Intervenciones para el abandono del consumo de tabaco en pacientes en tratamiento o recuperación de los trastornos por consumo de sustancias
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Country: Brazil Recruitment: alcohol‐dependent outpatient smokers enrolled at university treatment clinic Randomised controlled trial | |
| Participants | 103 male smokers aged 18 to 60 yr | |
| Interventions | Intervention: two arms combined; daily naltrexone (50 mg), 12 wk, or daily topiramate (dose escalating from 25 mg to 300 mg), 12 wk. (combined n = 65) Control: placebo, usual care (smoking behaviour monitored) (n = 38) | |
| Outcomes | Self‐reported abstinence at 12 wk Abstinence verification: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study, all participants received capsules of identical appearance manufactured in a different university division |
| Incomplete outcome data (attrition bias) | High risk | 45% of participants lost to follow‐up; authors reported statistically significant differences between dropout rates between placebo and topiramate groups Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: daily smokers enrolled at 4 residential alcohol treatment centres in central and western Nebraska Cluster randomised trial | |
| Participants | 90 smokers aged > 18 yr | |
| Interventions | Intervention: 10‐min counselling session based on trans‐theoretical model of readiness to change (n = 30) Control: usual care (n = 60) | |
| Outcomes | Self‐reported 7‐day abstinence at 1 and 6 months Abstinence verification: participants provided saliva COT samples by mail and a list of collateral contact references to verify use of alcohol, tobacco, and other drugs | |
| Notes | ICC not available; sensitivity analysis excluded this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomised: 4 treatment centres were blocked (2 treatment, 2 control), method not described |
| Allocation concealment (selection bias) | High risk | Intervention assignment determined by centre of residence |
| Blinding of participants and personnel (performance bias) | Low risk | Intervention and control conditions at different sites |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 3% in intervention and 13% in control group (not statistically significant), authors reported that respondents had completed more formal education than non‐respondents (12.4 yr vs 11.2 yr, P = 0.037) Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: smokers enrolled at 12 residential drug treatment centres in Iowa, Kansas, and Nebraska Cluster randomised trial | |
| Participants | 575 smokers aged > 18 yr | |
| Interventions | Intervention: 4 individualised 10‐ to 15‐min counselling sessions based on trans‐theoretical model of readiness to change (n = 288) Control: usual care (n = 287) | |
| Outcomes | Self‐reported 7‐day tobacco abstinence and 30‐day alcohol abstinence at 1, 6, and 12 months Abstinence verification: participants reporting tobacco abstinence provided saliva COT samples by mail, all participants provided a list of collateral contact references to verify use of alcohol, tobacco, and other drugs. 30% of respondents had references contacted for verification | |
| Notes | ICC not available; sensitivity analysis excluded this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomised: 12 treatment centres were paired based on state licensing authority assessment of comparability, sites within pairs randomised by coin‐toss; 2 centres declining to participate were replaced |
| Allocation concealment (selection bias) | High risk | Cluster randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Intervention and control conditions at different sites |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 22%, differences between intervention and control groups not reported Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: people aged > 18 yr enrolled in an urban recovery community organisation Randomised controlled trial | |
| Participants | 151 current cigarette smokers (> 100 lifetime, > 1 day for the past 7 days and > 10/week, expired carbon monoxide level ≥ 6 ppm) in recovery from addiction to alcohol and other drugs (self‐reported) | |
| Interventions | Intervention: 30‐min computerised brief motivational intervention (5‐A framework) + information/referral sheet, offer of NRT (n = 82) Control: information/referral sheet, offer of NRT (n = 69) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 4 and 6 wk Abstinence verification: breath carbon monoxide (< 8 ppm) at 4 wk | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated urn randomisation stratified by gender and cigarettes smoked/day |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 34% in treatment group and 38% in control group Participants lost to follow‐up were separately analysed as non‐abstinent and excluded |
| Methods | Country: USA Recruitment: male veterans enrolled in inpatient substance abuse treatment at a California medical centre Randomised controlled trial | |
| Participants | 39 smokers in residence for at least 1 month | |
| Interventions | Intervention: computer‐guided nicotine fading, daily 15‐min counselling, and self‐administered contingency contract (n = 19) Control: waiting list with usual care (n = 20) | |
| Outcomes | Tobacco abstinence: self‐report and carbon monoxide levels ≤ 8 ppm; other drugs: self‐reported 30‐day abstinence, follow‐up at 3 and 6 months Abstinence verification: carbon monoxide assessment of air samples (tobacco); other drugs: breath and urine sample for onsite follow‐ups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up reporting incomplete Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: drug and alcohol‐dependent smokers in residential rehabilitation programme for homeless veterans at a California medical centre Randomised controlled trial | |
| Participants | 200 daily smokers in residence for at least 1 month | |
| Interventions | Intervention 1: computer‐guided nicotine fading, daily 30‐ to 45‐min counselling, self‐administered contingency contract (smoking oriented) (n = 50) Intervention 2: computer‐guided nicotine fading, daily 30‐ to 45‐min counselling, self‐administered contingency contract (generalised from smoking to other drugs) (n = 50) Control 1: usual care (n = 50) Control 2: treatment refusers (n = 50, not included in meta‐analysis) | |
| Outcomes | Self‐reported 7‐day tobacco abstinence, self‐reported 30‐day abstinence from other drugs at 1, 3, 6, and 12 months after discharge Abstinence verification: breath and urine samples (cut‐off measures not reported) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up reporting incomplete (authors reported 80% to 90% follow‐up rate) Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: smokers enrolled in residential and outpatient programmes at a non‐profit substance abuse treatment agency in Oregon Randomised controlled trial | |
| Participants | 112 smokers | |
| Interventions | Intervention: 4 daily group counselling sessions followed by 15 weekly group counselling sessions, free transdermal nicotine patches, payment for participation and continued abstinence, individual counselling on request (n = 90) Control: waiting list with usual care (n = 21) | |
| Outcomes | Self‐reported abstinence at 1 day, 4 and 16 wk Abstinence verification: expired air carbon monoxide sample analysis < 10 ppm (tobacco) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | High risk | Wait list control |
| Blinding of participants and personnel (performance bias) | High risk | Wait list control |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up reporting incomplete (authors reported 17% follow‐up rate for treatment group) Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: alcohol‐dependent daily‐smoker veterans enrolled in drug and alcohol treatment programmes at 2 California medical centres Randomised controlled trial | |
| Participants | 162 smokers (≥ 5 cigarettes/day) abstinent from alcohol for ≥ 7 days, aged > 18 yr | |
| Interventions | Intervention: 16 sessions of individual CBT and combination NRT over 26 wk (n = 82) Control: usual care (referral to a free‐standing smoking cessation programme) (n = 80) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco and 30‐day abstinence alcohol at 12, 26, and 52 wk Abstinence verification: exhaled carbon monoxide sample analysis < 10 ppm (tobacco) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment list, stratified by number of cigarettes smoked/day, depression, and abuse of other drugs |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up was 24% in intervention group, 29% in control group, authors reported that missing data may not have been random Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: alcohol‐dependent daily smokers enrolled in substance abuse treatment outpatient programmes for veterans Randomised controlled trial | |
| Participants | 118 daily smokers (≥ 10 cigarettes/day) aged ≥ 18 yr who met DSM‐IV criteria for alcohol dependence in the prior 3 months | |
| Interventions | Intervention: 3 × 60‐min behavioural smoking cessation counselling sessions, 8 wk of transdermal nicotine patches (n = 44) Control: 15‐min advice intervention, 5‐min follow‐up (n = 47) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco and 30‐day abstinence from alcohol at 14 days, and 3 and 6 months Abstinence verification: breath carbon monoxide levels < 10 ppm (tobacco) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 20% overall, with attrition rates higher in the control group; early quit rates were comparable across both groups Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: people with current alcohol abuse or dependence recruited through university health clinics and radio/newspaper advertisements Randomised controlled trial | |
| Participants | 96 alcohol‐dependent daily smokers (≥ 15 cigarettes/day) aged ≥ 18 yr willing to attend outpatient treatment for substance abuse | |
| Interventions | Intervention: nicotine patch and nicotine gum plus usual care behavioural counselling for alcohol and smoking (16 sessions) (n = 45) Control: nicotine patch and placebo gum plus usual care behaviour counselling for alcohol and tobacco dependence (16 sessions) (n = 51) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco and 90‐day abstinence from alcohol at 2 wk, and 3, 6, and 12 months Abstinence verification: breath carbon monoxide levels < 10 ppm (tobacco), alcohol breathalyser reading = 0 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn‐randomised computer program that balanced groups by history of previous substance abuse, treatment, age, sex, baseline drinks/drinking day and baseline cigarettes/day |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded research design All participants were asked whether they believed they were in the treatment or control conditions; 80% reported "don't know", remaining 20% identified the gum's content with 50% accuracy |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 28% overall, with no between‐treatment differences in participants retained (P > 0.05) Participants lost to follow‐up had abstinence status independently verified |
| Methods | Country: USA Recruitment: people enrolled in an intensive 3‐wk outpatient alcohol treatment programme Randomised controlled trial | |
| Participants | 151 alcohol‐dependent smokers (alcohol use in past 30 days, 1+ cigarettes smoked/day, 3‐yr smoking history) | |
| Interventions | Intervention: 12 × 15‐min individual counselling treatment twice daily before and after substance abuse treatment days using centralised therapist supervision and progressive contingency management rewards, and 8 to 20 wk of combination NRT (patch + gum/lozenge) Control: smoking cessation treatment delayed until 3 months after enrolment in alcohol dependence treatment and 8 to 20 wk of combination NRT (patch + gum/lozenge) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at treatments and 2 and 13 wk Abstinence verification: breath carbon monoxide (< 5 ppm) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised urn‐randomisation stratified by cigarette craving, alcohol self‐efficacy, alcohol dependence, nicotine dependence, and gender at 2:1 treatment:control ratio |
| Allocation concealment (selection bias) | High risk | Waiting list control |
| Blinding of participants and personnel (performance bias) | High risk | Waiting list control |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 19% and comparable across treatment and control groups Participants lost to follow‐up were significantly younger than those retained; analysis assumed non‐abstinence |
| Methods | Country: USA Recruitment: people enrolled in inpatient substance abuse treatment at a veterans medical centre Randomised controlled trial | |
| Participants | 64 substance‐dependent daily smokers (≥ 10 cigarettes/day) | |
| Interventions | Intervention: 1 individual counselling session and encouragement to attend daily group session screening films addressing quitting, nicotine patch, referral to outside clinic on request (n = 34) Control: usual care (nicotine patch, referral to outside clinic on request) (n = 30) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco and 30‐day abstinence from tobacco and other drugs at 6 months Abstinence verification: breath carbon monoxide (< 9 ppm) and BAC (0.000 ppm) samples, urine samples (COT < 50 ng/mL), qualitative assessment by technician | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by primary substance type, method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 12% overall (excluding 2 deaths), differences between groups not reported Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: veterans enrolled in an outpatient substance abuse treatment programme Randomised controlled trial | |
| Participants | 40 alcohol‐dependent daily smokers (≥ 10 cigarettes/day) | |
| Interventions | Intervention: 5 × 30‐min weekly education and group therapy sessions addressing nicotine dependence followed by 60‐min group therapy session, carbon monoxide assessments, 8 weeks of NRT offered (gum or patch) (n = 20) Control: usual care (access to 1 educational session and NRT) (n = 20) | |
| Outcomes | Self‐reported abstinence from alcohol, tobacco, and other drugs at 1, 6, and 12 months Abstinence verification: 2 collateral informants contacted at 6‐month follow‐up for confirmation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 43% overall (55% in treatment group, 30% in control group) Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: people enrolled in outpatient alcohol treatment in community and veterans centre programmes Randomised controlled trial | |
| Participants | 58 alcohol‐dependent daily smokers | |
| Interventions | Intervention: nicotine patch and bupropion, smoking cessation lecture and group therapy session (n = 192) Control: nicotine patch, smoking cessation lecture and group therapy session (n = 191) | |
| Outcomes | Self‐reported abstinence from alcohol, tobacco, and other drugs at 4 and 9 wk, and 6 months Abstinence verification: 2 collateral informants contacted at 6‐month follow‐up for confirmation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 31% overall (40% in treatment group, 21% in control group) Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: people in community alcohol and drug treatment programmes recruited by news releases, advertisements, and notices Randomised controlled trial | |
| Participants | 110 daily smokers (≥ 20 cigarettes/day) aged ≥ 18 yr and abstinent from alcohol and other drugs at least 1 yr | |
| Interventions | Intervention: bupropion SF 150 mg/day for 3 days followed by 150 mg twice daily, < 10 min behavioural counselling per visit (n = 35) Control: placebo, < 10 min behavioural counselling per visit (n = 32) | |
| Outcomes | Self‐reported abstinence from alcohol, tobacco and other drugs at 1 wk, 3 and 6 months Abstinence verification: urine screening | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind reported, method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 34% in treatment group, 37% in control group, with no significant differences between groups Participants lost to follow‐up were excluded |
| Methods | Country: Iran Recruitment: men with opiate dependence referred to 1 of 4 urban drug abuse treatment centres to undergo methadone maintenance treatment Randomised controlled trial | |
| Participants | 424 men aged 15 to 88 yr with a history of drug abuse (opiates, hashish, other recreational drugs) for 1 yr and 1 yr habitual tobacco consumption (cigarettes or hookah) | |
| Interventions | Intervention: 6 wk step‐down NRT patches (30 mg, 20 mg, 10 mg) + supply of NRT gum/pills, behavioural therapy to aid in smoking cessation (5‐As) (n = 212) Control: behavioural therapy to aid in smoking cessation (5‐As) (n = 212) | |
| Outcomes | Self‐reported abstinence from tobacco and other drugs at 1 and 6 months Abstinence verification: breath carbon monoxide, rapid opiate test, thin‐layer chromatography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up in study |
| Methods | Country: USA Recruitment: smokers with a history of alcohol dependence recruited by advertisements, notices at outpatient clinics, and Alcoholics Anonymous meetings Randomised controlled trial | |
| Participants | 115 daily smokers (≥ 20 cigarettes/day) and abstinent from alcohol and other drugs for ≥ 30 days | |
| Interventions | Intervention: nicotine patch 21 mg (for 6 wk), reduced to 14 mg (for 2 wk), 7 mg (for 2 wk), placebo (for 2 wk), stop smoking booklet, 7 × 60‐min group therapy sessions, 3 × 15‐min individual sessions (n = 61) Control: placebo patch 12 weeks, stop smoking booklet, 7 × 60‐min group therapy sessions, 3 × 15‐min individual sessions (n = 54) | |
| Outcomes | Self‐reported abstinence at 16 wk, 6 months Abstinence verification: breath carbon monoxide reading < 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up 73% overall, differences between groups not reported Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: smokers in treatment for alcohol dependence or abuse in 3 centres (2 private, 1 VAMC) in Minneapolis‐St Paul area Randomised controlled trial | |
| Participants | 499 alcohol‐dependent daily smokers (≥ 5 cigarettes/day) aged 21 to 75 yr expressing interest in quitting (score > 2 on Contemplation Ladder) | |
| Interventions | Intervention: 60‐min individual counselling session, 3 follow‐up session, nicotine patches (21 mg/6 wk, 14 mg/2 wk, 7 mg/2 wk), reminders of treatment available on request every 3 months for following 12 months (n = 251) Control: usual care, 6‐month delayed enrolment to intervention (n = 248) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco, 30‐day abstinence from alcohol at 6, 12, and 18 months Abstinence verification: biochemical testing (expired carbon monoxide, BAC), collateral interviews, or both | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. stratified by substance use disorder treatment site and blocked within site in groups of 10 |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random sequence was concealed from study personnel; research assistants ready to enrol an eligible person consulted the study co‐ordinator, who obtained the treatment assignment from an independent office holding the master list |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up 22% in treatment group, 17% in control group Participants lost to follow‐up were assumed to be non‐abstinent |
| Methods | Country: USA Recruitment: smokers in inpatient treatment at a VAMC for alcohol dependence Randomised controlled trial | |
| Participants | 36 alcohol‐dependent male daily smokers (≥ 10 cigarettes/day) who expressed readiness to quit | |
| Interventions | Intervention: 3 × 45‐min individual smoking cessation counselling session, nicotine patches (n = 16) Control: 1 counselling session, nicotine patch delayed to 1 wk post‐discharge (n = 13) | |
| Outcomes | Self‐reported alcohol and tobacco abstinence at 12, 16, and 20 wk Abstinence verification: participants reporting abstinence returned to clinic for biochemical verification (carbon monoxide testing, COT analysis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Outcomes assessed by a research assistant blinded to study condition |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors reported loss to follow‐up was not significantly different between groups, no further discussion |
| Methods | Country: USA Recruitment: alcohol‐dependent smokers enrolled in residential and community substance abuse treatment programmes Randomised controlled trial | |
| Participants | 148 daily smokers (≥ 10 cigarettes/day) with a history of alcohol dependence/abuse abstinent from alcohol for 2 to 12 months | |
| Interventions | Intervention: bupropion 150 mg twice daily 7 wk, nicotine patch 7 wk (21 mg/4 wk, 14 mg/2 wk, 7 mg/1 wk), 8 weekly counselling sessions (n = 73) Control: placebo twice daily 7 wk, nicotine patch 7 wk (21 mg/4 wk, 14 mg/2 wk, 7 mg/1 wk), 8 weekly counselling sessions (n = 70) | |
| Outcomes | Self‐reported 7‐day tobacco abstinence 7, 11, and 24 wk Abstinence verification: biochemical testing (salivary COT < 15 mg/mL) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation stratified by gender, severity of nicotine dependence, depressive symptoms, substance use history |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Active and placebo tablets were identical in appearance |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up 40% in treatment group, 36% in control group Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers in treatment for alcohol‐dependence at a university outpatient addictions treatment programme Randomised controlled trial | |
| Participants | 11 alcohol‐dependent daily smokers (≥ 10 cigarettes/day) abstinent from alcohol between 6 wk and 6 months | |
| Interventions | Intervention: bupropion 150 mg daily 1 wk, twice daily 7 wk, smoking cessation booklet, 10‐min counselling session (n = 6) Control: placebo daily 1 wk, twice daily 7 wk, smoking cessation booklet, 10‐min counselling session (n = 5) | |
| Outcomes | Self‐reported tobacco abstinence at 4 and 8 wk Abstinence verification: expired carbon monoxide, BAC testing, urine drug screen, collateral informants contacted at follow‐up for confirmation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up reported |
| Methods | Country: USA Recruitment: smokers recruited through advertising directed to Alcoholics Anonymous programmes Randomised controlled trial | |
| Participants | 205 daily smokers (≥ 10 cigarettes/day) aged ≥ 18 yr with a history of alcohol dependence, ≥ 3 months' alcohol and other drug abstinence | |
| Interventions | Intervention 1: 8 wk 60‐ to 75‐min group behavioural counselling sessions, aerobic exercise prescription increasing from 15 min to 45 min (n = 73) Intervention 2: 8 wk behavioural counselling, nicotine gum 2 mg with advice to chew 1 to 6 pieces/day up to 6 months (n = 62) Control: 8 wk 60‐ to 75‐min group behavioural counselling sessions, American Lung Association 20‐day quit programme (n = 70) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 6 months and 1 yr Abstinence verification: expired carbon monoxide < 10 ppm, 1 collateral informant contacted at follow‐up if carbon monoxide data unavailable | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation into cohorts dependent on time of enrolment (6 consecutive cohorts in groups of 36) |
| Allocation concealment (selection bias) | High risk | No concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up rates not reported Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: opioid and nicotine‐dependent smokers enrolled in a veterans' outpatient substance abuse treatment programme Randomised controlled trial | |
| Participants | 40 opiate and nicotine‐dependent daily smokers (≥ 10 cigarettes/day) aged 18 to 65 yr Men and women opioid‐dependent smokers stabilised on buprenorphine 24 mg/day; 20 assigned to treatment and 20 assigned to control | |
| Interventions | Intervention: buprenorphine (increasing to 24 mg/day) and bupropion (150 mg daily for 3 days, 150 mg twice daily thereafter, last week taper) 12 wk, weekly 60‐min counselling sessions (n = 19) Control: buprenorphine (increasing to 24 mg/day) and placebo 12 wk, weekly 60‐min counselling sessions (n = 20) (Test of Bupropion (300 mg/day) versus placebo) | |
| Outcomes | Tobacco abstinence assessed 3 times weekly by expired carbon monoxide (< 10 ppm), other drug abstinence by urine assay for opiates < 200 ng/mL, benzoylecgonine < 300 ng/mL at weekly intervals over 12 wk | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An urn randomisation procedure was used to ensure balance distribution across race and sex |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Bupropion pills were over‐encapsulated to match placebo pills |
| Incomplete outcome data (attrition bias) | High risk | Overall loss to follow‐up was 42%, treatment group retention was significantly lower than control group retention P = 0.0241) Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: Switzerland Recruitment: people enrolled in a 21‐day inpatient alcohol detoxification treatment programme Randomised controlled trial | |
| Participants | 103 alcohol‐dependent smokers aged 18 to 65 yr with stay long enough to complete 10‐day treatment programme; excluded if using pharmacotherapy for smoking cessation | |
| Interventions | Intervention: 5 × 30‐min group CBT sessions focused on smoking cessation (CBT) (n = 53) Control: autogenic training (n = 50) | |
| Outcomes | Self‐reported 7‐day abstinence from alcohol and tobacco at end of intervention, 6 months Abstinence verification: breath carbon monoxide, urine sample | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 53% in intervention group, 34% in control group Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers interested in quitting enrolled in 1 of 3 urban methadone maintenance programs in New York City Randomised controlled trial | |
| Participants | 112 smokers (≥ 5 cigarettes/day) maintained on methadone for at least 3 months without psychiatric disorders, suicidal ideation, or recent suicide attempts English‐speaking with no psychiatric disorders | |
| Interventions | Intervention: 12 wk varenicline (1 mg) twice daily, with inpatient or telephone counselling (n = 57) Control: matched placebo, with inpatient or telephone counselling (n = 55) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 2, 4, 8, 12, and 24 wk Abstinence verification: breath carbon monoxide (< 8 ppm) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated and stratified by 3 clinic sites in blocks of 6 by stratum |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by central data manager using a password‐protected file; medication orders faxed to pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | All study participants and staff blinded to treatment condition; pharmacist compounded identical varenicline and placebo tablets |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 10% Participants lost to follow‐up were assumed to be non‐abstinent; sensitivity tests for differences conducted using Fisher's exact |
| Methods | Country: Spain Recruitment: smokers enrolled in a university outpatient alcohol dependence treatment clinic Randomised controlled trial | |
| Participants | 92 alcohol‐dependent daily smokers (≥ 5 cigarettes/day) aged 18 to 65 yr | |
| Interventions | Intervention: 10 × 30‐to 45‐min individual counselling sessions based on CBT, nicotine patch/gum/lozenge for 3 months (n = 51) Control: treatment delayed 6 months with usual care (n = 41) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 1, 2, 3, and 6 months Abstinence verification: expired carbon monoxide < 9 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | High risk | Waiting list control |
| Blinding of participants and personnel (performance bias) | High risk | Waiting list control |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 72.5% in treatment group, 61% in control group, difference between groups was not significant; authors reported treatment adherence unrelated to sociodemographic or baseline clinical data Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers recruited from the community through advertising directed to Alcoholics Anonymous programmes Randomised controlled trial | |
| Participants | 29 daily smokers (≥ 10 cigarettes/day) aged ≥ 18 yr with a history of alcohol dependence and major depression | |
| Interventions | Intervention: behavioural counselling + cognitive‐behavioural mood management, 8 weekly 120‐min group sessions (n = 13) Control: behavioural counselling, 8 weekly 120‐min group sessions (n = 16) | |
| Outcomes | Self‐reported abstinence from tobacco at 1, 3, and 12 months Abstinence verification: expired carbon monoxide, 2 collateral informants contacted at follow‐up for confirmation (3 and 12 months) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation into cohorts dependent on time of enrolment (consecutive cohorts in groups of 8) |
| Allocation concealment (selection bias) | High risk | No concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 55% in treatment group, 70% in control group Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers recruited from 7 methadone‐maintenance/drug and alcohol treatment programmes using person to person communication, flyers, clinical referrals Randomised controlled trial | |
| Participants | 225 daily smokers (≥ 10 cigarettes/day) with a history of drug/alcohol dependence enrolled in substance abuse treatment ≥ 30 days | |
| Interventions | Intervention: usual substance abuse treatment, smoking cessation treatment consisting of 8 weeks of group counselling and transdermal nicotine patches (21 mg/day in wk 1 to 6, 14 mg/day in wk 7 and 8) (n = 140) Control: usual substance abuse treatment (n = 68) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco, alcohol, and other drug use at 13 and 26 wk Abstinence verification: expired carbon monoxide < 10 ppm, urine drug screen, alcohol breathalyser test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated using permuted blocks of 6, stratified by site and sex |
| Allocation concealment (selection bias) | Low risk | A study statistician who had no other contact with site study staff, performed the randomisation and staff were blind as to stratification and block size strategies |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Low risk | Overall loss to follow‐up was 7%, no significant difference in time to dropout between groups or between methadone and non‐methadone study sites Participants lost to follow‐up were excluded |
| Methods | Country: USA Recruitment: smokers recruited from a state‐funded inner‐city residential substance abuse treatment programme Randomised controlled trial | |
| Participants | 165 alcohol‐dependent daily smokers (≥ 10 cigarettes/day for 6 months) recently admitted to a 45‐day residential alcohol dependence treatment centre | |
| Interventions | Intervention: motivational interviewing based therapy (with and without boosters) and free access to NRT, smoking cessation information (n = 80) Control: brief advice (with and without boosters) and free access to NRT, smoking cessation information (n = 85) | |
| Outcomes | Self‐reported alcohol, tobacco, and other drug use, carbon monoxide levels at 1, 3, 6, and 12 months Abstinence verification: breath carbon monoxide (< 10 ppm), collateral contacts, urine drug screens | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned using random numbers table |
| Allocation concealment (selection bias) | Low risk | Assignment placed in a sealed envelope opened immediately before 1st treatment session |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment content described as informational to participants and took place during unscheduled time so no reduction in other programme activities; personnel conducting assessments blinded to assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 34% in treatment group, 29% in control group Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers recruited from a state‐funded inner‐city residential substance abuse treatment programme Randomised controlled trial | |
| Participants | 184 smokers (≥ 10 cigarettes/day for 6 months) in substance abuse treatment, excluding those with psychiatric disorders | |
| Interventions | Intervention: motivational interviewing based therapy (7 sessions), crossed with contingent vouchers (outcomes not included), and free access to NRT, smoking cessation information (n = 97) Control: brief advice (7 sessions), crossed with contingent vouchers (outcomes not included) and free access to NRT, smoking cessation information (n = 86) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 1, 3, 6, and 12 months Abstinence verification: breath carbon monoxide (< 4 ppm) or salivary COT (≤ 15 ng/mL) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation stratifying for gender, nicotine dependence severity, motivation to change |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants in all groups informed they would receive "informational sessions about smoking;" personnel conducting assessments blinded to assignment |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 22% in treatment group, 27% in control group Multiple imputation methods used to assess sensitivity for missing values; 1 participant who died before first follow‐up excluded from the analysis |
| Methods | Country: USA Recruitment: smokers recruited from 3 narcotic treatment centres in Los Angeles using on‐site flyers and staff referrals Randomised controlled trial | |
| Participants | 175 daily smokers (≥ 10 cigarettes/day) aged 18 to 65 yr in good standing in a methadone maintenance programme | |
| Interventions | Intervention 1: 12 wk of NRT patch (21 mg/day 8 wk, 14 mg/day 2 wk, 7 mg/day 2 wk) and weekly 60‐min relapse prevention group counselling (n = 42) Intervention 2: 12 wk of NRT patch (dose as above) and contingency management vouchers worth USD2 for providing initial verification samples, increasing by USD0.5 consecutively with a USD5 for each third consecutive sample; relapse restarted voucher payments at USD2, total of USD447.50 available for 100% abstinent breath samples (n = 43) Intervention 3: 12 wk of NRT patch (dose as above) and relapse prevention counselling/contingency management vouchers (n = 47) Control: 12 wk of NRT patch only (dose as above) (n = 43) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco and other drugs at 6 and 12 months Abstinence verification: expired carbon monoxide < 9 ppm, urine samples analysed for COT (< 30 ng/mL) and metabolites of opiates (< 300 ng/mL) and cocaine (< 300 ng/mL) | |
| Notes | Only counselling and control arms included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to 1 of 4 smoking cessation interventions using urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall loss to follow‐up was 27%, multiple imputation applied to intermittent missing data; dropouts determined to be non‐random and not covariate‐dependent random Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: smokers enrolled at 5 methadone maintenance treatment programme clinics in Rhode Island Randomised controlled trial | |
| Participants | 383 English‐speaking daily smokers (≥ 10 cigarettes/day) aged ≥ 18 yr in methadone maintenance for ≥ 6 months | |
| Interventions | Intervention: 8 to 12 wk nicotine patch (< 2 pack/day smokers: 21 mg/day 4 wk, 14 mg/day 2 wk, 7 mg/day 2 wk; 2 pack/day smokers: 42 mg/day 4 wk, 35 mg/day 2 wk, 28 mg/day 2 wk, 14 mg/day 1 wk, 7 mg/day 1 wk), 3 counselling sessions based on motivational interviewing, quit date counselling, follow‐up session reinforcing skills training (n = 192) Control: brief advice using the National Cancer Institute 4As model (n = 191) | |
| Outcomes | Self‐reported 7‐day abstinence from tobacco at 1, 3, and 6 months Abstinence verification: expired carbon monoxide < 8 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Assignments made by a separate study interventionist |
| Blinding of participants and personnel (performance bias) | Low risk | Follow‐up research assessments performed by staff blinded to participant group assignment |
| Incomplete outcome data (attrition bias) | Low risk | Overall loss to follow‐up was 18.5%, authors reported follow‐up rates were similar in both groups and no association between attrition and covariates Participants lost to follow‐up were classified as non‐abstinent |
| Methods | Country: USA Recruitment: methadone‐maintained participants from 9 treatment centres in New England Randomised controlled trial | |
| Participants | 315 daily smokers (≥ 10 cigarettes/day) in methadone maintenance for ≥ 4 wk, not pregnant or nursing or unwilling to set quit date | |
| Interventions | Intervention 1: 6 months varenicline 1 mg treatment, brief advice (n = 137) Intervention 2: 6 months NRT prescription patch + ad libitum nicotine rescue, brief advice (n = 133) Control: placebo, brief advice (5‐As) (n = 45) | |
| Outcomes | Self‐reported 7‐day tobacco abstinence at 6 months Abstinence verification: breath carbon monoxide (< 8 ppm), urinary COT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Follow‐up research assessments performed by staff blinded to participant group assignment; placebo group given capsules identical to varenicline capsules; NRT arm unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 18% in intervention arms, 22% in control arm Participants lost to follow‐up were classified as non‐abstinent; sensitivity analysis conducted on missing data |
| Methods | Country: USA Recruitment: adults recruited from 1 of 12 substance use disorder treatment programmes at treatment start Randomised controlled trial | |
| Participants | 538 cocaine or methamphetamine (or both)‐dependent smokers (≥ 7 cigarettes/day for 3 months, carbon monoxide ≥ 8 ppm) interested in quitting, excluded for conditions that could make participation unsafe (e.g. pregnancy), use of non‐cigarette tobacco products | |
| Interventions | Intervention: weekly individual smoking cessation counselling and extended release bupropion 300 mg/day for 10 wk, nicotine inhaler and contingency management during post‐quit treatment, substance abuse treatment (n = 267) Control: substance abuse treatment (n = 271) | |
| Outcomes | Self‐reported 7‐day tobacco and other drug abstinence at 3 and 6 months Abstinence verification: breath carbon monoxide (< 8 ppm), urinary drug screen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was 10% in intervention group, 9% in control group Participants lost to follow‐up were classified as non‐abstinent |
BAC: blood alcohol concentration; CBT: cognitive behavioural therapy; COT: cotinine; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth Edition; ICC: intraclass correlation coefficient; min: minute; n: number of participants; NRT: nicotine replacement therapy; ppm: parts per million; VAMC: Veterans Affairs Medical Center; wk: week; yr: year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention of contingency management | |
| Intervention of contingency management | |
| Intervention of contingency management | |
| Not a trial in relevant population; comparison of outcomes for people with and without substance use disorders | |
| Measured reduction in smoking rather than abstinence | |
| Intervention of contingency management | |
| Intervention of contingency management | |
| Measured reduction in smoking rather than abstinence | |
| Completed clinical trial; outcomes not described, no published results | |
| Control group did not receive placebo | |
| Measured reduction in smoking rather than abstinence | |
| Measured reduction in smoking rather than abstinence | |
| Measured reduction in smoking rather than abstinence | |
| Measured reduction in smoking rather than abstinence | |
| Intervention of contingency management | |
| Control group did not receive placebo | |
| Intervention of increased methadone | |
| Completed clinical trial with a planned enrolment of 75 participants; outcomes not described, no published results | |
| Measured reduction in smoking rather than abstinence |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 1/2‐Multi‐Site Study: Varenicline Treatment of Alcohol Dependence in Smokers |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: aged 18 to 70 years, alcohol dependent and seeking treatment, report smoking 100 lifetime cigarettes and smoke twice weekly in the past 90 days with urinary cotinine of > 30 ng/mL, report heavy drinking at least 2/days week in the past 90 days Exclusion criteria: current clinically significant physical disease or abnormality, history of cancer, history of sensitivity to varenicline, psychiatric illness, suicidal ideation, psychotropic drug use, drug dependence other than nicotine or alcohol, at risk for alcohol withdrawal, used another investigational drug within 30 days, intention to donate blood or blood products, body mass index < 15 or > 39.99 or weigh < 45 kg, women of childbearing potential who is pregnant, nursing, or not practicing effective contraception |
| Interventions | Intervention: varenicline 0.5 mg once per day for days 1 to 3, 0.5 mg twice daily for days 4 to 7, 2 × 0.5 mg tablets twice daily following Control: placebo comparator on same schedule |
| Outcomes | Primary outcome: percentage of heavy drinking days in last 8 weeks of treatment for weeks 11 to 17 Secondary: 30‐day self‐reported smoking abstinence at weeks 13 to 17 |
| Starting date | September 2012 |
| Contact information | Stephanie O'Malley, Connecticut Mental Health Center Substance Abuse Treatment Unit, New Haven, CT, USA 06511 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Counselling Show forest plot | 11 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.95] |
| Analysis 1.1 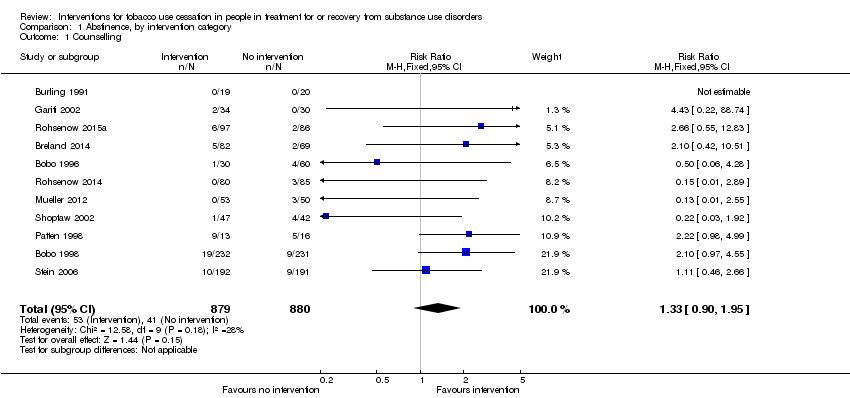 Comparison 1 Abstinence, by intervention category, Outcome 1 Counselling. | ||||
| 2 Pharmacotherapy Show forest plot | 11 | 1808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.37, 2.57] |
| Analysis 1.2  Comparison 1 Abstinence, by intervention category, Outcome 2 Pharmacotherapy. | ||||
| 2.1 Nicotine replacement therapy (NRT) | 3 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.74 [3.00, 19.94] |
| 2.2 Other pharmacotherapy or combined NRT and other pharmacotherapy | 8 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.77] |
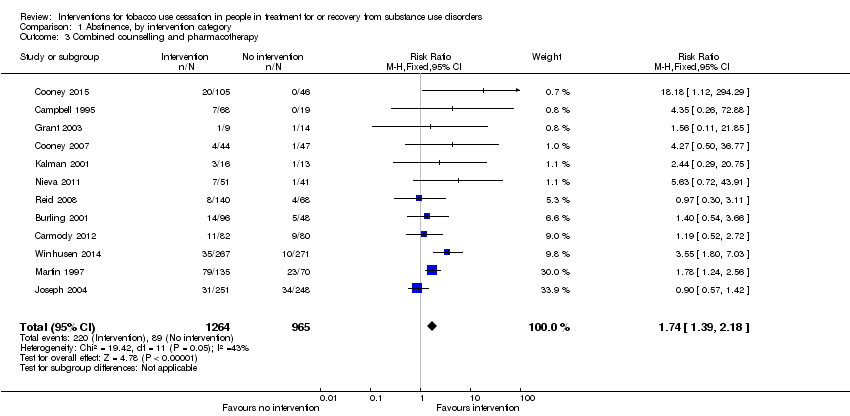
| 3 Combined counselling and pharmacotherapy Show forest plot | 12 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.39, 2.18] |
| Analysis 1.3  Comparison 1 Abstinence, by intervention category, Outcome 3 Combined counselling and pharmacotherapy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
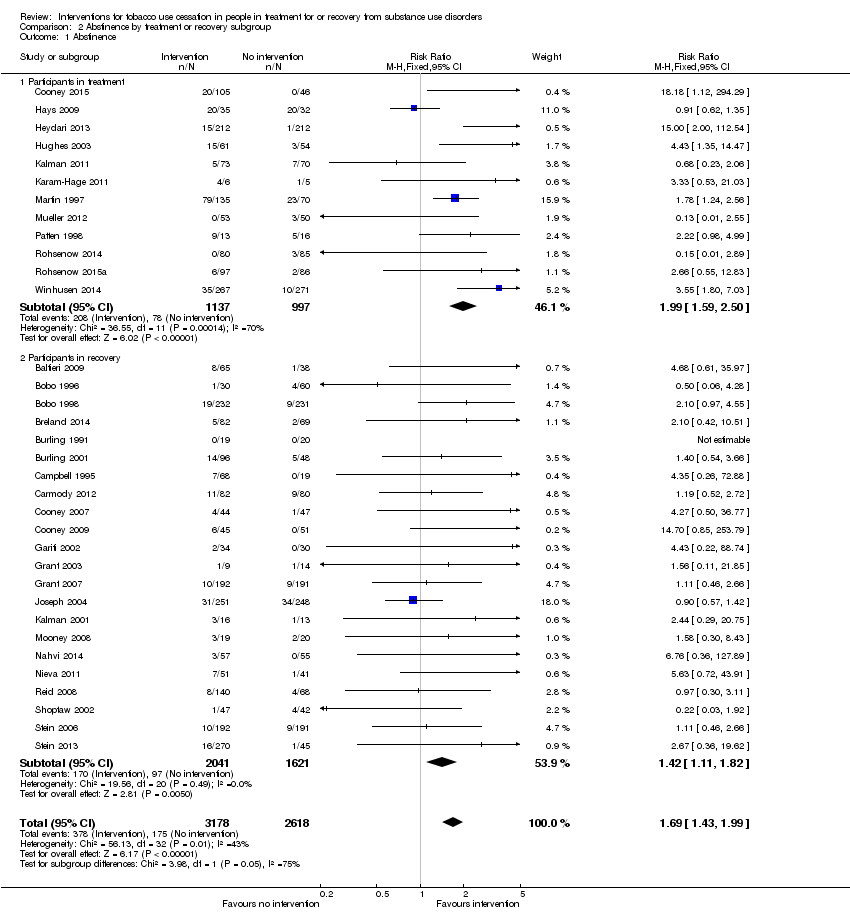
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| Analysis 2.1 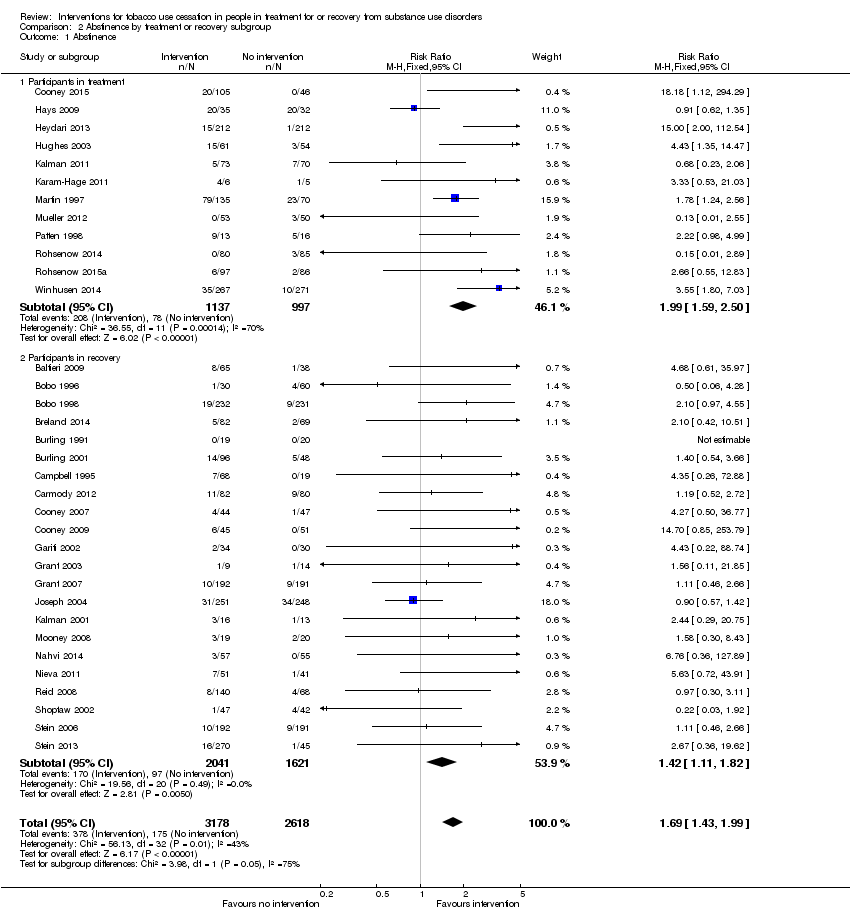 Comparison 2 Abstinence by treatment or recovery subgroup, Outcome 1 Abstinence. | ||||
| 1.1 Participants in treatment | 12 | 2134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.59, 2.50] |
| 1.2 Participants in recovery | 22 | 3662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.11, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
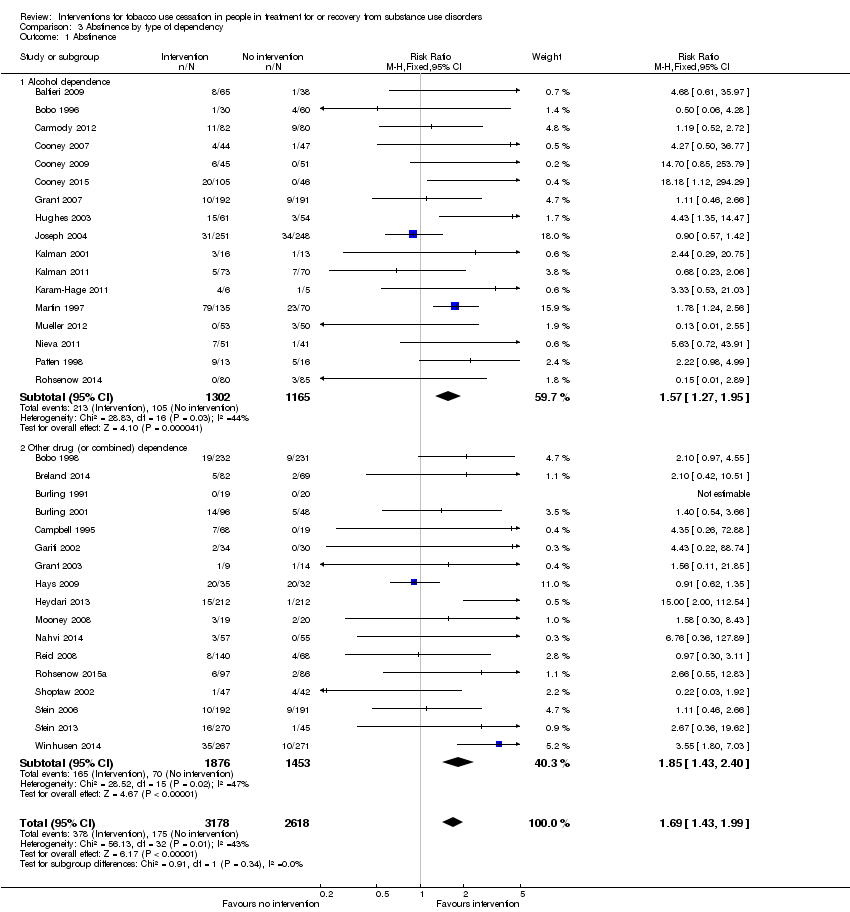
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| Analysis 3.1  Comparison 3 Abstinence by type of dependency, Outcome 1 Abstinence. | ||||
| 1.1 Alcohol dependence | 17 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.95] |
| 1.2 Other drug (or combined) dependence | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.43, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
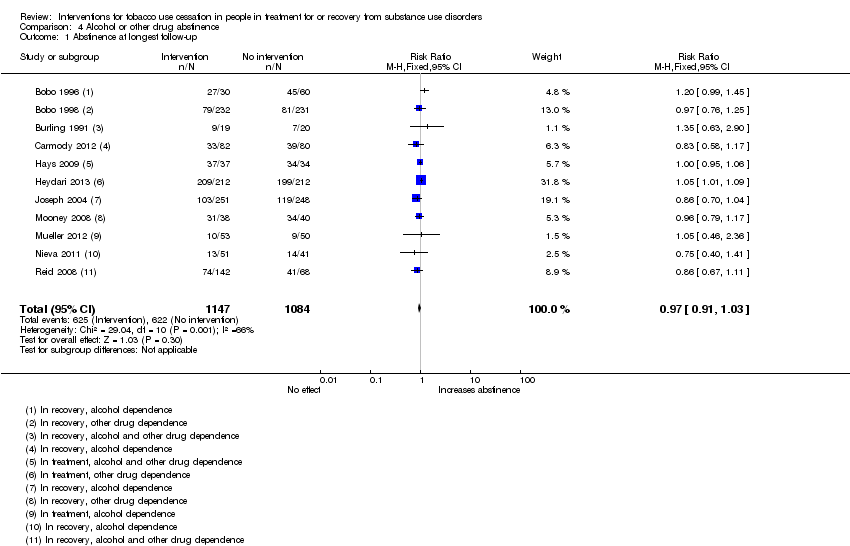
| 1 Abstinence at longest follow‐up Show forest plot | 11 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| Analysis 4.1  Comparison 4 Alcohol or other drug abstinence, Outcome 1 Abstinence at longest follow‐up. | ||||
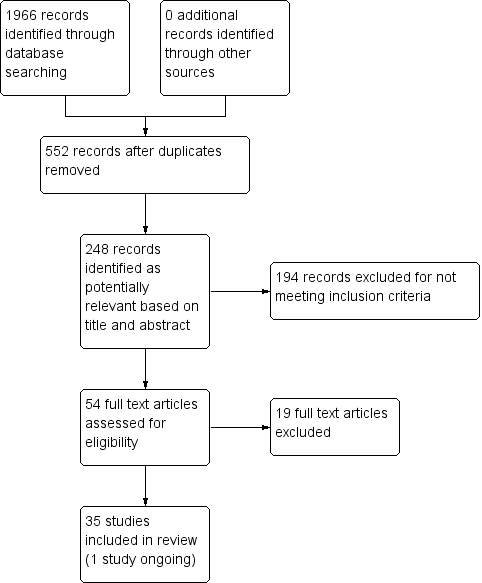
Study flow diagram.

Comparison 1 Abstinence, by intervention category, Outcome 1 Counselling.
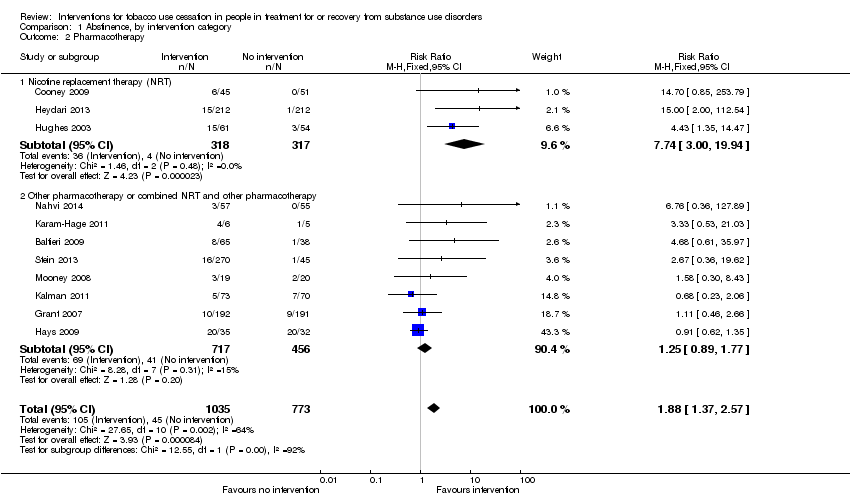
Comparison 1 Abstinence, by intervention category, Outcome 2 Pharmacotherapy.
Comparison 1 Abstinence, by intervention category, Outcome 3 Combined counselling and pharmacotherapy.
Comparison 2 Abstinence by treatment or recovery subgroup, Outcome 1 Abstinence.
Comparison 3 Abstinence by type of dependency, Outcome 1 Abstinence.
Comparison 4 Alcohol or other drug abstinence, Outcome 1 Abstinence at longest follow‐up.
| Tobacco cessation interventions compared to placebo or usual care for people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Patient or population: people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with tobacco cessation interventions | |||||
| Tobacco abstinence after counselling (counselling) | Study population | RR 1.33 | 1759 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 47 per 1000 | 62 per 1000 | |||||
| Tobacco abstinence after pharmacotherapy (pharmacotherapy) | Study population | RR 1.88 | 1808 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 58 per 1000 | 109 per 1000 | |||||
| Tobacco abstinence after combined counselling and pharmacotherapy (combined) | Study population | RR 1.74 | 2229 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 92 per 1000 | 160 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limited information provided regarding study designs; some cluster‐randomised studies and waiting list controls. 2 Clinical interventions had substantial variance, ranging from one‐time to daily counselling sessions and individual or group therapy. 3 Evidence of publication bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Counselling Show forest plot | 11 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.95] |
| 2 Pharmacotherapy Show forest plot | 11 | 1808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.37, 2.57] |
| 2.1 Nicotine replacement therapy (NRT) | 3 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.74 [3.00, 19.94] |
| 2.2 Other pharmacotherapy or combined NRT and other pharmacotherapy | 8 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.77] |
| 3 Combined counselling and pharmacotherapy Show forest plot | 12 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.39, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| 1.1 Participants in treatment | 12 | 2134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.59, 2.50] |
| 1.2 Participants in recovery | 22 | 3662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.11, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| 1.1 Alcohol dependence | 17 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.95] |
| 1.2 Other drug (or combined) dependence | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.43, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence at longest follow‐up Show forest plot | 11 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |

