Comparación de surfactantes de origen animal para la prevención y el tratamiento del síndrome de dificultad respiratoria en lactantes prematuros
Resumen
Antecedentes
Los surfactantes de origen animal han mostrado tener varias ventajas sobre los surfactantes sintéticos de primera generación y son las preparaciones de surfactantes utilizadas con más frecuencia. Los surfactantes de origen animal en uso clínico se obtienen a partir de pulmones bovinos o porcinos triturados o lavados y modificados o purificados. No está claro si existen diferencias significativas en el resultado clínico entre los extractos de surfactantes bovino (triturado o lavado modificado) y porcino (triturado o lavado) disponibles.
Objetivos
Comparar el efecto de la administración de diferentes extractos de surfactantes de origen animal sobre el riesgo de mortalidad, enfermedad pulmonar crónica y otras morbilidades asociadas con la prematurez en lactantes prematuros con riesgo de presentar o que presentan síndrome de dificultad respiratoria (SDR).
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología (Cochrane Neonatal Review group) para buscar en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL 2015, número 7), MEDLINE vía PubMed (1966 hasta 31 julio 2015), EMBASE (1980 hasta 31 julio 2015) y en CINAHL (1982 hasta 31 julio 2015). También se buscaron ensayos controlados aleatorios y ensayos cuasialeatorios en las bases de datos de ensayos clínicos, las actas de congresos y las listas de referencias de los artículos recuperados.
Criterios de selección
Ensayos controlados aleatorios o cuasialeatorios que compararon el efecto del tratamiento con extractos de surfactantes de origen animal administrados a los lactantes prematuros con riesgo de presentar o que presentan SDR para prevenir las complicaciones de la prematurez y la mortalidad.
Obtención y análisis de los datos
Los autores de la revisión extrajeron datos relacionados con resultados clínicos a partir de los informes de ensayos clínicos. Se realizaron análisis de subgrupos según la edad gestacional, la dosis y el régimen de surfactante, la intensidad del tratamiento y la estrategia de tratamiento. El análisis de los datos se realizó de acuerdo con las normas del Grupo de Revisión Cochrane de Neonatología (Cochrane Neonatal Review Group).
Resultados principales
Dieciséis ensayos controlados aleatorios se incluyeron en el análisis.
Extracto de surfactante de pulmones bovinos lavados versus extracto de surfactante modificado de pulmones bovinos triturados: Siete estudios de tratamiento y dos estudios de prevención compararon extracto de surfactante de pulmones bovinos lavados con extracto de surfactante modificado de pulmones bovinos triturados. El metanálisis no demostró diferencias significativas en la muerte o la enfermedad pulmonar crónica en los ensayos de prevención (CR típico 1,02; IC del 95%: 0,89 a 1,17; DR típica 0,01; IC del 95%: ‐0,05 a 0,06; dos estudios y 1123 lactantes; pruebas de alta calidad) ni en los ensayos de tratamiento (CR típico 0,95; IC del 95%: 0,86 a 1,06; DR típica ‐0,02; IC del 95%: ‐0,06 a 0,02; tres estudios y 2009 lactantes; pruebas de alta calidad)
Extracto de surfactante modificado de pulmones bovinos triturados comparado con extracto de surfactante de pulmones porcinos triturados: Nueve estudios de tratamiento compararon extracto de surfactante modificado de pulmones bovinos triturados con extracto de surfactante de pulmones porcinos triturados. El metanálisis de estos ensayos demuestra un aumento significativo en el riesgo de mortalidad antes del alta hospitalaria (CR típico 1,44; IC del 95%: 1,04 a 2,00; DR típica 0,05; IC del 95%: 0,01 a 0,10; NNTD 20; IC del 95%: 10 a 100; nueve estudios y 901 lactantes; pruebas de calidad moderada), muerte o necesidad de oxígeno a las 36 semanas de edad posmenstrual (CR típico 1,30; IC del 95%: 1,04 a 1,64; DR típica 0,11; IC del 95%: 0,02 a 0,20; NNTD 9; IC del 95%: 5 a 50; tres estudios y 448 lactantes; pruebas de calidad moderada), necesidad de recibir más de una dosis de surfactante (CR típico 1,57; IC del 95%: 1,29 a 1,92; DR típica 0,14; IC del 95%: 0,08 a 0,20; NNTD 7; IC del 95%: 5 a 13; seis estudios y 786 lactantes) y conducto arterioso permeable (CAP) que requirió tratamiento (CR típico 1,86; IC del 95%: 1,28 a 2,70; DR típica 0,28; IC del 95%: 0,13 a 0,43; NNTD 4; IC del 95%: 2 a 8; tres estudios y 137 lactantes) en los lactantes tratados con extracto de surfactante modificado de pulmones bovinos triturados en comparación con extracto de surfactante de pulmones porcinos triturados. En el análisis de subgrupos según la dosis inicial de surfactante, la mejora en la mortalidad antes del alta (CR típico 1,62; IC del 95%: 1,11 a 2,38; DR típica 0,06; IC del 95%: 0,01 a 0,11; NNTH 16; IC del 95%: 9 a 100) y el riesgo de muerte o la necesidad de oxígeno a las 36 semanas de edad posmenstrual (CR típico 1,39; IC del 95%: 1,08 a 1,79; DR típica 0,13; IC del 95%: 0,03 a 0,23; NNTH 7; IC del 95%: 4 a 33) estuvo limitada a la dosis inicial mayor de surfactante de pulmones porcinos triturados (> 100 mg/kg).
Otras comparaciones: No se observaron diferencias en el resultado entre el extracto de surfactante de pulmones bovinos lavados versus el extracto de surfactante de pulmones porcinos triturados. No hubo estudios que compararan el extracto de surfactante de pulmones bovinos lavados versus el surfactante de pulmones porcinos lavados; o el extracto de surfactante de pulmones porcinos triturados versus surfactante de pulmones porcinos lavados.
Conclusiones de los autores
Se observaron diferencias significativas en el resultado clínico en los ensayos que compararon el extracto de surfactante modificado de los pulmones triturados (beractant) en comparación con el extracto de surfactante de pulmones porcinos triturados (poractant alfa), que incluyeron un aumento significativo en el riesgo de mortalidad antes del alta, muerte o necesidad de oxígeno a las 36 semanas de edad posmenstrual, CAP que requirió tratamiento y "necesidad de recibir > 1 dosis de surfactante" en los lactantes tratados con extracto de surfactante modificado de pulmones bovinos triturados en comparación con el extracto de surfactante de pulmones porcinos triturados. La diferencia en estos resultados estuvo limitada a los estudios que utilizaron una dosis inicial mayor de extracto de surfactante de pulmones porcinos triturados. No está claro si las diferencias observadas se deben a diferencias en la dosis o en la fuente de extracción (porcino versus bovino) debido a la ausencia de grupos de comparación de dosis equivalentes con tamaños de la muestra apropiados. No se observaron diferencias en los resultados clínicos en los ensayos comparativos entre el surfactante de pulmones bovinos lavados y los surfactantes modificados de pulmones bovinos triturados.
PICO
Resumen en términos sencillos
Comparación de surfactantes de origen animal para la prevención y el tratamiento del síndrome de dificultad respiratoria en lactantes prematuros
Pregunta de la revisión: ¿El uso de una preparación de surfactante de origen animal en comparación con una preparación alternativa de surfactante de origen animal da lugar a una mejoría en el resultado en los lactantes con riesgo de presentar o que presentan síndrome de dificultad respiratoria?
Antecedentes: Para prevenir o tratar el síndrome de dificultad respiratoria (SDR) en los lactantes prematuros se utiliza una amplia variedad de preparaciones de surfactante. Todos los productos de surfactante de origen animal comercialmente disponibles son eficaces para la prevención y el tratamiento del síndrome de dificultad respiratoria en los lactantes prematuros. Sin embargo, no está claro si existen diferencias significativas en el resultado clínico entre los extractos de surfactantes de origen animal disponibles. Esta revisión comparó diferentes productos de surfactantes de origen animal según su fuente (bovino versus porcino) y método de extracción (pulmones triturados versus pulmones lavados).
Características de los estudios:16 ensayos controlados aleatorios cumplieron con los criterios de inclusión.
Resultados: Se encontró una mejoría en el riesgo de muerte antes del alta hospitalaria y el riesgo del resultado combinado de muerte o necesidad de oxígeno a las cuatro semanas antes de la fecha de nacimiento prevista con la administración de la dosis mayor de surfactante porcino (un producto surfactante derivado de los pulmones del cerdo) en comparación con un surfactante bovino triturado (surfactante obtenido de triturar los pulmones de la vaca). Según las pruebas disponibles, no se puede señalar con certeza si la mejoría en el resultado con la dosis alta del surfactante porcino se debe al efecto de la dosis (mayores niveles de fosfolípidos con el uso de una dosis inicial mayor) o al efecto de la fuente de extracción del surfactante (cerdo versus vaca). Los ensayos que compararon los surfactantes obtenidos a partir de fuentes bovinas que utilizaron diferentes métodos de extracción o modificaciones no mostraron diferencias en los resultados clínicos.
Authors' conclusions
Summary of findings
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for prevention of RDS (Comparision 1: Prevention studies) | ||||||
| Patient or population: Preterm infants for prevention of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge (from any cause) | 107 per 1000 | 133 per 1000 | RR 1.24 | 1123 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and and the total number of events does not meet the optimal information size (OIS). |
| Oxygen requirement at 36 weeks' postmenstrual age | 341 per 1000 | 331 per 1000 | RR 0.97 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 409 per 1000 | 418 per 1000 | RR 1.02 | 1133 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the no effect. The total number of events meets the OIS |
| Pneumothorax. | 67 per 1000 | 51 per 1000 | RR 0.76 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Pulmonary hemorrhage | 44 per 1000 | 63 per 1000 | RR 1.44 | 1123 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Severe IVH in infants receiving neuroimaging | 87 per 1000 | 112 per 1000 | RR 1.28 | 1087 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) 95% CI includes both no effect and appreciable harm.2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 2000). |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefit, no effect and appreciable harm and the total number of events does not meet the optimal information size 2 95% CI includes benefit, no effect and appreciable harm 3 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 3000 4 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 2000 | ||||||
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 131 per 1000 | 128 per 1000 | RR 0.98 | 2231 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the OIS. |
| Oxygen requirement at 36 weeks' postmenstrual age (all infants) | 312 per 1000 | 297 per 1000 | RR 0.95 | 1564 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 421 per 1000 | 400 per 1000 | RR 0.95 | 2009 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Pneumothorax | 73 per 1000 | 83 per 1000 | RR 1.14 | 2224 | ⨁⨁◯◯ | Downgraded two levels due to: 1) Serious imprecision (95% CI includes both no effect and appreciable harm). 2) Inconsistency: Unexplained heterogeneity, with point estimates widely different; 95% CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) |
| Pulmonary hemorrhage | 44 per 1000 | 48 per 1000 | RR 1.08 | 2138 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Severe IVH in infants receiving neuroimaging | 125 per 1000 | 108 per 1000 | RR 0.86 | 2040 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes benefits, no effect and appreciable harm). The optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefits, no effect and appreciable harm, and the total number of events does not meet the OIS 2 Unexplained heterogeneity, with point estimates widely different and CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) 3 95% CI of the pooled effect crosses 1 and the optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 | ||||||
| Bovine lung lavage surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 185 per 1000 | 259 per 1000 | RR 1.40 | 54 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Oxygen requirement at 36 weeks' postmenstrual age | 148 per 1000 | 111 per 1000 | RR 0.75 | 54 | ⨁⨁◯◯ | Downgraded two levels due to: 1. Serious imprecision (95% CI includes both no effect and appreciable harm). 2. Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | see comments | see comments | Not reported in any of the studies | |||
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | 222 per 1000 | 184 per 1000 | RR 0.83 | 54 | ⨁◯◯◯ | Downgraded three levels due to: 1. potential risk of bias (lack of blinding of outcome assessment) 2. very serious imprecision: (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 2 We downgraded because lack of blinding of patients, providers and blinding of outcome assessment 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 113 per 1000 | 162 per 1000 | RR 1.44 | 901 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Oxygen requirement at 36 weeks' postmenstrual age | 282 per 1000 | 293 per 1000 | RR 1.04 (0.83 to 1.31) | 773 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 380 per 1000 | 494 per 1000 | RR 1.30 | 448 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (total number of events does not meet the OIS) |
| Pneumothorax | 63 per 1000 | 78 per 1000 | RR 1.24 | 669 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Pulmonary hemorrhage | 72 per 1000 | 92 per 1000 | RR 1.28 | 871 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Severe intraventricular hemorrhage in infants who received neuroimaging | 97 per 1000 | 124 per 1000 | RR 1.28 | 705 | ⨁◯◯◯ | Downgraded three levels due to: 1. Potential risk of bias and 2. serious imprecision: 1) the 95% CI includes both no effect and appreciable harm; and 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The total number of events does not meet the OIS 2 95% CI includes benefit, no effect and appreciable harm. Total number of events does not meet the optimal information size. 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 5000 4 Studies that carried large weight for the overall effect estimate are classified as high or unclear risk of bias due to lack of blinding in patients, and outcome assessment 5 95% CI of the pooled effect widely crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 3000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine lung lavage surfactant in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine lung lavage surfactant | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 476 per 1000 | 524 per 1000 | RR 1.10 | 44 | ⨁⨁◯◯ | Downgraded two levels due to serious imprecision: 1) The 95% CI includes both no effect and appreciable harm. 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 700). |
| Oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | 429 per 1000 | 43 per 1000 | RR 0.10 | 44 | ⨁⨁⨁⨁ | |
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | see comments | see comments | Not reported in any of the studies | |||
| Neurodevelopmental outcome at approximately two years corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95 % CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 700 | ||||||
Background
Description of the condition
Respiratory distress syndrome (RDS) is caused by a deficiency or dysfunction of pulmonary surfactant (Avery 1959). Surfactant lines the alveolar surface and prevents atelectasis at end expiration. Pulmonary surfactant is predominantly dipalmitoylphosphatidylcholine (DPPC) with lesser amounts of other phospholipids including phosphatidylglycerol (PG), phosphatidylethanolamine, and phosphatidylinositol. Pulmonary surfactant also contains neutral lipids and four distinct surfactant proteins: SP‐A, SP‐B, SP‐C, and SP‐D. The physiologic functions of surfactant include the ability to lower surface tension, and the ability to rapidly adsorb, spread, and reform a monolayer in the dynamic conditions associated with the respiratory cycle (Jobe 1993).
Description of the intervention
Investigators in the 1960s attempted to administer aerosolized DPPC to infants with, or at risk of developing, RDS (Chu 1967; Robillard 1964). These investigators could not demonstrate any beneficial effect of surfactant replacement. In part, the poor results were because of an incomplete understanding of what constitutes pulmonary surfactant, and in part because of the inefficiency of the aerosolization. The first successful animal model of surfactant replacement therapy was developed by Enhorning and Robertson in the 1970s (Enhorning 1972). They administered a crude animal‐derived surfactant extract obtained from lavage of the lungs of mature rabbits directly into the trachea of immature rabbits. Improvement in lung compliance and alveolar expansion was noted. Success in animal models led to further clinical trials in newborn infants. The first successful experience with surfactant replacement therapy for infants with established RDS was reported in 1980 (Fujiwara 1980). These Japanese researchers studied a series of 10 preterm infants with severe RDS requiring assisted ventilation. The infants improved dramatically following treatment with Surfactant TA, a modified bovine surfactant extract, containing SP‐B and SP‐C.
Since the initial experience of Fujiwara and coworkers, multiple randomized trials of surfactant in the treatment of established RDS have been conducted (Seger 2009; Soll 1997; Soll 1998; Soll 2010).
A wide variety of surfactant products have been formulated and studied in clinical trials (Halliday 2008). These include synthetic (protein‐free) surfactants and animal‐derived surfactant extracts. Currently available animal‐derived surfactant extracts are mammalian in origin. These can be further classified as either modified or unmodified surfactant extracts; modified animal‐derived surfactant extract is supplemented with phospholipids or other surface‐active material, while unmodified animal‐derived surfactant extract contains only the components remaining after the extraction process. A further classification is based upon whether the surfactant is extracted from minced lungs (for example, beractant and poractant alfa) or lung lavage (for example, calfactant and bovactant). All these animal‐derived surfactants contain phospholipids and SP‐B and SP‐C in differing amounts.
Trials of surfactant replacement have either tried to prevent the development of RDS in high‐risk preterm infants (Soll 1997; Soll 2010); or treat established RDS in preterm infants (Seger 2009; Soll 1998). Using either approach, surfactant has been shown to decrease the risk of pneumothorax, decrease mortality, and improve survival without bronchopulmonary dysplasia (at 28 days of life) (Engle 2008). Animal‐derived surfactants have been compared with synthetic surfactants, and the advantages reported include lower mortality rates (typical risk ratio (RR) 0.86; 95% confidence interval (CI) 0.76 to 0.98; number needed to treat for an additional beneficial outcome (NNTB) 40), lower inspired oxygen and ventilation requirements early in the course of RDS, and fewer pneumothoraces (typical RR 0.63, 95% CI 0.53 to 0.75; NNTB 22) (Engle 2008; Soll 2001). First‐generation protein‐free synthetic surfactants (such as colfosceril palmitate and pumactant) are no longer widely available (Engle 2008). New synthetic surfactants containing proteins or peptides that mimic SP activity are under investigation (Pfister 2007; Pfister 2009).
How the intervention might work
Animal‐derived surfactants in clinical use are obtained by organic extraction of lung lavage fluid or minced lung and contain surfactant proteins SP‐B and SP‐C. SP‐A and SP‐D are extremely hydrophilic and do not remain in the preparation of any commercial natural surfactant. SP‐B and SP‐C are thought to be crucial in promoting the adsorption and spread of monolayers of dipalmitoylphosphatidylcholine (DPPC) (Hawgood 1985; Whitsett 1995). Different animal‐derived surfactants may differ in their source (bovine vs. porcine), method of extraction (minced vs. lavage), composition (viscosity, phospholipid content, amount of surfactant protein (SP B&C), and plasmalogen content), and dosing volume. It is unclear if significant differences in clinical outcomes exist among the available products (Engle 2008; Ramanathan 2009).
The following animal‐derived surfactants are known to be available and were included in the review:
1. Bovine lung lavage surfactant extract (calfactant, CLSE, also known as BLES or SF‐RI 1, also known as bovactant):
-
Calfactant (Infasurf/Forest Pharmaceuticals Inc. St Louis. MO, USA) contains high surfactant protein (SP B&C) and phospholipid (33.3 mg/ml); and is given at a dose of 100 mg phospholipid/kg.
-
Bovine Lung Expanding Substance (BLES/BLES Biochemicals, London, ON, Canada) contains 1% SP B&C; 27 mg/ml phospholipid; and is given at a dose of 137 mg phospholipid/kg.
-
Bovactant (Alveofact/Boehringer Ingelheim, Germany; (marketed and manufactured by Lyomark Pharma, Germany) contains 1% SP B&C; 41.7 mg/ml phospholipid and is given at a dose of 50 mg phospholipid/kg (Sweet 2013).
2 . Modified bovine minced lung surfactant extract (beractant or surfactant TA):
-
Beractant (Survanta/ Abbott Laboratories, Abbott Park, IL, USA) contains DPPC, tripalmitin and palmitic acid; less than 0.5% SP B&C; 25 mg/ml phospholipid; and is given at a dose of 100 mg phospholipid/kg.
-
Surfactant TA (Surfacten/ Tokyo Tanabe Co, Tokyo, Japan) contains DPPC, tripalmitin and palmitic acid; less than 0.5% SP B&C; 30 mg/ml phospholipid; and is given at a dose of 120mg phospholipid/kg.
3. Porcine minced lung surfactant extract (poractant alfa):
-
Poractant alfa (Curosurf/Chiesi Farmaceutici Parma, Italy) contains approximately 1% SP B&C; 80 mg/ml phospholipid; and is given at a dose of 100 to 200 mg phospholipid/kg. This surfactant extract undergoes an additional step during preparation called liquid gel chromatography. As a result, it contains only polar lipids and is more concentrated than any other animal‐derived surfactant preparation.
4. Porcine lung lavage surfactant extract (Surfacen):
-
Surfacen is an inexpensive porcine lung lavage surfactant developed in Cuba (Surfacen/Censa, Cuba).
Other animal‐derived products are being developed and tested, particularly in developing countries. At least two other surfactant preparations (Newfacten (Newfacten/Yuhan Co Ltd, Korea) and HL‐10 (HL‐10/Leo Pharma, Denmark)) were identified during our search; however, no study compared their efficacy with another surfactant preparation. Surfactants derived from human amniotic fluid are not included in this review as they are unavailable and have never been approved for use by regulatory agencies.
Why it is important to do this review
Cochrane reviews that address trials of pulmonary surfactant in neonates
Multiple systematic reviews have addressed the use of animal‐derived surfactant preparations or synthetic surfactant preparations in the prevention or treatment of respiratory distress syndrome. Meta‐analyses of the original randomized controlled trials of surfactant for the treatment and prevention of RDS were first published in Effective Care of the Newborn Infant (Soll 1992).
Since then, multiple systematic reviews have been published in The Cochrane Library, including reviews of protein‐free synthetic surfactant for the prevention and treatment of RDS (Soll 1998; Soll 2010); and reviews of animal‐derived surfactant for the prevention and treatment of RDS (Seger 2009; Soll 1997). Trials that compare animal‐derived surfactant extract to protein‐containing synthetic surfactant and trials that compare protein‐free synthetic surfactant to protein‐containing synthetic surfactant are addressed by other reviews (Pfister 2007; Pfister 2009). Comparison trials of animal‐derived products compared with protein‐free synthetic products have been reviewed by Soll and Blanco (Soll 2001). Prophylactic administration of surfactant to infants at high risk of developing RDS, compared with selective use of surfactant in infants with established RDS, has been shown to improve some clinical outcomes (Rojas‐Reyes 2012).
Clinical trials that compare various animal‐derived surfactant extract preparations to each other are included in this systematic review. The differences in composition of the animal‐derived surfactant preparations could potentially lead to differences in clinical efficacy.
The analysis included all randomized controlled trials in which animal‐derived surfactant extracts were compared with other animal‐derived products in the prevention or treatment of RDS. If similar in effect, differences in cost may be appropriate to consider in deciding which product to use. A previous review addressing only comparisons of bovine surfactant (beractant and calfactant) with porcine surfactant has been published by Singh and colleagues, but did not address comparisons between bovine‐derived products (Singh 2011).
Objectives
To compare the effect of administration of different animal‐derived surfactant extracts on the risk of mortality, chronic lung disease, and other morbidities associated with prematurity in preterm infants at risk for or having RDS.
Comparisons:
-
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) versus modified bovine minced lung surfactant extract (beractant or surfactant TA).
-
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF RI 1 (bovactant)) versus porcine minced lung surfactant extract (poractant alfa).
-
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) versus porcine lung lavage surfactant (Surfacen).
-
Modified bovine minced lung surfactant extract (beractant or surfactant TA) versus porcine minced lung surfactant extract (poractant alfa).
-
Modified bovine minced lung surfactant extract (beractant or surfactant TA) versus porcine lung lavage surfactant (Surfacen).
-
Porcine minced lung surfactant extract (poractant alfa) versus porcine lung lavage surfactant (Surfacen).
Subgroup analyses
-
Gestational age:
-
prevention trials: infants born at less than 28 weeks' gestation;
-
treatment trials: infants born at less than 30 weeks' gestation.
-
-
Surfactant dosage (initial dose up to 100 mg/kg)
-
Surfactant dosing schedule (single dose, multiple dose)
-
Treatment strategy (prevention versus treatment of established disease)
-
For treatment trials: disease severity (moderate to severe disease defined as need for assisted ventilation and supplemental oxygen concentration greater than 40% necessary to maintain adequate oxygenation).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials, quasi‐randomized controlled clinical trials, and cluster randomized trials for this review.
Types of participants
Prevention studies
Preterm infants (less than 32 weeks' gestation) at risk for developing RDS.
Treatment studies
Preterm infants (less than 37 weeks' gestation) with clinical and radiologic evidence of RDS requiring assisted ventilation.
Types of interventions
Prevention
Preterm infants at risk for RDS randomized to receive an animal‐derived surfactant preparation versus a different animal‐derived surfactant product.
Treatment
Preterm infants with established RDS randomized to receive an animal‐derived surfactant preparation versus a different animal‐derived surfactant product.
All included studies utilized surfactant products derived from mammalian sources (bovine or calf lung surfactant extract, modified bovine surfactant extract, and porcine surfactant extract).
In either comparison, any dosing regimen (single dose or multiple dose) and dosage were included.
Types of outcome measures
Primary outcomes
-
Neonatal mortality (mortality < 28 days of age) from any cause.
-
Mortality prior to hospital discharge (from any cause).
-
Chronic lung disease (in all infants):
-
oxygen requirement at 28 to 30 days of age;
-
oxygen requirement at 36 weeks' postmenstrual age.
-
-
Death or chronic lung disease:
-
death or oxygen requirement at 28 to 30 days of age;
-
death or oxygen requirement at 36 weeks' postmenstrual age.
-
Secondary outcomes
-
Doses of surfactant.
-
Pneumothorax.
-
Air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum).
-
Pulmonary hemorrhage.
-
Patent ductus arteriosus (PDA) (that has been treated with cyclo‐oxygenase inhibitor or surgery).
-
Culture‐confirmed bacterial sepsis.
-
Culture‐confirmed fungal sepsis.
-
Necrotizing enterocolitis (defined as Bell Stage II or greater) (Bell 1978).
-
Periventricular leukomalacia.
-
Retinopathy of prematurity in infants examined (all stages and severe (stage 3 or greater)) (ICCROP 2005).
-
Intraventricular hemorrhage (any grade and severe (grade 3 to 4)) (Papile 1978).
-
Cerebral palsy at approximately two years corrected age (as defined by the study authors).
-
Neurodevelopmental outcome at approximately two years corrected age (acceptable range 18 months to 28 months) including: cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity), and hearing deficit (aided or < 60 dB on audiometric testing). The composite outcome 'neurodevelopmental impairment' was defined as having any one of the aforementioned deficits.
We considered post hoc analyses for any unexpected adverse effects reported by the studies. Post hoc analysis was performed for the outcome "received > one dose of surfactant." For studies that did not report NEC based on Bell's criteria, we reported "any NEC."
We identified critical outcomes for decision making (primary and main secondary outcomes) including mortality prior to hospital discharge, chronic lung disease (defined as supplemental oxygen at 36 weeks' gestation), death or chronic lung disease (CLD) at 36 weeks' postmenstrual age, pneumothorax, pulmonary hemorrhage, severe intraventricular hemorrhage, and neurodevelopmental outcome at approximately two years' corrected age for inclusion in the 'Summary of findings' tables.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). The full search strategies for each database are included in Appendix 1.
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, issue 7 2015; MEDLINE via PubMed (1996 to July 2015); EMBASE (1980 to July 2015); CINAHL (1982 to July 2015).
Search terms: {surfactant OR pulmonary surfactant}, limited to humans and further limited to the age group of newborn infants (infant, newborn) and type of publication (clinical trial). No language restrictions were applied. We searched the reference lists of any articles selected for inclusion in this review. From the resulting studies, randomized controlled studies that fulfilled the inclusion criteria were selected. To identify long‐term neurodevelopmental sequelae, a second search using the following keywords was performed: (outcome OR sequelae OR follow‐up OR mental retardation OR cerebral palsy OR hearing OR visual OR motor OR mental OR psychological) AND (surfactant OR pulmonary surfactant) not limited to any age group or language. We searched the bibliography cited in each publication obtained in order to identify additional relevant articles.
Searching other resources
Published abstracts: We searched by hand the abstracts of the Society for Pediatric Research (US) (published in Pediatric Research) for the years 1985 to 1999 using the following key words: [surfactant OR pulmonary surfactant] AND [respiratory distress syndrome]. We electronically searched the abstracts from 2000 to 2014 through the PAS web site (abstractsonline).
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry). We also searched for conference abstracts from Pediatric Academic Societies (PAS) and European Society for Paediatric Research (ESPR). Searches were carried out in Abstracts to View (2000 to 2014) and Pediatric Research.
Data collection and analysis
Information was collected regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was single or multicenter for each included study. We noted the information regarding trial participants including gestational age criteria, birth weight criteria, and other inclusion or exclusion criteria. We analyzed the information on clinical outcomes including pneumothorax, pulmonary interstitial emphysema, PDA, necrotizing enterocolitis, intraventricular hemorrhage (any intraventricular hemorrhage and severe intraventricular hemorrhage), chronic lung disease (bronchopulmonary dysplasia), retinopathy of prematurity, neonatal mortality, mortality prior to hospital discharge, and chronic lung disease (bronchopulmonary dysplasia) or death.
Selection of studies
We included all randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section. Both superiority trials and non‐inferiority trials were eligible for inclusion. All review authors reviewed the results of the search and separately selected the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
NS and RS extracted, assessed, and coded all data for each study, using a form designed specifically for this review. Any standard error of the mean was replaced by the corresponding standard deviation. We resolved any disagreement by discussion. For each study, final data was entered into Review Manager (RevMan) 5 (RevMan 2014) by one review author (NS) and then checked by the other review author (RS). All authors reviewed the protocol, analysis and draft manuscript.
Assessment of risk of bias in included studies
We employed the standard methods of the Cochrane Neonatal Group. The methodologic quality of the studies was assessed using the following key criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. For each criterion, assessment was 'yes', 'no', 'cannot determine.' This information was included in the table 'Characteristics of included studies'.
In addition, the review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The methodological quality of the studies was assessed using the following criteria:
-
Sequence generation (evaluating possible selection bias). For each included study, we described the method used to generate the allocation sequence as: adequate (any truly random process e.g. random number table; computer random number generator); inadequate (any nonrandom process e.g. odd or even date of birth; hospital or clinic record number); or unclear;
-
Allocation concealment (evaluating possible selection bias). For each included study, we described the method used to conceal the allocation sequence as: adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes); inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or unclear;
-
Blinding (evaluating possible performance bias). For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We assessed the methods as: adequate, inadequate, or unclear for participants; adequate, inadequate, or unclear for study personnel; and adequate, inadequate, or unclear for outcome assessors;
-
Incomplete outcome data (evaluating possible attrition bias through withdrawals, drop‐outs, protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as: adequate (< 20% missing data); inadequate (> 20% missing data), or unclear;
-
Selective reporting bias. For each included study where the protocol is available (through trials registers), we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported); inadequate (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or unclear;
-
Other sources of bias. We noted other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes; no; or unclear.
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a). This methodological approach considers evidence from randomized controlled trials as high quality that may be downgraded based on consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades: 1) High: We are very confident that the true effect lies close to that of the estimate of the effect; 2) Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; 3) Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect; 4) Very Low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect (Schünemann 2013).
Two review authors (MXR, RS) independently assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making: mortality prior to hospital discharge, chronic lung disease (defined as supplemental oxygen at 36 weeks' gestation), death or CLD at 36 weeks' postmenstrual age, pneumothorax, pulmonary hemorrhage, severe intraventricular hemorrhage, and neurodevelopmental outcome at approximately two years' corrected age.
In cases where we considered the risk of bias arising from inadequate concealment of allocation, randomized assignment, complete follow‐up or blinded outcome assessment to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). Consistency was evaluated by similarity of point estimates, extent of overlap of confidence intervals and statistical criteria including measurement of heterogeneity (I²). The quality of evidence was downgraded when inconsistency across studies' results was present, being large and unexplained (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011d). Precision was assessed based on the width of the 95% confidence interval (CI) and by calculating the optimal information size (OIS). If the total number of patients included in the pooled effect estimation was less than the number of patients generated by a conventional sample size calculation for a single adequately powered trial, we considered rating down for imprecision (Guyatt 2011c). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e).
Data (i.e. pooled estimates of the effects and corresponding 95% CI) and explicit judgments for each of the above aspects assessed were entered into the Guideline Development Tool, the software used to create 'Summary of findings' tables (GradePro 2008). All judgements involving the assessment of the study characteristics described above are explained in foot notes or comments in the 'Summary of findings' tables.
Measures of treatment effect
We performed the statistical analyses using Review Manager 5 software (RevMan 2014). We analyzed categorical data using risk ratio (RR), and risk difference (RD). For statistically significant outcomes we calculated the number needed to treat for an additional participant with a beneficial outcome (NNTB) or number needed to treat for an additional participant with a harmful outcome (NNTH). We analyzed continuous data using weighted mean difference (WMD) and the standardized mean difference (SMD). We reported the 95% CI on all estimates.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as: less than 25% no heterogeneity; 25% to 49% low heterogeneity; 50% to 75% moderate heterogeneity; more than 75% substantial heterogeneity. If statistical heterogeneity (I² > 50%) was noted, the possible causes were explored (for example, differences in study quality, participants, intervention regimens, or outcome assessments).
Data synthesis
If multiple studies were identified and they were thought to be sufficiently similar, meta‐analysis was done using RevMan 2014, supplied by Cochrane. For categorical outcomes the typical estimates of RR and RD, each with its 95% CI, was calculated; and for continuous outcomes the WMD or a summary estimate for the SMD, each with its 95% CI, was calculated. We used a fixed‐effect model for meta‐analysis. When meta‐analysis was judged to be inappropriate, individual trials were analyzed and interpreted separately.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
-
Treatment strategy (prevention vs. treatment of established disease)
-
Gestational age:
-
Prevention trials: infants born at less than 28 weeks' gestation;
-
Treatment trials: infants born at less than 30 weeks' gestation.
-
-
Surfactant dosage (initial dose ≤ 100 mg/kg).
-
Number of surfactant doses (single dose, multiple dose
5. For treatment trials: disease severity (moderate to severe disease defined as need for assisted ventilation and supplemental oxygen concentration greater than 40% necessary to maintain adequate oxygenation).
Results
Description of studies
See the two tables: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Studies included in this review comprised those that studied the effect of administration of different animal‐derived surfactant extracts on clinical outcomes in infants with RDS or at risk for RDS.
2354 studies retrieved by search. Overall 16 studies are included for analysis.
1. Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) vs. modified bovine minced lung surfactant extract (beractant or surfactant TA).
Prevention studies: Two studies were identified (Bloom 1997; Bloom 2005).
Treatment studies: Seven studies were identified (Attar 2004; Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005; Yalaz 2004).
2. Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF RI 1 (bovactant)) vs. porcine minced lung surfactant extract (poractant alfa).
Prevention studies: No studies identified.
Treatment studies: One study was identified (Baroutis 2003).
3. Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: No studies were identified.
4. Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine minced lung surfactant extract (poractant alfa).
Prevention studies: No studies were identified.
Treatment studies: Nine studies were identified. (Baroutis 2003; Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995).
5. Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: One study was identified (Sanchez‐Mendiola 2005).
6. Porcine minced lung surfactant extract (poractant alfa) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: No studies were identified.
Excluded studies: Four studies were excluded (Bozdağ 2015; Choi 2005; Proquitté 2007; Rebello 2009)
Studies awaiting classification:Eras 2014; Gharehbaghi 2014; Mercado 2010; Saeidi 2013; Terek 2015.
Included studies
Bovine lung lavage surfactant extract [calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)] vs. modified bovine minced lung surfactant extract (beractant or surfactant TA).
Prevention studies: Two studies were identified (Bloom 1997; Bloom 2005).
-
Bloom 1997: In this multicenter, double‐blinded randomized controlled trial, Bloom and colleagues compared relative safety and efficacy of calfactant versus beractant in reducing severity of RDS when given to 374 infants less than 1250 grams and less than 29 weeks' gestation at birth in the prevention arm; and to 608 infants less than 2000 grams with established RDS in the treatment arm. The primary outcome was a decrease in need of a second dose in the prevention arm and a need for a third dose of surfactant in the treatment arm. Thirteen units participated in the treatment arm and seven of those concurrently participated in the prevention arm. Adequate measures were taken to blind the personnel during surfactant administration and for outcome assessment.
-
Bloom 2005: In this multicenter prospective masked randomized controlled trial, Bloom and colleagues compared relative safety and efficacy of calfactant verus beractant in reducing severity of RDS when given at birth to 749 preterm infants 23 to 29 weeks' of gestation and to 1361 infants less than 2000 grams with established RDS. Forty‐two units participated in the trial (21 units participated only in treatment study and 19 units in both treatment and prophylaxis study). The primary outcome was per cent of infants alive without supplemental oxygen requirement at 36 weeks' postmenstrual age. Both trials were halted prematurely for not meeting enrollment targets.
Treatment studies: Seven studies were identified (Attar 2004; Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005; Yalaz 2004).
-
Bloom 1997: In this multicenter, double‐blinded randomized controlled trial, Bloom and colleagues compared relative safety and efficacy of calfactant versus beractant in reducing severity of RDS when given to 374 preterm infants less than 1250 grams and less than 29 weeks' gestation at birth in the prevention arm; and to 608 preterm infants less than 2000 grams with established RDS in the treatment arm. Thirteen units participated in the treatment arm and seven of those concurrently participated in the prevention arm. The primary outcome was a decrease in need for a second dose in the prevention arm and need for a third dose of surfactant in the treatment arm.
-
Hammoud 2004: In this single center double‐blinded randomized controlled trial from Kuwait, Hammoud and colleagues compared the efficacy of bovactant (Alveofact) (SF‐RI 1) and beractant in 109 preterm infants less than 34 weeks' gestation with established RDS who needed intubation and mechanical ventilation. The primary outcome was CLD (oxygen requirement at 28 days of age).
-
Attar 2004: In this single‐center randomized controlled trial, Attar and colleagues compared the efficacy of calfactant and beractant in 40 preterm infants less than 37 weeks' gestation with radiographic diagnosis of RDS. The two groups were comparable except for a significantly higher number of males in the calfactant group. Primary outcome was difference in changing dynamic compliance of lungs one hour after surfactant administration. Secondary outcomes included CLD and other complications of prematurity.
-
Yalaz 2004: In this single center study from Turkey, Yalaz and colleagues compared effectiveness and side effects of bovactant (Alveofact) and beractant in 50 preterm infants less than 36 weeks' gestation with established RDS. They gave standard dose of bovactant (Alveofact) (50 mg/kg) and beractant (100 mg/kg), repeating doses based on blood gases and chest X‐ray. The primary outcome was FiO₂ requirement in first 24 hours after surfactant administration. Secondary outcomes were complications of prematurity.
-
Lam 2005: In this single‐center randomized controlled trial from Hong Kong, Lam and colleagues compared the response pattern and treatment outcomes of BLES and beractant in 63 preterm infants less than 1800 grams with established RDS. The primary outcome was oxygenation index and secondary outcomes were complications of prematurity.
-
Bloom 2005: In this multicenter, prospective masked randomized controlled trial, Bloom and colleagues compared the safety and efficacy of calfactant versus beractant in reducing severity of RDS when given at birth to 749 preterm infants 23 to 29 weeks' gestation and to 1361 infants less than 2000 grams with established RDS. Forty‐two units participated in the trial (21 units participated only in treatment study and 19 units in both treatment and prophylaxis study). The primary outcome was the per cent of infants alive without supplemental oxygen requirement at 36 weeks' postmenstrual age. Both trials were halted prematurely for not meeting enrollment targets.
-
Baroutis 2003: In this single‐center randomized controlled trial, Baroutis and colleagues compared three animal‐derived surfactants (bovactant, poractant alfa, and beractant) for treatment of established RDS in 82 preterm infants up to 32 weeks' gestation and BW 2000 grams. The initial and repeat dose for all three surfactants was 100 mg/kg. The primary outcome was oxygen dependence at 36 weeks' postmenstrual age.
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF RI 1 (bovactant)) vs. porcine minced lung surfactant extract (poractant alfa).
Prevention studies: No studies identified.
Treatment studies: One study was identified (Baroutis 2003).
-
Baroutis 2003: In this single center study, Baroutis and colleagues compared three animal‐derived surfactants (bovactant, poractant alfa, and beractant) for the treatment of established RDS in 82 preterm infants up to 32 weeks' gestation and BW up to 2000 grams. The primary outcome was oxygen dependence at 36 weeks' postmenstrual age.
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: No studies were identified.
Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine minced lung surfactant extract (poractant alfa).
Prevention studies: No studies were identified.
Treatment studies: Nine studies were identified. (Baroutis 2003; Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995).
-
Speer 1995: In this multicenter randomized controlled study from Germany, Speer and colleagues compared the effectiveness of poractant alfa and beractant in treatment of 73 preterm infants less than 1500 grams with RDS. The primary outcome was gas exchange and ventilation requirement.
-
Halahakoon 1999: As part of her PhD thesis, Halahakoon evaluated the effects of poractant alfa (n = 17), beractant (n = 10) and colfosceril palmitate (Exosurf Neonatal) (n = 12) on cerebral function, hypoxanthine levels, and antioxidant levels in 24 to 32 weeks' gestation infants with RDS requiring assisted ventilation. In this single‐center study, 39 preterm infants between 24 to 32 weeks' gestation were randomized into three groups. The study was initially designed to compare only two surfactants (poractant alfa and colfosceril palmitate) and later included beractant, hence differences in number of patients in each group. Death or prolonged dependency on oxygen at 36 weeks’ postmenstrual age with radiological evidence of BPD was considered as primary outcome.
-
Baroutis 2003: In this single‐center study, Baroutis and colleagues compared three animal‐derived surfactants (bovactant, poractant alfa, and beractant) for treatment of established RDS in 82 preterm infants up to 32 weeks' gestation and BW up to 2000 grams. The primary outcome was oxygen dependence at 36 weeks' postmenstrual age.
-
Ramanathan 2004: In this multicenter masked randomized trial, Ramanathan and colleagues compared onset of clinical response and safety of high dose of poractant alfa (200 mg/kg) compared with low‐dose poractant alfa (100 mg/kg) and beractant (100 mg/kg) in the treatment of preterm infants less than 35 weeks' gestation with established RDS. A total of 293 preterm infants were randomized to high‐dose poractant alfa (n = 99), low‐dose poractant alfa (n = 96), and beractant (n = 98). If needed, the repeat dose was similar for all three groups (100 mg/kg). The primary outcome was FiO₂ requirement during six hours after first dose of surfactant.
-
Malloy 2005: In this single‐center study, Malloy and colleagues compared effects of poractant alfa and beractant in 58 preterm infants less than 37 weeks' gestation with clinical signs of RDS. The primary outcome was FiO₂ requirement at 48 hours after the first dose of surfactant administration.
-
Fujii 2010: Fujii and colleagues conducted a single‐center study assessing the short‐term treatment efficacy of poractant alfa and beractant in 58 preterm infants less than 30 weeks' gestation with RDS. The primary outcome of the study was effect on respiratory support (FiO₂ and MAP) and complications of prematurity.
-
Gharehbaghi 2010: In this single‐center quasi‐randomized study, Gharehbaghi and colleagues compared the complications with poractant alfa and beractant in 150 preterm infants with RDS. The primary outcome of the study was remaining without ventilator support through seven days of age.
-
Didzar 2012: In this single‐center randomized controlled study, Didzar and colleagues compared the differences in clinical response and short‐term outcome between poractant alfa and beractant in 126 infants less than 37 weeks' gestation with RDS. The primary outcome of interest was FiO₂ requirement at 24 hours after surfactant administration.
-
Karadag 2014 reported results from a randomized controlled trial comparing the perfusion index (PI) variability following administration of two different animal‐derived surfactant preparations (poractant alfa and beractant) in 92 preterm infants less than 32 weeks' gestation with RDS.
Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: One study was identified (Sanchez‐Mendiola 2005).
-
Sanchez‐Mendiola 2005: Sanchez‐Mendiola and colleagues conducted a randomized controlled trial to compare an inexpensive porcine‐derived surfactant, Surfacen, with beractant in 44 preterm infants. The primary outcome studied was oxygenation and ventilation index, days with supplemental oxygen, days on mechanical ventilation, and mortality.
Porcine minced lung surfactant extract (poractant alfa) vs. porcine lung lavage surfactant (Surfacen).
Prevention studies: No studies were identified.
Treatment studies: No studies were identified.
Excluded studies
Excluded studies: Four studies were excluded (Bozdağ 2015; Choi 2005; Rebello 2009; Proquitté 2007)
Choi 2005: In this multicenter study conducted in South Korea, a domestically developed bovine surfactant, Newfacen, was compared with Surfacten, another bovine derived surfactant for efficacy in 492 preterm infants with established RDS with birth weight less than 1500 grams. Short‐term responses to surfactant and acute complications such as total doses of surfactant administered and changes in respiratory parameters were studied.
This study was excluded because both of the surfactants studied belong to the same comparison group (bovine lung lavage surfactant).
Rebello 2009: In this multicenter study conducted in Brazil, Rebello and colleagues compared the beneficial effects of butantan (a porcine surfactant obtained by organic extraction) with other commercially available surfactants (either beractant or poractant alfa) in 327 preterm infants with RDS. The primary outcome was being alive at 28 days of life.
This study was excluded because the control group received both modified bovine lung lavage surfactant and porcine lung lavage surfactant.
Bozdağ 2015: A prospective randomized controlled trial to compare the efficacy of two animal‐derived surfactants for pulmonary hemorrhage in very low birth weight (VLBW) infants. 42 infants were divided into two groups, poractant alfa (n = 21) and beractant (n = 21).
This study was excluded because patient population was VLBW infants with pulmonary hemorrhage.
Proquitté 2007 : Proquitté and colleagues performed a retrospective, observational study comparing the effects of bovactant (Alveofact) and poractant alfa (Curosurf) on gas exchange and outcome in premature infants.
Studies awaiting classification: (Eras 2014; Gharehbaghi 2014; Mercado 2010; Saeidi 2013: Terek 2015)
Mercado 2010: Compared a porcine‐derived surfactant to a bovine‐derived lung extract. The exact products are not named in the report.
Saeidi 2013: Clinical trial performed during a 2‐year period in Ghaem Center's neonatal care unit. Method of allocation unknown.
Terek 2015: Randomized controlled trial. 30 preterm infants with RDS, treated with poractant alfa (n = 15) or beractant (n = 15); 18 preterm infants without RDS served as a control group. Reported physiologic variables.
Eras 2014: Prospective, longitudinal, single‐center cohort study of infants born at up to 1500 grams or up to 32 weeks' gestation between 2008 and 2009 who received either poractant alfa (n = 113) or beractant (n = 102) for RDS. Neurological and developmental assessments were performed at a corrected age of 18 to 24 months. It is unclear whether these patients are related to Didzar 2012 study.
Gharehbaghi 2014: Randomized clinical trial in Alzahra Hospital, Tabriz, Iran. Enrolled preterm newborn infants with gestation age less than 32 weeks with RDS. Poractant alfa (Curosurf) (N = 66) and bovactant (Alveofact) (N = 64). Surfactant was administered using the INSURE method (intubation, surfactant administration, extubation).
Ongoing studies: none identified.
Risk of bias in included studies
See 'Risk of bias' tables.
Randomized controlled trials that compared available bovine (modified minced or lavage) and porcine (minced or lavage) surfactant extracts in preterm infants at risk of or with established RDS were included in the analysis. Specific methodologic issues are addressed below:
Randomization and allocation concealment: Six studies did not report on method of randomization (Attar 2004; Baroutis 2003; Didzar 2012; Speer 1995; Sanchez‐Mendiola 2005; Yalaz 2004). Randomization was performed and reported adequately in other studies except for Gharehbaghi 2010 which used admission code for randomization (even‐odd numbers). Allocation concealment was adequately reported and appropriately performed in only half of the studies (Baroutis 2003; Bloom 1997; Bloom 2005; Halahakoon 1999; Karadag 2014; Ramanathan 2004;Speer 1995) .
Blinding of treatment and of outcome assessors: Blinding of treatment was adequately performed and reported by Bloom 1997 and Bloom 2005. Eight studies did not report any attempt at blinding of the treatment (Didzar 2012; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Lam 2005; Ramanathan 2004; Sanchez‐Mendiola 2005; Yalaz 2004). Others reported inability to blind the treatment because of differences in appearance or in the method of administration of surfactant product (Attar 2004; Baroutis 2003; Fujii 2010; Malloy 2005; Speer 1995).
Incomplete outcome data or selective reporting: There was complete follow‐up of all enrolled patients in the studies with minimal risk of attrition bias or selective reporting bias, with the exception of the study of Sanchez‐Mendiola 2005 with unclear risk of bias.
Effects of interventions
See: Summary of findings for the main comparison Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for prevention of RDS; Summary of findings 2 Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for treatment of RDS; Summary of findings 3 Bovine lung lavage surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS; Summary of findings 4 Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS; Summary of findings 5 Modified bovine minced lung surfactant extract compared with porcine lung lavage surfactant in preterm infants for treatment of RDS
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) vs. modified bovine minced lung surfactant extract (beractant or surfactant TA) (COMPARISON 1).
Prevention: Two studies were identified (Bloom 1997; Bloom 2005).
Treatment: Seven studies were identified (Attar 2004; Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005; Yalaz 2004).
Primary outcomes
Neonatal mortality (mortality < 28 days of age) from any cause (outcome 1.1):
-
Prevention (outcome 1.1.1): This outcome was reported by one prevention study (Bloom 2005), Bloom and colleagues reported no significant effect from prophylactic administration of bovine lung lavage surfactant or modified bovine minced lung surfactant on the risk of neonatal mortality at less than 28 days from any cause (RR 1.19, 95% CI 0.79 to 1.80; RD 0.02 , 95% CI −0.03 to 0.06; 1 study and 749 infants).
-
Treatment (outcome 1.1.2): This outcome was reported by three studies (Attar 2004; Bloom 2005; Yalaz 2004). None of the individual studies comparing these surfactant preparations showed any effect on neonatal mortality at less than 28 days. The meta‐analysis of treatment trials showed no significant difference in the risk of neonatal mortality between bovine lung lavage surfactant and modified bovine minced lung surfactant (typical RR 0.90, 95% CI 0.65 to 1.26; typical RD −0.01, 95% CI −0.04 to 0.02; 3 studies and 1451 infants). There was no heterogeneity among the studies (I² = 0%).
Mortality prior to hospital discharge (from any cause) (outcome 1.2) (Figure 1):

Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.2 Mortality prior to discharge.
-
Prevention (outcome 1.2.1): This outcome was reported by two studies (Bloom 1997 and Bloom 2005). Neither study individually showed any significant difference in mortality prior to discharge. The meta‐analysis of these studies showed no significant difference in mortality prior to discharge with prophylactic administration of bovine lung lavage surfactant or modified bovine minced lung surfactant (typical RR 1.24, 95% CI 0.90 to 1.71; typical RD 0.03, 95% CI −0.01 to 0.06; 2 studies and 1123 infants). Low heterogeneity was present between the studies (I² = 31%). The evidence was graded as moderate quality because of imprecision in estimates.
-
Treatment (outcome 1.2.2): This outcome was reported by six studies (Attar 2004; Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on the mortality prior to discharge (typical RR 0.98, 95% CI 0.79 to 1.21; typical RD −0.00, 95% CI −0.03 to 0.03; 6 studies and 2231 infants). No heterogeneity was noted among the studies (I² = 0%) and the quality of evidence was graded as moderate.
Chronic lung disease:
‐ Oxygen requirement at 28 to 30 days of age (outcome 1.3):
-
Prevention (outcome 1.3.1): This outcome was reported by one prevention study (Bloom 2005) which reported no significant effect of surfactant preparation on oxygen requirement at 28 to 30 days of age (RR 0.99, 95% CI 0.88 to 1.12; RD −0.00, 95% CI −0.07 to 0.07; 1 study and 749 infants).
-
Treatment (outcome 1.3.2): This outcome was reported by three studies (Attar 2004; Bloom 2005; Hammoud 2004). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on risk of oxygen requirement at 28 to 30 days of age (typical RR 1.09, 95% CI 0.98 to 1.21; typical RD 0.04, 95% CI −0.01 to 0.09; 3 studies and 1510 infants). Moderate heterogeneity was present between the studies (I² = 64%).
‐ Oxygen requirement at 36 weeks' postmenstrual age (outcome 1.4) (Figure 2):
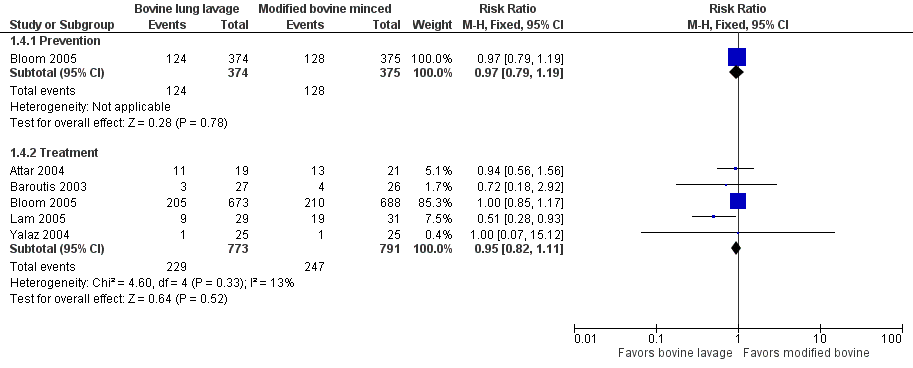
Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.4 Oxygen requirement at 36 weeks postmenstrual age (all infants).
-
Prevention (outcome 1.4.1): This outcome was reported by one prevention study (Bloom 2005), which reported no significant effect of surfactant preparation on oxygen requirement at 36 weeks' postmenstrual age (RR 0.97, 95% CI 0.79 to 1.19; RD −0.01, 95% CI −0.08 to 0.06; 1 study and 749 infants).
-
Treatment (outcome 1.4.2): This outcome was reported by five studies (Attar 2004; Baroutis 2003; Bloom 2005; Lam 2005; Yalaz 2004). Lam 2005 noted a significant decrease in oxygen requirement at 36 weeks' postmenstrual age (RR 0.51, 95% CI 0.28 to 0.93) favoring bovine lung lavage surfactant, while other studies did not report difference in the outcome. The meta‐analysis of treatment trials demonstrated no effect of surfactant preparation on risk of oxygen requirement at 36 weeks' postmenstrual age (typical RR 0.95, 95% CI 0.82 to 1.11; typical RD −0.01, 95% CI −0.06 to 0.03; 5 studies and 1564). No heterogeneity was noted among the studies (I² = 13%) and the quality of evidence was graded as high.
Death or chronic lung disease:
‐ Death or oxygen requirement at 28 to 30 days of age (outcome 1.5):
-
Prevention (outcome 1.5.1): This outcome was reported by one study (Bloom 2005), which showed no significant effect of surfactant preparation on risk of combined outcome of death or oxygen requirement at 28 to 30 days of age (RR 1.02, 95% CI 0.93 to 1.13; RD 0.02 , 95% CI −0.05 to 0.08; 1 study and 749 infants).
-
Treatment (outcome 1.5.2): This outcome was reported by two studies (Attar 2004; Bloom 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on the risk of the combined outcome of death or oxygen requirement at 28 to 30 days of age (typical RR 1.05, 95% CI 0.96 to 1.15; typical RD 0.03, 95% CI −0.02 to 0.08; 2 studies and 1401 infants). There was no heterogeneity among the studies (I² = 0%).
‐ Death or oxygen requirement at 36 weeks' postmenstrual age (outcome 1.6):
-
Prevention (outcome 1.6.1): This outcome was reported by two studies (Bloom 1997; Bloom 2005 ) no significant effect of surfactant preparation on risk of combined outcome of death or oxygen requirement at 36 weeks' postmenstrual age. None of the individual studies or the meta‐analysis of these prevention trials demonstrated any significant difference in the risk of combined outcome of death or oxygen requirement at 36 weeks' postmenstrual age between the surfactant preparations (typical RR 1.02, 95% CI 0.89 to 1.17; typical RD 0.01, 95% CI −0.05 to 0.06; 2 studies and 1123 infants). There was no statistical heterogeneity among the studies (I² = 0%) and the quality of evidence was graded as high (95% CI was narrow and precise around no effect and total number of events met optimal information size).
-
Treatment (outcome 1.6.2): This outcome was reported by three studies (Attar 2004; Bloom 1997; Bloom 2005). None of the individual studies showed any significant effect of surfactant preparation on combined outcome of death or oxygen requirement at 36 weeks' postmenstrual age. The meta‐analysis of these treatment trials did not demonstrate any significant difference in the risk of combined outcome of death or oxygen requirement at 36 weeks' postmenstrual age between the surfactant preparations (typical RR 0.95, 95% CI 0.86 to 1.06; typical RD −0.02, 95% CI −0.06 to 0.02; 3 studies and 2009 infants). There was no heterogeneity among the studies (I² = 0%) and the quality of evidence was graded as high (95% CI was narrow and precise around no effect and total number of events met optimal information size).
Secondary outcomes
Doses of surfactant.
Post hoc analysis: Received > one dose of surfactant (outcome 1.7):
-
Prevention (outcome 1.7.1): This outcome was reported by two studies (Bloom 1997; Bloom 2005). Neither the individual studies nor the meta‐analysis of the prevention trials demonstrated any significant difference in the risk of 'receiving more than one dose of surfactant' based on surfactant preparation used (typical RR 1.02, 95% CI 0.89 to 1.16; typical RD −0.01, 95% CI −0.05 to 0.07; 2 studies and 1123 infants). There was no heterogeneity among the studies (I² = 0%).
-
Treatment (outcome 1.7.2): This outcome was reported by five studies (Attar 2004; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant difference in the risk of 'receiving more than one dose of surfactant' based on surfactant preparation (typical RR 0.99, 95% CI 0.93 to 1.06; typical RD −0.01, 95% CI −0.05 to 0.04; 5 studies and 2178 infants). There was low heterogeneity among the studies (I² = 46%).
Pneumothorax (outcome 1.8):
-
Prevention (outcome 1.8.1): This outcome was reported by one study (Bloom 2005), which showed no effect of surfactant preparation on risk of pneumothorax (RR 0.76, 95% CI 0.43 to 1.36; one study and 749 infants) and the quality of evidence was graded as moderate.
-
Treatment (outcome 1.8.2): This outcome was reported by six studies (Attar 2004; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005; Yalaz 2004). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on risk of pneumothorax (typical RR 1.14, 95% CI 0.85 to 1.51; typical RD 0.01, 95% CI −0.01 to 0.03; 6 studies and 2224 infants). There was moderate heterogeneity among the studies (I² = 62%) and the quality of evidence is low (95% CI included both no effect and appreciable harm, and unexplained heterogeneity).
Air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum) (outcome 1.9):
-
Prevention (outcome 1.9.1): This outcome was reported by both Bloom 1997 and Bloom 2005. Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any significant effect of surfactant preparation on risk of air leak syndrome (typical RR 1.16, 95% CI 0.84 to 1.60; typical RD 0.02, 95% CI −0.02 to 0.05; 2 studies and 1123 infants). There was no heterogeneity among the studies (I² = 0%).
-
Treatment (outcome 1.9.2): This outcome was reported by three studies (Baroutis 2003; Bloom 1997; Bloom 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on air leak syndrome (typical RR 1.02, 95% CI 0.82 to 1.28; typical RD 0.00, 95% CI −0.03 to 0.03; 3 studies and 2011 infants). There was low heterogeneity among the studies (I² = 41%).
Pulmonary hemorrhage (outcome 1.10):
-
Prevention (outcome 1.10.1): This outcome was reported by both Bloom 1997 and Bloom 2005 . Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any significant effect of surfactant preparation on risk of pulmonary hemorrhage (typical RR 1.44, 95% CI 0.88 to 2.39; typical RD 0.02, 95% CI −0.01 to 0.05; 2 studies and 1123 infants). There was low heterogeneity among the studies (I² = 30%) and the "quality of evidence" is low (95% CI included both no effect and appreciable harm; and total number of events died not meet optimal information size).
-
Treatment (outcome 1.10.2): This outcome was reported by four studies (Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on risk of pulmonary hemorrhage (typical RR 1.08, 95% CI 0.74 to 1.59; typical RD 0.00, 95% CI −0.01 to 0.02; 4 studies and 2138 infants). There was no heterogeneity among the studies (I² = 0%) and the "quality of evidence" is moderate.
Patent ductus arteriosus (PDA) (that has been treated with cyclo‐oxygenase inhibitor or surgery) (outcome 1.11):
-
Prevention: This outcome was not reported by any study.
-
Treatment (outcome 1.11.1): This outcome was reported by one study. Attar 2004 reported no effect of surfactant preparation on risk of PDA requiring treatment with cyclo‐oxygenase inhibitor or surgery (RR 0.32, 95% CI 0.07 to 1.34).
Culture‐confirmed bacterial sepsis (outcome 1.12):
-
Prevention (outcome 1.12.1): This outcome was reported by both Bloom 1997 and Bloom 2005. Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any significant effect of surfactant preparation on the risk of culture‐confirmed bacterial sepsis (typical RR 1.08, 95% 0.91 to 1.28; typical RD 0.02, 95% CI −0.03 to 0.08; 2 studies and 1123 infant). There was no heterogeneity among the studies (I² = 0%).
-
Treatment (outcome 1.12.2): This outcome was reported by six studies (Attar 2004; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005; Yalaz 2004). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on risk of culture‐confirmed bacterial sepsis (typical RR 1.00, 95% CI 0.87 to 1.15; typical RD 0.00, 95% CI −0.04 to 0.04, 6 studies and 2228 infants). There was no heterogeneity among the studies (I² = 0%).
Culture‐confirmed fungal sepsis:
-
Prevention: This outcome was not reported by any study.
-
Treatment: This outcome was not reported by any treatment study.
Necrotizing enterocolitis (any stage) (Bell 1978) (outcome 1.13):
-
Prevention (outcome 1.13.1): This outcome was reported by both Bloom 1997 and Bloom 2005. Neither study reported NEC based on Bell's criteria. Both studies reported on "any NEC" (outcome 1.13). Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any significant effect of surfactant preparation on the risk of NEC (any stage) (typical RR 1.03, 95% CI 0.74 to 1.42; typical RD 0.00, 95% CI −0.03 to 0.04; 2 studies and 1123 infants). There was no heterogeneity among the studies (I² = 0%).
-
Treatment (outcome 1.13.2): This outcome (any NEC) was reported by five studies (Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on the risk of NEC (any stage) (typical RR 1.02, 95% CI 0.78 to 1.33, typical RD 0.00, 95% CI −0.02 to 0.02; 5 studies and 2191 infants). There was low heterogeneity among the studies (I² = 42%).
Periventricular leukomalacia (in infants who received neuroimaging) (outcome 1.14):
-
Prevention (outcome 1.14.1): This outcome was reported by one study (Bloom 2005), which showed no significant effect from prophylactic administration of bovine lung lavage surfactant or modified bovine minced lung surfactant on the risk of PVL (RR 0.61, 95% CI 0.29 to 1.26; RD −0.02, 95% CI −0.05 to 0.01; 1 study and 713 infants).
-
Treatment (outcome 1.14.2): This outcome was reported by one study (Bloom 2005 ) which showed no significant effect from treatment with bovine lung lavage surfactant or modified bovine minced lung surfactant on the risk of PVL (RR 1.01, 95% CI 0.59 to 1.73; RD 0.00, 95% CI −0.02 to 0.02; 1 study and 1275 infants).
Retinopathy of prematurity in subjects examined.
‐ ROP all stages (outcome 1.15):
-
Prevention (outcome 1.15.1): This outcome was reported by both Bloom 1997 and Bloom 2005. Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any significant effect of surfactant preparation on ROP (all stages) (typical RR 0.98, 95% CI 0.86 to 1.12; typical RD −0.01, 95% CI −0.7 to 0.05; 2 studies and 1011 infants).
-
Treatment (outcome 1.15.2): This outcome was reported by three studies (Baroutis 2003; Bloom 1997; Bloom 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on ROP (all stages) (typical RR 1.02, 95% CI 0.89 to 1.16; typical RD −0.01, 95% CI −0.07 to 0.05; 3 studies and 1662 infants).
‐ ROP stage 3 or greater (outcome 1.16):
-
Prevention (outcome 1.16.1): This outcome was reported by one study (Bloom 2005) which showed no significant effect from prophylactic administration of bovine lung lavage surfactant or modified bovine minced lung surfactant on the risk of ROP stage 3 or greater (RR 1.14, 95% CI 0.77 to 1.69; one study and 637 infants).
-
Treatment (outcome 1.16.2): This outcome was reported by one study (Bloom 2005) which showed no significant effect from bovine lung lavage surfactant or modified bovine minced lung surfactant treatment on the risk of ROP stage 3 or greater (RR 0.92, 95% CI 0.64 to 1.33; one study and 1001 infants).
Intraventricular hemorrhage (in infants who received neuroimaging).
‐ Intraventricular hemorrhage (any grade) (outcome 1.17)
-
Prevention (outcome 1.17.1): This outcome was reported by one study (Bloom 2005) which showed no significant effect from prophylactic administration of bovine lung lavage surfactant or modified bovine minced lung surfactant on the risk of IVH (any grade) (RR 1.04, 95% CI 0.87 to 1.24; RD 0.02 , 95% CI −0.06 to 0.09; one study and 713 infants).
-
Treatment (outcome 1.17.2): This outcome was reported by three studies (Bloom 2005; Hammoud 2004; Yalaz 2004 ). No individual trial demonstrated an effect on intraventricular hemorrhage of any grade and the meta‐analysis of treatment trials demonstrated no significant effect of surfactant preparation on the risk of IVH (any grade) (typical RR 1.14, 95% CI 0.98 to 1.33; typical RD 0.04, 95% CI −0.01 to 0.09; 3 studies and 1434 infants). There was no heterogeneity in the studies (I² = 0%).
‐ Severe intraventricular hemorrhage (grade 3 or greater) (outcome 1.18)
-
Prevention (outcome 1.18.1): This outcome was reported by two studies (Bloom 1997 and Bloom 2005). Neither the individual studies nor the meta‐analysis of these prevention trials demonstrated any effect of surfactant preparation on the risk of severe IVH (typical RR 1.28, 95% CI 0.89 to 1.83; typical RD 0.02, 95% CI −0.01 to 0.06; 2 studies and 1087 infants). There was no heterogeneity among the studies (I² = 0%) and the "quality of evidence" is low (95% CI included both no effect and appreciable harm; and total number of events died not meet optimal information size) .
-
Treatment (outcome 1.18.2): This outcome was reported by five studies (Baroutis 2003; Bloom 1997; Bloom 2005; Hammoud 2004; Lam 2005). Neither the individual studies nor the meta‐analysis of these treatment trials demonstrated any significant effect of surfactant preparation on risk of severe IVH (typical RR 0.86, 95% CI 0.68 to 1.09; typical RD −0.02, 95% CI −0.05 to 0.01; 5 studies and 2040 infants). There was no heterogeneity among the studies (I² = 0%) and the "quality of evidence" is moderate (95% CI included both no effect and appreciable harm; and total number of events died not meet optimal information size).
Cerebral palsy at approximately two years' corrected age (as defined by the study authors). Not reported.
Neurodevelopmental outcome at approximately two years' corrected age (acceptable range 18 months to 28 months) including: cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index less than 70), legal blindness (less than 20/200 visual acuity), and hearing deficit (aided or less than 60 dB on audiometric testing). The composite outcome 'neurodevelopmental impairment' was defined as having any one of the aforementioned deficits. Not reported by either study.
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF RI 1 (bovactant)) vs. porcine minced lung surfactant extract (poractant) (COMPARISON 2).
Prevention: No studies were identified.
Treatment: One study was identified (Baroutis 2003).
Primary outcome measures
Prevention Studies:
Not applicable as no studies were identified
Treatment Studies:
Mortality (outcome 2.1): Neonatal mortality (mortality at less than 28 days of age) from any cause was not reported. Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of mortality prior to hospital discharge (from any cause) (RR 1.40, 95% CI 0.51 to 3.87; RD 0.07, 95% CI −0.15 to 0.29; one study and 54 infants) . The "quality of evidence" is low because of serious imprecision in analysis and total number of events did not meet optimal information size.
Chronic lung disease (outcome 2.2): Oxygen requirement at 28 to 30 days of age was not reported. Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of oxygen requirement at 36 weeks' postmenstrual age (RR 0.75, 95% 0.19 to 3.04; RD −0.04, 95% CI −0.22 to 0.14; one study and 54 infants). The "quality of evidence" is low because of serious imprecision in analysis and total number of events did not meet optimal information size.
Death or chronic lung disease at 28 to 30 days of age or at 36 weeks' postmenstrual age: was not reported.
Secondary outcomes
Air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum) (outcome 2.3):Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of air leak syndrome (RR 0.67, 95% CI 0.12 to 3.68; RD −0.04 , 95% CI −0.19 to 0.12; one study and 54 infants).
Necrotizing enterocolitis (any stage) (Bell 1978) (outcome 2.4): Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of NEC (any stage) (RR 0.67, 95% CI 0.12 to 3.68; RD −0.04, 95% CI −0.19 to 0.12; one study and 54 infants).
Retinopathy of prematurity in subjects examined
‐ ROP all stages (outcome 2.5):Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of ROP (all stages) (RR 0.80, 95% CI 0.24 to 2.66; RD −0.04, 95% CI −0.24 to 0.16; one study and 54 infants).
‐ ROP stage 3 or greater: not reported.
Intraventricular hemorrhage (in infants who received neuroimaging)
‐ Intraventricular hemorrhage (any grade): not reported.
‐ Severe intraventricular hemorrhage (grade 3 or greater) (outcome 2.6):Baroutis 2003 reported no significant effect from treatment with bovine lung lavage surfactant or porcine minced lung surfactant on the risk of severe IVH (RR 0.83, 95% 0.29, 2.41; RD −0.04 , 95% CI −0.25 to 0.18; one study and 54 infants). The "quality of evidence" is very low because of serious imprecision in analysis, risk of bias from lack of blinding of outcome assessment and total number of events not meeting optimal information size.
No other comparison could be made for the remaining secondary outcomes, including doses of surfactant, post hoc analysis: received more than one dose of surfactant, pneumothorax, pulmonary hemorrhage, patent ductus arteriosus (PDA) (that has been treated with cyclo‐oxygenase inhibitor or surgery), culture‐confirmed bacterial sepsis, culture‐confirmed fungal sepsis, periventricular leukomalacia (in infants who received neuroimaging), cerebral palsy at approximately two years' corrected age and neurodevelopmental outcome at approximately two years' corrected age.
Bovine lung lavage surfactant extract (calfactant, CLSE (BLES) or SF‐RI 1 (bovactant)) vs. Porcine lung lavage surfactant (Surfacen).
Prevention: No studies were identified.
Treatment: No studies were identified.
Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine minced lung surfactant extract (poractant alfa) (COMPARISON 3).
Prevention: No studies were identified.
Treatment: Nine studies were identified. (Baroutis 2003; Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995).
Primary outcomes
Neonatal mortality (mortality < 28 days of age) from any cause (outcome 3.1): This outcome was reported by two studies (Halahakoon 1999; Ramanathan 2004). The meta‐analysis did not demonstrate any significant effect of surfactant preparation on the risk of neonatal mortality from any cause (typical RR 1.48, 95% CI 0.72 to 3.07; typical RD 0.03, 95% CI −0.03 to 0.10; 2 studies and 320 infants). There was no heterogeneity among the studies (I² = 0% ).
Mortality prior to hospital discharge (from any cause) (outcome 3.2): This outcome was reported by nine studies (Baroutis 2003; Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies showed any effect of surfactant preparation on the risk of mortality prior to hospital discharge. The meta‐analysis of these trials demonstrated an increased risk of mortality prior to hospital discharge associated with beractant treatment compared with poractant alfa treatment (typical RR 1.44, 95% CI 1.04 to 2.00; typical RD 0.05, 95% CI 0.01 to 0.10; NNTH 20, 95% CI 10 to 100; 9 studies and 901 infants). There was no heterogeneity among the studies (I² = 0% ) and the "quality of evidence" is moderate (imprecision in estimates).
Chronic lung disease:
‐ Oxygen requirement at 28 to 30 days of age (outcome 3.3): This outcome was reported by two studies (Halahakoon 1999; Ramanathan 2004). The meta‐analysis demonstrated no significant effect of surfactant preparation on risk of oxygen requirement at 28 to 30 days of age (typical RR 0.97, 95% CI 0.77 to 1.23; typical RD −0.01, 95 % CI −0.13 to 0.10; 2 studies and 320 infants). No heterogeneity was noted among the studies (I² = 0%).
‐ Oxygen requirement at 36 weeks' postmenstrual age (outcome 3.4): This outcome was reported by nine studies (Baroutis 2003; Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies showed an effect of surfactant preparation on the risk of oxygen requirement at 36 weeks' postmenstrual age. The meta‐analysis of these studies demonstrated no significant effect of surfactant preparation on risk of oxygen requirement at 36 weeks' postmenstrual age (typical RR 0.94, 95% CI 0.79 to 1.12; typical RD −0.02, 95% CI −0.08 to 0.04; 9 studies and 899 infants). No heterogeneity was noted among the studies (I² = 0%) and the "quality of evidence" is moderate.
Death or chronic lung disease:
‐ Death or oxygen requirement at 28 to 30 days of age: This outcome was not reported.
‐ Death or oxygen requirement at 36 weeks' postmenstrual age (outcome 3.5): This outcome was reported by three studies (Didzar 2012; Fujii 2010; Ramanathan 2004). Didzar 2012 showed increased risk of death or oxygen requirement at 36 weeks' postmenstrual age for modified bovine minced lung surfactant (RR 1.95, 95% CI 1.11 to 3.42). The meta‐analysis of these trials demonstrated a significant increase in combined outcome of death or oxygen requirement at 36 weeks' postmenstrual age for modified bovine minced lung surfactant compared with porcine minced lung surfactant (typical RR 1.30, 95% CI 1.04 to 1.64; typical RD 0.11, 95% CI 0.02 to 0.20; NNTH 9, 95% CI 5 to 50). There was low heterogeneity noted among the studies (I² = 45%), and the "quality of evidence" is moderate.
Secondary outcomes
Post hoc analysis: Received > one dose of surfactant (outcome 3.6): This outcome was reported by six studies (Didzar 2012; Fujii 2010; Gharehbaghi 2010; Karadag 2014; Ramanathan 2004; Speer 1995). Except for Gharehbaghi 2010, all other studies reporting this outcome showed a trend towards increase in risk of receiving more than one dose of surfactant for modified bovine minced lung surfactant. Ramanathan 2004, Karadag 2014 and Didzar 2012 reported a statistically significant increase in risk of receiving more than one dose of surfactant for modified bovine minced lung surfactant. Overall, the meta‐analysis of these trials demonstrated a significant increase in risk of receiving more than one dose of surfactant for modified bovine minced lung surfactant compared with porcine minced lung surfactant (typical RR 1.57, 95% CI 1.29 to 1.92; typical RD 0.14, 95% CI 0.08 to 0.20; NNTH 7, 95% CI 5 to 12; 6 studies and 786 infants). No heterogeneity was noted among the studies (I² = 19%).
Pneumothorax (outcome 3.7): This outcome was reported by six studies (Didzar 2012; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies or the meta‐analysis showed any effect of surfactant preparation on the risk of pneumothorax (typical RR 1.24, 95% CI 0.71 to 2.17; typical RD 0.02, 95% CI −0.02 to 0.05; 6 studies and 669 infants). No heterogeneity was noted among the studies (I² = 0%) and the quality of evidence was graded as low.
Air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum) (outcome 3.8): This outcome was reported by three studies (Baroutis 2003; Fujii 2010; Gharehbaghi 2010). None of the individual studies or the meta‐analysis of these studies demonstrated any effect of surfactant preparation on risk of air leak syndrome (typical RR 2.55, 95% 0.98 to 6.68; typical RD 0.07, 95% CI 0.00 to 0.13; 3 studies and 255 infants). No heterogeneity was noted among the studies (I² = 0%).
Pulmonary hemorrhage (outcome 3.9): This outcome was reported by eight studies (Didzar 2012; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies showed any effect of surfactant preparation on the risk of pulmonary hemorrhage except for Karadag 2014 which showed increased risk of pulmonary hemorrhage with beractant . The meta‐analysis of these studies demonstrated no significant effect of surfactant preparation on risk of pulmonary hemorrhage (typical RR 1.28, 95% CI 0.81 to 2.02; typical RD 0.02, 95% CI −0.02 to 0.06; 8 studies and 871 infants). No heterogeneity was noted among the studies (I² = 0%) and the quality of evidence was graded as low.
Patent ductus arteriosus (PDA) (that has been treated with cyclo‐oxygenase inhibitor or surgery) (outcome 3.10): This outcome was reported by three studies (Fujii 2010; Halahakoon 1999; Malloy 2005). Both Fujii 20100 and Malloy 2005 showed a significant increase in the risk of PDA requiring treatment with cyclo‐oxygenase inhibitor [Fujii 2010 (RR 2.20, 95% CI 1.18 to 4.09) and Malloy 2005 (RR 2.60, 95% CI 1.06 to 6.36)] with modified bovine minced lung surfactant while Halahakoon 1999 did not show such effect.
The meta‐analysis of these trials demonstrated a significant increase in risk of PDA requiring treatment with cyclo‐oxygenase inhibitor with modified bovine minced lung surfactant compared with porcine minced lung surfactant (typical RR 1.86, 95% 1.28 to 2.70; typical RD 0.28, 95% CI 0.13 to 0.43; NNTH 4, 95% CI 2 to 8). Moderate heterogeneity was noted (I² = 65%) which was statistically not significant
Culture‐confirmed bacterial sepsis (outcome 3.11): This outcome was reported by six studies (Didzar 2012; Gharehbaghi 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Speer 1995). Neither the individual studies nor the meta‐analysis of these studies demonstrated any significant effect of surfactant preparation on culture‐confirmed bacterial sepsis (typical RR 1.13, 95% CI 0.87 to 1.46; typical RD 0.03, 95% CI −0.04 to 0.10; 6 studies and 526 infants). No heterogeneity was noted among the studies (I² = 13%).
Culture‐confirmed fungal sepsis: This outcome was not reported.
Necrotizing enterocolitis (any stage) (Bell 1978) (outcome 3.12): This outcome was reported by seven studies (Baroutis 2003; Didzar 2012; Fujii 2010; Halahakoon 1999; Karadag 2014; Malloy 2005; Ramanathan 2004). None of the individual studies or meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the risk of NEC (typical RR 0.82, 95% 0.50 to 1.33; typical RD −0.02, 95% CI −0.06 to 0.02; 7 studies and 701 infants). No heterogeneity was noted among the studies (I² = 0%).
Periventricular leukomalacia (in infants who received neuroimaging). (outcome 3.13): This outcome was reported by one study (Fujii 2010). This study reported no significant effect of surfactant preparation on the risk of PVL (RR 1.04, 95% 0.07 to 15.72; one study and 47 infants).
Retinopathy of prematurity in subjects examined.
‐ ROP all stages (outcome 3.14): This outcome was reported by three studies (Baroutis 2003; Gharehbaghi 2010; Halahakoon 1999). None of the individual studies or the meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the risk of ROP (all stages) (typical RR 0.60, 95% CI 0.29 to 1.26; typical RD −0.06, 95% CI −0.13 to 0.02; 3 studies and 230 infants). No heterogeneity was noted among the studies (I² = 0%).
‐ ROP stage 3 or greater (outcome 3.15): This outcome was reported by four studies (Fujii 2010; Halahakoon 1999; Karadag 2014; Malloy 2005). None of the studies or the meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the risk of ROP stage 3 or greater (typical RR 1.02, 95% CI 0.57 to 1.80; typical RD 0.00, 95% CI −0.09 to 0.09; 4 studies and 444 infants). Low heterogeneity was noted among the studies (I² = 25%).
Intraventricular hemorrhage (in infants who received neuroimaging).
‐ Intraventricular hemorrhage (any grade) (outcome 3.16): This outcome was reported by four studies (Didzar 2012; Halahakoon 1999; Karadag 2014; Speer 1995). None of the individual studies or the meta‐analysis of these trials demonstrated any effect of surfactant preparation on risk of IVH (any grade) (typical RR 0.98, 95% CI 0.64 to 1.50; typical RD 0.00, 95% CI −0.09 to 0.08; 4 studies and 318 infants ). Low heterogeneity was noted among the studies (I² = 29%).
‐ Severe intraventricular hemorrhage (grade 3 or greater) (outcome 3.17): This outcome was reported by seven studies (Baroutis 2003; Fujii 2010; Gharehbaghi 2010; Halahakoon 1999; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies or the meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the risk of severe IVH (typical RR 1.28, 95% CI 0.83 to 1.97; typical RD 0.03, 95% CI −0.02 to 0.07; 7 studies and 705 infants). No heterogeneity was noted among the studies (I² = 0%) and the "quality of evidence" is very low.
Cerebral palsy at approximately two years' corrected age (as defined by the study authors): Not reported.
Neurodevelopmental outcome at approximately two years' corrected age: This outcome (acceptable range of assessment 18 months to 28 months corrected age) included: cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity), and hearing deficit (aided or < 60 dB on audiometric testing). The composite outcome 'neurodevelopmental impairment' was defined as having any one of the aforementioned deficits. None of the studies reported on this outcome.
Modified bovine minced lung surfactant extract (beractant or surfactant TA) vs. porcine lung lavage surfactant (Surfacen) (COMPARISON 4).
Prevention: No studies were identified.
Treatment: One study was identified (Sanchez‐Mendiola 2005).
Primary outcome
Mortality prior to hospital discharge (from any cause) (outcome 4.1):Sanchez‐Mendiola 2005 reported no effect of surfactant preparation on mortality prior to discharge (RR 1.10, 95% CI 0.60 to 1.99). The quality of evidence is low (95% CI including both no effect and appreciable harm, and total number of events not meeting optimal information size).
Secondary outcome
Pneumothorax (outcome 4.2):Sanchez‐Mendiola 2005 reported highly significant decrease in modified bovine minced lung surfactant compared with porcine lung lavage surfactant (Surfacen) on the risk of pneumothorax (RR 0.10, 95% CI 0.01 to 0.73; typical RD −0.39, 95% CI −0.61 to −0.16; NNTB 2, 95% CI 1 to 6).
No other comparison could be made for the remaining outcomes including neonatal mortality (mortality < 28 days of age) from any cause, chronic lung disease, oxygen requirement at 28 to 30 days of age, oxygen requirement at 36 weeks' postmenstrual age, death or chronic lung disease, received > one dose of surfactant, air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum), pulmonary hemorrhage, patent ductus arteriosus (PDA) (that has been treated with cyclo‐oxygenase inhibitor or surgery), culture‐confirmed bacterial sepsis, culture‐confirmed fungal sepsis, necrotizing enterocolitis (defined as Bell Stage II or greater), periventricular leukomalacia (in infants who received neuroimaging), retinopathy of prematurity in subjects examined, intraventricular hemorrhage (in infants who received neuroimaging), cerebral palsy at approximately two years corrected age (as defined by the study authors), and neurodevelopmental outcome at approximately two years corrected age.
Porcine minced lung surfactant extract (poractant alfa) vs. porcine lung lavage surfactant (Surfacen) .
Prevention: No studies were identified.
Treatment: No studies were identified.
SUBGROUP ANALYSES
Subgroup analyses based on gestational age and surfactant dosing schedule (single dose, multiple dose) were not performed because of lack of sufficient data. The subgroup analysis based on treatment strategy (prevention vs. treatment of established disease) was performed (as noted above) for bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract; however, subgroup analysis based on disease severity could not be performed because of lack of data.
Subgroup analysis based on initial surfactant dosing (initial dose ≤ 100 mg/kg of porcine minced lung and initial dose > 100 mg/kg of porcine minced lung compared with beractant 100 mg/kg) was performed for modified bovine minced lung vs. porcine minced lung surfactant.
Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage)(COMPARISON 5)
Primary outcome measures:
Neonatal mortality (mortality < 28 days of age) from any cause (outcome 5.1): This outcome was reported by two studies (Halahakoon 1999 and Ramanathan 2004). In this subgroup analysis based on initial surfactant dosing, no significant difference was noted on risk of neonatal mortality for initial dose ≤ 100 mg/kg porcine minced lung (typical RR 1.20, 95% CI 0.55 to 2.62; typical RD 0.02; 95% CI −0.06 to 0.09; 2 studies and 221 infants) or initial dose > 100 mg/kg porcine minced lung (typical RR 2.69, 95% 0.74 to 9.86; 1 study and 197 infants) compared with 100 mg/kg beractant.
Mortality prior to hospital discharge (from any cause) (outcome 5.2): Three studies reported the effect on mortality prior to discharge (from any cause) forinitial dose ≤ 100 mg/kg porcine minced lung surfactant (Baroutis 2003; Halahakoon 1999; Ramanathan 2004). None of the individual studies or the meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the mortality prior to discharge at initial dose ≤ 100 mg/kg porcine minced lung surfactant compared with 100 mg/kg beractant (typical RR 1.10, 95% CI 0.61 to 1.96; typical RD 0.01, 95% CI −0.07 to 0.10, 3 studies and 255 infants). No heterogeneity was noted among the studies (I² = 0%).
In the subgroup analysis for initial dose based on initial dose >100 mg/kg of porcine minced lung surfactant, seven studies reported the risk of mortality prior to discharge (Didzar 2012; Fujii 2010; Gharehbaghi 2010; Karadag 2014; Malloy 2005; Ramanathan 2004). None of the individual studies showed any significant effect of higher initial dose of porcine minced lung surfactant on this outcome. However, in the meta‐analysis of these trials, there was a significantly higher mortality prior to discharge in the modified bovine minced lung surfactant (beractant 100mg/kg) compared with those receiving initial dose > 100 mg/kg of porcine minced lung surfactant (typical RR 1.62, 95% 1.11 to 2.38; typical RD 0.06, 95% CI 0.01 to 0.11; NNTH 16, 95% CI 9 to 100). There is low heterogeneity among the studies (I² = 30%).
Chronic lung disease:
‐ Oxygen requirement at 28 to 30 days of age (outcome 5.3): This outcome was reported by two studies (Halahakoon 1999;Ramanathan 2004). In the subgroup analysis based on initial surfactant dosing, no significant difference was noted on risk of chronic lung disease at 28 to 30 days of age forinitial dose ≤ 100 mg/kg of porcine minced lung surfactant (typical RR 0.96, 95% CI 0.73 to 1.25; typical RD −0.02, 95% CI −0.15 to 0.11; 2 studies and 221 infants) or > 100 mg/kg porcine minced lung surfactant (RR 1.01, 95% 0.76 to 1.34; RD 0.01, 95% CI −0.13 to 0.14; one study and 197 infants).
‐ Oxygen requirement at 36 weeks' postmenstrual age (outcome 5.4): Three studies (Baroutis 2003; Halahakoon 1999; Ramanathan 2004) reported the effect on oxygen requirement at 36 weeks' postmenstrual age for initial dose ≤ 100 mg/kg of porcine minced lung surfactant. None of the individual studies or the meta‐analysis of these trials demonstrated any significant effect of surfactant preparation on the risk of oxygen requirement at 36 weeks' postmenstrual age at initial dose ≤ 100 mg/kg of porcine minced lung surfactant (typical RR 0.94, 95% CI 0.65 to 1.37; typical RD −0.02, 95% CI −0.13 to 0.09; 3 studies and 255 infants). No heterogeneity was noted among the studies (I² = 0%).
In the subgroup analysis for initial dose > 100 mg/kg of porcine minced lung surfactant dose, six studies reported the risk of oxygen requirement at 36 weeks' postmenstrual age (Fujii 2010; Gharehbaghi 2010; Karadag 2014; Malloy 2005; Ramanathan 2004; Speer 1995). None of the individual studies showed any significant effect of higher initial dose of porcine minced lung surfactant on this outcome. The meta‐analysis of these trials demonstrated no significant effect of initial dose > 100 mg/kg of porcine minced lung surfactant compared with 100mg/kg beractant (typical RR 1.08, 95% CI 0.84 to 1.38; typical RD 0.02, 95% CI −0.05 to 0.09; 6 studies and 608 infants) on the risk of oxygen requirement at 36 weeks' postmenstrual age. No heterogeneity was noted among the studies (I² = 0%).
Death or chronic lung disease:
‐ Death or oxygen requirement at 36 weeks' postmenstrual age (outcome 5.5): One study (Ramanathan 2004) reported the effect of initial dose ≤100 mg/kg of porcine minced lung surfactant on this outcome. In the subgroup analysis based on initial surfactant dosing, no significant difference was noted on risk of death or oxygen requirement at 36 weeks' postmenstrual age for initial dose ≤ 100 mg/kg of porcine minced lung surfactant (RR 1.04, 95% CI 0.76 to 1.43; RD 0.02, 95% CI −0.13 to 0.17; one study and 175 infants).
Three studies (Didzar 2012; Fujii 2010; Ramanathan 2004) reported the effect of initial dose > 100 mg/kg of porcine minced lung surfactant on the risk of death or oxygen requirement at 36 weeks' postmenstrual age. Didzar 2012 showed a significant increase in risk of death or oxygen requirement at 36 weeks' postmenstrual age for the modified bovine minced lung surfactant compared with initial dose > 100 mg/kg of porcine minced lung surfactant (RR 1.95, 95% CI 1.11 to 3.42), while the other two trials (Fujii 2010; Ramanathan 2004) did not show such difference. The meta‐analysis of these three trials showed a significant increase in risk of death or oxygen requirement at 36 weeks' postmenstrual age for the modified bovine minced lung surfactant (100 mg/kg beractant) compared with initial dose > 100 mg/kg of porcine minced lung surfactant (typical RR 1.39, 95% 1.08 to 1.79; typical RD 0.13, 95% 0.03 to 0.23; NNTH 7, 95% CI 4 to 33). Low heterogeneity was noted among the studies (I² = 23%).
Discussion
A wide variety of surfactant products have been formulated and studied in clinical trials (Halliday 2008). Animal‐derived surfactants seem to confer an advantage over first generation synthetic surfactants (Soll 2001). Differences exist among the commercially available animal‐derived surfactant products with respect to their source of extraction (bovine or porcine), method of extraction (minced or lavage), composition (viscosity, phospholipid content, amount of surfactant protein (SP B&C)), and dosing volume (Ramanathan 2009). A variety of animal‐derived surfactant products have been studied around the world in randomized or quasi‐randomized studies. Widely used and well‐studied surfactants included the following: bovine lung lavage surfactant extracts (calfactant, bovactant and BLES); modified bovine minced lung surfactant extracts (beractant and Surfactant TA); and porcine minced lung surfactant extract (poractant alfa). However, whether there are clinically important differences among these animal‐derived surfactant products has not been determined unequivocally.
This review compared the effect of administration of different animal‐derived surfactant extracts on the risk of mortality, chronic lung disease, and other morbidities associated with prematurity in preterm infants at risk for or having RDS. Sixteen randomized or quasi‐randomized studies eligible for inclusion compared bovine lung lavage surfactant extract, modified bovine minced lung surfactant extract, porcine minced lung surfactant, and porcine lung lavage surfactant. Only two prevention studies comparing bovine lung lavage surfactant extract with modified bovine minced lung surfactant extract were identified. Seven treatment studies compared modified bovine minced lung surfactant extract with bovine lung lavage surfactant extract, and seven treatment studies compared modified bovine minced lung surfactant extract with porcine minced lung surfactant extract. Most of the studies were of small sample size with the exception of Bloom 1997, Bloom 2005 and Ramanathan 2004. They varied with respect to range of gestational age (24 to 37 wk), birth weight (400 to 2000 g) and age at enrollment of the included infants. There were differences in the timing of surfactant administration (variable or not reported) and dosing schedule. Most studies reported the primary outcome (mortality and chronic lung disease) and complications associated with prematurity. None of the studies reported any long‐term neurodevelopmental outcome.
This meta‐analysis demonstrates a significant increase in the risk of mortality prior to discharge, death or oxygen requirement at 36 weeks' postmenstrual age, receiving more than one dose of surfactant, and PDA requiring treatment with modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract. In the subgroup analysis based on the initial dose of poractant alfa used, the improvement in risk of pre‐discharge mortality and risk of death or oxygen requirement at 36 weeks' postmenstrual age was observed in the sub‐group of infants receiving an initial dose of more than 100 mg/kg of porcine minced lung surfactant but not in the sub‐group receiving an initial dose of less than 100mg/kg of porcine minced lung surfactant (all infants in the bovine surfactant comparison arm received the same dose of bovine surfactant).
The meta‐analysis of studies comparing modified bovine minced lung surfactant extract with bovine lung lavage surfactant extract did not demonstrate significant differences in the primary outcomes. No meaningful comparison could be made for bovine lung lavage surfactant extract versus porcine lung lavage surfactant; bovine lung lavage surfactant extract versus porcine minced lung surfactant extract; or porcine minced lung surfactant extract versus porcine lung lavage surfactant extract because of the lack of adequate studies.
In summary, significant differences were noted between modified bovine minced lung surfactant extract (beractant) and porcine minced lung surfactant extract (poractant alfa), but not between other comparison groups or subgroups. The clinically meaningful differences include increase in the risk of mortality prior to discharge, risk of death or oxygen requirement at 36 weeks' postmenstrual age, risk of PDA requiring treatment, and risk of "receiving > 1 dose of surfactant" with modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract. The quality of evidence is moderate for primary outcomes because of imprecision.
The improved outcome noted with porcine minced lung surfactant extract compared with modified bovine minced lung surfactant extract could be either due to differences in biophysical/biochemical composition of porcine minced lung surfactant extract or from the higher phospholipid content associated with higher initial dose of porcine surfactant. The subgroup analysis based on initial dose of surfactant showed improvement in outcomes limited to the use of higher initial phospholipid/kg dose of porcine minced lung surfactant extract. This suggests possible dose effect from higher phospholipid administered with higher initial dose of porcine minced lung extract. However, our inability to detect any improvement with the lower initial dose of porcine minced lung surfactant extract from a Type II error (smaller sample size) cannot be ruled out. Also, in absence of adequately powered studies comparing dose equivalent porcine and modified bovine lung surfactant, the effect of dose or biophysical/biochemical profile of surfactant obtained from different animal sources on the clinical outcome cannot be determined with certainty.
A major limitation of this review was absence of dose equivalent trials comparing animal‐derived surfactants from different sources or by different method of extractions. Another limitation was the small sample size for individual studies (except for Bloom 1997 and Bloom 2005) and the pooled data which was not powered to detect significant differences for the primary outcomes. Lack of blinding in majority of the studies might have introduced potential for bias and affected the results.
Insufficient data are available to demonstrate any cost benefit with use of one animal‐derived surfactant over another. The outcome relevant to economic impact included in this review was "received > 1 dose surfactant." A significant increase in the outcome "receiving > 1 dose of surfactant" with modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract was observed in this review which is in agreement with the review by Singh 2011.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with other systematic reviews comparing animal‐derived surfactants. A recent systematic review of five studies comparing modified bovine minced lung surfactant extract with porcine minced lung surfactant found decrease in mortality prior to discharge with high dose poractant compared to beractant (Singh 2011), which is similar to the results of the current review. A meta‐analysis of five randomized trials by Halliday 2008 also supports the beneficial effect of higher initial dose of porcine minced lung surfactant extract compared with modified minced lung surfactant. Other reviews limited to comparing specific animal‐derived surfactant products also showed differences in mortality favoring porcine minced lung surfactant, but no significant difference between modified bovine minced lung surfactant extract and bovine lung lavage surfactant (Ramanathan 2009; Moya 2009).

Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.2 Mortality prior to discharge.
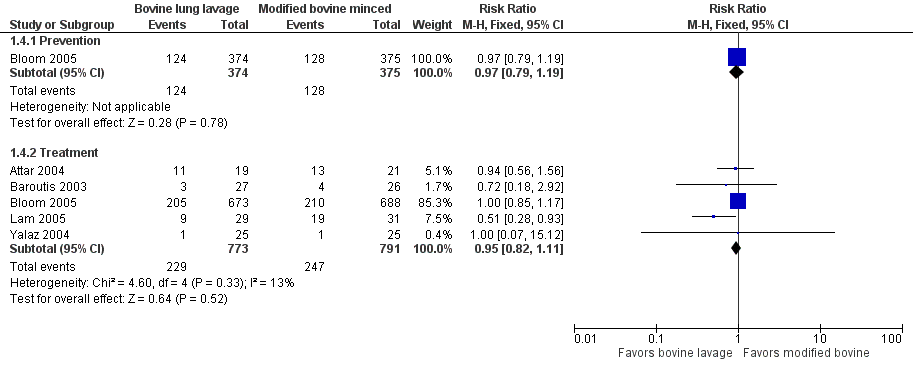
Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.4 Oxygen requirement at 36 weeks postmenstrual age (all infants).
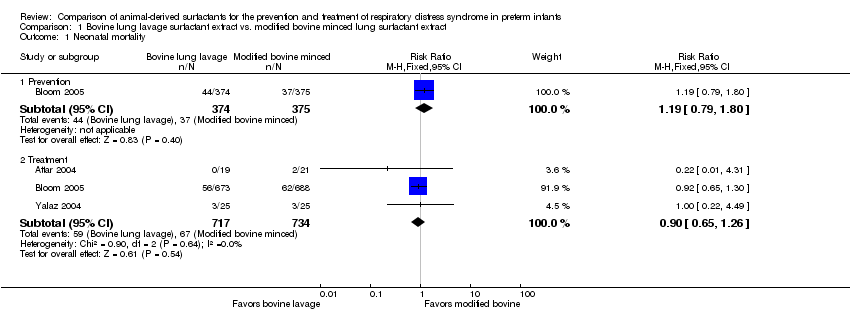
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 1 Neonatal mortality.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 2 Mortality prior to discharge.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 3 Oxygen requirement at 28 to 30 days of age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 5 Death or oxygen requirement at 28 to 30 days of age.
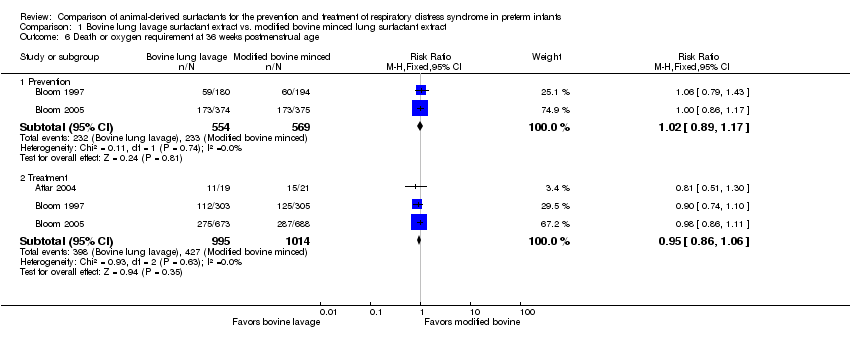
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 6 Death or oxygen requirement at 36 weeks postmenstrual age.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 7 Received > one dose of surfactant.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 8 Pneumothorax.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 9 Air leak syndromes.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 10 Pulmonary hemorrhage.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 11 Treated patent ductus arteriosus (PDA).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 12 Culture‐confirmed bacterial sepsis.
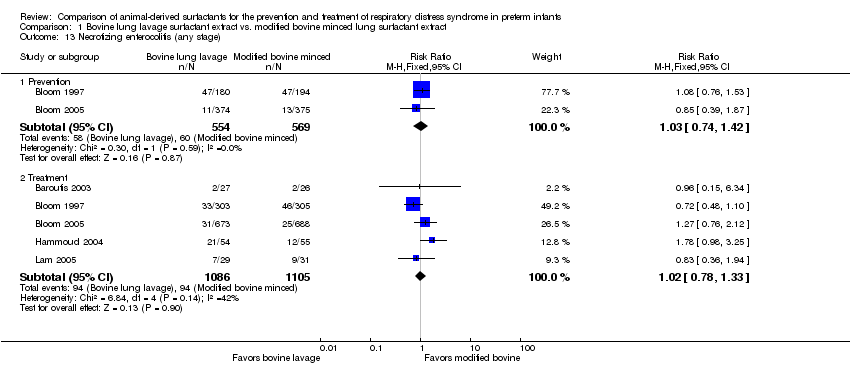
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 13 Necrotizing enterocolitis (any stage).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 14 Periventricular leukomalacia.
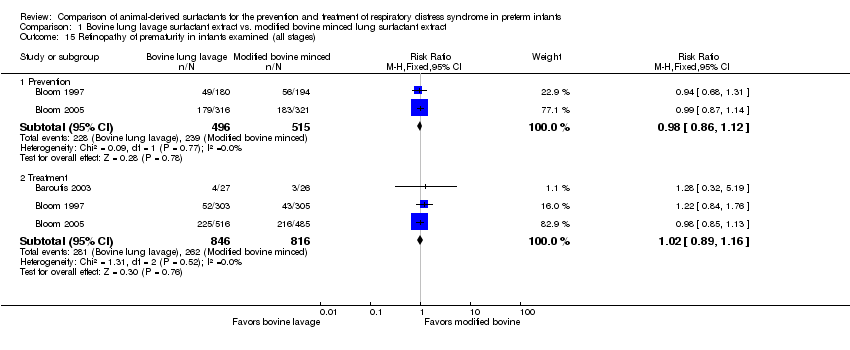
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 15 Retinopathy of prematurity in infants examined (all stages).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 18 Severe IVH in infants receiving neuroimaging.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 1 Mortality prior to discharge.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 2 Oxygen requirement at 36 weeks postmenstrual age.
Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 3 Air leak syndromes.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 4 Necrotizing enterocolitis (any stage).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 5 Retinopathy of prematurity in infants examined (all stages).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 6 Severe IVH.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 1 Neonatal mortality.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 2 Mortality prior to discharge.
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
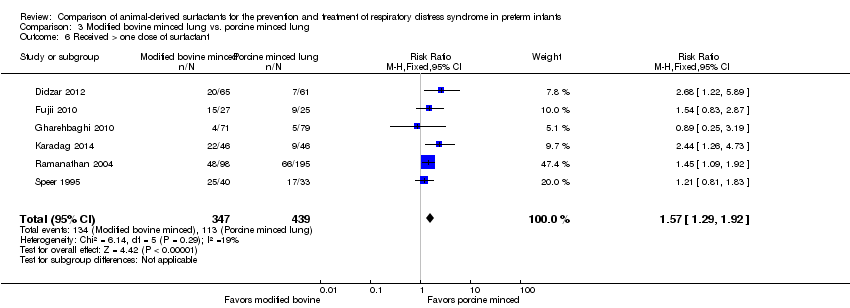
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 6 Received > one dose of surfactant.
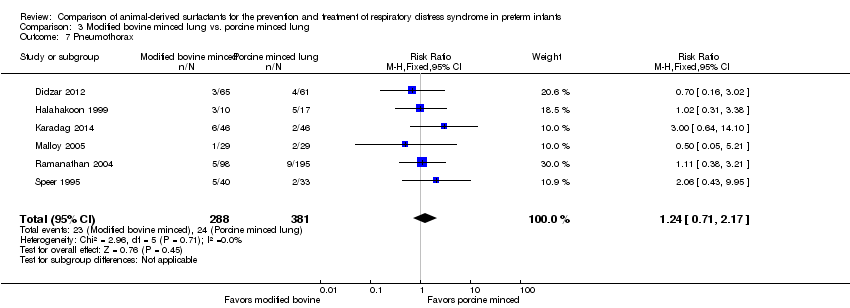
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 7 Pneumothorax.
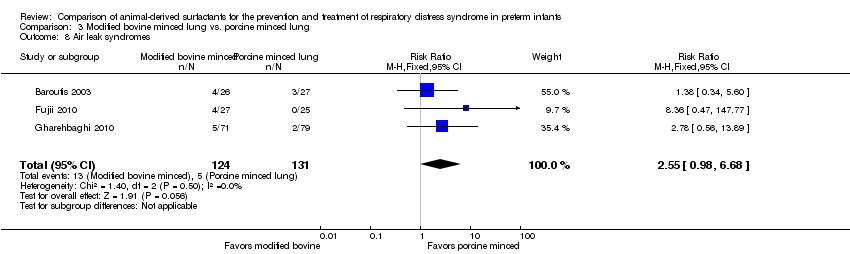
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 8 Air leak syndromes.
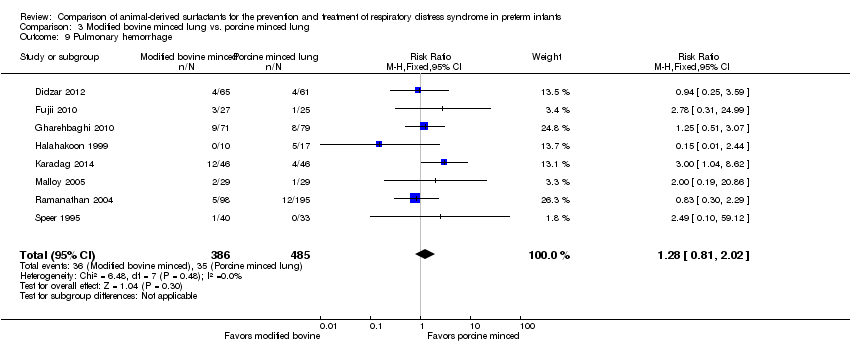
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 9 Pulmonary hemorrhage.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 10 Treated patent ductus arteriosus (PDA).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 11 Culture‐confirmed bacterial sepsis.
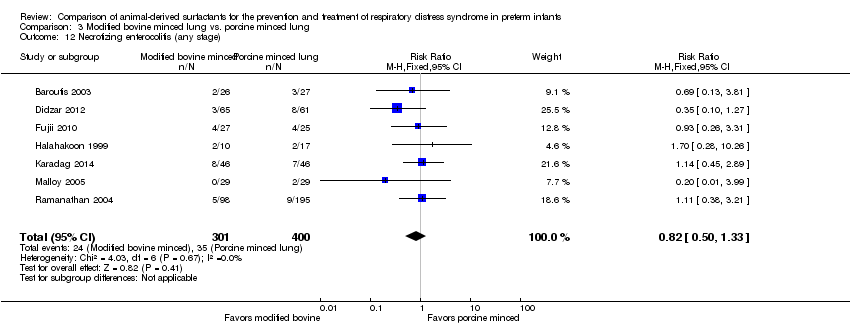
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 12 Necrotizing enterocolitis (any stage).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 13 Periventricular leukomalacia.
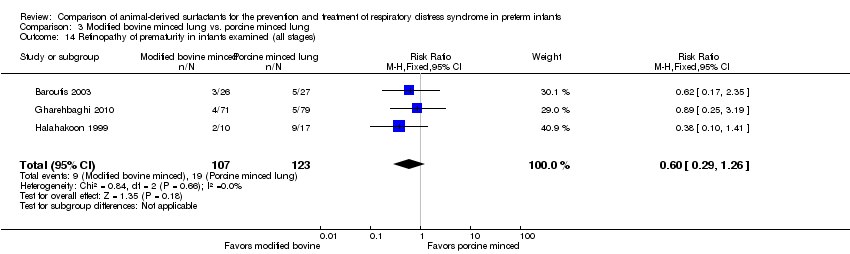
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 14 Retinopathy of prematurity in infants examined (all stages).
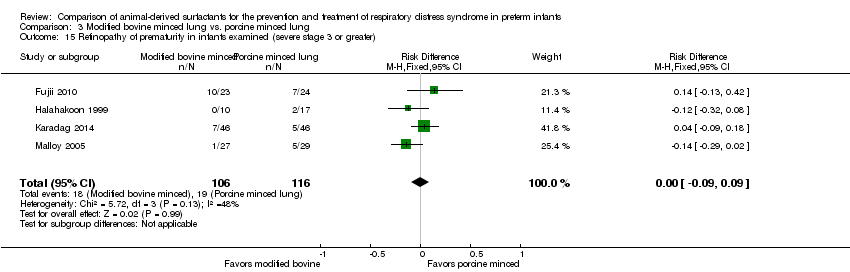
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 16 Intraventricular hemorrhage (all grades).
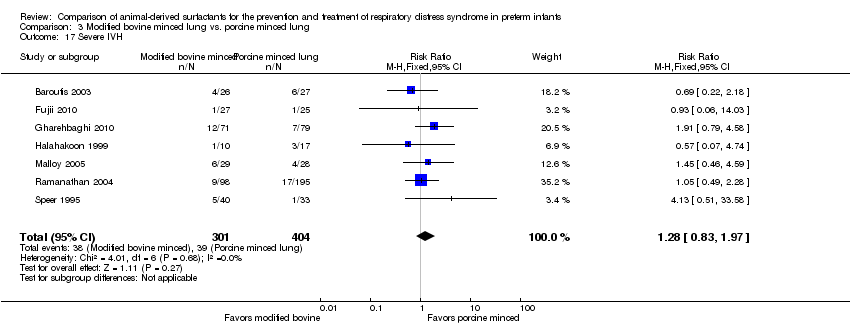
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 17 Severe IVH.

Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 1 Mortality prior to discharge.
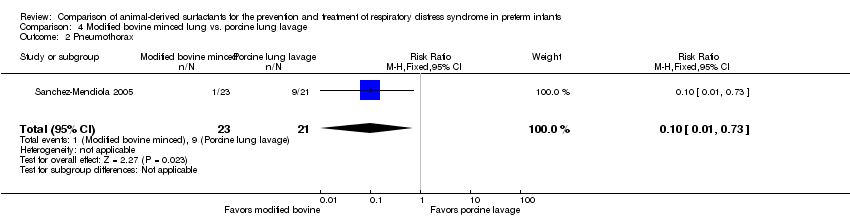
Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 2 Pneumothorax.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 1 Neonatal mortality.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 2 Mortality prior to discharge.
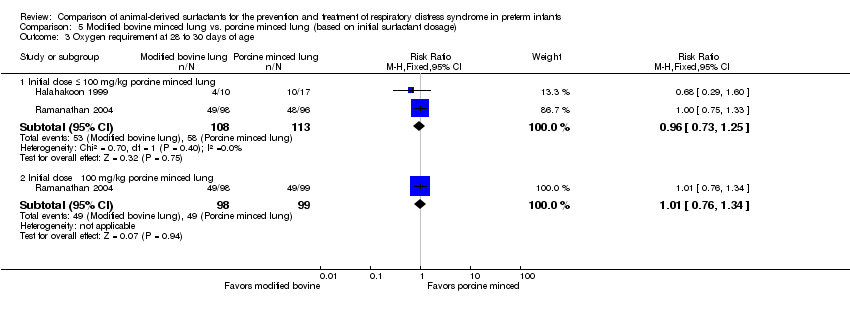
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.
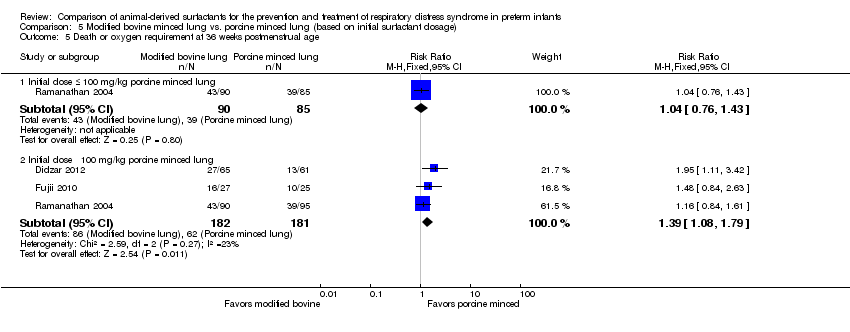
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for prevention of RDS (Comparision 1: Prevention studies) | ||||||
| Patient or population: Preterm infants for prevention of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge (from any cause) | 107 per 1000 | 133 per 1000 | RR 1.24 | 1123 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and and the total number of events does not meet the optimal information size (OIS). |
| Oxygen requirement at 36 weeks' postmenstrual age | 341 per 1000 | 331 per 1000 | RR 0.97 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 409 per 1000 | 418 per 1000 | RR 1.02 | 1133 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the no effect. The total number of events meets the OIS |
| Pneumothorax. | 67 per 1000 | 51 per 1000 | RR 0.76 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Pulmonary hemorrhage | 44 per 1000 | 63 per 1000 | RR 1.44 | 1123 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Severe IVH in infants receiving neuroimaging | 87 per 1000 | 112 per 1000 | RR 1.28 | 1087 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) 95% CI includes both no effect and appreciable harm.2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 2000). |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefit, no effect and appreciable harm and the total number of events does not meet the optimal information size 2 95% CI includes benefit, no effect and appreciable harm 3 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 3000 4 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 2000 | ||||||
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 131 per 1000 | 128 per 1000 | RR 0.98 | 2231 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the OIS. |
| Oxygen requirement at 36 weeks' postmenstrual age (all infants) | 312 per 1000 | 297 per 1000 | RR 0.95 | 1564 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 421 per 1000 | 400 per 1000 | RR 0.95 | 2009 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Pneumothorax | 73 per 1000 | 83 per 1000 | RR 1.14 | 2224 | ⨁⨁◯◯ | Downgraded two levels due to: 1) Serious imprecision (95% CI includes both no effect and appreciable harm). 2) Inconsistency: Unexplained heterogeneity, with point estimates widely different; 95% CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) |
| Pulmonary hemorrhage | 44 per 1000 | 48 per 1000 | RR 1.08 | 2138 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Severe IVH in infants receiving neuroimaging | 125 per 1000 | 108 per 1000 | RR 0.86 | 2040 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes benefits, no effect and appreciable harm). The optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefits, no effect and appreciable harm, and the total number of events does not meet the OIS 2 Unexplained heterogeneity, with point estimates widely different and CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) 3 95% CI of the pooled effect crosses 1 and the optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 | ||||||
| Bovine lung lavage surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 185 per 1000 | 259 per 1000 | RR 1.40 | 54 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Oxygen requirement at 36 weeks' postmenstrual age | 148 per 1000 | 111 per 1000 | RR 0.75 | 54 | ⨁⨁◯◯ | Downgraded two levels due to: 1. Serious imprecision (95% CI includes both no effect and appreciable harm). 2. Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | see comments | see comments | Not reported in any of the studies | |||
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | 222 per 1000 | 184 per 1000 | RR 0.83 | 54 | ⨁◯◯◯ | Downgraded three levels due to: 1. potential risk of bias (lack of blinding of outcome assessment) 2. very serious imprecision: (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 2 We downgraded because lack of blinding of patients, providers and blinding of outcome assessment 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 113 per 1000 | 162 per 1000 | RR 1.44 | 901 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Oxygen requirement at 36 weeks' postmenstrual age | 282 per 1000 | 293 per 1000 | RR 1.04 (0.83 to 1.31) | 773 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 380 per 1000 | 494 per 1000 | RR 1.30 | 448 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (total number of events does not meet the OIS) |
| Pneumothorax | 63 per 1000 | 78 per 1000 | RR 1.24 | 669 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Pulmonary hemorrhage | 72 per 1000 | 92 per 1000 | RR 1.28 | 871 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Severe intraventricular hemorrhage in infants who received neuroimaging | 97 per 1000 | 124 per 1000 | RR 1.28 | 705 | ⨁◯◯◯ | Downgraded three levels due to: 1. Potential risk of bias and 2. serious imprecision: 1) the 95% CI includes both no effect and appreciable harm; and 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The total number of events does not meet the OIS 2 95% CI includes benefit, no effect and appreciable harm. Total number of events does not meet the optimal information size. 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 5000 4 Studies that carried large weight for the overall effect estimate are classified as high or unclear risk of bias due to lack of blinding in patients, and outcome assessment 5 95% CI of the pooled effect widely crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 3000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine lung lavage surfactant in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine lung lavage surfactant | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 476 per 1000 | 524 per 1000 | RR 1.10 | 44 | ⨁⨁◯◯ | Downgraded two levels due to serious imprecision: 1) The 95% CI includes both no effect and appreciable harm. 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 700). |
| Oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | 429 per 1000 | 43 per 1000 | RR 0.10 | 44 | ⨁⨁⨁⨁ | |
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | see comments | see comments | Not reported in any of the studies | |||
| Neurodevelopmental outcome at approximately two years corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95 % CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 700 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.80] |
| 1.2 Treatment | 3 | 1451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 2 Mortality prior to discharge Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 2.2 Treatment | 6 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 3 Oxygen requirement at 28 to 30 days of age (all infants) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.2 Treatment | 3 | 1510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.21] |
| 4 Oxygen requirement at 36 weeks postmenstrual age (all infants) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.19] |
| 4.2 Treatment | 5 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.11] |
| 5 Death or oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
| 5.2 Treatment | 2 | 1401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 6 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 6.2 Treatment | 3 | 2009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 7 Received > one dose of surfactant Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7.2 Treatment | 5 | 2178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 8 Pneumothorax Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.36] |
| 8.2 Treatment | 6 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.51] |
| 9 Air leak syndromes Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 9.2 Treatment | 3 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 10 Pulmonary hemorrhage Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.88, 2.39] |
| 10.2 Treatment | 4 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.59] |
| 11 Treated patent ductus arteriosus (PDA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.34] |
| 12 Culture‐confirmed bacterial sepsis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 12.2 Treatment | 6 | 2228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 13 Necrotizing enterocolitis (any stage) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 13.2 Treatment | 5 | 2191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 14 Periventricular leukomalacia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.26] |
| 14.2 Treatment | 1 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.59, 1.73] |
| 15 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Prevention | 2 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 15.2 Treatment | 3 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Prevention | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 16.2 Treatment | 1 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 17.2 Treatment | 3 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 18 Severe IVH in infants receiving neuroimaging Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Prevention | 2 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.89, 1.83] |
| 18.2 Treatment | 5 | 2040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.51, 3.87] |
| 2 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.04] |
| 3 Air leak syndromes Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 4 Necrotizing enterocolitis (any stage) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 5 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.24, 2.66] |
| 6 Severe IVH Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.29, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.72, 3.07] |
| 2 Mortality prior to discharge Show forest plot | 9 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 2.00] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.64] |
| 6 Received > one dose of surfactant Show forest plot | 6 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.29, 1.92] |
| 7 Pneumothorax Show forest plot | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| 8 Air leak syndromes Show forest plot | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.98, 6.68] |
| 9 Pulmonary hemorrhage Show forest plot | 8 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| 10 Treated patent ductus arteriosus (PDA) Show forest plot | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.28, 2.70] |
| 11 Culture‐confirmed bacterial sepsis Show forest plot | 6 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| 12 Necrotizing enterocolitis (any stage) Show forest plot | 7 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.33] |
| 13 Periventricular leukomalacia Show forest plot | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.72] |
| 14 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 4 | 222 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| 16 Intraventricular hemorrhage (all grades) Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.50] |
| 17 Severe IVH Show forest plot | 7 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.83, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.60, 1.99] |
| 2 Pneumothorax Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.62] |
| 1.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.74, 9.86] |
| 2 Mortality prior to discharge Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.96] |
| 2.2 Initial dose ˃ 100 mg/kg porcine minced lung | 7 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.11, 2.38] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.25] |
| 3.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.37] |
| 4.2 Initial dose ˃ 100 mg/kg porcine minced lung | 6 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Initial dose ≤ 100 mg/kg porcine minced lung | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.76, 1.43] |
| 5.2 Initial dose ˃ 100 mg/kg porcine minced lung | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.08, 1.79] |

