Perencat aromatase (letrozole) untuk wanita yang mengalami masalah kesuburan (subfertiliti) dengan sindrom ovari polisistik (PCOS)
Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common cause of infrequent periods (oligomenorrhoea) and absence of periods (amenorrhoea). It affects about 4% to 8% of women worldwide and often leads to anovulatory subfertility. Aromatase inhibitors (AIs) are a class of drugs that were introduced for ovulation induction in 2001. Since about 2001 clinical trials have reached differing conclusions as to whether the AI letrozole is at least as effective as the first‐line treatment clomiphene citrate (CC).
Objectives
To evaluate the effectiveness and safety of aromatase inhibitors for subfertile women with anovulatory PCOS for ovulation induction followed by timed intercourse or intrauterine insemination (IUI).
Search methods
We searched the following sources from inception to November 2017 to identify relevant randomised controlled trials (RCTs): the Cochrane Gynaecology and Fertility Group Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, PsycINFO, Pubmed, LILACS, Web of Knowledge, the World Health Organization (WHO) clinical trials register and Clinicaltrials.gov. We also searched the references of relevant articles. We did not restrict the searches by language or publication status.
Selection criteria
We included all RCTs of AIs used alone or with other medical therapies for ovulation induction in women of reproductive age with anovulatory PCOS.
Data collection and analysis
Two review authors independently selected trials, extracted the data and assessed risks of bias. We pooled studies where appropriate using a fixed‐effect model to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for most outcomes, and risk differences (RDs) for ovarian hyperstimulation syndrome (OHSS). The primary outcomes were live birth and OHSS. Secondary outcomes were clinical pregnancy, miscarriage and multiple pregnancy. We assessed the quality of the evidence for each comparison using GRADE methods.
Main results
This is a substantive update of a previous review. We identified 16 additional studies for the 2018 update. We include 42 RCTs (7935 women). The aromatase inhibitor letrozole was used in all studies.
Letrozole compared to clomiphene citrate (CC) with or without adjuncts followed by timed intercourse
Live birth rates were higher with letrozole (with or without adjuncts) compared to clomiphene citrate (with our without adjuncts) followed by timed intercourse (OR 1.68, 95% CI 1.42 to 1.99; 2954 participants; 13 studies; I2 = 0%; number needed to treat for an additional beneficial outcome (NNTB) = 10; moderate‐quality evidence). There is high‐quality evidence that OHSS rates are similar with letrozole or clomiphene citrate (0.5% in both arms: risk difference (RD) −0.00, 95% CI −0.01 to 0.00; 2536 participants; 12 studies; I2 = 0%; high‐quality evidence). There is evidence for a higher pregnancy rate in favour of letrozole (OR 1.56, 95% CI 1.37 to 1.78; 4629 participants; 25 studies; I2 = 1%; NNTB = 10; moderate‐quality evidence). There is little or no difference between treatment groups in the rate of miscarriage by pregnancy (20% with CC versus 19% with letrozole; OR 0.94, 95% CI 0.70 to 1.26; 1210 participants; 18 studies; I2 = 0%; high‐quality evidence) and multiple pregnancy rate (1.7% with CC versus 1.3% with letrozole; OR 0.69, 95% CI 0.41 to 1.16; 3579 participants; 17 studies; I2 = 0%; high‐quality evidence). However, a funnel plot showed mild asymmetry, indicating that some studies in favour of clomiphene might be missing.
Letrozole compared to laparoscopic ovarian drilling
There is low‐quality evidence that live birth rates are similar with letrozole or laparoscopic ovarian drilling (OR 1.38, 95% CI 0.95 to 2.02; 548 participants; 3 studies; I2 = 23%; low‐quality evidence). There is insufficient evidence for a difference in OHSS rates (RD 0.00, 95% CI −0.01 to 0.01; 260 participants; 1 study; low‐quality evidence). There is low‐quality evidence that pregnancy rates are similar (OR 1.28, 95% CI 0.94 to 1.74; 774 participants; 5 studies; I2 = 0%; moderate‐quality evidence). There is insufficient evidence for a difference in miscarriage rate by pregnancy (OR 0.66, 95% CI 0.30 to 1.43; 240 participants; 5 studies; I2 = 0%; moderate‐quality evidence), or multiple pregnancies (OR 3.00, 95% CI 0.12 to 74.90; 548 participants; 3 studies; I2 = 0%; low‐quality evidence).
Additional comparisons were made for Letrozole versus placebo, Selective oestrogen receptor modulators (SERMS) followed by intrauterine insemination (IUI), follicle stimulating hormone (FSH), Anastrozole, as well as dosage and administration protocols.
There is insufficient evidence for a difference in either group of treatment due to a limited number of studies. Hence more research is necessary.
Authors' conclusions
Letrozole appears to improve live birth and pregnancy rates in subfertile women with anovulatory polycystic ovary syndrome, compared to clomiphene citrate. There is high‐quality evidence that OHSS rates are similar with letrozole or clomiphene citrate. There is high‐quality evidence of no difference in miscarriage rates or multiple pregnancy rates. There is low‐quality evidence of no difference in live birth and pregnancy rates between letrozole and laparoscopic ovarian drilling, although there were few relevant studies. For the 2018 update, we added good‐quality trials, upgrading the quality of the evidence.
PICO
Ringkasan bahasa mudah
Perencat aromatase (letrozole) untuk rawatan subfertiliti bagi wanita dengan sindrom ovari polisistik (PCOS)
Soalan ulasan: Para penulis Cochrane telah memeriksa bukti tentang perencat aromatasae (AIs) untuk wanita yang mengalami masalah kesuburan (subfertiliti) dengan sindrom ovari polisistik (PCOS).
Latar belakang: PCOS merupakan sebab utama jarang atau tiada haid, dan dialami oleh 4% hingga 8% wanita sedunia. Ia kerap menyebabkan masalah kesuburan tanpa ovulasi (subfertiliti berkaitan dengan kegagalan untuk berovulasi). AIs digunakan untuk menghasilkan ovulasi. Sejak tahun 2001, kajian‐kajian klinikal telah memberikan kesimpulan yang berbeza sama ada AI letrozole adalah sama berkesan untuk merawat subfertiliti dengan kaedah rawatan paling biasa digunakan, clomiphene citrate (CC).
Ciri‐ciri kajian: Ulasan ini melibatkan kajian‐kajian kilinikal di mana peserta telah dibahagikan secara rawak kepada kumpulan intervensi atau kumpulan perbandingan (kajian terkawal rawak, RCTs). Ulasan kami melibatkan 42 RCTs dengan 7935 wanita. Dalam semua kajian, perencat aromatase yang digunakan adalah letrozole. Ajen pembanding termasuklah CC, yang digunakan dalam 25 daripada RCTs, dan penggerudian ovari laparoskopik (teknik pembedahan untuk menusuk membran keliling ovari) yang digunakan dalam lima RCTs. Beberapa kajian melibatkan rawatan yang lain dalam satu atau kedua‐dua kumpulan.
Keputusan‐keputusan utama: Letrozole berkemungkinan meningkatkan kadar kelahiran hidup dan kadar kehamilan berbanding CC apabila digunakan untuk mengalakkan ovulasi dan persetubuhan secara berjadual. Kualiti bukti ini adalah sederhana dan kelihatan boleh dipercayai. Didapati tiada perbezaan antara kadar keguguran atau kadar kehamilan kembar. Didapati tiada perbezaan antara letrozole atau penggerudian ovari laparoskopik bagi apa‐apa hasil yang diperhatikan, walaupun wujudnya beberapa kajian yang relevan. Sindrom ovari terlebih rangsangan (OHSS), adalah kesan buruk rangsangan hormon yang serius, merupakan peristiwa yang sangat jarang berlaku dan tidak berlaku dalam kebanyakan kajian. Bukti adalah terkini sehingga Januari 2018.
Kualiti bukti: Kualiti bukti keseluruhan adalah berkisar dari sederhana hingga tinggi. Beberapa kajian yang lebih memihak kepada clomiphene citrate berkemungkinan tidak pernah diterbitkan. Didapati kajian yang melaporkan kelahiran hidup melaporkan kadar kehamilan klinikal yang lebih tinggi dalam kumpulan letrozole berbanding kajian yang gagal untuk melaporkan kelahiran hidup. Ini mencadangkan bahawa keputusan tersebut berkemungkinan tidak memihak kepada letrozole jika semua kajian melaporkan kelahiran hidup.
Authors' conclusions
Summary of findings
| Letrozole with or without adjuncts compared to clomiphene citrate (CC) with or without adjuncts for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with CC with or without adjuncts | Risk with letrozole with or without adjuncts | |||||
| Live birth rate | 214 per 1000 | 314 per 1000 | OR 1.68 | 2954 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 5 per 1000 | 5 per 1000 | RD 0.00 | 2536 | ⊕⊕⊕⊕ | |
| Clinical pregnancy rate | 264 per 1000 | 359 per 1000 | OR 1.56 | 4629 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 201 per 1000 | 191 per 1000 | OR 0.94 | 1210 | ⊕⊕⊕⊕ | |
| Multiple pregnancy rate | 17 per 1000 | 13 per 1000 | OR 0.69 | 3579 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious risk of bias associated with potential selective reporting: Studies that reported live birth tended to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, suggesting that results might be less favourable to letrozole if all studies reported live birth. | ||||||
| Letrozole compared to laparoscopic ovarian drilling compared to placebo for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: Subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with LOD | Risk with letrozole | |||||
| Live birth rate | 236 per 1000 | 299 per 1000 | OR 1.38 | 548 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 0 per 1000 | 0 per 1000 | RD 0.00 | 260 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 284 per 1000 | 336 per 1000 | OR 1.28 | 774 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 145 per 1000 | 101 per 1000 | OR 0.66 | 240 | ⊕⊕⊕⊝ | |
| Multiple pregnancy rate | 0 per 1000 | 0 per 1000 | OR 3.00 | 548 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aInsufficient data to allow judgement of risk of bias in some studies ‐ downgraded one level for serious risk of bias. | ||||||
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is the most common cause of infrequent periods (oligomenorrhoea) and absence of periods (amenorrhoea), affecting about 4% to 8% of women worldwide in their fertile years (Abu 2012). Many of these women are subfertile, but for most of them it just takes longer to become pregnant naturally and only a small percentage need fertility treatment.
The mechanisms causing PCOS are very complex and the exact pathogenesis remains unknown, but some of the symptoms are believed to be caused by abnormal levels of the pituitary hormones luteinizing hormone (LH) and of the male hormones (androgens) which interfere with the normal function of the ovaries (Azziz 2006).
The diagnosis can be made based on the 'Rotterdam criteria 2003', jointly proposed by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine (Rotterdam 2003). The woman must have two of the following three criteria to be diagnosed with PCOS:
-
Oligoovulation (infrequent ovulation) or anovulation (absence of ovulation), or both
-
High male hormone levels (hyperandrogenism) diagnosed either clinically (excessive hair growth, hirsutism) or biochemically (raised serum testosterone levels)
-
Ovaries which appear to be polycystic on vaginal sonogram, defined by the presence of 12 or more antral follicles in an ovary or an ovarian volume of more than 10 mL. Antral follicles are defined as measuring between 2 and 9 mm in diameter.
Other definitions of PCOS include the National Institutes of Health Criteria (NIH), defined in 1990. They include only the presence of clinical and/or biochemical hyperandrogenism and oligo/amenorrhoea anovulation (Zawadski 1992).
The Androgen Excess Society defines PCOS as hyperandrogenism with ovarian dysfunction or polycystic ovaries (Azziz 2006).
Description of the intervention
There are many possible options for treatment of subfertility in women with anovulatory PCOS.
Clomiphene citrate (CC) is a selective oestrogen receptor modulator (SERM), and is the most common medication used for treating the condition. It was first introduced in 1960 for treatment of World Health Organization (WHO) type II anovulation (a type of subfertility where hormone levels stay normal) in subfertile women, and has been the first‐line treatment ever since. CC is given orally and is relatively safe and inexpensive, but there are also adverse effects associated with it, such as negative changes in endometrium and cervical mucus due to the down‐regulation of oestrogen receptors that might impair implantation after successful induction of ovulation (Casper 2006).
Aromatase inhibitors (AIs) are a newer class of drugs that were introduced for ovulation induction in 2001 by Mitwally and Casper (Mitwally 2001). Since about 2001 data from many clinical trials have been collected and there is evidence that the AI letrozole might be as effective as CC, but the outcome data vary. AIs are administered orally, but due to their short half‐life elimination time of 48 hours there are fewer adverse effects on oestrogen target tissues such as the endometrium and cervix compared with CCs (Baruah 2009; Jirge 2010; Samani 2009). Despite evidence of effectiveness and safety in well‐designed large randomised controlled trials (RCTs), letrozole is still used off‐label for ovulation induction, since it has not been approved by the US Food and Drug Administration (FDA) for this indication (Amer 2017; Legro 2014). A 2005 study (Biljan 2005), including 150 babies, raised some concerns about the teratogenicity of letrozole, but there were major methodological flaws in this study as the intervention group was not well controlled. Two other large studies, including 911 and 470 infants respectively, compared the use of letrozole to CC and spontaneously‐conceiving women. Both reported no higher levels of minor or major congenital malformations or cardiac abnormalities in newborns after use of letrozole for ovulation induction (Forman 2007; Tulandi 2006).
Due to the short half‐life elimination time of letrozole it should be completely cleared out of the system before implantation takes place. Some clinicians recommend testing the blood levels of ß‐hCG prior to treatment with letrozole to exclude pregnancy (Casper 2011). CC and AIs are usually both given for five days, starting on day three of the cycle. The dose for CC ranges from 50 mg to 150 mg a day, and for letrozole from 2.5 mg to 7.5 mg a day (Lee 2011).
Since many women with PCOS experience insulin resistance or impaired glucose tolerance, metformin and other insulin‐sensitising agents were thought to be a superior drug for treatment of ovulation induction (Velázquez 1997). However, the latest version of the Cochrane Review on oral agents for ovulation induction concludes that the use of metformin and other insulin sensitising agents as an adjunct is limited and might be favourable only in women who are resistant to CC alone (Brown 2016).
Human menopausal gonadotropins (hMG) were introduced into clinical practice in 1961 for ovulation induction. They exert a central role in ovulation induction in CC‐resistant subfertile normogonadotropic anovulatory women (Lunenfeld 2004). However, women with PCOS are at particular risk for complications such as ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies, and a low‐dose step‐up protocol was introduced to reach the follicle stimulating hormone (FSH) threshold gradually in order to minimise the risks of OHSS and multiple pregnancies (White 1996). Use of FSH for ovulation induction in women with PCOS appears to be safe and effective (Homburg 2011).
For all the above‐mentioned drugs for ovulation induction, follicular growth should be monitored during a stimulation cycle to reassure effectiveness and also to minimise the occurrence of adverse events, such as multiple pregnancy (Von Hofe 2015).
Finally, another possible option for ovulation induction in cases of CC resistance is laparoscopic ovarian diathermy (or drilling, LOD), during which the damaging of localised areas in the ovarian cortex and stroma seems to have similar success rates compared with gonadotropin therapy (Farquhar 2002). It is not fully understood how the partial destruction of the ovary results in follicle development and ovulation induction (Farquhar 2012). However, long‐term outcomes of a study with eight to 12 years of follow‐up indicate that LOD is safe and effective (Nahuis 2011).
How the intervention might work
AIs down‐regulate the production of oestrogen by inhibiting the cytochrome P450 isoenzymes 2A6 and 2C19 of the aromatase enzyme complex (Cole 1990). They inhibit the negative feedback loop of oestrogen in the hypothalamus, and result in stronger gonadotropin‐releasing hormone (GnRH) pulses. The elevated levels of GnRH stimulate the pituitary gland to produce more FSH, which induces development of follicles in the ovaries. Because AIs do not deplete oestrogen receptors, in contrast to CC, the central feedback mechanism remains intact and as the dominant follicle grows and oestrogen levels rise, normal negative feedback occurs centrally. This results in suppression of FSH and the smaller‐growing follicles will undergo atresia, leading to a single dominant follicle and mono‐ovulation (ovulation of a single egg) in most cases (Casper 2006; Lee 2011). Therefore, by leaving the central mechanism intact, the AIs might lower the risk of high multiple ovulation and OHSS compared to CC.
Why it is important to do this review
Because evidence for and against the effectiveness and safety of AIs has fluctuated over the last decade, and new data based on recent RCTs have become available, an update of the existing Cochrane Review was necessary to provide up‐to‐date information for daily practice.
This review evaluates the effectiveness and safety of AIs compared to other agents for ovulation induction or laparoscopic ovarian drilling, to provide evidence about whether or not AIs should be used in subfertile women with PCOS who are trying to conceive.
Objectives
To evaluate the effectiveness and safety of aromatase inhibitors for subfertile women with anovulatory PCOS for ovulation induction followed by timed intercourse or intrauterine insemination (IUI).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) for inclusion in the review. We excluded cross‐over trials unless phase one data were available separately.
Types of participants
Women of reproductive age with anovulatory PCOS (WHO type II anovulation in women with normogonadotropic normoestrogenic anovulation), diagnosed according to the Rotterdam Criteria (Rotterdam 2003), the NIH consensus criteria (Zawadski 1992) or the AES criteria (Azziz 2009).
Exclusion criteria
We excluded RCTs of women with hyperprolactinaemia or Cushing’s syndrome, or both. We also excluded trials covering women with WHO type I anovulation (hypogonadotropic hypogonadal anovulation). Women in this group have amenorrhoea, low or low‐normal serum FSH concentrations and low serum estradiol concentrations due to decreased hypothalamic secretion of gonadotropin‐releasing hormone (GnRH) or pituitary unresponsiveness to GnRH. We excluded studies using methods other than ovulation induction followed by intercourse or IUI, for example 'in vitro fertilisation' (IVF).
Types of interventions
We considered for inclusion aromatase inhibitors for ovulation induction, alone or in conjunction with medical adjuncts, e.g. metformin or FSH, followed by sexual intercourse or IUI in women with anovulatory subfertility. AIs were compared to each other and to other choices of treatment, including CC, tamoxifen, recombinant and urinary gonadotropin (FSH), insulin‐sensitising agents such as metformin, and laparoscopic ovarian drilling.
Types of outcome measures
Primary outcomes
Effectiveness:
1. Live birth rate by woman randomised, defined as delivery of a live fetus after 20 completed weeks of gestational age.
Adverse effects:
2. OHSS rate by woman randomised, as defined by the study authors.
Secondary outcomes
3. Clinical pregnancy rate by woman randomised, defined as the presence of a gestational sac on ultrasound.
4. Miscarriage rate by woman randomised, defined as the involuntary loss of a clinical pregnancy before 20 weeks of gestation, including partial loss of a multiple pregnancy.
5. Miscarriage rate by pregnancies, defined as the involuntary loss of a clinical pregnancy before 20 weeks of gestation, including partial loss of a multiple pregnancy.
6. Multiple pregnancy rate by woman randomised, defined as more than one intrauterine pregnancy, confirmed by ultrasound or delivery.
Search methods for identification of studies
We searched for all published and unpublished RCTs of the use of AIs for ovulation induction in anovulatory women with PCOS. We consulted the Cochrane Gynaecology and Fertility Group Information Specialist. We used the following search strategy, without language restriction:
Electronic searches
1. Cochrane Gynaecology and Fertility Group Specialised Register (CGFG) (inception to 6 November 2017; Appendix 1)
2. The Cochrane Central Register of Controlled Trials (CENTRAL) (inception to 6 November 2017; Appendix 2)
3. MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (inception to 6 November 2017; Appendix 3)
4. Embase (inception to 6 November 2017; Appendix 4)
5. PsycINFO (inception to 6 November 2017; Appendix 5)
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials which appears in theCochrane Handbook of Systematic Reviews of Interventions (Cochrane Handbook) (Version 5.1.0, Section 6.4.11). We combined the Embase search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random). There was no language restriction in these searches.
Searching other resources
We checked the references of relevant systematic reviews and RCTs obtained by the search and contacted experts in the field and manufacturers of aromatase inhibitors, to pick up any additional, relevant data. We also searched the databases of the WHO, clinicaltrials.gov, Web of Knowledge, PubMed and LILACS, Google and Google Scholar, up to January 2018.
Data collection and analysis
We conducted data collection and analysis in accordance with theCochrane Handbook (Higgins 2011).
Selection of studies
For this update of the review, two review authors (SF and SE ) independently selected the trials to be included, in accordance with the aforementioned criteria. We excluded trials from the review if they made comparisons other than those specified above. Studies from non‐English language journals were translated if necessary. If a trial was published more than once, we only included the most complete and up‐to‐date data. We contacted authors of primary studies if papers did not contain enough information to enable an accurate assessment of eligibility for inclusion. We provide a list of excluded studies, showing the reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
For this update of the review, two review authors (SF and SE) independently extracted the data, resolving any disagreements by recourse to a third party. We used a data extraction form designed and piloted by the review authors. All data collected for our analyses were dichotomous. If studies had multiple publications, we included only the main trial report. The review authors contacted study investigators to resolve any data queries, as required.
Assessment of risk of bias in included studies
We assessed the included studies for risks of bias, using the Cochrane 'Risk of bias' tool. We evaluated seven domains of possible biases:
-
Random sequence generation
-
Allocation concealment
-
Blinding of participants and personnel
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Selective reporting
-
Other potential bias
We judged the different types of bias using the criteria from the Cochrane Handbook Table 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool (Higgins 2011). Two review authors (SF and SE) checked these domains of bias independently and rated them as being at high, low or unclear risk of bias. The assessments were compared and any disagreements resolved by consensus or by discussion with a third review author (CF). The conclusions are presented in the ’Risk of bias’ table and were incorporated into the interpretation of the review findings by means of sensitivity analyses.
Measures of treatment effect
Where dichotomous data measures were used, we have expressed the results in the control and intervention groups of each study as odds ratios (ORs) with 95% confidence intervals (CIs). For the very rare outcome OHSS we have used a risk difference (RD) analysis to allow CIs for the difference in percentage points. Based on the specified outcomes, there were no continuous data measures.
Unit of analysis issues
The primary analysis was by woman randomised. The secondary outcome of miscarriage rate was also analysed by pregnancies. We contacted authors of studies that used cycles as the denominator rather than women, for additional information; if we could not obtain it we did not include the trial in the analysis. If there were multiple cycles, the unit of analysis remained as the woman randomised. We used only the first phase of cross‐over‐trials in the analysis, as successful treatment prevents a cross‐over. We treated multiple live births as one event.
Dealing with missing data
If data were missing from included studies, we contacted the investigators to request the relevant missing data. If this was not possible, we imputed individual values for the primary and secondary outcomes. In participants without a reported outcome we assumed that live births had not occurred. For other outcomes, we analysed only the available data. We subjected any imputation to sensitivity analysis. We analysed the data on an intention‐to‐treat (ITT) basis, as far as possible.
Assessment of heterogeneity
We tested the results of the included studies for heterogeneity by measuring the scatter in the data points on the graph and the overlap in their CIs. We used the I2 statistic, which describes the percentage of total variation across the trials that is due to heterogeneity rather than to chance (Higgins 2011). The values of the I2 statistic lie between 0% (no heterogeneity) and 100% (extreme heterogeneity). We take values above 50% to indicate moderate heterogeneity and we explored them within sensitivity and subgroup analyses.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. We compared all outcome measures stated in the Methods section to the outcomes reported in the Results section, to ensure comparability. If there were more than 10 trials included in a comparison, we produced a funnel plot to test for reporting bias.
Data synthesis
We used a fixed‐effect model to combine the data from the primary studies if they were sufficiently similar. We conducted statistical analysis with Review Manager 5, in accordance with the guidelines for statistical analysis developed by Cochrane (Higgins 2011).
Our comparisons were:
-
Letrozole compared to placebo;
-
Letrozole compared to other ovulation induction agents followed by intercourse;
-
Letrozole compared to other ovulation induction agents followed by IUI;
-
Letrozole compared to laparoscopic ovarian drilling;
-
Letrozole compared to FSH;
-
Letrozole compared to anastrozole;
-
Comparison of different administration protocols of letrozole;
-
Dosage studies of letrozole.
Increases in the odds of an outcome, either beneficial (e.g. live birth) or detrimental (e.g. OHSS) are shown in the forest plots of the meta‐analysis to the right of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis for primary outcomes only, to evaluate the evidence for a study population with an average body mass index (BMI) above 25 compared to women with an average BMI below 25 within their study group. We conducted a further subgroup analysis comparing women with no previous treatment for ovulation induction to women that were CC‐resistant. We intended to perform subgroup analyses on other parameters, such as the age of the woman, the duration of subfertility and the duration and drug dosages, but this was not possible due to the lack of data.
Sensitivity analysis
We conducted a sensitivity analysis for the primary outcomes to evaluate whether the conclusions are robust to arbitrary decisions made about the eligibility and analysis of studies. This analysis includes consideration of whether the review conclusions would have differed if:
-
Eligibility was restricted to studies without high or unclear risk of bias;
-
We had used a random‐effects model;
-
We had implemented alternative imputation strategies;
-
The summary effect measure had been the risk ratio instead of the odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We generated 'Summary of findings' tables using GRADEpro software. The first table evaluates the overall quality of the body of evidence for the main review comparison (aromatase inhibitors compared to selective oestrogen receptor modulators, with or without adjuncts), using GRADE criteria, i.e. study limitations (risk of bias), consistency of effect, imprecision, indirectness and publication bias. Judgements about evidence quality (high, moderate, low or very low) were justified, documented and incorporated into the reporting of results for each outcome.Two review authors independently evaluated the overall quality of the evidence for the main outcomes of the review (live birth rate, miscarriage rate, clinical pregnancy rate, multiple pregnancy rate, and OHSS by woman randomised). We produced a second ’Summary of findings’ table for the comparison 'Letrozole compared to laparoscopic ovarian drilling'. There are no 'Summary of findings' tables for the remaining comparisons of the review, because we considered them less clinically important. The results of these comparisons are discussed within the text of the review.
Results
Description of studies
Results of the search
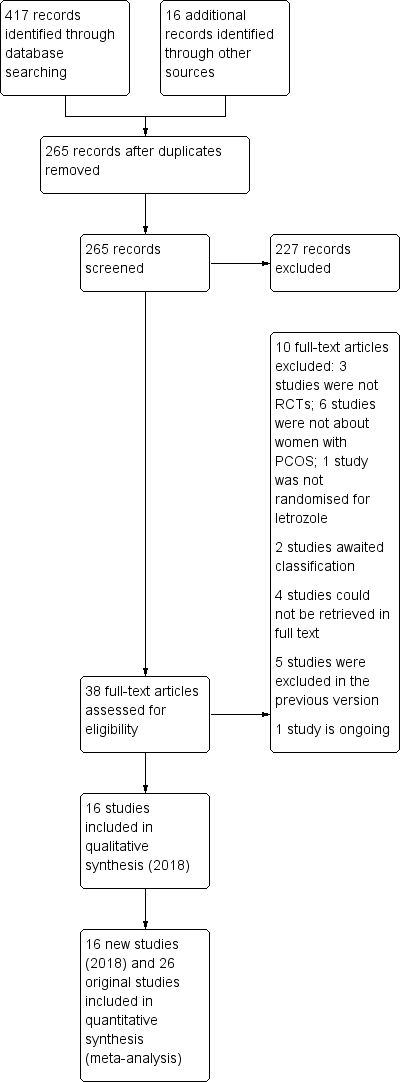
The previous version of this review included 26 trials. The searches for the 2018 review update resulted in the retrieval of 38 full‐text papers (Figure 1). We included 16 new studies (Characteristics of included studies). We excluded 10 new studies (Characteristics of excluded studies). Two new studies are awaiting classification (Characteristics of studies awaiting classification); we have contacted their authors and still await a response. We have moved five studies from ongoing to excluded (NCT01679574; NCT01793038; NCT01431352;Palihawadana 2015; Sarvi 2010), and three studies from ongoing to included (Amer 2017; Liu 2017; Wu 2016). Four studies have been moved from awaiting classification to excluded (Al‐Hussaini 2014; NCT01577017; NCT00610077; Sharma 2010). We classify one new study as ongoing (NCT03009838; Characteristics of ongoing studies).
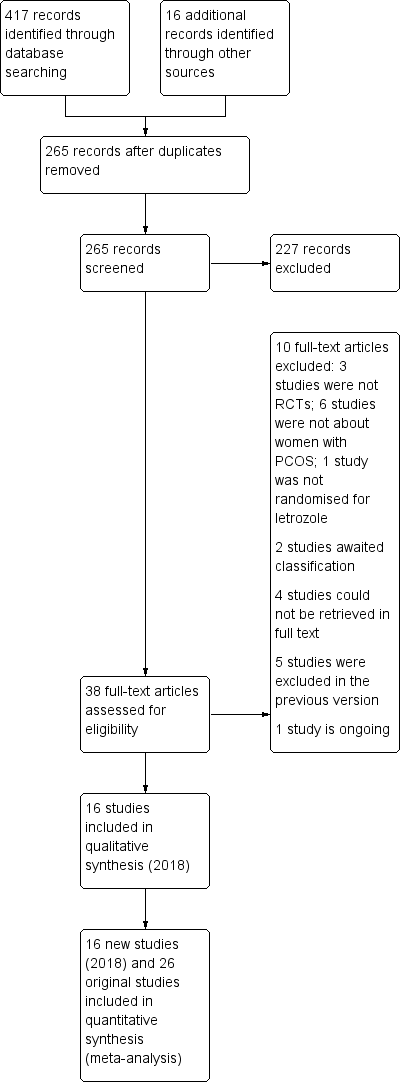
Study flow diagram for update 2018
Included studies
Study design and setting
We include 42 parallel‐designed randomised controlled trials (RCTs) in this 2018 updated review.
The studies were done in different parts of the world:
-
Egypt (Abdellah 2011; Abu Hashim 2010a; Abu Hashim 2010b; Badawy 2008; Badawy 2009a; Badawy 2009b; El‐Gharib 2015; El‐Khayat 2016; Elgafor 2013; Hassan 2017; Hendawy 2011; Ibrahim 2017; Moussa 2016; Selim 2012)
-
India (Begum 2009; Ganesh 2009; Kamath 2010; Kar 2012; Ray 2012; Roy 2012; Sh‐El‐Arab Elsedeek 2011)
-
Iraq (Al‐Omari 2004; Sharief 2015)
-
Iran (Davar 2011; Dehbashi 2009; Foroozanfard 2011; Ghahiri 2016; Ghomian 2015; Ramezanzadeh 2011; Seyedoshohadaei 2016; Sohrabvand 2006; Zarei 2015; Zeinalzadeh 2010)
-
Turkey (Atay 2006; Bayar 2006; Nazik 2012)
-
United Kingdom (Amer 2017)
-
USA (Legro 2014)
The following different settings recruited women into the trials:
-
Not stated (Atay 2006; Ray 2012; Roy 2012; Selim 2012).
-
Subfertility clinic (Amer 2017; Begum 2009; Davar 2011; Dehbashi 2009; Foroozanfard 2011; Ghahiri 2016; Ganesh 2009; Kar 2012; Nazik 2012; Ramezanzadeh 2011; Sh‐El‐Arab Elsedeek 2011; Seyedoshohadaei 2016; Sohrabvand 2006; Zeinalzadeh 2010).
-
Outpatient clinic (Abu Hashim 2010a; Abu Hashim 2010b; Badawy 2008; Badawy 2009a; Badawy 2009b; Bayar 2006; El‐Gharib 2015; Ibrahim 2017).
-
Department of obstetrics and gynaecology (Al‐Omari 2004; Chen 2016; Elgafor 2013; Legro 2014).
-
Division of reproductive endocrinology (Kamath 2010).
-
Maternity and child hospital (Seyedoshohadaei 2016; Zarei 2015).
-
University hospital (El‐Khayat 2016; Ghomian 2015; Hassan 2017; Hendawy 2011; Liu 2015; Liu 2017; Moussa 2016; Wu 2016).
-
Women's health institute (Abdellah 2011).
Drs Abu Hasim and Badawy confirmed to us that their five studies, conducted from 2008 until 2010, were independent and did not include the same women.
Participants
The studies included 7935 women who were subfertile due to anovulatory PCOS. The ages of the women ranged from 18 to 40 years.
Interventions
-
2/42 studies compared letrozole versus placebo (Kamath 2010; Zarei 2015).
-
26/42 studies compared letrozole to other ovulation induction agents followed by intercourse (Abu Hashim 2010b; Amer 2017; Atay 2006; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Gharib 2015; El‐Khayat 2016; Foroozanfard 2011; Ghahiri 2016; Hendawy 2011; Legro 2014; Liu 2017; Moussa 2016; Nazik 2012; Ray 2012; Roy 2012; Selim 2012; Seyedoshohadaei 2016; Sharief 2015; Sh‐El‐Arab Elsedeek 2011; Sohrabvand 2006; Wu 2016).
-
3/42 studies compared letrozole to other ovulation induction agents followed by IUI (Ganesh 2009; Kar 2012; Zeinalzadeh 2010).
-
5/42 studies compared letrozole versus laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Elgafor 2013; Ibrahim 2017; Liu 2015).
-
1/42 studies compared letrozole versus FSH (Hassan 2017).
-
2/42 studies compared letrozole versus anastrozole (Al‐Omari 2004; Badawy 2008).
-
2/42 studies compared different administration protocols of letrozole (Badawy 2009a; Ghomian 2015).
-
1/26 studies compared different doses of letrozole (Ramezanzadeh 2011).
Outcomes
-
16/42 studies reported live birth rate by woman randomised (Abdellah 2011; Abu Hashim 2010a; Abu Hashim 2010b; Amer 2017; Bayar 2006; Begum 2009; Dehbashi 2009; Foroozanfard 2011; Legro 2014; Liu 2015; Liu 2017; Kamath 2010; Ray 2012; Roy 2012; Seyedoshohadaei 2016; Wu 2016).
-
21/42 studies reported OHSS rate by woman randomised (Abu Hashim 2010a; Abu Hashim 2010b; Badawy 2008; Badawy 2009a; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; El‐Khayat 2016; Foroozanfard 2011; Ganesh 2009; Ghahiri 2016; Hassan 2017; Kamath 2010; Legro 2014; Nazik 2012; Ramezanzadeh 2011; Roy 2012; Selim 2012; Zarei 2015; Zeinalzadeh 2010).
-
41/42 studies reported clinical pregnancy rate by woman randomised (Abdellah 2011; Abu Hashim 2010a; Abu Hashim 2010b; Al‐Omari 2004; Amer 2017; Atay 2006; Badawy 2008; Badawy 2009a; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Gharib 2015; El‐Khayat 2016; Elgafor 2013; Foroozanfard 2011; Ganesh 2009; Ghahiri 2016; Ghomian 2015; Hassan 2017; Ibrahim 2017; Kamath 2010; Kar 2012; Legro 2014; Liu 2015; Liu 2017; Moussa 2016; Nazik 2012; Ramezanzadeh 2011; Ray 2012; Roy 2012; Selim 2012; Sh‐El‐Arab Elsedeek 2011; Seyedoshohadaei 2016; Sharief 2015; Sohrabvand 2006; Wu 2016; Zarei 2015; Zeinalzadeh 2010).
-
30/42 studies reported miscarriage rate by woman randomised and by pregnancies (Abdellah 2011; Abu Hashim 2010a; Abu Hashim 2010b; Badawy 2008; Badawy 2009a; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Khayat 2016; Elgafor 2013; Foroozanfard 2011; Ganesh 2009; Ghahiri 2016; Hassan 2017; Ibrahim 2017; Kamath 2010; Kar 2012; Liu 2015; Liu 2017; Nazik 2012; Ramezanzadeh 2011; Ray 2012; Roy 2012; Seyedoshohadaei 2016; Sohrabvand 2006; Wu 2016; Zarei 2015).
-
30/42 studies reported multiple pregnancy rate by woman randomised (Abdellah 2011; Abu Hashim 2010a; Abu Hashim 2010b; Al‐Omari 2004; Amer 2017; Atay 2006; Badawy 2008; Badawy 2009a; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Dehbashi 2009; El‐Khayat 2016; Foroozanfard 2011; Ganesh 2009; Hassan 2017; Hendawy 2011Kamath 2010; Kar 2012; Legro 2014; Liu 2015; Nazik 2012; NCT00610077; Ramezanzadeh 2011; Roy 2012; Selim 2012; Sharief 2015; Wu 2016; Zeinalzadeh 2010).
Excluded studies
We exclude 30 trials from the review. Sixteen of these were identified for the 2018 update (Al‐Hussaini 2014; Al‐Shaikh 2017; Angel 2014; Azmoodeh 2015; Khanna 2013; Li 2016; NCT01431352; NCT01577017; NCT01679574; NCT01793038; Pakrashi 2014; Palihawadana 2015; Pourali 2017; Sharma 2010; Xi 2015; Yun 2015). The primary reasons for exclusion of the studies were inclusion criteria, interventions, inability to obtain study data and no randomisation. (See Characteristics of excluded studies)
Risk of bias in included studies
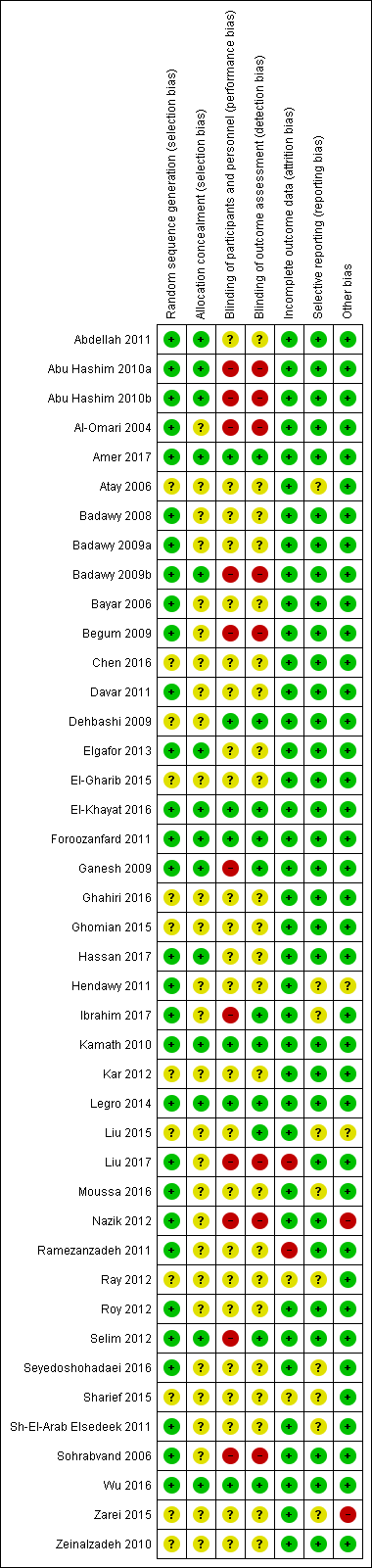
See Characteristics of included studies; Figure 2; Figure 3.
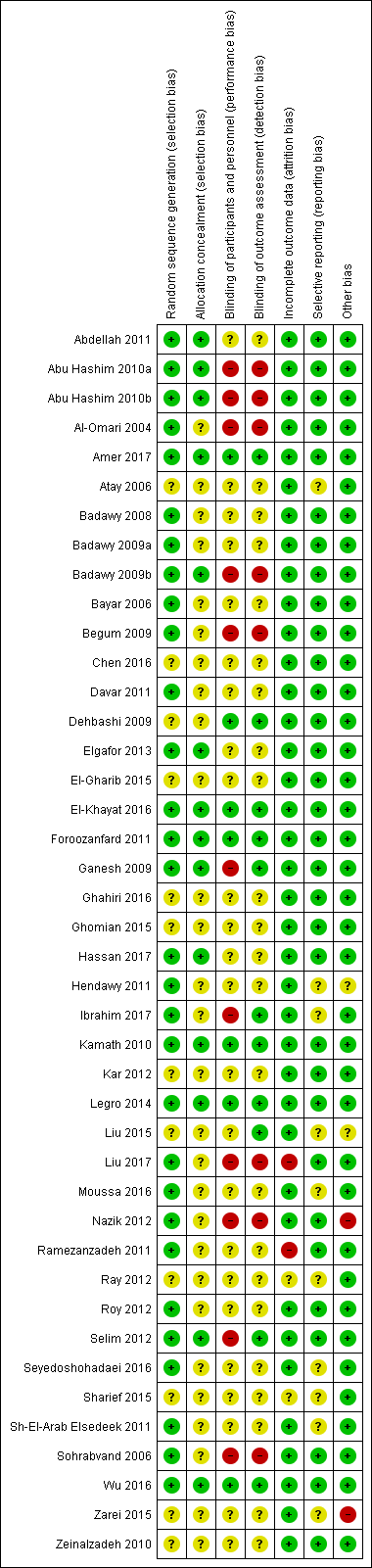
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Thirty studies were at low risk of selection bias related to sequence generation. They used computer randomisation, a random‐number table or lottery. The remaining 12 studies did not fully describe their method of randomisation and the contacted authors did not respond, so we rated them at unclear risk of this bias (Figure 2).
Fourteen studies were at low risk of selection bias related to allocation concealment. They used sequentially‐numbered, sealed (opaque) envelopes and the list was kept by a third party during the procedure. The other 28 studies did not describe allocation concealment sufficiently and the authors did not respond to our emails, so we rated them at unclear risk of bias (Figure 2).
Blinding
Seven out of 42 studies described the blinding of participants and personnel, and were thus rated tat low risk of performance bias. Twenty‐five studies did not mention blinding of participants of personnel and the authors did not respond to our emails, so we rated them at unclear risk of bias. Ten studies stated that there was no blinding of participants or personnel or both, and were at high risk of bias (Figure 2).
Eleven of 42 studies reported that the outcome assessors were blinded and were therefore at low risk of bias. Twenty‐three studies did not mention blinding of outcome assessors and the authors did not respond to our email contact, so were rated at unclear risk of bias. Four studies were at high risk of detection bias, because they reported that the outcome assessors were not blinded. Another four studies were also at high risk of bias because the participants were not blinded and it is therefore not plausible that the outcome assessors were blinded. (Figure 2).
Incomplete outcome data
Thirty‐eight of 42 studies included all or nearly all women they had randomised (more than 90%) and were therefore at low risk of attrition bias. Two studies were at unclear risk of attrition bias. One study had peculiar group numbers and none of the other biases were addressed, so we tried without success to contact the authors (Ray 2012). Another study did not report how many women were originally randomised (Sharief 2015). Two studies were at high risk of attrition bias: one study because 13 of 80 women were not analysed. Four women were excluded after randomisation due to an ovarian cyst on sonography on day three. Nine more women were lost to follow‐up without any reasons given (Ramezanzadeh 2011). The second study excluded 28 of 268 women from analysis; 13 were lost to follow‐up, three were excluded, and 12 had complications (Liu 2017; Figure 2).
Selective reporting
Thirty‐nine of the 42 included studies reported the outcomes they had stated in the Methods section, and we therefore judged them to be at low risk of bias. In three of the 42 studies only a few outcomes were presented and the contacted authors did not respond, so we rated them at unclear risk of reporting bias (Figure 2).
Other potential sources of bias
In one study there were substantial baseline differences in age and duration of infertility between the two groups and we deemed the risk of bias to be high (Nazik 2012). In another study, the methods were not well described and the clinical trial registration number led to a different trial (Zarei 2015). We found no potential sources of within‐study bias in the other 40 studies (Figure 2).
Effects of interventions
See: Summary of findings for the main comparison Letrozole with or without adjuncts compared to clomiphene citrate (CC) with or without adjuncts for subfertile women with polycystic ovary syndrome; Summary of findings 2 Letrozole compared to laparoscopic ovarian drilling for subfertile women with polycystic ovary syndrome
1. Letrozole compared to placebo
Two trials including 167 participants compared an aromatase inhibitor (letrozole) and placebo (Kamath 2010; Zarei 2015). Only one trial reported live birth rate, and there was insufficient evidence to suggest a difference in live birth rate (OR 3.17, 95% CI 0.12 to 83.17; 36 participants; 1 study; Analysis 1.1). A risk difference analysis for OHSS rate showed insufficient evidence of a difference in frequency of this adverse event (RR 0.00, 95% CI −0.05 to 0.05; 167 participants; 2 studies; I2 = 0%; Analysis 1.2). Pregnancy rate was higher using letrozole compared to placebo (OR 2.88, 95% CI 1.08 to 7.66; 167 participants; 2 studies; I2 = 0%; Analysis 1.3). A risk difference analysis for miscarriage rate showed insufficient evidence of a difference in frequency of this adverse event (OR 1.60, 95% CI 0.26 to 9.89; 167 participants; 2 studies; Analysis 1.4). Multiple pregnancy rate was not estimable because there were no cases reported.
2. Letrozole compared to clomiphene citrate (CC) with or without adjuncts, followed by intercourse
25 trials including 4629 women compared letrozole to selective oestrogen receptor modulators with or without adjuncts (Abu Hashim 2010b; Amer 2017; Atay 2006; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Gharib 2015; El‐Khayat 2016; Foroozanfard 2011; Ghahiri 2016; Legro 2014; Liu 2017; Moussa 2016; Nazik 2012; Ray 2012; Roy 2012; Selim 2012; Seyedoshohadaei 2016; Sharief 2015; Sh‐El‐Arab Elsedeek 2011; Sohrabvand 2006; Wu 2016).
-
Letrozole (2.5 mg to 7.5 mg/day) versus clomiphene citrate (50 mg to 150 mg/day), either alone or in combination with metformin (1000 mg to 1500 mg daily); 75 to 150 IU hMG in one or both arms; estradiol valerate 4 mg/day or berberine 1.5 g for 6 months.
Primary outcomes
2.1 Live birth
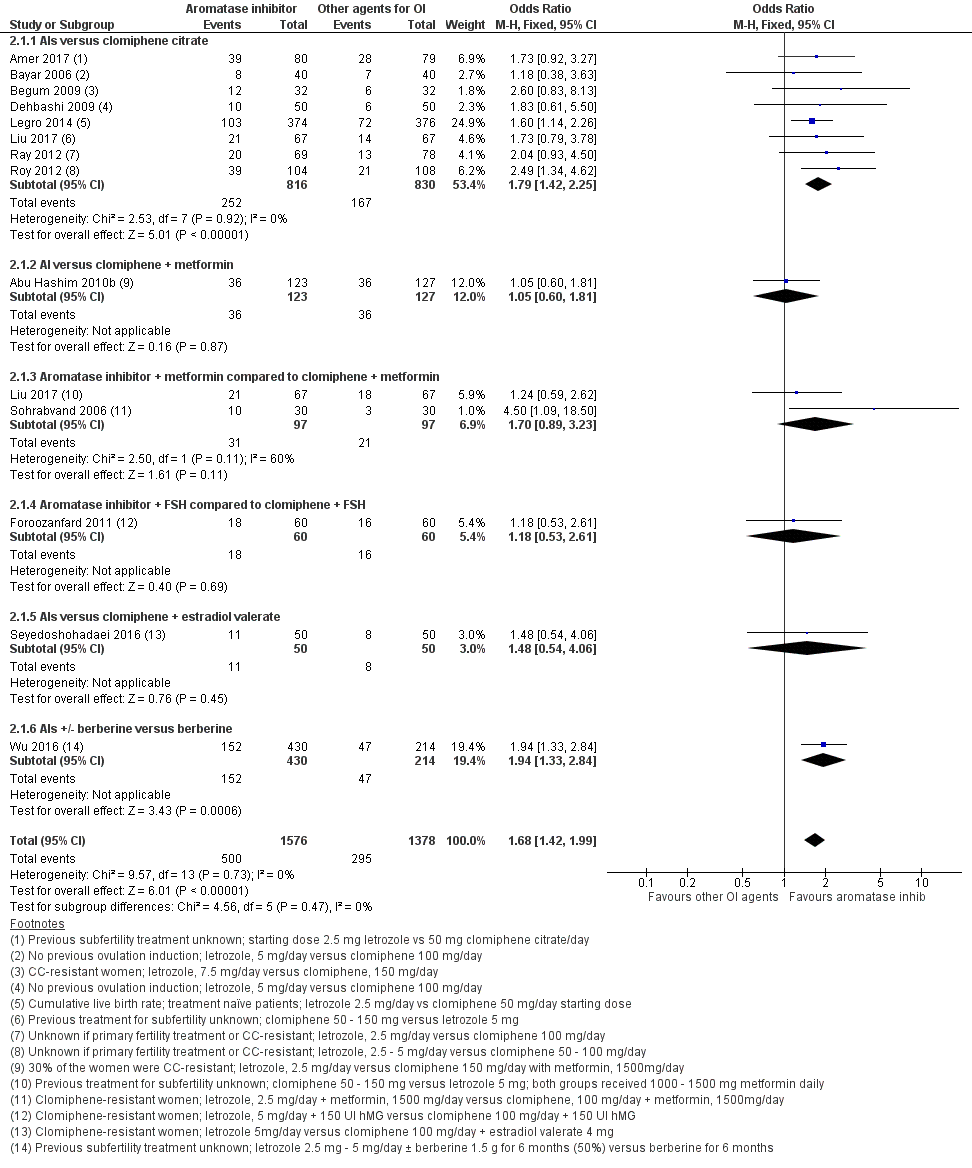
Thirteen studies including 2954 women compared letrozole to CC, with or without adjuncts in one or both arms, and reported live birth (Abu Hashim 2010b; Amer 2017; Bayar 2006; Begum 2009; Dehbashi 2009; Foroozanfard 2011; Legro 2014; Liu 2017; Ray 2012; Roy 2012; Seyedoshohadaei 2016; Sohrabvand 2006; Wu 2016). Letrozole resulted in an increased live birth rate compared to CC for ovulation induction (OR 1.68, 95% CI 1.42 to 1.99; 2954 participants; 13 studies; I2 = 0%; number needed to treat for an additional beneficial outcome (NNTB) = 10; moderate‐quality evidence; Figure 4; Analysis 2.1).

Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.1 Live birth rate.
Subgroup analysis showed insufficient evidence to suggest a difference by mean BMI (P = 0.87) or in study populations that were CC‐resistant or had no previous treatment for ovulation induction (P = 0.85) (analysis not shown). Sensitivity analysis excluding one study (Begum 2009) with high risk of detection bias showed no substantive influence on the treatment effect. A sensitivity analysis comparing studies with unclear and low risk for allocation bias also showed insufficient evidence for a difference in treatment effect between the two subgroups (P = 0.34). Subgroup analysis by CC resistance showed insufficient evidence for a difference in treatment effect for live birth.
In our sensitivity analyses, findings for live birth were not influenced by the use of a random‐effects model, alternative imputation strategies, or risk ratio rather than odds ratio. An additional sensitivity analysis showed that studies that reported live births tended to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, suggesting that results might be less favourable to letrozole if all studies reported live birth, with a more modest treatment effect. However, a funnel plot for live birth rate was symmetrical, indicating that our findings might not be influenced by publication bias (Figure 5).

Funnel plot of comparison: 2 Aromatase inhibitors compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, outcome: 2.1 Live birth rate.
2.2 Ovarian hyperstimulation syndrome (OHSS)
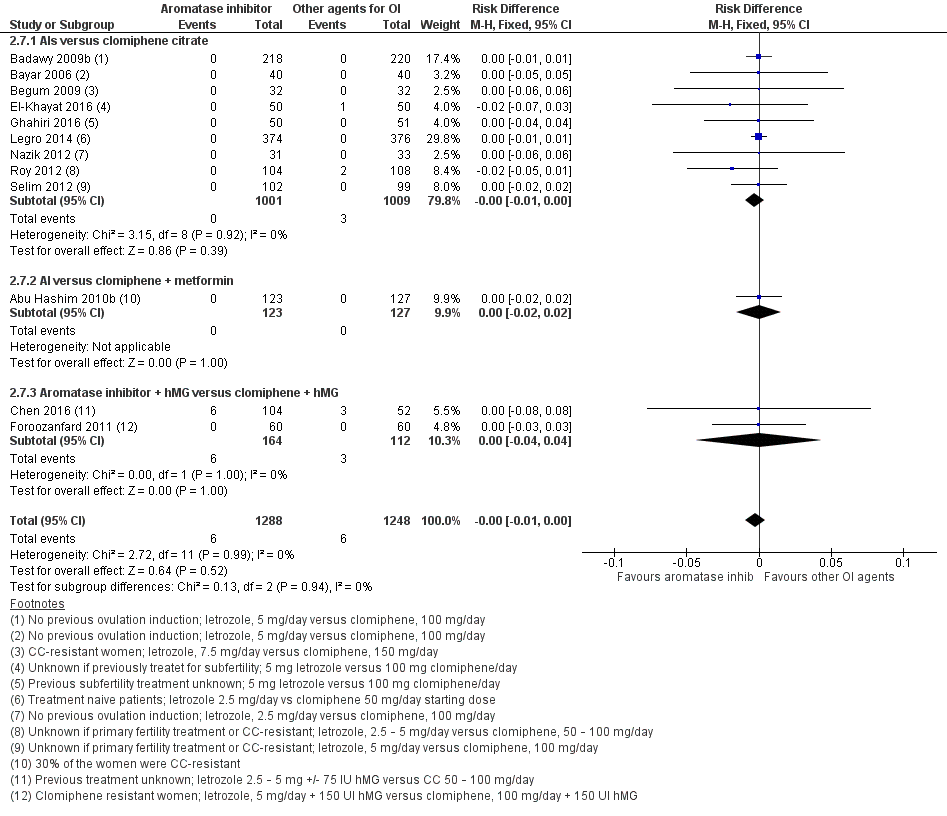
Twelve studies including 2536 women compared letrozole to CC, with or without adjuncts in one or both arms, and reported the occurrence of ovarian hyperstimulation syndrome (Abu Hashim 2010b; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; El‐Khayat 2016; Foroozanfard 2011; Ghahiri 2016; Legro 2014; Nazik 2012; Roy 2012; Selim 2012). Our risk‐difference analysis showed that there is high‐quality evidence of a similar frequency of this adverse event in both groups (RD −0.00; 95% CI −0.01 to 0.00; 2536 participants; 12 studies; I2 = 0%; high‐quality evidence; Figure 6; Analysis 2.7). A subgroup analysis showed insufficient evidence to suggest a difference by BMI mean (P = 0.79) (analysis not shown). A funnel plot for OHSS was insufficient for detection of a potential publication bias because there were no events in most of the studies (analysis not shown).

Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.6 Ovarian hyperstimulation syndrome rate.
Secondary outcomes
2.3 Clinical pregnancy
Clinical pregnancy rate was reported in 25 studies, including 4629 women (Abu Hashim 2010b; Amer 2017; Atay 2006; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Gharib 2015; El‐Khayat 2016; Foroozanfard 2011; Ghahiri 2016; Legro 2014; Liu 2017; Moussa 2016; Nazik 2012; Ray 2012; Roy 2012; Selim 2012; Seyedoshohadaei 2016; Sharief 2015; Sh‐El‐Arab Elsedeek 2011; Sohrabvand 2006; Wu 2016). Use of letrozole resulted in a higher clinical pregnancy rate compared to clomiphene citrate, with or without adjuncts in one or both arms (OR 1.56, 95% CI 1.37 to 1.78; 4629 participants; 25 studies; I2 = 1%; NNTB = 10; moderate‐quality evidence; Analysis 2.9).
2.4 Miscarriage rate by woman randomised and by pregnancies
Miscarriage rate was reported in 18 studies, including 3754 women (Abu Hashim 2010b; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Davar 2011; Dehbashi 2009; El‐Khayat 2016; Foroozanfard 2011; Ghahiri 2016; Legro 2014; Liu 2017; Nazik 2012; Ray 2012; Roy 2012; Seyedoshohadaei 2016; Sohrabvand 2006; Wu 2016). The analysis of miscarriage rate by woman randomised showed little evidence for a difference in favour of clomiphene citrate, with or without adjuncts in one or both arms (OR 1.39, 95% CI 1.07 to 1.81; 3754 participants; 18 studies; I2 = 0%; Analysis 2.11). However, the results of the analysis of miscarriage rate by pregnancies showed no evidence of a difference between the groups (OR 0.94, 95% CI 0.70 to 1.26; 1210 participants; 18 studies; I2 = 0%; high‐quality evidence; Analysis 2.12).
2.5 Multiple pregnancy rate
Multiple pregnancy rate was reported in 16 studies, including 3519 women (Abu Hashim 2010b; Amer 2017; Atay 2006; Badawy 2009b; Bayar 2006; Begum 2009; Chen 2016; Dehbashi 2009; El‐Khayat 2016; Foroozanfard 2011; Legro 2014; Nazik 2012; Roy 2012; Selim 2012; Sharief 2015; Wu 2016). The analysis of multiple pregnancy rate by woman randomised showed high‐quality evidence of no difference in multiple pregnancy rate for letrozole compared to CC (OR 0.73, 95% CI 0.43 to 1.24; 3519 participants; 16 studies; I2 = 0%; Analysis 2.13).
Publication bias
We produced a funnel plot for both primary outcomes and for the outcome of pregnancy rate. A funnel plot for live birth rate was symmetrical, indicating that our findings might not be influenced by publication bias (Figure 5). A funnel plot for OHSS was insufficient for detection of a potential publication bias because there were no events in most of the studies (analysis not shown). The funnel plot for the secondary outcome pregnancy rate showed some asymmetries, with a gap on the left side. This indicates that there were possibly some studies with significant effects in favour of CC which were not reported, and therefore the results of our meta‐analysis might have overestimated the effect of letrozole on pregnancy rate.
3. Letrozole compared to clomiphene citrate for ovulation induction followed by IUI
Three studies including 1597 women compared use of letrozole with or without adjuncts to other agents for ovulation induction followed by IUI (Ganesh 2009; Kar 2012; Zeinalzadeh 2010).
-
Letrozole (2.5 mg to 5 mg daily, cycle days 3 to 7 or 2 to 6) versus clomiphene citrate (50 mg to 150 mg daily, cycle days 3 to 7 or 2 to 6) with or without adjuncts or rFSH only (rFSH 75 IU to 100 IU from day 2 until the day of hCG administration).
Primary outcomes
3.1 Live birth
No studies comparing letrozole to clomiphene reported live birth rate.
3.2 Ovarian hyperstimulation syndrome
Two studies reported ovarian hyperstimulation syndrome rate comparing use of letrozole to CC (Ganesh 2009; Zeinalzadeh 2010). Our risk‐difference analysis showed insufficient evidence of a difference in frequency of this adverse event between the two treatment groups (RD −0.00, 95% CI −0.01 to 0.00; 1494 participants; 2 studies; I2 = 0%; Analysis 3.1). Subgroup analyses were not possible because there were too few studies.
Secondary outcomes
3.3 Clinical pregnancy
Clinical pregnancy rate was reported in three studies comparing letrozole to CC (Ganesh 2009; Kar 2012; Zeinalzadeh 2010). The analysis showed evidence in favour of letrozole compared to CC for ovulation induction followed by IUI (OR 1.71, 95% CI 1.30 to 2.25; 1597 participants; 3 studies; I2 = 0%; Analysis 3.2).
3.4 Miscarriage rate by woman randomised and by pregnancies
Miscarriage rate was reported in two studies comparing letrozole to CC (Ganesh 2009; Kar 2012). There was insufficient evidence of a difference between the two groups for miscarriage rate by woman randomised (OR 1.22, 95% CI 0.62 to 2.40; 1490 participants; 2 studies; I2 = 0%; Analysis 3.3) or by pregnancies (OR 0.76, 95% CI 0.37 to 1.57; 260 participants; 2 studies; I2 = 30%; Analysis 3.4).
3.5 Multiple pregnancy rate
Multiple pregnancy rate was reported in three studies comparing letrozole to CC, with or without adjuncts (Ganesh 2009; Kar 2012; Zeinalzadeh 2010). There was insufficient evidence of a difference between the two groups (OR 1.03, 95% CI 0.49 to 2.13; 1597 participants; 3 studies; I2 = 0%; Analysis 3.5).
4. Letrozole compared to laparoscopic ovarian drilling
Five studies including 774 women compared letrozole with or without metformin to laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Elgafor 2013; Ibrahim 2017; Liu 2015).
-
Letrozole (2.5 mg to 5 mg daily, cycle days 3 to 7) with or without metformin (850 mg to 1700 mg daily for 6 to 8 weeks) versus laparoscopic ovarian drilling.
Primary outcomes
4.1 Live birth
Live birth rate was reported in three studies including 548 women, comparing letrozole to laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Liu 2015). There was low‐quality evidence of no difference in live birth rate between the two treatment groups (OR 1.38, 95% CI 0.95 to 2.02; 548 participants; 3 studies; I2 = 23%; Analysis 4.1). Subgroup analyses were not possible because there were only three studies.
4.2 Ovarian hyperstimulation syndrome
Only one study including 260 women comparing use of letrozole to laparoscopic ovarian drilling reported ovarian hyperstimulation syndrome rate (Abu Hashim 2010a). Our risk‐difference analysis showed insufficient evidence of a difference in frequency of this adverse event for the two treatment groups (RD 0.00; 95% CI −0.01 to 0.01; 260 participants; 1 study; Analysis 4.2).
Secondary outcomes
4.3 Clinical pregnancy
Clinical pregnancy rate was reported in five studies including 774 women, comparing letrozole with or without metformin to laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Elgafor 2013; Ibrahim 2017; Liu 2015). There was low‐quality evidence of no difference between the two groups (OR 1.28, 95% CI 0.94 to 1.74; 774 participants; 5 studies; I2 = 0%; Analysis 4.3).
4.4 Miscarriage rate by woman randomised and by pregnancies
Miscarriage rate was reported in five studies including 774 women, comparing letrozole with or without metformin to laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Elgafor 2013; Ibrahim 2017; Liu 2015). There was moderate‐quality evidence of no difference between the two groups for miscarriage rate by woman randomised (OR 0.81, 95% CI 0.38 to 1.70; 774 participants; 5 studies; I2 = 0%; Analysis 4.4) and by pregnancy (OR 0.66; 95% CI 0.30 to 1.43; 240 participants; 5 studies ; I2 = 0%; Analysis 4.5).
4.5 Multiple pregnancy rate
Multiple pregnancy rate was reported in three studies including 548 women, comparing letrozole to laparoscopic ovarian drilling (Abdellah 2011; Abu Hashim 2010a; Liu 2015). There was low‐quality evidence of no difference between the two groups for multiple pregnancy rate by woman randomised (OR 3.00, 95% CI 0.12 to 74.90; 548 participants; 3 studies; I2 = 0%; Analysis 4.6).
5. Letrozole compared to follicle stimulating hormone (FSH)
One study including 140 women compared use of letrozole to FSH (Hassan 2017).
-
Letrozole 2.5 mg twice daily for 5 days versus uFSH 75 IU a day for 7 days, both groups starting on the third day of menstruation.
Primary outcomes
5.1 Live birth
No studies comparing letrozole to FSH reported live birth rate.
5.2 Ovarian hyperstimulation syndrome
Only one study comparing letrozole to FSH reported no occurrence of ovarian hyperstimulation syndrome (Hassan 2017). A risk‐difference analysis showed insufficient evidence of a difference between the two treatment groups (RD 0.00, 95% CI −0.03 to 0.03, 140 participants; 1 study; Analysis 5.1)
Secondary outcomes
5.3 Clinical pregnancy
Clinical pregnancy rate was reported in one study comparing letrozole to FSH (Hassan 2017). There was insufficient evidence of a difference between the two groups (OR 0.82, 95% CI 0.40 to 1.67; 140 participants; 1 study; Analysis 5.2)
5.4 Miscarriage rate by woman randomised and by pregnancies
Miscarriage rate was reported in one study comparing letrozole to FSH (Hassan 2017). There was insufficient evidence of a difference between the two groups for miscarriage rate by woman randomised (OR 0.66, 95% CI 0.11 to 4.06; 140 participants; 1 study; Analysis 5.3) or by pregnancies (OR 0.74, 95% CI 0.11 to 4.90; 140 participants; 1 study; Analysis 5.4).
5.5 Multiple pregnancy rate
Multiple pregnancy rate was reported in one study comparing letrozole to FSH (Hassan 2017). There was insufficient evidence to suggest a difference between the two treatment groups (OR 0.19, 95% CI 0.01 to 4.12; 140 participants; 1 study; Analysis 5.5).
6. Letrozole compared to anastrozole
Two studies including 270 women compared letrozole to the AI anastrozole (Al‐Omari 2004; Badawy 2008).
-
Letrozole 2.5 mg/day versus anastrozole 1 mg/day for 5 days, both starting on cycle day 3.
Primary outcomes
6.1 Live birth
No studies comparing letrozole to anastrozole reported live birth rate.
6.2 Ovarian hyperstimulation syndrome
Only one study comparing letrozole to anastrozole reported the occurrence of ovarian hyperstimulation syndrome (Badawy 2008). A risk difference analysis showed insufficient evidence of a difference between the two treatment groups (RD 0.00, 95% CI −0.02 to 0.02; 220 participants; 1 study; Analysis 6.1)
Secondary outcomes
6.3 Clinical pregnancy
Clinical pregnancy rate was reported in two studies comparing letrozole to anastrozole (Al‐Omari 2004; Badawy 2008). There was insufficient evidence of a difference between the two groups (OR 0.85, 95% CI 0.51 to 1.43; 260 participants; 2 studies; I2 = 12%; Analysis 6.2).
6.4 Miscarriage rate by woman randomised and by pregnancies
Miscarriage rate was reported in one study comparing letrozole to anastrozole (Badawy 2008). There was insufficient evidence of a difference between the two groups for miscarriage rate by woman randomised (OR 0.98, 95% CI 0.24 to 4.03; 220 participants; 1 study; Analysis 6.3) or by pregnancies (OR 1.19, 95% CI 0.27 to 5.13; 220 participants; 1 study; Analysis 6.4).
6.5 Multiple pregnancy rate
Multiple pregnancy rate was reported in two studies comparing letrozole to anastrozole (Al‐Omari 2004; Badawy 2008). One study did not report any cases of multiple pregnancies and an odds ratio was therefore not estimable (Al‐Omari 2004). The other study showed insufficient evidence to suggest a difference between the two treatment groups (OR 5.00, 95% CI 0.24 to 105.35; 220 participants; 1 study; Analysis 6.5).
7. Different administration protocols of letrozole
7.1 Five days compared to 10 days administration protocol of letrozole
There was one trial comparing a five‐day letrozole administration protocol to a 10‐day letrozole administration protocol (Badawy 2009a). This study did not report live birth rate. A risk‐difference analysis on the OHSS rate showed insufficient evidence to suggest a difference in occurrence of OHSS between the two treatment groups (RD 0.00, 95% CI −0.02 to 0.02; 220 participants; 1 study; Analysis 7.1). The analysis showed insufficient evidence of a difference between the groups in clinical pregnancy rate (OR 0.63, 95% CI 0.35 to 1.13; 220 participants; 1 study; Analysis 7.2), miscarriage rate by woman randomised (OR 0.69, 95% CI 0.21 to 2.24; 220 participants; 1 study; Analysis 7.3), miscarriage rate by pregnancies (OR 0.96, 95% CI 0.27 to 3.42; 220 participants; 1 study; Analysis 7.4) or multiple pregnancy rate (OR 0.32, 95% CI 0.01 to 8.05; 220 participants; 1 study; Analysis 7.5).
7.2 Letrozole day 3 ‐ 7 administration versus day 5 ‐ 9 administration
There was one trial including 70 women comparing starting letrozole on day 3 versus day 5 administration protocol (Ghomian 2015). This study did not report live birth rate, OHSS rate, miscarriage rate or multiple pregnancy rate. The analysis showed insufficient evidence of a difference between the two groups in clinical pregnancy rate (OR 1.38, 95% CI 0.28 to 6.66; 70 participants, 1 study; Analysis 7.2).
8. Dosage studies of letrozole
There was only one trial comparing a 5 mg/day administration of letrozole to a 7.5 mg/day administration protocol (Ramezanzadeh 2011). This study did not report live birth rate. A risk‐difference analysis on OHSS rate showed insufficient evidence to suggest a difference in occurrence of OHSS between the two treatment groups (RD 0.00, 95% CI −0.05 to 0.05; 80 participants; 1 study; Analysis 8.1). Their results show insufficient evidence of a difference between the groups in clinical pregnancy rate (OR 1.00, 95% CI 0.32 to 3.17; 80 participants; 1 study; Analysis 8.2), miscarriage rate by woman randomised (OR 0.33, 95% CI 0.01 to 8.22; 80 participants; 1 study; Analysis 8.3), miscarriage rate by pregnancies (OR 0.29, 95% CI 0.01 to 8.39; 80 participants; 1 study; Analysis 8.4) or multiple pregnancy rate (OR 1.00, 95% CI 0.06 to 16.56; 80 participants; 1 study; Analysis 8.5).
Discussion
Summary of main results
Letrozole compared to placebo
Two trials compared Letrozole to placebo. There is a lack of adequate studies with a large number of participants and a low risk of bias.
Letrozole compared to clomiphene citrate followed by timed intercourse
The results of our analysis of 25 trials comparing letrozole to CC followed by timed intercourse suggest that letrozole improves the live birth rate and pregnancy rate compared to CC (summary of findings Table for the main comparison).
However, we note that studies that reported live birth tended to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, with insufficient evidence of a difference in miscarriage rates by pregnancies. This suggests that findings might be less favourable to letrozole if all studies reported live birth. However, a funnel plot for live birth rate was symmetrical, indicating that our findings might not be influenced by publication bias (Figure 5).
A risk‐difference analysis suggested that letrozole and clomiphene citrate are equally safe in terms of ovarian hyperstimulation and miscarriage. (summary of findings Table for the main comparison).
The funnel plot for clinical pregnancy rate was asymmetrical, suggesting that our findings might be influenced by publication bias in favour of letrozole. A funnel plot investigating the impact of possible allocation bias on clinical pregnancy rate also showed some asymmetry, suggesting that the results might be influenced by allocation bias in favour of letrozole.
All analyses had absent or low levels of statistical heterogeneity (I2 less than 25%).
Seven of our 25 studies in this analysis included women that were resistant to clomiphene citrate (Abu Hashim 2010b; Begum 2009; Davar 2011; El‐Gharib 2015; Foroozanfard 2011; Seyedoshohadaei 2016; Sohrabvand 2006); the other 18 studies included women who were not resistant to clomiphene citrate (Badawy 2009b; Bayar 2006; Dehbashi 2009; Legro 2014; Nazik 2012; Sh‐El‐Arab Elsedeek 2011) or it was not mentioned (Amer 2017; Atay 2006; Chen 2016; El‐Khayat 2016; Ghahiri 2016; Liu 2017; Moussa 2016; Selim 2012; Sharief 2015; Ray 2012; Roy 2012; Wu 2016).
Data based on findings from Legro 2014 found that the interventions had comparable treatment costs. This suggests that, given its higher effectiveness, letrozole is more cost‐effective than clomiphene citrate (Reproductive Medicine Network 2013).
Letrozole compared to other agents for ovulation induction followed by IUI
Three trials compared letrozole to clomiphene citrate for ovulation induction followed by IUI. None reported live birth. Two reported OHSS: only three cases occurred and there was insufficient evidence of a difference despite a study population of 1494 women. Clinical pregnancy rates were increased in women treated with letrozole, compared to CC and FSH. There was insufficient evidence of a difference in rates of miscarriage or multiple pregnancy.
Letrozole compared to laparoscopic ovarian drilling
Five trials compared letrozole to laparoscopic ovarian drilling in clomiphene citrate‐resistant women (summary of findings Table 2). OHSS was reported only in Abu Hashim 2010a , but no cases of OHSS were found despite a study population of 260 women. There was low‐ to moderate‐quality evidence of no difference in rates of live birth, pregnancy, miscarriage or multiple pregnancy rate.
Letrozole compared to FSH
A single study including 140 women compared use of letrozole to FSH (Hassan 2017). Live birth rate was not reported and there were no events of OHSS. There was insufficient evidence of a difference for clinical pregnancy, miscarriage or multiple pregnancy rate.
Letrozole compared to anastrozole
Letrozole was compared to anastrozole in two studies including 260 women (Al‐Omari 2004; Badawy 2008). Neither study reported live birth and OHSS was only reported in Badawy 2008 but with no events. Rates of clinical pregnancy and multiple pregnancies were compared in both trials, but there was insufficient evidence of a difference. Miscarriage rates were reported only in Badawy 2008, with insufficient evidence of a difference between the groups.
Different administration protocols of letrozole
Five days compared to 10 days letrozole administration protocol
A single study including 218 women compared a five‐day administration protocol to a 10‐day administration protocol for letrozole. There was insufficient evidence of increased effectiveness or reduced side effects for any of our outcomes.
Letrozole day 3‐7 administration versus day 5‐9 administration protocol
A single study including 70 women compared a day 3 to 5 versus day 5 to 9 administration protocol. Only pregnancy rate was reported and there was insufficient evidence for a difference.
Dosage studies of letrozole
We intended to analyse different doses of letrozole in the range from 2.5 to 5 mg/day, but we found only one study including 80 women comparing a dosage of 5 mg/day to 7.5 mg/day. There was insufficient evidence of a difference in effectiveness as only seven pregnancies were reported in each group. There was also insufficient evidence of a difference in adverse events, but the size of the study population might have been too small because only one or no cases were reported in each group for OHSS, miscarriage and multiple pregnancy rate.
Overall completeness and applicability of evidence
For our main comparison of letrozole compared to other agents for ovulation induction, we found sufficient studies for our analysis of live birth and OHSS to answer our research question.
Most of the studies included were conducted in Egypt or the Middle East. There are, however, two large studies from the USA and Europe confirming the results (Amer 2017; Legro 2014).
Quality of the evidence
We included 42 studies with 7935 women. The overall quality of the evidence varied and was rated as moderate to high for our main comparison (summary of findings Table for the main comparison). The reasons for downgrading the evidence included possible publication bias. Moreover, there was a tendency for studies that reported live birth to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, suggesting that results might be somewhat less favourable to letrozole if all studies reported live birth. However, based on the large numbers of participants and the addition of trials at low risk of bias in this update, it is unlikely that additional studies are going to alter the effect estimates of our main comparison of letrozole versus clomiphene citrate, with or without adjuncts.
The other comparisons included only one to five studies. We downgraded much of the evidence for risks of bias and imprecision (summary of findings Table 2).
Potential biases in the review process
We conducted a comprehensive search with the help of an experienced Information Specialist, and ran extensive manual searches in order to identify all relevant studies and in an effort to minimise the risk of publication bias. However, we generated a funnel plot for the outcome of pregnancy rate in the comparison of aromatase inhibitors versus other ovulation induction agents, which indicated that there might be some studies not published that reported results in favour of clomiphene citrate. There are five studies awaiting classification, which could also have an influence on our results. There might therefore be some publication bias in this review.
We followed Cochrane guidelines to select studies, extract data and assess the quality and potential risks of different types of biases in all our included studies, in order to minimise the chance of error and bias by the review authors.
Agreements and disagreements with other studies or reviews
Our meta analysis shows evidence for increased live birth rates in favour of letrozole when compared to clomiphene citrate in women with PCOS. This differs from a previous review, which did not detect a difference (Misso 2012). This is most likely due to the limited number of studies included in the previous review. Another recent meta analysis (Roque 2015) is in accordance with our findings of increased live birth and pregnancy rates, as well as no difference for miscarriage and multiple pregnancy rates in our meta‐analysis. In addition, our meta‐analysis showed no evidence for a difference in effect between letrozole and laparoscopic ovarian drilling for subfertility treatment in women who are resistant to clomiphene citrate, which is in agreement with the results of an earlier meta‐analysis (Misso 2012).
Study flow diagram for update 2018
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.1 Live birth rate.

Funnel plot of comparison: 2 Aromatase inhibitors compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, outcome: 2.1 Live birth rate.
Forest plot of comparison: 2 Aromatase inhibitors compared to other ovulation induction agents, outcome: 2.6 Ovarian hyperstimulation syndrome rate.
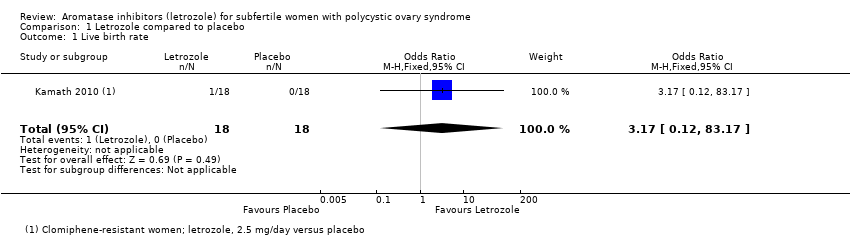
Comparison 1 Letrozole compared to placebo, Outcome 1 Live birth rate.

Comparison 1 Letrozole compared to placebo, Outcome 2 Ovarian hyperstimulation syndrome rate.

Comparison 1 Letrozole compared to placebo, Outcome 3 Clinical pregnancy rate.

Comparison 1 Letrozole compared to placebo, Outcome 4 Miscarriage rate by woman randomised.

Comparison 1 Letrozole compared to placebo, Outcome 5 Miscarriage rate by pregnancies.
Comparison 1 Letrozole compared to placebo, Outcome 6 Multiple pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 1 Live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 2 Live birth rate by BMI.
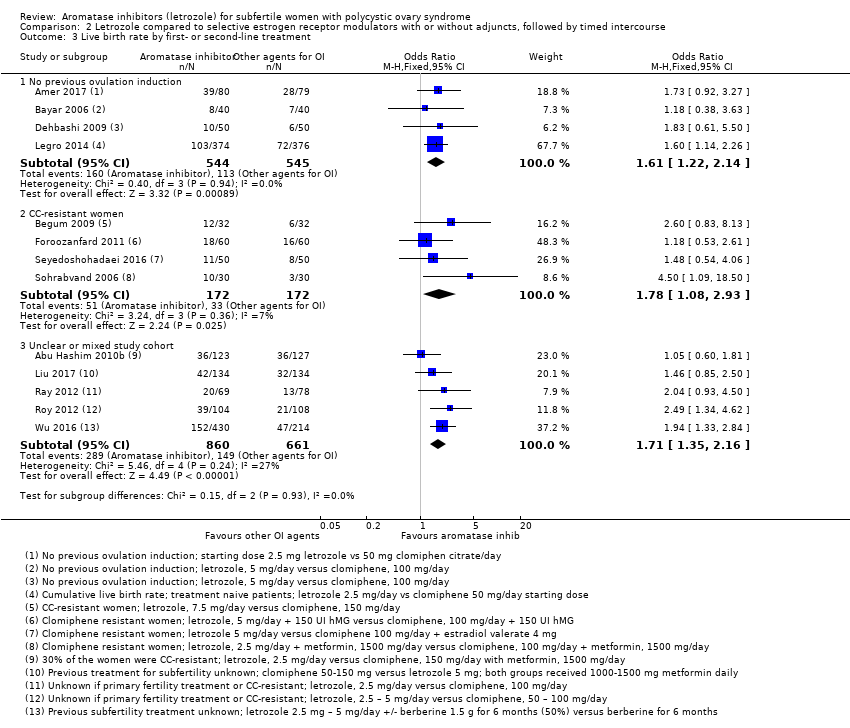
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 3 Live birth rate by first‐ or second‐line treatment.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 4 Impact of allocation bias for live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 5 Impact of detection bias for live birth rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 6 Impact of attrition bias for live birth rate.
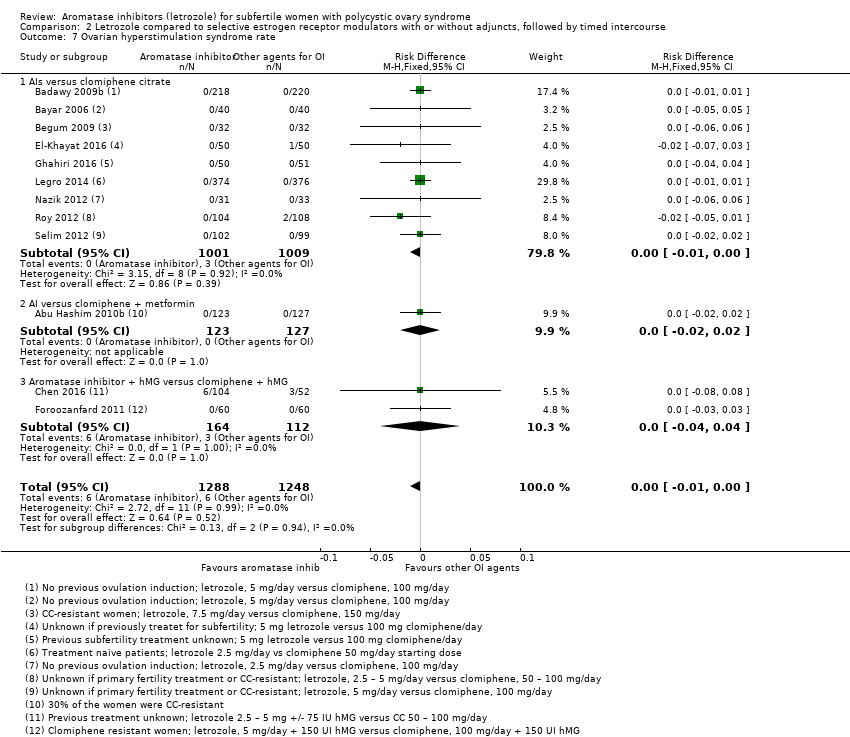
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 7 Ovarian hyperstimulation syndrome rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 8 Ovarian hyperstimulation syndrome rate per BMI.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 9 Clinical pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 10 Impact of allocation bias for clinical pregnancy rate.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 11 Miscarriage rate by woman randomised.

Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 12 Miscarriage rate by pregnancies.
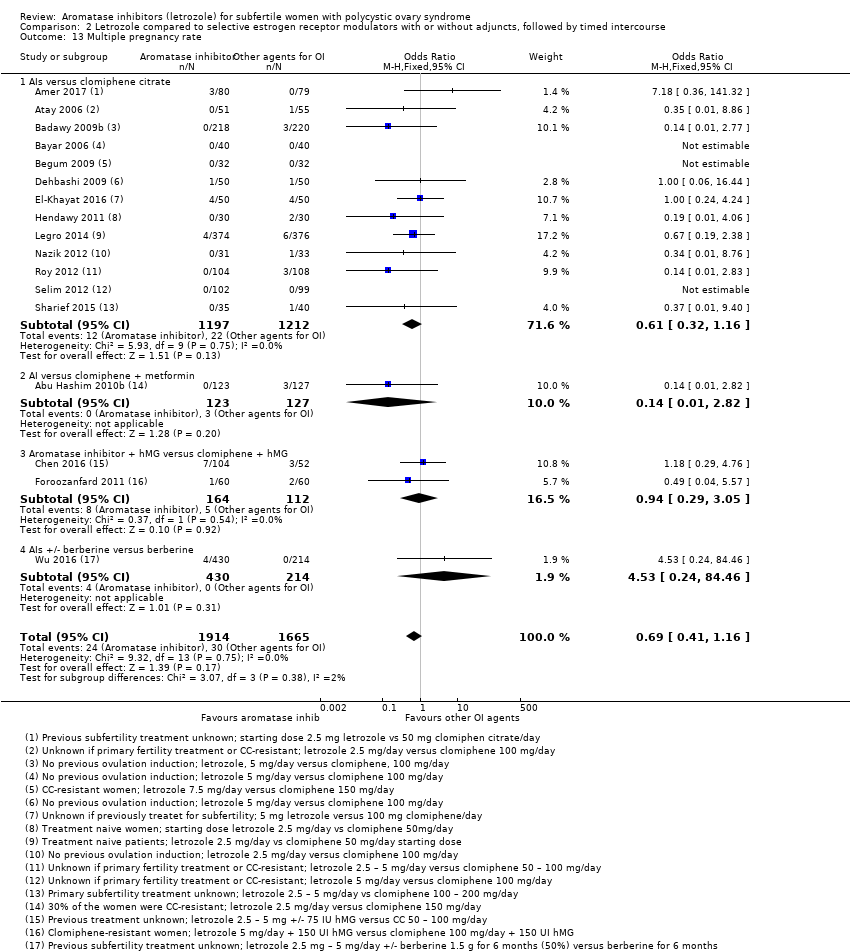
Comparison 2 Letrozole compared to selective estrogen receptor modulators with or without adjuncts, followed by timed intercourse, Outcome 13 Multiple pregnancy rate.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 2 Clinical pregnancy rate.
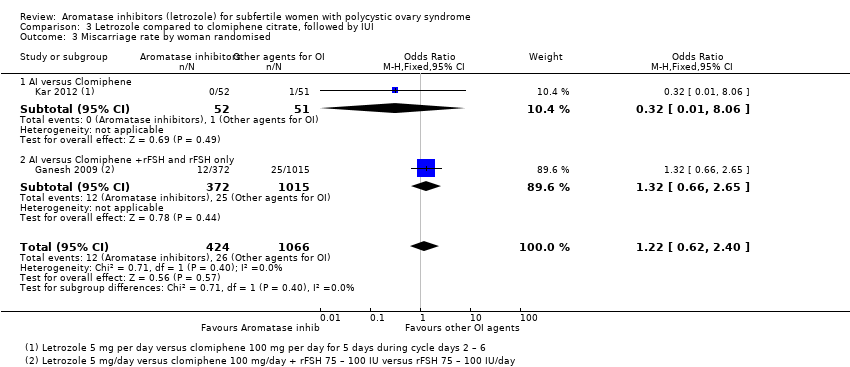
Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 3 Miscarriage rate by woman randomised.
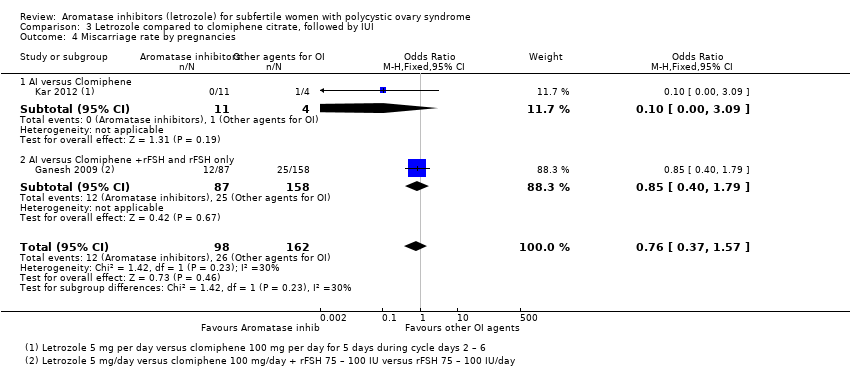
Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 4 Miscarriage rate by pregnancies.

Comparison 3 Letrozole compared to clomiphene citrate, followed by IUI, Outcome 5 Multiple pregnancy rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 1 Live birth rate.
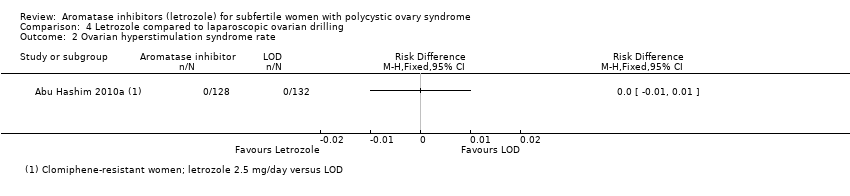
Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 2 Ovarian hyperstimulation syndrome rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 3 Clinical pregnancy rate.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 4 Miscarriage rate by woman randomised.
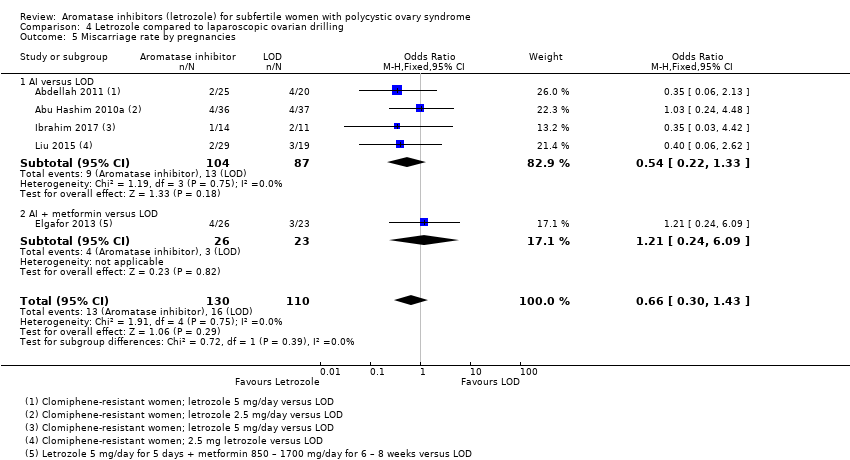
Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 5 Miscarriage rate by pregnancies.

Comparison 4 Letrozole compared to laparoscopic ovarian drilling, Outcome 6 Multiple pregnancy rate.
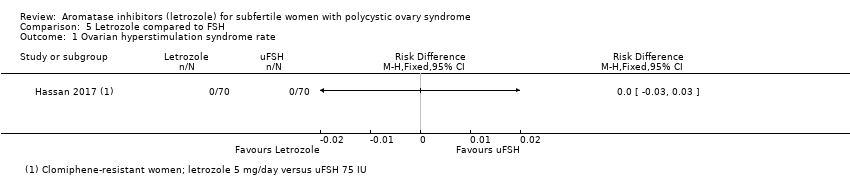
Comparison 5 Letrozole compared to FSH, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 5 Letrozole compared to FSH, Outcome 2 Clinical pregnancy rate.
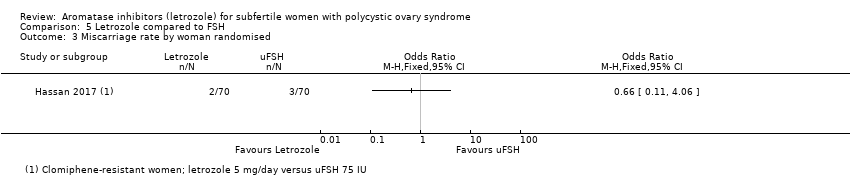
Comparison 5 Letrozole compared to FSH, Outcome 3 Miscarriage rate by woman randomised.
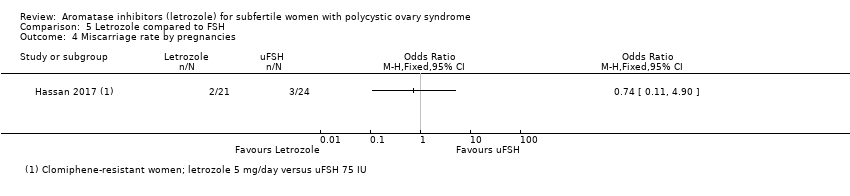
Comparison 5 Letrozole compared to FSH, Outcome 4 Miscarriage rate by pregnancies.

Comparison 5 Letrozole compared to FSH, Outcome 5 Multiple pregnancy rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 2 Clinical pregnancy rate.

Comparison 6 Letrozole compared to anastrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 6 Letrozole compared to anastrozole, Outcome 4 Miscarriage rate by pregnancies.
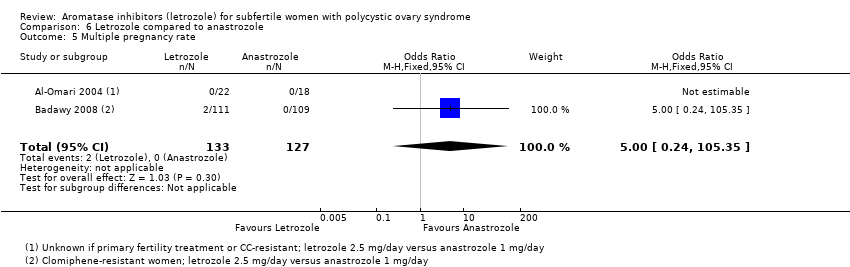
Comparison 6 Letrozole compared to anastrozole, Outcome 5 Multiple pregnancy rate.
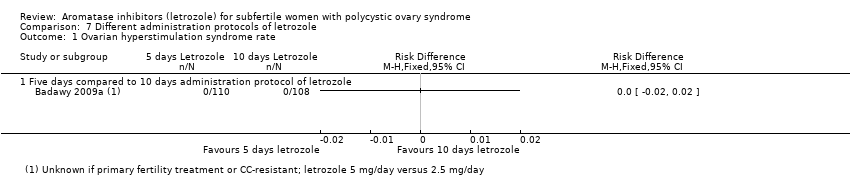
Comparison 7 Different administration protocols of letrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 7 Different administration protocols of letrozole, Outcome 2 Clinical pregnancy rate.

Comparison 7 Different administration protocols of letrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 7 Different administration protocols of letrozole, Outcome 4 Miscarriage rate by pregnancies.

Comparison 7 Different administration protocols of letrozole, Outcome 5 Multiple pregnancy rate.

Comparison 8 Dosage studies of letrozole, Outcome 1 Ovarian hyperstimulation syndrome rate.

Comparison 8 Dosage studies of letrozole, Outcome 2 Clinical pregnancy rate.

Comparison 8 Dosage studies of letrozole, Outcome 3 Miscarriage rate by woman randomised.

Comparison 8 Dosage studies of letrozole, Outcome 4 Miscarriage rate by pregnancies.
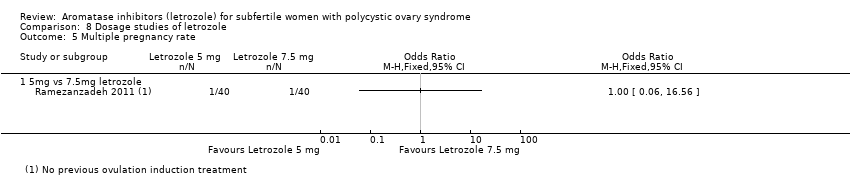
Comparison 8 Dosage studies of letrozole, Outcome 5 Multiple pregnancy rate.
| Letrozole with or without adjuncts compared to clomiphene citrate (CC) with or without adjuncts for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with CC with or without adjuncts | Risk with letrozole with or without adjuncts | |||||
| Live birth rate | 214 per 1000 | 314 per 1000 | OR 1.68 | 2954 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 5 per 1000 | 5 per 1000 | RD 0.00 | 2536 | ⊕⊕⊕⊕ | |
| Clinical pregnancy rate | 264 per 1000 | 359 per 1000 | OR 1.56 | 4629 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 201 per 1000 | 191 per 1000 | OR 0.94 | 1210 | ⊕⊕⊕⊕ | |
| Multiple pregnancy rate | 17 per 1000 | 13 per 1000 | OR 0.69 | 3579 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious risk of bias associated with potential selective reporting: Studies that reported live birth tended to report higher clinical pregnancy rates in the letrozole group than studies that failed to report live birth, suggesting that results might be less favourable to letrozole if all studies reported live birth. | ||||||
| Letrozole compared to laparoscopic ovarian drilling compared to placebo for subfertile women with polycystic ovary syndrome | ||||||
| Patient or population: Subfertile women with polycystic ovary syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Risk with LOD | Risk with letrozole | |||||
| Live birth rate | 236 per 1000 | 299 per 1000 | OR 1.38 | 548 | ⊕⊕⊕⊝ | |
| Ovarian hyperstimulation syndrome rate | 0 per 1000 | 0 per 1000 | RD 0.00 | 260 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate | 284 per 1000 | 336 per 1000 | OR 1.28 | 774 | ⊕⊕⊕⊝ | |
| Miscarriage rate by pregnancies | 145 per 1000 | 101 per 1000 | OR 0.66 | 240 | ⊕⊕⊕⊝ | |
| Multiple pregnancy rate | 0 per 1000 | 0 per 1000 | OR 3.00 | 548 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aInsufficient data to allow judgement of risk of bias in some studies ‐ downgraded one level for serious risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.12, 83.17] |
| 2 Ovarian hyperstimulation syndrome rate Show forest plot | 2 | 167 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 3 Clinical pregnancy rate Show forest plot | 2 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.08, 7.66] |
| 4 Miscarriage rate by woman randomised Show forest plot | 2 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.26, 9.89] |
| 5 Miscarriage rate by pregnancies Show forest plot | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.07, 4.56] |
| 6 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 13 | 2954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.42, 1.99] |
| 1.1 AIs versus clomiphene citrate | 8 | 1646 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.42, 2.25] |
| 1.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.60, 1.81] |
| 1.3 Aromatase inhibitor + metformin compared to clomiphene + metformin | 2 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.89, 3.23] |
| 1.4 Aromatase inhibitor + FSH compared to clomiphene + FSH | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.53, 2.61] |
| 1.5 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.54, 4.06] |
| 1.6 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.33, 2.84] |
| 2 Live birth rate by BMI Show forest plot | 11 | 2774 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.42, 2.02] |
| 2.1 BMI > 25 | 7 | 1678 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.34, 2.09] |
| 2.2 BMI < 25 | 4 | 1096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.31, 2.28] |
| 3 Live birth rate by first‐ or second‐line treatment Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No previous ovulation induction | 4 | 1089 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.14] |
| 3.2 CC‐resistant women | 4 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.08, 2.93] |
| 3.3 Unclear or mixed study cohort | 5 | 1521 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.35, 2.16] |
| 4 Impact of allocation bias for live birth rate Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Unclear risk of allocation | 8 | 1031 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.42, 2.54] |
| 4.2 Low risk of allocation | 5 | 1923 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.29, 1.95] |
| 5 Impact of detection bias for live birth rate Show forest plot | 13 | 2954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.42, 1.99] |
| 5.1 High risk of detection | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.83, 8.13] |
| 5.2 Low risk of detection | 7 | 2083 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.33, 1.99] |
| 5.3 Unclear risk of detection | 5 | 807 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.27, 2.44] |
| 6 Impact of attrition bias for live birth rate Show forest plot | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Unclear risk of attrition | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.93, 4.50] |
| 6.2 Low risk of attrition | 11 | 2539 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.03] |
| 6.3 High risk of attrition | 1 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.85, 2.50] |
| 7 Ovarian hyperstimulation syndrome rate Show forest plot | 12 | 2536 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 7.1 AIs versus clomiphene citrate | 9 | 2010 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 7.2 AI versus clomiphene + metformin | 1 | 250 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8 Ovarian hyperstimulation syndrome rate per BMI Show forest plot | 11 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 BMI > 25 | 6 | 1851 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 8.2 BMI < 25 | 5 | 605 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 9 Clinical pregnancy rate Show forest plot | 25 | 4629 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| 9.1 AIs versus clomiphene citrate | 17 | 2930 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.28, 1.76] |
| 9.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.71] |
| 9.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.05, 3.29] |
| 9.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.82, 2.27] |
| 9.5 AIs versus tamoxifen | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.64, 3.90] |
| 9.6 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.94, 6.46] |
| 9.7 AIs ± berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.48, 3.13] |
| 10 Impact of allocation bias for clinical pregnancy rate Show forest plot | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Unclear risk of allocation | 16 | 1907 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.43, 2.18] |
| 10.2 Low risk of allocation | 7 | 1912 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.12, 1.65] |
| 11 Miscarriage rate by woman randomised Show forest plot | 18 | 3754 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.07, 1.81] |
| 11.1 AIs versus clomiphene citrate | 11 | 2190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.97, 1.93] |
| 11.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.25, 4.23] |
| 11.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.52, 2.82] |
| 11.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.31, 2.27] |
| 11.5 AIs versus clomiphene + estradiol valerate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.21 [0.66, 226.97] |
| 11.6 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.87, 3.04] |
| 12 Miscarriage rate by pregnancies Show forest plot | 18 | 1210 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| 12.1 AIs versus clomiphene citrate | 11 | 705 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| 12.2 AI versus clomiphene + metformin | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.24, 4.40] |
| 12.3 Aromatase inhibitor + metformin versus clomiphene + metformin | 3 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.02] |
| 12.4 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.23, 1.96] |
| 12.5 AIs versus clomiphene + estradiol valerate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.13 [0.39, 167.90] |
| 12.6 AIs +/‐ berberine versus berberine | 1 | 213 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 13 Multiple pregnancy rate Show forest plot | 17 | 3579 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.16] |
| 13.1 AIs versus clomiphene citrate | 13 | 2409 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.32, 1.16] |
| 13.2 AI versus clomiphene + metformin | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.82] |
| 13.3 Aromatase inhibitor + hMG versus clomiphene + hMG | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.29, 3.05] |
| 13.4 AIs +/‐ berberine versus berberine | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.53 [0.24, 84.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 2 | 1494 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 1.1 AI versus Clomiphene | 1 | 107 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 1.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 2 Clinical pregnancy rate Show forest plot | 3 | 1597 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.30, 2.25] |
| 2.1 AI versus Clomiphene | 2 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.97, 4.53] |
| 2.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.23, 2.22] |
| 3 Miscarriage rate by woman randomised Show forest plot | 2 | 1490 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.62, 2.40] |
| 3.1 AI versus Clomiphene | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.06] |
| 3.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.66, 2.65] |
| 4 Miscarriage rate by pregnancies Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.57] |
| 4.1 AI versus Clomiphene | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 3.09] |
| 4.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.79] |
| 5 Multiple pregnancy rate Show forest plot | 3 | 1597 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.13] |
| 5.1 AI versus Clomiphene | 2 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.48 [0.14, 87.49] |
| 5.2 AI versus Clomiphene +rFSH and rFSH only | 1 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.44, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.95, 2.02] |
| 2 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Clinical pregnancy rate Show forest plot | 5 | 774 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.94, 1.74] |
| 3.1 AI versus LOD | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.93, 1.83] |
| 3.2 AI + metformin versus LOD | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.60, 2.39] |
| 4 Miscarriage rate by woman randomised Show forest plot | 5 | 774 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.70] |
| 4.1 AI versus LOD | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.63] |
| 4.2 AI + metformin versus LOD | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.29, 6.27] |
| 5 Miscarriage rate by pregnancies Show forest plot | 5 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.43] |
| 5.1 AI versus LOD | 4 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.33] |
| 5.2 AI + metformin versus LOD | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.24, 6.09] |
| 6 Multiple pregnancy rate Show forest plot | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 74.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy rate Show forest plot | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.67] |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Multiple pregnancy rate Show forest plot | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy rate Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.43] |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Multiple pregnancy rate Show forest plot | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 105.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Five days compared to 10 days administration protocol of letrozole | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy rate Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Letrozole day 3‐7 administratio versus day 5‐9 administration | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Five days compared to 10 days administration protocol of letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 5mg vs 7.5mg letrozole | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Miscarriage rate by woman randomised Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate by pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 5mg vs 7.5mg letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

