Ингибиторы ароматазы (летрозол) в лечении репродуктивной недостаточности (субфертильности) у женщин с синдромом поликистозных яичников
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010287.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SF wrote the protocol and drafted the full review.
JK and CF acted as clinical experts and commented on the protocol and full review.
SF, SE and LK updated the 2018 version of the review.
CF acted as clinical expert and commented on the full review update in 2018.
Sources of support
Internal sources
-
Cochrane Gynaecology and Fertility Group, New Zealand.
editorial support
External sources
-
None, Other.
Declarations of interest
No declarations of interest.
Acknowledgements
We would like to thank Marian Showell (Information Specilaist) for writing and running the search, Vanessa Jordan for assistance with methodological questions, Helen Nagels (Managing Editor of CGFG) for helping us to develop the protocol and answering our questions, and Julie Brown for assistance with writing the full review. We would like to thank our peer reviewers for giving very constructive feedback, leading to great improvement of the review.
The authors of the 2018 update thank Dr. Willianne Nelen for her contributions to the previous version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Sep 27 | Aromatase inhibitors (letrozole) for ovulation induction in infertile women with polycystic ovary syndrome | Review | Sebastian Franik, Quang-Khoi Le, Jan AM Kremer, Ludwig Kiesel, Cindy Farquhar | |
| 2018 May 24 | Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome | Review | Sebastian Franik, Stephanie M Eltrop, Jan AM Kremer, Ludwig Kiesel, Cindy Farquhar | |
| 2014 Feb 24 | Aromatase inhibitors for subfertile women with polycystic ovary syndrome | Review | Sebastian Franik, Jan AM Kremer, Willianne LDM Nelen, Cindy Farquhar | |
| 2012 Dec 12 | Aromatase inhibitors for subfertile women with polycystic ovary syndrome | Protocol | Sebastian Franik, Jan AM Kremer, Willianne LDM Nelen, Cindy Farquhar | |
Differences between protocol and review
A new secondary outcome has been added as an amendment: Miscarriage rate by pregnancies.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abortion, Spontaneous [epidemiology];
- Anastrozole;
- Aromatase Inhibitors [*therapeutic use];
- Birth Rate;
- Clomiphene [therapeutic use];
- Coitus;
- Fertility Agents, Female [therapeutic use];
- Infertility, Female [*drug therapy, etiology];
- Letrozole;
- Live Birth [epidemiology];
- Nitriles [*therapeutic use];
- Ovarian Hyperstimulation Syndrome [epidemiology];
- Ovary [surgery];
- Ovulation Induction [methods];
- Polycystic Ovary Syndrome [*complications];
- Pregnancy Outcome;
- Pregnancy, Multiple [statistics & numerical data];
- Randomized Controlled Trials as Topic;
- Triazoles [*therapeutic use];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
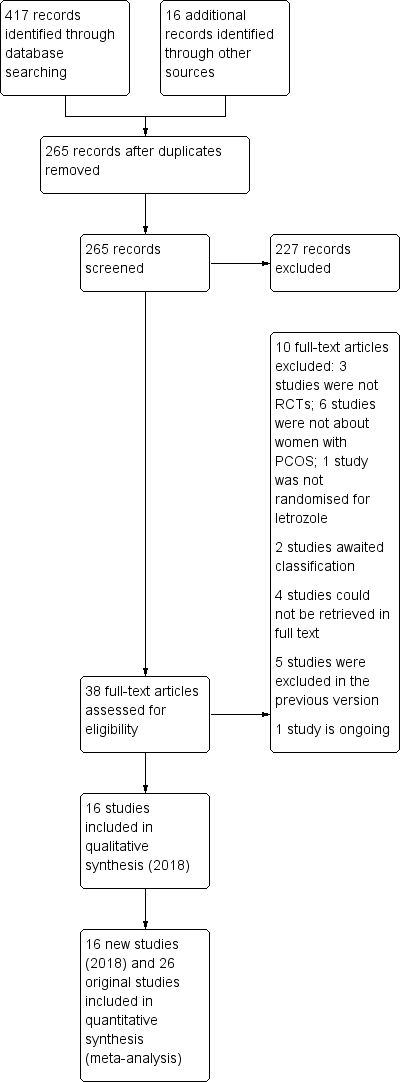
Study flow diagram for update 2018
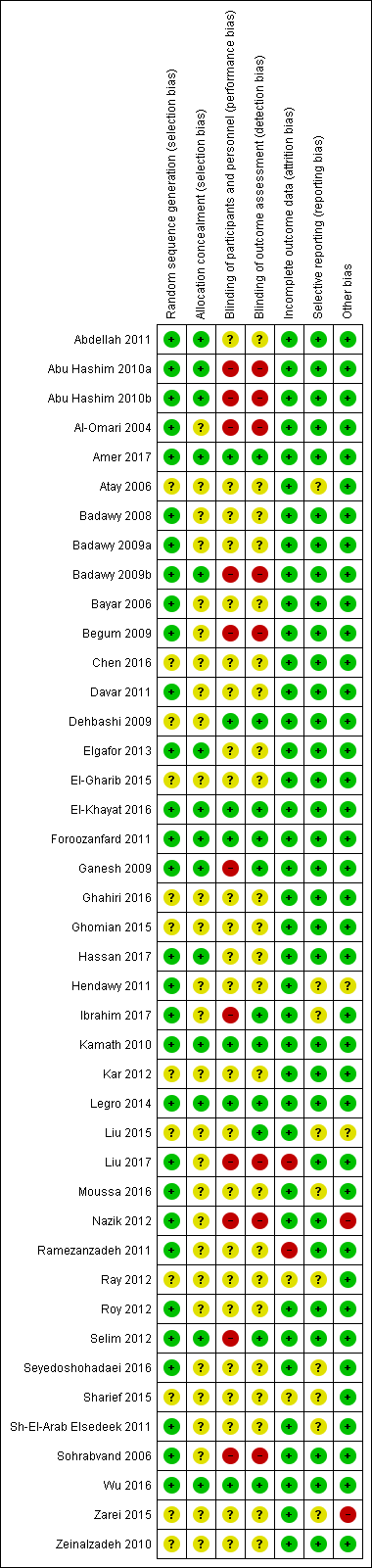
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
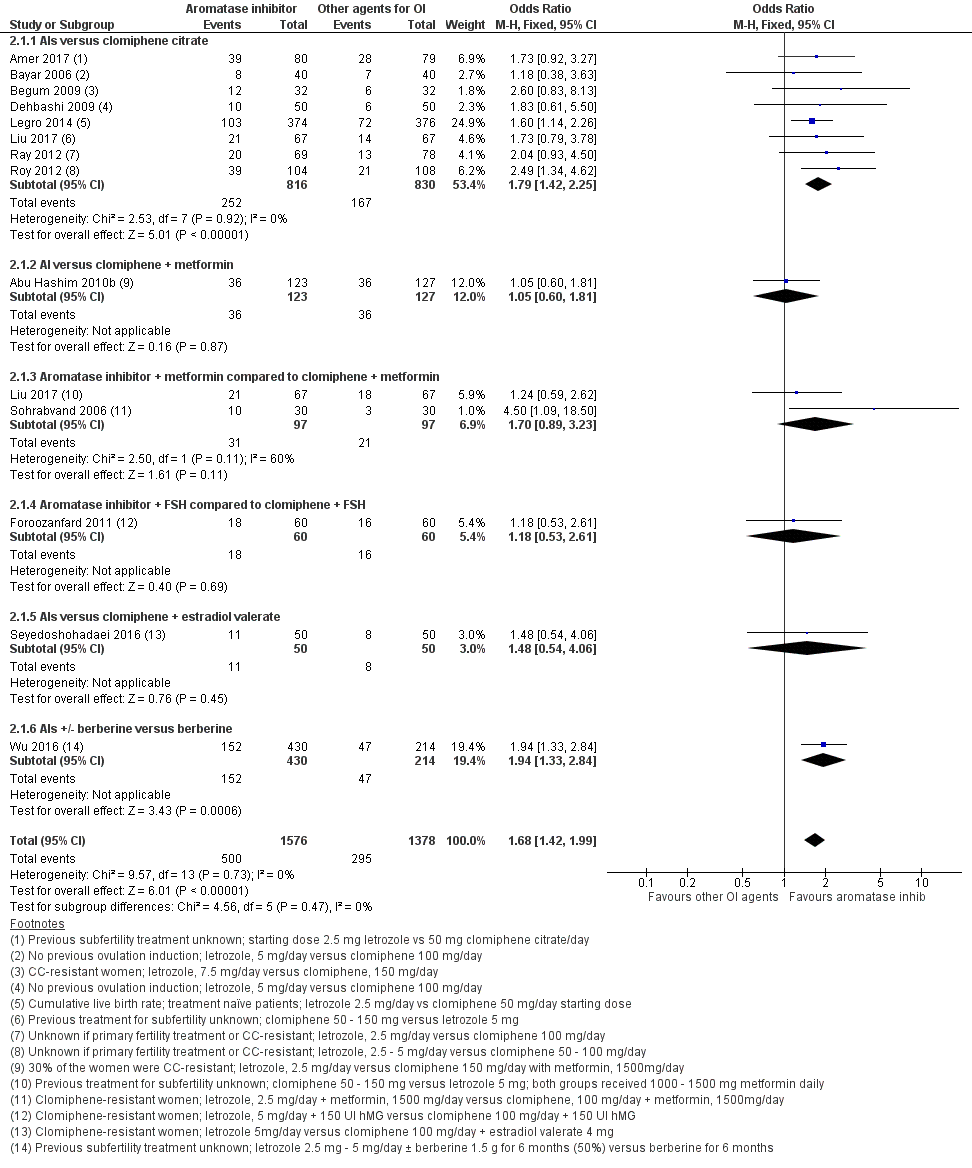
Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.1 Live birth rate.

Funnel plot of comparison: 2 Aromatase inhibitors compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, outcome: 2.1 Live birth rate.
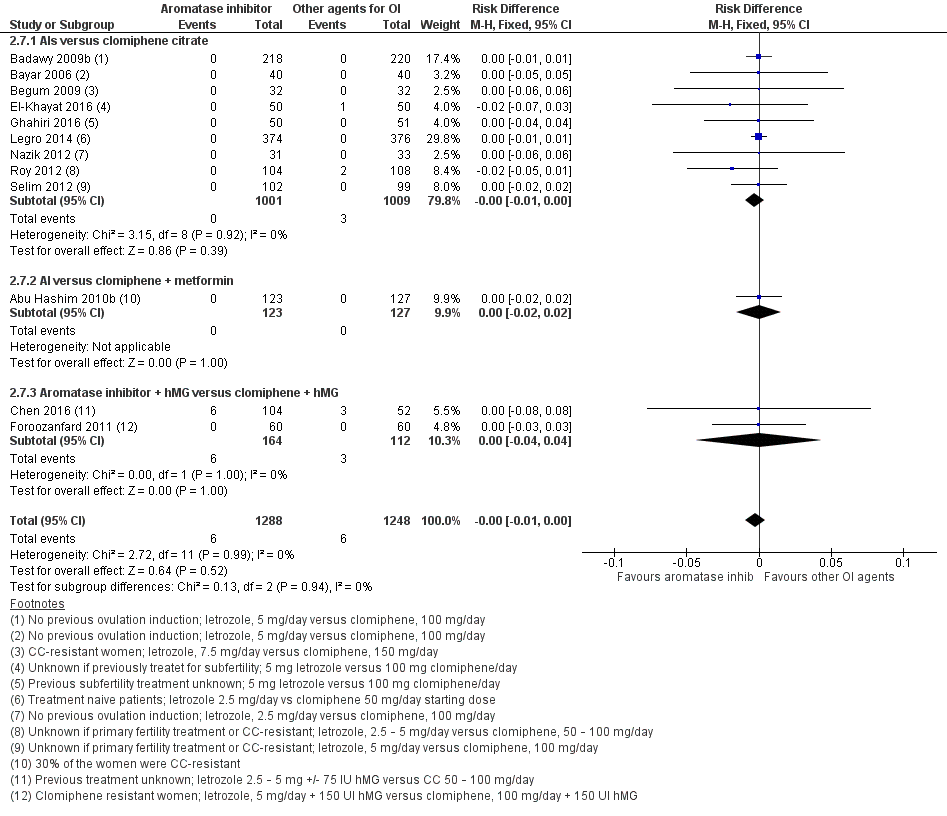
Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.6 Ovarian hyperstimulation syndrome rate.
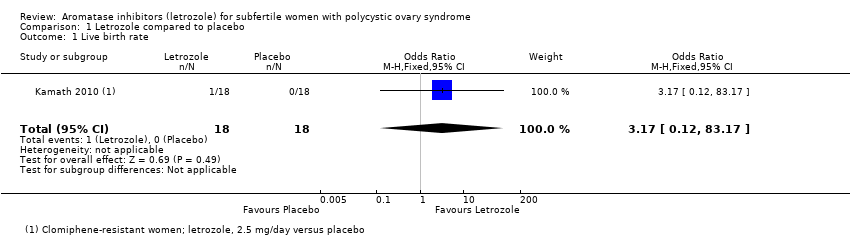
Comparison 1 Letrozole compared to placebo, Outcome 1 Live birth rate.

Comparison 1 Letrozole compared to placebo, Outcome 2 Ovarian hyperstimulation syndrome rate.

Comparison 1 Letrozole compared to placebo, Outcome 3 Clinical pregnancy rate.

Comparison 1 Letrozole compared to placebo, Outcome 4 Miscarriage rate by woman randomised.

Comparison 1 Letrozole compared to placebo, Outcome 5 Miscarriage rate by pregnancies.
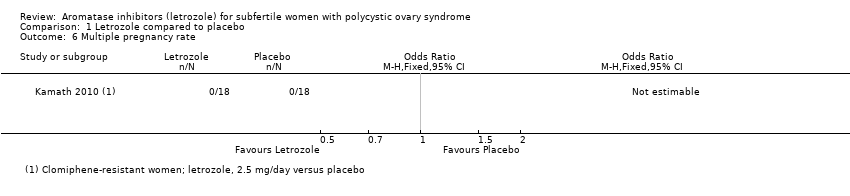
Comparison 1 Letrozole compared to placebo, Outcome 6 Multiple pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 1 Live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 2 Live birth rate by BMI.
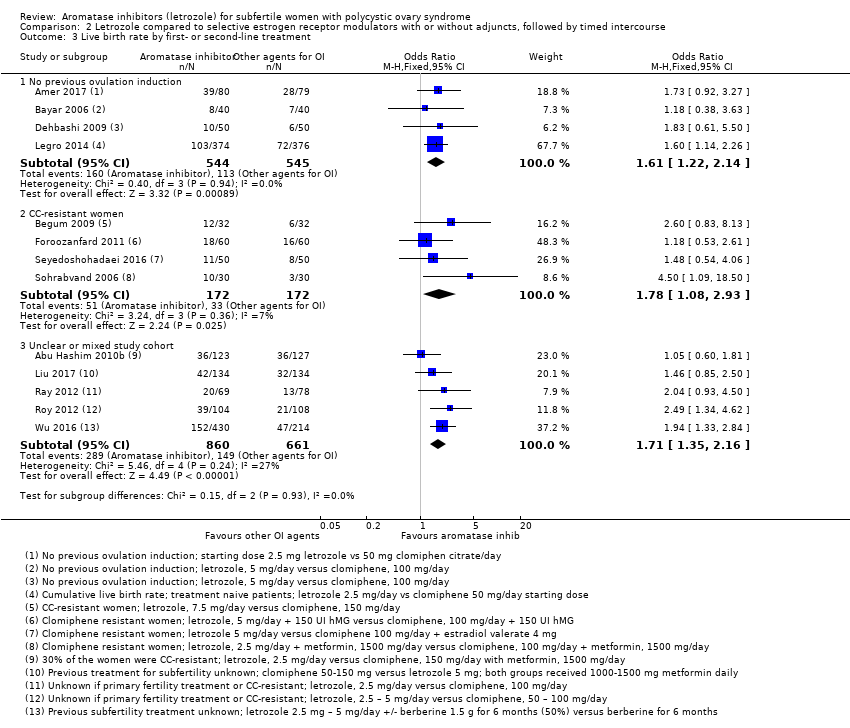
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 3 Live birth rate by first‐ or second‐line treatment.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 4 Impact of allocation bias for live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 5 Impact of detection bias for live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 6 Impact of attrition bias for live birth rate.
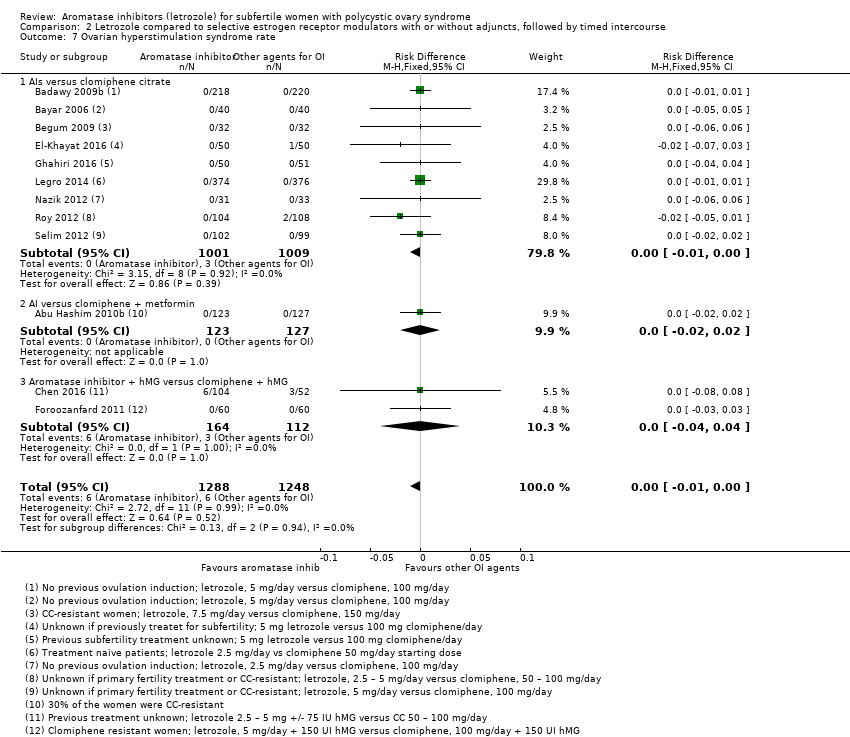
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 7 Ovarian hyperstimulation syndrome rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 8 Ovarian hyperstimulation syndrome rate per BMI.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 9 Clinical pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 10 Impact of allocation bias for clinical pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 11 Miscarriage rate by woman randomised.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 12 Miscarriage rate by pregnancies.
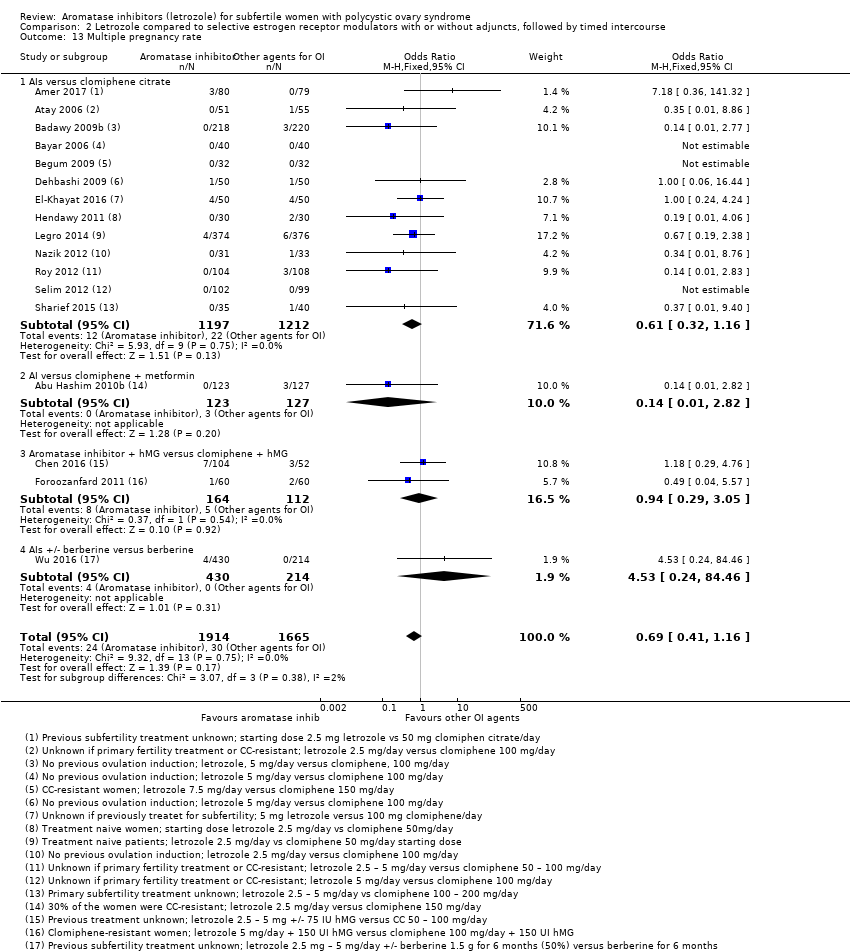
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 13 Multiple pregnancy rate.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 2 Clinical pregnancy rate.
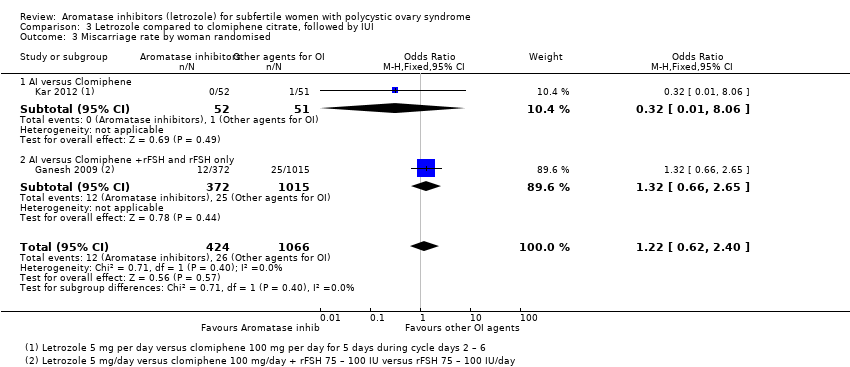
Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 3 Miscarriage rate by woman randomised.
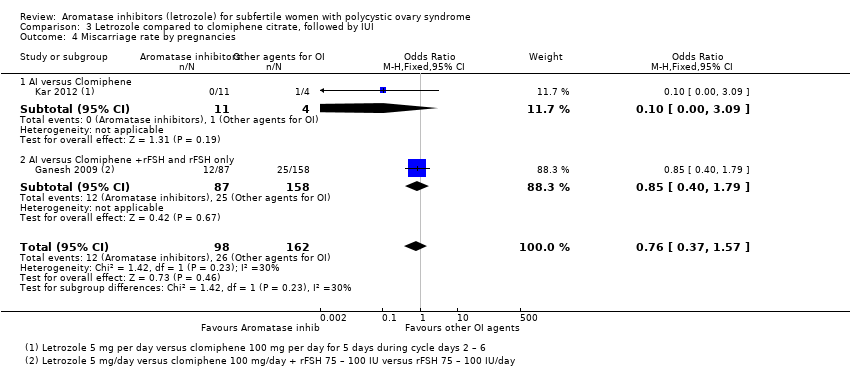
Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 4 Miscarriage rate by pregnancies.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 5 Multiple pregnancy rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 1 Live birth rate.
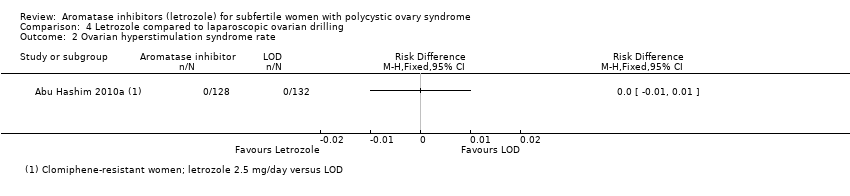
Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 2 Ovarian hyperstimulation syndrome rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 3 Clinical pregnancy rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 4 Miscarriage rate by woman randomised.
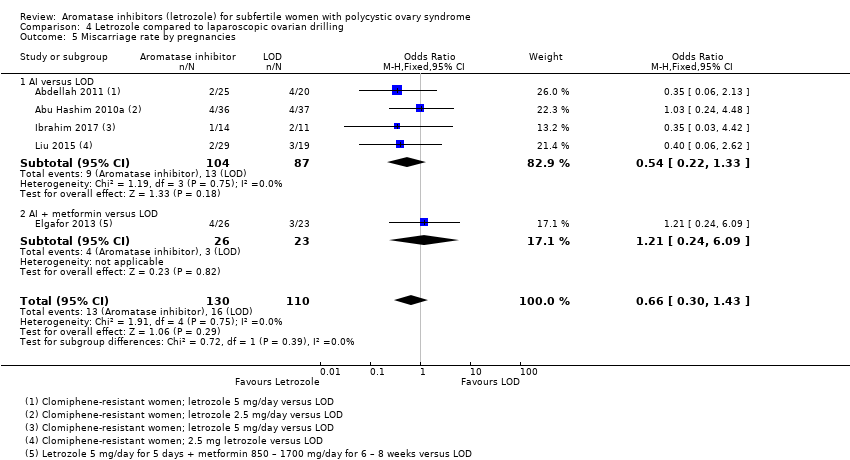
Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 5 Miscarriage rate by pregnancies.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 6 Multiple pregnancy rate.
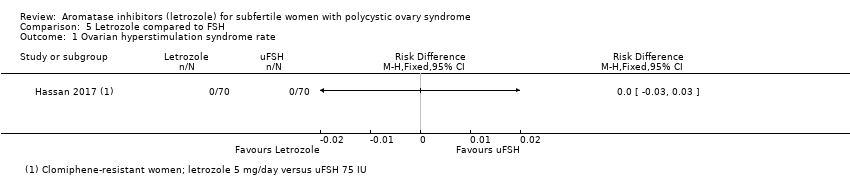
Comparison 5 Letrozole compared to FSH, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 5 Letrozole compared to FSH, Outcome 2 Clinical pregnancy rate.
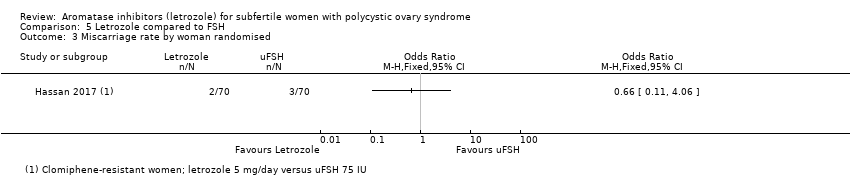
Comparison 5 Letrozole compared to FSH, Outcome 3 Miscarriage rate by woman randomised.
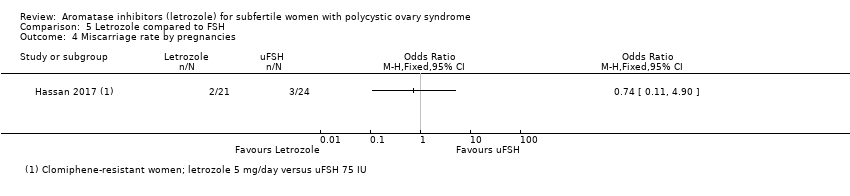
Comparison 5 Letrozole compared to FSH, Outcome 4 Miscarriage rate by pregnancies.

Comparison 5 Letrozole compared to FSH, Outcome 5 Multiple pregnancy rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 2 Clinical pregnancy rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 6 Letrozole compared to anastrozole, Outcome 4 Miscarriage rate by pregnancies.
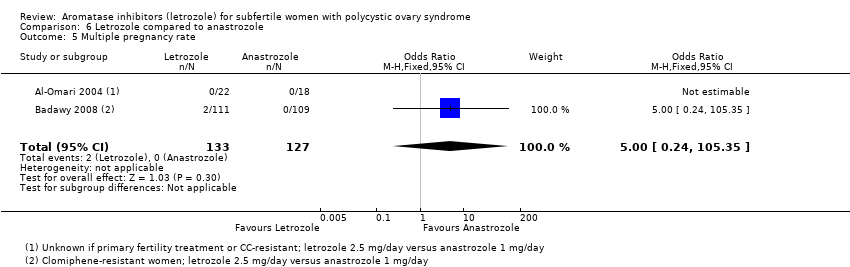
Comparison 6 Letrozole compared to anastrozole, Outcome 5 Multiple pregnancy rate.
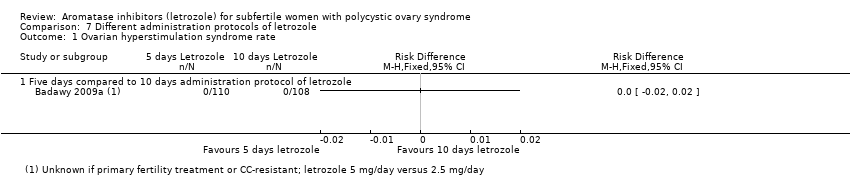
Comparison 7 Different administration protocols of letrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 7 Different administration protocols of letrozole, Outcome 2 Clinical pregnancy rate.

Comparison 7 Different administration protocols of letrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 7 Different administration protocols of letrozole, Outcome 4 Miscarriage rate by pregnancies.

Comparison 7 Different administration protocols of letrozole, Outcome 5 Multiple pregnancy rate.

Comparison 8 Dosage studies of letrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 8 Dosage studies of letrozole, Outcome 2 Clinical pregnancy rate.

Comparison 8 Dosage studies of letrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 8 Dosage studies of letrozole, Outcome 4 Miscarriage rate by pregnancies.
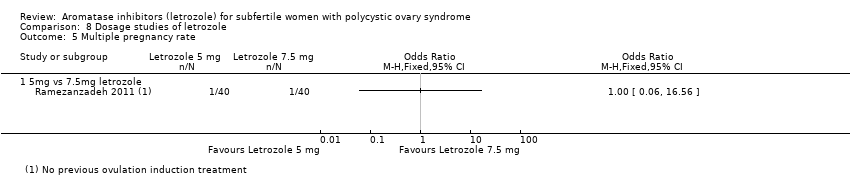
Comparison 8 Dosage studies of letrozole, Outcome 5 Multiple pregnancy rate.
| Letrozole with or without adjuncts compared to clomiphene citrate (CC) with or without adjuncts for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with CC with or without adjuncts | Risk with letrozole with or without adjuncts | |||||
| Live birth rate | 214 per 1000 | 314 per 1000 | OR 1.68 | 2954 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 5 per 1000 | 5 per 1000 | RD 0.00 | 2536 | ⊕⊕⊕⊕ | |
| Clinical pregnancy rate | 264 per 1000 | 359 per 1000 | OR 1.56 | 4629 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 201 per 1000 | 191 per 1000 | OR 0.94 | 1210 | ⊕⊕⊕⊕ | |
| Multiple pregnancy rate | 17 per 1000 | 13 per 1000 | OR 0.69 | 3579 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious risk of bias associated with potential selective reporting: Studies that reported live birth tended to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, suggesting that results might be less favourable to letrozole if all studies reported live birth. | ||||||
| Letrozole compared to laparoscopic ovarian drilling compared to placebo for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: Subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with LOD | Risk with letrozole | |||||
| Live birth rate | 236 per 1000 | 299 per 1000 | OR 1.38 | 548 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 0 per 1000 | 0 per 1000 | RD 0.00 | 260 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 284 per 1000 | 336 per 1000 | OR 1.28 | 774 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 145 per 1000 | 101 per 1000 | OR 0.66 | 240 | ⊕⊕⊕⊝ | |
| Multiple pregnancy rate | 0 per 1000 | 0 per 1000 | OR 3.00 | 548 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aInsufficient data to allow judgement of risk of bias in some studies ‐ downgraded one level for serious risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.12, 83.17] |
| 2 Ovarian hyperstimulation syndrome rate Show forest plot | 2 | 167 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 3 Clinical pregnancy rate Show forest plot | 2 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.08, 7.66] |
| 4 Miscarriage rate by woman randomised Show forest plot | 2 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.26, 9.89] |
| 5 Miscarriage rate by pregnancies Show forest plot | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.07, 4.56] |
| 6 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 13 | 2954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.42, 1.99] |
| 1.1 AIs versus clomiphene citrate | 8 | 1646 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.42, 2.25] |
| 1.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.60, 1.81] |
| 1.3 Aromatase inhibitor + metformin compared to clomiphene + metformin | 2 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.89, 3.23] |
| 1.4 Aromatase inhibitor + FSH compared to clomiphene + FSH | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.53, 2.61] |
| 1.5 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.54, 4.06] |
| 1.6 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.33, 2.84] |
| 2 Live birth rate by BMI Show forest plot | 11 | 2774 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.42, 2.02] |
| 2.1 BMI > 25 | 7 | 1678 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.34, 2.09] |
| 2.2 BMI < 25 | 4 | 1096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.31, 2.28] |
| 3 Live birth rate by first‐ or second‐line treatment Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No previous ovulation induction | 4 | 1089 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.14] |
| 3.2 CC‐resistant women | 4 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.08, 2.93] |
| 3.3 Unclear or mixed study cohort | 5 | 1521 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.35, 2.16] |
| 4 Impact of allocation bias for live birth rate Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Unclear risk of allocation | 8 | 1031 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.42, 2.54] |
| 4.2 Low risk of allocation | 5 | 1923 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.29, 1.95] |
| 5 Impact of detection bias for live birth rate Show forest plot | 13 | 2954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.42, 1.99] |
| 5.1 High risk of detection | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.83, 8.13] |
| 5.2 Low risk of detection | 7 | 2083 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.33, 1.99] |
| 5.3 Unclear risk of detection | 5 | 807 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.27, 2.44] |
| 6 Impact of attrition bias for live birth rate Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Unclear risk of attrition | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.93, 4.50] |
| 6.2 Low risk of attrition | 11 | 2539 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.03] |
| 6.3 High risk of attrition | 1 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.85, 2.50] |
| 7 Ovarian hyperstimulation syndrome rate Show forest plot | 12 | 2536 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 7.1 AIs versus clomiphene citrate | 9 | 2010 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 7.2 AI versus clomiphene + metformin | 1 | 250 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8 Ovarian hyperstimulation syndrome rate per BMI Show forest plot | 11 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 BMI > 25 | 6 | 1851 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 8.2 BMI < 25 | 5 | 605 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 9 Clinical pregnancy rate Show forest plot | 25 | 4629 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| 9.1 AIs versus clomiphene citrate | 17 | 2930 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.28, 1.76] |
| 9.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.71] |
| 9.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.05, 3.29] |
| 9.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.82, 2.27] |
| 9.5 AIs versus tamoxifen | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.64, 3.90] |
| 9.6 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.94, 6.46] |
| 9.7 AIs ± berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.48, 3.13] |
| 10 Impact of allocation bias for clinical pregnancy rate Show forest plot | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Unclear risk of allocation | 16 | 1907 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.43, 2.18] |
| 10.2 Low risk of allocation | 7 | 1912 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.12, 1.65] |
| 11 Miscarriage rate by woman randomised Show forest plot | 18 | 3754 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.07, 1.81] |
| 11.1 AIs versus clomiphene citrate | 11 | 2190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.97, 1.93] |
| 11.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.25, 4.23] |
| 11.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.52, 2.82] |
| 11.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.31, 2.27] |
| 11.5 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.21 [0.66, 226.97] |
| 11.6 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.87, 3.04] |
| 12 Miscarriage rate by pregnancies Show forest plot | 18 | 1210 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| 12.1 AIs versus clomiphene citrate | 11 | 705 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| 12.2 AI versus clomiphene + metformin | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.24, 4.40] |
| 12.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.02] |
| 12.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.23, 1.96] |
| 12.5 AIs versus clomiphene + estradiol valerate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.13 [0.39, 167.90] |
| 12.6 AIs +/‐ berberine versus berberine | 1 | 213 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 13 Multiple pregnancy rate Show forest plot | 17 | 3579 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.16] |
| 13.1 AIs versus clomiphene citrate | 13 | 2409 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.32, 1.16] |
| 13.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.82] |
| 13.3 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.29, 3.05] |
| 13.4 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.53 [0.24, 84.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 2 | 1494 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 1.1 AI versus Clomiphene | 1 | 107 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 1.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 2 Clinical pregnancy rate Show forest plot | 3 | 1597 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.30, 2.25] |
| 2.1 AI versus Clomiphene | 2 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.97, 4.53] |
| 2.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.23, 2.22] |
| 3 Miscarriage rate by woman randomised Show forest plot | 2 | 1490 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.62, 2.40] |
| 3.1 AI versus Clomiphene | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.06] |
| 3.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.66, 2.65] |
| 4 Miscarriage rate by pregnancies Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.57] |
| 4.1 AI versus Clomiphene | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 3.09] |
| 4.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.79] |
| 5 Multiple pregnancy rate Show forest plot | 3 | 1597 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.13] |
| 5.1 AI versus Clomiphene | 2 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.48 [0.14, 87.49] |
| 5.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.44, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.95, 2.02] |
| 2 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Clinical pregnancy rate Show forest plot | 5 | 774 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.94, 1.74] |
| 3.1 AI versus LOD | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.93, 1.83] |
| 3.2 AI + metformin versus LOD | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.60, 2.39] |
| 4 Miscarriage rate by woman randomised Show forest plot | 5 | 774 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.70] |
| 4.1 AI versus LOD | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.63] |
| 4.2 AI + metformin versus LOD | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.29, 6.27] |
| 5 Miscarriage rate by pregnancies Show forest plot | 5 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.43] |
| 5.1 AI versus LOD | 4 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.33] |
| 5.2 AI + metformin versus LOD | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.24, 6.09] |
| 6 Multiple pregnancy rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 74.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy rate Show forest plot | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.67] |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Multiple pregnancy rate Show forest plot | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy rate Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.43] |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Multiple pregnancy rate Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 105.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Five days compared to 10 days administration protocol of letrozole | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy rate Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Letrozole day 3‐7 administratio versus day 5‐9 administration | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 5mg vs 7.5mg letrozole | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

