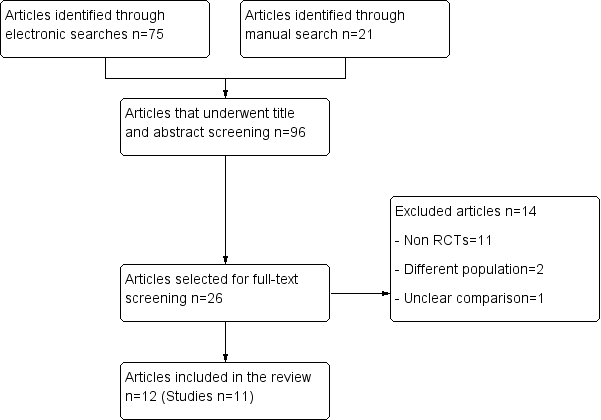
پروفیلاکسی آنتیبیوتیکی در پیشگیری از بروز عوارض عفونی در جراحی ارتوگناتیک
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 34 Setting: Oral and Maxillofacial Surgery Department, Faculty of Dentistry, University of Jordan, Amman, Jordan Sex: 32.35% male Age: mean 27 years (range 18‐48 years) Inclusion criteria: "Patients listed to undergo orthognathic operations" Exclusion criteria: use of antibiotics in the month before the operation, lactose intolerance (because the placebo was lactose‐based) and previous orthognathic operations | |
| Interventions | Long‐term group: amoxicillin 1 g intravenously at induction, followed by 500 mg intravenously 3 hours postoperatively and amoxicillin 500 mg orally every 8 hours for 5 days. Participants allergic to penicillin were given clindamycin 300 mg intravenously at induction and 150 mg 3 hours postoperatively, and continued taking clindamycin 150 mg orally every 6 hours for a total of 5 days Short‐term group: amoxicillin 1 g intravenously at induction, followed by 500 mg intravenously 3 hours postoperatively and placebo orally every 8 hours for 5 days. Participants allergic to penicillin were given clindamycin 300 mg intravenously at induction and 150 mg 3 hours postoperatively and placebo for the following 5 days | |
| Outcomes | SSI: measured up to 30 days after surgery. Seven variables from a previously validated system (according to study authors, 4 references given) were used to audit postoperative infection, including the following.
The total achievable score for severe infection per participant was 52. Study authors reported the sum of scores across participants, per group | |
| Notes | No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | List of random numbers was prepared by the clinical pharmacist (no explanation how) |
| Allocation concealment (selection bias) | Low risk | List of random numbers was kept by the clinical pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Dispensed capsules were unmarked, so that neither the participant nor the assessor knew the regimen that was being administered |
| Blinding of outcome assessment (detection bias) | Low risk | Dispensed capsules were unmarked so that neither the participant nor the assessor knew the regimen |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | High risk | No explicit report described the infected number or proportion of participants. Only the numbers of participants who required extra antibiotics were reported in the discussion section of the article |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of patients enrolled: 30 Setting: Oral and Maxillofacial Surgery Service at Montreal General Hospital, Montreal, Canada Sex: not reported Age: not reported Inclusion criteria: healthy male and female patients who were to undergo intraoral or combined intraoral and extraoral orthognathic surgical procedures, including those requiring autogenous bone grafts Exclusion criteria: not reported | |
| Interventions | Arm 1, 5‐day regimen: 2 million units aqueous penicillin G intravenously (TV) immediately preoperatively, 1 million units lV every 3 hours intraoperatively, and then 1 million units IV postoperatively 3 hours after the last intraoperative dose. Then, aqueous penicillin G, 1 million units IV every 6 hours for 8 doses, then a suspension of benzathine penicillin V 300 mg given orally every 6 hours for 8 doses Arm 2, 1 day‐regimen: 2 million units aqueous penicillin G intravenously (TV) immediately preoperatively, 1 million units lV every 3 hours intraoperatively, and then 1 million units IV postoperatively 3 hours after the last intraoperative dose. Then placebo and oral placebo according to the same schedule | |
| Outcomes | SSI: measured daily in the hospital and at 1, 2 and 4 postoperative weeks Criteria for an infected wound were based on the Centers for Disease Control and Prevention definition of infection, that is, infection must occur at the operative site within 30 days of surgery and must be based on the existence of any 1 of the following conditions.
| |
| Notes | No use of plates, only wire fixation No report about adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 2 groups, no explanation how |
| Allocation concealment (selection bias) | Unclear risk | No information was provided about allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information was provided about blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators were not aware of the randomisation codes until 4 weeks after the last surgical procedure was performed |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported and details per participant provided |
| Other bias | High risk | The trial was stopped early because of harm (33.4% increased risk of infection), which may overestimate the treatment effect |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 150 Setting: Department of Oral and Maxillofacial Surgery, College of Dental Surgery, Saveetha University, Chennai, India Sex: 38% male Age: mean 24 years (15‐37 years) Inclusion criteria: not reported Exclusion criteria: patients who had received antibiotics 1 month before surgery; patients who had a history of allergy to ampicillin; signs of active infection; immunocompromised patients | |
| Interventions | Arm 1, single dose: saline solution intravenously every 6 hours for 24 hours and ampicillin 1 g intravenously at induction. Arm 2, single day: ampicillin 500 mg intravenously every 6 hours for 24 hours and ampicillin 1 g intravenously at induction | |
| Outcomes | SSI: measured 1, 2, 3 and 4 postoperative days until discharge and then at the 2nd, 3rd and 4th week postoperatively. Criteria for infection were based on any of the following conditions.
| |
| Notes | No report about adverse reactions to drugs was provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 2 groups, with no explanation how |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Low risk | Both participant and assessor were blinded to the antibiotic protocol |
| Blinding of outcome assessment (detection bias) | Low risk | Both participant and assessor were blinded to the antibiotic protocol |
| Incomplete outcome data (attrition bias) | Unclear risk | No information about withdrawals was provided, and reporting does not allow us to judge whether losses to follow‐up occurred |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 30 Setting: Department of Hospital Dentistry, Division of Oral and Maxillofacial Surgery, University Iowa Hospitals and Clinics, Iowa City, USA Sex: 53.33% male Age: mean 27.2 years (15‐55 years) Inclusion criteria: not reported Exclusion criteria: not reported | |
| Interventions | Arm 1, 1 week: penicillin G 2 million U IV preoperatively and continued every 4 hours until the IV was discontinued on postoperative day 1. 500 mg penicillin VK was continued 4 times daily for 1 week. Cefazolin or clindamycin was used in allergic participants in comparable doses, intervals and duration Arm 2, 1 day: penicillin G 2 million U IV, preoperatively and continued every 2 hours until participants reached the recovery room, where the final dose was given. Cefazolin or clindamycin was used in allergic participants in comparable doses, intervals and duration | |
| Outcomes | SSI: measured up to 8 weeks after the surgery. No further details provided | |
| Notes | No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 2 groups, with no explanation how |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of assessors was provided |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned previously in the methods section were reported with sufficient details |
| Other bias | High risk | Both groups used drainage tubes, which in arm 2 may increase the rate of infection because participants were not using antibiotic coverage |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 122 Setting: Faculty of Dentistry, Chulalolongkorn University, Thailand Sex: mean 26.52 years (17.10‐47.60 years) Age: 34.42% male Inclusion criteria: not reported Exclusion criteria: patients with metabolic disease or low resistance to infection; need for a bone graft for correction of dentofacial deformities; patients who sustained perioperative complications that made antibiotic usage crucial, such as unfavourable fractures or excessive bleeding that could cause a large haematoma; patients who had received an antibiotic within 4 weeks of surgery; patients who had been treated by a distraction osteogenesis device or surgically assisted rapid palatal expansion; patients who had a history of allergy to penicillin | |
| Interventions | Arm 1, short‐term amoxicillin‐clavulanic acid: 1.2 g of intravenous amoxicillin‐clavulanic acid 30 minutes preoperatively and every 8 hours during the operation. then 1 more single dose 8 hours postoperatively (33 participants) Arm 2, short‐term penicillin: 2 million units of aqueous penicillin G (IV) 30 minutes preoperatively, which was continued every 4 hours during surgery. then 1 more single dose 4 hours after surgery (29 participants) Arm 3, long‐term amoxicillin‐clavulanic acid: 1.2 g of intravenous amoxicillin‐clavulanic acid 30 minutes preoperatively and every 8 hours during the operation, followed by a 625‐mg tablet amoxicillin‐clavulanic acid orally every 8 hours postoperatively for 5 days (28 participants) Arm 4, long‐term penicillin: 2 million units of aqueous penicillin G (IV) 30 minutes preoperatively, which was continued every 4 hours during surgery. then postoperative antibiotic of 500 mg oral amoxicillin every 8 hours for 5 days (32 participants) | |
| Outcomes | SSI: measured daily at the hospital and at 1, 2, 4, 6, 8 and 12 weeks The criteria for postoperative infection were based on the definition of the infection provided by the Centers
| |
| Notes | No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 2 groups, with no explanation how |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Low risk | The study was double‐blind. It is likely that participants were blinded to the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of surgeons and personnel was provided. Study authors mentioned only that the study was double‐blind, and it cannot be inferred who was the second party blinded |
| Incomplete outcome data (attrition bias) | High risk | Mention is made of 15 participants excluded from the analysis for different reasons. Six participants were excluded because of intraoperative complications, and 1 was lost |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 56 Setting: Department of Oral and Maxillofacial Surgery, College of Dentistry, Yonsei University, Seoul; Korea National Health Insurance Corporation Ilsan Hospital Gyeonggi; Wonju Christian Hospital, Kangwon, South Korea Sex: 40% male Age: arm 1: mean 23.9 years (SD 5.84); arm 2: mean 24.3 years (SD 6.33) Inclusion criteria: not reported Exclusion criteria: not reported | |
| Interventions | Arm 1, short term: 1.0 g of a third‐generation cephalosporin (cefpiramide) intravenously 30 minutes before surgery (28 participants) Arm 2, long term: 1.0 g of Cefpiramide 30 minutes before surgery, as well as twice daily until 3 days after surgery (28 participants) | |
| Outcomes | SSI: measured every day during the first 3 days and at the end of the first and second weeks after surgery for any postoperative infection. Postoperative wound infection was defined by at least 1 of the following criteria.
| |
| Notes | Both groups used a closed intraoral suction, which in arm 2 may increase the rate of infection because participants are not using postoperative antibiotic coverage No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated using Microsoft Excel |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of participants was provided |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | High risk | Both groups used drainage tubes, which in arm 2 may increase the rate of infection because participants were not using postoperative antibiotic coverage |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 70 Setting: Medical Center and Academic Center for Dentistry, University of Amsterdam, The Netherlands Sex: 25.71% male Age: mean 29.9 years (19‐54 years) Inclusion criteria: 70 consecutive patients who underwent a bilateral sagittal ramus osteotomy of the mandible. All participants were Angle Class II retrognathia patients, and all had received preoperative orthodontic treatment to optimise the shape of the dental arches Exclusion criteria: patients who had received antibiotics within 2 weeks before surgery; history of allergy to clindamycin; signs and symptoms of active infection; additional surgical procedures (i.e. chin or maxillary osteotomies) indicated; and participants suffering from severe underlying illness associated with compromised host defences | |
| Interventions | Arm 1: single dose of 600 mg clindamycin and saline solution intravenously 15 minutes before surgery (35 participants) Arm 2: 4 doses of 600 mg clindamycin and saline solution intravenously (1 every 6 hours for 24 hours; 35 participants) | |
| Outcomes | SSI: After surgery and until hospital discharge, all participants were observed daily and at 1, 2, and 4 weeks and at 3 months. Early infection was defined as infection occurring within 1 week postoperatively Drug adverse reactions: All undesirable reactions such as skin rashes or gastrointestinal disorders occurring in connection with the antibiotic prophylaxis were noted | |
| Notes | Both groups used chlorhexidine and were seen by a dental hygienist preoperatively and postoperatively. Bethamethasone 8 mg preoperatively and 4 mg postoperatively, and, during the next 3 days, in a slow, tapering fashion was administered to both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A list of random numbers was used |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding of participants was provided |
| Blinding of outcome assessment (detection bias) | Low risk | One clinician was blinded to the antibiotic protocol |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 40 Setting: Medical University of South Carolina, College of Dental Medicine, Department of Oral and Maxillofacial Surgery, USA Sex: 77.5% male Age: mean 23 years (19 to 39 years) Inclusion criteria: not reported Exclusion criteria: history of allergy to penicillin or other beta‐lactam antibiotics, a compromised immune defense and a history of receiving antibiotic therapy within 14 days before the planned surgery | |
| Interventions | Arm 1, 2 days: intramuscular dose of 600,000 units procaine penicillin G and 400,000 units aqueous penicillin G 1 hour preoperatively. Two million units aqueous penicillin G was administered intravenously over 30 minutes every 3 hours during the operation, and another 2 million units aqueous penicillin G was administered intravenously over 30 minutes 3 hours after the last intraoperative dose was given. Aqueous penicillin G intravenously over 30 minutes every 4 hours for a total of 12 doses postoperatively (2 days of antibiotic prophylaxis, 20 participants) Arm 2, 1 day: intramuscular dose of 600,000 units procaine penicillin G and 400,000 units aqueous penicillin G 1 hour preoperatively. Two million units aqueous penicillin G was administered intravenously over 30 minutes every 3 hours during the operation, and another 2 million units aqueous penicillin G was administered intravenously over 30 minutes 3 hours after the last intraoperative dose was given. Then placebo was administered (20 participants) | |
| Outcomes | SSI: All participants were observed postoperatively. Not reported how many days or weeks The diagnosis of postoperative infection was made when 3 of the following criteria were met.
| |
| Notes | No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to divide the 40 participants into 2 groups of 20 |
| Allocation concealment (selection bias) | Low risk | The code was not revealed to the investigators until 6 weeks after the last surgical procedure was performed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of assessors was provided |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 160 Setting: Department of Oral and Maxillofacial Surgery, University of Hong Kong, Hong Kong, China Sex: not reported Age: not reported Inclusion criteria: not reported Exclusion criteria: not reported | |
| Interventions | Arm 1: penicillin, 1 intravenous dose at induction Arm 2: penicillin, 2 IV doses 6 hours apart Arm 3: penicillin, 8 IV doses over 2 days Arm 4: penicillin, 8 IV doses over 2 days with additional 5 days of oral penicillin | |
| Outcomes | SSI: followed for 6 months and assessed for incidence of clinically significant postoperative infection | |
| Notes | This is an abstract from a conference No report described adverse reactions to drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 4 groups, with no explanation how |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding of participants and personnel was provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of assessors was provided |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up are reported |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 42 Setting: University of Hong Kong/Hospital, China Sex: 33.33% male Age: mean 26 years (18‐34 years, SD 4,2) Inclusion criteria: patients who underwent bimaxillary OS Exclusion criteria: History of any type of previous surgery to the head and neck area, including previous OS; patients who were having distraction osteogenesis as part of the OS; history of malignancy of the head and neck region and/or history of radiation to the head and neck region; known hypersensitivity to amoxicillin, ampicillin or other beta‐lactamic antibiotics; known history of lactose intolerance; patients who had used any antibiotics in the 14 days before surgery; patients with compromised host defences (e.g. diabetes mellitus, autoimmune disease, end‐stage renal disease, severe alcoholic cirrhosis, neutropenia); and patients who were receiving immunosuppressive drugs that interfere with host defences (e.g. cyclosporine, steroids, cancer chemotherapeutic agents) | |
| Interventions | Arm 1: oral amoxicillin 500 mg 3 times daily and intravenous placebo (normal saline) injection 4 times daily in the first 2 days after OS. Intravenous ampicillin 1 g during anaesthetic induction and 500 mg every 6 hours during the operation.Oral amoxicillin 500 mg 3 times daily for 3 days. (21 participants) Arm 2: intravenous ampicillin 1 g 4 times daily and oral lactose (placebo) 3 times daily for the first 2 days after OS. Intravenous ampicillin 1 g during anaesthetic induction and 500 mg every 6 hours during the operation.Oral amoxicillin 500 mg 3 times daily for 3 days (21 participants) | |
| Outcomes | SSI: Participants were evaluated daily during their hospital stay. Subsequently, they were assessed at 1, 2, 4 and 6 weeks after the operation in the outpatient clinic. Blinded clinical assessors evaluated all participants for infection based on the presence of the following clinical criteria, similar to previous studies:
| |
| Notes | No adverse drug events were observed in this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomly assigned in blocks of 4 to 2 groups, corresponding to a list of computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and surgeons were blinded to the postoperative prophylactic antibiotic regimen |
| Blinding of outcome assessment (detection bias) | Low risk | Assesors were blinded to the postoperative prophylactic antibiotic regimen |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
| Methods | Randomised controlled trial | |
| Participants | Number of participants enrolled: 54 Setting: University Hospital Vrije Universiteit, Amsterdam, The Netherlands Sex: mean 25.5 years (18‐40 years) Age: 24.07% male Inclusion criteria: not reported Exclusion criteria: history of allergy to penicillin or other beta‐lactam antibiotics, any long‐term medication use, use of antibiotics in the 4 weeks preceding admission and serum creatinine exceeding 110 mmol/L as an indication of renal dysfunction | |
| Interventions | Arm 1: placebo (19 participants) 30 minutes before surgery Arm 2: 2200 mg amoxicillin‐clavulanic acid 30 minutes before surgery (18 participants) Arm 3: 1500 mg cefuroxime 30 minutes before surgery (17 participants) | |
| Outcomes | SSI: postoperatively and after 1 month The following criteria for infection were used.
| |
| Notes | No adverse drug events were reported in this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 3 groups, with no explanation how |
| Allocation concealment (selection bias) | Low risk | Code was maintained by the pharmacist during the entire period of the study |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinding is suggested |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific information about blinding of outcome assessors was provided |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported with sufficient details |
| Other bias | Low risk | No other biases were detected |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No evidence suggested random allocation of participants | |
| Systematic review | |
| Participants underwent many types of maxillofacial surgery, and results for participants who underwent OS are not provided separately | |
| Narrative review | |
| Discussion of a randomised controlled trial | |
| Retrospective study | |
| Narrative review | |
| Retrospective study | |
| No evidence of random allocation of participants | |
| Participants underwent any type of oral surgery | |
| Case series | |
| Systematic review | |
| No clear description of the control arm. Study authors claim that participants received the standard of care; however, no standard of care is known, and additional details are not available. | |
| No evidence of random allocation of participants |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Orthognathic Surgery and Postoperative Antibiotic Use |
| Methods | Double‐blind randomised controlled trial |
| Participants | Estimated enrolment: 300 Setting: QEII VG hospital (Queen Elizabeth II Victoria General Hospital) Inclusion criteria: over 16 years old undergoing OS Exclusion criteria: use of antibiotics in past 2 weeks, active oral or odontogenic infection, significant medical condition, immunocompromised |
| Interventions | Arm 1: Group will be receiving 1 day of IV cefazolin or clindamycin followed by 2 days of oral cephalexin or clindamycin. Clindamycin will be used in participants with allergy Arm 2: Group will receive 1 day IV cefazolin or clindamycin followed by 2 days of oral placebo. Clindamycin will be used if participant has allergy |
| Outcomes | Primary outcome measures: rate of infection (time frame: 4 weeks following surgery), investigation of the incidence of postoperative infection following surgery in each of the 2 groups Secondary outcome measures: side effect from antibiotic use (time frame: 4 weeks), investigation of the incidence of side effects from an extended antibiotic regimen |
| Starting date | June 2013 |
| Contact information | Clayton Davis, DDS 902‐473‐2070 [email protected] Victoria General Hospital Halifax, Nova Scotia, Canada, B3H 1W2 Canada |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 472 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.24, 0.74] |
| Analysis 1.1  Comparison 1 Short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 6 | 438 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.22, 0.75] |
| Analysis 2.1 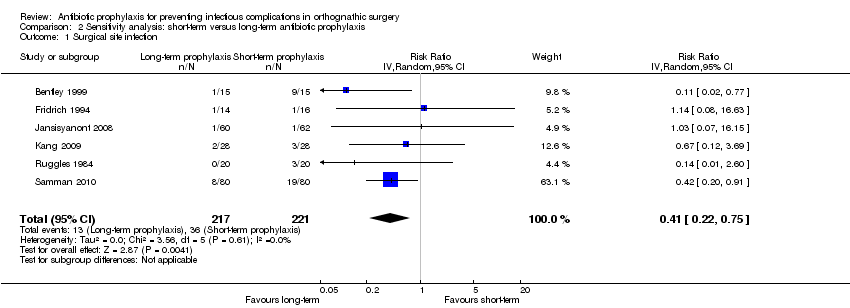 Comparison 2 Sensitivity analysis: short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 220 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| Analysis 3.1 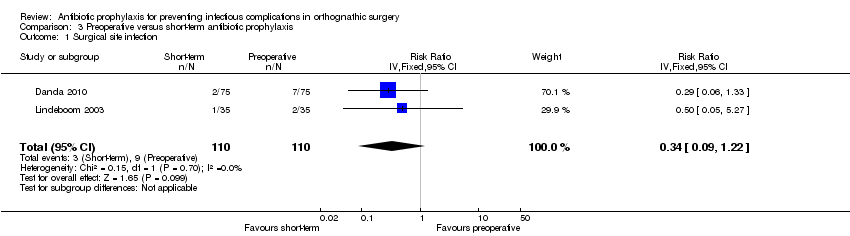 Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 1 Surgical site infection. | ||||
| 2 Adverse events Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.2  Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 2 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Amoxicillin versus ampicillin, Outcome 1 Surgical site infection. | ||||
| 2 Adverse events Show forest plot | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.2  Comparison 4 Amoxicillin versus ampicillin, Outcome 2 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Amoxicillin and clavulanic acid versus cefuroxime, Outcome 1 Surgical site infection. | ||||
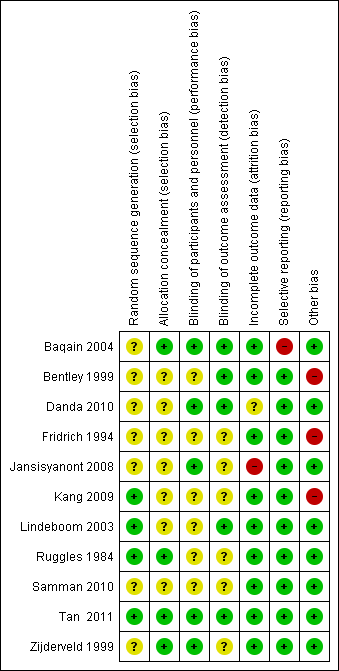
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.

Comparison 2 Sensitivity analysis: short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
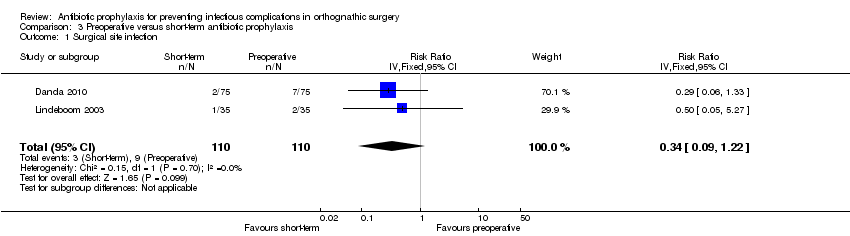
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
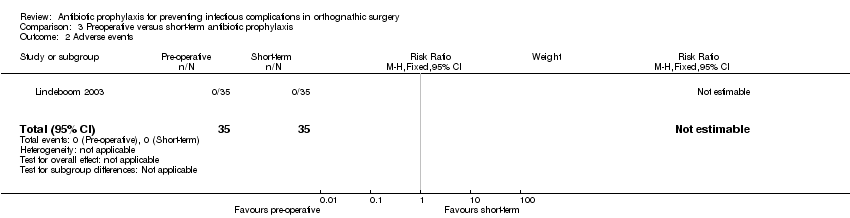
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 2 Adverse events.

Comparison 4 Amoxicillin versus ampicillin, Outcome 1 Surgical site infection.

Comparison 4 Amoxicillin versus ampicillin, Outcome 2 Adverse events.
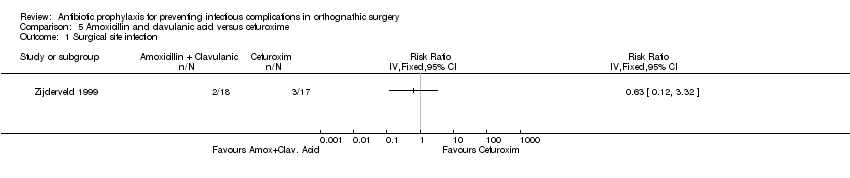
Comparison 5 Amoxicillin and clavulanic acid versus cefuroxime, Outcome 1 Surgical site infection.
| Short‐term antibiotic prophylaxis compared with long‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: short‐term antibiotic prophylaxis Comparison: long‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐term | Long‐term | |||||
| Surgical site infection Follow‐up: 2 to 36 weeks | 168 per 1000a | 71 per 1000 (41 to 125) | RR 0.42 (0.24 to 0.74) | 472 | ⊕⊕⊕⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Adverse events | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Preoperative antibiotic prophylaxis compared with short‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: preoperative antibiotic prophylaxis Comparison: short‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Preoperative | Short‐term | |||||
| Surgical site infection Follow‐up: 4 to 12 weeks | 82 per 1000a | 28 per 1000 | RR 0.34 (0.09 to 1.22) | 220 | ⊕⊕⊝⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Adverse events Follow‐up: up to 12 weeks | 0 per 35 See comment | 0 per 35 See comment | Not estimable | 70 | ⊕⊕⊝⊝ | No adverse events were reported in any of arms of the trial |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 472 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.24, 0.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 6 | 438 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.22, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 220 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| 2 Adverse events Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |