مایکوفنولات موفتیل در مقابل متوتروکسات برای پیشگیری از بیماری پیوند علیه میزبان در افراد دریافت کننده پیوند سلولهای بنیادی خونساز آلوژنیک
چکیده
پیشینه
پیوند سلولهای بنیادی خونساز آلوژنیک (allogeneic hematopoietic stem cell transplantation; allo‐HCT) با پیامدهای مثبتی برای افراد مبتلا به بیماریهای خونی مختلف همراه است؛ با این حال، عوارض و مرگومیر ناشی از بیماری حاد و متعاقبا بیماری مزمن پیوند علیه میزبان (chronic graft‐versus‐host disease; GVHD) یک چالش جدی برای کاربرد گستردهتر allo‐HCT به شمار میآید. متوتروکسات (methotrexate) داخل وریدی در ترکیب با یک مهارکننده کلسینورین (calcineurin inhibitor)، سیکلوسپورین (cyclosporine) یا تاکرولیموس (tacrolimus)، یک رژیم پرکاربرد برای پیشگیری از GVHD حاد است، اما تجویز متوتروکسات با تعدادی از عوارض جانبی همراه است. مایکوفنولات موفتیل (mycophenolate mofetil)، در ترکیب با یک مهار کننده کلسینورین، بهطور گستردهای در افراد تحت allo‐HCT استفاده شده است. هنگام مقایسه رژیمهای مبتنی بر مایکوفنولات موفتیل با رژیمهای مبتنی بر متوتروکسات برای پیشگیری از GVHD حاد، نتایج متناقضی در مورد پیامدهای بالینی مختلف به دنبال allo‐HCT مشاهده شده است.
اهداف
هدف اولیه: ارزیابی تاثیر مایکوفنولات موفتیل در مقابل متوتروکسات برای پیشگیری از GVHD حاد در افرادی که تحت allo‐HCT قرار میگیرند.
اهداف ثانویه: ارزیابی تاثیر مایکوفنولات موفتیل در مقابل متوتروکسات برای بقای کلی (overall survival; OS)، پیشگیری از GVHD مزمن، بروز عود، آسیبهای مربوط به درمان، مرگومیر بدون عود، و کیفیت زندگی.
روشهای جستوجو
پایگاه مرکزی ثبت کارآزماییهای کنترلشده کاکرین (CENTRAL) و MEDLINE را از ابتدا تا مارچ 2014 جستوجو کردیم. چکیدههای کنفرانس را از دو نشست علمی گذشته (2011 و 2012) مجامع علمی مرتبط در این زمینه به صورت دستی جستوجو کردیم. ما ClinicalTrials.gov، بانک اطلاعاتی کارآزماییهای بالینی نوارتیس (www.novctrd.com)، پایگاه ثبت پروتکل کارآزمایی بالینی روش (www.roche‐trials.com)، پایگاه ثبت کارآزماییهای بالینی استرالیایی نیوزیلند (ANZCTR)، و متارجیستر کارآزماییهای کنترلشده را برای یافتن کارآزماییهای در حال انجام جستوجو کردیم.
معیارهای انتخاب
دو نویسنده مرور بهطور مستقل از هم همه عناوین/چکیدهها را بررسی کرده و مقالات دارای متن کامل را برای گنجاندن انتخاب کردند. تمام منابعی را وارد کردیم که نتایج کارآزماییهای تصادفیسازی و کنترلشده (randomised controlled trials; RCTs) مایکوفنولات موفتیل را در مقابل متوتروکسات برای پیشگیری از GVHD میان افراد تحت allo‐HCT گزارش کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل دادههای مربوط به پیامدهای همه مطالعات را استخراج کرده و قبل از ورود و آنالیز دادهها، آنها را مقایسه کردند. نتایج را در قالب خطر نسبی (RR) و 95% فاصله اطمینان (CI) برای پیامدهای دو حالتی (dichotomous outcome) و نسبت خطر (HR) و CIs %95 برای پیامدهای زمان‐تا‐رویداد (time‐to‐event) بیان کردیم. تاثیرات مطالعه فردی را با استفاده از مدل اثرات تصادفی (random‐effects model) ادغام کردیم. تخمینهای کمتر از یک نشان میدهند که مایکوفنولات موفتیل نسبت به متوتروکسات ارجح است.
نتایج اصلی
سه کارآزمایی را شامل 177 شرکتکننده (174 شرکتکننده آنالیز شده) وارد کردیم. همه شرکتکنندگان در کارآزماییهای Keihl et al و Bolwell et al سیکلوسپورین دریافت کردند در حالی که همه شرکتکنندگان در کارآزمایی Perkins et al تاکرولیموس گرفتند. با این حال، نتایج بر اساس نوع مهارکننده کلسینورین مورد استفاده (سیکلوسپورین در مقابل تاکرولیموس) تفاوتی نداشتند. هیچ شواهدی مبنی بر وجود تفاوت میان مایکوفنولات موفتیل و متوتروکسات برای پیامدهای بروز GVHD حاد (RR: 1.25؛ 95% CI؛ 0.75 تا 2.09؛ P = 0.39، شواهد با کیفیت بسیار پائین)، بقای کلی (HR: 0.73؛ 95% CI؛ 0.45 تا 1.17؛ P = 0.19، شواهد با کیفیت پائین)، میانه (median) روزهای سپریشده تا پیوند نوتروفیل (HR: 0.77؛ 95% CI؛ 0.51 تا 1.17؛ P = 0.23، شواهد با کیفیت پائین)، بروز عود (RR: 0.84؛ 95% CI؛ 0.52 تا 1.38؛ P = 0.50، شواهد با کیفیت پائین)، مرگومیر بدون عود (RR: 1.21؛ 95% CI؛ 0.62 تا 2.36؛ P = 0.57، شواهد با کیفیت پائین)، و بروز GVHD مزمن (RR: 0.92؛ 95% CI؛ 0.65 تا 1.30؛ P = 0.62، شواهد با کیفیت پائین) وجود نداشت. شواهدی با کیفیت پائین وجود داشت مبنی بر اینکه مایکوفنولات موفتیل در مقایسه با متوتروکسات دوره پیوند پلاکت را بهبود بخشید (HR: 0.87؛ 95% CI؛ 0.81 تا 0.93؛ P < 0.0001، شواهد با کیفیت پائین). شواهدی با کیفیت پائین نشان داد که مایکوفنولات موفتیل در مقایسه با متوتروکسات منجر به کاهش بروز موکوزیت (mucositis) شدید (RR: 0.48؛ 95% CI؛ 0.32 تا 0.73؛ P = 0.0006، شواهد با کیفیت پائین)، استفاده از تغذیه تزریقی (RR: 0.48؛ 95% CI؛ 0.26 تا 0.91؛ P = 0.02، شواهد با کیفیت پائین)، و نیاز به دارو درمانی برای کنترل درد (RR: 0.76؛ 95% CI: 0.63 تا 0.91؛ P = 0.002، شواهد با کیفیت پائین) شد. ناهمگونی (heterogeneity) کلی در آنالیز به جز برای پیامد پیوند نوتروفیل تشخیص داده نشد. هیچ یک از مطالعات واردشده در مورد پیامدهای مرتبط باکیفیت زندگی گزارش ندادند. سطح کیفیت کلی شواهد، پائین بود.
نتیجهگیریهای نویسندگان
به نظر میرسد استفاده از مایکوفنولات موفتیل در مقایسه با متوتروکسات برای پیشگیری اولیه از GVHD با مشخصات مطلوبتری از نظر مسمومیت دارویی همراه باشد، بدون اینکه خطری در عود بیماری، مرگومیر مرتبط با پیوند، یا بقای کلی داشته باشد. تاثیرات مداخله روی بروز GVHD میان افرادی که مایکوفنولات موفتیل دریافت کردند در مقایسه با متوتروکسات، نامشخص بودند. برای تعیین استراتژی مطلوب پیشگیری از GVHD نیاز به انجام RCTهایی با کیفیت بالا وجود دارد. مطالعات آینده باید دیدگاه جامعی را از مزایای بالینی، از جمله معیارهای عوارض، بار (burden) نشانه بیماری، و استفاده از منابع مراقبت سلامت مرتبط با مداخلات، در نظر بگیرند.
PICO
خلاصه به زبان ساده
مایکوفنولات موفتیل در مقابل متوتروکسات برای پیشگیری از بیماری پیوند علیه میزبان پس از دریافت پیوند سلولهای بنیادی خونساز آلوژنیک
پیشینه
پیوند سلولهای بنیادی خونساز آلوژنیک روشی است که در آن بخشی از سلولهای بنیادی (سلولهایی که میتوانند به انواع سلولهای خونی تبدیل شوند) اهدا کننده سالم یا مغز استخوان برای انفوزیون داخل وریدی تهیه و آماده میشوند. سلولهای بنیادی خونساز از یک اهدا کننده سالم گرفته شده و به بیمار (گیرنده) پیوند داده میشود. افرادی که تحت پیوند سلولهای بنیادی خونساز آلوژنیک قرار میگیرند، با خطر ابتلا به بیماری پیوند علیه میزبان (graft‐versus‐host disease; GVHD) روبهرو هستند. GVHD زمانی حاصل میشود که سلولهای پیوند شده از اهدا کننده (گرافت) به سلولهای بدن گیرنده (میزبان) حمله میکنند زیرا بدن گیرنده را خارجی میدانند. مایکوفنولات موفتیل (mycophenolate mofetil) و متوتروکسات (methotrexate) دو دارویی هستند که اغلب برای سرکوب واکنش بدن انسان در برابر پیوند (پاسخ ایمنی) و پیشگیری از GVHD استفاده میشوند. ما یک مرور سیستماتیک را از سه کارآزمایی تصادفیسازی و کنترلشده (RCT، که مطالعات بالینی هستند که در آن افراد بهطور تصادفی در یکی از دو یا چند گروه درمانی قرار میگیرند) انجام دادیم که مایکوفنولات موفتیل را در مقابل متوتروکسات برای استفاده در پیشگیری از GVHD میان 174 شرکتکننده مقایسه کردند. برای یافتن مطالعات مربوطه در مارچ 2014 جستوجو کردیم.
ویژگیهای مطالعه
همه شرکتکنندگان در این RCTها دارویی را با هدف سرکوب پاسخ ایمنی (سیکلوسپورین یا تاکرولیموس) دریافت کردند. مطالعه Perkins و همکاران توسط منابع عمومی و صنعتی تامین مالی شد. مطالعه Kiehl و همکاران توسط منابع عمومی تامین شد. منبع بودجه برای مطالعه Bolwell و همکاران مشخص نشد.
نتایج کلیدی
نتایج ما هیچ تفاوت بالینی معنیداری را میان مایکوفنولات موفتیل و متوتروکسات بر طول بقا (survival)، بروز GVHD، عود بیماری، یا مرگ ناشی از درمان نشان نمیدهند. افراد درمانشده با مایکوفنولات موفتیل در مقایسه با افرادی که تحت درمان با متوتروکسات قرار گرفتند، زمان کوتاهتری برای ساخت پلاکتهای جدید (سلولهایی که به لخته شدن خون کمک میکنند) از سلولهای اهدا کننده داشتند. علاوه بر این، از نظر عوارض جانبی، افرادی که تحت درمان با مایکوفنولات موفتیل قرار گرفتند، کمتر با موکوزیت (التهاب غشاهای مخاطی) شدید، نیاز به تغذیه تزریقی (تغذیه از طریق ورید)، یا نیاز به داروهای ضددرد مواجه شدند.
هیچ یک از مطالعات واردشده هیچ داده مرتبط با کیفیت زندگی را گزارش نکردند.
بهطور خلاصه، مایکوفنولات موفتیل و متوتروکسات هر دو داروهای قابل قبولی برای پیشگیری از GVHD هستند؛ با این حال، به نظر میرسد مایکوفنولات موفتیل با بروز کمتری از عوارض مانند موکوزیت شدید و مراقبتهای حمایتی مرتبط، همراه باشد.
کیفیت شواهد
کیفیت کلی شواهد، پائین بود.
Authors' conclusions
Summary of findings
| Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Patient or population: people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Methotrexate | Mycophenolate mofetil | ||||
| Overall survival | HR 0.73 (0.45 to 1.17) | 129 | ⊕⊕⊝⊝ | ||
| Prevention of acute GVHD grade II to IV | Study population | RR 1.25 | 174 | ⊕⊝⊝⊝ | |
| 595 per 1000 | 744 per 1000 | ||||
| Moderate5 | |||||
| 368 per 1000 | 460 per 1000 | ||||
| Incidence of relapse | Study population | RR 0.84 | 129 | ⊕⊕⊝⊝ | |
| 348 per 1000 | 293 per 1000 | ||||
| Moderate5 | |||||
| 386 per 1000 | 324 per 1000 | ||||
| Nonrelapse mortality | Study population | RR 1.21 | 89 | ⊕⊕⊝⊝ | |
| 255 per 1000 | 309 per 1000 | ||||
| Moderate5 | |||||
| 255 per 1000 | 309 per 1000 | ||||
| Prevention of chronic GVHD | Study population | RR 0.92 | 129 | ⊕⊕⊝⊝ | |
| 500 per 1000 | 460 per 1000 | ||||
| Moderate5 | |||||
| 539 per 1000 | 496 per 1000 | ||||
| Incidence of severe mucositis | Study population | RR 0.48 | 174 | ⊕⊕⊝⊝ | |
| 557 per 1000 | 267 per 1000 | ||||
| Moderate5 | |||||
| 579 per 1000 | 278 per 1000 | ||||
| Use of total parenteral nutrition | Study population | RR 0.48 | 129 | ⊕⊕⊝⊝ | |
| 606 per 1000 | 291 per 1000 | ||||
| Moderate5 | |||||
| 614 per 1000 | 295 per 1000 | ||||
| Incidence of narcotic use for pain control | Study population | RR 0.76 | 129 | ⊕⊕⊝⊝ | |
| 909 per 1000 | 691 per 1000 | ||||
| Moderate5 | |||||
| 921 per 1000 | 700 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Only one of the included articles described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). The trials conducted by Kiehl et al. and Perkins et al. were open‐label trials. 5 Generated by GRADEpro software based on event rate in the control arm of included studies. | |||||
Background
Allogeneic hematopoietic stem cell transplantation (allo‐HCT) is associated with improved outcomes for people with various hematologic diseases (Bensinger 2006; Kharfan‐Dabaja 2012; Koreth 2009). Despite improved understanding of the pathophysiology of acute graft‐versus‐host disease (GVHD) and introduction of newer immunosuppressive agents, the morbidity and mortality resulting from acute and subsequently chronic GVHD pose a serious challenge to wider applicability of allo‐HCT (Ferrara 2009). Effects of allo‐HCT are particularly significant when we consider that an increasing number of allo‐HCTs are being performed in populations with known risk factors for development of acute or chronic GVHD (Flowers 2011; Kollman 2001). Specifically, more people are receiving unrelated donor hematopoietic stem cells, which are either human leukocyte antigen (HLA)‐matched or mismatched, and more older people who generally receive stem cell allografts from older siblings are undergoing allo‐HCT (Flowers 2011; Kollman 2001).
Description of the condition
Acute GVHD is a clinico‐pathologic syndrome that affects a significant proportion of allo‐HCT recipients. Approximately 35% to 50% of people undergoing allo‐HCT are expected to develop grade II to IV acute GVHD (Jacobsohn 2007). This syndrome is driven by alloreactive donor T cells that recognize disparate minor histocompatibility antigens. Organs targeted by acute GVHD are largely the skin, liver, and gastrointestinal tract. Diagnosis is made on a clinical basis, but confirmatory pathologic findings on tissue biopsy can help to confirm clinical diagnosis. Severity of the syndrome is associated with increased risk of mortality. Despite pharmacologic immune suppression prophylaxis, many people will still develop the syndrome and experience the attendant morbidity and mortality. The established primary therapy of high‐dose prednisone offers complete remission in 30% to 50% of cases; however, people with steroid‐refractory acute GVHD have poor long‐term survival (Pidala 2010).
While severe acute GVHD is a major source of early post‐allo‐HCT mortality, chronic GVHD constitutes a major threat in terms of late HCT‐associated morbidity, impaired quality of life, symptom burden, disability, and mortality. The majority of people alive beyond 100 days post‐HCT will develop chronic GVHD. In contrast to acute GVHD, chronic GVHD has protean manifestations, many of which have parallels to allied human immune‐mediated disorders. The most commonly involved organ sites are the skin, mouth, eyes, and liver. Following a 2005 National Institutes of Health (NIH) Consensus Conference, major changes were proposed to the diagnosis, classification, and severity scoring of the syndrome (Filipovich 2005). It is distinguished from acute GVHD by the diagnosis of chronic GVHD manifestations and is not based solely on the time from allo‐HCT. The previously used limited/extensive severity classification (Shulman 1980) has also been replaced by a scoring system that takes into account the number and severity of organs involved to produce a global score of mild, moderate, or severe.(Filipovich 2005)
Currently, the evidence supports high‐dose prednisone as primary therapy, but this has limited effectiveness, with most affected people requiring second‐line immune suppressive therapy to control the syndrome.
As survival rates associated with acute GVHD have increased over the past decades, so also have the costs associated with treatment (Svahn 2006). One review by Khera et al. found the costs of allo‐HCT to range from USD 96,000 to USD 204,000 in 2012 and multiple studies agree that major drivers of these costs are post‐transplantation complications such as acute GVHD (Khera 2012; Svahn 2012). Developing effective regimens for the prevention of both acute and chronic GVHD is of paramount importance due to the risk of morbidity and mortality associated with established GVHD and its adverse impact on patient symptom burden, functional ability, and quality of life.
Description of the intervention
As of 2014, no single acute GVHD prophylaxis regimen is considered the standard of care. Intravenous (IV) methotrexate in combination with a calcineurin inhibitor, cyclosporine or tacrolimus, is a widely used regimen for the prophylaxis of acute GVHD. However, administration of methotrexate is associated with a number of adverse events such as severe mucositis, delayed hematopoietic recovery, and organ toxicity (Bolwell 2004; Cutler 2005; Neumann 2005; Perkins 2010; Pinana 2010).
How the intervention might work
Mycophenolate mofetil is an ester prodrug of mycophenolic acid and a known inhibitor of inosine monophosphate dehydrogenase. By inhibition of de novo purine biosynthesis, mycophenolate mofetil selectively targets activated lymphocytes and suppresses the primary antibody response (Allison 2000). In canine models, investigators have established that stable mixed chimeras, organisms composed of a mixture of two or more genetically distinct cells, are achievable with administration of mycophenolate mofetil plus cyclosporine following a sublethal dose of total body irradiation and dog‐leukocyte antigen compatible marrow transplantation (Storb 1997; Yu 1998).
Mycophenolate mofetil has been useful in preventing graft rejection in the field of organ transplantation. Specifically, it is effective in reducing GVHD among people undergoing kidney transplant, and has been evaluated in people undergoing heart, lung, and liver transplants (Knight 2009; Schmeding 2011; Zuk 2009). In addition, mycophenolate mofetil, in combination with a calcineurin inhibitor, has been used extensively in people undergoing allo‐HCT. Several observational studies have evaluated the combination of mycophenolate mofetil with a calcineurin inhibitor as a possible alternative to methotrexate and shown this combination to be well tolerated in both nonmyeloablative and ablative settings with acceptable rates of GVHD (McSweeney 2003; Nieto 2006; Osunkwo 2004).
Why it is important to do this review
Preference for a particular regimen, mycophenolate mofetil or methotrexate, for acute GVHD prophylaxis is largely based on uncontrolled, observational studies, and physician or transplant center preference. Conflicting results regarding various clinical outcomes following allo‐HCT have been observed when comparing mycophenolate mofetil‐based regimens against methotrexate‐based regimens for acute GVHD prophylaxis. These comparisons are further limited by the heterogeneity of participant, disease, and treatment‐related characteristics among studies. Heterogeneity is also introduced by donor and cell source, ablative intensity of preparative regimens, and dosing and schema of administration of acute GVHD prophylaxis agents. With an increasing number of allo‐HCTs being performed in people at high risk of developing acute GVHD, we believe it is important to evaluate the comparative efficacy of the two commonly used prophylactic agents, methotrexate versus mycophenolate mofetil, in the prevention of acute GVHD.
Objectives
Primary objective: to assess the effect of mycophenolate mofetil versus methotrexate for the prevention of acute GVHD in people undergoing allo‐HCT.
Secondary objectives: to evaluate the effect of mycophenolate mofetil versus methotrexate for overall survival, prevention of chronic GVHD, incidence of relapse, treatment‐related harms, nonrelapse mortality, and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We considered all prospective, randomized controlled trials (RCTs) of mycophenolate mofetil versus methotrexate utilizing a parallel study design for inclusion in this systematic review. We excluded all other study designs.
Types of participants
We included studies that enrolled participants who were at risk of developing GVHD as a result of undergoing allo‐HCT. We excluded studies that enrolled participants with an existing diagnosis of acute or chronic GVHD. We applied no restrictions on participant gender, ethnic group, or age. We described the disease type and stage of the included participants.
Types of interventions
Included studies reported on the direct comparison of any mycophenolate mofetil‐based regimen versus any methotrexate‐based regimen administered as prophylaxis for acute GVHD in people undergoing allo‐HCT. Specifically, we considered a regimen to be used as prophylaxis for acute GVHD if 1. the investigators specifically stated mycophenolate mofetil or methotrexate was used as prophylaxis for acute GVHD or 2. if the study inclusion/exclusion criteria excluded people with an existing diagnosis of acute or chronic GVHD. Supportive care and other GVHD prophylaxis/therapies, if any, were similar in both arms. In addition, since mycophenolate mofetil and methotrexate were commonly administered in combination with a calcineurin inhibitor (e.g. cyclosporine or tacrolimus), we included all regimens containing mycophenolate mofetil versus all regimens containing methotrexate, regardless of co‐therapies, in the review.
Types of outcome measures
Primary outcomes
-
Incidence of acute GVHD.
-
Overall survival.
Secondary outcomes
-
Engraftment kinetics evaluated as median days to neutrophil engraftment and median days to platelet engraftment.
-
Incidence of relapse.
-
Incidence of non‐relapse mortality (any death occurring without disease relapse/recurrence).
-
Incidence of chronic GVHD.
-
Quality of life (if measured using a validated tool for the assessment of quality of life).
-
Any grade III or IV adverse events of treatment.
-
Pain evaluated by incidence of narcotic use for pain control.
Search methods for identification of studies
Electronic searches
We conducted an electronic search of Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) using the search strategy in Appendix 1 and MEDLINE using search strategy in Appendix 2 from inception to 17 March 2014. We applied no date or language limits.
Searching other resources
In order to identify any recently completed studies that had not been published in full, we searched conference abstracts from the last two meetings (2011 and 2012) of the American Society of Clinical Oncology (ASCO), American Society of Hematology (ASH), European Group of Blood and Marrow Transplantation (EBMT), and BMT tandem meetings of the American Society of Blood and Marrow Transplantation (ASABM), Center for International Blood and Marrow Transplant Research (CIBMTR), and European Hematology Association (EHA). We also handsearched references of all identified review articles and included studies. In order to identify unpublished or ongoing studies, we searched ClinicalTrials.gov (www.clinicaltrials.gov/), Novartis Clinical Trial Results Database (www.novctrd.com), Roche Clinical Trial Protocol Registry (www.roche‐trials.com), Australian New Zealand Clinical Trials Registry (ANZCTR), and the metaRegister of Controlled Trials.
Data collection and analysis
Selection of studies
Two review authors (RM and TR) reviewed all titles, abstracts, and full‐text reports independently. We included studies that met the following criteria in the review.
-
Prospective clinical trial.
-
Parallel study design.
-
Participants randomized to prophylaxis with mycophenolate mofetil versus methotrexate.
-
Participants undergoing allo‐HCT.
We matched references on author names, location and setting, specific intervention details, and participants to avoid inclusion of duplicate publications. We resolved any disagreements between review authors during the study selection by consensus with a third review author (MAK‐D or AK).
Data extraction and management
Two review authors independently extracted data according to Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions using a standardized data extraction form containing the following items (Higgins 2011):
-
general information: study title, authors, source;
-
study characteristics: study design, setting, duration of follow‐up;
-
participant characteristics: number of participants enrolled, number of participants included in the analysis, specific disease diagnosis, donor status (related or unrelated donor), HLA‐mismatch, participant age;
-
interventions: name, dose, route, administration schedule, and associated therapies;
-
outcomes: incidence of acute GVHD (grades II to IV and III to IV GVHD), overall survival, median days to neutrophil engraftment, median days to platelet engraftment, incidence of relapse, incidence of chronic GVHD, grade III or IV adverse events, nonrelapse mortality (any death occurring without disease relapse/recurrence), pain;
-
risk of bias.
For studies that had multiple publications, we used the publication with longest follow‐up for extracting data on outcomes. We used earlier publications to extract data on methodology and baseline characteristics. In cases where the method of analysis was not specified by the investigators and only the number of events was reported, we used the number randomized as the denominator. That is, we recorded results according to intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
Two review authors (RM and TR) independently assessed the risk of bias in the included studies using The Cochrane Collaboration's tool for assessing the risk of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions based on extracted information (Higgins 2011a). A third author (MAK‐D or AK) resolved any disagreements between the two review authors. In addition to risk of bias, we evaluated the risk of random error by extracting data on the investigator's predetermined effect difference, alpha, power, and sample size.
Specifically, for assessment of risk of bias, we graded each component of methodologic quality as low, high, or unclear. We evaluated selection bias by assessing the investigators' description of method of randomization and allocation concealment. The method of randomization was:
-
low risk if the investigators described a random component in the sequence generation process (i.e. refer to a random number table, use a computer random number generator, coin toss);
-
high risk if the investigators described a nonrandom component in the sequence generation process (sequence generated by odd or even date of birth, some rule based on date (or day) of admission, or some rule based on hospital or clinic record number); and
-
unclear risk if there was insufficient information about the sequence generation process to permit judgment of 'low risk' or 'high risk'.
We considered allocation concealment to be:
-
low risk if participants and investigators enrolling participants could not foresee assignment (i.e. use of central allocation, sequentially numbered identical drug containers or sequentially numbered, opaque, sealed envelopes);
-
high risk if participants or investigators enrolling participants could possibly foresee assignments (allocation based on date of birth, case record number, using an open random allocation schedule); and
-
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We evaluated performance bias by assessing the investigators' description of blinding of participants and investigators. Performance bias was:
-
low risk if no blinding was used, but the outcome was not likely to be influenced by lack of blinding or participants and key study personnel were blinded;
-
high risk if no blinding or incomplete blinding was used, and the outcome was likely to be influenced by lack of blinding; and
-
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We judged detection bias due to knowledge of the allocated interventions by outcome assessors to be:
-
low risk if no blinding of outcome assessment was used, but the outcome measurement was not likely to be influenced by lack of blinding or blinding of outcome assessment was ensured;
-
high risk if there was no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; and
-
unclear risk if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
We judged attrition bias due to the amount, nature, or handling of incomplete outcome data to be:
-
low risk if there were no missing outcome data, reasons for missing outcome data were unlikely to be related to true outcome, or missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups;
-
high risk if the reasons for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate, or uses 'as‐treated' analysis with substantial departure of the intervention received from that assigned at randomization or uses potentially inappropriate application of simple imputation;
-
unclear risk if there was insufficient reporting of attrition/exclusions to permit judgment of 'low risk' or 'high risk' (e.g. number randomized not stated, no reasons for missing data provided).
We considered reporting bias due to selective outcome reporting to be:
-
low risk if the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review had been reported in the prespecified way or the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified;
-
high risk if the study's prespecified primary outcomes had not been reported, primary outcomes were reported using measurements that were not prespecified, primary outcomes were not prespecified, outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis, or the study report did not include results for a key outcome that would be expected to have been reported for such a study; and
-
unclear if there was insufficient information to permit judgment of 'low risk' or 'high risk'.
For the evaluation of risk or random error, we captured whether investigators report predetermined effect difference, alpha, power, and sample size calculation (yes/no and reported values) and if they were able to enroll the prespecified number of participants (prespecified sample size versus total number enrolled per arm).
Measures of treatment effect
Dichotomous data
We summarized dichotomous data (i.e. incidence of acute/chronic GVHD, incidence of relapse, nonrelapse mortality, adverse events, narcotic use) using risk ratio (RR) pooled using the random‐effects model and reported with 95% confidence intervals (CI).
Time‐to‐event data
In cases of time‐to‐event data (i.e. overall survival and days to neutrophil/platelet engraftment), for each included study we calculated the observed minus expected events (O minus E) and variance from the reported time‐to‐event estimates to obtain the log hazard ratio (LnHR) and standard error (SE) of LnHR for imputation using Review Manager 5 (RevMan 2011). In cases where time‐to‐event estimates were not reported, we extracted data from papers using the methods described by Tierney et al. (Tierney 2007). This method allowed calculation of the hazard ratio (HR) from different parameters using indirect calculation of the variance and the number of O minus E events. We pooled time‐to‐event estimates using the random‐effects model and reported with 95% CI using the generic inverse variance method.
Unit of analysis issues
The unit of analysis for this review was individual study. In the case of repeated follow‐up (e.g. reporting of survival at three and six months), we used the longest follow‐up from each study. We treated recurring events (e.g. adverse events) as a single event occurring in one participant (e.g. we considered four instances of nausea in one participant as one participant with nausea). In the case of multiple intervention arms, we combined arms together to create a single pair‐wise comparison.
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), in the case of missing outcome data, we attempted to contact the principal investigator or corresponding author (or both) of the study. If the corresponding author was unable to provide the missing data for an outcome, we included the study in the systematic review but excluded it from the meta‐analysis for the outcome with missing data. We undertook no imputation of missing individual participant data.
Assessment of heterogeneity
To evaluate heterogeneity between pooled studies, we calculated the Chi2 and I2 statistics (Deeks 2011). We considered an I2 statistic > 50% to indicate substantial heterogeneity or a Chi2 test with a significance level at P value < 0.1 to indicate statistically significant heterogeneity.
Assessment of reporting biases
We planned to assess publication bias using a funnel plot if more than 10 studies were included in the review (Egger 1997; Sterne 2011). We evaluated selective reporting of outcomes within studies by comparing outcomes reported with outcomes specified in protocols, when available.
Data synthesis
We performed pooled analysis using Review Manager 5 (RevMan 2011). We employed a random‐effects model using the DerSimonian‐Laird approach to pool studies for all analyses (DerSimonian 1986).
We constructed a 'Summary of findings' table using the most clinically and participant‐relevant outcomes (Guyatt 2011). These outcomes included: overall survival, incidence of relapse, incidence of grade II to IV acute GVHD, incidence of chronic GVHD, nonrelapse mortality, and incidence of any grade III to IV adverse events. In addition, we evaluated and reported the quality of evidence for each outcome according to GRADE guidelines (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e).
Subgroup analysis and investigation of heterogeneity
Originally we planned to conduct subgroup analyses on prognostically relevant factors including gender, age (adult versus child), disease stage, previous treatment, differences in therapy regimen (co‐therapies), remission status prior to conditioning (complete remission), and type of donor (sibling versus unrelated). However, due to the small number of studies identified and lack of reporting by subgroup, we did not perform any subgroup analyses.
Sensitivity analysis
Originally we planned to conduct a sensitivity analysis on all aspects of methodological quality. Due to the small number of studies identified by this systematic review, we have not performed any sensitivity analyses proposed in the protocol.
Results
Description of studies
Results of the search
The electronic search retrieved 263 references and the abstract search retrieved 609 references. In all, we screened 841 unique references by title and abstract. Of these, we selected 25 references for full‐text review. Three studies met the inclusion criteria. Selection flow diagram and reasons for exclusion are provided in Figure 1.
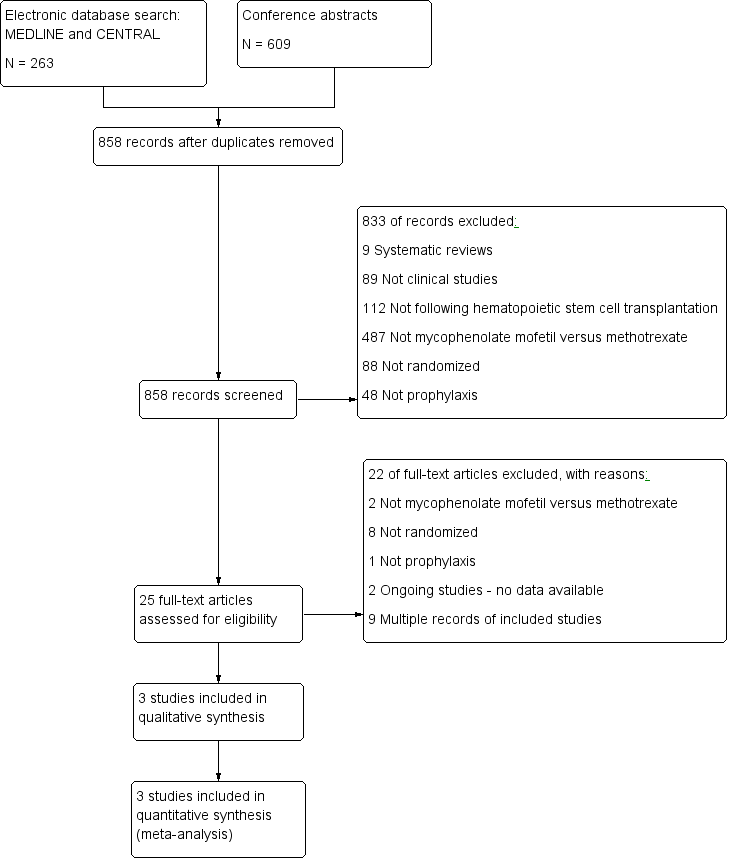
Study flow diagram.
Included studies
The review includes three studies (see Characteristics of included studies) (Bolwell 2004; Kiehl 2002; Perkins 2010).
The study by Kiehl et al. was a multicenter RCT comparing GVHD prophylaxis among three treatment arms; mycophenolate mofetil twice daily at 1 g per dose IV for the duration of the study; mycophenolate mofetil twice daily at 1.5 g per dose IV for the duration of the study; and methotrexate on days one, three, and six (total dose on day one was 15 mg IV, and on days three and six was 10 mg IV for the duration of the study). In addition to randomized treatment, all participants received cyclosporine and prednisolone supportive care. Cyclosporine was begun on day +1 and adjusted to trough plasma levels of 250 to 300 ng/L. At the time of publication of interim findings, 45 participants were randomized to receive either the methotrexate‐containing regimen or mycophenolate mofetil in a dosage of 1 g twice daily or 1.5 g twice daily IV. Eligible participants had received stem cells from a mismatched related or a mismatched or a matched unrelated donor. (Kiehl 2002). The results of the two mycophenolate mofetil arms in this study were pooled for this review.
In the study by Bolwell et al., participants were prospectively randomized 1:1 to receive either cyclosporine plus mycophenolate mofetil or cyclosporine plus methotrexate for GVHD prophylaxis. All participants were required to have a 6/6 HLA‐matched related donor. All donors were required to undergo a bone marrow harvest. Study end points included the incidence of acute GVHD, severity of mucositis, time to engraftment of neutrophils and platelets, and 100‐day survival. In this study, 21 participants received mycophenolate mofetil and 19 participants received methotrexate (Bolwell 2004).
The study by Perkins et al. was a single‐center, randomized phase II trial comparing tacrolimus plus mycophenolate mofetil versus tacrolimus plus methotrexate. ITT analysis included 42 participants randomized to tacrolimus plus mycophenolate mofetil and 47 participants randomized to tacrolimus plus methotrexate (Perkins 2010).
In summary, two RCTs used cyclosporine as the calcineurin inhibitor (Bolwell 2004; Kiehl 2002), and one RCT used tacrolimus as the calcineurin inhibitor (Perkins 2010). The results did not differ based on the type of the calcineurin inhibitor. See Characteristics of included studies table.
Excluded studies
We excluded four observational studies that reported data on mycophenolate mofetil versus methotrexate because they were not RCTs (Neumann 2005; Ostronoff 2009; Piñana 2010; Wang 2002). These four studies were included in the eight non‐randomized trials excluded at the full‐text manuscript phase. We have chosen to specifically report these four since they resemble our included studies more closely than the rest of the excluded studies. See Characteristics of excluded studies table.
Risk of bias in included studies
Results of risk of bias assessment are presented in Figure 2.
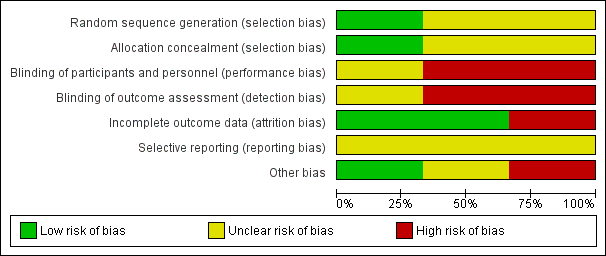
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Only one of the included RCTs described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). We judged the potential risk for selection bias as unclear.
Blinding
The trials conducted by Kiehl et al. and Perkins et al. were open label trials (Kiehl 2002; Perkins 2010). We were unable to determine whether the trial conducted by Bolwell et al. used blinding (Bolwell 2004). We judged the potential risk for detection and performance bias as high.
Incomplete outcome data
An ITT analysis was performed in all trials (Bolwell 2004; Kiehl 2002; Perkins 2010). The trial by Bolwell et al. had less than 50% of planned sample size accrual (Bolwell 2004). We judged the potential risk for attrition bias as low.
Selective reporting
The trial conducted by Perkins et al. reported all major outcomes (Perkins 2010). However, since we did not have access to the trial protocol(s), we could not investigate the potential for selective reporting bias based only on trial publications. We judged the potential risk for selection bias as unclear.
Other potential sources of bias
A sample size was pre‐planned in two trials (Bolwell 2004; Perkins 2010), but the planned number was not reached in one trial (Bolwell 2004). Since the trial by Kiehl et al. was published as an abstract, data regarding a priori sample size calculation, and alpha and beta error were not reported (Kiehl 2002). We judged the potential risk for other sources bias as low.
Effects of interventions
We included three trials with 174 participants in the analysis. Ninety‐five participants were randomized to the mycophenolate mofetil group and 79 participants to the methotrexate group. The effects of mycophenolate mofetil versus methotrexate are summarized in summary of findings Table for the main comparison.
Benefits
Prevention of acute graft‐versus‐host disease grade II to IV
Data on incidence of acute GVHD grade II to IV were extractable from three RCTs (three comparisons, 174 participants) (Bolwell 2004; Kiehl 2002; Perkins 2010). The pooled analysis found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on prevention of grade II to IV acute GVHD (RR 1.25; 95% CI 0.75 to 2.09; P value = 0.39) (Analysis 1.1). Substantial heterogeneity was detected in the analysis (P value = 0.12, I2 = 52%).
Overall survival
Data on overall survival could be extracted from two trials with 129 participants (Bolwell 2004; Perkins 2010). The meta‐analysis found no statistically significant benefit favoring mycophenolate mofetil use (HR 0.73; 95% CI 0.45 to 1.17; P value = 0.19) (Analysis 1.2). No heterogeneity was detected in the analysis (P value = 0.60, I2 = 0%).
Time to neutrophil engraftment
Data on median time to neutrophil engraftment were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no significant difference in the median time to neutrophil engraftment between the mycophenolate mofetil group and the methotrexate group (HR 0.77; 95% CI 0.51 to 1.17; P value = 0.23) (Analysis 1.3). Substantial heterogeneity was detected in the analysis (P value = 0.008, I2 = 86%).
Time to platelet engraftment
Data on median time to platelet engraftment were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found a statistically significant benefit in time to platelet engraftment in the mycophenolate mofetil group compared with the methotrexate group (HR 0.87; 95% CI 0.81 to 0.93; P value < 0.0001) (Analysis 1.4). No heterogeneity was detected in the analysis (P value = 0.43, I2 = 0%).
Incidence of relapse
Data on incidence of relapse were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no benefit with use of mycophenolate mofetil versus methotrexate on incidence of relapse (RR 0.84; 95% CI 0.52 to 1.38; P value = 0.50) (Analysis 1.5). No heterogeneity was detected in the analysis (P value = 0.86, I2 = 0%).
Nonrelapse mortality
Data on non‐relapse mortality were extractable from one RCT enrolling 89 participants (Perkins 2010). The data from this RCT found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on nonrelapse mortality (RR 1.21; 95% CI 0.62 to 2.36; P value = 0.57) (Analysis 1.6).
Prevention of chronic graft‐versus‐host disease
Data on incidence of chronic GVHD were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis found no statistically significant benefit with use of mycophenolate mofetil versus methotrexate on prevention of chronic GVHD (RR 0.92; 95% CI 0.65 to 1.30; P value = 0.62) (Analysis 1.7). No heterogeneity was detected in the analysis (P value = 0.69, I2 =0%).
Quality of life
None of the included studies reported any data related to quality of life.
Harms
Incidence of severe mucositis
Data on incidence of severe mucositis were extractable from three RCTs (three comparisons, 174 participants) (Bolwell 2004; Kiehl 2002; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate in reduced incidence of severe mucositis (RR 0.48; 95% CI 0.32 to 0.73; P value = 0.0006) (Analysis 1.8). Low heterogeneity was detected in the analysis (P value = 0.33, I2 = 10%).
Use of total parenteral nutrition
Data on use of total parenteral nutrition were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate suggesting decreased need of total parenteral nutrition with mycophenolate mofetil use (RR 0.48; 95% CI 0.26 to 0.91; P value = 0.02) (Analysis 1.9). Moderate heterogeneity was detected in the analysis (P value = 0.20, I2 = 40%).
Incidence of narcotic use for pain control
Data on incidence of narcotic use for pain control were extractable from two RCTs enrolling 129 participants (Bolwell 2004; Perkins 2010). The pooled analysis showed a statistically significant benefit with use of mycophenolate mofetil versus methotrexate suggesting a lower narcotic use for pain control with the use of mycophenolate mofetil (RR 0.76; 95% CI 0.63 to 0.91; P value = 0.002) (Analysis 1.10). No heterogeneity was detected in the analysis (P value = 0.53, I2 = 0%).
Discussion
Summary of main results
The use of mycophenolate mofetil for acute GVHD prophylaxis appears to result in significantly faster platelet engraftment and a lower incidence of severe mucositis compared with methotrexate (summary of findings Table for the main comparison). As a result, hematopoietic allograft recipients receiving mycophenolate mofetil for acute GVHD prophylaxis are likely to require less total parenteral nutrition or narcotics for pain control compared with people treated with methotrexate. This might translate into lower rates of complications that result from use of total parenteral nutrition or narcotics with the use of mycophenolate mofetil instead of methotrexate and, in turn, could reduce the length of hospital stay. However, we did not detect a statistically significant difference between mycophenolate mofetil and methotrexate in regards to risk of relapse, nonrelapse mortality, or overall survival based.
Overall completeness and applicability of evidence
Despite the fact that a better toxicity profile and faster engraftment are always desirable outcomes after allo‐HCT, the absence of a survival advantage or a favorable effect on prevention of acute or chronic GVHD or a lower transplant‐associated mortality with the use of mycophenolate mofetil limits our ability to recommend mycophenolate mofetil over methotrexate. In addition, we found a statistically significant heterogeneity for the outcome of time to neutrophil engraftment and since there were only two trials reporting this outcome, it was not possible to conduct subgroup or sensitivity analyses to explore the heterogeneity. The findings for this outcome need to be interpreted with caution. In addition, it is important to emphasize that the overall number of relevant trials and included participants is small. Accordingly, it is likely that a greater number of studies would be needed to have sufficient power to detect differences in certain outcomes. A major objective in future research will be to incorporate new trials in this analysis if they become available. We are not aware of any currently ongoing trials comparing mycophenolate mofetil versus methotrexate for the prevention of GVHD in people undergoing allo‐HCT. Nevertheless, there is significant diversity in current investigational approaches for prevention of GVHD. However, in our opinion it is highly unlikely that additional high‐quality trials comparing mycophenolate mofetil and methotrexate will be performed in the near future.
Quality of the evidence
We assessed the quality of the included trials according to the previously described quality domains, and these are represented in Figure 2 and Figure 3. Overall methodologic quality of included studies was either low or very low. Two included RCTs had a high risk of performance and detection bias. Two included RCTs reported analyses according to the principle of ITT. None of the included RCTs reported data on quality of life and hence we were not able to perform a meta‐analysis on this outcome. Overall, for majority of the outcomes the quality of evidence was low. For the outcome of prevention of acute GVHD grade II to IV the quality of evidence was very low.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Potential biases in the review process
We did not find any methodologic issues in the preparation of the review that could put it at risk for bias. We are confident that we have identified all eligible studies. We searched multiple electronic databases and conference proceedings. Once we had compiled the final list of included studies, we consulted content experts to ensure no unpublished studies were missed. We obtained all available data from included studies. We contacted study investigators in an attempt to obtain missing information. Two review authors performed study selection and data extraction. All included studies were RCTs. No subgroup analyses, planned in the protocol (see Methods section) were conducted due to lack of individual participant data. Due to the small number of included studies, we decided not to perform any sensitivity analyses.

Study flow diagram.
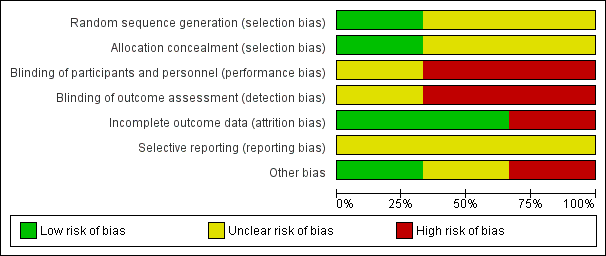
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
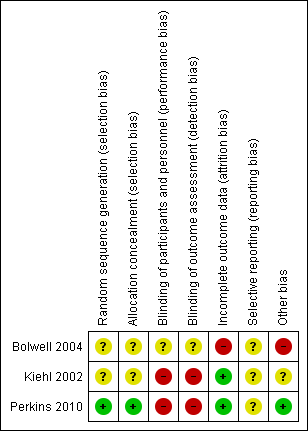
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
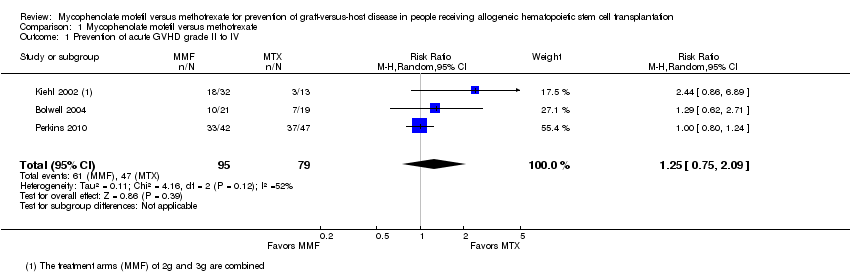
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 1 Prevention of acute GVHD grade II to IV.
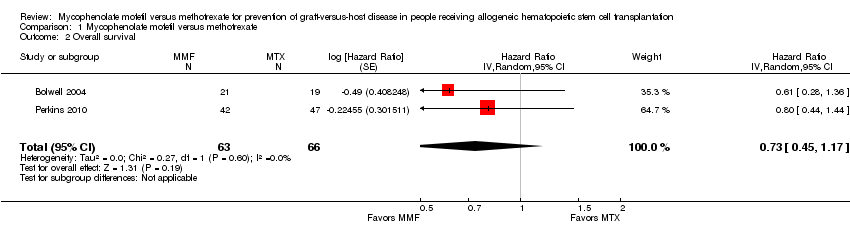
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 2 Overall survival.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 3 Time to neutrophil engraftment.
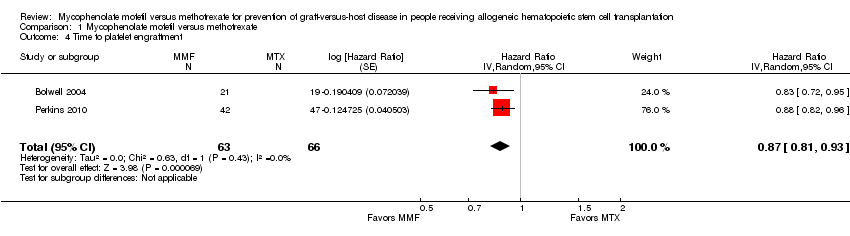
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 4 Time to platelet engraftment.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 5 Incidence of relapse.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 6 Nonrelapse mortality.
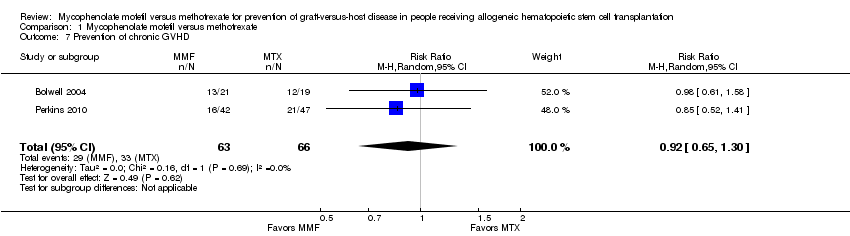
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 7 Prevention of chronic GVHD.
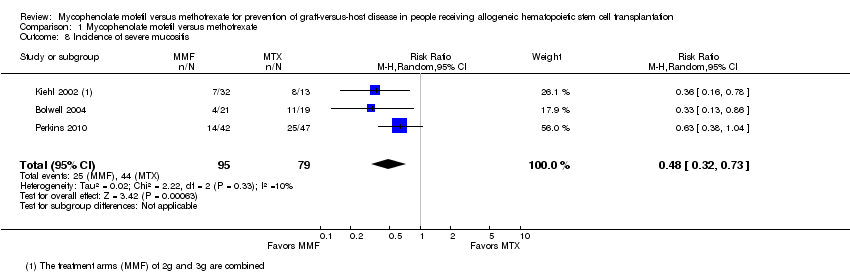
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 8 Incidence of severe mucositis.
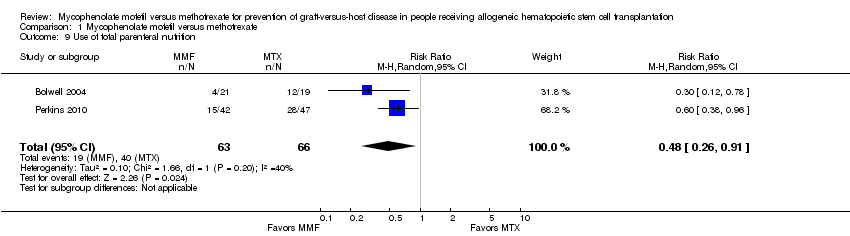
Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 9 Use of total parenteral nutrition.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 10 Incidence of narcotic use for pain control.
| Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Patient or population: people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Methotrexate | Mycophenolate mofetil | ||||
| Overall survival | HR 0.73 (0.45 to 1.17) | 129 | ⊕⊕⊝⊝ | ||
| Prevention of acute GVHD grade II to IV | Study population | RR 1.25 | 174 | ⊕⊝⊝⊝ | |
| 595 per 1000 | 744 per 1000 | ||||
| Moderate5 | |||||
| 368 per 1000 | 460 per 1000 | ||||
| Incidence of relapse | Study population | RR 0.84 | 129 | ⊕⊕⊝⊝ | |
| 348 per 1000 | 293 per 1000 | ||||
| Moderate5 | |||||
| 386 per 1000 | 324 per 1000 | ||||
| Nonrelapse mortality | Study population | RR 1.21 | 89 | ⊕⊕⊝⊝ | |
| 255 per 1000 | 309 per 1000 | ||||
| Moderate5 | |||||
| 255 per 1000 | 309 per 1000 | ||||
| Prevention of chronic GVHD | Study population | RR 0.92 | 129 | ⊕⊕⊝⊝ | |
| 500 per 1000 | 460 per 1000 | ||||
| Moderate5 | |||||
| 539 per 1000 | 496 per 1000 | ||||
| Incidence of severe mucositis | Study population | RR 0.48 | 174 | ⊕⊕⊝⊝ | |
| 557 per 1000 | 267 per 1000 | ||||
| Moderate5 | |||||
| 579 per 1000 | 278 per 1000 | ||||
| Use of total parenteral nutrition | Study population | RR 0.48 | 129 | ⊕⊕⊝⊝ | |
| 606 per 1000 | 291 per 1000 | ||||
| Moderate5 | |||||
| 614 per 1000 | 295 per 1000 | ||||
| Incidence of narcotic use for pain control | Study population | RR 0.76 | 129 | ⊕⊕⊝⊝ | |
| 909 per 1000 | 691 per 1000 | ||||
| Moderate5 | |||||
| 921 per 1000 | 700 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Only one of the included articles described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). The trials conducted by Kiehl et al. and Perkins et al. were open‐label trials. 5 Generated by GRADEpro software based on event rate in the control arm of included studies. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevention of acute GVHD grade II to IV Show forest plot | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.75, 2.09] |
| 2 Overall survival Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.73 [0.45, 1.17] |
| 3 Time to neutrophil engraftment Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.77 [0.51, 1.17] |
| 4 Time to platelet engraftment Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.87 [0.81, 0.93] |
| 5 Incidence of relapse Show forest plot | 2 | 129 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.52, 1.38] |
| 6 Nonrelapse mortality Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.62, 2.36] |
| 7 Prevention of chronic GVHD Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| 8 Incidence of severe mucositis Show forest plot | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.73] |
| 9 Use of total parenteral nutrition Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.91] |
| 10 Incidence of narcotic use for pain control Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |

