three authors |
Sources of support
Internal sources
-
Government Medical College Hospital, Chandigarh, India.
Support from library regarding literature search and getting full text articles.
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None known.
Acknowledgements
We thank Nikki Jahnke for support in drafting the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Sep 22 | Anti‐IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis | Review | Kana R Jat, Dinesh K Walia, Anju Khairwa | |
| 2018 Mar 18 | Anti‐IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis | Review | Kana R Jat, Dinesh K Walia, Anju Khairwa | |
| 2015 Nov 04 | Anti‐IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis | Review | Kana R Jat, Dinesh K Walia, Anju Khairwa | |
| 2013 Sep 17 | Anti‐IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis | Review | Kana R Jat, Dinesh K Walia, Anju Khairwa | |
| 2012 Dec 12 | Anti‐IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis | Protocol | Kana R Jat, Dinesh K Walia, Anju Khairwa | |
Differences between protocol and review
None.
Notes
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti‐Allergic Agents [adverse effects, *therapeutic use];
- Antibodies, Anti‐Idiotypic [adverse effects, *therapeutic use];
- Antibodies, Monoclonal, Humanized [adverse effects, *therapeutic use];
- Antifungal Agents [therapeutic use];
- Aspergillosis, Allergic Bronchopulmonary [*drug therapy, etiology];
- Cystic Fibrosis [*complications];
- Early Termination of Clinical Trials;
- Itraconazole [therapeutic use];
- Omalizumab [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
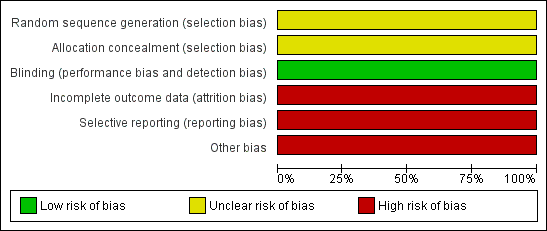
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
| AE: adverse event | ||

