مقایسه درمان جراحی در برابر درمان غیر‐جراحی برای تنگی مهرههای کمری
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | 100 patients with symptomatic lumbar spinal stenosis, 54 men and 46 women whose median age was 59 years (range 16 to 77 years). From these patients, a group S (n = 19) was selected for surgical treatment because of the severity of their symptoms, and a group C (n = 50), with milder pain, was selected for conservative treatment. Remaining patients, group R (n = 31), whose severity of pain left the physician in doubt concerning which treatment to recommend, was randomly assigned to surgical treatment (group RS (n = 13) or conservative treatment (group RC (n = 18) Inclusion criteria: sciatic pain in the leg(s), with or without pain in the back, together with radiological signs of stenosis and compression of clinically afflicted nerve root(s) Exclusion criteria: bulging or herniated disc, spondylolysis, coxarthrosis, gonarthrosis, arterial insufficiency in the legs, polyneuropathy, concomitant serious disease, previous surgery on the back | |
| Interventions | Surgical procedure: standardised for the purpose of nerve decompression by partial or total laminectomy, medial facetectomy, discectomy and/or removal of osteophytes from the vertebral margins or facet joints. Hypertropic ligamenta flava were removed if necessary. No fusions were performed Conservative treatment: fitted with an orthosis and transferred to the rehabilitation department for 1 month. No regular physiotherapy was given, except for instruction and "back school" | |
| Outcomes | Outcomes: Visual Analogue Pain Scale, Verbal Rating Scale, Subjective Change (better, worse or unchanged), Work Status, Subjective Physician Rating (excellent, fair, unchanged, worse) Time points: 6 months, 12 months, 4 years, 10 years | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation using tables of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants not possible for the types of interventions compared |
| Incomplete outcome data (attrition bias) | High risk | Only completers included |
| Selective reporting (reporting bias) | High risk | Data not fully reported |
| Group similarity at baseline (selection bias) | Low risk | Similar characteristics between groups at baseline |
| Co‐interventions (performance bias) | Low risk | No co‐intervention unbalance |
| Compliance (performance bias) | High risk | Compliance not monitored for conservative treatment group |
| Intention‐to‐treat‐analysis | Low risk | ITT performed |
| Timing of outcome assessments (detection bias) | Low risk | Similar timing for both groups |
| Other bias | High risk | High dropout rate |
| Methods | Double‐blind randomised prospective study | |
| Participants | 38 participants were randomly assigned to 2 treatment groups, with 21 included in the mild group and 17 in the ESI group Inclusion criteria: patients with symptomatic LSS with painful lower limb neurogenic claudication and hypertrophic ligamentum flavum as a contributing factor. All patients were at least 18 years of age, had previously failed conservative therapy and presented with an Oswestry Disability Index (ODI) score > 20. Radiological evidence showed LSS (L3–L5), ligamentum flavum > 2.5 mm confirmed by preoperative MRI or CT, central canal cross‐sectional area £ 100 mm2 and anterior listhesis confirmed at £ 5.0 mm for all patients. All were able to walk at least 10 feet unaided before they were limited by pain Exclusion criteria: prior surgery at the intended treatment level or previous treatment with epidural steroids. History of recent spinal fracture, disabling back or leg pain from causes other than LSS, fixed spondylolisthesis > grade 1, disc protrusion or osteophyte formationor excessive facet hypertrophy. Patients with bleeding disorders, current use of anticoagulants or wound healing pathologies deemed to compromise outcomes, such as diabetes, cancer, and severe COPD; and those who had used ASA or NSAID within 5 days of treatment were not eligible | |
| Interventions | Conservative treatment (epidural steroid treatment): Participants received 80 mg of triamcinolone acetate (40 mg in diabetic patients) mixed with 6 mL of preservative‐free saline injected in divided doses at treated levels. Injections were delivered at the level of pathology with fluoroscopy and radiographic contrast used to document accurate placement of the steroid into the epidural space. In addition, skin anaesthesia and a small incision, followed by trocar placement under fluoroscopy as with the mild procedure, were performed. No bone or tissue was removed, and thus, no decompression procedure was performed. Wounds were dressed and cared for postoperatively identically to those in the mild treatment group. Individuals randomly assigned to mild received no steroid Surgical treatment: mild lumbar decompression procedure performed. Mild devices are designed to access the interlaminar space from the posterior lumbar spine, enabling removal of small portions of lamina and hypertrophic ligamentum flavum, thereby achieving lumbar decompression. Initially, the mild patient is placed in the prone position for posterior spinal access. Frequently, a bolster is used to open the spinal anatomy for treatment. An epidurogram is performed at the beginning of the procedure for the purpose of identifying the border of the dural and epidural space relative to the ligamentum flavum and interlaminar space. On the basis of these visual landmarks, the mild trocar and portal system is advanced under manual control and is positioned under fluoroscopic guidance. The trocar is then removed, and the 6‐gauge mild portal is secured in place, with the portal stabiliser becoming the percutaneous working port for the procedure. The bone sculpter rongeur is advanced through the secured portal to the laminar bone surface. This device is used to precisely cut and remove very small pieces of bone until access to the ligamentous tissue has been created. The mild tissue sculpter is then placed through the portal and through the laminotomy to excise portions of hypertrophic ligamentum flavum. Progressive tissue cuts are performed to the inferior edge and under the ventral surface of the lamina, under fluoroscopic guidance. The amount of decompression is assessed through visual observation of epidurogram contrast flow, as the flow becomes thicker and straighter. Once the procedure is complete, the mild portal and stabiliser assembly is removed. No implants are left behind, and the site is closed with a sterile adhesive strip. The mild procedure is usually performed with only light sedation and local anaesthetic | |
| Outcomes | Outcomes: Visual Analogue Pain Scale, Oswestry Disability Index, Zurich Claudication Questionnaire Time points: 6 weeks, 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation determined by the independent statistician in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Neither enrolling physician nor participant was aware of the participant's ultimate treatment group |
| Blinding (performance bias and detection bias) | Low risk | Both groups received skin anaesthesia and a small incision. Wounds were dressed and cared for identically postoperatively. Participants and raters were blinded |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Group similarity at baseline (selection bias) | Low risk | Similar characteristics between groups at baseline |
| Co‐interventions (performance bias) | Low risk | No co‐intervention unbalance |
| Compliance (performance bias) | Low risk | Similar compliance for both groups |
| Intention‐to‐treat‐analysis | Unclear risk | ITT performed |
| Timing of outcome assessments (detection bias) | Unclear risk | Similar timing for both groups |
| Other bias | Unclear risk | No further details available |
| Methods | Randomised controlled trial | |
| Participants | 94 participants were randomly assigned to a surgical or non‐operative treatment group: 50 (age 62 ± 9) and 44 participants (63 ± 9), respectively Inclusion criteria: back pain radiation to lower limbs or buttocks; fatigue or loss of sensation in the lower limbs aggravated by walking; persistent pain without progressive neurological dysfunction; imaging techniques: spinal canal narrowing, sagittal diameter of the dural sac < 10 mm2 or planimetrically assessed cross‐sectional dural area < 75 mm; duration of symptoms and signs > 6 months; clinical signs and symptoms corresponding to segmental radiographic level of stenosis; severity of the disease justifying surgical or non‐operative treatment Exclusion criteria: severe LSS with intractable pain and progressive neurological dysfunction, suggesting forthcoming surgical treatment; mild LSS, characterised by radiographic narrowing of the lumbar spinal canal, but clinical signs and symptoms feeble enough to exclude surgical intervention; spinal stenosis not caused by degeneration, e.g. congenital spinal stenosis; spondylolysis and spondylolytic spondylolisthesis; previous back operation due to spinal stenosis or instability; lumbar herniated disc diagnosed during last 12 months; another specific spinal disorder, e.g. ankylosing spondylitis, neoplasm or metabolic disease; intermittent claudication due to atherosclerosis; severe osteoarthrosis or arthritis causing dysfunction of the lower limbs; neurological disease causing impaired function of the lower limbs, including diabetic neuropathy; psychiatric disorders; alcoholism | |
| Interventions | Surgical group: segmental decompression and an undercutting facetectomy of the affected area performed. Presence or risk of lumbar instability was, at the surgeon’s discretion, treated by fusion of the lumbar spine, if necessary, augmented by transpedicular instrumentation. Treated individuals also received a brochure and instructions about pain relief and management Conservative treatment: non‐steroidal anti‐inflammatory drugs prescribed when indicated and individuals referred to physiotherapists. Participants were seen 1 to 3 times by a physiotherapist, in addition to the standard visit at each follow‐up occasion. The physiotherapist gave all participants a printed brochure describing the nature of spinal stenosis, characteristic symptoms and signs of the disease and the principles of activation and physical training | |
| Outcomes | Outcomes: 11‐point numerical pain scale for leg and back, Oswestry Disability Index, walking ability (distance without a break measured by treadmill), General Health Status (very good, quite good, average, quite poor, very poor) Time points: 6, 12 and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random blocks of variable size separate for each hospital |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants not possible for the types of interventions compared |
| Incomplete outcome data (attrition bias) | High risk | Analysis performed only for completers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Group similarity at baseline (selection bias) | Unclear risk | Similar characteristics between groups at baseline |
| Co‐interventions (performance bias) | High risk | Co‐intervention unbalanced in control groups as 24% performed supplementary exercise |
| Compliance (performance bias) | High risk | Compliance not monitored in the control group |
| Intention‐to‐treat‐analysis | Low risk | ITT performed |
| Timing of outcome assessments (detection bias) | Low risk | Similar timing of outcome assessments for both groups |
| Other bias | Unclear risk | Insufficient details given |
| Methods | Multi‐centre randomised controlled trial and prospective observational study | |
| Participants | 289 participants with a history of neurogenic claudication or radicular leg symptoms ≥ 12 weeks and confirmatory cross‐sectional imaging showing lumbar spinal stenosis at ≥ 1 level were included in the randomly assigned arm. Mean age 65.5 ± 10.5, females 38%. Patients with degenerative spondylolisthesis on instability were excluded. 138 participants were assigned to the surgical group, and 151 to the non‐surgical group | |
| Interventions | The protocol surgery was standard posterior decompressive laminectomy. The non‐surgical protocol consisted of “usual care”, which was recommended to include at least active physical therapy, education or counselling with home exercise instruction and administration of non‐steroidal anti‐inflammatory drugs, if tolerated | |
| Outcomes | Outcomes: SF‐36, Oswestry Disability Index (MODEMS version), Low Back Pain Bothersomeness Scale, Leg Pain Bothersomeness Scale, Stenosis Bothersomeness Index, Self‐Reported Satisfaction Time points: 6 weeks, 3 months, 6 months, and 1, 2 and 4 years | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with variable clock size stratified according to centre |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants not possible because of the types of interventions compared |
| Incomplete outcome data (attrition bias) | High risk | Large number of cross‐overs made ITT impossible after the first phase |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Group similarity at baseline (selection bias) | High risk | Worst pain, function and disability at baseline in surgery group |
| Co‐interventions (performance bias) | Unclear risk | No co‐intervention unbalance |
| Compliance (performance bias) | High risk | Compliance not monitored in the control group |
| Intention‐to‐treat‐analysis | High risk | Large number of cross‐overs made ITT impossible after first phase data from the randomly assigned arm were mixed with those from the observational arm. Data from the randomly assigned arm were presented separately only for the as‐treated protocol |
| Timing of outcome assessments (detection bias) | Low risk | Similar for both groups |
| Other bias | Unclear risk | Insufficient details given |
| Methods | Multi‐centre randomised clinical trial | |
| Participants | Nine centres randomly assigned 200 participants between May of 2000 and July of 2001, in a prospective, controlled trial. Of 200 participants enrolled in this study, 191 were treated: 100 in the X STOP group and 91 in the conservative group. Most of the 9 patients from conservative group who withdrew from the study before receiving their initial epidural injection entered the study with the hope of being randomly assigned to the X STOP group Inclusion criteria: ≥ 50 years of age with leg, buttock or groin pain, with or without back pain, that could be relieved during flexion; able to sit for 50 minutes without pain and to walk 50 or more feet; completed ≥ 6 months of non‐operative therapy. Stenosis was confirmed by CT or MRI scans at 1 or 2 levels Primary exclusion criteria: fixed motor deficit, cauda equina syndrome, | |
| Interventions | Participants enrolled in the X STOP group underwent surgery for implantation of the interspinous implant. Those randomly assigned to the conservative group received ≥ 1 epidural steroid injection and could receive non‐steroidal anti‐inflammatory drugs, analgesics and physical therapy. Physical therapy consisted of back school and modalities such as ice packs, heat packs, massage, stabilisation exercises and pool therapy. Braces, such as abdominal binders and corsets, were permitted, but body jackets and chair‐back braces were not allowed | |
| Outcomes | Outcomes: SF‐36, Zurich Claudication Questionnaire (ZCQ), Oswestry Disabilty Index, Worker's Compensation Claim, radiographic changes Time points: 6 weeks, 6 months, 1 year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation by surgical centre |
| Allocation concealment (selection bias) | Low risk | After baseline examination and questionnaires were completed, treating physician phoned the central office, gave participant identification data and received treatment allocation |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants not possible for the types of interventions compared |
| Incomplete outcome data (attrition bias) | High risk | Only data from completers used |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Group similarity at baseline (selection bias) | Low risk | Similar groups at baseline |
| Co‐interventions (performance bias) | High risk | Co‐interventions not standardised and not properly described |
| Compliance (performance bias) | High risk | Compliance not monitored for the control group |
| Intention‐to‐treat‐analysis | Unclear risk | Not described |
| Timing of outcome assessments (detection bias) | Unclear risk | Similar timing for both groups |
| Other bias | Unclear risk | Not clear |
Abbreviations:
ASA: acetylsalicylic acid.
COPD: chronic obstructive pulmonary disease.
CT: computed tomography.
ESI: epidural steroid injections
ITT: intention‐to‐treat.
LSS: lumbar spinal stenosis.
MRI: magnetic resonance imaging.
ODI: Oswestry Disability Index.
SF‐36: Short Form 36.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomly assigned | |
| Not randomly assigned | |
| Not randomly assigned | |
| Review, not original data | |
| Not randomly assigned | |
| Protocol of a cohort study | |
| Commentary, no data | |
| Not randomly assigned | |
| Not randomly assigned | |
| Not randomly assigned | |
| Mixed population including spondylolisthesis | |
| Cost‐effectiveness analysis of mixed population including spondylolisthesis and disc herniation |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multi‐site randomised controlled trial |
| Participants | 169 participants with lumbar spinal stenosis (LSS) 50 years of age or older. 87 underwent surgery and 82 physical therapy (PT) |
| Interventions | Surgical decompression vs physical therapy |
| Outcomes | Mean improvement in physical function for surgery and PT groups was 22.4 (95% confidence interval (CI) 16.9 to 27.9) and 19.2 (95% CI 13.6 to 24.8), respectively Intention‐to‐treat analyses revealed no differences between groups (24‐month difference 0.9, 95% CI ‐7.9 to 9.6). Sensitivity analyses using causal‐effects methods to account for the high proportion of cross‐overs from PT to surgery (57%) showed no significant differences in physical function between groups |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Verbiest |
| Methods | Multi‐centre randomised controlled trial |
| Participants | Patients (> 50 years of age) with ≥ 3 months of complaints of neurogenic intermittent claudication and considering surgical treatment are eligible for inclusion |
| Interventions | Prolonged conservative treatment vs surgery |
| Outcomes | Primary
Secondary
|
| Starting date | N/A |
| Contact information | |
| Notes | NTR2216 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Decompression ± fusion vs usual conservative care for Oswestry Disability Index, Outcome 1 Oswestry Disability Index. | ||||
| 1.1 6 months | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐3.66 [‐10.12, 2.80] |
| 1.2 1 year | 2 | 340 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐15.02, 2.67] |
| 1.3 2 years | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐7.91, ‐0.96] |
| 2 Pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Decompression ± fusion vs usual conservative care for Oswestry Disability Index, Outcome 2 Pain. | ||||
| 2.1 3 months | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.22, 8.59] |
| 2.2 4 years | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.5 [1.00, 56.48] |
| 2.3 10 years | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [0.95, 17.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.7 [0.57, 10.83] |
| Analysis 2.1  Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 1 Oswestry Disability Index. | ||||
| 1.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.7 [0.57, 10.83] |
| 2 Visual Analogue Scale Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [1.92, 2.88] |
| Analysis 2.2 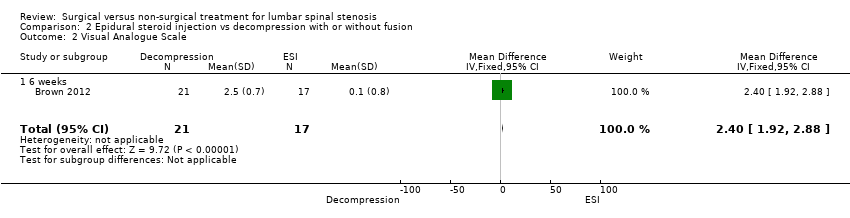 Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 2 Visual Analogue Scale. | ||||
| 2.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [1.92, 2.88] |
| 3 Zurich Claudication Questionnaire Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.77, ‐0.43] |
| Analysis 2.3  Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 3 Zurich Claudication Questionnaire. | ||||
| 3.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.77, ‐0.43] |
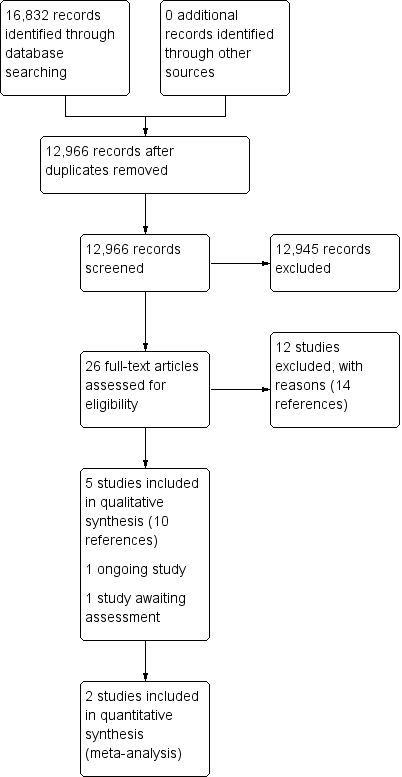
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
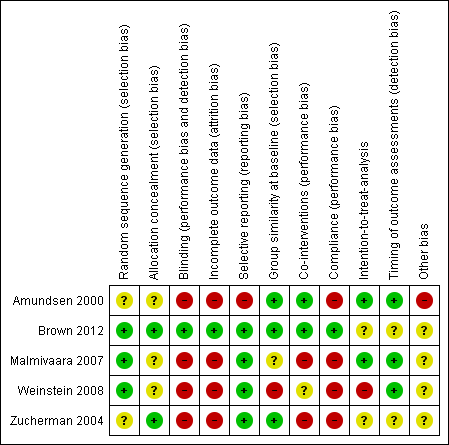
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Decompression ± fusion vs usual non‐operative care for Oswestry Disability Index, outcome: 1.1 Oswestry Disability Index [%].](/es/cdsr/doi/10.1002/14651858.CD010264.pub2/media/CDSR/CD010264/image_n/nCD010264-AFig-FIG04.png)
Forest plot of comparison: 1 Decompression ± fusion vs usual non‐operative care for Oswestry Disability Index, outcome: 1.1 Oswestry Disability Index [%].

Forest plot of comparison: 1 Decompression ± fusion versus usual non‐operative care for adverse events.

Comparison 1 Decompression ± fusion vs usual conservative care for Oswestry Disability Index, Outcome 1 Oswestry Disability Index.

Comparison 1 Decompression ± fusion vs usual conservative care for Oswestry Disability Index, Outcome 2 Pain.
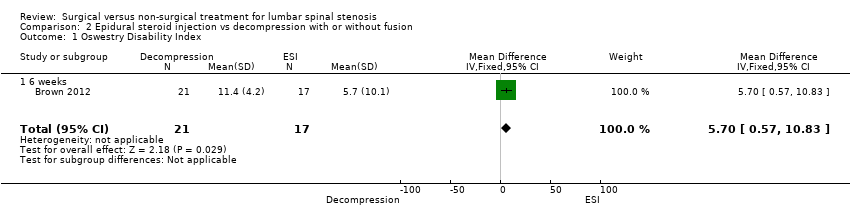
Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 1 Oswestry Disability Index.

Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 2 Visual Analogue Scale.

Comparison 2 Epidural steroid injection vs decompression with or without fusion, Outcome 3 Zurich Claudication Questionnaire.
| Decompression ±fusion vs usual conservative care for Oswestry Disabilty Index and Visual Analogue Pain Scale (VAS) for lumbar spinal stenosis | |||||
| Patient or population: lumbar spinal stenosis Intervention: decompression ± fusion Comparison: usual conservative care | |||||
| Outcomes | Relative effect | Outcome means | Number of participants | Quality of the evidence | |
| Oswestry Disability Index ‐ 6 months (0 to 100%) | (MD ‐3.66%, 95% CI ‐10.12 to 2.80) | Decompression range: 20.7 to 28.1 Usual conservative care range: 28.3 to 29.0 | 349 (2) | ⊕⊕⊝⊝ | |
| Oswestry Disability Index ‐ 1 year (0 to 100%) | (MD ‐6.17%, 95% CI ‐15.02 to 2.67) | Decompression range: 18.9 to 27.8 Usual conservative care range: 30.0 to 30.2 | 340 (2) | ⊕⊕⊝⊝ | |
| Oswestry Disability Index ‐ 2 years (0 to 100%) | (MD ‐4.43%, 95% CI ‐7.91 to ‐0.96) | Decompression range: 21.2 to 26.3 Usual conservative care range: 29 to 29.8 | 315 (2) | ⊕⊕⊝⊝ | |
| Pain ‐ 3 months (0 to 10) | (RR 1.38, 95% CI 0.22 to 8.59) | Decompression: 5.45 Usual conservative care: 2.81 | 31 (1) | ⊕⊕⊝⊝ | |
| Pain ‐ 4 years (0 to 10) | (RR 7.50, 95% CI 1.00 to 56.48) | Decompression: 5.05 Usual conservative care: 2.72 | 30 (1) | ⊕⊕⊝⊝ | |
| Pain ‐ 10 years (0 to 10) | (RR 4.09, 95% CI 0.95 to 17.58) | Decompression: 4.87 Usual conservative care: 2.74 | 29 (1) | ⊕⊕⊝⊝ | |
| CI: confidence interval; MD: mean difference; RR: risk ratio | |||||
| Studies failed on 3 of 5 GRADE factors, including:
| |||||
| Epidural steroid injection vs mild decompression ±fusion for lumbar spinal stenosis | |||||
| Patient or population: lumbar spinal stenosis Intervention: epidural steroid injection Comparison: decompression ± fusion | |||||
| Outcomes | Relative effect | Outcome means | Number of participants | Quality of the evidence | Comments |
| Oswestry Disability Index ‐ 6 weeks | (MD 5.70, 95% CI 0.57 to 10.83) | Epidural injection: 34.8 Mild decompression: 27.4 | 38 (1) | ⊕⊕⊝⊝ | |
| Visual Analogue Scale (VAS) ‐ 6 weeks | (MD 2.40, 95% CI 1.92 to 2.88) | Epidural injection: 6.3 Mild decompression: 3.8 | 38 (1) | ⊕⊕⊝⊝ | |
| Zurich Claudication Questionnaire ‐ 6 weeks | (MD ‐0.60, 95% CI ‐0.77 to ‐0.43) | Epidural injection: 2.8 Mild decompression: 2.2 | 38 (1) | ⊕⊕⊝⊝ | |
| CI: confidence interval; MD: mean difference | |||||
| Although this study had low risk of bias, this was the only study examined. Further research is very likely to have an impact on our confidence | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐3.66 [‐10.12, 2.80] |
| 1.2 1 year | 2 | 340 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐15.02, 2.67] |
| 1.3 2 years | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐7.91, ‐0.96] |
| 2 Pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.22, 8.59] |
| 2.2 4 years | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.5 [1.00, 56.48] |
| 2.3 10 years | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [0.95, 17.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.7 [0.57, 10.83] |
| 1.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.7 [0.57, 10.83] |
| 2 Visual Analogue Scale Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [1.92, 2.88] |
| 2.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [1.92, 2.88] |
| 3 Zurich Claudication Questionnaire Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.77, ‐0.43] |
| 3.1 6 weeks | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.77, ‐0.43] |

