Usporedba učinkovitosti životinjskog surfaktanta za prevenciju i liječenje sindroma respiratornoga poremećaja (distresa, RDS) u prijevremeno rođene djece
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010249.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Drs Singh, Halliday, Soll, and Stevens all participated in drafting and reviewing the protocol for the systematic review of 'Comparison of animal‐derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants'. Dr. Suresh performed an independent literature search, and reviewed the content and the language of the review. Dr. Rojas performed the assessment of the evidence quality following the GRADE approach, developed the 'Summary of findings' tables and included the quality‐of‐evidence‐related aspects to the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
Dr Soll has previously acted as a consultant for several of the pharmaceutical companies that manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Farmaceutici, Dey Laboratories, Burroughs Wellcome). Dr. Soll has not acted as a paid consultant for the past nine years.
Dr Halliday is currently a consultant for Chiesi Farmaceutici, a pharmaceutical company that manufactures a porcine‐derived surfactant preparation; and has been an invited speaker at meetings supported by Abbott Laboratories, Ross Laboratories and Burroughs Wellcome.
Dr Stevens has no known conflicts of interest. This will be further clarified prior to publication of the review.
Dr Singh has no conflict of interest to report.
Dr Suresh has no conflict of interest to report.
Dr. Rojas has no conflict of interest to report.
Acknowledgements
We would like to thank Paola Orrego, medical student participant of the "Seed Research Program in Systematic Reviews" at the Department of Clinical Epidemiology and Biostatistics, Pontificia Universidad Javeriana, Bogotá, Colombia for input and assistance in creating the 'Summary of Findings' tables.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 21 | Comparison of animal‐derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants | Review | Neetu Singh, Henry L Halliday, Timothy P Stevens, Gautham Suresh, Roger Soll, Maria Ximena Rojas‐Reyes | |
| 2012 Nov 14 | Comparison of animal‐derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants | Protocol | Neetu Singh, Henry L Halliday, Timothy P Stevens, Roger Soll | |
Differences between protocol and review
Methodology for 'Summary of findings' tables added.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Animals; Humans; Infant, Newborn;
PICO

Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.2 Mortality prior to discharge.
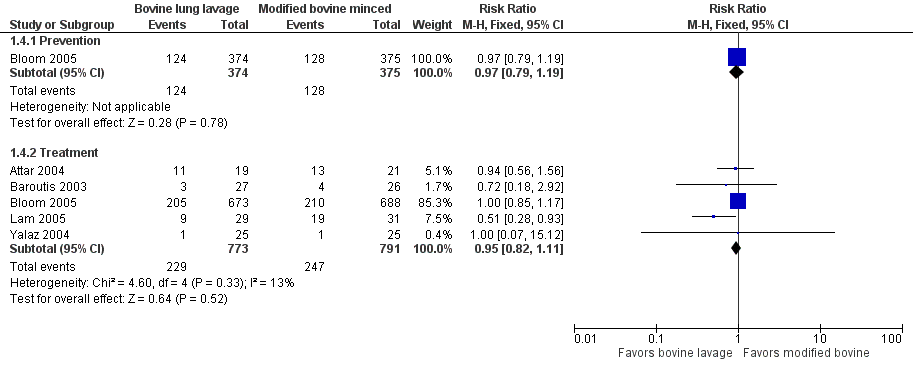
Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.4 Oxygen requirement at 36 weeks postmenstrual age (all infants).
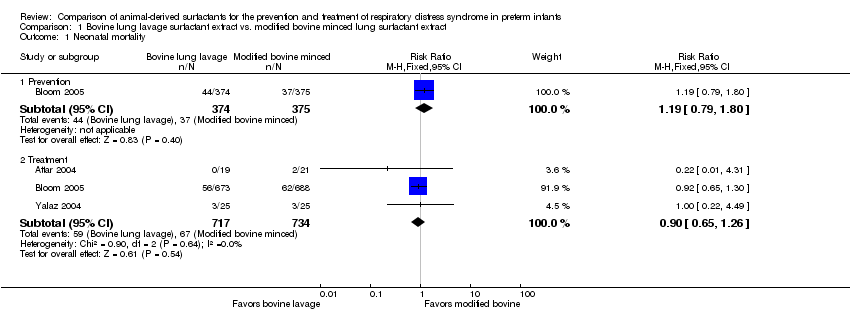
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 1 Neonatal mortality.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 2 Mortality prior to discharge.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 3 Oxygen requirement at 28 to 30 days of age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 5 Death or oxygen requirement at 28 to 30 days of age.
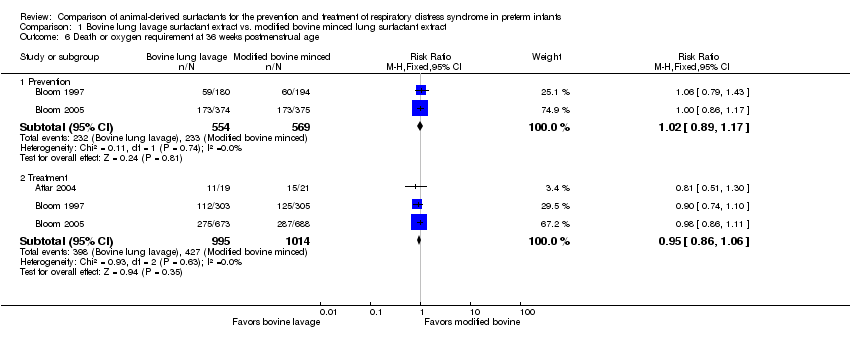
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 6 Death or oxygen requirement at 36 weeks postmenstrual age.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 7 Received > one dose of surfactant.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 8 Pneumothorax.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 9 Air leak syndromes.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 10 Pulmonary hemorrhage.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 11 Treated patent ductus arteriosus (PDA).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 12 Culture‐confirmed bacterial sepsis.
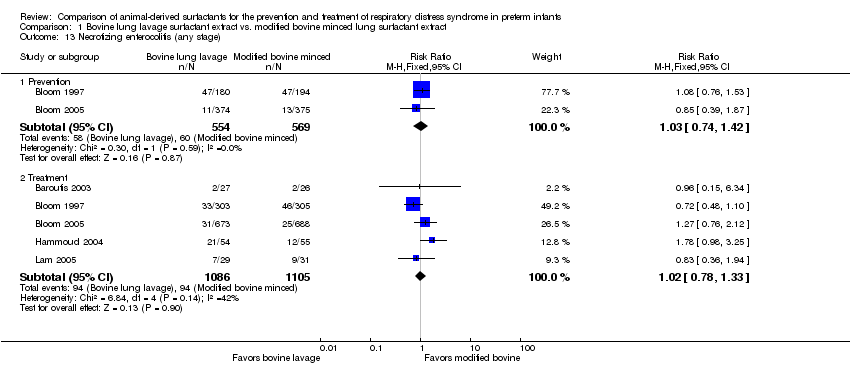
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 13 Necrotizing enterocolitis (any stage).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 14 Periventricular leukomalacia.
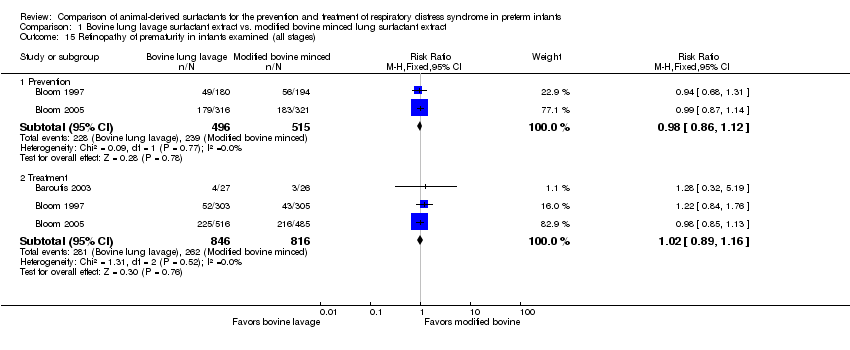
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 15 Retinopathy of prematurity in infants examined (all stages).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 18 Severe IVH in infants receiving neuroimaging.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 1 Mortality prior to discharge.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 2 Oxygen requirement at 36 weeks postmenstrual age.
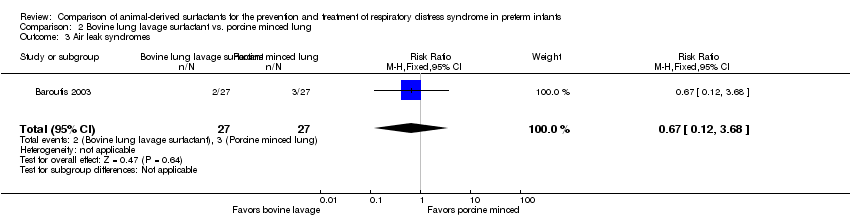
Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 3 Air leak syndromes.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 4 Necrotizing enterocolitis (any stage).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 5 Retinopathy of prematurity in infants examined (all stages).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 6 Severe IVH.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 1 Neonatal mortality.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 2 Mortality prior to discharge.
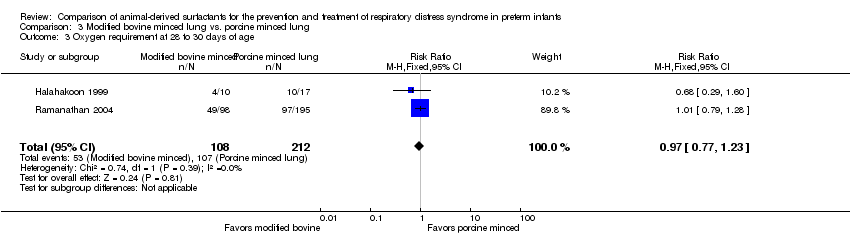
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
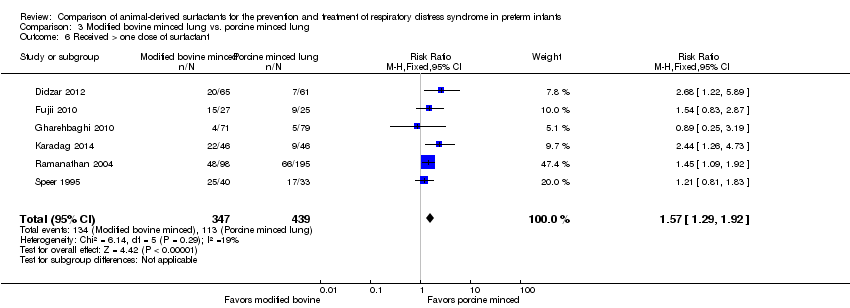
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 6 Received > one dose of surfactant.
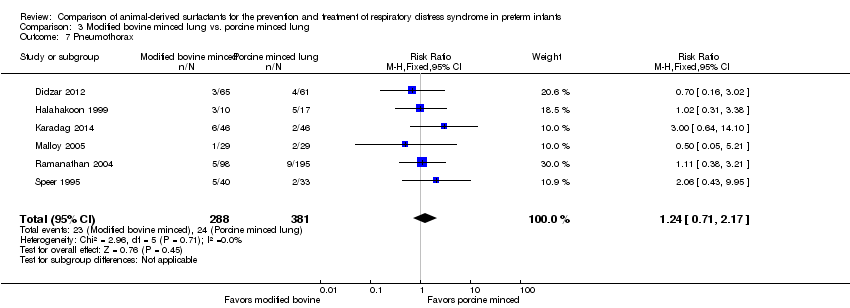
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 7 Pneumothorax.
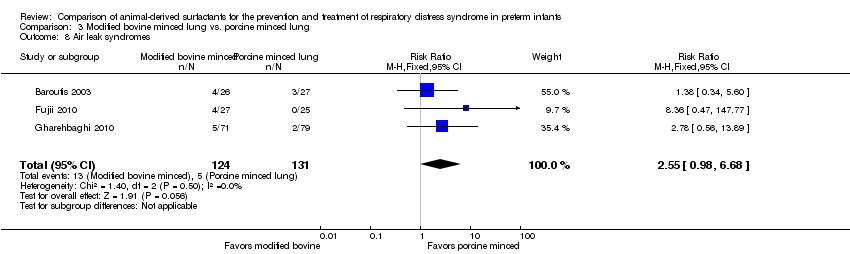
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 8 Air leak syndromes.
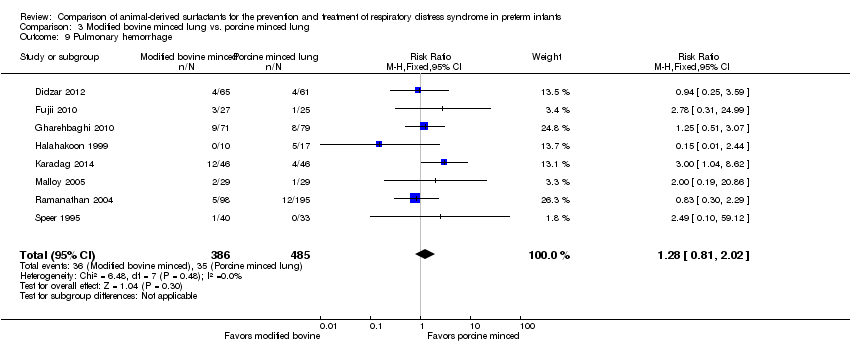
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 9 Pulmonary hemorrhage.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 10 Treated patent ductus arteriosus (PDA).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 11 Culture‐confirmed bacterial sepsis.
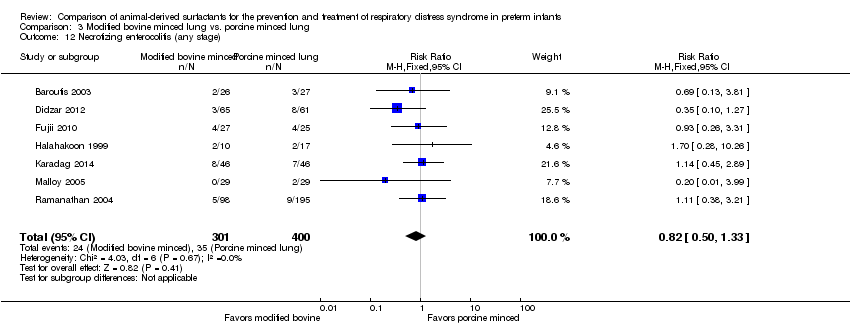
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 12 Necrotizing enterocolitis (any stage).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 13 Periventricular leukomalacia.
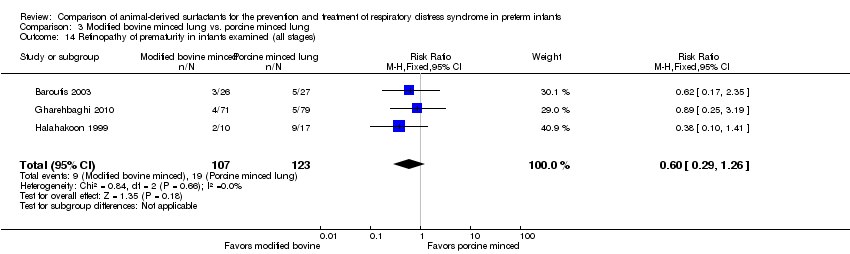
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 14 Retinopathy of prematurity in infants examined (all stages).
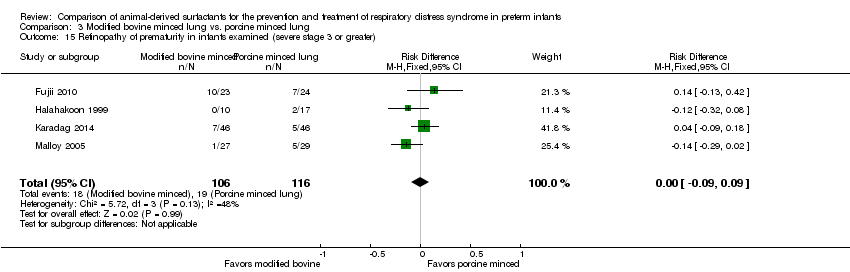
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 16 Intraventricular hemorrhage (all grades).
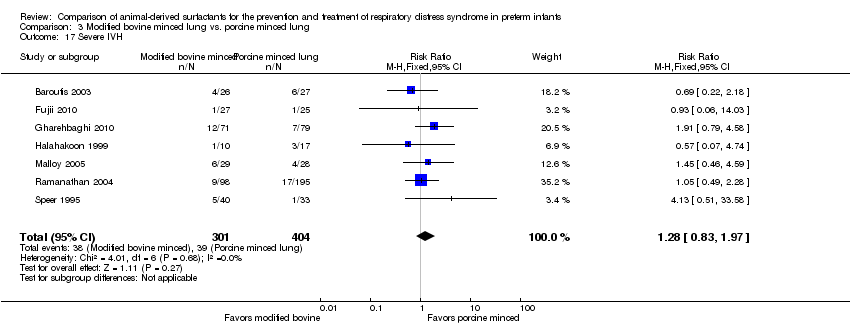
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 17 Severe IVH.

Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 1 Mortality prior to discharge.
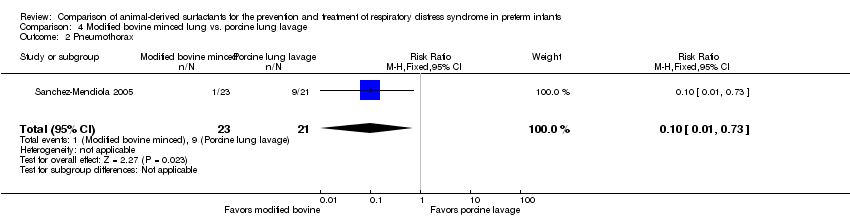
Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 2 Pneumothorax.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 1 Neonatal mortality.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 2 Mortality prior to discharge.
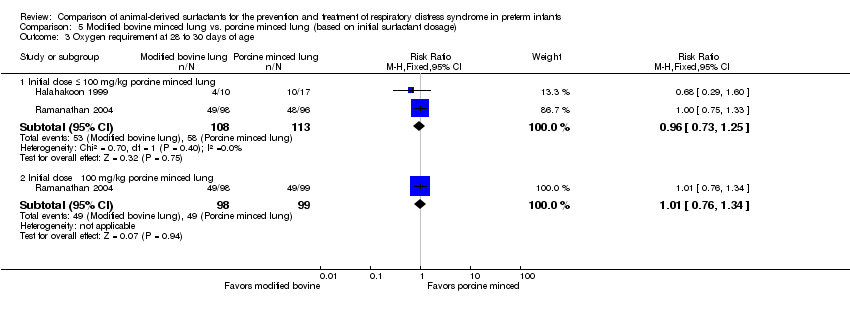
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.
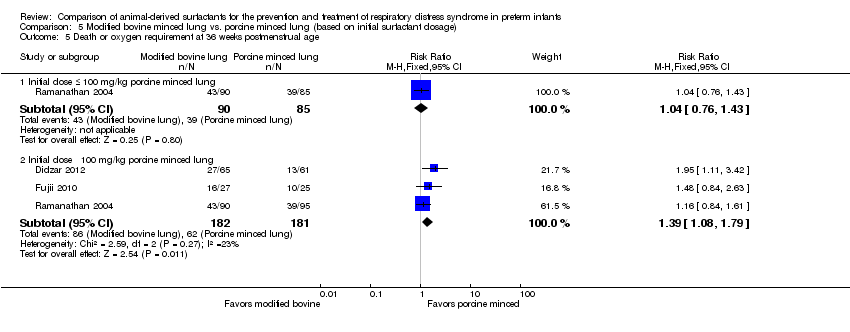
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for prevention of RDS (Comparision 1: Prevention studies) | ||||||
| Patient or population: Preterm infants for prevention of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge (from any cause) | 107 per 1000 | 133 per 1000 | RR 1.24 | 1123 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and and the total number of events does not meet the optimal information size (OIS). |
| Oxygen requirement at 36 weeks' postmenstrual age | 341 per 1000 | 331 per 1000 | RR 0.97 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 409 per 1000 | 418 per 1000 | RR 1.02 | 1133 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the no effect. The total number of events meets the OIS |
| Pneumothorax. | 67 per 1000 | 51 per 1000 | RR 0.76 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Pulmonary hemorrhage | 44 per 1000 | 63 per 1000 | RR 1.44 | 1123 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Severe IVH in infants receiving neuroimaging | 87 per 1000 | 112 per 1000 | RR 1.28 | 1087 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) 95% CI includes both no effect and appreciable harm.2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 2000). |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefit, no effect and appreciable harm and the total number of events does not meet the optimal information size 2 95% CI includes benefit, no effect and appreciable harm 3 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 3000 4 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 2000 | ||||||
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 131 per 1000 | 128 per 1000 | RR 0.98 | 2231 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the OIS. |
| Oxygen requirement at 36 weeks' postmenstrual age (all infants) | 312 per 1000 | 297 per 1000 | RR 0.95 | 1564 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 421 per 1000 | 400 per 1000 | RR 0.95 | 2009 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Pneumothorax | 73 per 1000 | 83 per 1000 | RR 1.14 | 2224 | ⨁⨁◯◯ | Downgraded two levels due to: 1) Serious imprecision (95% CI includes both no effect and appreciable harm). 2) Inconsistency: Unexplained heterogeneity, with point estimates widely different; 95% CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) |
| Pulmonary hemorrhage | 44 per 1000 | 48 per 1000 | RR 1.08 | 2138 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Severe IVH in infants receiving neuroimaging | 125 per 1000 | 108 per 1000 | RR 0.86 | 2040 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes benefits, no effect and appreciable harm). The optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefits, no effect and appreciable harm, and the total number of events does not meet the OIS 2 Unexplained heterogeneity, with point estimates widely different and CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) 3 95% CI of the pooled effect crosses 1 and the optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 | ||||||
| Bovine lung lavage surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 185 per 1000 | 259 per 1000 | RR 1.40 | 54 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Oxygen requirement at 36 weeks' postmenstrual age | 148 per 1000 | 111 per 1000 | RR 0.75 | 54 | ⨁⨁◯◯ | Downgraded two levels due to: 1. Serious imprecision (95% CI includes both no effect and appreciable harm). 2. Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | see comments | see comments | Not reported in any of the studies | |||
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | 222 per 1000 | 184 per 1000 | RR 0.83 | 54 | ⨁◯◯◯ | Downgraded three levels due to: 1. potential risk of bias (lack of blinding of outcome assessment) 2. very serious imprecision: (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 2 We downgraded because lack of blinding of patients, providers and blinding of outcome assessment 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 113 per 1000 | 162 per 1000 | RR 1.44 | 901 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Oxygen requirement at 36 weeks' postmenstrual age | 282 per 1000 | 293 per 1000 | RR 1.04 (0.83 to 1.31) | 773 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 380 per 1000 | 494 per 1000 | RR 1.30 | 448 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (total number of events does not meet the OIS) |
| Pneumothorax | 63 per 1000 | 78 per 1000 | RR 1.24 | 669 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Pulmonary hemorrhage | 72 per 1000 | 92 per 1000 | RR 1.28 | 871 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Severe intraventricular hemorrhage in infants who received neuroimaging | 97 per 1000 | 124 per 1000 | RR 1.28 | 705 | ⨁◯◯◯ | Downgraded three levels due to: 1. Potential risk of bias and 2. serious imprecision: 1) the 95% CI includes both no effect and appreciable harm; and 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The total number of events does not meet the OIS 2 95% CI includes benefit, no effect and appreciable harm. Total number of events does not meet the optimal information size. 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 5000 4 Studies that carried large weight for the overall effect estimate are classified as high or unclear risk of bias due to lack of blinding in patients, and outcome assessment 5 95% CI of the pooled effect widely crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 3000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine lung lavage surfactant in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine lung lavage surfactant | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 476 per 1000 | 524 per 1000 | RR 1.10 | 44 | ⨁⨁◯◯ | Downgraded two levels due to serious imprecision: 1) The 95% CI includes both no effect and appreciable harm. 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 700). |
| Oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | 429 per 1000 | 43 per 1000 | RR 0.10 | 44 | ⨁⨁⨁⨁ | |
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | see comments | see comments | Not reported in any of the studies | |||
| Neurodevelopmental outcome at approximately two years corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95 % CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 700 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.80] |
| 1.2 Treatment | 3 | 1451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 2 Mortality prior to discharge Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 2.2 Treatment | 6 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 3 Oxygen requirement at 28 to 30 days of age (all infants) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.2 Treatment | 3 | 1510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.21] |
| 4 Oxygen requirement at 36 weeks postmenstrual age (all infants) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.19] |
| 4.2 Treatment | 5 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.11] |
| 5 Death or oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
| 5.2 Treatment | 2 | 1401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 6 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 6.2 Treatment | 3 | 2009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 7 Received > one dose of surfactant Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7.2 Treatment | 5 | 2178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 8 Pneumothorax Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.36] |
| 8.2 Treatment | 6 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.51] |
| 9 Air leak syndromes Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 9.2 Treatment | 3 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 10 Pulmonary hemorrhage Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.88, 2.39] |
| 10.2 Treatment | 4 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.59] |
| 11 Treated patent ductus arteriosus (PDA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.34] |
| 12 Culture‐confirmed bacterial sepsis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 12.2 Treatment | 6 | 2228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 13 Necrotizing enterocolitis (any stage) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 13.2 Treatment | 5 | 2191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 14 Periventricular leukomalacia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.26] |
| 14.2 Treatment | 1 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.59, 1.73] |
| 15 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Prevention | 2 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 15.2 Treatment | 3 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Prevention | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 16.2 Treatment | 1 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 17.2 Treatment | 3 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 18 Severe IVH in infants receiving neuroimaging Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Prevention | 2 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.89, 1.83] |
| 18.2 Treatment | 5 | 2040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.51, 3.87] |
| 2 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.04] |
| 3 Air leak syndromes Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 4 Necrotizing enterocolitis (any stage) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 5 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.24, 2.66] |
| 6 Severe IVH Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.29, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.72, 3.07] |
| 2 Mortality prior to discharge Show forest plot | 9 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 2.00] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.64] |
| 6 Received > one dose of surfactant Show forest plot | 6 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.29, 1.92] |
| 7 Pneumothorax Show forest plot | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| 8 Air leak syndromes Show forest plot | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.98, 6.68] |
| 9 Pulmonary hemorrhage Show forest plot | 8 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| 10 Treated patent ductus arteriosus (PDA) Show forest plot | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.28, 2.70] |
| 11 Culture‐confirmed bacterial sepsis Show forest plot | 6 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| 12 Necrotizing enterocolitis (any stage) Show forest plot | 7 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.33] |
| 13 Periventricular leukomalacia Show forest plot | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.72] |
| 14 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 4 | 222 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| 16 Intraventricular hemorrhage (all grades) Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.50] |
| 17 Severe IVH Show forest plot | 7 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.83, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.60, 1.99] |
| 2 Pneumothorax Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.62] |
| 1.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.74, 9.86] |
| 2 Mortality prior to discharge Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.96] |
| 2.2 Initial dose ˃ 100 mg/kg porcine minced lung | 7 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.11, 2.38] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.25] |
| 3.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.37] |
| 4.2 Initial dose ˃ 100 mg/kg porcine minced lung | 6 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Initial dose ≤ 100 mg/kg porcine minced lung | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.76, 1.43] |
| 5.2 Initial dose ˃ 100 mg/kg porcine minced lung | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.08, 1.79] |

