Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010280.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 25 July 2014see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Haematology Group
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Mohamed Kharfan‐Dabaja (MK‐D), Joseph Pidala (JAP), Janelle B Perkins (JBP), Benjamin Djulbegovic (BD), Ambuj Kumar (AK), and Rahul Mhaskar (RM) contributed to the initiation and design of this review.
Tea Reljic (TR) and RM conducted the search, study selection, and data extraction.
MK‐D, JAP, and AK resolved any disagreements during the conduct of the review.
MK‐D and AK performed a random data check prior to analysis.
TR and RM performed all analyses.
MK‐D, JAP, JBP, and BD contributed clinical expertise.
BD, RM, TR, and AK contributed statistical and methodological expertise.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
The review authors have no conflicts of interest to report.
Acknowledgements
We would like to thank the Cochrane Haematological Malignancies Group for critical reading of our protocol and review, and helpful feedback.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Jul 25 | Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation | Review | Mohamed Kharfan‐Dabaja, Rahul Mhaskar, Tea Reljic, Joseph Pidala, Janelle B Perkins, Benjamin Djulbegovic, Ambuj Kumar | |
| 2012 Dec 12 | Mycophenolate mofetil versus methotrexate for prevention of acute graft‐versus‐host disease in patients receiving allogeneic hematopoietic stem cell transplantation | Protocol | Mohamed Kharfan‐Dabaja, Rahul Mhaskar, Tea Reljic, Joseph Pidala, Janelle B Perkins, Benjamin Djulbegovic, Ambuj Kumar | |
Differences between protocol and review
Due to the small number of studies identified by this systematic review and the lack of data reported by subgroup, we have not performed any subgroup analyses or sensitivity analyses proposed in the protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Allografts;
- Calcineurin Inhibitors;
- Cyclosporine [therapeutic use];
- Graft vs Host Disease [mortality, *prevention & control];
- Hematopoietic Stem Cell Transplantation [*adverse effects];
- Immunosuppressive Agents [adverse effects, *therapeutic use];
- Methotrexate [adverse effects, *therapeutic use];
- Mycophenolic Acid [adverse effects, *analogs & derivatives, therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Tacrolimus [therapeutic use];
Medical Subject Headings Check Words
Humans;
PICOs
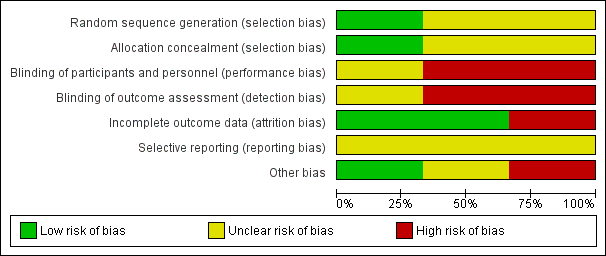
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 1 Prevention of acute GVHD grade II to IV.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 2 Overall survival.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 3 Time to neutrophil engraftment.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 4 Time to platelet engraftment.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 5 Incidence of relapse.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 6 Nonrelapse mortality.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 7 Prevention of chronic GVHD.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 8 Incidence of severe mucositis.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 9 Use of total parenteral nutrition.

Comparison 1 Mycophenolate mofetil versus methotrexate, Outcome 10 Incidence of narcotic use for pain control.
| Mycophenolate mofetil versus methotrexate for prevention of graft‐versus‐host disease in people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Patient or population: people receiving allogeneic hematopoietic stem cell transplantation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Methotrexate | Mycophenolate mofetil | ||||
| Overall survival | HR 0.73 (0.45 to 1.17) | 129 | ⊕⊕⊝⊝ | ||
| Prevention of acute GVHD grade II to IV | Study population | RR 1.25 | 174 | ⊕⊝⊝⊝ | |
| 595 per 1000 | 744 per 1000 | ||||
| Moderate5 | |||||
| 368 per 1000 | 460 per 1000 | ||||
| Incidence of relapse | Study population | RR 0.84 | 129 | ⊕⊕⊝⊝ | |
| 348 per 1000 | 293 per 1000 | ||||
| Moderate5 | |||||
| 386 per 1000 | 324 per 1000 | ||||
| Nonrelapse mortality | Study population | RR 1.21 | 89 | ⊕⊕⊝⊝ | |
| 255 per 1000 | 309 per 1000 | ||||
| Moderate5 | |||||
| 255 per 1000 | 309 per 1000 | ||||
| Prevention of chronic GVHD | Study population | RR 0.92 | 129 | ⊕⊕⊝⊝ | |
| 500 per 1000 | 460 per 1000 | ||||
| Moderate5 | |||||
| 539 per 1000 | 496 per 1000 | ||||
| Incidence of severe mucositis | Study population | RR 0.48 | 174 | ⊕⊕⊝⊝ | |
| 557 per 1000 | 267 per 1000 | ||||
| Moderate5 | |||||
| 579 per 1000 | 278 per 1000 | ||||
| Use of total parenteral nutrition | Study population | RR 0.48 | 129 | ⊕⊕⊝⊝ | |
| 606 per 1000 | 291 per 1000 | ||||
| Moderate5 | |||||
| 614 per 1000 | 295 per 1000 | ||||
| Incidence of narcotic use for pain control | Study population | RR 0.76 | 129 | ⊕⊕⊝⊝ | |
| 909 per 1000 | 691 per 1000 | ||||
| Moderate5 | |||||
| 921 per 1000 | 700 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Only one of the included articles described an adequate method of generation of randomization sequence and reported an adequate concealment of the sequence of allocation (Perkins 2010). The trials conducted by Kiehl et al. and Perkins et al. were open‐label trials. 5 Generated by GRADEpro software based on event rate in the control arm of included studies. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevention of acute GVHD grade II to IV Show forest plot | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.75, 2.09] |
| 2 Overall survival Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.73 [0.45, 1.17] |
| 3 Time to neutrophil engraftment Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.77 [0.51, 1.17] |
| 4 Time to platelet engraftment Show forest plot | 2 | 129 | Hazard Ratio (Random, 95% CI) | 0.87 [0.81, 0.93] |
| 5 Incidence of relapse Show forest plot | 2 | 129 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.52, 1.38] |
| 6 Nonrelapse mortality Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.62, 2.36] |
| 7 Prevention of chronic GVHD Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| 8 Incidence of severe mucositis Show forest plot | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.73] |
| 9 Use of total parenteral nutrition Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.91] |
| 10 Incidence of narcotic use for pain control Show forest plot | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |